Abstract
Cell motility and migration play pivotal roles in numerous physiological and pathophysiological processes including development and tissue repair. Cell migration is regulated through external stimuli such as platelet-derived growth factor-AA (PDGF-AA), a key regulator in directional cell migration during embryonic development and a chemoattractant during postnatal migratory responses including wound healing. We previously showed that PDGFRα signaling is coordinated by the primary cilium in quiescent cells. However, little is known about the function of the primary cilium in cell migration. Here we used micropipette analysis to show that a normal chemosensory response to PDGF-AA in fibroblasts requires the primary cilium. In vitro and in vivo wound healing assays revealed that in ORPK mouse (IFT88Tg737Rpw) fibroblasts, where ciliary assembly is defective, chemotaxis towards PDGF-AA is absent, leading to unregulated high speed and uncontrolled directional cell displacement during wound closure, with subsequent defects in wound healing. These data suggest that in coordination with cytoskeletal reorganization, the fibroblast primary cilium functions via ciliary PDGFRα signaling to monitor directional movement during wound healing.
Key Words: Fibroblasts, Cell migration, Primary cilia, Wound healing, PDGFRα, PDGF-AA
Introduction
Directional cell migration plays a critical role in embryonic development and in maintenance of tissue homeostasis. Migration relies heavily on the concerted action of chemosensory stimuli; the dynamic reorganization of the cytoskeleton, particularly the actin cytoskeleton [1-6]; the formation and release of cell-matrix contacts and the local ion homeostasis across the plasma membrane [7, 8]. Migration is directed when a chemotactic gradient is imposed onto the cells. Platelet-derived growth factors (PDGFs) are important chemosensory regulators of the migratory response during development and in wound healing [9, 10]. PDGFs are essential chemoattractants that promote distribution of oligodendrocyte progenitors throughout the developing CNS in vivo [11, 12], while PDGF receptors (PDGFRs) are strongly expressed in the neural crest mesenchyme and are mandatory for spreading/migration of various populations of cells [11-13]. Additionally, PDGFs are significant chemoattractants during postnatal migratory responses such as in wound healing [14]. As part of the mechanisms that control speed and directionality of migrating cells, the Na+/H+-exchanger, NHE1, which is activated by PDGF-signaling, localizes to the leading edge of migrating fibroblasts to develop and maintain polarization along the front to rear axis of the cells [15-19].
In NIH3T3 fibroblasts and mouse embryonic fibroblasts (MEFs), PDGFRβ is localized at the cell membrane [20]. In fibroblasts, PDGFRβ signaling operates in part via the Nck family of Src homology (SH) 2/SH3 domain adaptors to regulate downstream modulators of actin dynamics [2, 4]. Nck adaptors are required for cytoskeletal reorganization and chemotaxis stimulated by PDGF-BB. Nck-deficient cells fail to display cytoskeletal rearrangements, including the formation of membrane ruffles and the disassembly of F-actin, typically shown by their wild type (wt) counterparts in response to PDGF-BB. Other proteins, such as Akt and Rac-1, controlling actin polymerization, bundling and disassembly, are also influenced by PDGFRβ signals [3, 5, 6].
PDGFRα signaling, along with PDGFR-β signaling, regulates cell migration [10]. PDGF-AA acts exclusively through the PDGFRα homodimer, PDGFRαα. We previously showed that during growth arrest (in the G0/G1 phase) in fibroblasts, PDGFRα expression is up-regulated and the receptor is targeted to the primary cilium where ligand-dependent activation of the receptor and the Mek1/2-Erk1/2 pathway occurs, indicating that PDGF-AA-mediated signaling via the PDGFRα homodimer in cell cycle entry is coordinated by the primary cilium [20].
The primary cilium is a microtubule-based organelle that emanates from the mother centriole into the extracellular environment as an antenna-like structure. In most cultured cells, primary cilia emerge during growth arrest, i.e., at G0/G1, following centrosomal docking to the plasma membrane. In most cases, the cilium is disassembled in late G2, so that the engaged centrioles are available for mitotic spindle formation [21-23]. In the present work, we have used NIH 3T3 cells and primary cultures of MEFs, which we previously showed form primary cilia only during growth arrest after serum starvation for 24–48 h, and cilia are absent in subconfluent cells grown in the presence of serum [20]. As a control we used fibroblasts from the ORPK (IFT88Tg737Rpw) mouse, which we will refer to as Tg737 MEFs. Tg737 encodes the protein polaris/IFT88, which is part of the intraflagellar transport (IFT) protein complex responsible for assembly and maintenance of the primary cilium [24]. Consequently, Tg737 MEFs form no or very short cilia [20]. A single primary cilium contains many different signal transduction systems in order to carry out diverse signaling processes during development and in tissue homeostasis [25], and it is likely that the composition of signal systems closely reflects the functionality of the cell type in different tissues, i.e., that some ciliary signal systems are tissue specific. Some signaling interactions can be unique features of primary cilia on fibroblasts or mesenchymal cells, versus cilia that protrude from the apical surface into a lumen as seen on epithelial and endothelial cells.
Emerging evidence indicates that cell migration is directly or indirectly related to primary cilia assembly and/or ciliary signaling [26]. Originally, Albrecht Buehler discovered that primary cilia in migrating 3T3 fibroblasts were oriented predominantly in parallel to the substrate and to the current movement direction [27]. In various cell systems previous work has shown that the centrosome and Golgi apparatus come to lie in front of the nucleus and towards the direction of eventual cell migration [28-32]. In a more detailed study, Katsumoto et al. demonstrated that reorientation of primary cilia together with the centrosome and stable cytoplasmic microtubules in 3Y1 rat cells occurs prior to initiation of migration [33]. They proposed that the direction of migration is determined by the orientation of the centrioles, which is controlled by the primary cilium. More recently, in vitro wound healing assays provided other examples of orientation of primary cilia towards the leading edge in cultures of smooth muscle cells [34, 35].
Here we show that in response to PDGF-AA, migration of quiescent fibroblasts in culture is regulated by their primary cilium, such that cells from wt mice with normal primary cilia show chemotaxis towards a PDGF-AA gradient with an increase in migration speed and directional cell movement in wound closure in vitro. During migration the primary cilium is oriented parallelly to the direction of migration, often pointing towards the leading edge of the migrating cell. In contrast, Tg737 MEFs do not show chemotaxis, nor do they respond to PDGF-AA by regulating speed or direction during wound closure. Further, Akt is phosphorylated at the base of the primary cilium in wt MEFs in the presence of PDGF-AA, but this is blocked in mutant MEFs. The ORPK mice show a reduced rate of wound repair and defects in wound closure in vivo. These results indicate that signaling through the primary cilium, probably continuing via the PI3 kinase-Akt pathway, activates directional migration of tissue fibroblasts. In coordination with actin and microtubule cytoskeletal reorganization mediated through PDGF or other cell membrane-based signaling pathways, signaling through the primary cilium is necessary for the sensing of a PDGF-AA-mediated chemotactic gradient in wound healing.
Materials and Methods
Cell culturing
NIH3T3 mouse fibroblasts were grown in Dulbeccos modified Eagles medium (DMEM) supplemented with 10% fetal calf serum (FBS) and 100 U/ml penicillin-streptomycin at 37°C, 5% CO2, 95% humidity. Primary cell cultures of Mouse Embryonic Fibroblasts (MEFs) from wt and Tg737orpk mice were grown in 45% DMEM and 45% F12-ham supplemented with 10% FBS and 10ml 1−1 penicillin-streptomycin. Cells were serum starved for 48h to induce growth arrest. About 90% of the cells were ciliated after serum starvation. In some experiments cells were stimulated with 50ng/ml PDGF-AA (R&D Systems, 221-AA).
Immunofluorescence (IF) Microscopy
Cells were grown on glass cover slips to 100% confluence, serum starved for 48h and fixed in 4% formaldehyde or methanol [36], permeabilized in 0.2% triton X100, quenched in PBS with 2% BSA, and incubated with primary antibodies at room temperature for 2h. Cells were washed in PBS and incubated with secondary antibodies for 1h. Fluorescence was visualized on Microphox-FXA and Eclipse E600 microscopes (Nikon, Tokyo, Japan). Primary antibodies: Primary cilia were detected with mouse acetylated alpha-tubulin antibody (1:5000 Sigma, T6793) or anti-detyrosinated-tubulin (glu-tub;, 1:1000, Abcam, AB3201); phospho-Akt (1:5000, Cell Signaling, 587F11). Secondary antibodies: (1:600, GAR488 A11070, GAM568 A11019 Molecular Probes). Nuclei were stained with DAPI (Molecular Probes, D1306).
Construction of GFP-fusion protein (GFP-PDGFRα)
GFP-tagged PDGFRα was made by recombination cloning. pEGFP-C1 vector (GenBank, Accession, U55763) was linearized by digesting with HindIII (Biolabs, R0104S) and Age1 (Biolabs, R0145S). PDGFRα DNA was tagged with vector homology regions, generated by PCR (Primers containing additional 21 bases of the vector sequence 5’ to the PDGFRα-specific sequence, Sense TCT CGA GCT CAA GCT TTG GGG ACC TCC CAC C, antisense GAT CAG TTA TCT AGA T TAC AGG AAG CTG TCC; Amplification: Sense CAA GTC CGG ACT CAG ATC TCG AGC TCAAGC, Antisense GTG GTA TGG CTG ATT ATG ATC AGT TAT CTA G). PCR products were subcloned into a linearized vector and transformed into One Shot TOP10 Competent Cells (Invitrogen, C4040-10). Colonies were selected for kanamycin resistance. The GFP-PDGFRα construct was transfected using lipofectamine for 24h.
SDS PAGE, Immunoprecipitation and Western Blotting Analysis
Cells were grown in petri dishes and washed in PBS, 150 μl 0.1% SDS lysis buffer was added and cells were scraped off and transferred 10 times through a 27 gauge needle, followed by centrifugation at 16.000xg. The protein concentrations were calculated using a BCA protein kit (Pierce, 23209). Rabbit anti-PDGFRα was added to lysates for IP in RIPA buffer with no SDS and incubated overnight at 4°C. Protein A- and G-conjugated sepharose (1:1) equilibrated in RIPA was added and incubated for 2 h at room temperature. The beads were washed with RIPA and the precipitate was dissolved in sample buffer. Proteins from whole cell lysates and immunoprecipitates were separated by SDS PAGE on 10% NuPAGE Bis-Tris gels using NuPAGE MOPS SDS running buffer (NP0002), Fermentas protein standards and Novex XCell (E19001) system, and electrophoretically transferred to nitrocellulose membranes using XCell II blot module (Novex). Membranes were blocked for 2h at room temperature prior to incubation with primary antibodies in blocking buffer over night at 4°C: anti-PDGFRα (1:600, Santa Cruz, sc-338); anti-phospho-PDGFRα Y754 (1:200, Santa Cruz, sc-12911-R), anti-Akt (1:500, Cell Signaling, 9272); anti-phospho-Akt (1:200, Cell signaling 587F11) and anti-Phospho-Tyr (1:300, Santa Cruz, sc-18182), anti-pericentrin (1:500, Santa Cruz, sc-28145), anti-β-actin (1:10,000, SigmaAldrich, A5441). Primary antibodies were detected using alkaline phosphatase-coupled secondary antibodies in blocking buffer for 1h (GAR & GAM 1:1200, A3937 & A12293) and visualized with a 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium solution, BCIP/NBT (Kirkegaard and Perry Laboratories, Gaithersburg, MD). The developed blots were scanned and band intensity was estimated from arbitrary densitometric values obtained using UN-SCAN-IT software. The data were tested for significance using analysis of variance (ANOVA) or Kruskal Wallis Test (nonparametric ANOVA). The level of significance was set at p< 0.05; (∗∗∗): p<0.001; (∗∗): p<0.01.
Wound healing assays
Cells were grown to confluence in growth media and serum starved for 48h. A wound was made using a pipette tip and the culture medium was changed to fresh serum-free medium, and the cells were allowed to recover for 1 h in the incubator after the scratch was made. Subsequently, the cells were incubated with and without 50ng/ml PDGF-AA or 10% FBS and placed in a heating chamber (37°C) on the stage of an inverted microscope (Axiovert 25 or Axiovert 40C; 10x or 20x; Zeiss, Oberkochen, Germany). Migration was monitored for ∼4 h with a video camera (Hamamatsu, Hersching, Germany) controlled by HiPic software (Hamamatsu). Images were taken at 5 min intervals and stored as stacks of Tiff-files. The circumferences of individual cells were marked at each time step throughout the entire image stacks with Amira software (TGS, France; http://www.amiravis.com/) as described [37, 38]. These segmentation data were used for further analysis. Migration was quantified as the movement of the cell centre per time unit. All experiments were repeated at least three times and data in graphs are presented as the mean values +/− S.E.M. The data were tested for significance using analysis of variance (ANOVA) or Kruskal Wallis Test (nonparametric ANOVA). The level of significance was set at p< 0.05; (∗∗∗): p<0.001; (∗∗): p<0.01.
Micropipette Analysis
Cells were grown to 30–40% confluence and serum starved for 48 h. Chemotaxis experiments were performed by continuously ejecting small amounts of PDGF-AA from a micropipette (0.5 μg/ml in the pipette) to create a gradient in the vicinity of growth-arrested wt and Tg737orpk MEFs. Cell movement in the presence of a PDGF-AA gradient was monitored with time lapse video microscopy, taking images at 5 min intervals. For system specifications please see: http://www.aecom.yu.edu/aif/instructions/ccd4/index.htm. A Femtojet Micromanipulator 5171 (Eppendorf-Brinkman Instruments) and a pump (model Femtojet; Eppendorf) were used to control the position of the micropipette and the pressure required for the chemoattractant flow. Femptotip II micropipettes (defined opening with 0.5 μm inner diameter and 0.7 μm outer diameter (± μm) as per http://www.eppendorfna.com/products/ECET_tips_de.asp) were positioned within 1 μm of the cover slip and pressure set at 30 to 45 hPa [39]. The data were tested for significance using ANOVA or Kruskal Wallis Test: (∗∗∗): p<0.001: (∗∗): p<0.01. Symbols: (O) marks the tip of the micropipette.
In vivo Wound Healing of Wt and ORPK (IFT88Tg737Rpw) mutant mice
P15 ORPK mutant (mt) mice and corresponding wild type (wt) littermates were wounded via 4 mm full thickness punch biopsy and assessed for rate of wound closure by secondary intention. Wound closure was monitored by digital imaging or by using digital calipers (Fisher). Wound edges and areas were analyzed using Image J (Find Edges) algorithm and Image J scale and measurement tools or by calculating the wound ellipse area from caliper measurements. Significance was tested using a two-tailed student's t-test. Wound closure was defined as no scab or a scab < 2mm2. All animals in this study were maintained in AALAC certified mouse facilities at UAB and in accordance with IACUC regulations and protocols at the University of Alabama at Birmingham. The data were tested for significance using analysis of variance (ANOVA) or Kruskal Wallis Test (nonparametric ANOVA). The level of significance was set at p< 0.05; (∗∗): p<0.001; (∗): p<0.05.
Results
Orientation of primary cilia during in vitro wound healing
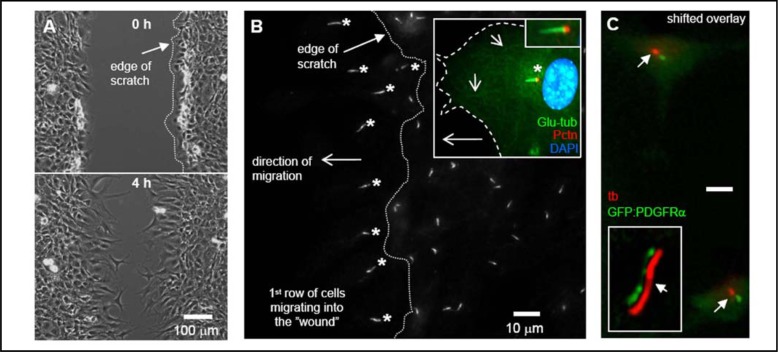
To investigate the role of the primary cilium in cell migration, we initially studied the orientation of the cilium during wound healing in cell cultures. Scratch assays were performed followed by immunofluorescence analysis on cultures of confluent, growth arrested NIH3T3 fibroblasts (Figure 1A). We found that in serum-free medium primary cilia in the first row of cells facing the wound often oriented towards the wound within 30–60 min after the scratch was made (Figure 1B). The primary cilium emanates from the centrosome that was found predominantly in front of the nucleus (Figure 1B, inset). Stable cytoplasmic microtubules marked with Glu-tub were also detected towards the leading edge (Figure 1B, inset). Occasionally, cilia were observed pointing towards the trailing edge, albeit still oriented parallelly to the direction of migration. Similar observations were reported in 3T3 cells [27], in 3Y1 cells [33], and in cultures of smooth muscle cells (SMC) [34, 35]. In contrast, primary cilia on non-migrating cells in subsequent rows were oriented in all directions and not specifically towards the wound (Figure 1B). IF analysis was performed to show GFP-tagged PDGFRα localization to the primary cilium in cultures of growth arrested MEFs (Figure 1C). This subcellular distribution is similar to that of the endogenous PDGFRα detected with antibodies [20].
Fig. 1.
Primary cilia orient in the direction of cell movement during wound healing. NIH 3T3 cells were grown to 100% confluency on glass cover slips and serum starved for 24–48h. Wounds were made in confluent layers of growth-arrested cells using a fine pipette tip (A). Immunofluorescence (IF) was used to detect orientation of the primary cilia pointing in the direction of cell migration (asterisks) during wound healing in the first row of cells facing the wound. Primary cilia were detected using mouse acetylated alpha-tubulin primary antibody (B). Insets in figure 1B show the orientation of the primary cilium detected with rabbit detyrosinated tubulin antibody (Glu-tub (green), asterisk) emerging from the centrosome detected with goat pericentrin antibody (Pctn, red) pointing towards the leading edge of a migrating cell (stippled line). The nucleus was stained with DAPI (blue) (B, insets). The shorter arrows in inset B show stable microtubule structures that points in the direction of the leading edge. Ciliary localization of GFP-tagged PDGFRα in cells serum starved to induce growth arrest and formation of primary cilia (C) [20]. The inset shows a high resolution image of GFP-PDGFRα in the cilium (shifted overlay). Primary cilia were detected with mouse acetylated alpha-tubulin antibody (tb (red), arrows) using IF microscopy.
Primary cilia coordinate PDGF-AA-mediated migration speed and directionality
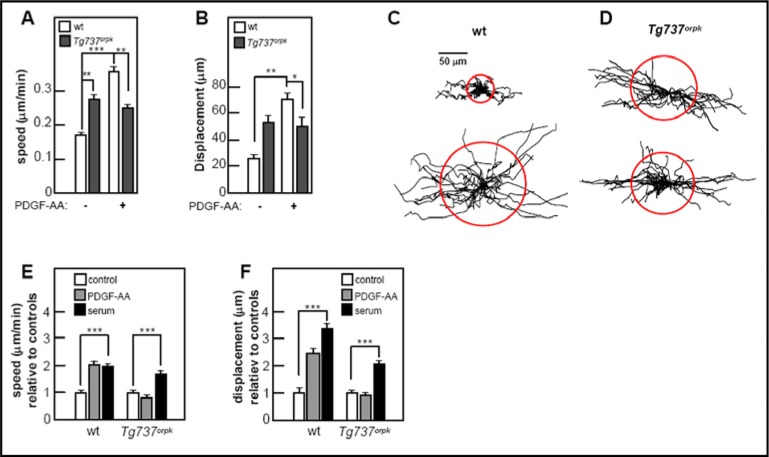
Next we determined the migratory speed and displacement of growth-arrested cells in scratch assays in the presence and in the absence of PDGF-AA, the specific ligand for dimerization of PDGFRα and activation of the homodimeric receptor [20, 40]. In these experiments we used primary cultures of mouse embryonic fibroblasts (MEF); wt MEF and Tg737orpk MEF. In the absence of ligand, Tg737orpk MEFs had a significantly higher migratory speed than wt MEFs (p<0.01); however, the mutant cells were unresponsive to PDGF-AA (2A). In contrast, the migratory speed of wt MEFs, initially lower than that of Tg737orpk MEFs, increased by about 40% (p<0.001) to a level higher than that of Tg737orpk MEFs (p<0.01) upon PDGF-AA addition (Figure 2A). Figures 2C & 2D show trajectories of growth arrested MEFs in the presence and absence of PDGF-AA. All trajectories are normalized to a common starting point and the radii of the red circles represent the mean distances covered within appr. 4 h. These data are summarized in Figure 2A. The mean displacement of wt MEFs increased three-fold upon incubation with the ligand (p<0.01), whereas mutant cells were unaffected by addition of PDGF-AA. In non-arrested cells, there were no differences in migratory speed or displacement between mutant and wt cells.
Fig. 2.
Primary cilia regulate PDGF-AA-mediated migratory speed and directional cell movement. Scratch assays were performed on confluent layers of serum starved wt and Tg737orpk MEFs serum with and without PDGF-AA or 10% FBS. Migration was monitored for ∼4 h for calculation of migration speed (μm/min) and cellular displacement (μm) with and without PDGF-AA (A & B). The displacement is the distance between the cells′ positions at the beginning and at the end of the experiment (B). Cell migration for growth-arrested wt and Tg737orpk MEFs was also illustrated by trajectories (C & D). The trajectories of 20–30 cells for each condition were normalized to common starting points. Each track represents the movement of one cell during the 4h period described in A & B. The speed (E) and displacement (F) of wt and Tg737orpk MEFs was also monitored in the presence of either PDGF-AA or serum relative to control cells.
To investigate whether migration in serum starved and growth-arrested wt and Tg737orpk MEFs can be stimulated by signals other than PDGF-AA, we performed scratch assays re-adding serum as a chemoattractant (Figures 2E and 2F). While wt cells responded equally well to PDGF-AA and serum, only serum increased the speed and displacement of mutant cells. The relative levels of response of mutant cells is lower than that of wt cells, since mutant cells have a higher initial migratory speed and displacement than wt without stimulus (Figure 2A & 2B). The migratory speed of wt and mutant cells in the presence of serum was identical (0.21±0.01 and 0.21±0.02 μm/min, respectively), and the displacement in the presence of serum was not significantly different (49.49± 3.59 and 51.84± 5.66 (μm).
PDGF-AA mediated chemotaxis is controlled by the primary cilium
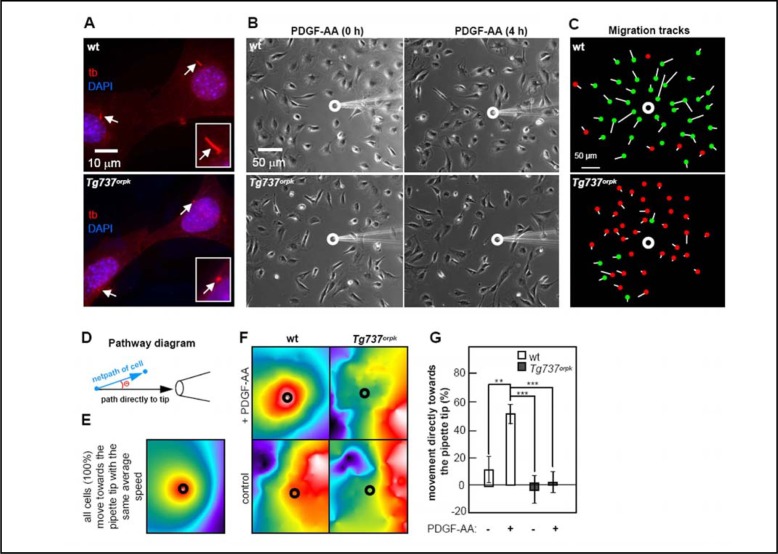
In order to directly investigate the role of fibroblast primary cilia in coordinated cell movement directly, we analysed the significance of the cilium for chemotaxis towards gradients of PDGF-AA. The gradient was established by continuously ejecting the ligand from a micropipette placed in the vicinity of the cells. We used growth-arrested cells at a confluence of ca. 50%, so that the cells could move in any direction along the surface of the culture dish. We observed that wt MEFs, which had primary cilia under these conditions (Figure 3A), were strongly affected by the gradient of PDGF-AA and moved directly towards the pipette tip. In sharp contrast, Tg737orpk MEFs, which have significantly shortened nonfunctional primary cilia (Figure 3A), did not respond to the gradient, and continued to move randomly (Figure 3B & 3C). Figure 3C summarizes the movement of individual wt or Tg737orpk cells monitored during a 4 h period. Starting and end-point positions of each cell are indicated by the white line and the green/red dots, respectively. Green dots represent the end-point of cells that moved directly towards the gradient; red dots label cells not moving towards the pipette. Before addition of PDGF-AA, Tg737orpk MEFs moved with a high frequency of “running on spot” with a high turnover of ruffling and formation of lamellipodia in different directions, but little cell displacement. A similar pattern of movement was observed for wt cells albeit at a lower frequency.
Fig. 3.
PDGF-AA mediated chemotaxis is controlled by the primary cilium. IF analysis of growth arrested wt and Tg737orpk MEFs shows defects in ciliary assembly in mutant cells (A). Primary cilia were detected with mouse acetylated alpha-tubulin antibody (tb (red), arrows). The nucleus was stained with DAPI (blue). PDGF-AA mediated chemotaxis was investigated using a micropipette to create a PDGF-AA gradient in the centre of growth-arrested wt and Tg737orpk MEF cultures. Cell movement in the presence of a gradient was monitored with time lapse video microscopy (B). Figure 3C shows data of growth arrested wt and Tg737orpk MEFs in the presence of PDGF-AA gradient. Green dots represent cells moving towards the pipette and red dots illustrate cells not moving towards the pipette. Thus, overall distance and direction of migration are visualized in this way. Figure 3D outlines the set of connections used to produce color scales of cells moving towards the tip of the micropipette. The net path is the straight line distance the cell′s centroid travels from the first to the final time point. The angle measured (Θ) is the angle this path deviates from a straight line directly to the pore of the micropipette. The less the deviation, the more red shifted a color. Figure 3E shows a simulated color scale for all cells moving uniformly towards the source of a chemoattractant, and Figure 3F shows quantified color scale analysis of wt and Tg737orpk MEF movement in the presence of PBS (negative control) and in the presence of the PDGF-AA gradient. The percentage of wt and Tg737orpk MEF moving towards the pipette tip in the presence or absence of PDGF-AA is illustrated in Figure 3G.
To visualize the overall patterns of migration in a systemic way, we developed a colorimetric analysis (Figure 3D). The coordinates of cell movement are converted into a color scale that shows the level of coordinated migration in the presence of a stimulus gradient. For comparison, we simulated the color scale for all cells moving uniformly towards the source of a chemoattractant (Figure 3E). This color scale was used as reference for our experimental analysis on migration of wt and Tg737orpk MEFs. In the presence of a PDGF-AA gradient, cells in cultures of wt MEFs produced a color scale similar to that of the reference (Figure 3F, upper left panel), whereas cells in the absence of the PDGF-AA gradient produced a scale visibly different from the reference, with no uniform migration towards the tip of the pipette (Figure 3F, lower left panel). Tg737orpk MEFs, both in the presence and absence of the PDGF-AA gradient, produced a color scale almost identical to that of wt MEFs with no gradient of PDGF-AA (Figure 3F, right panels). In sharp contrast to cells in cultures of Tg737orpk MEFs, where essentially no cells moved towards the gradient of PDGF-AA (p<0.001), quantitatively, about 50% of the cells in cultures of wt MEFs (p<0.01) moved uniformly and directly towards the gradient of PDGF-AA (Figure 3G).
PDGF-AA signals through Akt at the ciliary base
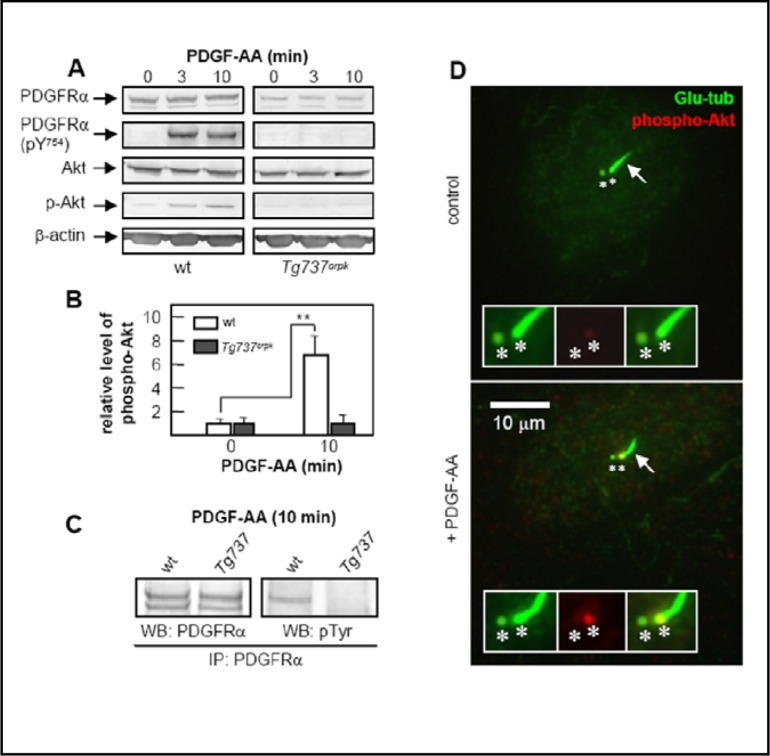
To explore the mechanism by which ciliary signaling via PDGFRα affects the speed and direction of migration, we investigated the activation of the receptor and the Akt-pathway upon stimulation with PDGF-AA in wt and Tg737orpk MEFs. Western blot analysis showed that PDGFRα and Akt are activated, with the level of Akt phosphorylation increasing about 7-fold in wt MEFs (Figure 4A & 4B). This increase was absent in Tg737orpk MEFs. PDGFRα is a growth arrest specific protein in fibroblasts [41], and we previously demonstrated that up-regulation of PDGFRα in quiescent cells is inhibited in Tg737orpk MEFs [20]. In order to investigate whether lack of activation of the receptor and Akt in Tg737orpk MEFs is due to lower levels of PDGFRα, equal amounts of PDGFRα were analyzed after immunoprecipitation from PDGF-AA-stimulated wt and Tg737orpk MEFs. Activation of the receptor occurred only in wt cells (Figure 4C). Then we used immunofluorescence microscopy to examine the localization of increased Akt phosphorylation in wt MEFs after PDGF-AA stimulation. We show that the level of phospho-Akt increases at the base of the cilium, i.e. at the mother centriole (Figure 4D). There is little or no increase in background staining of phospho-Akt in the cytoplasm.
Fig. 4.
PDGF-AA signals through the primary cilium and activates Akt at the ciliary basal body in the centrosome. Western blotting analysis of phospho-PDGFRα (phosphorylated at tyrosine in position 754) and phospho-Akt upon PDGF-AA stimulation in wt and Tg737orpk MEFs (A). Quantification of Akt phosphorylation in wt and Tg737orpk MEFs before and after stimulation with PDGF-AA (B), shows that the level of Akt phosphorylation increases approximately 7-fold in wt MEFs (p<0.01). Figure 4C shows PDGFRα activation in wt and Tg737orpk MEFs. Immunoprecipitated PDGFRα was stimulated for 10 min with 50 ng/ml PDGF-AA, and the level of phospho-receptor was detected with anti-phosphotyrosine antibody (p-Tyr). (D) IF analysis on the localization of phospho-Akt (second panel, red) before and after three minutes stimulation of growth-arrested wt and Tg737orpk MEFs with 50 ng/ml PDGF-AA. The primary cilium was stained with anti-detyrosinated-tubulin (glu-tub (first panel, green), arrows), which marks both the primary cilium and the two centrioles [24] (asterisks). The third panel of inserts shows the merged images.
Wound Healing defects in ORPK (IFT88Tg737Rpw) mutant mice
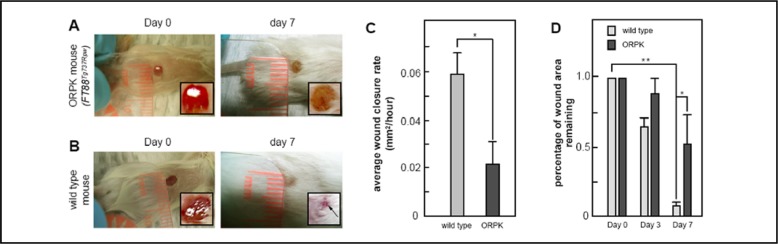
The in vitro data suggest that cilia have important in vivo roles in regulating wound healing. To assess this possibility, we conducted small punch biopsy wound healing assays in wild type and IFT88Tg737Rpw (ORPK) mutants from which the MEF cells described above were derived (Figure 5). The wounds formed fibrin clots at approximately the same time in both mutants and wild types, suggesting that there was no defect in platelet clotting or subsequent PDGF release. However, Tg737orpk animals analyzed had defects in wound closure, with wounded ORPK mutant animals being unable to achieve a significant wound closure by seven days post wounding (Figure 5A & 5B). This effect was quantified by measuring the rate of closure, where the average wound closure rate in mm2 per hour during seven days after wounding was significantly reduced in mutant cells (p<0.05) (Figure 5C). Further, the average percentage of wound area remaining was found to be significantly reduced only in wild type cells (p<0.01) (Figure 5D).
Fig. 5.
Wound Healing Defects in ORPK (IFT88Tg737Rpw) mutant mice. P15 ORPK mutant (mt) mice (A) and corresponding wild type (wt) littermates (B) were wounded and assessed for rate of wound closure by secondary intention. All wt animals achieved complete wound closure by day 7 post wounding (B, right panel). In contrast, most ORPK mutants did not have wound closure by this time period (A, right panel). The effect was further quantified by measuring the rate of closure measured at 3 and 7 days post wounding. (C) shows average wound closure rates (mm2/hour) during seven days of wound healing of five wild type and four mutant mice. The average wound closure rate was found to be significantly reduced in mutant cells (p<0.05). (D) shows average percentage of wound area remaining three and seven days after wounding relative to day of wounding (day 0). The percentage of wound area remaining was found to be significantly reduced in only wild type cells (p<0.01).
Discussion
Primary cilia orient in the direction of wound healing
We have investigated the orientation of primary cilia during wound healing in vitro and the role of primary cilia in PDGF-AA mediated cell migration. We show that the primary cilium during migration orients predominantly in parallel to the direction of migration, and often towards the leading edge when the centrosome lies in front of the nucleus. Similar observations were made in a variety of other cell types [27, 33, 34, 35], indicating that ciliary reorientation is a general phenomenon of the migratory response in growth arrested cells, in which reorientation of the centrosome towards the leading edge seem to occur upon wounding before the cells move [34, 42]. In fibroblasts the primary cilium may therefore move with its basal body, projecting forward as the nucleus moves rearward and the basal body tilts [35, 42]. Whether the primary cilium is the follower of the centrosome or vice-versa, and the exact mechanisms underlying these processes remain to be elucidated. However, these observations support the model that orientation of the primary cilium is linked to cellular mechanisms that control directional cell migration. Presumably, this orientation is controlled in concert with not only centrosomes but also the dynamic reorganization of the cytoskeleton as demonstrated by Katsumoto et al. in 3Y1 rat cells [33]. As will be discussed in the following, formation of a primary cilium from the centrosome in growth-arrested cells may act as a unique sensory site from which changes in environmental cues are relayed to the centrosome to control the migratory response
Primary cilia control PDGF-AA mediated migration speed, directionality and chemotaxis in mouse fibroblasts
We further investigated the migratory speed and directionality of growth-arrested wt and Tg737orpk MEFs in the presence and absence of PDGF-AA in scratch assays. We showed that PDGF-AA significantly increases both migration speed and displacement of growth arrested wt MEFs. In contrast, Tg737orpk MEFs were unaffected by PDGF-AA, suggesting an essential role of primary cilia in PDGF-AA-mediated regulation of the migratory response. Without the ligand, however, growth arrested mutant cells generally have a higher speed and subsequently higher displacement than their wt counterparts. These results imply that loss of the cilium, and potentially the lack of a cilium orienting parallelly to the direction of migration in quiescent fibroblasts, leads to a lack of regulatory control of the migratory speed and displacement of cells; this is a characteristic of fibrosis and tumor cell invasion. However, in the absence of PDGF-AA, PDGFRα-signaling is minimal in both wt and Tg737orpk MEFs [20], indicating that the observed increased migration speed of mutant cells is not related to aberrant PDGFRα-signaling. In non-arrested cells, there were no differences in migratory speed or displacement between mutant and wt cells, suggesting that PDGF-AA signaling is a key regulator of cell migration specifically during cellular growth arrest. We further demonstrated that both quiescent wt and Tg737orpk MEFs can be stimulated equally by serum. These data show that quiescent cells lacking the primary cilium are able to respond to factors other than PDGF-AA, but that signaling through the cilium plays a major role in PDGF-AA-mediated migration. This is an important observation since PDGF-AA is an essential mediator during development and in the early processes of wound repair in vivo [10, 14, 43-45].
In order to measure the function of the primary cilium in the chemotactic response to PDGF-AA, we performed micropipette analysis in serum-starved cultures of MEFs at a low confluency. This allows the cells to move in any direction in two dimensions in the culture dish. Wt cells moved directly towards the gradient of PDGF-AA, whereas mutant cells moved randomly and unaffected by PDGF-AA. These data illustrate an essential role of the primary cilium in PDGFRα-mediated cell migration and directional cell movement in quiescent fibroblasts. The overall displacement of mutant MEFs in the presence of PDGF-AA was reduced relative to wt MEFs but was characterized by a high frequency of “running on spot”, as evidenced by a high turnover of ruffling and formation of lamellipodia in different directions, exactly as if PDGF-AA had not been added. Thus, the observed increase in both migration speed and displacement of confluent mutant MEFs in scratch assays relative to wt MEFs in the absence of PDGF-AA could be a result of passive migration promoted by expanding cells in subsequent rows of cells, pushing on the first row of cells directly facing towards the wound, coupled with a high rate of lamellipod formation. Evidently, this physical interaction between cells that results in actual migration was absent at low confluency of cells in the micropipette analysis.
Activation of Akt at the ciliary base is part of the PDGF-AA-mediated response
We observed that in the presence of PDGF-AA, Akt is phosphorylated at the base of the primary cilium in wt MEFs. This corresponds an increase in the level of phospho-Akt in western blot analysis of PDGF-AA stimulated wt MEFs that is blocked in mutant MEFs. Lack of Akt phosphorylation in PDGF-AA stimulated mutant cells is not caused by reduced levels of PDGFRα, which suggests that activation of PDGFRα occurs in wt cells, because the receptor needs to localize to the primary cilium in order to become activated. One possibility is that PDGFRα only dimerizes to PDGFRαα after it reaches the cilium. This suggests that ciliary PDGF-AA-mediated signaling leading to directional migration in wt MEFs involves the activation of Akt, initially at the ciliary base. Increased levels of phospho-Akt are blocked in mutant MEFs. Additionally, we show that activation of PDGFRα only occurs in wt cells, supporting the conclusion that lack of Akt phosphorylation in mutant cells may not be caused by reduced levels of PDGFRα, but a mistranslocation of PDGFRα.
Akt is known to affect cell migration and cell motility by a variety of molecular mechanisms including transcriptional regulation of motility genes, actin cytoskeletal reorganization and dynamics, and control of cellular interactions with the extracellular matrix [46]. The Akt/PI3 kinase pathway also promotes stabilization of the microtubule cytoskeleton at the leading edge in migrating fibroblasts after PDGF addition. Onishi et al. [47] and Vidali et al. [5] showed that an Akt/PI3 kinase pathway remains functional upon PDGF stimulation (presumably including PDGFRαα stimulation) in Rac-1 null MEFs, which do not organize their actin into lamellipodia, but migrate nonetheless. Taken together, these results support the conclusion that the primary cilium is critical for PDGFRαα-mediated signaling via Akt and other pathways to control microtubule stabilization at the leading edge in cell migration, producing directional cell movement. Further, in the absence of PDGFRα, activation the overall level of Akt phosphorylation is kept at a low and comparable level in both wt and Tg737orpk MEFs, indicating that increased directional migration speed in mutant cells is not caused by aberrant Akt signaling, but by changes in the activity of regulatory components downstream or independent of Akt.
Defective wound healing in ORPK (IFT88Tg737Rpw) mutant mice
By investigating the possible role of primary cilia in regulating wound healing in vivo, we found that Tg737orpk animals had significant defects in wound closure compared to wt animals. These data suggest that primary cilia play a role in physiological wound repair, and that PDGFRα signaling in primary cilia in fibroblasts is part of this repair process, since impaired wound healing is associated with defects in PDGF-AA signaling in diabetic mouse models [48]. It is important to note that the analysis of wound repair in vivo is complex and multiple signaling pathways, in addition to PDGFRα, may be impaired by loss of cilia function. The ORPK mutants on the FVB/N inbred background have multiple health problems including growth retardation, hydrocephalus, polycystic kidney, liver and pancreatic diseases and hyperkeratosis in the skin that may contribute to an impaired wound healing response [49]. To fully address this issue in the absence of other potentially confounding phenotypes will require the use of conditional mutants that will specifically disrupt ciliary function in the skin fibroblasts. However, our in vitro model is consistent with the observed defects in in vivo wound healing, with PDGFRαα contributing to directional migration of fibroblasts. The mutants likely achieved partial closure via fibroblast migration in response to additional signals, much as the in vitro fibroblasts migrate in response to serum stimulus. At in vivo wound healing conditions, the establishment of gradients of chemotactic mediators for directional cell migration, including PDGF-AA, is coordinated by platelets, whereas serum released to the wound serves to form the fibrin/fibrinonectin network in clotting that provides the provisional ECM, which supports migration of fibroblasts and immune cells into the wound [14]. Consequently, fibroblasts that lack the primary cilium are blind to PDGF-AA and move more randomly.
Conclusions and perspectives
In tissue fibroblasts, the primary cilium may be part of the positioning machinery that coordinates cell polarity, which is essential for directed migration in wound healing and developmental processes [50]. In the absence of chemosensory stimuli, signals from the cilium may restrain excessive cell migration to prevent the uncontrolled and/or incorrect displacement of cells that is seen in Tg737orpk MEFs in scratch assays. During the establishment of a gradient of chemosensory stimuli and initiation of migration, the primary cilium may function as a cellular GPS [51] to monitor the directional movement and organize the coordinated actions of chemosensory stimuli and the dynamic reorganization of the cytoskeleton. It seems probable that signaling molecules generated within the primary cilium leave the cilium and impinge on the centrosome, surrounding the ciliary basal body [52], where proteins such as activated Akt could modulate actin, microtubule and cell junction reorganization and generalized cell motility. A second PDGFRαα-mediated signaling pathway that could affect the migratory response is the Mek1/2-Erk1/2 pathway, which is specifically activated in the primary cilium upon PDGF-AA stimulation in fibroblasts [20].
A possible link between PDGFRα signaling and leading edge events was recently suggested to involve the activation of the Na+/H+ exchanger 1 (NHE1) [53], which is located mainly in the leading edge of the cell where it works as a central player in the regulation of cell migration [15, 54]. Both the PI3K-Akt and the Mek1/2-Erk1/2 pathways activate NHE1[53-55]; the Mek1/2/Erk1/2 pathway acting via p90Ribosomal S6 kinase (p90Rsk) to phoshorylate the C-terminal part of NHE1 [56, 57]. Recently PDGF-BB was shown to mediate activation of NHE1 and actin cytoskeleton remodeling via Akt in fibroblasts [58]. One hypothesis is that PDGF-AA-mediated activation of Akt at the centrosome affects both NHE1, microtubule cytoskeleton stabilization and actin reorganization towards the leading edge, which in turn affects the generation of leading edge lamellipodia [52, 26]. In this scenario the cilium/centrosome axis could coordinate the reorientation and remodeling of stable microtubules, which is known to regulate turnover of focal adhesions and trafficking of membrane proteins to the leading edge [59]. In smooth muscle cells, primary cilia containing EGFR, integrins and polycystins 1 and 2 orient towards the leading edge during migration [34, 35], supporting the model in which the primary cilium coordinate a whole series of different signal transduction pathways that are critical in reorientation and remodeling of stable microtubules towards the leading edge in directional cell migration.
In terms of tumor cell invasion, signaling systems that may be coordinated by the primary cilium besides PDGFRα signaling include the Hedgehog (Hh) and Wingless/Int (Wnt) pathways, which are important regulators of the migratory response in a variety of cell types and which, when mutated, cause cancer [60-63]. Indeed, several types of cancer cells are recognized by a significantly lower frequency of primary cilia [64, 65], and changes in primary cilium signaling has been linked to increased progression of various types of cancers [64, 66-68]. As an example, Wong et al. [67] and Han et al. [68] showed that the primary cilium has a dual role as a unique signaling organelle that can either mediate or suppress tumorigenesis depending on the nature of the oncogenic initiating event. It would be interesting to know if primary cilia dysfunction causes a change in the migratory behavior in cancer cells similar to that of Tg737 mutant MEFs, which migrate with disrupted directionality and increased speed compared with the wt MEFs.
Even though many signaling pathways may be involved in the responses to PDGF and other serum factors, our results emphasize that in fibroblasts, PDGFRα signaling originating in the primary cilium is a component of the response, essential for efficient directional migration in the presence of PDGF-AA, and important for chemotaxis in wound healing. Probably, directional migration of fibroblasts is similarly regulated through interactions between the cilium and ECM; potentially in concert with chemoattractants in embryonic patterning and adult tissue reorganization. This may be a unique feature of primary cilia on cells deep within tissues such as in fibroblasts versus cilia that protrude from the apical surface into a lumen as seen on epithelial and endothelial cells. In fibroblasts, defects in building the primary cilium or in the targeting of PDGFRα to it have crucial consequences on cellular and physiological levels and may be responsible for human diseases and migration-related disorders involving PDGF pathway mutations [69, 70].
Abbreviations
GPS (Global positioning system); MEF (Mouse embryonic fibroblast); ORPK (Oak Ridge polycystic kidney); PDGF-AA (Platelet-derived growth factor AA); PDGFR (Platelet-derived growth factor receptor).
Acknowledgements
This work was supported by grants to S.T.C. and L.S. (Lundbeck Foundation grant number R9-A969, Fonden af 1870, funds from the University of Copenhagen), S.K.N. (Novo Nordic foundation), E.K.H. and S.T.C (The Danish Natural Science Research Council, 21-04-0535 and The Danish Cancer Society, DP05072), A.S. (Deutsche Forschungsgemeinschaft Schw 407/9-3, and 10-1 and IZKF Münster), C.S. (“Innovative Medical Research” Fund of the University of Münster Medical School, ST 2 1 06 01), and by a Pilot and Feasibility award to B.K.Y. from the UAB Skin Diseases Research Center (SDRC) supported by an NIH P30 (AR050948-03, C. Elmets). We thank Anni Bech Nielsen, Sabine Mally and Dr. Jacco Van Rheenen for excellent technical assistance.
References
- 1.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen M, She H, Kim A, Woodley DT, Li W. Nckbeta adapter regulates actin polymerization in NIH 3T3 fibroblasts in response to platelet-derived growth factor bb. Mol Cell Biol. 2000;20:7867–7880. doi: 10.1128/mcb.20.21.7867-7880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antón IM, Saville SP, Byrne MJ, Curcio C, Ramesh N, Hartwig JH, Geha RS. WIP participates in actin reorganization and ruffle formation induced by PDGF. J Cell Sci. 2003;116:2443–2451. doi: 10.1242/jcs.00433. [DOI] [PubMed] [Google Scholar]
- 4.Rivera GM, Antoku S, Gelkop S, Shin NY, Hanks SK, Pawson T, Mayer BJ. Requirement of Nck adaptors for actin dynamics and cell migration stimulated by platelet-derived growth factor B. Proc Natl Acad Sci USA. 2006;103:9536–9541. doi: 10.1073/pnas.0603786103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidali L, Chen F, Cicchetti G, Ohta Y, Kwiatkowski DJ. Rac1-null mouse embryonic fibroblasts are motile and respond to platelet-derived growth factor. Mol Biol Cell. 2006;17:2377–2390. doi: 10.1091/mbc.E05-10-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legg JA, Bompard G, Dawson J, Morris HL, Andrew N, Cooper L, Johnston SA, Tramountanis G, Machesky LM. N-WASP involvement in dorsal ruffle formation in mouse embryonic fibroblasts. Mol Biol Cell. 2007;18:678–687. doi: 10.1091/mbc.E06-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwab A. Functional and spatial distribution of ion channels and transporters in cell migration. Am J Physiol Renal Physiol. 2001;280:F739–F747. doi: 10.1152/ajprenal.2001.280.5.F739. [DOI] [PubMed] [Google Scholar]
- 8.Schwab A, Nechyporuk-Zloy V, Fabian A, Stock CM. Cells move when ions and water flow. Pflügers Arch. 2007;453:421–432. doi: 10.1007/s00424-006-0138-6. [DOI] [PubMed] [Google Scholar]
- 9.Jones AV, Cross NC. Oncogenic derivatives of platelet-derived growth factor receptors. Cell Mol Life Sci. 2004;61:2912–2923. doi: 10.1007/s00018-004-4272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes and Devl. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frost EE, Zhou Z, Krasnesky K, Amstrong RC. Initiation of Oligodendrocyte Progenitor cell migration by a PDGF-A activated extracellular regulated kinase (ERK) signaling pathway. Neurochem Res. 2009;34(1):169–81. doi: 10.1007/s11064-008-9748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruttiger M, Karlsson L, Hall AC, Abrahamsson A, Calver AR, Bostrom H, Willetts K, Bertold CH, Heath JK, Betholtz C, Richardson W. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–467. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- 13.Ho L, Symes K, Yordan C, Gudas LJ, Mercola M. Localization of PDGF A and PDGFR alpha mRNA in Xenopus embryos suggests signaling from neural ectoderm and pharyngeal endoterm to neural crest cells. Mech Dev. 1994;48:165–174. doi: 10.1016/0925-4773(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 14.Singer AJ, Clark RH. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 15.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159:1087–1096. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grinstein S, Woodside M, Waddell TK, Downey GP, Orlowski J, Pouyssegur J, Wong DC, Foskett JK. Focal localization of NHE-1 isoform of the Na+/H+ antiport: Assessment of effects on intracellular pH. EMBO J. 1993;12:5290–5218. doi: 10.1002/j.1460-2075.1993.tb06216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagana A, Vadnais J, Le PU, Nguyen TN, Laprade R, Nabi IR, Noel J. Regulation of the formation of tumor cell pseudopodia by the Na+/H+ exchanger NHE1. J Cell Sci. 2000;113:3649–3662. doi: 10.1242/jcs.113.20.3649. [DOI] [PubMed] [Google Scholar]
- 18.Stock CM, Mueller M, Kraehling H, Mally S, Noel J, Eder C, Schwab A. pH nanoenvironment at the surface of single melanoma cells. Cell Physiol Biochem. 2007;20:679–686. doi: 10.1159/000107550. [DOI] [PubMed] [Google Scholar]
- 19.Yan W, Nehrke K, Choi J, Barber DL. The Nck-interacting kinase (NIK) phosphorylates the Na+-H+ exchanger NHE1 and regulates NHE1 activation by platelet-derived growth factor. J Biol Chem. 2001;276:31349–31356. doi: 10.1074/jbc.M102679200. [DOI] [PubMed] [Google Scholar]
- 20.Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRαα signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Santos N, Reiter JF. Building it up and taking it down: the regulation of vertebrate ciliogenesis. Dev Dyn. 2008;237:1972–1981. doi: 10.1002/dvdy.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen LB, Veland IR, Schroder JM, Christensen ST. Assembly of primary cilia. Dev Dyn. 2008;237(8):1993–2006. doi: 10.1002/dvdy.21521. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum JL, Witman G. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 25.Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and intergration of signal transduction in human health and disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 26.Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol. 2008;85:261–301. doi: 10.1016/S0070-2153(08)00810-7. [DOI] [PubMed] [Google Scholar]
- 27.Albrecht-Buhler GA. Phagokinetic tracks of 3T3 cells: Parallels between the orientation of track segments and of cellular structures which contain actin or tubulin. Cell. 1977;12:333–339. doi: 10.1016/0092-8674(77)90109-x. [DOI] [PubMed] [Google Scholar]
- 28.Gotlieb AI, May LM, Subrahmanyan L, Kalnins VI. Distribution of microtubule-organizing centers in migrating sheets of endothelial cells. J Cell Biol. 1981;91:589–594. doi: 10.1083/jcb.91.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gotlieb AI, Subrahmanyan L, Kalnins VI. Microtubule-organizing centers and cell migration: Effect of inhibition of migration and microtubule disruption in endothelial cells. J Cell Biol. 1983;96:1266–1272. doi: 10.1083/jcb.96.5.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kupfer A, Louvard D, Singer SJ. Polarization of the golgi apparatus and the microtubule-organising center in cultured fibroblasts at the edge of a experimental wound. Proc Natl Acad Sci USA. 1982;79:2603–2607. doi: 10.1073/pnas.79.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gundersen GG, Bulinski JC. Selective stabilization of micro tubule oriented toward the direction of cell migration. Proc Natl Acad Sci USA. 1988;85:5946–5950. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42 MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Katsumoto T, Higaki K, Ohno K, Onodera K. The orientation of the primary cilia during the wound response in 3YI cells. Biol Cell. 1994;81(1):17–21. doi: 10.1016/0248-4900(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 34.Lu CJ, Du H, Wu J, Jansen DA, Jordan KL, Xu N, Sieck GC, Qian Q. Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press Res. 2008;31:171–184. doi: 10.1159/000132462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Du H, Wang X, Mei C, Sieck GC, Qian Q. Characterisation of primary cilia in human airway smooth muscle cells. Chest. 2009;136(2):561–570. doi: 10.1378/chest.08-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrøder JM, Schneider L, Christensen ST, Pedersen LB. EB1 is required for primary cilia assembly in fibroblasts. Curr Biol. 2007;17:1134–1139. doi: 10.1016/j.cub.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 37.Dieterich P, Klages R, Preuss R, Schwab A. Anomalous dynamics of cell migration. Proc Natl Acad Sci USA. 2008;105:459–463. doi: 10.1073/pnas.0707603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider L, Klausen TK, Stock C, Mally S, Christensen ST, Pedersen SF, Hoffmann EK, Schwab A. H-ras transformation sensitizes volume-activated anion channels and increases migratory activity of NIH3T3 fibroblasts. Pflugers Arch. 2008;455:1055–1062. doi: 10.1007/s00424-007-0367-3. [DOI] [PubMed] [Google Scholar]
- 39.Mouneimne G, Soon L, DesMarais V, Sidani M, Song X, Yip SC, Ghosh M, Eddy R, Backer JM, Condeelis J. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J Cell Biol. 2004;166(5):697–708. doi: 10.1083/jcb.200405156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heldin CH, Westermark B. Mechanisms of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 41.Lih CJ, Cohen SN, Wang C, Lin-Chao S. The platelet derived growth factor á receptor is encoded by a growth arrest specific (gas) gene. Proc Natl Acad Sci USA. 1996;93:4617–4622. doi: 10.1073/pnas.93.10.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers KA, McKee NH, Kalnins VI. Preferential orientation of centrioles toward the heart in endothelial cells of major blood vessels is re-established after reversal of a segment. Proc Natl Acad Sci USA. 1985;82(10):3272–3276. doi: 10.1073/pnas.82.10.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeh YL, Kang YM, Chaibi MS, Xie JF, Graves DT. IL-1 and transforming growth factor-b inhibit platelet-derived growth factor-AA binding to osteoblastic cells by reducing platelet-derived growth factor-alpha receptor expression. J Immunol. 1993;150:5625–5632. [PubMed] [Google Scholar]
- 44.Xie JF, Stroumza J, Graves DT. IL-1 down-regulates platelet-derived growth factor-a receptor gene expression at the transcriptional level in human osteoblastic cells. J Immunol. 1994;153:378–383. [PubMed] [Google Scholar]
- 45.Soma Y, Mizoguchi M, Yamane K, Yazawa N, Kubo M, Ihn H, Kikuchi K, Tamaki K. Specific inhibition of human skin fibroblast chemotaxis to platelet-derived growth factor A-chain homodimer by transforming growth factor-(1. Arch Dermatol Res. 2002;293:609–613. doi: 10.1007/s00403-001-0279-6. [DOI] [PubMed] [Google Scholar]
- 46.Stambolic V, Woodgett JR. Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends Cell Biol. 2006;16:461–466. doi: 10.1016/j.tcb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Onishi K, Higuchi M, Asakura T, Masuyama N, Gotoh Y. The PI3K-Akt pathway promotes microtubule stabilization in migrating fibroblasts. Genes Cells. 2007;12:535–546. doi: 10.1111/j.1365-2443.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 48.Beer HD, Longaker MT, Werner S. Reduced expression of PDGF and PDGF receptors during impaired wound healing. J Invest Dermatol. 1997;109:132–138. doi: 10.1111/1523-1747.ep12319188. [DOI] [PubMed] [Google Scholar]
- 49.Lehman JM, Michaud EJ, Schoeb TR, Aydin-Son Y, Miller M, Yoder BK. The Oak Ridge Polycystic Mouse: Modeling Ciliopathies of Mice and Men. Dev Dyn. 2008;237(8):1960–71. doi: 10.1002/dvdy.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider IC, Haugh KM. Mechanisms of gradient sensing and chemotaxis: Conserved pathways, diverse regulation. Cell Cycle. 2006;5:1130–1134. doi: 10.4161/cc.5.11.2770. [DOI] [PubMed] [Google Scholar]
- 51.Benzing T, Walz G. Cilium-generated signaling: A cellular GPS? Curr Opin Nephrol Hypertens. 2006;15:245–249. doi: 10.1097/01.mnh.0000222690.53970.ca. [DOI] [PubMed] [Google Scholar]
- 52.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in cilia assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 53.Schneider L, Stock CM, Dieterich P, Jensen BH, Pedersen LB, Satir P, Schwab A, Christensen ST, Pedersen SF. The Na+/H+ exchanger NHE1 is required for directional migration stimulated via PDGFRα in the primary cilium. J Cell Biol. 2009;185(1):163–176. doi: 10.1083/jcb.200806019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stock CM, Schwab A. Role of Na+/H+ exchanger NHE1 in cell migration. Acta Physiol. (Oxf). 2006;187(1–2):149–157. doi: 10.1111/j.1748-1716.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen SF. The Na+/H+ excanger NHE1 in stress-induced signal transduction: Implications for cell proliferation and cell death. Pflugers Arch. 2006;452(3):249–259. doi: 10.1007/s00424-006-0044-y. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi E, Abe J, Gallis B, Aebersold R, Spring DJ, Krebs EG, Berk BC. p90(RSK) is a serum-stimulated Na+/H+ exchanger isoform-1 kinase. Regulatory phosphorylation of serine 703 of Na+/H+ exchanger isoform-1. J Biol Chem. 1999;16(29):20206–20214. doi: 10.1074/jbc.274.29.20206. 274. [DOI] [PubMed] [Google Scholar]
- 57.Luo J, Sun D. Physiology and pathophysiology of Na+/H+ exchange isoform 1 in the central nervous system. Curr NeuroVasc Res. 2007;4(3):205–215. doi: 10.2174/156720207781387178. [DOI] [PubMed] [Google Scholar]
- 58.Meima ME, Webb BA, Witkowska HE, Barber D. The Na-H Exchanger NHE1 is an Akt Substrate necessary for actin filament reorganization by growth factors. J Biol Chem. 2009;284:26666–26675. doi: 10.1074/jbc.M109.019448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wehrle-Haller B, Imhof BA. Actin, microtubules and focal adhesion dynamics during cell migration. J Pathol. 2003;35(1):39–50. doi: 10.1016/s1357-2725(02)00071-7. [DOI] [PubMed] [Google Scholar]
- 60.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1(4):e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Dev. 2005;132(13):3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 62.Tobin JL, Di Franco M, Eichers E, May-Simera H, Garcia M, Yan J, Quinlan R, Justice MJ, Hennekam RC, Briscoe J, Tada M, Mayor R, Burns AJ, Lupski JR, Hammond P, Beales PL. Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung's disease in Bardet-Biedl syndrome. Proc Natl Acad Sci USA. 2008;105(18):6714–6719. doi: 10.1073/pnas.0707057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wada N, Javidan Y, Nelson S, Carney TJ, Kelsh RN, Schilling TF. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Dev. 2005;132(17):3977–3988. doi: 10.1242/dev.01943. [DOI] [PubMed] [Google Scholar]
- 64.Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20(1):73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- 65.Nielsen SK, Møllgård K, Clement CA, Veland IR, Awan A, Yoder BK, Novak I, Christensen ST. Characterization of primary cilia and Hedgehog signaling during development of the human pancreas and in human pancreatic duct cancer cell lines. Dev Dyn. 2008;237(8):2039–2052. doi: 10.1002/dvdy.21610. [DOI] [PubMed] [Google Scholar]
- 66.Plotnikova OV, Golemis EA, Pugacheva EN. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008;68(7):2058–2061. doi: 10.1158/0008-5472.CAN-07-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Jr, Dlugosz AA, Reiter JF. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15(9):1055–1061. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15(9):1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu J, Moon A, Kim HR. Both Platelet-Derived Growth Factor Receptor (PDGFR)-α and PDGFR-β Promote Murine Fibroblast Cell Migration. Biochem Biophys Res Commun. 2001;282:697–700. doi: 10.1006/bbrc.2001.4622. [DOI] [PubMed] [Google Scholar]
- 70.Michaud EJ, Yoder BK. The Primary Cilium in Cell Signaling and Cancer. Cancer Research. 2006;66:6463–6467. doi: 10.1158/0008-5472.CAN-06-0462. [DOI] [PubMed] [Google Scholar]