Abstract
Fertilization is a multistep process requiring spermatozoa with unique cellular structures and numerous germ cell-specific molecules that function in the various steps. In the highly coordinated process of male germ cell development, RNA splicing and polyadenylation help regulate gene expression to assure formation of functional spermatozoa. Male germ cells express tauCstF-64 (Cstf2t gene product), a paralog of the X-linked CstF-64 protein that supports polyadenylation in most somatic cells. We previously showed that loss of tauCstF-64 causes male infertility because of major defects in mouse spermatogenesis. Surprisingly, although Cstf2t−/− males produce very few recognizable spermatozoa, some of the spermatozoa produced are motile. This led us to ask whether these Cstf2t−/− sperm were fertile. A motile cell-enriched population of spermatozoa from Cstf2t-null males dispersed cumulus cells of cumulus-oocyte complexes normally. However, motile spermatozoa from Cstf2t-null males failed to fertilize cumulus-intact mouse eggs in vitro. In addition, sperm adhesion to the zona pellucida (ZP) of cumulus-free eggs was significantly decreased, indicating tauCstF-64 is required for production of spermatozoa capable of ZP interaction. Acrosomal proteins involved in sperm-ZP recognition, including zonadhesin, proacrosin, SPAM1/PH-20, and ZP3R/sp56, were normally distributed in the apical head of Cstf2t−/− spermatozoa. We conclude that tauCstF-64 is required not only for expression of genes involved in morphological differentiation of spermatids but also for genes having products that function during interaction of motile spermatozoa with eggs. To our knowledge, this is the first demonstration that a gene involved in polyadenylation has a negative consequence on sperm-ZP adhesion.
Keywords: fertilization, gamete biology, infertility, oligoasthe-noteratozoospermia, polyadenylation, sperm, sperm-egg adhesion
Motile spermatozoa from mice deficient for a gene involved in polyadenylation: Cstf2t−/−; these cells possessed normal ability to disperse cumulus cells, but are unable to interact with the zona pellucida of a mature egg in vitro.
INTRODUCTION
Adhesion of sperm cells to the egg's extracellular envelope is an important and essential early event in fertilization [1]. Failure in this first physical contact between sperm cells and a mature oocyte will result in infertility because of the inability to conceive. The number of clinical consultations related to infertility is rising year after year worldwide. Internationally, procedures involving assisted reproductive technology have increased constantly, reaching a 25% increase between 2000 and 2002 [2]. Idiopathic male infertility is a growing condition among the human population, but to our knowledge, no sperm protein has been identified as a cause of this medical condition. In the last decade, several sperm proteins have been proposed as sperm-egg adhesion molecules, including zonadhesin, proacrosin, SPAM1/PH-20, galactosyltransferase, ZP3R/sp56, and others [3]. Each plays a role in fertilization by interacting with zona pellucida (ZP) glycoproteins, though none by itself has been shown to be essential for fertilization [4, 5].
Cstf2t encodes τCstF-64 (official symbol CSTF2T) [6–8], the testis-expressed paralog of the CstF-64 (official symbol CSTF2) RNA-binding protein (gene name Cstf2) that is important for the regulation of eukaryotic mRNA polyadenylation and 3′ end formation. Surprisingly, polyadenylation in male germ cells appears to be different than that in somatic cells: 1) The upstream hexamer AAUAAA, which is important for polyadenylation in somatic cell mRNAs, is less represented in germ cell mRNAs [6, 9, 10]; 2) the use of alternative polyadenylation sites is more frequent in germ cell mRNAs [11, 12]; and 3) downstream sequence elements that are evident in somatic cell mRNAs disappear as spermatogenesis progresses [10]. Together, these differences result in pervasive changes in germ cell mRNA 3′ ends [10, 12]. Possibly contributing to these differences, several tissue-specific nuclear polyadenylation factors are expressed in testis [13–15], including τCstF-64 [6–8]. Both CstF-64 and τCstF-64 are expressed in germ cells, but the patterns of expression are different in mice: CstF-64 is expressed in germ cells before and after meiosis but is absent during pachynema [6] because of male sex chromosome inactivation [16]. Conversely, τCstF-64 expression commences in pachytene spermatocytes and continues through meiosis until halting in postmeiotic elongating spermatids. Therefore, during most of pachynema, τCstF-64 is the only form of CstF-64 that is expressed, whereas in round and elongating spermatids, both CstF-64 and τCstF-64 are expressed.
The pattern of τCstF-64 expression suggested this protein supports polyadenylation and, therefore, gene expression during spermatogenesis. In transgenic mice lacking τCstF-64, produced by disrupting the Cstf2t gene, Cstf2ttm1Ccma/tm1Ccma (hereafter Cstf2t−/−), males showed an infertility phenotype resembling oligoasthenoteratozoospermia [17]; oligoasthenoteratozoospermia is characterized by low sperm counts, poor sperm motility, and a high number of abnormal cells [18]. Idiopathic oligoasthenoteratozoospermia is the most common cause of male subfertility [19]. Because of the complexity of mammalian fertilization, however, only a few mouse models mimicking human infertility have yet been produced. Therefore, Cstf2t−/− mice are appealing model animals for examining fertilization molecules in the context of male infertility. Despite the dramatic impairment of spermatogenesis in Cstf2t−/− mice, a number of motile spermatozoa were present in the epididymal lumen [17]. In addition, another type of cell was observed within cauda epididymal fluid from Cstf2t−/− mice, identified previously as round spermatids. Whereas our previous study determined in vivo male infertility, to our knowledge, spermatozoa from Cstf2t−/− males have not been examined in vitro to determine if infertility is related not only to a low number of sperm cells at the site of fertilization during in vivo mating but also to a physiologic malfunction during a specific step of fertilization.
Here, we assessed the function of motile spermatozoa from Cstf2t−/− males. These cells possessed normal ability to disperse cumulus cells in vitro but were unable to interact with the ZP of a mature egg. These results suggested that lack of τCstF-64 resulted in molecular defects that decrease sperm fertility. Together, these results support a model in which τCstF-64 functions during spermatogenesis by modulating both expression of developmental genes (e.g., transcription factors and signaling proteins) required for spermatid differentiation and expression of genes encoding critical fertilization proteins.
MATERIALS AND METHODS
Animal Studies
Animal studies were performed in accordance with protocols according to National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee. The Cstf2ttm1Ccma mice used in these studies were of mixed C57BL/6–129SvEv background. All genotyping was done as described previously [17].
Sperm Cell Preparation
Sperm cells from mouse epididymides (>60 days postpartum) were dispersed in PBS (10 mM phosphate and 137 mM NaCl; pH 7.4) after mincing the cauda and incubating at 37°C for a period of 15 min (two cauda into 2 ml). Following cell dispersion, sperm concentration was evaluated by using a cell-counter chamber.
Immunofluorescence
Sperm proteins were detected in methanol-fixed and permeabilized mouse spermatozoa using anti-zonadhesin D3p18 domain (1 μg/ml) affinity-purified antibodies [20], hyaluronidase antiserum (1:400) [21], proacrosin antiserum (1:500) [22], or anti-CST8 (CRES) (5 μg/ml) affinity-purified antibodies [23]. Anti-glutathione S-transferase (GST) [24] served as negative control. Bound antibodies were detected with a goat anti-rabbit immunoglobulin G conjugated to Alexa Flour 594 (3 μg/ml; Invitrogen). Acrosomes were labeled with biotinylated lectin from Arachis hypogaea (peanut agglutinin [PNA]; 0.1 mg/ml; L-6135; Sigma) and then detected with Alexa Flour 488-streptavidin conjugated (3 μg/ml; Invitrogen). Cells were viewed by epifluorescence and phase-contrast microscopy at 60× magnification.
Mouse In Vitro Fertilization and Sperm Capacitation
Mouse in vitro fertilization (IVF) was performed as previously described [20, 25]. Spermatozoa were preincubated under capacitating conditions (1.8 mM CaCl2, 25 mM NaHCO3, and 0.5% bovine serum albumin) for a period of 90 min under 5% CO2 at 37°C. Ovulated oocytes were obtained from supraovulated mice 13–15 h after i.p. injection of human chorionic gonadotropin (8 IU/mouse; C-1063; Sigma) and 63 h after synchronization by i.p. injection of pregnant mare serum gonadotropin (8 IU/mouse; G-4877; Sigma). Insemination was performed by adding approximately 5000 spermatozoa preincubated under capacitation conditions into a 50-μl drop containing 15–20 cumulus-intact oocytes under mineral oil followed by coincubation for 3 h (5% CO2 at 37°C). The remaining cumulus cells were removed by testicular hyaluronidase for 10 min (2.5 mg/ml; H-3506; Sigma), then washed in PBS. Eggs were fixed with 10% formalin in PBS, and fertilization was scored by either epifluorescence of Hoechst 33258-stained male and female pronuclei within the egg cytoplasm or by expulsion of the second polar body.
Cstf2t−/− Motile Sperm Enrichment
After dispersion of caudal spermatozoa from two mice in PBS (as described above), the sperm solution was loaded on a Percoll gradient and centrifuged in swinging bucket rotor. After centrifugation (14 min at 12 000 × g) the enriched sperm cells were recovered as a band at two thirds of the distance from of the bottom (80% Percoll); a higher band was harvested that was mainly constituted by round cells (52% Percoll). Percoll gradients were prepared as follows: Percoll solution (Sigma P-1644) made isosmotic using 10-fold concentrated PBS (nine parts of Percoll for one part of 10× PBS) was used to prepare 52% and 80% Percoll solution (diluted with 1× PBS) and then carefully layered into 2-ml phases. Once the sperm fraction was recovered, it was diluted 10-fold (v/v) with PBS (pH 7.4), followed by recentrifugation (10 min at 265 × g) and redilution to 2 × 107 cells/ml in IVF medium. Following motile sperm enrichment, motility was evaluated using the computer-assisted sperm motility (CASA) system (Hamilton-Thorne). Three independent experiments were performed using six mice in total, and motility evaluation by the CASA system used more than 600 spermatozoa and was performed in duplicates for each genotype.
Sperm-ZP Adhesion Assays
Sperm-ZP adhesion was assessed by gamete coincubation. The capacitation state of porcine spermatozoa was tested by the ability of the cells to undergo acrosome reaction after 3 h of incubation induced by calcium ionophore for 10 min (C-7522; A23187, 10 μM; Sigma) as previously shown [26, 27]. Mouse spermatozoa do not display ZP-adhesion on 2-cell embryos or on porcine eggs. Sperm-ZP adhesion is known as a species-specific process related to sperm capacitation [28]; thus, 2-cell embryo and porcine eggs were used as negative control.
Electron Microscopy
Electron microscopy was performed as described previously [29, 30], Epididymides were fixed with 4% formaldehyde and 0.25% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.4). Fixed cells were then rinsed in phosphate buffer, dehydrated through an ethanol series, and embedded in LR White Resin (Electron Microscopy Sciences). Thin sections (60–70 nm) were mounted on nickel grids, washed in PBS, and rinsed with water.
Statistical Analysis
Significance of differences in sperm-egg adhesion was determined by ANOVA using general linear model procedures (SAS software; Statistical Analysis System Institute Inc.). The Fisher protected least significant difference test was conducted when the main effect was significant (P < 0.01) to determine which treatments were significantly different.
RESULTS
Abnormal Spermatozoa Are Observed in Cstf2t−/− Mice
To investigate the biological consequence of loss of Cstf2t during spermatogenesis, spermatozoa were isolated from caudae epididymides of wild-type (Cstf2t+/+), heterozygous (Cstf2t+/−), and Cstf2t-null mice (Fig. 1). Few sperm cells were found with defective head shapes in Cstf2t+/− (Fig. 1B), and such cells were rare or absent in Cstf2t+/+ (Fig. 1A).
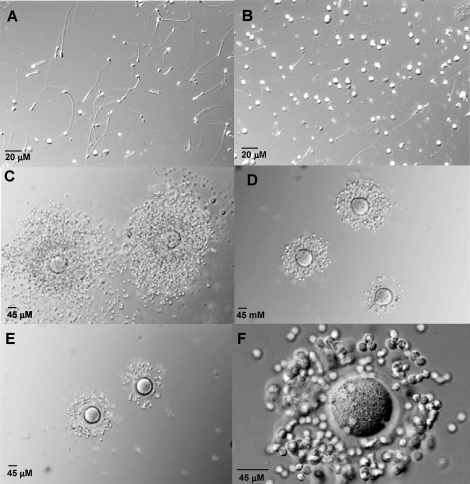
FIG. 1.
Overview of sperm population from Cstf2t+/+ (A), Cstf2t+/− (B), and Cstf2t−/− (C) mice. Caudal epididymal content is shown. The dilution factor of spermatozoa from Cstf2t+/+ and Cstf2t+/− was twofold greater than Cstf2t−/− cells. Note that round cells were always found in the Cstf2t−/− epididymal fluid but never in the Cstf2t+/+ sperm population. Sperm population from Cstf2t+/− and Cstf2t−/− mice displayed abnormal cells (arrowheads). Photomicrographs represented are in Normarsky interference microscopy at an original magnification of ×60.
When caudal spermatogenic cells from Cstf2t−/− mice (Fig. 1C) were compared to those from Cstf2t+/+ spermatozoa (Fig. 1A) or Cstf2t+/− (Fig. 1B) mice, cellular differences were more noticeable. Cstf2t−/− epididymides contained approximately one tenth as many recognizable spermatozoa as did wild-type mice [17]. Caudal fluid contained predominantly round cells (Fig. 1C). In examining the recognizable spermatozoa (identified by their elongated head shapes and tails), heads were often severely malformed in Cstf2t−/− mice. The hook shape characteristic of wild-type spermatozoa was generally absent, and the heads were more angular in shape (see Fig. 4B), suggesting that Cstf2t affected expression of genes that control normal development related to sperm head morphology.
FIG. 4.
Localization at acrosomal proteins (zonadhesin, proacrosin, SPAM1/PH-20, CST8 [CRES], and ZP3R/sp56) in spermatozoa. Using Cstf2t+/+ (A) and Cstf2t−/− (B) spermatogenic cells, the first column of the panels in A and B shows sperm cells from the cauda in phase contrast. The same sperm cells were labeled with an antibody to the protein described in the left margin (middle column), and the last column of images shows the same previous spermatozoa but labeled that time with PNA lectin. Note that each spermatozoon was positive for the PNA lectin and for the five sperm antibodies, with the exception of the negative control (GST or CREM, irrelevant antibody to sperm cells). Original magnification ×96.
Sperm Cells from Cstf2t−/− Mice Promote Normal Cumulus Cell Dispersion
In vitro fertilization was performed with motile Percoll-enriched spermatozoa from Cstf2t−/− mice on wild-type mouse eggs. No significant difference were observed between Cstf2t+/+ versus Cstf2t−/− on motility parameters as evaluated by the CASA system (Table 1). No 2-cell embryos or fertilized eggs were produced either in the presence or in the absence of cumulus cells, indicating that IVF was not successful (Table 2). Therefore, we next examined individual steps of IVF using spermatozoa from Cstf2t−/− mice. As a first step in normal gamete interactions, sperm cells cause dispersion of cumulus cells from the cumulus-oocyte complex (COC). Sperm fractions from Cstf2t−/− mice were enriched using Percoll gradients. Examination of spermatozoa (Fig. 2A) and rounds cell fractions (Fig. 2B) after Percoll enrichment from Cstf2t−/− epididymal fluid demonstrated that the procedure removed the majority of round cells from the sample (compare Fig. 2A to Fig. 2B).
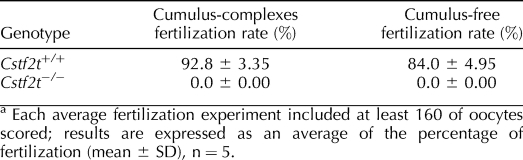
TABLE 1.
Motility parameters of sperm population enriched by Percoll procedure from Cstf2t+/+ and Cstf2t−/− mice evaluated by the CASA system.a
TABLE 2.
In vitro fertilization assay.a
FIG. 2.
Percoll-enriched cells from Cstf2t−/− mice disperse COCs. A) Spermatozoa from Cstf2t−/− caudal epididymal fluid depleted of round cells after Percoll procedure. B) Round cells fraction from Percoll enrichment procedure. C–F) Ovulated oocytes were recovered from CD-1 mice, then eggs were incubated in IVF medium with no spermatozoa (C), coincubated medium with 100 000 Cstf2t+/+ motile spermatozoa/ml IVF medium, coincubated with 100 000 Cstf2t−/− motile spermatozoa/ml IVF medium, and coincubated with 100 000 Cstf2t−/− motile spermatozoa/ml IVF medium depleted of round cells. Original magnification ×20 (A, B), ×30 (C–E), and ×60 (F).
Incubation of COCs in IVF medium did not disperse cumulus cells (Fig. 2C). When COCs were incubated with IVF medium containing sperm cells from Cstf2t+/+ mice, a high percentage of the cumulus mass was largely dispersed (Fig. 2D), typical of normal cumulus cell dispersion with spermatozoa from wild-type mice. Similarly, when COCs were incubated with sperm cells from Cstf2t−/− mice (Fig. 2, E and F), cumulus cell expansion was comparable to that for Cstf2t+/+ mice. This finding suggested that spermatozoa from Cstf2t−/− mice produced COC-dispersing components in quantities sufficient to support fertilization. The dispersion of cumulus cells with Cstf2t−/− spermatozoa likely resulted from spermatozoa rather than from the round cells found in the epididymal lumen, because when spermatozoa were enriched and round cells depleted by the Percoll procedure before incubation with COCs, the cumulus cell expansion was identical to that without sperm enrichment (Fig. 2F).
Cstf2t−/− Sperm Cells Do Not Adhere Normally to the Egg Extracellular Matrix
The next step in fertilization is adhesion of spermatozoa to the egg's ZP. To examine this step, sperm-ZP adhesion assays were performed using cumulus-free but zona-intact mouse eggs. When incubated with zona-intact eggs, spermatozoa from Cstf2t+/+ mice attached firmly to the ZP surface even after extensive washing (Fig. 3, A and C). In contrast, far fewer spermatozoa from Cstf2t−/− mice were able to adhere to mouse ZP (Fig. 3, B and C); this number resembled the low rate of Cstf2t+/+ sperm adhesion to 2-cell embryos or of pig sperm that adhered to the ZP of mouse eggs, interactions that served as negative controls representing nonspecific adhesion (Fig. 3C). Together, these data suggest that although sperm cells from Cstf2t−/− mice produced cumulus-dispersing components, they lacked one or more key components required for specific adhesion to the ZP.
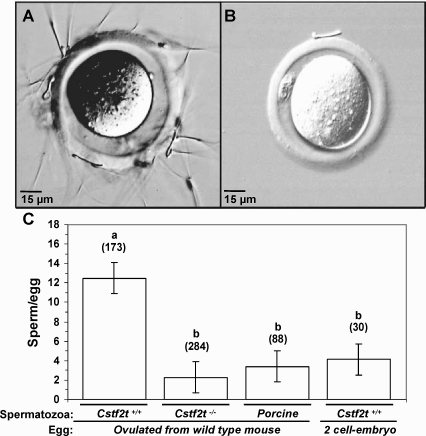
FIG. 3.
Percoll-enriched spermatozoa from Cstf2t−/− mice fail to adhere to ZP. A and B) Representative images from sperm-egg adhesion assay using Cstf2t+/+ spermatozoa (A) and Cstf2t−/−spermatogenic cells (B). Original magnification ×40. C) Average of sperm cells remaining bound to the ZP after extensive washing from sperm-ZP adhesion assay using Cstf2t+/+ (12.5 spermatozoa/egg), Cstf2t−/− (2.3 spermatozoa/egg), or porcine spermatozoa (3.4 spermatozoa/egg). Note that sperm-ZP adhesion assay using porcine spermatozoa with mouse eggs as well as the mouse 2-cell embryo coincubated with Cstf2t+/+ spermatozoa (4.1 spermatozoa/egg) were used as negative controls. The average number of sperm cells bound per egg was significantly decreased in sperm-ZP adhesion assay using Cstf2t−/− compared to Cstf2t+/+ cells (P < 0.001), and the number of Cstf2t−/− cells bound was similar to the average obtained by the negative controls. Lowercase letters indicate significant difference based on the number of spermatozoa bound per egg (n = 7, P < 0.001, SEM ± mean 1.6). Numbers directly above bars indicate the number of oocytes counted in each group.
Acrosomal Proteins Are Localized in Cstf2t−/− Sperm Cells
Because cumulus cells were dispersed normally during Cstf2t−/− fertilization and null spermatozoa did not interact correctly with ZP glycoproteins, the presence of acrosomal proteins involved in sperm-egg interaction was investigated (zonadhesin, proacrosin, SPAM1/PH-20, and CST8 [CRES]). Localization of these proteins (chosen because of their importance to sperm physiology and their presence in the acrosomal matrix) was performed on Cstf2t+/+ and Cstf2t−/− spermatozoa. Moreover, a marker of round spermatids, cAMP response element modulator (CREM) [31, 32], was introduced in immunolocalization experiments. Sperm cells from Cstf2t+/+ (Fig. 4A) and Cstf2t−/− (Fig. 4B) mice were methanol-fixed and permeabilized before immunolocalization. Figure 4A (panels A, D, G, J, M, P, and S) shows phase-contrast images of normal-appearing caudal spermatozoa from Cstf2t+/+ mice. In Figure 4A, antibodies directed against zonadhesin, proacrosin, SPAM1/PH-20, CST8 (CRES), and ZP3R/sp56 were used to localize each protein in sperm cells. All five acrosomal targets were localized in the acrosomal matrix of Cstf2t+/+ spermatozoa (Fig. 4A, panels B, E, H, K, and N). The acrosomes of those spermatozoa were visualized by PNA labeling, which detects carbohydrates associated to the intra-acrosomal membrane (Fig. 4A, panels C, F, I, L, and O). In contrast, CREM, a protein mostly associated with round spermatids, was not seen in sperm heads (Fig. 4A, panel Q). An irrelevant antibody (anti-GST) resulted in no visible staining of spermatozoa from Cstf2t+/+ mice (Fig. 4A, panel U).
In Figure 4B, acrosomal proteins were localized in spermatozoa from Cstf2t−/− mice. These spermatozoa were noticeably irregular in shape, with misshapen heads and broken hooks (Fig. 4B, panels A, D, G, J, M, and P), in agreement with our earlier assessment (Fig. 1C). However, despite the morphological abnormalities in these sperm heads, each stained with PNA in a pattern that resembled that seen in the wild-type cells (Fig. 4B, panels C, F, I, L, O, and R). Similarly, each of the chosen acrosomal proteins was present and seemed to be localized in a pattern associated with the acrosome (Fig. 4B, panels B, E, H, K, and N), although the shapes of these structures were uneven (panels A, D, G, J, M and P). These results suggest that despite the developmental and morphological anomalies seen in sperm cells from the Cstf2t−/− mice, pathways resulting in the production of several acrosomal proteins are still largely intact.
Round Cells in Caudal Epididymal Fluid from Cstf2t−/− Mice Contain Markers for Spermatozoa and Round Spermatids
Dass et al. [17] speculated that the round cells prominent in the epididymal contents of the Cstf2t−/− mice were round spermatids that had sloughed off prematurely into the testicular lumen. To better determine the origins of the round cells, we used the same markers as in Figure 4 to test whether they had contents usually associated with round spermatids or with later-stage spermatogenic cells. Zonadhesin [30], proacrosin [33], SPAM1/PH-20 [34], and CST8 (CRES) [35] are expressed in late-stage spermatogenic cells (round spermatids) but not in spermatogonia. Antibodies to each of these proteins showed an uneven distribution in the round cells (Fig. 5, B, E, H, K, and N), distributing as punctate (Fig. 5H), polarized (Fig. 5E), or peripheral (Fig. 5K) patterns. An antibody to GST resulted in no detectable labeling (Fig. 5Q). The PNA lectin staining mirrors those of the individual proteins (Fig. 5, C, F, I, L, and O). The presence of these acrosomal protein markers suggests that the round cells were derived from elongating spermatids or spermatozoa. Therefore, it was surprising that CREM expression was also seen in these cells (Fig. 5Q), because CREM is mostly associated with round spermatids and not with later-stage germ cells [31, 32]. This observation suggested that the round cells were neither round spermatids nor later-stage spermatogenic cells but, instead, were an abnormal developmental amalgam of both cell types.
FIG. 5.
Localization at acrosomal proteins (zonadhesin, proacrosin, SPAM1/PH-20, and ZP3R/sp56) and CREM in round cells found in Cstf2t−/− caudal epididymal fluid. The first column shows round cells from the cauda in phase contrast. The same cell was labeled with an antibody to the protein described in the left margin (middle column), and the last column of images shows the same previous cell but labeled that time with PNA lectin. Note that all round cells were positive for PNA and for all antibodies used, with the exception of the negative control (GST). Original magnification ×96.
To determine if the round cells were mostly empty vesicles, we used propidium iodide and SYBR-14 to label DNA associated with these cells. The majority of the round cells demonstrated the representative labeling pattern shown in Figure 6, B and C. Spermatozoa with rounded heads (Fig. 6, D–F), including some with externally coiled tails (Fig. 6F), were observed in caudal lumen. Therefore, it appears that some of the round cells may be immature, elongating spermatids in which the tail coiled over the partially developed sperm head and then fused with the plasma membrane to form a single entity. To investigate the putative explanation to the formation of the round cells, the structures of these cells were visualized by electron microscopy. Figure 6, G and H, shows representative findings. Overall, degraded spermatozoa, spermatids, and other unknown cell types were observed in the cauda epididymal fluid from Cstf2t−/− males and were present in null mice but absent in heterozygous and wild-type animals (data not shown). Notably, the aberrations differ from cell to cell and include disorganized or missing internal structures (Fig. 6G, arrow), coiled tails within the plasma membrane (Fig. 6, G and H, arrowheads), and disordered nuclear chromatin (Fig. 6G).
FIG. 6.
Round cells found in Cstf2t−/− caudal epididymal fluid showed DNA contents and remnant tail structures. The same representative round cells isolated from Cstf2t−/− caudal epididymal fluid is showed in phase contrast (A), labeled with propidium iodide (B), and labeled with SYBR-14 (C). Examples of spermatogenic cells in phase contrast with a round head (D–E) and including externally coiled flagella (F) are also shown. Electron micrographic images of this deficient spermatogenic cells showing disorganized or missing internal structure indicated by an arrow (G) and coiled tail within the plasma membrane indicated by arrowheads (G and H) are shown as well. Original magnification ×96.
DISCUSSION
Oligoasthenoteratozoospermia is one of the most common causes of male subfertility in human populations [19]. Disruption of the gene encoding the testis-expressed τCstF-64 polyadenylation protein, Cstf2t, in mice results in oligoasthenoteratozoospermia [17]. Cstf2t−/− sperm cells display a very poor motility, and sperm morphology is extremely abnormal, potentially explaining the infertility of Cstf2t−/− males. Various mouse models of infertility have been produced using gene knockout technology. In each of these models, spermatozoa were often found in the epididymal fluid, and the phenotype of these mice was rarely a complete block of fertilization [36, 37]. However, only a few mouse models display both abnormal sperm morphology and impaired progressive motility. For instance, Cnot7- and Tex18-null mice represent these kinds of deficient sperm population [38, 39], but both have produced offspring during breeding experiments. In contrast, no offspring have ever been sired by Cstf2t−/−males. Consistent with the complete block of in vivo fertility in Cstf2t−/− males, the present study is, to our knowledge, the first to demonstrate that a gene involved in the regulation of gene expression and polyadenylation can have a direct effect on sperm-ZP adhesion. In this circumstance, it seems likely that more than one gene product involved in ZP adhesion may be affected by the lack of polyadenylation activity involving τCstF-64.
Although τCstF-64 is expressed in other tissues [6, 40], the phenotype of Cstf2t−/− mice is restricted to male germ cells; to date, we have seen no phenotype in any other tissue or in females [17] (data not shown). This observation suggests that the primary function of τCstF-64 is to support polyadenylation during spermatogenesis and that its role elsewhere is redundant. Interestingly, in male germ cells, Cstf2ttm1Ccma showed variable expressivity, with many cells displaying defects in elongating and mature spermatids but with a few motile spermatozoa present in the caudae epididymides. This amalgam of cells reflects the various defects in spermatogenesis produced by loss of τCstF-64, and it is reminiscent of oligoasthenoteratozoospermia, an infertility condition diagnosed in the human population.
In the present study, Cstf2t−/− spermatozoa had a number of head defects but showed progressive motility for some cells. This last observation raised the question of whether assisted reproductive techniques might allow fertilization from this motile population. Upon testing, Cstf2t−/− spermatozoa enriched for motile cells failed to produce fertilized eggs in vitro, demonstrating that the infertile phenotype of the Cstf2t−/− spermatozoa in vitro could be attributed not only to low sperm count but also to failure of gamete interactions. Incubation of COCs with Cstf2t−/− sperm cells resulted in normal dispersion of cumulus cells, indicating that Cstf2t−/− sperm cells expressed hyaluronidase genes. Six hyaluronidase-like genes have been determined in the mouse genome, and in regions syntenic with the human genome [41]. Recently, arylsulfatase A, a protein previously reported to have a role in sperm-ZP adhesion [42], was shown to be involved in cumulus-cell dispersion [43]. Arylsulfatase A and other components necessary for this action, such as hyal5 and SPAM1/PH-20, were more likely to be moderately affected by the lack of polyadenylation via τCstF-64. Therefore, the block to fertilization seen in Cstf2t−/− spermatozoa was not at this early step.
The structure and morphology of Cstf2t−/− spermatozoa are markedly abnormal. Therefore, it is possible that at least part of the block to fertilization is caused by structural defects in these cells. Localization of zonadhesin, proacrosin, SPAM1/PH-20, CST8 (CRES), and ZP3R/sp56 in the apical head was consistent with the results of previous studies in which they were shown to be present in the acrosomal matrix of wild-type mice [4, 5, 23, 44, 45]. Although sperm head morphology was mostly incorrect within Cstf2t−/− spermatozoa, each antibody revealed specific proteins colocalized with PNA staining in the crescent shape associated with an intact acrosome, thus failing to identify mislocalization or absence of any of the proteins tested. However, CST8 (CRES) was shown to be associated with the acrosomal matrix of Cstf2t−/−; an additional subpopulation was detected at the postacrosomal region for certain sperm cells, as shown in Figure 4B. Moreover, with the exception of CST8 (CRES), each of the acrosomal proteins examined (zonadhesin, acrosin, SPAM1/PH-20, and ZP3R/sp56) in Cstf2t+/+ or Cstf2t−/− spermatozoa were previously shown to interact directly with ZP glycoproteins. Nevertheless, CST8 (CRES) is believed to play an important role in the regulation of protein processing, because it has been shown to inhibit prohormone convertase (PC) activity, suggesting a role in proprotein processing [46, 47]. Certain PCs are exclusively found in the testis. Therefore, PC4 is a sperm-specific molecule [48], and the lack of this protein produced a severe subfertility phenotype in the absence of sperm defect or impaired motility associated to these null spermatozoa [49]. Thus, the absence or mislocalization of acrosomal components tested was inconclusive for the failure of Cstf2t−/− sperm infertility.
These localization results imply that the presence of acrosomal proteins are perhaps less critical than their processing or their posttranslational modifications during spermiogenesis or fertilization. One or several of the acrosomal molecules could be localized correctly yet be completely nonfunctional as a consequence of aberrant processing. Zonadhesin, a sperm-ZP adhesion molecule that confers species-specificity during fertilization, is a target of proteolytic enzymes during the acrosome reaction (Tardif et al., unpublished results). ZP3R/sp56 is another acrosomal protein that was shown to be a target for sperm proteolytic enzyme but was dramatically reduced in noncapacitating conditions [50]. The impaired ZP adhesion in Cstf2t−/− spermatozoa could also be related to defective biosynthesis, processing, or trafficking of other acrosomal molecules. Additional characterization will be necessary to determine whether the mature forms of different acrosomal proteins are correctly synthesized and completely functional in the acrosomal matrix of Cstf2t−/− sperm cells.
Finally, characterization of the round cells observed prominently in the Cstf2t−/− mice suggests these cells contained developmental remnants of earlier spermatogenic stages. These micrographic images suggest problems in tail development, including, but not limited to, incorrectly formed radial spokes and nexin links. Therefore, the round cells may have at least three possible origins: 1) round spermatids that sloughed into the seminiferous tubule lumen prematurely, 2) incorrectly developed spermatozoa that lost tails or had the tails coil inside, and 3) empty cells, the contents of which were lost because of a loss of structural integrity, cell permeability, or osmotic selectivity.
Because polyadenylation is essential for gene expression, it was perhaps not surprising that the germ cell-expressed τCstF-64 proved to be important for spermatogenesis. However, it was surprising that targeted deletion of Cstf2t resulted in germ cell development that was impeded but not halted. The present results indicate that loss of τCstF-64 results in a large number of structural, biochemical, and developmental defects during spermatogenesis in Cstf2t−/− mice, probably because of both large and small changes in the expression of multiple genes rather than an absolute block. This hypothesis is consistent with earlier microarray results for testes from Cstf2t−/− mice in which levels of many mRNAs were altered but not eliminated [17]. Overall, we believe these changes in gene expression will be important for understanding the mechanisms of polyadenylation as well as the intricacies of spermatogenesis.
Acknowledgments
We thank Kathy J. Hockert and Stephen White for mouse husbandry and genotyping of the Cstf2ttm1Ccma colony, Gary Olson and Virginia Winfrey for electron micrography, Carlos Molina (UMDNJ New Jersey Medical School) for the anti-CREM antibody, George L. Gerton (University of Pennsylvania School of Medicine) for the anti-ZP3R/sp56 antibody, and Gail Cornwall (Texas Tech University Health Sciences Center) for the anti-CST8 (CRES) antibody. We also thank Gail Cornwall, Neil Pithadia, and Daniel Webster for constructive comments on the manuscript.
Footnotes
Supported by a postdoctoral fellowship from the Lalor Foundation and by Fonds Québécois de la Recherche sur la Nature et les Technologies to S.T., by a grant from the Texas Dept. of Agriculture to D.M.H., and by grants from the NIH (HD-037109), the South Plains Foundation, and the TTUHSC School of Medicine to C.C.M.
REFERENCES
- Yanagimachi R.The physiology of reproduction. Knobil E, Neill J.Mammalian Fertilization. New York:Raven Press;1994: 189–317. [Google Scholar]
- de Mouzon J, Lancaster P, Nygren KG, Sullivan E, Zegers-Hochschild F, Mansour R, Ishihara O, Adamson D.World collaborative report on assisted reproductive technology, 2002. Hum Reprod 2009; 24: 2310–2320. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Shi X, Burkin H.Molecular basis of mammalian gamete binding. Recent Prog Horm Res 2002; 57: 37–73. [DOI] [PubMed] [Google Scholar]
- Baba T, Azuma S, Kashiwabara S, Toyoda Y.Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem 1994; 269: 31845–31849. [PubMed] [Google Scholar]
- Baba D, Kashiwabara S, Honda A, Yamagata K, Wu Q, Ikawa M, Okabe M, Baba T.Mouse sperm lacking cell surface hyaluronidase PH-20 can pass through the layer of cumulus cells and fertilize the egg. J Biol Chem 2002; 277: 30310–30314. [DOI] [PubMed] [Google Scholar]
- Wallace AM, Dass B, Ravnik SE, Tonk V, Jenkins NA, Gilbert DJ, Copeland NG, MacDonald CC.Two distinct forms of the 64,000 Mr protein of the cleavage stimulation factor are expressed in mouse male germ cells. Proc Natl Acad Sci U S A 1999; 96: 6763–6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dass B, McMahon KW, Jenkins NA, Gilbert DJ, Copeland NG, MacDonald CC.The gene for a variant form of the polyadenylation protein CstF-64 is on chromosome 19 and is expressed in pachytene spermatocytes in mice. J Biol Chem 2001; 276: 8044–8050. [DOI] [PubMed] [Google Scholar]
- Dass B, McDaniel L, Schultz RA, Attaya E, MacDonald CC.The gene CSTF2T encoding the human variant CstF-64 polyadenylation protein τCstF-64 is intronless and may be associated with male sterility. Genomics 2002; 80: 509–514. [PubMed] [Google Scholar]
- MacDonald CC, Redondo J-L.Reexamining the polyadenylation signal: were we wrong about AAUAAA? Mol Cell Endocrinol 2002; 190: 1–8. [DOI] [PubMed] [Google Scholar]
- Liu D, Brockman JM, Dass B, Hutchins LN, Singh P, McCarrey JR, MacDonald CC, Graber JH.Systematic variation in mRNA 3′-processing signals during mouse spermatogenesis. Nucleic Acids Res 2007; 35: 234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwalds-Gilbert G, Veraldi KL, Milcarek C.Alternative poly(A) site selection in complex transcription units: means to an end? Nucleic Acids Res 1997; 25: 2547–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lee JY, Tian B.Biased alternative polyadenylation in human tissues. Gen Biol 2005; 6: R100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Lee Y, Chung JH.An intronless gene encoding a poly(A) polymerase is specifically expressed in testis. FEBS Lett 2000; 487: 287–292. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kim H, Chung JH, Lee Y.Testis-specific expression of an intronless gene encoding a human poly(A) polymerase. Mol Cells 2001; 11: 379–385. [PubMed] [Google Scholar]
- Sartini BL, Wang H, Wang W, Millette CF, Kilpatrick DL.Premessenger RNA cleavage factor I (CFIm): potential role in alternative polyadenylation during spermatogenesis. Biol Reprod 2008; 78: 472–482. [DOI] [PubMed] [Google Scholar]
- Handel MA.The XY body: a specialized meiotic chromatin domain. Exp Cell Res 2004; 296: 57–63. [DOI] [PubMed] [Google Scholar]
- Dass B, Tardif S, Park JY, Tian B, Weitlauf HM, Hess RA, Carnes K, Griswold MD, Small CL, MacDonald CC.Loss of polyadenylation protein tCstF-64 causes spermatogenic defects and male infertility. Proc Natl Acad Sci U S A 2007; 104: 20374–20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Mallidis C, Ma K.The genetic basis of infertility in men. Baillieres Best Pract Res Clin Endocrinol Metab 2000; 14: 363–388. [DOI] [PubMed] [Google Scholar]
- Hirsh A.Male subfertility. BMJ 2003; 327: 669–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif S, Wilson MD, Wagner R, Hunt P, Gertsenstein M, Nagy A, Lobe C, Koop BF, Hardy DM.Zonadhesin is essential for species specificity of sperm adhesion to the egg's zona pellucida. J Biol Chem 2010; (in press). published online ahead of print 7 June 2010;DOI 10.1074/jbc.M110.123125. [DOI] [PMC free article] [PubMed]
- Hardy DM, Oda MN, Friend DS, Huang TT., JrA mechanism for differential release of acrosomal enzymes during the acrosome reaction. Biochem J 1991; 275; 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DM, Wild GC, Tung KS.Purification and initial characterization of proacrosins from guinea pig testes and epididymal spermatozoa. Biol Reprod 1987; 37: 189–199. [DOI] [PubMed] [Google Scholar]
- Syntin P, Cornwall GA.Immunolocalization of CRES (cystatin-related epididymal spermatogenic) protein in the acrosomes of mouse spermatozoa. Biol Reprod 1999; 60: 1542–1552. [DOI] [PubMed] [Google Scholar]
- Hickox JR, Bi M, Hardy DM.Heterogeneous processing and zona pellucida binding activity of pig zonadhesin. J Biol Chem 2001; 276: 41502–41509. [DOI] [PubMed] [Google Scholar]
- Fraser LR.In vitro capacitation and fertilization. Methods Enzymol 1993; 225: 239–253. [DOI] [PubMed] [Google Scholar]
- Tardif S, Dube C, Chevalier S, Bailey JL.Capacitation is associated with tyrosine phosphorylation and tyrosine kinase-like activity of pig sperm proteins. Biol Reprod 2001; 65: 784–792. [DOI] [PubMed] [Google Scholar]
- Tardif S, Dube C, Bailey JL.Porcine sperm capacitation and tyrosine kinase activity are dependent on bicarbonate and calcium but protein tyrosine phosphorylation is only associated with calcium. Biol Reprod 2003; 68: 207–213. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Jovine L, Litscher ES.A profile of fertilization in mammals. Nat Cell Biol 2001; 3: E59–E64. [DOI] [PubMed] [Google Scholar]
- Bi M, Hickox JR, Winfrey VP, Olson GE, Hardy DM.Processing, localization and binding activity of zonadhesin suggest a function in sperm adhesion to the zona pellucida during exocytosis of the acrosome. Biochem J 2003; 375: 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson GE, Winfrey VP, Bi M, Hardy DM, NagDas SK.Zonadhesin assembly into the hamster sperm acrosomal matrix occurs by distinct targeting strategies during spermiogenesis and maturation in the epididymis. Biol Reprod 2004; 71: 1128–1134. [DOI] [PubMed] [Google Scholar]
- Delmas V, van der Hoorn F, Mellstrom B, Jegou B, Sassone-Corsi P.Induction of CREM activator proteins in spermatids: down-stream targets and implications for haploid germ cell differentiation. Mol Endocrinol 1993; 7: 1502–1514. [DOI] [PubMed] [Google Scholar]
- Weinbauer GF, Behr R, Bergmann M, Nieschlag E.Testicular cAMP responsive element modulator (CREM) protein is expressed in round spermatids but is absent or reduced in men with round spermatid maturation arrest. Mol Hum Reprod 1998; 4: 9–15. [DOI] [PubMed] [Google Scholar]
- Kashiwabara S, Baba T, Takada M, Watanabe K, Yano Y, Arai Y.Primary structure of mouse proacrosin deduced from the cDNA sequence and its gene expression during spermatogenesis. J Biochem 1990; 108: 785–791. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Martin-Deleon PA.The murine Spam1 gene: RNA expression pattern and lower steady-state levels associated with the Rb(6.16) translocation. Mol Reprod Dev 1997; 46: 252–257. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Hann SR.Transient appearance of CRES protein during spermatogenesis and caput epididymal sperm maturation. Mol Reprod Dev 1995; 41: 37–46. [DOI] [PubMed] [Google Scholar]
- Adham IM, Nayernia K, Burkhardt-Gottges E, Topaloglu O, Dixkens C, Holstein AF, Engel W.Teratozoospermia in mice lacking the transition protein 2 (Tnp2). Mol Hum Reprod 2001; 7: 513–520. [DOI] [PubMed] [Google Scholar]
- Neesen J, Kirschner R, Ochs M, Schmiedl A, Habermann B, Mueller C, Holstein AF, Nuesslein T, Adham I, Engel W.Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum Mol Genet 2001; 10: 1117–1128. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yao R, Ogawa T, Suzuki T, Ito C, Tsunekawa N, Inoue K, Ajima R, Miyasaka T, Yoshida Y, Ogura A, Toshimori K, et al. Oligoasthenoteratozoospermia in mice lacking Cnot7, a regulator of retinoid X receptor beta. Nat Genet 2004; 36: 528–533. [DOI] [PubMed] [Google Scholar]
- Jaroszynski L, Dev A, Li M, Meinhardt A, de Rooij DG, Mueller C, Bohm D, Wolf S, Adham IM, Wulf G, Engel W, Nayernia K.Asthenoteratozoospermia in mice lacking testis expressed gene 18 (Tex18). Mol Hum Reprod 2007; 13: 155–163. [DOI] [PubMed] [Google Scholar]
- Wallace AM, Denison TL, Attaya EN, MacDonald CC.Developmental distribution of the polyadenylation protein CstF-64 and the variant tauCstF-64 in mouse and rat testis. Biol Reprod 2004; 70: 1080–1087. [DOI] [PubMed] [Google Scholar]
- Csoka AB, Frost GI, Stern R.The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol 2001; 20: 499–508. [DOI] [PubMed] [Google Scholar]
- Tantibhedhyangkul J, Weerachatyanukul W, Carmona E, Xu H, Anupriwan A, Michaud D, Tanphaichitr N.Role of sperm surface arylsulfatase A in mouse sperm-zona pellucida binding. Biol Reprod 2002; 67: 212–219. [DOI] [PubMed] [Google Scholar]
- Wu A, Anupriwan A, Iamsaard S, Chakrabandhu K, Santos DC, Rupar T, Tsang BK, Carmona E, Tanphaichitr N.Sperm surface arylsulfatase A can disperse the cumulus matrix of cumulus oocyte complexes. J Cell Physiol 2007; 213: 201–211. [DOI] [PubMed] [Google Scholar]
- Gao Z, Garbers DL.Species diversity in the structure of zonadhesin, a sperm-specific membrane protein containing multiple cell adhesion molecule-like domains. J Biol Chem 1998; 273: 3415–3421. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Foster JA, Gerton GL.The role of the acrosomal matrix in fertilization. Int J Dev Biol 2008; 52: 511–522. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, von Horsten HH, Swartz D, Johnson S, Chau K, Whelly S.Extracellular quality control in the epididymis. Asian J Androl 2007; 9: 500–507. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Cameron A, Lindberg I, Hardy DM, Cormier N, Hsia N.The cystatin-related epididymal spermatogenic protein inhibits the serine protease prohormone convertase 2. Endocrinology 2003; 144: 901–908. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Kim WS, Torii S, Hosaka M, Nakagawa T, Ikemizu J, Baba T, Murakami K.Identification of the fourth member of the mammalian endoprotease family homologous to the yeast Kex2 protease. Its testis-specific expression. J Biol Chem 1992; 267: 5897–5900. [PubMed] [Google Scholar]
- Mbikay M, Tadros H, Ishida N, Lerner CP, De Lamirande E, Chen A, El-Alfy M, Clermont Y, Seidah NG, Chretien M, Gagnon C, Simpson EM.Impaired fertility in mice deficient for the testicular germ-cell protease PC4. Proc Natl Acad Sci U S A 1997; 94: 6842–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, Kim KS, Doak BJ, Rodriguez-Miranda E, Gerton GL.Functional consequences of cleavage, dissociation and exocytotic release of ZP3R, a c4bp-related protein, from the mouse sperm acrosomal matrix. J Cell Sci 2009; 22: 3153–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]