Abstract
Objective
Reduction in arterial elasticity marks progression toward cardiovascular morbidity and mortality. Variability in arterial elasticity may help account for race/ethnic and gender differences in cardiovascular risk.
Design
Cross-sectional study.
Setting
Whites, African Americans, Hispanics and Chinese aged 45–84 years free of clinically recognized cardiovascular disease were recruited in six US communities.
Participants
We examined 3,316 women and 3,020 men according to race/ethnicity and sex.
Main Outcome Measures
Large (LAE) and small artery (SAE) elasticity, derived from radial artery diastolic pulse wave contour registration in all subjects in a supine position using tonometry. LAE and SAE were adjusted for ethnicity, age, clinical site, height, heart rate, blood pressure, antihypertensive medication and body mass index, diabetes, smoking, and circulating lipids.
Results
Much of the sex difference in arterial elasticity was explained by height. After adjustment, LAE did not differ by race/ethnicity, but mean SAE in African Americans was 4.2 mL/mm Hg × 100 and 4.4 mL/mm Hg × 100 in Hispanics compared to means of 4.6 mL/mm Hg × 100 in Whites, and 4.8 mL/mm Hg × 100 in Chinese.
Conclusions
Reduced SAE may indicate earlier vascular disease in African Americans and Hispanics than other groups.
Keywords: Blood Pressure, Arterial Elasticity, MESA Study
INTRODUCTION
A diagnostic challenge is to detect abnormal structure and function in the vascular system before the development of symptoms or signs of cardiovascular disease (CVD).1,2 Variability in arterial elasticity may help account for race/ethnic and sex differences in cardiovascular risk; and knowledge of arterial elasticity might improve risk stratification, and identify individuals with early vascular damage who are predisposed to future vascular events, by providing a direct assessment of abnormal structure or tone in the arterial vasculature.3,4 Arterial elasticity is related to, but conceptually broader than, systemic blood pressure. However, the physiologic link between vascular elasticity and arterial pressure makes it difficult to separate the adverse effects of pressure from those of the vascular functional and structural alterations that characterize loss of elasticity. Since endothelial dysfunction and nitric oxide deficiency are characteristic features of hypertension and of other risk factors for morbid events, it has been proposed that blood pressure elevation may be viewed, in part, as a complication of functional and structural changes in the microcirculation, and that structural changes in the large arteries leading to morbid events may be viewed as a complication of both pressure elevation and endothelial dysfunction.5
Current evidence indicates that arterial stiffness is a predictor for CVD events in the general population,6–9 in patients with hypertension,10 end-stage renal disease,11 impaired glucose intolerance,12 and coronary artery disease.13 Several noninvasive techniques are available to assess arterial elasticity based on analysis of the arterial pulse wave regarding either the systolic or the diastolic part.14 The CR-2000 (HDI, Inc., Eagan, MN) provides information about the pools of large and small arteries derived from the radial artery pulse waveform using a mathematical model based on a modified Windkessel model.15 The Multi-Ethnic Study of Atherosclerosis (MESA), an epidemiologic cohort study that aimed to study a variety of subclinical CVD measures, elected to include the CR-2000 device to obtain information about arterial elasticity. The Windkessel model leads to two measures, namely large artery elasticity (LAE), pertaining to the pool of large arteries, and small artery elasticity (SAE), pertaining to the pool of small arteries, and was validated in an animal model.16 Besides the finding that SAE was inversely associated with incident CVD,9 randomized trials have shown that valsartan17,18 as well as a combination of amlodipine and atorvastatin increased both LAE and SAE.19 Aging,20 smoking,21 diabetes,22 and hyperlidemia23 have a negative effect on SAE and/or LAE, implying stiffer arteries in these conditions. LAE and SAE have high reproducibility in healthy subjects24 and correlate with other parameters that can be derived from the radial pulse waveform.25
Although CVD risk factor levels are substantially different in Whites, African Americans, Hispanics, and Chinese, the relative rates of coronary artery disease in these groups are not consistent with these differences.27
Differences in arterial elasticity may help explain race/ethnicity and sex differences in frequency of different CVD events.26,27 Women appear to exhibit a different pattern of events than men, even with similar blood pressures.28,29 African American men appear to experience more cardiovascular morbidity than white men, but with less large artery disease, probably as a consequence of more small vessel disease.30 Although CVD risk factor levels are substantially different in Whites, African Americans, Hispanics, and Chinese, the relative rates of coronary artery disease in these groups are not consistent with these differences.27 Therefore, we aimed to study race and sex differences in LAE and SAE among the large MESA cohort.
METHODS
Study Sample
MESA investigates the prevalence, correlates, and progression of subclinical CVD.31 In brief, between July 2000 and August 2002, 6814 men and women who identified themselves as White, African American, Hispanic, or Chinese and were aged 45 to 84 years, free of clinically apparent CVD (including prior heart attack, angina pectoris, stroke, transient ischemic attack, heart failure or CVD-related procedure such as balloon angioplasty or current atrial fibrillation), and never treated for cancer were recruited from six US communities. Each field site was located in a university and recruited broadly from the general population using locally available sources, with institutional review board approval at each center and after obtaining informed consent.31
Data Collection
We included 3,020 men and 3,316 women aged 45–84 years in whom arterial elasticity was measured. Participant age, race/ethnicity, and sex were self-reported. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight (kilograms)/height (meters squared). A central laboratory measured total cholesterol, high-density lipoprotein cholesterol triglycerides, and glucose levels from blood samples obtained after a 12-hour fast. Seated resting blood pressure was measured three times with a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL). The average of the last two measurements was used in analysis. Hypertension was defined as systolic pressure ≥140 mm Hg, diastolic pressure ≥90 mm Hg, or current use of antihypertensive medication. Diabetes was defined as a fasting glucose value ≥126 mg/dL or current use of antidiabetes medication. We obtained information about medication use by direct examination of medication bottles.
Large (LAE) and Small (SAE) Artery Elasticity Measurements
Arterial wave forms are recorded using the HDI/PulseWave CR2000 (Hypertension Diagnostics, Inc., Eagan, Minnesota).15 A solid-state pressure transducer array (a tonometer) was placed over the radial artery of the dominant arm to record the pulse contour. Once a stable measurement was achieved, a 30 second analog tracing of the radial waveform, excluding the dicrotic notch, was digitized at 200 samples per second, with accompanying automated, oscillatory blood pressure measurement. Measures of small and large artery elasticity (change in arterial volume per change in arterial pressure) are produced by the device. Briefly, the estimates of arterial elasticity are based on the asymptotic behavior of a Windkessel model,15,20 in which, heuristically, a viscous liquid (blood) is poured freely into a sink (pool of large arteries), draining slowly due to resistance in the drain (pool of small arteries). Mathematically (CR-2000 operator’s manual), the pulse waveform P(t), where t is the time elapsed since the beginning of diastole, is modeled as:
The modified Windkessel model then uses the parameters a1–a6 to estimate:
SVR is the systemic vascular resistance = mean arterial blood pressure/cardiac output. Cardiac output (L/min) is estimated as HR*(−6.6+(0.25 * (ET-35) − (0.62 * HR) + (40.4 * BSA)−(0.51*Age))/1000, where ET is ejection time in milliseconds, HR is heart rate in beats per minute, and BSA is body surface area in millimeters squared (estimated as 0.007184 * WT 0.425 *HT 0.725). ET in milliseconds is directly observable from the pulse waveform. Zimlichman et al24 reported repeatability correlations for LAE and SAE, both r=0.7 after 5 minutes and r=0.5 after 4 weeks. The 5 minute correlation coefficients are similar to those in the study of Rietzschel et al32, which may be computed from information presented by them as r=0.90 for SAE and r=0.72 for LAE.
Data Analysis
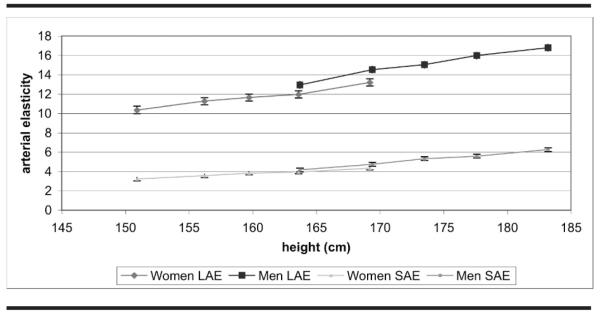
LAE and SAE were treated as separate dependent variables. Sex was examined in relation to height in Figure 1. The adjusted linear regression model included demographic variables (race/ethnicity, sex, age, and clinical center), correlates of LAE and SAE that are part of the estimation formulae (height, BMI, heart rate, and blood pressure), diabetes, pack years of cigarette smoking, and circulating levels of total cholesterol, high density lipoprotein cholesterol and triglycerides. For descriptive purposes, the continuous independent variables were divided into equal sized categories (quintiles) or categories based on meaningful cut points and examined in the fully adjusted model. Height cut points were gender-specific. A P value for trend was reported for continuous variables, while a multiple degree of freedom test was used for discrete predictor variables. Statistical significance to predict LAE and SAE was declared at P<.05. All analyses were performed using the SAS statistical software, version 9.0 (SAS Institute, Cary, NC).
Fig 1.
Mean ± 1.96 standard error of large artery elasticity (LAE, ml/mm Hg × 10) and small artery elasticity (SAE, ml/mm Hg × 100) by height and sex, adjusted for age, race, and clinical center. Multi-ethnic Study of Atherosclerosis 2000–2002
RESULTS
White and African American men were taller than the Chinese and Hispanic males (Table 1). The Chinese men had a lower BMI than the other races/ethnicities. African American men had the highest systolic and diastolic blood pressure and the highest percentage of hypertension and use of antihypertensive medication. Diabetes mellitus was most present in African American and Hispanic men. Among women, Chinese and Hispanics were shorter than Whites and African Americans. African American women had the highest systolic and diastolic blood pressure and the highest incidence of hypertension and antihypertensive treatment. Both African American and Hispanic women had more diabetes and a greater BMI than White and Chinese women. Pack years of cigarette smoking were highest among White men and lowest in Chinese and Hispanic women. Circulating lipids had the most adverse profile in Hispanic men and women.
Table 1.
Race/ethnicity/sex-specific descriptive statistics. Multi-ethnic Study of Atherosclerosis 2000–2002
| Male | White | Chinese | African American | Hispanic | P |
|---|---|---|---|---|---|
| Sample size | 1173 | 374 | 781 | 692 | |
| Age, years | 62.7 ± 10.2 | 62.3 ± 10.2 | 61.9 ± 10.2 | 61.1 ± 10.3 | .01 |
| Height, cm | 176.3 ± 6.9 | 168.0 ± 6.0 | 176.1 ± 6.9 | 168.9 ± 6.6 | <.0001 |
| Body mass index, kg/m2 | 28.0 ± 4.1 | 24.0 ± 3.1 | 28.7 ± 4.6 | 28.8 ± 4.3 | <.0001 |
| Systolic blood pressure, mm Hg | 124.3 ± 18.4 | 123.6 ± 18.8 | 130.1 ± 19.1 | 125.8 ± 20.0 | <.0001 |
| Diastolic blood pressure, mm Hg | 74.0 ± 9.1 | 74.8 ± 9.3 | 77.2 ± 9.5 | 75.0 ± 9.4 | <.0001 |
| Blood pressure medication (Rx), % users | 27% | 24% | 44% | 26% | <.0001 |
| Hypertension (% ≥140/90 or Rx) | 39% | 35% | 57% | 38% | <.0001 |
| Heart rate, beats/min | 61.4 ± 10.0 | 62.7 ± 8.7 | 61.5 ± 10.0 | 62.4 ± 9.8 | .03 |
| Diabetes (% ≥126 mg/dl or Rx) | 10% | 16% | 22% | 22% | <.0001 |
| Cigarette smoking (pack years) | 18.4 ± 34.2 | 9.2 ± 18.4 | 14.4 ± 20.4 | 11.9 ± 19.9 | <.0001 |
| Total cholesterol (mg/dL) | 189 ± 34.4 | 189.3 ± 31.6 | 181.7 ± 34.1 | 193.4 ± 37.1 | <.0001 |
| HDL cholesterol (mg/dL) | 45.3 ± 12.3 | 45.6 ± 10.8 | 46.7 ± 12.3 | 42.5 ± 10.1 | <.0001 |
| Triglycerides (mg/dL) | 135 ± 103.8 | 140.3 ± 79.3 | 108.3 ± 66.4 | 164.5 ± 106.8 | <.0001 |
| Female | White | Chinese | African American | Hispanic | P |
|---|---|---|---|---|---|
| Sample size | 1241 | 395 | 944 | 736 | |
| Age, years | 62.2 ± 10.4 | 62.2 ± 10.4 | 61.9 ± 9.9 | 61.4 ± 10.3 | .32 |
| Height, cm | 162.4 ± 6.5 | 155.5 ± 5.7 | 162.1 ± 6.6 | 155.4 ± 6.1 | <.0001 |
| Body mass index, kg/m2 | 27.6 ± 5.8 | 23.9 ± 3.5 | 31.3 ± 6.4 | 30.1 ± 5.7 | <0001 |
| Systolic blood pressure, mm Hg | 122.6 ± 21.8 | 124.9 ± 23.3 | 133.0 ± 23.3 | 127.3 ± 23.3 | <.0001 |
| Diastolic blood pressure, mm Hg | 66.9 ± 9.6 | 69.2 ± 10.7 | 72.6 ± 10.3 | 68.4 ± 9.5 | <0001 |
| Blood pressure medication, % users | 26% | 31% | 51% | 33% | <.0001 |
| Hypertension (% ≥140/90 or Rx) | 37% | 41% | 62% | 45% | <.0001 |
| Heart rate, beats/min | 64.3 ± 9.0 | 63.6 ± 8.6 | 64.4 ± 10.1 | 64.4 ± 9.1 | .49 |
| Diabetes (% ≥126 mg/dl or Rx) | 6% | 15% | 19% | 19% | <.0001 |
| Cigarette smoking (pack years) | 11.7 ± 19.7 | 0.7 ± 6 | 9.7 ± 18.4 | 3.7 ± 11.2 | <.0001 |
| Total cholesterol (mg/dL) | 202 ± 34.7 | 195.3 ± 32.4 | 196.1 ± 35.7 | 202.3 ± 37.7 | <.0001 |
| HDL cholesterol (mg/dL) | 58.7 ± 15.5 | 53 ± 13.2 | 56.9 ± 15.7 | 52.3 ± 13.8 | <.0001 |
| Triglycerides (mg/dL) | 131 ± 75.4 | 144.6 ± 84 | 101.7 ± 70.7 | 150.2 ± 80.2 | <.0001 |
P for any race/ethnic difference.
Tabulated descriptive statistics are either mean ± standard deviation or percentage.
HDL: high density lipoprotein.
Unadjusted mean values for LAE were 11.8 ± 5.1 mL/mm Hg × 10 in 3,316 women and 15.1 ± 5.7 mL/mm Hg × 10 in 3,020 men; corresponding values for SAE were 3.8 ± 2.3 mL/mm Hg × 100 in women and 5.2 ± 3.1 mL/mm Hg × 100 in men. This observed gender difference in LAE and SAE was primarily attributable to the lesser height of women than men (Figure 1). Although LAE and SAE were significantly (P<.0001) lower with decreasing height (Figure 1 and Table 2), little difference in elasticity existed between men and women in height-matched categories. In models adjusting for age, race, clinical center, and height as a continuous variable, LAE was 1.25 mL/mm Hg × 10 greater in men than women, while SAE was 0.42 mL/mm Hg × 100 greater in men than women (P<.001 for both comparisons). However, this model should be interpreted with caution because it implicitly extrapolated the linear association of arterial elasticity with male height to the low female height, and with female height to the high male height.
Table 2.
Multiple regression analysis of arterial elasticity (dependent variable) with demographic and anthropometric variables in the model
| Large artery elasticity (mL/mm Hg × 10) |
Small artery elasticity (mL/mm Hg × 100) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted * |
P trend/ categorical |
Unadjusted |
Adjusted |
P trend/ categorical |
||||||
| N | Mean | Std Dev | Mean | Std Error | Mean | Std Dev | Mean | Std Error | |||
| Race/ethnicity | |||||||||||
| African American | 1713 | 13.5 | 5.8 | 13.4 | 0.12 | .52 | 4.2 | 2.5 | 4.2 | 0.06 | <.0001 |
| Chinese | 769 | 12.9 | 5.4 | 13.4 | 0.20 | 4.1 | 2.8 | 4.6 | 0.10 | ||
| Hispanic | 1429 | 12.8 | 5.2 | 13.1 | 0.14 | 4.4 | 2.8 | 4.3 | 0.07 | ||
| White | 2386 | 13.7 | 5.7 | 13.2 | 0.10 | 4.9 | 3.0 | 4.7 | 0.05 | ||
| Clinical Site | |||||||||||
| New York | 1014 | 13.5 | 5.5 | 13.7 | 0.15 | <.0001 | 4.5 | 2.9 | 4.6 | 0.08 | <.0001 |
| Maryland | 854 | 12.0 | 5.2 | 11.8 | 0.17 | 4.2 | 2.5 | 4.2 | 0.08 | ||
| Minnesota | 991 | 13.5 | 5.1 | 12.9 | 0.15 | 5.4 | 3.1 | 5.1 | 0.08 | ||
| Illinois | 1110 | 14.3 | 6.1 | 14.1 | 0.14 | 4.6 | 3.0 | 4.4 | 0.07 | ||
| California | 1284 | 12.9 | 5.5 | 13.4 | 0.14 | 4.1 | 2.7 | 4.3 | 0.07 | ||
| North Carolina | 1044 | 13.8 | 5.8 | 13.6 | 0.15 | 4.2 | 2.5 | 4.1 | 0.08 | ||
| Height (cm) quintiles, female/male | |||||||||||
| 136.9–154/148.9–167 | 1263 | 11.2 | 5.0 | 11.7 | 0.14 | <.0001 | 3.4 | 2.2 | 3.6 | 0.07 | <.0001 |
| 154.1–158/167.1–171.4 | 1260 | 12.7 | 5.4 | 12.9 | 0.13 | 4.0 | 2.6 | 4.1 | 0.07 | ||
| 158.1–161.5/167.2–175.4 | 1256 | 13.4 | 5.1 | 13.2 | 0.13 | 4.6 | 2.8 | 4.5 | 0.06 | ||
| 161.6–166/175.5–179.9 | 1262 | 14.0 | 5.8 | 13.8 | 0.13 | 4.7 | 2.8 | 4.7 | 0.07 | ||
| 166.1–188.4/180–202.5 | 1252 | 15.5 | 5.8 | 14.8 | 0.14 | 5.6 | 3.2 | 5.3 | 0.07 | ||
| Age (years) | |||||||||||
| 44–54 | 1828 | 15.8 | 5.2 | 14.9 | 0.11 | <.0001 | 6.1 | 3.2 | 5.6 | 0.06 | <.0001 |
| 55–64 | 1739 | 13.7 | 5.2 | 13.6 | 0.11 | 4.6 | 2.7 | 4.5 | 0.06 | ||
| 65–74 | 1851 | 12.0 | 5.3 | 12.4 | 0.11 | 3.5 | 2.0 | 3.8 | 0.05 | ||
| 75–84 | 875 | 10.3 | 5.6 | 11.3 | 0.16 | 2.9 | 1.8 | 3.4 | 0.08 | ||
Notes: P trend for age and height, P-categorical with df corresponding to number of classes shown for race, sex, site. Specific pairwise comparisons are not presented.
Adjusted for all variables in Table 1, including race/ethnicity.
The adjusted SAE was significantly (P<0.0001) lower in the African American and Hispanic samples in comparison to the other race/ethnicities (Table 2), however there was no difference in the adjusted LAE among the different races/ethnicities. There was a significant (P<.0001) difference in adjusted LAE and SAE among the different clinical sites. African Americans had the lowest SAE among race/ethnic groups in four of the five clinics in which they were sampled; Hispanics had the second lowest SAE at each of the three clinics in which they were sampled (data not shown). In Table 2, after adjustment, there was a highly significant (P<.0001) decrease of LAE and SAE as a function of age.
With a higher heart rate, LAE and to a lesser extent SAE were significantly lower (P<.0001). LAE was mainly correlated with systolic blood pressure (Table 3). LAE was significantly (P<.0001) lower with higher systolic blood pressure, but was much less correlated with diastolic blood pressure (P<.04). Adjusted SAE was significantly (P<.0001) lower with higher systolic and diastolic blood pressure. Both LAE and SAE were significantly (P=.02) higher in medication users than in untreated hypertensives, achieving mean elasticity levels similar to untreated people whose blood pressure was in the range 130–139 mm Hg systolic or 85–89 mm Hg diastolic. Both LAE and SAE increased significantly (P<.0001) with a higher BMI. SAE decreased substantially with increasing pack years of cigarette smoking (P<.0001), while the decrease in LAE with pack years was more shallow. There was little association of LAE or SAE with circulating lipids (data not shown).
Table 3.
Multiple regression analysis of arterial elasticity (dependent variable) with demographic and anthropometric variables in the model
| Large artery elasticity (mL/mm Hg 3 10) |
Small artery elasticity (mL/mm Hg 3 100) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted* |
P trend | Unadjusted |
Adjusted |
P trend | ||||||
| N | Mean | Std Dev |
Mean | Std Error |
Mean | Std Dev |
Mean | Std Error |
|||
| Heart rate quintiles, beats/min | |||||||||||
| 36–55 | 1339 | 15.6 | 6.4 | 15.1 | 0.13 | <.0001 | 4.6 | 2.9 | 4.4 | 0.06 | .004 |
| 56–60 | 1312 | 14.2 | 5.6 | 14.0 | 0.13 | 4.8 | 3.1 | 4.7 | 0.06 | ||
| 61–64 | 1068 | 13.2 | 5.3 | 13.2 | 0.14 | 4.5 | 2.8 | 4.5 | 0.07 | ||
| 65–70 | 1287 | 12.5 | 4.7 | 12.5 | 0.13 | 4.4 | 2.8 | 4.5 | 0.06 | ||
| 71–130 | 1287 | 11.1 | 4.7 | 11.4 | 0.13 | 4.0 | 2.5 | 4.2 | 0.07 | ||
| Systolic/diastolic blood pressure< mm Hg | |||||||||||
| <110/75 | 1190 | 16.0 | 5.4 | 15.4 | 0.14 | Systolic <.0001 | 5.9 | 3.2 | 5.5 | 0.07 | Systolic <.0001 |
| ≥110/75 and <120/80 | 985 | 14.9 | 5.5 | 14.2 | 0.15 | Diastolic .09 | 5.3 | 3.1 | 4.9 | 0.07 | Diastolic <.0001 |
| ≥120/80 and <130/85 | 732 | 13.9 | 5.5 | 13.4 | 0.17 | Medication .97 | 4.7 | 2.7 | 4.4 | 0.09 | Medication .04 |
| ≥130/85 and <140/90 | 569 | 12.3 | 4.7 | 12.4 | 0.19 | 4.1 | 2.4 | 4.1 | 0.10 | ||
| ≥140/90 | 736 | 10.5 | 4.5 | 11.0 | 0.17 | 3.2 | 1.8 | 3.5 | 0.09 | ||
| Takes medication< all blood pressure values |
2081 | 12.2 | 5.5 | 12.6 | 0.11 | 3.8 | 2.4 | 4.1 | 0.05 | ||
| Body mass index< kg/m2 | |||||||||||
| < 23 | 913 | 12.6 | 5.7 | 12.1 | 0.16 | <.0001 | 4.1 | 2.7 | 3.9 | 0.08 | <.0001 |
| 23 to <25 | 889 | 13.4 | 5.4 | 12.8 | 0.15 | 4.4 | 3.0 | 4.1 | 0.08 | ||
| 25 to <30 | 2469 | 13.6 | 5.8 | 13.3 | 0.09 | 4.4 | 2.8 | 4.4 | 0.05 | ||
| 30 to <35 | 1322 | 13.5 | 5.4 | 13.8 | 0.13 | 4.7 | 2.9 | 4.8 | 0.07 | ||
| ≥ 35 | 700 | 13.0 | 5.3 | 14.2 | 0.18 | 4.8 | 2.6 | 5.3 | 0.09 | ||
| Diabetes | |||||||||||
| No | 5450 | 13.6 | 5.7 | 13.3 | 0.06 | .32 | 4.6 | 2.9 | 4.5 | 0.03 | .08 |
| Yes | 847 | 12.0 | 5.1 | 13.0 | 0.16 | 4.0 | 2.4 | 4.4 | 0.08 | ||
| Cigarette smoking (pack years) | |||||||||||
| 0 | 3268 | 12.9 | 5.5 | 13.3 | 0.08 | .009 | 4.4 | 2.8 | 4.6 | 0.04 | <.0001 |
| >0 but <10 | 1048 | 14.0 | 5.5 | 13.3 | 0.14 | 4.8 | 2.9 | 4.5 | 0.07 | ||
| 10 to <25 | 841 | 14.0 | 5.9 | 13.4 | 0.16 | 4.5 | 2.8 | 4.3 | 0.08 | ||
| 25 to <50 | 688 | 13.6 | 5.5 | 13.0 | 0.17 | 4.4 | 2.8 | 4.1 | 0.09 | ||
| ≥50 | 353 | 13.0 | 5.8 | 13.0 | 0.24 | 3.9 | 2.4 | 3.9 | 0.12 | ||
Notes: Blood pressure classified based on the highest category for the given systolic and diastolic levels.
P trend for heart rate and body mass index. P trend for systolic and diastolic blood pressures and P categorical for blood pressure medication; all 3 terms mutually adjusted. Specific pairwise comparisons are not presented.
Adjusted for all variables in Table 1.
The main finding was that African Americans had the lowest SAE, with somewhat higher values in Hispanics.
DISCUSSION
The main finding was that African Americans had the lowest SAE, with somewhat higher values in Hispanics. SAE value were higher in Whites and Chinese. LAE did not differ by race/ethnicity. SAE is considered as a specific expression of the functional behavior of the small arteries.20 This observation emphasizes the possibility that African Americans free of overt CVD may have an abnormality of the small vessels that has not yet manifested in the large arteries. This concept is consistent with the observation of reduced endothelial function in African American vs White adolescents.33 There is clinical evidence that African Americans are more prone than other groups to small artery than large artery disease. Small-vessel stroke in contrast to large vessel stroke is one of the most frequent identifiable causes of first-ever ischemic stroke among African Americans.34 A paradox is that African Americans are less likely to have obstructive coronary disease morbidity,35 yet are more likely to die from coronary disease.36 African Americans had less coronary calcium than apparently clinically similar Whites in a sample with evidence of ischemia on nuclear imaging.35
Similar to the finding of reduced vasodilatory response using forearm plethysmography in African American vs White adolescents,33 a study of healthy middle-aged men and women found that both flow-mediated and nitroglycerin-mediated dilation were lower in African Americans than Whites, despite normal blood pressure that was only slightly higher in African Americans than in Whites.37 Using highly sensitive nano-sensors, Kalinowski et al38 studied the disposition of reactive oxygen and nitrogen species (ROS/RNS) associated with lower bioavailable nitric oxide (NO) in intact single umbilical vein endothelial cells obtained from African American and White women. They directly measured eNOS protein level and NAD(P)H oxidase activity. They concluded that, despite the increased eNOS protein level, increased ROS/RNS in African Americans compared to Whites resulted from upregulated NAD(P)H-oxidase activity followed by eNOS uncoupling, leading ultimately to reduced NO bioavailability.38 These cellular events may explain decreased hemodynamic reactivity, increased vascular tone, elevated blood pressure, and lower SAE.33,37
Women have lower LAE and SAE than men. The arterial wave reflection has a shorter distance to cover in women than men; Smulyan et al39 studied men and women and found that short stature was associated with earlier reflection waves and increased augmentation index, independent of mean arterial blood pressure. Height did explain much of the sex difference in both LAE and SAE in our data, yet we did see a small difference in both LAE and SAE even for men and women of similar height. One study matched elderly hypertensive men and women on height and found that women still had earlier wave reflection.40 Women also had a smaller aortic diameter and stiffer aortic arch than men.40 The Framingham study showed that in women short stature was found to be a risk factor for myocardial infarction, though not for CVD mortality.41 Height also played a role in ethnic differences in LAE and SAE, since Chinese and Hispanic men and women have shorter height than White and African American men.
A strong gradient is seen with age for LAE and SAE. Aging is associated with endothelial dysfunction, reduction of capillary density and thus consequently a decrease in SAE. Moreover in the large arteries aging is also accompanied by the development of atherosclerotic plaque and thus stiffening of the large arteries or a decrease in LAE.42
A limitation in this study is the cross-sectional design, restricting the capacity to make any statements about the temporal ordering of events that may relate changes in arterial elasticity to participant characteristics; it would be desirable to have a second measure of arterial elasticity. Although there was a clear study center difference, that difference is quite small compared to the age gradient (the size of the age gradient is particularly clear comparing the LAE and SAE values in this study to those obtained by Valappil et al23, who reported that mean LAE was 25.3 mL/ mm Hg*10 and mean SAE was 8.3 mL/mm Hg*100 in participants with the average age of 35). Strengths of the study include the standardized protocol and large sample size and the diversity of the sample, including a broad range of ages, both genders, and a range of ethnic backgrounds.
In conclusion, the MESA study cohort showed that African Americans and Hispanics have a lower SAE than Whites and Chinese, while LAE was similar across race/ethnic groups after adjustment for all variables including in the estimate of arterial elasticity and for other CVD risk factors.
REFERENCES
- 1.Mancini GBJ, Dahlof B, Diez J. Surrogate markers for cardiovascular disease: structural markers. Circulation. 2004;109(Suppl I):IV-22–30. doi: 10.1161/01.CIR.0000133443.77237.2f. [DOI] [PubMed] [Google Scholar]
- 2.Cohn JN, Quyyumi AA, Hollenberg NK, Jamerson KA. Surrogate markers for cardiovascular disease in asymptomatic disease: functional markers. Circulation. 2004;109(Suppl I):IV-31–46. doi: 10.1161/01.CIR.0000133442.99186.39. [DOI] [PubMed] [Google Scholar]
- 3.Cohn JN, Duprez DA, Grandits GA. Arterial elasticity as part of a comprehensive assessment of cardiovascular risk and drug treatment. Hypertension. 2005;46:217–220. doi: 10.1161/01.HYP.0000165686.50890.c3. [DOI] [PubMed] [Google Scholar]
- 4.Duprez DA, Cohn JN. Monitoring vascular health beyond blood pressure. Curr Hypertens Rep. 2006;8:287–291. doi: 10.1007/s11906-006-0066-z. [DOI] [PubMed] [Google Scholar]
- 5.Landmesser U, Drexler H. Endothelial function and hypertension. Curr Opin Cardiol. 2007;22:316–320. doi: 10.1097/HCO.0b013e3281ca710d. [DOI] [PubMed] [Google Scholar]
- 6.Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 7.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 8.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 9.Grey E, Bratteli C, Glasser SP, et al. Reduced small artery but not large artery elasticity is an independent risk marker for cardiovascular events. Am J Hypertens. 2003;16:265–269. doi: 10.1016/s0895-7061(02)03271-5. [DOI] [PubMed] [Google Scholar]
- 10.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 11.Pannier B, Guérin AP, Marchais SJ, Safar ME, London GM. Stiffness of capacitive and conduit arteries: prognostic significance for end-stage renal disease patients. Hypertension. 2005;45:592–596. doi: 10.1161/01.HYP.0000159190.71253.c3. [DOI] [PubMed] [Google Scholar]
- 12.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 13.Duprez DA, Cohn JN. Arterial stiffness as a risk factor for coronary atherosclerosis. Curr Atheroscler Rep. 2007;9:139–144. doi: 10.1007/s11883-007-0010-y. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton PK, Lockhart CJ, Quinn CE, McVeigh GE. Arterial stiffness: clinical relevance, measurement and treatment. Clin Sci. 2007;113:157–170. doi: 10.1042/CS20070080. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein SM, Cohn JN. First- and third-order models for determining arterial compliance. J Hypertens Suppl. 1992;10:S11–14. [PubMed] [Google Scholar]
- 16.Mock J, Finkelstein SM, Eaton J, Hatfield G, Cohn JN. Vascular compliance changes in hypertensive dogs during nitroprusside infusion as measured by pulse-contour-analysis. Biomed Sci Instrum. 1987;23:77–82. [PubMed] [Google Scholar]
- 17.Duprez DA, Florea ND, Jones K, Cohn JN. Beneficial effects of valsartan in asymptomatic individuals with vascular or cardiac abnormalities: the DETECTIV Pilot Study. J Am Coll Cardiol. 2007;5:835–839. doi: 10.1016/j.jacc.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 18.Shargorodsky M, Leibovitz E, Lubimov L, Gavish D, Zimlichman R. Prolonged treatment with the AT1 receptor blocker, valsartan, increases small and large artery compliance in uncomplicated essential hypertension. Am J Hypertens. 2002 Dec;15(12):1087–91. doi: 10.1016/s0895-7061(02)03134-5. [DOI] [PubMed] [Google Scholar]
- 19.Cohn JN, Wilson DJ, Neutel J, et al. Coadministered amlodipine and atorvastatin produces early improvements in arterial wall compliance in hypertensive patients with dyslipidemia. Am J Hypertens. 2009;22:137–144. doi: 10.1038/ajh.2008.325. [DOI] [PubMed] [Google Scholar]
- 20.McVeigh GE, Bratteli CW, Morgan DJ, et al. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension. 1999;33:1392–1398. doi: 10.1161/01.hyp.33.6.1392. [DOI] [PubMed] [Google Scholar]
- 21.McVeigh GE, Morgan DJ, Finkelstein SM, Lemay LA, Cohn JN. Vascular abnormalities associated with long-term cigarette smoking identified by arterial waveform analysis. Am J Med. 1997;102:227–231. doi: 10.1016/S0002-9343(96)00454-8. [DOI] [PubMed] [Google Scholar]
- 22.McVeigh G, Brennan G, Hayes R, Cohn J, Finkelstein S, Johnston D. Vascular abnormalities in non-insulin-dependent diabetes mellitus identified by arterial waveform analysis. Am J Med. 1993;95:424–430. doi: 10.1016/0002-9343(93)90313-e. [DOI] [PubMed] [Google Scholar]
- 23.Valappil NI, Jacobs DR, Duprez DA, Gross MD, Arnett DK, Glasser S. Association between endothelial biomarkers and arterial elasticity in young adults: the CARDIA Study. JASH. 2008;2:70–79. doi: 10.1016/j.jash.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimlichman R, Shargorodsky M, Boaz M, et al. Determination of arterial compliance using blood pressure waveform analysis with the CR-2000 system: Reliability, repeatability, and establishment of normal values for healthy European population–the seven European sites study (SESS) Am J Hypertens. 2005;18:65–71. doi: 10.1016/j.amjhyper.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Duprez DA, Kaiser DR, Whitwam W, et al. Determinants of radial artery pulse wave analysis in asymptomatic individuals. Am J Hypertens. 2004;17:647–653. doi: 10.1016/j.amjhyper.2004.03.671. [DOI] [PubMed] [Google Scholar]
- 26.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 27.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104:2855–2864. doi: 10.1161/hc4701.099488. [DOI] [PubMed] [Google Scholar]
- 28.Zucker DR, Griffith JL, Beshansky JR, Selker HP. Presentations of acute myocardial infarction in men and women. J Gen Intern Med. 1997;12:79–87. doi: 10.1046/j.1525-1497.1997.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merz CN Bairey, Shaw LJ, Reis SE, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:21–30. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 30.Budoff MJ, Yang TP, Shavelle RM, Lamont DH, Brundage BH. Ethnic differences in coronary atherosclerosis. J Am Coll Cardiol. 2002;39:408–412. doi: 10.1016/s0735-1097(01)01748-x. [DOI] [PubMed] [Google Scholar]
- 31.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 32.Rietzschel ER, Boeykens E, De Buyzere ML, Duprez DA, Clement DL. A comparison between systolic and diastolic pulse contour analysis in the evaluation of arterial stiffness. Hypertension. 2001;37(6):E15–22. doi: 10.1161/01.hyp.37.6.e15. [DOI] [PubMed] [Google Scholar]
- 33.Duck MM, Hoffman RP. Impaired endothelial function in healthy African American adolescents compared with Caucasians. J Pediatr. 2007;150:400–6. doi: 10.1016/j.jpeds.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo D, Gebel J, Miller R, et al. Incidence rates of first-ever ischemic stroke subtypes among blacks: a population-based study. Stroke. 1999;30:2517–2522. doi: 10.1161/01.str.30.12.2517. [DOI] [PubMed] [Google Scholar]
- 35.Whittle J, Kressin NR, Peterson ED, et al. Racial Differences in Prevalence of Coronary Obstructions Among Men With Positive Nuclear Imaging Studies. J Am Coll Cardiol. 2006;47:2034–2041. doi: 10.1016/j.jacc.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 36.Cooper R, Cutler J, Desvigne-Nickens P, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102:3137–3147. doi: 10.1161/01.cir.102.25.3137. [DOI] [PubMed] [Google Scholar]
- 37.Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol. 2002;40:754–760. doi: 10.1016/s0735-1097(02)02015-6. [DOI] [PubMed] [Google Scholar]
- 38.Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function. Predisposition of African Americans to vascular diseases. Circulation. 2004;109:2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 39.Smulyan H, Marchais SJ, Pannier B, Guerin AP, Safar ME, London GM. Influence of body height on pulsatile arterial hemodynamic data. J Am Coll Cardiol. 1998;31:1103–1109. doi: 10.1016/s0735-1097(98)00056-4. [DOI] [PubMed] [Google Scholar]
- 40.Gatzka CD, Kingwell BA, Cameron JD, et al. Gender differences in the timing of arterial wave reflection beyond differences in body height. J Hypertens. 2001;19:2197–2203. doi: 10.1097/00004872-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Kannam JP, Levy D, Larson M, Wilson PWF. Short stature and risk for mortality and cardiovascular events: the Framingham Heart Study. Circulation. 1994;90:2241–2247. doi: 10.1161/01.cir.90.5.2241. [DOI] [PubMed] [Google Scholar]
- 42.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]