Abstract
Objectives
The purpose of this study was to investigate the prevalence of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium, in women attending a sexually transmitted disease (STD) clinic, as well as the frequency of coinfections, and relationship of each organism to cervicitis.
Methods
In this cross-sectional study of 324 women attending Baltimore City STD Clinics, C. trachomatis, N. gonorrhoeae, T. vaginalis, and M. genitalium were detected using nucleic acid amplification tests. Demographic characteristics and risk factors were ascertained.
Results
Overall prevalence of infection with C. trachomatis, N. gonorrhoeae, T. vaginalis, and M. genitalium was found to be 11.1%, 4.6%, 15.3%, and 19.2%, respectively. Prevalence in women with cervicitis was 15.8%, 6%, 18.9%, and 28.6% for C. trachomatis, N. gonorrhoeae, T. vaginalis, and M. genitalium, respectively. Percentages of coinfections were high. C. trachomatis and M. genitalium were significantly associated with cervicitis in univariate analysis, but only M. genitalium was significantly associated with cervicitis (AOR: 2.5) in multiple logistic regression models.
Conclusion
Knowledge of the statistical association of M. genitalium with cervicitis in this study increases the need for further confirmation of the etiologic significance of this organism with cervicitis in more diverse populations. The high prevalence merits more study and may have implications for diagnosis and treatment of cervicitis.
Cervicitis is characterized by inflammation of the cervix and most commonly is caused by sexually transmitted diseases (STDs). In 2000, Weinstock et al. estimated that 18.9 million new cases of STDs occur each year in the United States, of which 9.1 million occurred in young people between 15 and 24 years of age.1,2 Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) are the causative agents of 2 of the most common STDs and are the most frequent causes of cervicitis.2,3 However, in many cases of cervicitis the etiology is unknown or unclear.4 In those cases, Trichomonas vaginalis (TV), Mycoplasma genitalium (MG), and others (such as those associated with bacterial vaginosis (BV), herpes simplex virus Type 1, and herpes simplex virus Type 2) have been implicated as potential causative pathogens of nonchlamydial, nongonococcal cervicitis.5,6
Prolonged cervicitis with infectious etiology from several different pathogens, such as Chlamydia and gonorrhea may result in pelvic inflammatory disease (PID), infertility, ectopic pregnancy, and chronic pelvic pain.6 Cervicitis caused by Chlamydia in pregnancy may result in miscarriage or early delivery, as well as infection of the newborn during delivery, which in turn may cause pneumonia or conjunctivitis. Exposure to STDs, intercourse at an early age, high-risk sexual behavior, multiple and new sexual partners, and a previous STD, such as Chlamydia, have been associated with an increased risk of cervicitis.7,8 Less clear is the association of genital mycoplasmas with cervicitis and PID.5,9 –12
Cervicitis may be persistent due to lack of clear understanding of the infectious etiology or noninfectious origin of disease. It is essential to determine the extent to which the various microorganisms contribute to cervicitis so that appropriate patient diagnosis and treatment can occur. Asymptomatic cases of cervicitis also represent a continuing challenge in the chain of transmission and persistence in the population because etiologic agents are often not detected or treated.4 In this study, our goal was to determine the contribution of CT, NG, TV, and MG to cervicitis. The contribution of CT, NG, and to a lesser extent TV are already known. However, the association of MG with cervicitis is not as clear. Recently, Hjorth et al. provided evidence that MG is sexually transmitted using DNA-based typing.13 Advances in nucleic acid amplification techniques (NAATs) now allow for the testing of all of these organisms with high sensitivity and specificity.
MATERIALS AND METHODS
Patients
There were 328 female subjects enrolled at 2 Baltimore City STD clinics (Druid and Eastern Health Clinics) as part of a study whose purpose was to examine the feasibility and accuracy of 2 new NAATs to detect TV and MG. The study reported herein, was designed to evaluate the microbial etiologies of cervicitis. The Johns Hopkins University (via the Western IRB) and the Baltimore City Health Department Institutional Review Boards approved the study. Written informed consent was obtained from all subjects. Every fifth patient was assigned to the study clinician as 5 clinicians worked in the STD clinic, so randomly every fifth patient was eligible for the study. For those approached by the study clinician the acceptance rate (estimated by the study clinician) for women was greater than 90%.
Upon enrollment and after consent, women were asked questions by the clinic staff about sociodemographic information, presence of symptoms (dysuria, discharge, etc.), sexual history, behavioral risk factors, and STD history. In addition, women were asked questions regarding preference for specimen type: endocervical, self-collected vaginal, or urine.
Clinic Procedures and Specimens Collected
All normal clinic procedures were conducted. All women enrolled in the study received the standard clinical evaluation, which included: physical and pelvic examination; examination of vaginal secretions for detection of BV, yeast, and TV; CT test; NG culture; counseling; and, treatment as needed. A structured behavioral assessment and focused clinical exam were performed by the trained clinician. In addition, each woman was instructed to self-collect 2 vaginal swabs and then a urine specimen before the pelvic examination. Genital examination and collection of the 3 endocervical specimens from the patient was done by staff clinicians. The first endocervical swab was used for GC culture as part of standard-of-care testing. A second endocervical swab was used for the standard-of-care NAAT test for Chlamydia. The remaining endocervical swab was placed in the appropriate GEN-PROBE Specimen Transport Media (STM) and used for study purposes. The vaginal swab collection order was randomized as to assay type and one of the vaginal swabs was stored dry for use in the in-house research-based PCR assays and the other was placed in STM for the APTIMA Combo 2 and APTIMA transcription mediated amplification (TMA)-based assays (GEN-PROBE, Inc, San Diego, CA). The endocervical swab in STM, the 2 vaginal swabs, and the urine were transported to the research laboratory for testing according to the protocol. An aliquot of the urine was placed in the GEN-PROBE Urine Collection Tube on receipt by the laboratory within 24 hours. The following tests were performed: (1) APTIMA Combo 2, (2) APTIMA assay for C. trachomatis (ACT), (3) APTIMA assay for N. gonorrhoeae (AGC), (4) APTIMA assay for T. vaginalis assay, (5) APTIMA assay for M. genitalium, (6) research-based TV-PCR assay (see later), and (7) research-based MG-PCR assay. (see later).
Sample Preparation
One of the vaginal swab specimens was extracted utilizing the Roche MagNA Pure LC® robotic instrument (Roche Molecular Diagnostics, Indianapolis, IN) for the research-based TV and MG PCR assays. Dry vaginal swabs were rehydrated in 1 mL of Tris-EDTA buffer, of which 200 μL was removed for DNA extraction. DNA extraction was carried out according to the instructions supplied for the MagNA Pure LC® program “DNA I Blood Cells High Performance Serum” protocol.
For the TMA-based assays (APTIMA Combo 2, ACT, AGC), the endocervical swab, other vaginal swab and urine, collected in the appropriate STM, were processed according to the manufacturer’s instructions. Residual processed specimens from the TMA-processed vaginal swab were used in the TMA-based TV and MG assays.
CT and NG Assays
The endocervical, vaginal, and urine specimens were processed and tested according to the APTIMA Combo 2 manufacturer’s package inserts for CT and NG. All positive CT and NG APTIMA Combo 2 results from any specimen source were confirmed using the APTIMA ACT and AGC assays according to instructions provided in the APTIMA ACT and AGC package inserts. For CT and NG, infected patient status was defined as having a positive test in 2 different sample types or a positive test in 1 sample type confirmed using an alternate target assay (ACT or AGC). Description and performance of these assays have been previously published.14 –16
TV Assays
The APTIMA vaginal swab was tested with the AP-TIMA TMA Research assay which targets TV rRNA using the same methodology as other TMA assays.17 For this study, the cutoff for positive samples was set at 40,000 relative light units. After extraction, the other vaginal swab was used in the real-time TV-PCR assay, which targets the B-Tubulin gene. Description of this PCR method has been previously published.18 A patient was considered to be infected with TV when a positive result was obtained with the 2 assays.
MG Assays
A research assay for MG was used to assess patient infectivity with MG. After the vaginal swab was extracted, a multitarget real-time PCR for MG utilizing 2 alternate gene targets, the MgPa gene and the 16S rRNA gene, was performed.19 –21 The APTIMA vaginal swab was tested using the GEN-PROBE TMA research assay in the same manner as for the other APTIMA assays. This assay targets MG rRNA for detection in genital specimens. For this study, the cutoff for positive samples was set at 40,000 relative light units. In a previously published article, Hardick et al. have reported good agreement between the 2 methods with a Kappa statistic of 0.94 (95% confidence interval [CI]: 0.907, 0.976).21 A patient was considered to be infected with MG when a positive result was obtained with the 2 assays.
Definitions and Clinic Methods
A patient was considered to have cervicitis if the clinician found: cervical discharge, including mucupurulent discharge, or cervical friability (easily induced bleeding on use of the swab to collect the cervical sample), or otherwise indicated a diagnosis of “cervicitis” in the medical record.
Amsel’s criteria were used for the diagnosis of BV. If 3 of the following clinical features were present, the subject was considered to have BV: (1) discharge; (2) vaginal fluid pH > 4.5; (3) a positive amine test; and (4) presence of “clue cells.”22
During the clinic visit, vaginal smears were examined for the presence of yeast (candidiasis) using microscopy. In addition, a wet preparation of vaginal secretions was examined for the presence of motile trichomonads.
Data Analysis and Statistical Methods
All participants enrolled in the study for which specimens were collected and available for testing were included in the analysis. For MG and TV, there were a few cases where results from both diagnostic methods were not available (4 women missing TV results and 2 missing MG results due to insufficient volume left for one or both of the assays). Therefore, those subjects were excluded from the analysis of TV and MG.
All data were analyzed using either SAS (SAS Statistical Software: Release 9.1, SAS Institute, Cary, NC). Microsoft Excel 2002 (Microsoft Corporation, Redmond, WA) was used to record laboratory data results.
For this study, prevalence of an organism was defined as the proportion of subjects with a positive test result for the organism. Prevalence of each organism and prevalence of coinfections (presence of multiple kinds of organisms in the same individual), with 95% CIs, were calculated. Descriptive statistics included comparison of proportions using χ2 tests and/or Fisher exact tests for categorical variables and t tests or Wilcoxon tests were performed to assess significance of associations for continuous variables (P <0.05). Multiple logistic regression models were developed to examine the association of CT, NG, TV, and MG with cervicitis. Odds ratios (ORs) were calculated. The dependent variable was cervicitis and the independent (predictor) variables included in the model were diagnostic test results for CT, NG, TV, or MG, coded as a binary variable (“0” = no infection by diagnostic tests; “1” = infection by diagnostic tests). Possible confounders identified a priori included age, referral to the clinic as a sexual contact of an infected partner (contact), and BV. All statistical tests were 2-sided and evaluated at a 0.05 level of significance. Two-way interactions were evaluated.
RESULTS
Clinical Characteristics
Of the 328 enrolled women, specimens from 324 women were included in the study. One woman was excluded because she did not meet the inclusion criteria for age (she was less than 18 years of age). For the 3 others not included, specimens were either not collected or were unavailable for analysis due to transport issues. Of the 324 participants, 133 were classified as having cervicitis and 191 as not having cervicitis (Table 1). Signs of cervicitis (cervical discharge and/or friability), which led to a diagnosis of cervicitis, were found in 41.0% of the women. The most frequent reasons listed as a cause for a visit to the STD clinic were symptoms (approximately 70%), followed by checkup (23%). Less than 10% were sexual partners of a person diagnosed with an STD (contact).
Table 1.
Demographic Characteristics and Sexual Behaviors of Women With and Without Cervicitis
| Female n = 324 n (%) | Cervicitis + n = 133 n (%) | Cervicitis − n = 191 n (%) | P | |
|---|---|---|---|---|
| Age (≤25 yr of age) | 206 (63.6) | 91 (68.4) | 115 (60.2) | 0.13 |
| Clinic | ||||
| Eastern | 136 (42.0) | 30 (22.6) | 106 (55.5) | <0.001 |
| Druid | 188 (58.8) | 103 (77.4) | 85 (49.2) | |
| Black race | 301 (92.6) | 129 (97.0) | 171 (90.0) | 0.02 |
| Ethnicity | ||||
| Hispanic/Latino | 5 (1.5) | 1 (0.75) | 4 (2.09) | 0.65 |
| Non-Hispanic/Latino | 319 (98.2) | 132 (99.2) | 186 (97.9) | |
| Reason for visit | ||||
| Any symptoms and contact | 9 (2.8) | 4 (3.0) | 5 (2.6) | 0.20 |
| Symptoms | 220 (67.9) | 98 (73.7) | 122 (63.9) | |
| Contact | 20 (6.2) | 5 (3.8) | 15 (7.8) | |
| Checkup | 75 (23.1) | 26 (19.5) | 49 (25.6) | |
| Repeat visit vs. first time | 179 (55.4) | 81 (61.4) | 98 (51.6) | 0.08 |
| High school graduate (No) | 72 (22.2) | 24 (18.2) | 48 (25.4) | 0.13 |
| Unemployed | 137 (42.6) | 56 (42.4) | 81 (42.8) | 0.94 |
| Reason for visit = Contact | 29 (8.9) | 9 (6.8) | 20 (10.5) | 0.25 |
| Symptomatic for cervicitis | 213 (65.5) | 97 (72.9) | 116 (60.7) | 0.02 |
| Tobacco use (yes) | 137 (42.3) | 55 (42.9) | 82 (43.8) | 0.88 |
| Condom use (no) | 193 (59.6) | 72 (54.1) | 121 (63.4) | 0.10 |
| Condom freq = not always | 272 (84.0) | 114 (85.7) | 158 (82.7) | 0.47 |
| Sex preference ≠ Opposite | 25 (7.7) | 14 (10.6) | 11 (5.8) | 0.11 |
| New partner | 58 (17.9) | 32 (24.1) | 26 (13.6) | 0.02 |
| >1 Regular partners | 36 (11.2) | 14 (10.6) | 22 (11.6) | 0.77 |
| High-risk sex* | 36 (11.1) | 6 (4.5) | 30 (15.7) | 0.002 |
| Prior CT | 140 (43.2) | 60 (45.1) | 80(41.9) | 0.56 |
| Prior NG | 132 (40.7) | 55 (41.4) | 75 (39.3) | 0.71 |
| Prior TV | 133 (41.0) | 50 (37.6) | 83 (43.5) | 0.29 |
| Prior STD | 251 (77.4) | 102 (76.7) | 149 (78.1) | 0.78 |
| Other pathogens | ||||
| BV | 174 (54.4) | 80 (60.2) | 94 (50.5) | 0.09 |
| Candida | 41 (12.8) | 18 (13.5) | 23 (12.3) | 0.76 |
| Continuous | Mean (range) | Mean (range) | Mean (range) | Wilcoxin |
| Age | 25.13 (18–49) | 24.56 (18–46) | 25.53 (18–49) | 0.18 |
| No. new partners | 0.20 (0–6) | 0.29 (0–6) | 0.14 (0.0–2) | 0.01 |
| No. regular partners | 1.00 (0–3) | 1.01 (0–3) | 1.01 (0–2) | 0.67 |
| No. new partners in past 3 mo | 0.86 (0–7) | 1.17 (0–7) | 0.64 (0–4) | <0.001 |
| No. partners in past 6 mo | 1.73 (0–9) | 1.86 (0–8) | 1.64 (0–9) | 0.004 |
Includes sex with drugs/alcohol, sex with MSM, etc.
Demographics and Risk Factors for Cervicitis
In Table 1, demographic characteristics and sexual behaviors are described. The mean age of the study participants was 25.1 ± 6.3 years; 92.6% of the participants were black. Only 10% of the subjects reported participating in high-risk sexual behavior (e.g., sex with the exchange of drugs/alcohol/money, sex with HIV risk, sex with men who have sex with men, etc.). In the study population, 78% of the subjects were high school graduates and 57.4% were employed. The percentage of women who reported always using condoms was 16%. The range for number of new partners in the last 3 months was 0 to 7, with an average of 0.86.
Women attending the Druid Clinic were more likely to be diagnosed with cervicitis than those attending the Eastern Clinic (54.7% 103/188 vs. 22.1% 30/136). Women with cervicitis were more likely to be black than those without cervicitis (97.0% vs. 90%; P = 0.02). Women with cervicitis were more likely to symptomatic than women without cervicitis (72.9% vs. 60.7%, P = 0.02). Age was not associated with cervicitis either as a continuous or a dichotomous variable. Education, employment status, tobacco use, condom use, and prior STDs were not found to be significantly associated with cervicitis. Women with cervicitis were more likely to have BV than women without cervicitis (60.2% vs. 50.5%); however, that difference was not significant (P = 0.09). Women with cervicitis were less likely to admit participation in high-risk sexual behavior than those without cervicitis (4.5% vs. 15.7%, P = 0.002). When analyzed as continuous variables, the number of new partners (P = 0.01), the number of new partners in the last 3 months (P < 0.001), and the number of partners in the last 6 months (P value = 0.004) were associated with an increased risk of cervicitis.
Preference for Specimen Collection Type
More women (46.7%) preferred collection of vaginal swabs than urine (24.8%) or cervical swabs (28.5%). Thus, self-obtained samples (vaginal and urine) were preferred by 71.5% of women. Most women reported that vaginal swabs were “easy” to collect (80.9%), whereas 16.1% rated them as “OK,” and only 3% rated them as “hard.”
Prevalence and Coinfection
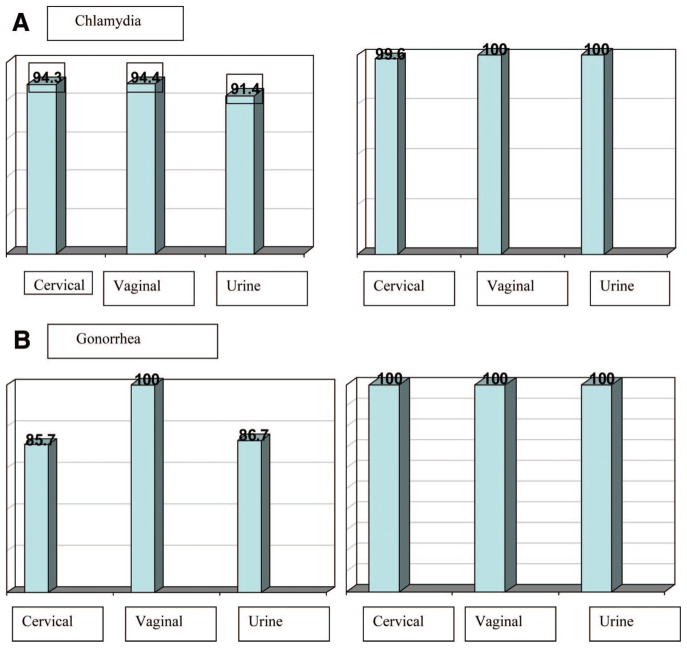
The overall prevalence for CT, NG, TV, and MG was 11.1%, 4.6%, 15.3%, and 19.2%, respectively (Table 2). Virtually all of the CT and GC infections were confirmed either by having 2 sample types positive or by the standalone ACT and AGC assay (Fig. 1A and B). In all cases, the prevalence for each organism was greater in those participants with cervicitis (15.8%, 6.0%, 18.9%, and 28.6% for CT, NG, TV, and MG, respectively) than those without cervicitis (7.8%, 3.7%, 12.8%, and 12.7% for CT, NG, TV, and MG, respectively). Overall, the percentage of coinfections with another organism for those infected with at least one organism ranged from 30.6% for TV-infected women to 73.3% for NG-infected women. For those with cervicitis, the percentage of those coinfected was not consistently greater as compared with participants without cervicitis.
Table 2.
Prevalence and Coinfection of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium in Women With and Without Cervicitis
| Prevalence |
%Coinfection |
||||||
|---|---|---|---|---|---|---|---|
| # | % | 95% CI | # | % | 95% CI | ||
| Overall N = 324 | CT | 36/324 | 11.1 | 7.9–15.0 | 21/36 | 58.3 | (40.8–74.5) |
| NG | 15/324 | 4.6 | 2.6–7.5 | 11/15 | 73.3 | (44.9–92.2) | |
| TV | 49/320* | 15.3 | 11.6–19.7 | 15/49 | 30.6 | (18.2–45.4) | |
| MG | 62/322* | 19.2 | 15.1–24.0 | 23/62 | 37.1 | (25.2–50.3) | |
| Cervicitis N = 133 | CT | 21/133 | 15.8 | 10.0–23.1 | 12/21 | 57.1 | (34.0–78.2) |
| NG | 8/133 | 6.0 | 2.6–11.5 | 6/8 | 75 | (34.9–96.8) | |
| TV | 25/132* | 18.9 | 12.6–26.7 | 9/25 | 36 | (18.0–57.5) | |
| MG | 38/133 | 28.6 | 21.1–37.1 | 13/38 | 34.2 | (19.6–51.4) | |
| No cervicitis N = 191 | CT | 15/191 | 7.8 | 4.5–12.6 | 9/15 | 60 | (32.3–83.7) |
| NG | 7/191 | 3.7 | 1.5–7.4 | 5/7 | 71.4 | (29.0–96.3) | |
| TV | 24/188* | 12.8 | 8.4–18.4 | 6/24 | 25 | (9.8–46.7) | |
| MG | 24/189* | 12.7 | 8.3–18.3 | 10/24 | 41.7 | (22.1–63.4) | |
For some women results for T. vaginalis and or M. genitalium were not available.
Figure 1.
A, Percent sensitivity and specificity according to infected patient status for Chlamydia trachomatis 11.1% (36/324). (N = 319 cervical swabs; 322 self-administered vaginal swabs; 324 urines). B, Percent sensitivity and specificity for infected patient status for Neisseria gonorrhoeae 4.6% (15/324). (N = 318 cervical swabs; 322 self-administered vaginal swabs; 324 urines).
The prevalence of BV and candidiasis (yeast) determined using routine clinic methods was 54.5% (174/319) and 12.8% (41/319), respectively (5 women were missing results for routine clinic methods) (Table 3). Sixteen women had positive TV NAAT test and negative wet-preparation results and 5 women had a positive wet-preparation but negative TV NAAT results (Table 3).
Table 3.
Prevalence of Bacterial Vaginosis, Candida, and Trichomonas Using Clinic Results
| Prevalence |
|||
|---|---|---|---|
| # | % | 95% CI | |
| BV | 174/319 | 54.5% | 48.9–60.1 |
| Candida | 41/319 | 12.8% | 9.38–17.03 |
| Trichomonas–wet preparation | 38/319 | 11.9% | 8.57–15.98 |
For 5 women, clinic results were not available.
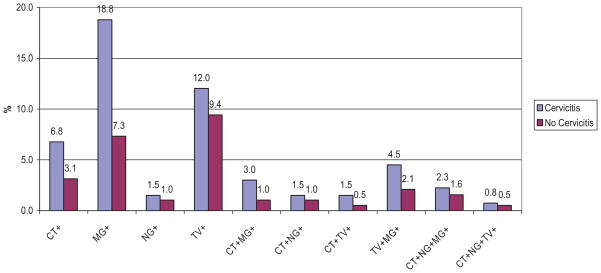
A summary of organisms detected for those with cervicitis and without cervicitis is shown in Table 4 and Figure 2. For those with cervicitis, at least one of the organisms (CT, NG, TV, or MG) was detected in 52.6% of the participants as compared with 29.3% of the participants who had at least one organism without cervicitis. The most frequent coinfection was CT with MG, which was found in 5.3% (7/133) of women with cervicitis and 2.1% (6/191) of women without cervicitis. Some of the CT and MG coinfections also included infection with either NG or TV. Coinfection of TV with MG, was found in 4.5% (6/133) of women with cervicitis and 2.6% (5/191) of women without cervicitis.
Table 4.
Summary of Organism Infection Status for Cervicitis and No Cervicitis
| CT | NG | TV | MG | All |
Cervicitis + |
Cervicitis − |
|||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||||
| N | N | N | N | 198 | 61.1 | 63 | 47.4 | 135 | 70.7 |
| N | N | N | P | 39 | 12.0 | 25 | 18.8 | 14 | 7.3 |
| N | N | P | N | 34 | 10.5 | 16 | 12.0 | 18 | 9.4 |
| N | N | P | P | 10 | 3.1 | 6 | 4.5 | 4 | 2.1 |
| N | N | X | N | 1 | 0.3 | 0 | 0.0 | 1 | 0.5 |
| N | N | X | X | 2 | 0.6 | 0 | 0.0 | 2 | 1.0 |
| N | P | N | N | 4 | 1.2 | 2 | 1.5 | 2 | 1.0 |
| P | N | N | N | 15 | 4.7 | 9 | 6.8 | 6 | 3.1 |
| P | N | N | P | 5 | 1.5 | 3 | 2.3 | 2 | 1.0 |
| P | N | P | N | 3 | 0.9 | 2 | 1.5 | 1 | 0.5 |
| P | N | P | P | 1 | 0.3 | 0 | 0.0 | 1 | 0.5 |
| P | N | X | P | 1 | 0.3 | 1 | 0.8 | 0 | 0.0 |
| P | P | N | N | 4 | 1.2 | 2 | 1.5 | 2 | 1.0 |
| P | P | N | P | 6 | 1.9 | 3 | 2.3 | 3 | 1.6 |
| P | P | P | N | 1 | 0.3 | 1 | 0.8 | 0 | 0.0 |
CT, C. trachomatis; NG, N. gonorrhoeae; TV, T. vaginalis; MG, M. genitalium; P, Positive; N, Negative; X, missing result.
Fig 2.
Percentage of Infections in women with (N = 133) and without (N = 191) cervicitis.
Univariate Analysis and Multiple Logistic Regression
Univariate analysis was performed to assess the association of each of the organisms with cervicitis (Table 5). CT was associated with cervicitis (OR: 2.20; 95% CI: 1.03– 4.44, P value = 0.03). Women with MG were over 2.5 times more likely to have cervicitis (OR: 2.75; 95% CI: 1.56 – 4.86, P value < 0.001) than women without cervicitis. Women with cervicitis were more likely to have NG and TV than women without cervicitis; however, NG and TV were not statistically significantly associated with cervicitis in this study. Age less than or equal to 25 years, BV, and contact status were not found to be significantly associated with cervicitis.
Table 5.
Univariate and Multiple Logistic Regression Models of Organisms Associated With Cervicitis
| n = 133 Cerv + | n = 191 Cerv − | Univariate |
Model 1 Multiple Logistic Regression |
Model 2 Multiple Logistic Regression |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |||
| CT | 15.8 | 7.8 | 2.20 | (1.09–4.45) | 0.02 | 1.83 | (0.82–4.12) | 0.14 | 1.81 | (0.79–4.14) | 0.16 |
| NG | 6.0 | 3.7 | 1.68 | (0.59–4.76) | 0.32 | 0.98 | (0.29–3.29) | 0.97 | 1.01 | (0.29–3.53) | 0.99 |
| TV | 18.9 | 12.8 | 1.60 | (0.87–2.94) | 0.13 | 1.57 | (0.84–2.94) | 0.15 | 1.74 | (0.92–3.310) | 0.09 |
| MG | 28.6 | 12.7 | 2.75 | (1.56–4.86) | <0.001 | 2.5 | (1.40–4.46) | 0.002 | 2.41 | (1.32–4.40) | 0.004 |
| Age ≥25 | 68.4 | 60.2 | 1.43 | (0.90–2.28) | 0.13 | 1.22 | (0.74–2.01) | 0.44 | |||
| Bacterial vaginosis | 60.2 | 50.5 | 1.48 | (0.94–2.32) | 0.09 | 1.51 | (0.95–2.41) | 0.08 | |||
| Reason for visit = contact | 6.8 | 10.5 | 0.62 | (0.27–1.41) | 0.25 | 0.46 | (0.19–1.10) | 0.08 | |||
No significant interactions were found.
Cerv indicate cervicitis.
Because of the concern over the impact of coinfections on the analysis, similar analyses were performed using data from those singly infected with CT, NG, TV, or MG and the results of these analyses yielded similar conclusions (data not shown). Therefore, women with a single infection and coinfected women were included in the multiple logistic regression modeling. In addition, a model was fit containing 2-way interaction terms for all of the organisms. Because none of the interaction terms were significant, they were not included in either model.
Two separate multiple logistic regression models were performed (Table 5). Model 1 contained CT, NG, TV, and MG. Two-way interactions were tested in the model. No significant interactions were found (data not shown). In model 1, only MG was strongly associated with cervicitis (OR: 2.50; 95% CI: 1.40 – 4.46; P value = 0.002). Model 2 contained CT, NG, TV, and MG as well as the possible confounders that were identified a priori - contact referral and BV. Only MG was found to be associated with cervicitis. CT was not found to be significantly associated with cervicitis in the multiple logistic models. The odds ratios for the association of CT with cervicitis in univariate and multiple logistic regression were 2.2 and 1.8, respectively.
DISCUSSION
Prevalence and Coinfection
Cervicitis was diagnosed in 41.0% (133/324) of the women. The prevalence of infection with CT and NG in this population was 11.1 and 4.6%, respectively, which is lower than the prevalence reported in some previous clinic studies. Typical prevalence for CT ranges from 5% for asymptomatic women to over 20% in women attending STD clinics, whereas prevalence for NG is generally less.23 In 1996, Quinn reported prevalence for Chlamydia detected by PCR of 15.8% in women attending Baltimore City STD clinics.24 In 1993, Lyss et al. in an analysis of data collected as part of Project RESPECT found an average prevalence of CT and NG for 5 participating STD clinics were 15% and 9%, respectively, whereas in Baltimore the prevalence of CT and NG was 18% and 14%, respectively.25 We speculate that the intensive universal Chlamydia screening programs associated with the Centers for Disease Control and Prevention’s Infertility Prevention Project in the past 10 years may be having a positive impact on lowering Chlamydia prevalence in women in Baltimore. We do not think the lower prevalence was due to our strict gold standard definition of requiring 2 positive tests or 2 positive specimens for CT and GC, as virtually most all positives for CT and GC were confirmed by the stand-alone test or by having more than one positive specimen per patient. In fact, since 3 specimen types (cervical, vaginal, and urine) were collected and tested for CT and GC, it may have added to the positivity rate observed for cervical samples, since more positive tests were observed and confirmed using vaginal samples. The women in our study also reported a high rate of satisfaction with self-collected vaginal swabs, which was important since all four organisms could be detected using vaginal swabs.
We found a prevalence of 15.3% (49) for TV in our study using NAAT compared with 11.9% (38) using wet-preparation microscopy. The prevalence compared well with previous studies of TV in our STD clinics.26 Sensitivity and specificity of wet-preparation microscopy for TV are estimated to be between 50% to 60% and >90%, respectively, whereas sensitivity and specificity of PCR for TV are both >90%, respectively.27–29 In this study, there was less difference between wet-preparation and NAAT than expected, but this might be explained by the fact that both NAAT tests used were required to be positive to diagnose a subject with TV infection. Because of the lower sensitivity of the wet-preparation method for diagnosis of TV, a significant percentage of infections may be routinely missed, which allows for sexual transmission to partners, as well as discomfort and possible harmful sequelae in untreated women. In a large study of over 3000 women attending 2 STD clinics, Kaydos et al. found a prevalence of 17% using a reference standard of wet-preparation microscopy and culture.30 Prevalence of MG, not previously measured in this population, was found to be 19.2%. MG was found in women both with (28.6%) and without cervicitis (12.7%). Earlier studies have reported lower prevalence of MG in cervicitis than that detected in this study.31,32 Explanations for these differences are not clear and require more study.
The percentage of subjects with coinfections with other organisms was greater than 30% for each pathogen. In women with cervicitis, 19.5% were infected with MG alone as compared with 3.9% infected with MG and CT. The observed percentage of coinfection of CT and MG was higher than in some studies, which have reported little or no coinfection of MG with CT.33
The prevalence of BV among women with cervicitis was 60.2% as compared with 50.3% in women without cervicitis. In this study BV, was not significantly associated with cervicitis (P = 0.08). There was also no association between BV and CT and/or BV and MG (data not shown).
Risk Factors for Cervicitis
Race, increased number of partners, and “high-risk sex” (sex with alcohol, sex with drugs, etc.) (protective) were all associated with cervicitis in univariate analysis. Race was significant when analyzed as a categorical or a binary variable. However, greater than 90% of the women were black, so the true effect of race was difficult to interpret. “High-risk sex” was not associated with cervicitis in multivariate analysis.
There was a marked difference in the number of cases of cervicitis between the 2 clinics. Reasons for differences in occurrence of cervicitis by clinic might be differences in patient population, selection bias in enrollment, or misclassification of cervicitis. Selection bias might occur if patients were more or less likely to participate based on another factor such as presence of symptoms. The diagnosis of cervicitis was based on the presence of signs as recorded by the clinician. If the clinician(s) varied in their assessment of these signs, the result could be misclassification. If the misclassification were nondifferential about disease status, then the estimates for clinic may be biased towards the null. Adjustment for clinic site in the model did not change the results.
In models using multiple logistic regression, MG was the only pathogen to be associated with cervicitis. Others have reported an association of MG with cervicitis.10,31,34 –36 We expected CT, NG, and possibly TV to be associated with cervicitis based on previously reported literature.2– 4,8 Although CT was associated with cervicitis in univariate models, CT was not found to be statistically associated with cervicitis in the multiple logistic models after controlling for NG, TV, and MG (OR = 1.8 vs. univariate OR = 2.2). However, the clinical interpretations of these findings are not different; an increased risk of cervicitis (i.e., higher OR) was found in those with CT infection.
The absence of association of NG with cervicitis could be due to the small number of women who tested positive for NG (only 15 women). However, the prevalence of CT, NG, TV, and MG was higher in women with cervicitis than in women without cervicitis. Neither BV nor candidiasis was also associated with cervicitis in this study. This is contrary to several recent studies that have shown an association of BV with cervicitis.37–39 No other demographic or behavioral variables were associated with cervicitis in multiple logistic regression models.
In conclusion, our study demonstrated a high prevalence of infections with CT, TV, and MG among women attending Baltimore City STD Clinics. In addition, we corroborated findings of others of a strong association of MG with cervicitis.10,34 –36 However, in contrast to our findings, one study of adolescent women reported that MG was associated with CT and recent sexual contact, but not with vaginal symptoms or signs of cervicitis.40 That study reported MG infection was as common as CT infection and TV and more common than gonorrhea.40
With regard to recent findings about MG associated PID sequelae, Haggerty et al. detected MG in 7 (14%) of 50 women with nongonococcal, nonchlamydial endometritis: 6 (12%) in cervical specimens and 4 (8%) in endometrial specimens, concluding that MG was prevalent in the endometrium of women with nongonococcal, nonchlamydial PID.41 This group also reported that the clinical presentation of PID associated with MG monoinfection, as compared with monoinfection, gonococcal PID, was milder with regard to symptoms and inflammatory signs, such as mucopurlent cervicitis, erythrocyte sedimentation rate, white cell count, and fever.42
High rates of coinfections were found in our study. These findings have important implications in the diagnosis and treatment of cervicitis. Some studies have shown success when treatment efforts for cervicitis are supplemented with metronidazole, which is effective in treating BV and TV.43
There are no current CDC recommended treatment guidelines for MG. Recent limited data indicate that antibiotics used to treat CT and NG may not be effective in all cases of MG. Paavonen et al. reported initial success with treatment of cervicitis with doxycycline and amoxicillin, but there was a high rate (>20%) of persistence or recurrence of cervicitis.44 Azithromycin was demonstrated to be effective at treating cervicitis caused by MG.35,45 However, more recently Bradshaw et al. found a failure rate of 28% for azithromycin when administered to men with MG positive, NGU whereas administration of moxyfloxacin resulted in symptom resolution and eradication of infection, but it is unknown if similar results occur in women.46 During the period of this study, women attending Baltimore City STD Clinics were routinely treated for GC with ciprofloxacin and for CT with doxycycline. More studies are needed to predict the best antibiotic for treating cervicitis caused by MG. In addition, more studies will be required to further confirm that clinical sequelae, such as PID,41 are caused by M. genitalium, a new cervicitis-associated pathogen.10,34 –36
Acknowledgments
The tests performed for Transcription Mediated Amplifications Tests were conducted using APTIMA reagents provided by GenProbe, Inc, San Diego, CA, and supported in part by The HIV Prevention Trials Network (HPTN) sponsored by the NIAID, National Institutes of Child Health and Human Development (NICH/HD), National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the NIH, DHHS (U01-AI-068613), and by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD.
References
- 1.Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among Am youth: Incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 2.Paavonen J, Critchlow CW, DeRouen T, et al. Etiology of cervical inflammation. Am J Obstet Gynecol. 1986;154:556–564. doi: 10.1016/0002-9378(86)90601-0. [DOI] [PubMed] [Google Scholar]
- 3.Brunham RC, Paavonen J, Stevens CE, et al. Mucopurulent cervicitis–the ignored counterpart in women of urethritis in men. N Engl J Med. 1984;311:1–6. doi: 10.1056/NEJM198407053110101. [DOI] [PubMed] [Google Scholar]
- 4.Marrazzo JM. Mucopurulent cervicitis: No longer ignored, but still misunderstood. Infect Dis Clin North Am. 2005;19:333–349. doi: 10.1016/j.idc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet. 2002;359:765–766. doi: 10.1016/S0140-6736(02)07848-0. [DOI] [PubMed] [Google Scholar]
- 6.Holmes K, Stamm WE. Lower genital tract infection syndromes in women. In: Homes KK, Sparling F, Mardh PA, et al., editors. Sexually Transmitted Diseases. 3. New York, NY: McGraw-Hill; 1999. pp. 761–781. [Google Scholar]
- 7.Marrazzo JM, Celum CL, Hillis SD, et al. Performance and cost-effectiveness of selective screening criteria for Chlamydia trachomatis infection in women. Implications for a national Chlamydia control strategy. Sex Transm Dis. 1997;24:131–141. doi: 10.1097/00007435-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Marrazzo JM, Handsfield HH, Whittington WL. Predicting chlamydial and gonococcal cervical infection: Implications for management of cervicitis. Obstet Gynecol. 2002;100:579–584. doi: 10.1016/s0029-7844(02)02140-3. [DOI] [PubMed] [Google Scholar]
- 9.Cohen CR, Mugo NR, Astete SG, et al. Detection of Mycoplasma genitalium in women with laparoscopically diagnosed acute salpingitis. Sex Transm Infect. 2005;81:463–466. doi: 10.1136/sti.2005.015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis. 2003;187:650–657. doi: 10.1086/367992. [DOI] [PubMed] [Google Scholar]
- 11.Schlicht MJ, Lovrich SD, Sartin JS, et al. High prevalence of genital mycoplasmas among sexually active young adults with urethritis or cervicitis symptoms in La Crosse, Wisconsin. J Clin Microbiol. 2004;42:4636–4640. doi: 10.1128/JCM.42.10.4636-4640.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Short VL, Totten PA, Ness RB, et al. Clinical presentation of Mycoplasma genitalium infection versus Neisseria gonorrhoeae infection among women with pelvic inflammatory disease. Clin Infect Dis. 2009;48:41–47. doi: 10.1086/594123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hjorth SV, Bjornelius E, Lidbrink P, et al. Sequence-based typing of Mycoplasma genitalium reveals sexual transmission. J Clin Microbiol. 2006;44:2078–2083. doi: 10.1128/JCM.00003-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaydos CA, Quinn TC, Willis D, et al. Performance of the APTIMA Combo 2 assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J Clin Microbiol. 2003;41:304–309. doi: 10.1128/JCM.41.1.304-309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chernesky MA, Martin DH, Hook EW, et al. Ability of new APTIMA CT and APTIMA GC assays to detect Chlamydia trachomatis and Neisseria gonorrhoeae in male urine and urethral swabs. J Clin Microbiol. 2005;43:127–131. doi: 10.1128/JCM.43.1.127-131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: Results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis. 2005;32:725–728. doi: 10.1097/01.olq.0000190092.59482.96. [DOI] [PubMed] [Google Scholar]
- 17.Hardick A, Hardick J, Wood BJ, et al. Comparison between the Prototype Gen-Probe TMA Trichomonas vaginalis assay and a Real-time PCR for Trichomonas vaginalis using the Roche Lightcycler Instrument in Female self-administered vaginal swabs and Male urine samples. J Clin Microbiol. 2006;44:4197–4199. doi: 10.1128/JCM.01447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardick J, Yang S, Lin S, et al. Use of the Roche LightCycler instrument in a real-time PCR for Trichomonas vaginalis in urine samples from females and males. J Clin Microbiol. 2003;41:5619–522. doi: 10.1128/JCM.41.12.5619-5622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida T, Deguchi T, Ito M, et al. Quantitative detection of Mycoplasma genitalium from first-pass urine of men with urethritis and asymptomatic men by real-time PCR. J Clin Microbiol. 2002;40:1451–1455. doi: 10.1128/JCM.40.4.1451-1455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen JS, Borre MB, Dohn B. Detection of Mycoplasma genitalium by PCR amplification of the 16S rRNA gene. J Clin Microbiol. 2003;41:261–266. doi: 10.1128/JCM.41.1.261-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardick J, Giles J, Hardick A, et al. Performance of the gen-probe transmission-mediated amplification research assay compared to that of a multitarget real-time PCR for Mycoplasma genitalium detection. J Clin Microbiol. 2006;44:1236–1240. doi: 10.1128/JCM.44.4.1236-1240.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 23.Stamm WE. Chlamydia trachomatis infections of the adult. In: Holmes KK, Sparling FP, Mardh P, et al., editors. Sexually Transmitted Diseases. 3. New York, NY: McGraw Hill; 1999. pp. 407–422. [Google Scholar]
- 24.Quinn TC. Association of sexually transmitted diseases and infection with the human immunodeficiency virus: Biological cofactors and markers of behavioral interventions. Int J STD AIDS. 1996;7(suppl 2):17–24. doi: 10.1258/0956462961917735. [DOI] [PubMed] [Google Scholar]
- 25.Lyss SB, Kamb ML, Peterman TA, et al. Chlamydia trachomatis among patients infected with and treated for Neisseria gonorrhoeae in sexually transmitted disease clinics in the United States. Ann Intern Med. 2003;139:178–185. doi: 10.7326/0003-4819-139-3-200308050-00007. [DOI] [PubMed] [Google Scholar]
- 26.Wendel KA, Erbelding EJ, Gaydos CA, et al. Trichomonas vaginalis polymerase chain reaction compared with standard diagnostic and therapeutic protocols for detection and treatment of vaginal trichomoniasis. Clin Infect Dis. 2002;35:576–580. doi: 10.1086/342060. [DOI] [PubMed] [Google Scholar]
- 27.Soper D. Trichomoniasis: Under control or undercontrolled? Am J Obstet Gynecol. 2004;190:281–290. doi: 10.1016/j.ajog.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Madico G, Quinn TC, Rompalo A, et al. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J Clin Microbiol. 1998;36:3205–3210. doi: 10.1128/jcm.36.11.3205-3210.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wendel KA, Erbelding EJ, Gaydos CA, et al. Use of urine polymerase chain reaction to define the prevalence and clinical presentation of Trichomonas vaginalis in men attending an STD clinic. Sex Transm Infect. 2003;79:151–153. doi: 10.1136/sti.79.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaydos SC, Swygard H, Wise SL, et al. Development and validation of a PCR-based enzyme-linked immunosorbent assay with urine for use in clinical research settings to detect Trichomonas vaginalis in women. J Clin Microbiol. 2002;40:89–95. doi: 10.1128/JCM.40.1.89-95.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uno M, Deguchi T, Komeda H, et al. Mycoplasma genitalium in the cervices of Japanese women. Sex Transm Dis. 1997;24:284–286. doi: 10.1097/00007435-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Moller BR, Taylor-Robinson D, Furr PM. Serological evidence implicating Mycoplasma genitalium in pelvic inflammatory disease. Lancet. 1984;1:1102–1103. doi: 10.1016/s0140-6736(84)92511-x. [DOI] [PubMed] [Google Scholar]
- 33.Simms I, Eastick K, Mallinson H, et al. Associations between Mycoplasma genitalium, Chlamydia trachomatis, and pelvic inflammatory disease. J Clin Pathol. 2003;56:616–618. doi: 10.1136/jcp.56.8.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falk L, Fredlund H, Jensen JS. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect. 2005;81:73–78. doi: 10.1136/sti.2004.010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor-Robinson D. Mycoplasma genitalium – an up-date. Int J STD AIDS. 2002;13:145–151. doi: 10.1258/0956462021924776. [DOI] [PubMed] [Google Scholar]
- 36.Anagrius C, Lore B, Jensen JS. Mycoplasma genitalium: prevalence, clinical significance, and transmission. Sex Transm Infect. 2005;81:458–462. doi: 10.1136/sti.2004.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keshavarz H, Duffy SW, Sadeghi-Hassanabadi A, et al. Risk factors for and relationship between bacterial vaginosis and cervicitis in a high risk population for cervicitis in Southern Iran. Eur J Epidemiol. 2001;17:89–95. doi: 10.1023/a:1010935723248. [DOI] [PubMed] [Google Scholar]
- 38.Peipert JF, Montagno AB, Cooper AS, et al. Bacterial vaginosis as a risk factor for upper genital tract infection. Am J Obstet Gynecol. 1997;177:1184–1187. doi: 10.1016/s0002-9378(97)70038-3. [DOI] [PubMed] [Google Scholar]
- 39.Willmott FE. Mucopurulent cervicitis: A clinical entity? Genitourin Med. 1988;64:169–171. doi: 10.1136/sti.64.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huppert JS, Mortensen JE, Reed JL, et al. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis. 2008;35:250–254. doi: 10.1097/OLQ.0b013e31815abac6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haggerty CL, Totten PA, Astete SG, et al. Mycoplasma genitalium among women with nongonococcal, nonchlamydial pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2006;2006:30184. doi: 10.1155/IDOG/2006/30184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Short VL, Totten PA, Ness RB, et al. Clinical presentation of Mycoplasma genitalium infection among women with pelvic inflammatory disease. Clin Infect Dis. 2009;48:41–47. doi: 10.1086/594123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwebke JR, Weiss HL. Interrelationships of bacterial vaginosis and cervical inflammation. Sex Transm Dis. 2002;29:59–64. doi: 10.1097/00007435-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Paavonen J, Roberts PL, Stevens CE, et al. Randomized treatment of mucopurulent cervicitis with doxycycline or amoxicillin. Am J Obstet Gynecol. 1989;161:128–135. doi: 10.1016/0002-9378(89)90249-4. [DOI] [PubMed] [Google Scholar]
- 45.Falk L, Fredlund H, Jensen JS. Tetracycline treatment does not eradicate Mycoplasma genitalium. Sex Transm Infect. 2003;79:318–319. doi: 10.1136/sti.79.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradshaw CS, Jensen JS, Tabrizi SN, et al. Azithromycin failure in Mycoplasma genitalium urethritis. Emerg Infect Dis. 2006;12:1149–152. doi: 10.3201/eid1207.051558. [DOI] [PMC free article] [PubMed] [Google Scholar]