Introduction
Many processes have been implicated in early atherogenesis. These include endothelial denudation, injury, or activation, including shear stress–related events; local adherence of platelets; lipoprotein oxidation; lipoprotein aggregation; macrophage chemotaxis and foam cell formation; and smooth muscle cell alterations. Which process, if any, could be regarded as the key event in early atherogenesis, ie, absolutely required, yet also sufficient as the sole pathological stimulus in an otherwise normal artery to provoke a cascade of events leading to lesion formation? The work of many investigators, which we summarize here, strongly supports subendothelial retention of atherogenic lipoproteins as the central pathogenic process in atherogenesis (for prior reviews, see References 1 through 61, 2, 3, 4, 5, 6). Our thesis is that other contributory processes are either not individually necessary or are not sufficient. Most often, they are merely normal, expected responses of otherwise-healthy tissue to the presence of retained lipoproteins.
Competing Hypotheses
It is instructive to catalog other processes that have been argued to be central to the initiation of atherogenesis. The first is endothelial denudation,7, 8, 9 injury,10 or activation,11, 12 as outlined in the “response-to-injury” hypothesis of Ross, Glomset, and coworkers. Although this important hypothesis has stimulated much of the work that we cite here, there is no definitive evidence in vivo that endothelial injury is either necessary or sufficient for lesion formation.
The response-to-injury hypothesis originally presupposed endothelial desquamation as the key event in atherogenesis.7, 8, 9 It is now clear, however, that developing atheromata are covered by an intact endothelial layer throughout most stages of lesion progression: lipoprotein retention, fatty streak formation, and formation of advanced lesions.5, 6, 12, 13, 14, 15 In humans, only the most complicated, ulcerated lesions lose their endothelial layer. Furthermore, in some experimental models, an intact endothelium is required for lesion initiation and development, which do not occur in adjacent areas of denudation.16, 17, 18 Gross endothelial denudation, though presumably important in restenosis after balloon injury19 and in very advanced complicated plaques, does not appear to be central to early atherogenesis.5
A refinement of the response-to-injury hypothesis states that endothelial injuries that are insufficient to cause gross denudation but severe enough to cause functional modifications are key to atherogenesis.10, 11, 12, 20 A major hypothesized change in endothelial function was increased permeability,21, 22 particularly to atherogenic lipoproteins.23, 24, 25, 26, 27 This idea is related to the lipid infiltration hypothesis,5 which originated with Anichkov and Khalatov28 (reviewed in References 29 and 3029, 30). Alterations in permeability or even microscopic losses of endothelial cells in excess of those due to normal cell turnover are not mechanistically required for atherogenesis, however, because normal, healthy endothelium transports31, 32 or “leaks”26, 33 many molecules, including lipoproteins (reviewed in Reference 2727). In fact, the rate of LDL entry into the normal, healthy arterial wall vastly exceeds the LDL accumulation rate.34 Most important, seminal studies by Schwenke and Carew35, 36 showed in vivo that the early prelesional accumulation of atherogenic lipoproteins within the arterial wall is focally concentrated in sites that are known to be prone to the later development of atheromata, but that the rates of lipoprotein entry into prelesional susceptible versus resistant sites were not different (cf Reference 22). These studies indicate that retention, not enhanced endothelial permeability to lipoprotein influx, is the key pathological event in this experimental model. Subsequent studies in several other animal models have demonstrated either increased23, 24, 25, 26 or decreased37 rates of lipoprotein entry into atherosclerosis-susceptible sites, suggesting a nonessential role for alterations in endothelial permeability. All studies agree, however, that prelesional susceptible arterial sites show enhanced retention of apoB-rich, atherogenic lipoproteins.35, 36, 38, 39, 40, 41 We conclude that alterations in endothelial permeability, though apparently not essential to lesion development, may play a contributory role, eg, in smoking,42 dyslipidemias27, 37 (cf Reference 3636), and possibly hypertension27 (cf Reference 4343), but only if some of the infiltrated material is retained.35
Several other functional modifications have been documented in the endothelial layer in vivo during atherogenesis, but these occur comparatively late. For example, in rabbits, cell adhesion molecules,44 the earliest known being vascular cell adhesion molecule–1 (VCAM-1), are expressed by endothelial cells that overlie lesions, but only after more than 4 days of severe hypercholesterolemia and resultant foam cell formation.45 In contrast, lipoprotein retention and aggregation are detectable within minutes to hours after the onset of hypercholesterolemia.31, 36, 41, 46 Furthermore, atherogenic lipoproteins and their components have been shown to regulate endothelial expression of cell-adhesion molecules.12, 47, 48, 49 The most straightforward conclusion is that the earliest known endothelial changes during atherogenesis in vivo, such as VCAM-1 expression, cannot be a cause, and are likely to be a consequence, of the initial retention of lipoproteins within the arterial wall (see “Future Directions”).
The effect of turbulent blood flow on the arterial wall deserves special comment, particularly because it can be such an early influence. Arterial segments that are subject to turbulent blood flow, such as those at branch points or during hypertension, show a predisposition to lesion development, though the precise relationship in vivo may be complicated.50 Because of the response-to-injury hypothesis, the connection between blood flow and atherogenesis has led to many studies on the effects of shear stress on the endothelium in cell culture experiments. Many alterations have been reported,12, 18, 51 such as intracellular calcium mobilization, ion channel activation, cytoskeletal changes, altered cellular alignment, cell surface streamlining,52 increased endothelial cell division,53 stimulation of specific transcription factors,54 and production of potentially atherogenic molecules, such as vasoactive,55 adhesive,56 and growth12, 20, 57 factors. Somewhat different results are obtained in vitro when shear is low instead of high, constant instead of pulsatile, laminar instead of turbulent, or spatially uniform instead of graded,12, 51, 53 but the overall findings in vitro strongly support a contributory role for shear stress–induced alterations of the endothelium during atherogenesis.
In vivo, however, it is clear that sheer stress–induced endothelial alterations are neither necessary nor sufficient for atherogenesis. In vivo lesion development at sites of turbulent flow shows an absolute requirement for high plasma concentrations of atherogenic lipoproteins relative to those that occur naturally in nonhuman, nonatherosclerotic mammals: the plasma concentration of LDL cholesterol must exceed 2 mmol/L (80 mg/dL) for atherogenesis, even at sites of high shear stress.58, 59 Furthermore, at sufficiently high plasma lipoprotein concentrations, lesions develop even at sites of low shear stress, such as at non–branch points6, 60 or within the pulmonary arteries.60, 61 Although stress-induced endothelial changes can play a contributory role in atherogenesis, the most directly relevant functional changes that have been documented at prelesional sites that are susceptible to atherogenesis, including those that are subject to turbulent blood flow, are altered proteoglycan structure62, 63, 64, 65, 66 and increased lipoprotein retention36, 39, 46, 67, 68, 69 (see above).
We therefore propose that the atherogenic effects of sheer stress in vivo are entirely dependent on lipoprotein retention within the arterial wall and are limited to increased local vulnerability to lipoprotein retention and the consequences thereof. Specifically, we hypothesize that the role of shear stress in early atherogenesis is mediated primarily through the stimulation of intramural synthesis of molecules, such as proteoglycans, that promote lipoprotein retention (see References 63, 64, 66, 70, and 7163, 64, 66, 70, 71). Later, once vessel segments have accumulated retained lipoproteins, the threshold for injury and activation from continued shear stress may be lowered. Many stimuli can activate endothelial cells, and synergy is likely between shear stress and, for example, oxidative breakdown products of retained lipoproteins. The same general lines of reasoning can be used to argue against a central role for other potential activators of the endothelium, such as viruses72 or homocysteine,9, 12, 20, 73 which are likewise neither necessary nor sufficient for lesion development in vivo. Note that these hypotheses about the central role of retained lipoproteins are testable (see “Future Directions”).
The second process that has been proposed to be central to atherogenesis is lipoprotein oxidation.74, 75, 76 Current evidence indicates, however, that pathophysiologically important oxidation can occur only after the retention of lipoproteins within the sequestered microenvironment of the arterial wall. Lipoprotein oxidation by cells or transition metals in vitro is blocked by small concentrations of plasma or plasma proteins,77 such as albumin78, 79, 80, 81, 82, 83 (cf Reference 8484), and any oxidized lipoproteins that might appear in the plasma in vivo would be rapidly removed by the liver,85, 86 rather than be deposited into developing lesions within the arterial wall.87 In fact, oxidation may be regarded as a normal, expected consequence of lipoprotein trapping: studies in vitro indicate that once lipoproteins are sequestered from the protective elements of plasma, nearby healthy arterial cells will cause oxidation,75 apparently through their efficient generation of thiols.88, 89 In vivo, myeloperoxidases may be involved.90 Note that adherence of LDL to arterial proteoglycans increases LDL’s susceptibility to oxidation in vitro71, 91 (see below), but that prior oxidation of LDL does not enhance its retention in arteries.87 Consistent with these results, apoB is retained in the human intima before it is detectably oxidized.92 The most straightforward conclusion is that oxidation is a normal, expected consequence of intramural sequestration of sufficient quantities of atherogenic lipoproteins within an otherwise healthy artery.
The importance of lipoprotein oxidation in lesion development is supported by discoveries of many biological consequences in vitro that are consistent with atherogenesis,48, 74, 75, 93 by demonstrations of antiatherogenic effects in experimental animals of compounds with antioxidant actions,94, 95, 96 and by the findings in humans of oxidized epitopes within lesions92, 97 (see Reference 9898) and of anti–oxidized LDL antibodies within lesions99 and in plasma.100 Nevertheless, there are also reports that show the ineffectiveness of antioxidants on atherogenesis. For example, recent reports of humans who consumed antioxidant vitamins101, 102 or who were given probucol103, 104 and of animals that were given a potent antioxidant analogue of probucol that does not affect plasma lipoprotein concentrations105, 106 (see Reference 107107) failed to find beneficial effects of these treatments on disease. Note that vitamin E and probucol, which appear to be antiatherogenic in humans102 and animals,94, 95 respectively, have many actions besides inhibition of lipoprotein oxidation.102, 104, 105, 108, 109 Because even minimal oxidation of LDL, which has been described in human lesions110 (see also Reference 111111), produces many potentially harmful biological effects,93 lipoprotein oxidation is unlikely to be a rate-limiting step in atherogenesis. Thus, nearly total inhibition of oxidation of retained lipoproteins may be required before there would be any substantial effect on lesion development in vivo (cf Reference 105105).
Evidence to Support Retention as the Key Event
Following rapid induction of hypercholesterolemia in rabbits due to injection of LDL, the earliest detectable change in the arterial wall is the intramural retention of LDL and of microaggregates of LDL, a change that occurs within 2 hours.46 Perfusion of arterial segments in situ has shown substantial accumulation of LDL within 5 minutes.31 Early arterial retention of injected LDL in vivo is focal, in sites that are known to be susceptible to the subsequent development of atheromata.35, 36 LDL retention in these sites is not the result of increased flux into the arterial wall but from reduced lipoprotein egress.36 These rapidly apparent differences in lipoprotein retention between prelesional susceptible versus resistant sites suggest a preexisting metabolic difference that, under the proper conditions of hypercholesterolemia, leads to differences in lipoprotein retention. This conclusion is supported by the observation that atherosclerosis cannot develop when plasma β-lipoprotein concentrations are truly low,58, 59, 112 even in the presence of other major risk factors.113
Several lines of evidence indicate that intramural retention of atherogenic lipoproteins involves extracellular matrix, chiefly proteoglycans1, 71 and perhaps other structural elements,114, 115, 116, 117, 118, 119 and lipolytic enzymes, chiefly lipoprotein lipase (LpL)120, 121, 122, 123, 124, 125, 126, 127 and sphingomyelinase (SMase).127, 128, 129, 130 First, all of these molecules are present to varying degrees within the normal arterial wall.70, 71, 120, 128, 131, 132, 133 Thus, they are available to participate in the earliest stages of atherogenesis. Second, retained apoB in extremely early46, 69 as well as advanced64, 71 lesions is closely associated with arterial proteoglycans. Purified arterial proteoglycans, particularly those from lesion-prone sites,66, 134 bind LDL in vitro, particularly LDL from patients with coronary artery disease.135, 136 This interaction involves several well-defined, positively charged segments of apoB.71, 137 Third, LpL enhances adherence of LDL in vitro to the matrix that is derived from normal endothelial125, 138 and smooth muscle127 cells and to normal cell-surface proteoglycans.122, 123, 124, 125, 126, 127, 139 This adherence is independent of LpL enzymatic activity122, 123, 125, 127 (cf References 120 and 121120, 121) and appears to occur in vessels enriched in situ with LpL.140 Fourth, a linkage between peritoneal macrophage production of LpL and susceptibility to atherosclerosis has been documented in recombinant inbred mice.141 Results in humans indicate a linkage between LpL polymorphisms and coronary artery disease, though without a linkage between LpL polymorphisms and specific plasma lipoprotein patterns, thus suggesting that the effects on lesion development are independent of plasma changes.142 A genetic absence of LpL in humans has long been known to cause hyperlipidemia without increased atherosclerosis,121 presumably because of poor generation of small, cholesteryl ester–rich particles143 that are able to enter the arterial wall27 and loss of LpL-facilitated binding to arterial proteoglycans.122, 123, 124, 125, 126, 127 More recent work in humans indicates that the single most important determinant of lesion development in homozygous familial hypercholesterolemia is the postheparin plasma concentration of LpL mass, not enzymatic activity; this is consistent with a structural effect.144 Fifth, SMase causes the formation of LDL microaggregates129 that morphologically resemble the intramural particles seen 2 hours after rapid induction of hypercholesterolemia,46 and LpL and SMase synergistically interact to cause massive retention and aggregation of LDL and Lp(a) in vitro to arterial cell proteoglycans and matrix.127 The arterial wall SMase128 was recently shown to act on the LDL retained in aortic strips ex vivo.130 At later stages, once atherogenesis has begun, the content of specific proteoglycans,64, 65, 66, 71, 145 LpL,120, 131, 132, 133, 146, 147, 148 and SMase128 increases in lesional areas, thereby apparently accelerating the disease.
The factors responsible for focal retention of lipoproteins and subsequent lesion development, however, are not clear. Because prelesional differences in lipoprotein retention between susceptible versus resistant arterial sites are so rapidly apparent after induction of hypercholesterolemia,35 there are likely to be preexisting local differences in apoB-retentive molecules. Proteoglycan variations alone may not explain the focal development of early lesions, because potentially atherogenic proteoglycans are abundant and ubiquitous throughout the arterial tree,70 though focal alterations in proteoglycans have been documented in prelesional63, 64, 65, 66 and lesional145, 149 sites. The enzymes LpL and SMase show enhanced expression in established human133, 146, 148 and animal133 lesions, and arterial contents of LpL were shown two decades ago to correlate strongly with the arterial accumulation of cholesteryl ester during atherogenesis in rabbits (r=0.9).121 Nevertheless, the status of proteoglycans, LpL, SMase, and possibly other apoB-retentive molecules in prelesional susceptible versus prelesional resistant sites remains an important area for study (see “Future Directions”).
Consequences of Retention
Following its retention by proteoglycans, LDL has been shown in vitro to undergo several modifications with important biological consequences. Proteoglycan-bound LDL in vitro forms aggregates71 and vesicular structures127 that resemble material seen in vivo.46, 67, 68, 150, 151 LDL-proteoglycan complexes show increased susceptibility to oxidation under typical serum-free, albumin-free pro-oxidative experimental conditions.91 Minimally oxidized LDL induces endothelial and smooth muscle cells to express monocyte chemotactic activity,152 and more extensively oxidized LDL is directly chemoattractive to monocytes,153, 154 smooth muscle cells,155 and T lymphocytes,154 largely because of its lysophosphatidylcholine content.154 Retained LDL would also be subject to arterial wall SMases,127, 128, 129, 130 which generate choline phosphate and ceramides. Ceramides are well documented to have many biological effects, such as induction of NF-κB and stimulation of apoptosis or mitogenesis.156, 157
LDL that has been aggregated or otherwise modified by arterial proteoglycans under a variety of conditions in vitro is avidly taken up by normal cultured macrophages and smooth muscle cells,134 leading to foam cell formation.136, 151, 158 This avid uptake may involve several different receptors,122, 123, 124, 125, 126, 134, 139, 158, 159 of which only the LDL receptors are known to be nonessential, in that their genetic absence does not impede arterial lipoprotein accumulation160 or atherogenesis60, 161 in vivo, in contrast to the situation with LpL deficiency (see above). The conversion of macrophages to foam cells stimulates the release of more LpL133, 146, 147, 148, 162, 163 and other potentially atherogenic factors164, 165, 166 and has been shown to alter proteoglycan metabolism.167 Retained, altered lipoproteins155, 168, 169 and nearby macrophages170, 171 can stimulate chemotaxis and transformation of smooth muscle cells from the contractile to the proliferative state, which in smooth muscle cells causes increased synthesis of proteoglycans,64, 172 including LDL-binding proteoglycans,173 and possibly LpL132, 133 (cf Reference 146146). Thus, retained lipoproteins can directly or indirectly provoke all known features of early lesions and, by stimulating local synthesis of proteoglycans and LpL, can accelerate further lipoprotein retention and aggregation.
Lesion development may be altered by local cellular expression of apoE within the arterial wall. As macrophages become foam cells in situ, they appear to increase their synthesis of apoE,148, 174 a molecule that has been shown in vitro to release lipoproteins from the extracellular matrix175 (see Reference 137137). The ultimate fate of these released lipoproteins, however, is unclear. They could leave the lesion, or they could be taken up by arterial cells, particularly through apoE-mediated binding.
The atherogenic nature of Lp(a) may originate from its extensive propensity for intramural retention.41, 136, 176, 177, 178 Despite plasma concentrations of Lp(a) that are far lower than those of other apoB-rich lipoproteins, Lp(a) may account for most of the apoB in human lesions.179, 180 In vitro, Lp(a) binds to arterial proteoglycans with greater affinity176 and capacity176, 181 than does LDL. Avid cellular uptake of Lp(a) by at least four processes may then occur: through scavenger receptors after oxidation86; through the heparan sulfate–proteoglycan pathway in the presence of intramural LpL122, 123; by phagocytosis following aggregation and cross-linking by LpL, SMase, and proteoglycans127; and by a specific Lp(a)/apo(a) receptor on cholesterol-enriched macrophages.182, 183 After Lp(a) retention, many other biological effects occur, including enhanced LDL retention,184 stimulation of smooth muscle cell proliferation,185 and, possibly, local inhibition of lysis of microthrombi186, 187, 188, 189 (cf References 190 through 192190, 191, 192).
Future Directions
A major mystery in atherogenesis is the well-known variation in lesion progression among individuals with similar plasma lipid profiles (see Reference 5959) and among different arterial sites within the same individual.6 All known risk factors account for merely 50% of coronary events and well under 50% of interindividual variability in the actual extent of coronary lesions.193 The remaining risk is at least partially attributable to poorly characterized properties of the vessel wall. The response-to-retention hypothesis predicts that these vessel wall factors include molecules involved in lipoprotein retention, which, at this state of our knowledge, means proteoglycans, LpL, SMase, apoE, apoB, and apo(a).
Tools now exist or can be developed to assess the roles of proteoglycans and arterial wall enzymes in very early atherogenesis. Many proteoglycan core proteins of endothelial194, 195, 196 and smooth muscle194, 195, 197, 198, 199, 200, 201, 202 cells have been cloned and sequenced, which would allow linkage studies in animals and humans and direct genetic manipulation, particularly at lesion-susceptible branch points. Enzymes that are involved in proteoglycan side-chain assembly are still being characterized,203, 204, 205, 206 but we suggest that polymorphisms might play a role in atherosclerosis susceptibility (see References 207 and 208207, 208). To establish a causal role for LpL in atherogenesis in vivo, direct stimulation and especially suppression of intramural arterial LpL must be done, followed by an examination of lesion progression (see Reference 140140). Because several functional domains within LpL have already been characterized,209 specific constructs can be engineered to separately examine in vivo the atherogenicity of its enzymatic121 versus structural122, 123, 125, 127 actions. Two decades ago, lesions were shown to be enriched with SMase, but detailed enzymatic or molecular characterization of this lipase activity is still lacking, thus preventing linkage studies or genetic manipulation. The mechanism of SMase-induced aggregation in vitro has been shown to depend on the generation of ceramide.130 Thus, to implicate SMase in atherogenesis, lesional lipoproteins will have to be shown to be enriched in ceramide.
Investigation into the local roles of apoproteins and other molecules in very early atherogenesis can also be performed. Transgenic mice that overexpress apoE in the arterial wall, among other sites, have shown reduced atherosclerosis in one preliminary report,210 consistent with the hypothesis of a local protective role for this molecule in vivo.175, 211 Transgenic mice that overexpress apoA-I show reduced lesion development,212, 213, 214 perhaps because of accelerated reverse cholesterol transport, but possibly because of the local inhibitory effects that apoA-I particles exert on aggregation215, 216 and oxidation77, 80, 84, 215, 217 of atherogenic lipoproteins. It will be interesting to develop animal models in which overexpression of apoE or apoA-I is cleanly confined to the arterial wall. The physical characteristics of LDL, including its size,218, 219 apoB conformation,220 or sialic acid content,221 may affect binding affinity to arterial proteoglycans135, 219, 221 and subsequent oxidation.220, 222 Apo(a) polymorphisms177 should also be examined.
Tools exist as well to investigate the proposed sequence of events in early atherogenesis, subsequent to lipoprotein retention. For example, on the basis of the apparent sequence of events in vivo, we predict that massive retention of LDL or Lp(a) by smooth muscle cells or matrix127 will stimulate nearby endothelial and possibly smooth muscle cells127, 152, 223 to express cell adhesion molecules, chemoattractants, and growth factors (see “Competing Hypotheses”). A likely mechanism would be oxidation of the retained lipoproteins, followed by release of biologically active components, such as lysophosphatidylcholine, which is known to stimulate the expression of VCAM-1, platelet-derived growth factor, and other molecules by otherwise-normal endothelium.224,The prediction can be tested by coculture in vitro,84 with specific emphasis on the search for lipoprotein retention as an initial event. In addition, we predict that one key atherogenic effect of turbulent blood flow on prelesional sites in vivo is the locally enhanced expression of apoB-retaining molecules, particularly by vascular smooth muscle cells. A search for these molecules in susceptible versus resistant prelesional arterial sites could be undertaken, the molecules genetically manipulated if already cloned, and the effects on atherogenesis examined. Possible mechanisms for altered expression of these molecules would include direct effects of hemodynamic forces acting through sheer stress response–elements in underlying smooth muscle cells18 or through endothelium-dependent effects,63 including direct electrical communication between the endothelium and smooth muscle18, 225 or humoral signals.63 For example, transforming growth factor–β is expressed by the endothelium under control of a shear stress–responsive element57 and is known to stimulate synthesis of chondroitin sulfate proteoglycans by smooth muscle cells.226, 227
Finally, the search for additional molecules or mechanisms that may be important in vivo to lipoprotein retention and responses to retention should continue. For example, collagen,114 fibrin,115, 116 fibronectin,117, 118, 119 and matrix-bound phospholipase A2228, 229 have been implicated in several studies. Also, the LDL receptor–related protein, which binds LpL230 and apoE231 on ligand blots in vitro, has recently been reported to be present in normal and lesional arteries.232, 233 Of particular interest is how an arterial segment might remain healthy after the entry of lipoproteins (see Reference 9292). For example, there is substantial and well-documented evidence for the egress of atherogenic lipoproteins from the normal arterial wall,27, 36 which has generally been assumed to be passive, though it may not be. Other processes that could blunt or enhance oxidative and inflammatory responses to retained lipoproteins show genetic variability in mice234 and merit further study in humans.
Arterial retention of atherogenic lipoproteins is a logical target for therapeutic intervention. So far, three strategies specifically directed against lipoprotein retention have been proposed in the literature. The first strategy is the local use of molecules that interfere with adherence of apoB or apo(a)-apoB to arterial proteoglycans. As noted before, apoE is a promising candidate, in vitro137, 175 and possibly in mice.210 Boosting local expression of apoE in human arterial segments, however, would be difficult at present and may require gene therapy, an approach still in its infancy. Other potential candidates include apoB fragments137 or other proteoglycan-binding peptides.235, 236, 237 The second strategy proposed in the literature is inhibition of intra-arterial SMase activity.129 The effects of SMase, unlike the effect of LpL, involve its enzymatic action.130 Specific enzymatic inhibitors could test its role in vivo and might provide therapeutic benefit. A third strategy to reduce arterial retention of LDL is the use of nifedipine,238 a calcium-channel blocker that alters many cellular functions,239 including arterial retention of autologous, but not human, LDL in normocholesterolemic rabbits.238 Note, however, that calcium-channel blockers do not appear to affect atherogenesis in LDL receptor–deficient rabbits, which exhibit nondietary hyperlipidemia.240 Other novel targets to consider would be inhibition of intramural production of proteoglycans or LpL, alteration of cytokine expression, such as transforming growth factor–β, and perhaps stimulation of ceramidase production.
Concluding Remarks
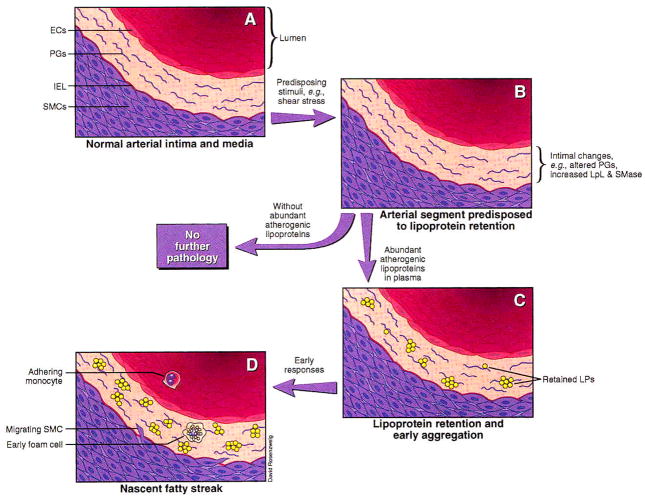
Although atherosclerosis is a complex and multifactorial process, we conclude that there exists a key pathogenic event, namely, lipoprotein retention, that is both necessary and sufficient to provoke lesion initiation in an otherwise-normal artery. Other potential contributors to early atherogenesis, such as hyperlipidemia; lipoprotein influx; lipoprotein modification; turbulent blood flow; and alterations in the endothelium, smooth muscle cells, and matrix, individually fail to meet this dual criterion of necessity and sufficiency. Lipoprotein retention, however, is an absolute requirement for lesion development and appears to be sufficient in most circumstances to provoke otherwise-normal cellular and matrix elements to participate in a cascade of events leading to atherosclerosis (see the Figure ↓). Essentially all later events can be traced to these early changes.
Figure 1.
Schematic of the response-to-retention model of early atherogenesis. Mild to moderate hyperlipidemia causes lesion development only in specific sites within the arterial tree, implying the existence of predisposing stimuli, such as sheer stress, that make these sites particularly lesion-prone by stimulating local synthesis of apoB-retentive molecules (B). Predisposing stimuli in the absence of abundant atherogenic lipoproteins (ie, <2 mmol LDL cholesterol/L) are insufficient to cause atherogenesis. Predisposing stimuli in the presence of abundant atherogenic lipoproteins result in lipoprotein retention (C). Evidence suggests that aggregation promptly follows or may be part of the retentive process. Once significant retention has occurred, a cascade of early responses, including lipoprotein oxidation and cellular chemotaxis, leads to lesion development (D). ECs indicates endothelial cells; PGs, proteoglycans; IEL, internal elastic lamina; SMCs, smooth muscle cells; LpL, lipoprotein lipase; SMase, sphingomyelinase; and LPs, lipoproteins.
Acknowledgments
Dr Williams is an Established Investigator of the American Heart Association and Genentech. During portions of this work, Dr Tabas was an Established Investigator of the American Heart Association and Boehringer-Ingelheim. Support is also acknowledged from National Institutes of Health grants HL38956, HL39703, and HL21006 and a cardiovascular research grant from the W.W. Smith Charitable Trust. We thank our colleagues for their helpful comments during the preparation of this article.
References
- 1.Faber M. The human aorta: sulfate-containing polyuronides and the deposition of cholesterol. Arch Pathol. 1949;48:342–350. [PubMed] [Google Scholar]
- 2.Smith EB. The relationship between plasma and tissue lipids in human atherosclerosis. Adv Lipid Res. 1974;12:1–49. doi: 10.1016/b978-0-12-024912-1.50008-9. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan SR, Radhakrishnamurthy B, Vijayagopal P, Berenson GS. Proteoglycans, lipoproteins, and atherosclerosis. Adv Exp Med Biol. 1991;285:373–381. doi: 10.1007/978-1-4684-5904-3_45. [DOI] [PubMed] [Google Scholar]
- 4.Simionescu N, Sima A, Dobrian A, Tirziu D, Simionescu M. Pathobiochemical changes of the arterial wall at the inception of atherosclerosis. Curr Top Pathol. 1993;87:1–45. doi: 10.1007/978-3-642-76849-1_1. [DOI] [PubMed] [Google Scholar]
- 5.Wissler RW. Morphological characteristics of the developing atherosclerotic plaque: animal studies and studies of lesions from young people. Atheroscler Rev. 1991;23:91–103. [Google Scholar]
- 6.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb. 1994;14:840–856. doi: 10.1161/01.atv.14.5.840. [DOI] [PubMed] [Google Scholar]
- 7.Ross R, Glomset JA. The pathogenesis of atherosclerosis. N Engl J Med. 1976;295:369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Glomset JA. The pathogenesis of atherosclerosis. N Engl J Med. 1976;295:420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- 9.Ross R, Glomset J, Harker L. Response to injury and atherogenesis. Am J Pathol. 1977;86:675–684. [PMC free article] [PubMed] [Google Scholar]
- 10.DiCorleto PE, Chisolm GM. Participation of the endothelium in the development of the atherosclerotic plaque. Prog Lipid Res. 1986;25:365–374. doi: 10.1016/0163-7827(86)90074-3. [DOI] [PubMed] [Google Scholar]
- 11.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 12.DiCorleto PE, Soyombo AA. The role of the endothelium in atherogenesis. Curr Opin Lipidol. 1993;4:364–372. [Google Scholar]
- 13.Taylor KE, Glagov S, Zarins CK. Preservation and structural adaptation of endothelium over experimental foam cell lesions: quantitative ultrastructural study. Arteriosclerosis. 1989;9:881–894. doi: 10.1161/01.atv.9.6.881. [DOI] [PubMed] [Google Scholar]
- 14.Katsuda S, Boyd HC, Fligner C, Ross R, Gown AM. Human atherosclerosis, III: immunocytochemical analysis of the cell composition of lesions of young adults. Am J Pathol. 1992;140:907–914. [PMC free article] [PubMed] [Google Scholar]
- 15.Simionescu N, Mora R, Vasile E, Lupu F, Filip DA, Simionescu M. Prelesional modifications of the vessel wall in hyperlipidemic atherogenesis: extracellular accumulation of modified and reassembled lipoproteins. Ann N Y Acad Sci. 1990;598:1–16. doi: 10.1111/j.1749-6632.1990.tb42271.x. [DOI] [PubMed] [Google Scholar]
- 16.Minick CR, Stemerman MG, Insull W., Jr Effect of regenerated endothelium on lipid accumulation in the arterial wall. Proc Natl Acad Sci U S A. 1977;74:1724–1728. doi: 10.1073/pnas.74.4.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang MY, Lees AM, Lees RS. Time course of 125I-labeled LDL accumulation in the healing, balloon-deendothelialized rabbit aorta. Arterioscler Thromb. 1992;12:1088–1098. doi: 10.1161/01.atv.12.9.1088. [DOI] [PubMed] [Google Scholar]
- 18.Davies PF, Robotewskyj A, Griem ML, Dull RO, Polacek DC. Hemodynamic forces and vascular cell communication in arteries. Arch Pathol Lab Med. 1992;116:1301–1306. [PubMed] [Google Scholar]
- 19.Jackson CL, Raines EW, Ross R, Reidy MA. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb. 1993;13:1218–1226. doi: 10.1161/01.atv.13.8.1218. [DOI] [PubMed] [Google Scholar]
- 20.Clinton SK, Libby P. Cytokines and growth factors in atherogenesis. Arch Pathol Lab Med. 1992;116:1292–1300. [PubMed] [Google Scholar]
- 21.McGill HC, Geer JC, Holman RL. Sites of vascular vulnerability in dogs demonstrated by Evans blue. Arch Pathol. 1957;64:303–311. [PubMed] [Google Scholar]
- 22.Ross R, Masuda J, Raines EW. Cellular interactions, growth factors, and smooth muscle proliferation in atherogenesis. Ann N Y Acad Sci. 1990;598:102–112. doi: 10.1111/j.1749-6632.1990.tb42282.x. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen LB, Nordestgaard BG, Stender S, Kjeldsen K. Aortic permeability to LDL as a predictor of aortic cholesterol accumulation in cholesterol-fed rabbits. Arterioscler Thromb. 1992;12:1402–1409. doi: 10.1161/01.atv.12.12.1402. [DOI] [PubMed] [Google Scholar]
- 24.Thubrikar MJ, Keller AC, Holloway PW, Nolan SP. Distribution of low density lipoprotein in the branch and non-branch regions of the aorta. Atherosclerosis. 1992;97:1–9. doi: 10.1016/0021-9150(92)90045-i. [DOI] [PubMed] [Google Scholar]
- 25.Fry DL, Herderick EE, Johnson DK. Local intimal-medial uptakes of 125I-albumin, 125I-LDL, and parenteral Evans blue dye protein complex along the aortas of normocholesterolemic minipigs as predictors of subsequent hypercholesterolemic atherogenesis. Arterioscler Thromb. 1993;13:1193–1204. doi: 10.1161/01.atv.13.8.1193. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann RA, Malinauskas RA, Truskey GA. Characterization of sites with elevated LDL permeability at intercostal, celiac, and iliac branches of the normal rabbit aorta. Arterioscler Thromb. 1994;14:313–323. doi: 10.1161/01.atv.14.2.313. [DOI] [PubMed] [Google Scholar]
- 27.Nordestgaard BG, Nielsen LB. Atherosclerosis and arterial influx of lipoproteins. Curr Opin Lipidol. 1994;5:252–257. doi: 10.1097/00041433-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Anitschkow N, Chalatow S. Über experimentelle Cholesterinsteatose und ihre Bedeutung für die Entstehung einiger pathologischer Prozesse. Zentralbl Allg Pathol. 1913;24:1–9. [Google Scholar]
- 29.Capron L. Pathogenesis of atherosclerosis: an update on the three main theories. Ann Cardiol Angeiol (Paris) 1989;38:631–634. [PubMed] [Google Scholar]
- 30.Hoeg JM, Klimov AN. Cholesterol and atherosclerosis: “the new is the old rediscovered”. Am J Cardiol. 1993;72:1071–1072. doi: 10.1016/0002-9149(93)90863-8. [DOI] [PubMed] [Google Scholar]
- 31.Vasile E, Simionescu M, Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. J Cell Biol. 1983;96:1677–1689. doi: 10.1083/jcb.96.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navab M, Hough GP, Berliner JA, Frank JA, Fogelman AM, Haberland ME, Edwards PA. Rabbit β-migrating very low density lipoprotein increases endothelial macromolecular transport without altering electrical resistance. J Clin Invest. 1986;78:389–397. doi: 10.1172/JCI112589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin S-J, Jan K-M, Weinbaum S, Chien S. Transendothelial transport of low density lipoprotein in association with cell mitosis in rat aorta. Arteriosclerosis. 1989;9:230–236. doi: 10.1161/01.atv.9.2.230. [DOI] [PubMed] [Google Scholar]
- 34.Carew TE, Pittman RC, Marchand ER, Steinberg D. Measurement in vivo of irreversible degradation of low density lipoprotein in the rabbit aorta: predominance of intimal degradation. Arteriosclerosis. 1984;4:214–224. doi: 10.1161/01.atv.4.3.214. [DOI] [PubMed] [Google Scholar]
- 35.Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits, I: focal increases in arterial LDL concentration precede development of fatty streak lesions. Arteriosclerosis. 1989;9:895–907. doi: 10.1161/01.atv.9.6.895. [DOI] [PubMed] [Google Scholar]
- 36.Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits, II: selective retention of LDL vs. selective increases in LDL permeability in susceptible sites of arteries. Arteriosclerosis. 1989;9:908–918. doi: 10.1161/01.atv.9.6.908. [DOI] [PubMed] [Google Scholar]
- 37.Schwenke DC, St Clair RW. Influx, efflux, and accumulation of LDL in normal arterial areas and atherosclerotic lesions of white Carneau pigeons with naturally occurring and cholesterol-aggravated aortic atherosclerosis. Arterioscler Thromb. 1993;13:1368–1381. doi: 10.1161/01.atv.13.9.1368. [DOI] [PubMed] [Google Scholar]
- 38.Falcone DJ, Hajjar DP, Minick CR. Lipoprotein and albumin accumulation in reendothelialized and deendothelialized aorta. Am J Pathol. 1984;114:112–120. [PMC free article] [PubMed] [Google Scholar]
- 39.Spring PM, Hoff HF. LDL accumulation in the grossly normal human iliac bifurcation and common iliac arteries. Exp Mol Pathol. 1989;51:179–185. doi: 10.1016/0014-4800(89)90018-x. [DOI] [PubMed] [Google Scholar]
- 40.Schwenke DC, St Clair RW. Accumulation of 125I-tyramine cellobiose-labeled low density lipoprotein is greater in the atherosclerosis-susceptible region of White Carneau pigeon aorta and further enhanced once atherosclerotic lesions develop. Arterioscler Thromb. 1992;12:446–460. doi: 10.1161/01.atv.12.4.446. [DOI] [PubMed] [Google Scholar]
- 41.Kreuzer J, Lloyd MB, Bok D, Fless GM, Scanu AM, Lusis AJ, Haberland ME. Lipoprotein (a) displays increased accumulation compared with low-density lipoprotein in the murine arterial wall. Chem Phys Lipids. 1994;67/68:175–190. doi: 10.1016/0009-3084(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 42.Lin S-J, Hong C-Y, Chang M-S, Chiang BN, Chien S. Long-term nicotine exposure increases aortic endothelial cell death and enhances transendothelial macromolecular transport in rats. Arterioscler Thromb. 1992;12:1305–1312. doi: 10.1161/01.atv.12.11.1305. [DOI] [PubMed] [Google Scholar]
- 43.Fry DL, Haupt MW, Pap JM. Effect of endothelial integrity, transmural pressure, and time on the intimal-medial uptake of serum 125I-albumin and 125I-LDL in an in vitro porcine arterial organ-support system. Arterioscler Thromb. 1992;12:1313–1328. doi: 10.1161/01.atv.12.11.1313. [DOI] [PubMed] [Google Scholar]
- 44.Johnson-Tidey RR, McGregor JL, Taylor PR, Poston RN. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques: coexpression with intercellular adhesion molecule-1. Am J Pathol. 1994;144:952–961. [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Cybulsky MI, Gimbrone MA, Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993;13:197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- 46.Nievelstein PFEM, Fogelman AM, Mottino G, Frank JS. Lipid accumulation in rabbit aortic intima 2 hours after bolus infusion of low density lipoprotein: a deep-etch and immunolocalization study of ultrarapidly frozen tissue. Arterioscler Thromb. 1991;11:1795–1805. doi: 10.1161/01.atv.11.6.1795. [DOI] [PubMed] [Google Scholar]
- 47.Kume N, Cybulsky MI, Gimbrone MA., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JA, Territo MC, Wayner E, Carlos TM, Parhami F, Smith CW, Haberland ME, Fogelman AM, Berliner JA. Partial characterization of leukocyte binding molecules on endothelial cells induced by minimally oxidized LDL. Arterioscler Thromb. 1994;14:427–433. doi: 10.1161/01.atv.14.3.427. [DOI] [PubMed] [Google Scholar]
- 49.Chisolm GM. Oxidized lipoproteins and leukocyte-endothelial interactions: growing evidence for multiple mechanisms. Lab Invest. 1993;68:369–371. [PubMed] [Google Scholar]
- 50.Zand T, Majno G, Nunnari JJ, Hoffman AH, Savilonis BJ, MacWilliams B, Joris I. Lipid deposition and intimal stress and strain: a study in rats with aortic stenosis. Am J Pathol. 1991;139:101–113. [PMC free article] [PubMed] [Google Scholar]
- 51.Davies PF, Tripathi SC. Mechanical stress mechanisms and the cell: an endothelial paradigm. Circ Res. 1993;72:239–245. doi: 10.1161/01.res.72.2.239. [DOI] [PubMed] [Google Scholar]
- 52.Barbee KA, Davies PF, Lal R. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ Res. 1994;74:163–171. doi: 10.1161/01.res.74.1.163. [DOI] [PubMed] [Google Scholar]
- 53.DePaola N, Gimbrone MA, Jr, Davies PF, Dewey CF., Jr Vascular endothelium responds to fluid shear stress gradients. Arterioscler Thromb. 1992;12:1254–1257. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 54.Lan Q, Mercurius KO, Davies PF. Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem Biophys Res Commun. 1994;201:950–956. doi: 10.1006/bbrc.1994.1794. [DOI] [PubMed] [Google Scholar]
- 55.Shen J, Gimbrone MA, Jr, Luscinskas FW, Dewey CF., Jr Regulation of adenine nucleotide concentration at endothelium-fluid interface by viscous shear flow. Biophys J. 1993;64:1323–1330. doi: 10.1016/S0006-3495(93)81498-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagel T, Resnick N, Atkinson WJ, Dewey CF, Jr, Gimbrone MA., Jr Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest. 1994;94:885–891. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Resnick N, Collins T, Atkinson W, Bonthron DT, Dewey CF, Jr, Gimbrone MA., Jr Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci U S A. 1993;90:4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mills GL, Taylaur CE. The distribution and composition of serum lipoproteins in eighteen animals. Comp Biochem Physiol [B] 1971;40:489–501. doi: 10.1016/0305-0491(71)90234-3. [DOI] [PubMed] [Google Scholar]
- 59.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 60.Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 62.Manley G, Hawksworth J. Distribution of mucopolysaccharides in the human vascular tree. Nature. 1965;206:1152–1153. doi: 10.1038/2061152a0. [DOI] [PubMed] [Google Scholar]
- 63.Wight TN, Curwen KD, Litrenta MM, Alonso DR, Minick CR. Effect of endothelium on glycosaminoglycan accumulation in injured rabbit aorta. Am J Pathol. 1983;113:156–164. [PMC free article] [PubMed] [Google Scholar]
- 64.Hoff HF, Wagner WD. Plasma low density lipoprotein accumulation in aortas of hypercholesterolemic swine correlates with modifications in aortic glycosaminoglycan composition. Atherosclerosis. 1986;61:231–236. doi: 10.1016/0021-9150(86)90143-7. [DOI] [PubMed] [Google Scholar]
- 65.Ylä-Herttuala S, Pesonen E, Kaprio E, Rapola J, Soveri T, Viikari J, Savilahti E, Oksanen H, Nikkari T. Effect of repeated endotoxin treatment and hypercholesterolemia on preatherosclerotic lesions in weaned pigs, part II: lipid and glycosaminoglycan analysis of intima and inner media. Atherosclerosis. 1988;72:173–181. doi: 10.1016/0021-9150(88)90078-0. [DOI] [PubMed] [Google Scholar]
- 66.Cardoso LE, Mouraõ PA. Glycosaminoglycan fractions from human arteries presenting diverse susceptibilities to atherosclerosis have different binding affinities to plasma LDL. Arterioscler Thromb. 1994;14:115–124. doi: 10.1161/01.atv.14.1.115. [DOI] [PubMed] [Google Scholar]
- 67.Chao FF, Amende LM, Blanchette-Mackie EJ, Skarlatos SI, Gamble W, Resau JH, Mergner WT, Kruth HS. Unesterified cholesterol-rich lipid particles in atherosclerotic lesions of human and rabbit aortas. Am J Pathol. 1988;131:73–83. [PMC free article] [PubMed] [Google Scholar]
- 68.Simionescu N, Vasile E, Lupu F, Popescu G, Simionescu M. Prelesional events in atherogenesis: accumulation of extracellular cholesterol-rich liposomes in the arterial intima and cardiac valves of the hyperlipidemic rabbit. Am J Pathol. 1986;123:109–125. [PMC free article] [PubMed] [Google Scholar]
- 69.Ylä-Herttuala S, Solakivi T, Hirvonen J, Laaksonen H, Mottonen M, Pesonen E, Raekallio J, Akerblom HK, Nikkari T. Glycosaminoglycans and apolipoproteins B and A-I in human aortas: chemical and immunological analysis of lesion-free aortas from children and adults. Arteriosclerosis. 1987;7:333–340. doi: 10.1161/01.atv.7.4.333. [DOI] [PubMed] [Google Scholar]
- 70.Camejo G, Hurt-Camejo E, Bondjers G. Effect of proteoglycans on lipoprotein-cell interactions: possible contribution to atherogenesis. Curr Opin Lipidol. 1990;1:431–436. [Google Scholar]
- 71.Camejo G, Hurt-Camejo E, Olsson U, Bondjers G. Proteoglycans and lipoproteins in atherosclerosis. Curr Opin Lipidol. 1993;4:385–391. [Google Scholar]
- 72.Etingin OR, Silverstein RL, Hajjar DP. von Willebrand factor mediates platelet adhesion to virally infected endothelial cells. Proc Natl Acad Sci U S A. 1993;90:5153–5156. doi: 10.1073/pnas.90.11.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsai JC, Perrella MA, Yoshizumi M, Hsieh CM, Haber E, Schlegel R, Lee ME. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proc Natl Acad Sci U S A. 1994;91:6369–6373. doi: 10.1073/pnas.91.14.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chisolm GM. Antioxidants and atherosclerosis: a current assessment. Clin Cardiol. 1991;14(2 suppl 1):I25–I30. doi: 10.1002/clc.4960141304. [DOI] [PubMed] [Google Scholar]
- 75.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steinberg D. Antioxidants in the prevention of human atherosclerosis: summary of the proceedings of a National Heart, Lung, and Blood Institute Workshop, September 5–6, 1991, Bethesda, Maryland. Circulation. 1992;85:2337–2344. doi: 10.1161/01.cir.85.6.2337. [DOI] [PubMed] [Google Scholar]
- 77.van Hinsbergh VW, Scheffer M, Havekes L, Kempen HJ. Role of endothelial cells and their products in the modification of low-density lipoproteins. Biochim Biophys Acta. 1986;878:49–64. doi: 10.1016/0005-2760(86)90343-7. [DOI] [PubMed] [Google Scholar]
- 78.Halliwell B. Albumin—an important extracellular antioxidant? Biochem Pharmacol. 1988;37:569–571. doi: 10.1016/0006-2952(88)90126-8. [DOI] [PubMed] [Google Scholar]
- 79.Zawadzki Z, Milne RW, Marcel YL. Cu2(+)-mediated oxidation of dialyzed plasma: effects on low and high density lipoproteins and cholesteryl ester transfer protein. J Lipid Res. 1991;32:243–250. [PubMed] [Google Scholar]
- 80.Kalant N, McCormick S. Inhibition by serum components of oxidation and collagen-binding of low-density lipoprotein. Biochim Biophys Acta. 1992;1128:211–219. doi: 10.1016/0005-2760(92)90310-r. [DOI] [PubMed] [Google Scholar]
- 81.Thomas CE. The influence of medium components on Cu(2+)-dependent oxidation of low-density lipoproteins and its sensitivity to superoxide dismutase. Biochim Biophys Acta. 1992;1128:50–57. doi: 10.1016/0005-2760(92)90256-u. [DOI] [PubMed] [Google Scholar]
- 82.Dobrian A, Mora R, Simionescu M, Simionescu N. In vitro formation of oxidatively-modified and reassembled human low-density lipoproteins: antioxidant effect of albumin. Biochim Biophys Acta. 1993;1169:12–24. doi: 10.1016/0005-2760(93)90076-l. [DOI] [PubMed] [Google Scholar]
- 83.Wagner JR, Motchnik PA, Stocker R, Sies H, Ames BN. The oxidation of blood plasma and low density lipoprotein components by chemically generated singlet oxygen. J Biol Chem. 1993;268:18502–18506. [PubMed] [Google Scholar]
- 84.Navab M, Imes SS, Hama SY, Hough GP, Ross LA, Bork RW, Valente AJ, Berliner JA, Drinkwater DC, Laks H, Fogelman AM. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991;88:2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Berkel TJ, de Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats: recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem. 1991;266:2282–2289. [PubMed] [Google Scholar]
- 86.de Rijke YB, Jurgens G, Hessels EM, Hermann A, van Berkel TJ. In vivo fate and scavenger receptor recognition of oxidized lipoprotein[a] isoforms in rats. J Lipid Res. 1992;33:1315–1325. [PubMed] [Google Scholar]
- 87.Chang MY, Lees AM, Lees RS. Low-density lipoprotein modification and arterial wall accumulation in a rabbit model of atherosclerosis. Biochemistry. 1993;32:8518–8524. doi: 10.1021/bi00084a018. [DOI] [PubMed] [Google Scholar]
- 88.Heinecke JW, Rosen H, Suzuki LA, Chait A. The role of sulfur-containing amino acids in superoxide production and modification of low density lipoprotein by arterial smooth muscle cells. J Biol Chem. 1987;262:10098–10103. [PubMed] [Google Scholar]
- 89.Sparrow CP, Olszewski J. Cellular oxidation of low density lipoprotein is caused by thiol production in media containing transition metal ions. J Lipid Res. 1993;34:1219–1228. [PubMed] [Google Scholar]
- 90.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hurt-Camejo E, Camejo G, Rosengren B, López F, Ahlstrom C, Fager G, Bondjers G. Effect of arterial proteoglycans and glycosaminoglycans on low density lipoprotein oxidation and its uptake by human macrophages and arterial smooth muscle cells. Arterioscler Thromb. 1992;12:569–583. doi: 10.1161/01.atv.12.5.569. [DOI] [PubMed] [Google Scholar]
- 92.Jürgens G, Chen Q, Esterbauer H, Mair S, Ledinski G, Dinges HP. Immunostaining of human autopsy aortas with antibodies to modified apolipoprotein B and apoprotein(a) Arterioscler Thromb. 1993;13:1689–1699. doi: 10.1161/01.atv.13.11.1689. [DOI] [PubMed] [Google Scholar]
- 93.Liao F, Berliner JA, Mehrabian M, Navab M, Demer LL, Lusis AJ, Fogelman AM. Minimally modified low density lipoprotein is biologically active in vivo in mice. J Clin Invest. 1991;87:2253–2257. doi: 10.1172/JCI115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kita T, Nagano Y, Yokode M, Ishii K, Kume N, Ooshima A, Yoshida H, Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987;84:5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carew TE, Schwenke DC, Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987;84:7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sparrow CP, Doebber TW, Olszewski J, Wu MS, Ventre J, Stevens KA, Chao YS. Low density lipoprotein is protected from oxidation and the progression of atherosclerosis is slowed in cholesterol-fed rabbits by the antioxidant N,N′-diphenyl phenylenediamine. J Clin Invest. 1992;89:1885–1891. doi: 10.1172/JCI115793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ylä-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haberland ME, Fong D, Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988;241:215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- 99.Ylä-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 100.Salonen JT, Ylä-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssonen K, Palinski W, Witztum JL. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 101.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers: the Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 102.Gaziano JM. Antioxidant vitamins and coronary artery disease risk. Am J Med. 1994;97:3A-18S–3A-21S. doi: 10.1016/0002-9343(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 103.Lund G, Beisiegel U, Seeber N, Nikutta P, Hamm CW. Abstracts Submitted to the Council on Arteriosclerosis for the 66th Scientific Sessions of the American Heart Association. Dallas, Tex: American Heart Association Inc; 1993. Effect of probucol on progression/regression of coronary artery disease; p. 124. [Google Scholar]
- 104.Elinder LS, Walldius G. Antioxidants and atherosclerosis progression: unresolved questions. Curr Opin Lipidol. 1994;5:265–268. doi: 10.1097/00041433-199408000-00004. [DOI] [PubMed] [Google Scholar]
- 105.Fruebis J, Steinberg D, Dresel HA, Carew TE. A comparison of the antiatherogenic effects of probucol and of a structural analogue of probucol in low density lipoprotein receptor-deficient rabbits. J Clin Invest. 1994;94:392–398. doi: 10.1172/JCI117334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stalenhoef AFH, Armstrong VW, Humphries S, Schmitz G, Soutar AK, Taskinen M-R. The 16th annual meeting of the European Lipoprotein Club. Arterioscler Thromb. 1994;14:831–837. doi: 10.1161/01.atv.14.5.831. [DOI] [PubMed] [Google Scholar]
- 107.Mao SJ, Yates MT, Parker RA, Chi EM, Jackson RL. Attenuation of atherosclerosis in a modified strain of hypercholesterolemic Watanabe rabbits with use of a probucol analogue (MDL 29,311) that does not lower serum cholesterol. Arterioscler Thromb. 1991;11:1266–1275. doi: 10.1161/01.atv.11.5.1266. [DOI] [PubMed] [Google Scholar]
- 108.Mori Y, Wada H, Nagano Y, Deguchi K, Kita T, Shirakawa S. Hypercoagulable state in the Watanabe heritable hyperlipidemic rabbit, an animal model for the progression of atherosclerosis: effect of probucol on coagulation. Thromb Haemost. 1989;61:140–143. [PubMed] [Google Scholar]
- 109.Ku G, Doherty NS, Schmidt LF, Jackson RL, Dinerstein RJ. Ex vivo lipopolysaccharide-induced interleukin-1 secretion from murine peritoneal macrophages inhibited by probucol, a hypocholesterolemic agent with antioxidant properties. FASEB J. 1990;4:1645–1653. doi: 10.1096/fasebj.4.6.2318380. [DOI] [PubMed] [Google Scholar]
- 110.Steinbrecher UP, Lougheed M. Scavenger receptor–independent stimulation of cholesterol esterification in macrophages by low density lipoprotein extracted from human aortic intima. Arterioscler Thromb. 1992;12:608–625. doi: 10.1161/01.atv.12.5.608. [DOI] [PubMed] [Google Scholar]
- 111.Hoff HF, O’Neil J. Extracts of human atherosclerotic lesions can modify low density lipoproteins leading to enhanced uptake by macrophages. Atherosclerosis. 1988;70:29–41. doi: 10.1016/0021-9150(88)90097-4. [DOI] [PubMed] [Google Scholar]
- 112.Kahn JA, Glueck CJ. Familial hypobetalipoproteinemia: absence of atherosclerosis in a postmortem study. JAMA. 1978;240:47–48. doi: 10.1001/jama.240.1.47. [DOI] [PubMed] [Google Scholar]
- 113.Li H, Reddick RL, Maeda N. Lack of apoA-I is not associated with increased susceptibility to atherosclerosis in mice. Arterioscler Thromb. 1993;13:1814–1821. doi: 10.1161/01.atv.13.12.1814. [DOI] [PubMed] [Google Scholar]
- 114.Nievelstein-Post P, Mottino G, Fogelman A, Frank J. An ultrastructural study of lipoprotein accumulation in cardiac valves of the rabbit. Arterioscler Thromb. 1994;14:1151–1161. doi: 10.1161/01.atv.14.7.1151. [DOI] [PubMed] [Google Scholar]
- 115.Smith EB, Staples EM. Haemostatic factors in human aortic intima. Lancet. 1981;1:1171–1174. doi: 10.1016/s0140-6736(81)92346-1. [DOI] [PubMed] [Google Scholar]
- 116.Harpel PC, Gordon BR, Parker TS. Plasmin catalyzes binding of lipoprotein (a) to immobilized fibrinogen and fibrin. Proc Natl Acad Sci U S A. 1989;86:3847–3851. doi: 10.1073/pnas.86.10.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cseh K, Karadi I, Rischak K, Szollar L, Janoki G, Jakab L, Romics L. Binding of fibronectin to human lipoproteins. Clin Chim Acta. 1989;182:75–85. doi: 10.1016/0009-8981(89)90151-4. [DOI] [PubMed] [Google Scholar]
- 118.Labat-Robert J, Gruber E, Bihari-Varga M. Interaction between fibronectin, proteoglycans and lipoproteins. Int J Biol Macromol. 1990;12:50–54. doi: 10.1016/0141-8130(90)90081-k. [DOI] [PubMed] [Google Scholar]
- 119.Ehnholm C, Jauhiainen M, Metso J. Interaction of lipoprotein(a) with fibronectin and its potential role in atherogenesis. Eur Heart J. 1990;11(suppl E):190–195. doi: 10.1093/eurheartj/11.suppl_e.190. [DOI] [PubMed] [Google Scholar]
- 120.Corey JE, Zilversmit DB. Effect of cholesterol feeding on arterial lipolytic activity in the rabbit. Atherosclerosis. 1977;27:201–212. doi: 10.1016/0021-9150(77)90057-0. [DOI] [PubMed] [Google Scholar]
- 121.Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60:473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]
- 122.Williams KJ, Fless GM, Petrie K, Snyder ML, Brocia RW, Swenson TL. Lipoprotein lipase enhances cellular catabolism of lipoprotein(a) Circulation. 1991;84(suppl II):II-566. [Google Scholar]
- 123.Williams KJ, Fless GM, Petrie KA, Snyder ML, Brocia RW, Swenson TL. Mechanisms by which lipoprotein lipase alters cellular metabolism of lipoprotein(a), low density lipoprotein, and nascent lipoproteins: roles for low density lipoprotein receptors and heparan sulfate proteoglycans. J Biol Chem. 1992;267:13284–13292. [PubMed] [Google Scholar]
- 124.Rumsey SC, Obunike JC, Arad Y, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase-mediated uptake and degradation of low density lipoproteins by fibroblasts and macrophages. J Clin Invest. 1992;90:1504–1512. doi: 10.1172/JCI116018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eisenberg S, Sehayek E, Olivecrona T, Vlodavsky I. Lipoprotein lipase enhances binding of lipoproteins to heparan sulfate on cell surfaces and extracellular matrix. J Clin Invest. 1992;90:2013–2021. doi: 10.1172/JCI116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mulder M, Lombardi P, Jansen H, van Berkel TJ, Frants RR, Havekes LM. Low density lipoprotein receptor internalizes low density and very low density lipoproteins that are bound to heparan sulfate proteoglycans via lipoprotein lipase. J Biol Chem. 1993;268:9369–9375. [PubMed] [Google Scholar]
- 127.Tabas I, Li Y, Brocia RW, Xu SW, Swenson TL, Williams KJ. Lipoprotein lipase and sphingomyelinase synergistically enhance the association of atherogenic lipoproteins with smooth muscle cells and extracellular matrix: a possible mechanism for low density lipoprotein and lipoprotein(a) retention and macrophage foam cell formation. J Biol Chem. 1993;268:20419–20432. [PubMed] [Google Scholar]
- 128.Portman OW, Alexander M. Metabolism of sphingolipids by normal and atherosclerotic aorta of squirrel monkeys. J Lipid Res. 1970;11:23–30. [PubMed] [Google Scholar]
- 129.Xu X-X, Tabas I. Sphingomyelinase enhances low density lipoprotein uptake and ability to induce cholesteryl ester accumulation in macrophages. J Biol Chem. 1991;266:24849–24858. [PubMed] [Google Scholar]
- 130.Schissel SL, Williams KJ, Tabas I. Sphingomyelinase (SMase)-induced LDL aggregation: mechanism and relevance. Circulation. 1994;90(pt 2):I-403. [Google Scholar]
- 131.Howard AN, Patelski J, Bowyer DE, Gresham GA. Atherosclerosis induced in hypercholesterolaemic baboons by immunological injury; and the effects of intravenous polyunsaturated phosphatidyl choline. Atherosclerosis. 1971;14:17–29. doi: 10.1016/0021-9150(71)90035-9. [DOI] [PubMed] [Google Scholar]
- 132.Jonasson L, Bondjers G, Hansson GK. Lipoprotein lipase in atherosclerosis: its presence in smooth muscle cells and absence from macrophages. J Lipid Res. 1987;28:437–445. [PubMed] [Google Scholar]
- 133.Ylä-Herttuala S, Lipton BA, Rosenfeld ME, Goldberg IJ, Steinberg D, Witztum JL. Macrophages and smooth muscle cells express lipoprotein lipase in human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991;88:10143–10147. doi: 10.1073/pnas.88.22.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ismail NA, Alavi MZ, Moore S. Lipoprotein-proteoglycan complexes from injured rabbit aortas accelerate lipoprotein uptake by arterial smooth muscle cells. Atherosclerosis. 1994;105:79–87. doi: 10.1016/0021-9150(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 135.Linden T, Bondjers G, Camejo G, Bergstrand R, Wilhelmsen L, Wiklund O. Affinity of LDL to a human arterial proteoglycan among male survivors of myocardial infarction. Eur J Clin Invest. 1989;19:38–44. doi: 10.1111/j.1365-2362.1989.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 136.Kostner GM, Bihari-Varga M. Is the atherogenicity of Lp(a) caused by its reactivity with proteoglycans? Eur Heart J. 1990;11(suppl E):184–189. doi: 10.1093/eurheartj/11.suppl_e.184. [DOI] [PubMed] [Google Scholar]
- 137.Camejo G, Olofsson S-O, Lopez F, Carlsson P, Bondjers G. Identification of Apo B-100 segments mediating the interaction of low density lipoproteins with arterial proteoglycans. Arteriosclerosis. 1988;8:368–377. doi: 10.1161/01.atv.8.4.368. [DOI] [PubMed] [Google Scholar]
- 138.Saxena U, Klein MG, Vanni TM, Goldberg IJ. Lipoprotein lipase increases low density lipoprotein retention by subendothelial cell matrix. J Clin Invest. 1992;89:373–380. doi: 10.1172/JCI115595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Williams KJ, Swenson TL, Brocia RW, Rothman VL, Tuszynski GP, Fisher EA. Involvement of the syndecan family of heparan sulfate proteoglycans (HSPGs) in cellular uptake of complexes of lipoprotein lipase (LpL) with lipoproteins. Circulation. 1993;88(suppl I):I-365. [Google Scholar]
- 140.Rutledge JC, Goldberg IJ. Lipoprotein lipase (LpL) affects low density lipoprotein (LDL) flux through vascular tissue: evidence that LpL increases LDL accumulation in vascular tissue. J Lipid Res. 1994;35:1152–1160. [PubMed] [Google Scholar]
- 141.Renier G, Skamene E, DeSanctis JB, Radzioch D. High macrophage lipoprotein lipase expression and secretion are associated in inbred murine strains with susceptibility to atherosclerosis. Arterioscler Thromb. 1993;13:190–196. doi: 10.1161/01.atv.13.2.190. [DOI] [PubMed] [Google Scholar]
- 142.Peacock RE, Hamsten A, Nilsson-Ehle P, Humphries SE. Associations between lipoprotein lipase gene polymorphisms and plasma correlations of lipids, lipoproteins and lipase activities in young myocardial infarction survivors and age-matched healthy individuals from Sweden. Atherosclerosis. 1992;97:171–185. doi: 10.1016/0021-9150(92)90130-9. [DOI] [PubMed] [Google Scholar]
- 143.Zambon A, Torres A, Bijvoet S, Gagne C, Moorjani S, Lupien PJ, Hayden MR, Brunzell JD. Prevention of raised low-density lipoprotein cholesterol in a patient with familial hypercholesterolaemia and lipoprotein lipase deficiency. Lancet. 1993;341:1119–1121. doi: 10.1016/0140-6736(93)93129-o. [DOI] [PubMed] [Google Scholar]
- 144.Dugi KA, Feuerstein IM, Santamarina-Fojo S, Brewer HB, Jr, Hoeg JM. Lipoprotein lipase may contribute to the variant degree of atherosclerosis in homozygous familial hypercholesterolemia. Circulation. 1994;90(pt 2):I-405. doi: 10.1161/01.atv.17.2.354. [DOI] [PubMed] [Google Scholar]
- 145.Ylä-Herttuala S, Sumuvuori H, Karkola K, Mottonen M, Nikkari T. Glycosaminoglycans in normal and atherosclerotic human coronary arteries. Lab Invest. 1986;54:402–407. [PubMed] [Google Scholar]
- 146.O’Brien KD, Gordon D, Deeb S, Ferguson M, Chait A. Lipoprotein lipase is synthesized by macrophage-derived foam cells in human coronary atherosclerotic plaques. J Clin Invest. 1992;89:1544–1550. doi: 10.1172/JCI115747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mattsson L, Johansson H, Ottosson M, Bondjers G, Wiklund O. Expression of lipoprotein lipase mRNA and secretion in macrophages isolated from human atherosclerotic aorta. J Clin Invest. 1993;92:1759–1765. doi: 10.1172/JCI116764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.O’Brien KD, Deeb SS, Ferguson M, McDonald TO, Allen MD, Alpers CE, Chait A. Apolipoprotein E localization in human coronary atherosclerotic plaques by in situ hybridization and immunohistochemistry and comparison with lipoprotein lipase. Am J Pathol. 1994;144:538–548. [PMC free article] [PubMed] [Google Scholar]
- 149.Wagner WD, Salisbury GJ, Rowe HA. A proposed structure of chondroitin 6-sulfate proteoglycan of human normal and adjacent atherosclerotic plaque. Arteriosclerosis. 1986;6:407–417. doi: 10.1161/01.atv.6.4.407. [DOI] [PubMed] [Google Scholar]
- 150.Hoff HF, Morton RE. Lipoproteins containing apo B extracted from human aortas: structure and function. Ann N Y Acad Sci. 1985;454:183–194. doi: 10.1111/j.1749-6632.1985.tb11857.x. [DOI] [PubMed] [Google Scholar]
- 151.Vijayagopal P, Srinivasan SR, Radhakrishnamurthy B, Berenson GS. Lipoprotein-proteoglycan complexes from atherosclerotic lesions promote cholesteryl ester accumulation in human monocytes/macrophages. Arterioscler Thromb. 1992;12:237–249. doi: 10.1161/01.atv.12.2.237. [DOI] [PubMed] [Google Scholar]
- 152.Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987;84:2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McMurray HF, Parthasarathy S, Steinberg D. Oxidatively modified low density lipoprotein is a chemoattractant for human T lymphocytes. J Clin Invest. 1993;92:1004–1008. doi: 10.1172/JCI116605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Autio I, Jaakkola O, Solakivi T, Nikkari T. Oxidized low-density lipoprotein is chemotactic for arterial smooth muscle cells in culture. FEBS Lett. 1990;277:247–249. doi: 10.1016/0014-5793(90)80857-f. [DOI] [PubMed] [Google Scholar]
- 156.Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- 157.Joseph CK, Wright SD, Bornmann WG, Randolph JT, Kumar ER, Bittman R, Liu J, Kolesnick RN. Bacterial lipopolysaccharide has structural similarity to ceramide and stimulates ceramide-activated protein kinase in myeloid cells. J Biol Chem. 1994;269:17606–17610. [PubMed] [Google Scholar]
- 158.Hurt E, Bondjers G, Camejo G. Interaction of LDL with human arterial proteoglycans stimulates its uptake by human monocyte-derived macrophages. J Lipid Res. 1990;31:443–454. [PubMed] [Google Scholar]
- 159.Vijayagopal P, Srinivasan SR, Radhakrishnamurthy B, Berenson GS. Human monocyte-derived macrophages bind low-density-lipoprotein-proteoglycan complexes by a receptor different from the low-density-lipoprotein receptor. Biochem J. 1993;289:837–844. doi: 10.1042/bj2890837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rosenfeld ME, Carew TE, von Hodenberg E, Pittman RC, Ross R, Steinberg D. Autoradiographic analysis of the distribution of 125I-tyramine-cellobiose-LDL in atherosclerotic lesions of the WHHL rabbit. Arterioscler Thromb. 1992;12:985–995. doi: 10.1161/01.atv.12.8.985. [DOI] [PubMed] [Google Scholar]
- 161.Rosenfeld ME, Tsukada T, Chait A, Bierman EL, Gown AM, Ross R. Fatty streak expansion and maturation in Watanabe heritable hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1987;7:24–34. doi: 10.1161/01.atv.7.1.24. [DOI] [PubMed] [Google Scholar]
- 162.Mori N, Yamada N, Ishibashi S, Kawakami M, Takahashi K, Shimano H, Fujisawa M, Takaku F, Murase T. High-cholesterol diet-induced lipoproteins stimulate lipoprotein lipase secretion in cultured rat alveolar macrophages. Biochim Biophys Acta. 1987;922:103–110. doi: 10.1016/0005-2760(87)90143-3. [DOI] [PubMed] [Google Scholar]
- 163.Murata Y, Behr SR, Kraemer FB. Regulation of macrophage lipoprotein lipase secretion by the scavenger receptor. Biochim Biophys Acta. 1988;972:17–24. doi: 10.1016/0167-4889(88)90097-3. [DOI] [PubMed] [Google Scholar]
- 164.Lesnik P, Rouis M, Skarlatos S, Kruth HS, Chapman MJ. Uptake of exogenous free cholesterol induces upregulation of tissue factor expression in human monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1992;89:10370–10374. doi: 10.1073/pnas.89.21.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Falcone DJ, McCaffrey TA, Haimovitz-Friedman A, Vergilio JA, Nicholson AC. Macrophage and foam cell release of matrix-bound growth factors: role of plasminogen activation. J Biol Chem. 1993;268:11951–11958. [PubMed] [Google Scholar]
- 166.Katsuda S, Coltrera MD, Ross R, Gown AM. Human atherosclerosis, IV: immunocytochemical analysis of cell activation and proliferation in lesions of young adults. Am J Pathol. 1993;142:1787–1793. [PMC free article] [PubMed] [Google Scholar]
- 167.Owens RT, Wagner WD. Metabolism and turnover of cell surface–associated heparan sulfate proteoglycan and chondroitin sulfate proteoglycan in normal and cholesterol-enriched macrophages. Arterioscler Thromb. 1991;11:1752–1758. doi: 10.1161/01.atv.11.6.1752. [DOI] [PubMed] [Google Scholar]
- 168.Tertov VV, Orekhov AN, Ryong LH, Smirnov VN. Intracellular cholesterol accumulation is accompanied by enhanced proliferative activity of human aortic intimal cells. Tissue Cell. 1988;20:849–854. doi: 10.1016/0040-8166(88)90026-2. [DOI] [PubMed] [Google Scholar]
- 169.Sachinidis A, Locher R, Steiner A, Mengden T, Vetter W. Effect of low-density lipoprotein on intracellular calcium, intracellular pH and DNA synthesis in cultured vascular smooth muscle cells. J Hypertens Suppl. 1989;7:S116–S117. doi: 10.1097/00004872-198900076-00054. [DOI] [PubMed] [Google Scholar]
- 170.Martin BM, Gimbrone MA, Jr, Unanue ER, Cotran RS. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981;126:1510–1515. [PubMed] [Google Scholar]
- 171.Rennick RE, Campbell JH, Campbell GR. Vascular smooth muscle phenotype and growth behaviour can be influenced by macrophages in vitro. Atherosclerosis. 1988;71:35–43. doi: 10.1016/0021-9150(88)90300-0. [DOI] [PubMed] [Google Scholar]
- 172.Merrilees MJ, Campbell JH, Spanidis E, Campbell GR. Glycosaminoglycan synthesis by smooth muscle cells of differing phenotype and their response to endothelial cell conditioned medium. Atherosclerosis. 1990;81:245–254. doi: 10.1016/0021-9150(90)90072-q. [DOI] [PubMed] [Google Scholar]
- 173.Camejo G, Fager G, Rosengren B, Hurt-Camejo E, Bondjers G. Binding of low density lipoproteins by proteoglycans synthesized by proliferating and quiescent human arterial smooth muscle cells. J Biol Chem. 1993;268:14131–14137. [PubMed] [Google Scholar]
- 174.Rosenfeld ME, Butler S, Ord VA, Lipton BA, Dyer CA, Curtiss LK, Palinski W, Witztum JL. Abundant expression of apoprotein E by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb. 1993;13:1382–1389. doi: 10.1161/01.atv.13.9.1382. [DOI] [PubMed] [Google Scholar]
- 175.Saxena U, Ferguson E, Bisgaier CL. Apolipoprotein E modulates low density lipoprotein retention by lipoprotein lipase anchored to the subendothelial matrix. J Biol Chem. 1993;268:14812–14819. [PubMed] [Google Scholar]
- 176.Dahlén G, Ericson C, Berg K. In vitro studies of the interaction of isolated Lp(a) lipoprotein and other serum lipoproteins with glycosaminoglycans. Clin Genet. 1978;14:36–42. doi: 10.1111/j.1399-0004.1978.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 177.Scanu AM, Fless GM. Lipoprotein (a): heterogeneity and biological relevance. J Clin Invest. 1990;85:1709–1715. doi: 10.1172/JCI114625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Brown MS, Goldstein JL. Plasma lipoproteins: teaching old dogmas new tricks. Nature. 1987;330:113–114. doi: 10.1038/330113a0. [DOI] [PubMed] [Google Scholar]
- 179.Smith EB, Cochran S. Factors influencing the accumulation in fibrous plaques of lipid derived from low density lipoprotein, II: preferential immobilization of lipoprotein (a) (Lp(a)) Atherosclerosis. 1990;84:173–181. doi: 10.1016/0021-9150(90)90088-z. [DOI] [PubMed] [Google Scholar]
- 180.Beisiegel U. Lipoprotein (a) in the arterial wall. Curr Opin Lipidol. 1991;2:317–323. [Google Scholar]
- 181.Bihari-Varga M, Gruber E, Rotheneder M, Zechner R, Kostner GM. Interaction of lipoprotein Lp(a) and low density lipoprotein with glycosaminoglycans from human aorta. Arteriosclerosis. 1988;8:851–857. doi: 10.1161/01.atv.8.6.851. [DOI] [PubMed] [Google Scholar]
- 182.Bottalico LA, Keesler GA, Fless GM, Tabas I. Cholesterol loading of macrophages leads to marked enhancement of native lipoprotein(a) and apoprotein(a) internalization and degradation. J Biol Chem. 1993;268:8569–8573. [PubMed] [Google Scholar]
- 183.Keesler GA, Li Y, Skiba PJ, Fless GM, Tabas I. Macrophage foam cell lipoprotein(a)/apoprotein(a) receptor: cell-surface localization, dependence of induction on new protein synthesis, and ligand specificity. Arterioscler Thromb. 1994;14:1337–1345. doi: 10.1161/01.atv.14.8.1337. [DOI] [PubMed] [Google Scholar]
- 184.Yashiro A, O’Neil J, Hoff HF. Insoluble complex formation of lipoprotein (a) with low density lipoprotein in the presence of calcium ions. J Biol Chem. 1993;268:4709–4715. [PubMed] [Google Scholar]
- 185.Grainger DJ, Kirschenlohr HL, Metcalfe JC, Weissberg PL, Wade DP, Lawn RM. Proliferation of human smooth muscle cells promoted by lipoprotein(a) Science. 1993;260:1655–1658. doi: 10.1126/science.8503012. [DOI] [PubMed] [Google Scholar]
- 186.Miles LA, Fless GM, Levin EG, Scanu AM, Plow EF. A potential basis for the thrombotic risks associated with lipoprotein(a) Nature. 1989;339:301–303. doi: 10.1038/339301a0. [DOI] [PubMed] [Google Scholar]
- 187.Hajjar KA, Gavish D, Breslow JL, Nachman RL. Lipoprotein(a) modulation of endothelial cell surface fibrinolysis and its potential role in atherosclerosis. Nature. 1989;339:303–305. doi: 10.1038/339303a0. [DOI] [PubMed] [Google Scholar]
- 188.Loscalzo J, Weinfeld M, Fless GM, Scanu AM. Lipoprotein(a), fibrin binding, and plasminogen activation. Arteriosclerosis. 1990;10:240–245. doi: 10.1161/01.atv.10.2.240. [DOI] [PubMed] [Google Scholar]
- 189.Etingin OR, Hajjar DP, Hajjar KA, Harpel PC, Nachman RL. Lipoprotein (a) regulates plasminogen activator inhibitor-1 expression in endothelial cells: a potential mechanism in thrombogenesis. J Biol Chem. 1991;266:2459–2465. [PubMed] [Google Scholar]
- 190.Halvorsen S, Skjonsberg OH, Berg K, Ruyter R, Godal HC. Does Lp(a) lipoprotein inhibit the fibrinolytic system? Thromb Res. 1992;68:223–232. doi: 10.1016/0049-3848(92)90080-t. [DOI] [PubMed] [Google Scholar]
- 191.Terres W, Krewitt M, Hamm CW. Effects of lipoprotein(a) on in vitro lysis of whole blood thrombi from healthy volunteers. Thromb Res. 1993;69:479–487. doi: 10.1016/0049-3848(93)90236-h. [DOI] [PubMed] [Google Scholar]
- 192.Edelberg J, Pizzo SV. Lipoprotein (a) regulates plasmin generation and inhibition. Chem Phys Lipids. 1994;67/68:363–368. doi: 10.1016/0009-3084(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 193.Crouse JR., III Progress in coronary artery disease risk-factor research: what remains to be done? Clin Chem. 1984;30:1125–1127. [PubMed] [Google Scholar]
- 194.Järveläinen HT, Kinsella MG, Wight TN, Sandell LJ. Differential expression of small chondroitin/dermatin sulfate proteoglycans, PG-I/biglycan and PG-II/decorin, by vascular smooth muscle and endothelial cells in culture. J Biol Chem. 1991;266:23274–23281. [PubMed] [Google Scholar]
- 195.Kojima T, Shworak NW, Rosenberg RD. Molecular cloning and expression of two distinct cDNA-encoding heparan sulfate proteoglycan core proteins from a rat endothelial cell line. J Biol Chem. 1992;267:4870–4877. [PubMed] [Google Scholar]
- 196.Mertens G, Cassiman JJ, Van den Berghe H, Vermylen J, David G. Cell surface heparan sulfate proteoglycans from human vascular endothelial cells: core protein characterization and antithrombin III binding properties. J Biol Chem. 1992;267:20435–20443. [PubMed] [Google Scholar]
- 197.Asundi V, Cowan K, Matzura D, Wagner W, Dreher KL. Characterization of extracellular matrix proteoglycan transcripts expressed by vascular smooth muscle cells. Eur J Cell Biol. 1990;52:98–104. [PubMed] [Google Scholar]
- 198.Carey DJ, Evans DM, Stahl RC, Asundi VK, Conner KJ, Garbes P, Cizmeci-Smith G. Molecular cloning and characterization of N-syndecan, a novel transmembrane heparan sulfate proteoglycan. J Cell Biol. 1992;117:191–201. doi: 10.1083/jcb.117.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Asundi VK, Dreher KL. Molecular characterization of vascular smooth muscle decorin: deduced core protein structure and regulation of gene expression. Eur J Cell Biol. 1992;59:314–321. [PubMed] [Google Scholar]
- 200.Cizmeci-Smith G, Asundi V, Stahl RC, Teichman LJ, Chernousov M, Cowan K, Carey DJ. Regulated expression of syndecan in vascular smooth muscle cells and cloning of rat syndecan core protein cDNA. J Biol Chem. 1992;267:15729–15736. [PubMed] [Google Scholar]
- 201.Cizmeci-Smith G, Stahl RC, Showalter LJ, Carey DJ. Differential expression of transmembrane proteoglycans in vascular smooth muscle cells. J Biol Chem. 1993;268:18740–18747. [PubMed] [Google Scholar]
- 202.Nikkari ST, Jarvelainen HT, Wight TN, Ferguson M, Clowes AW. Smooth muscle cell expression of extracellular matrix genes after arterial injury. Am J Pathol. 1994;144:1348–1356. [PMC free article] [PubMed] [Google Scholar]
- 203.Eriksson I, Sandback D, Ek B, Lindahl U, Kjellen L. cDNA cloning and sequencing of mouse mastocytoma glucosaminyl N-deacetylase/N-sulfotransferase, an enzyme involved in the biosynthesis of heparin. J Biol Chem. 1994;269:10438–10443. [PubMed] [Google Scholar]
- 204.Orellana A, Hirschberg CB, Wei Z, Swiedler SJ, Ishihara M. Molecular cloning and expression of a glycosaminoglycan N-acetylglucosaminyl N-deacetylase/N-sulfotransferase from a heparin-producing cell line. J Biol Chem. 1994;269:2270–2276. [PubMed] [Google Scholar]
- 205.Zhang L, Esko JD. Amino acid determinants that drive heparan sulfate assembly in a proteoglycan. J Biol Chem. 1994;269:19295–19299. [PubMed] [Google Scholar]
- 206.Shworak NW, Shirakawa M, Mulligan RC, Rosenberg RD. Characterization of ryudocan glycosaminoglycan acceptor sites. J Biol Chem. 1994;269:21204–21214. [PubMed] [Google Scholar]
- 207.Quentin E, Gladen A, Roden L, Kresse H. A genetic defect in the biosynthesis of dermatan sulfate proteoglycan: galactosyltransferase I deficiency in fibroblasts from a patient with a progeroid syndrome. Proc Natl Acad Sci U S A. 1990;87:1342–1346. doi: 10.1073/pnas.87.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ishihara M, Guo Y, Wei Z, Yang Z, Swiedler SJ, Orellana A, Hirschberg CB. Regulation of biosynthesis of the basic fibroblast growth factor binding domains of heparan sulfate by heparan sulfate-N-deacetylase/N-sulfotransferase expression. J Biol Chem. 1993;268:20091–20095. [PubMed] [Google Scholar]
- 209.Santamarina-Fojo S, Dugi KA. Structure, function and role of lipoprotein lipase in lipoprotein metabolism. Curr Opin Lipidol. 1994;5:117–125. doi: 10.1097/00041433-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 210.Shimano H, Yamada N, Ohsuga J, Shimada M, Yazaki Y. Anti-atherogenic effect of apolipoprotein E is mediated through enhanced cholesterol efflux in transgenic mice. Circulation. 1993;88(suppl I):I-421. [Google Scholar]
- 211.Carew TE, Koschinsky T, Hayes SB, Steinberg D. A mechanism by which high-density lipoproteins may slow the atherogenic process. Lancet. 1976;1:1315–1317. doi: 10.1016/s0140-6736(76)92650-7. [DOI] [PubMed] [Google Scholar]
- 212.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 213.Pászty C, Maeda N, Verstuyft J, Rubin EM. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994;94:899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Klimov AN, Kozhemiakin LA, Pleskov VM, Andreeva LI. Antioxidant effect of high-density lipoproteins in the peroxidation of low-density lipoproteins. Biull Eksp Biol Med. 1987;103:550–552. [PubMed] [Google Scholar]
- 216.Khoo JC, Miller E, McLoughlin P, Steinberg D. Prevention of low density lipoprotein aggregation by high density lipoprotein or apolipoprotein A-I. J Lipid Res. 1990;31:645–652. [PubMed] [Google Scholar]
- 217.Ohta T, Takata K, Horiuchi S, Morino Y, Matsuda I. Protective effect of lipoproteins containing apoprotein A-I on Cu2+-catalyzed oxidation of human low density lipoprotein. FEBS Lett. 1989;257:435–438. doi: 10.1016/0014-5793(89)81590-x. [DOI] [PubMed] [Google Scholar]
- 218.Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype: a proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495–506. doi: 10.1161/01.cir.82.2.495. [DOI] [PubMed] [Google Scholar]
- 219.Camejo G, Hurt E, Thubrikar M, Bondjers G. Modification of low density lipoprotein association with the arterial intima: a possible environment for the antiatherogenic action of beta-blockers. Circulation. 1991;84(suppl VI):VI-17–VI-22. [PubMed] [Google Scholar]
- 220.Stalenhoef AF, Defesche JC, Kleinveld HA, Demacker PN, Kastelein JJ. Decreased resistance against in vitro oxidation of LDL from patients with familial defective apolipoprotein B-100. Arterioscler Thromb. 1994;14:489–493. doi: 10.1161/01.atv.14.3.489. [DOI] [PubMed] [Google Scholar]
- 221.Camejo G, Lopez A, Lopez F, Quinones J. Interaction of low density lipoproteins with arterial proteoglycans: the role of charge and sialic acid content. Atherosclerosis. 1985;55:93–105. doi: 10.1016/0021-9150(85)90169-8. [DOI] [PubMed] [Google Scholar]
- 222.Tribble DL, van den Berg JJ, Motchnik PA, Ames BN, Lewis DM, Chait A, Krauss RM. Oxidative susceptibility of low density lipoprotein subfractions is related to their ubiquinol-10 and alpha-tocopherol content. Proc Natl Acad Sci U S A. 1994;91:1183–1187. doi: 10.1073/pnas.91.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li H, Cybulsky MI, Gimbrone MA, Jr, Libby P. Inducible expression of vascular cell adhesion molecule-1 by vascular smooth muscle cells in vitro and within rabbit atheroma. Am J Pathol. 1993;143:1551–1559. [PMC free article] [PubMed] [Google Scholar]
- 224.Kume N, Gimbrone MA., Jr Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human endothelial cells. J Clin Invest. 1994;93:907–911. doi: 10.1172/JCI117047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Navab M, Liao F, Hough GP, Ross LA, Van Lenten BJ, Rajavashisth TB, Lusis AJ, Laks H, Drinkwater DC, Fogelman AM. Interaction of monocytes with cocultures of human aortic wall cells involves interleukins 1 and 6 with marked increases in connexin43 message. J Clin Invest. 1991;87:1763–1772. doi: 10.1172/JCI115195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen J-K, Hoshi H, McKeehan WL. Stimulation of human arterial smooth muscle cell chondroitin sulfate proteoglycan synthesis by transforming growth factor-beta. In Vitro Cell Dev Biol. 1991;27A:6–12. doi: 10.1007/BF02630888. [DOI] [PubMed] [Google Scholar]
- 227.Merrilees MJ, Scott L. Endothelial cell stimulation of smooth muscle glycosaminoglycan synthesis can be accounted for by transforming growth factor beta activity. Atherosclerosis. 1990;81:255–265. doi: 10.1016/0021-9150(90)90073-r. [DOI] [PubMed] [Google Scholar]
- 228.Kurihara H, Nakano T, Takasu N, Arita H. Intracellular localization of group II phospholipase A2 in rat vascular smooth muscle cells and its possible relationship to eicosanoid formation. Biochim Biophys Acta. 1991;1082:285–292. doi: 10.1016/0005-2760(91)90204-u. [DOI] [PubMed] [Google Scholar]
- 229.Hurt-Camejo E, Camejo G, Rosengren B, Bondjers G. LDL binding to matrix proteoglycans from smooth muscle cells is modulated by cell secreted and martix bound phospholipase A2. Circulation. 1994;90(pt 2):I-403. [Google Scholar]
- 230.Beisiegel U, Weber W, Bengtsson-Olivecrona G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein-related protein. Proc Natl Acad Sci U S A. 1991;88:8342–8346. doi: 10.1073/pnas.88.19.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kowal RC, Herz J, Goldstein JL, Esser V, Brown MS. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc Natl Acad Sci U S A. 1989;86:5810–5814. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Luoma J, Hiltunen T, Särkioja T, Moestrup SK, Gliemann J, Kodama T, Nikkari T, Ylä-Herttuala S. Expression of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein and scavenger receptor in human atherosclerotic lesions. J Clin Invest. 1994;93:2014–2021. doi: 10.1172/JCI117195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lupu F, Heim D, Bachmann F, Kruithof EKO. Expression of low density lipoprotein receptor–related protein A2-macroglobulin receptor in human normal and atherosclerotic arteries. Arterioscler Thromb. 1994;14:1438–1444. doi: 10.1161/01.atv.14.9.1438. [DOI] [PubMed] [Google Scholar]
- 234.Liao F, Andalibi A, Qiao JH, Allayee H, Fogelman AM, Lusis AJ. Genetic evidence for a common pathway mediating oxidative stress, inflammatory gene induction, and aortic fatty streak formation in mice. J Clin Invest. 1994;94:877–884. doi: 10.1172/JCI117409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kempen HJ, Buytenhek M, Gruber E, Bihari-Varga M. Factor, present in plasma, inhibiting the interaction of low density lipoprotein with arterial proteoglycan. Atherosclerosis. 1989;78:137–144. doi: 10.1016/0021-9150(89)90217-7. [DOI] [PubMed] [Google Scholar]
- 236.Wiklund O, Camejo G, Mattsson L, Lopez F, Bondjers G. Cationic polypeptides modulate in vitro association of low density lipoprotein with arterial proteoglycans, fibroblasts, and arterial tissue. Arteriosclerosis. 1990;10:695–702. doi: 10.1161/01.atv.10.5.695. [DOI] [PubMed] [Google Scholar]
- 237.Cardin AD, Weintraub HR. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 238.Görög P, Born GVR. Nifedipine inhibits accumulation of LDL and cholesterol in the aorta of the normocholesterolemic rabbit. Arterioscler Thromb. 1993;13:637–639. doi: 10.1161/01.atv.13.5.637. [DOI] [PubMed] [Google Scholar]
- 239.Phair RD. Cellular calcium and atherosclerosis: a brief review. Cell Calcium. 1988;9:275–284. doi: 10.1016/0143-4160(88)90008-5. [DOI] [PubMed] [Google Scholar]
- 240.Tilton GD, Buja LM, Bilheimer DW, Apprill P, Ashton J, McNatt J, Kita T, Willerson JT. Failure of a slow channel calcium antagonist, verapamil, to retard atherosclerosis in the Watanabe heritable hyperlipidemic rabbit: an animal model of familial hypercholesterolemia. J Am Coll Cardiol. 1985;6:141–144. doi: 10.1016/s0735-1097(85)80265-5. [DOI] [PubMed] [Google Scholar]