Abstract
Thymic stromal lymphopoietin (TSLP) is an interleukin 7 (IL-7)-like cytokine originally characterized by its ability to promote the activation of B cells and dendritic cells (DCs). Subsequent studies have shown that TSLP promotes T helper type 2 (TH2) cell responses associated with immunity to some helminth parasites and the pathogenesis of many inflammatory diseases, including atopic dermatitis and asthma. This review will focus on recent findings indicating that in addition to influencing B cell and DC function, TSLP can promote TH2 cytokine–associated inflammation by directly promoting the effector functions of CD4+ TH2 cells, basophils and other granulocyte populations while simultaneously limiting the expression of DC-derived proinflammatory cytokines and promoting regulatory T cell responses in peripheral tissues.
Thymic stromal lymphopoietin (TSLP) is a cytokine with structural and functional similarities to the hematopoietin family of cytokines. As the name suggests, it was originally isolated from a mouse thymic stromal cell line and characterized as a lymphocyte growth factor1–3. A TSLP homolog was subsequently identified in humans by in silico methods4,5. Similarly, several groups isolated a TSLP-binding protein in both humans and mice (referred to as the ‘TSLP receptor’ (TSLPR)), most closely related to the common γ-chain, which bound TSLP with low affinity6. It is now known that the functional, high- affinity TSLPR complex is a heterodimer of TSLPR and interleukin 7 receptor-α (IL-7Rα)6,7. Cross-species homology for both the cytokine and its receptor is relatively low (~40% for each), although, as described below, functionally they seem to be quite similar. Finally, it is not clear how the TSLPR complex transmits signals. There are abundant data showing that receptor engagement can activate the STAT5 transcription factor4,7–9; however, no Jak kinases are activated by the intact receptor complex8.
The similarity of TSLP to IL-7 and the homology of TSLPR to the common γ-chain suggested that TSLP may have a role in regulating lymphocyte development and/or function. Indeed, early studies did show that TSLP was able to influence the development and proliferation of both T cells and B cells, both in vitro and in vivo2,3,10. However, the effects were modest, which suggested that the influence of TSLP on lymphocyte development is redundant.
Analysis of the expression profiles of the two receptor subunits in human cell populations provided important insights into the primary biological role of TSLP. The cell populations with the highest known coexpression of TSLPR and IL-7Rα are myeloid dendritic cells (DCs)4. In confirmation of the expression data, treatment of human DCs with TSLP induces several phenotypic changes, including improved survival, upregulation of major histocompatibility complex class II and costimulatory molecules (CD86 and CD40), and the production of a variety of chemokines, most notably the chemokine receptor CCR4 ligands CCL17 and CCL22 (refs. 4,11). Mouse bone marrow–derived DCs acquire a similar activated phenotype after TSLP stimulation12.
Cellular targets and biological properties of TSLP
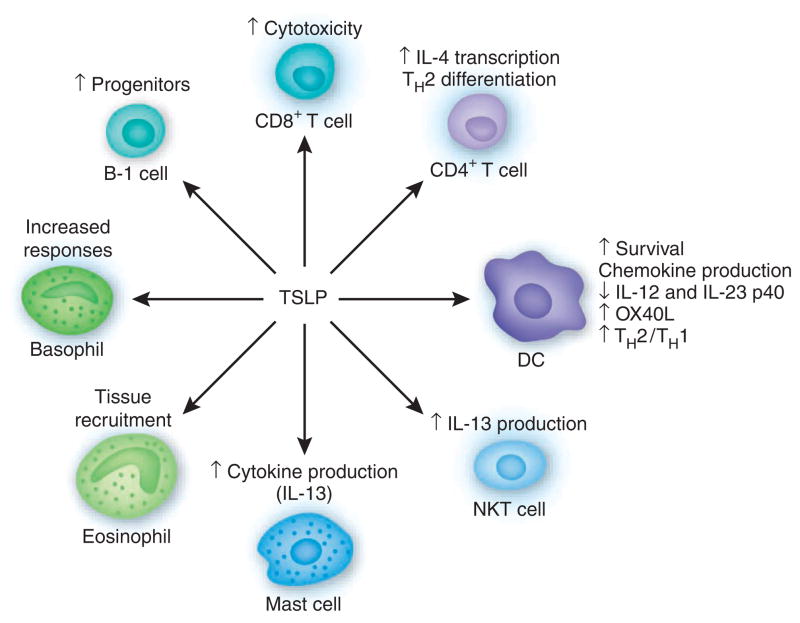
Several cellular targets of TSLP have been identified, including DCs, lymphocytes and granulocytes (Fig. 1). TSLP-stimulated DCs are able to activate CD4+ T cells. Culture of TSLP-activated DCs together with naive syngeneic CD4+ T cells leads to T cell proliferation but no differentiation, which suggests a role for TSLP in CD4+ T cell homeostasis13. However, when TSLP-stimulated DCs prime CD4+ T cells in an antigen-specific manner (for example, in an allogeneic culture), the resulting T cells show characteristic features of T helper type 2 (TH2)-differentiated cells (production of IL-4, IL-5, IL-13 and tumor necrosis factor), with the exception that IL-10 production is not evident11. These data suggest that TSLP-activated DCs prime for inflammatory TH2 cell differentiation. Interestingly, TSLP, in the absence of IL-12, induces expression of OX40L, the ligand for the cell survival factor OX40, on DCs, and OX40-OX40L interactions are critical for the ability of the DCs to drive TH2 cell differentiation14. Consistent with a role in regulating TH2 cytokine responses, TSLP-activated DCs are also able to support the maintenance and further polarization of CRTH2+ TH2 effector-memory cells15.
Figure 1.
Pantheon of TSLP-responsive cells. NKT, natural killer T.
In addition to promoting chemokine production and expression of OX40L, TSLP has a potent immunoregulatory effect on human and mouse DCs. For example, TSLP inhibits expression of the IL-12 (and IL-23) p40 subunit in human monocyte-derived DCs16,17. In addition, pretreatment of mouse bone marrow–derived DCs with recombinant TSLP inhibits p40 expression induced by Toll-like receptor ligands18,19 and impairs their ability to promote antigen-specific TH1 differentiation19. DCs isolated from TSLPR-deficient mice have higher expression of the IL-12p40 subunit, which supports the idea of a role for TSLP in limiting the expression of proinflammatory responses19. Coupled with the induction of OX40L expression on DCs, the ability of TSLP to limit expression of the p40 subunit suggests that TSLP may indirectly promote a microenvironment permissive for TH2 cell differentiation by limiting the proinflammatory functions of DCs.
DCs have a critical role in promoting TH2 cytokine responses20–23, and the ability of TSLP to limit p40 expression may be essential in this. Basophils also show antigen-presenting-cell functions in the context of helminth- or allergen-induced TH2 cytokine responses24–26. Importantly, TSLP seems to promote basophil responses in vivo24, which suggests that in addition to influencing cytokine expression in DCs, the TH2-promoting properties of TSLP may be mediated, at least in part, through basophils. It has been shown that bone marrow–derived basophils, but not bone marrow–derived dendritic cells, can induce IL-4 production in antigen-specific CD4+ T cells. In addition, exposure to papain, a protease allergen, induces the activation of major histocompatibility complex class II–positive basophils and their recruitment into the draining lymph nodes. Basophils that have endocytosed fluorescein isothiocyanate–labeled ovalbumin upregulate the expression of major histocompatibility complex class II and costimulatory molecules and form immunologic synapses with ovalbumin- specific T cells25. Basophils can also capture immunoglobulin E (IgE) complexes and promote TH2 cytokine responses in a mouse model of antigen-IgE-mediated inflammation26. In the context of helminth infection, exposure to schistosome eggs elicits robust recruitment of major histocompatibility complex class II–positive basophils into the draining lymph nodes, and depletion of basophils impairs TH2 cytokine–dependent expulsion of the gastrointestinal helminth Trichuris muris24. Published studies have shown that TSLP-TSLPR interactions are essential for immunity to Trichuris19,27. The importance of the TSLP pathway and basophils in protective immunity to Trichuris, coupled with the demonstration that delivery of recombinant TSLP can augment basophil numbers in the periphery24, suggest that coordinated TSLP-dependent regulation of DCs and basophils may have an important role in developing TH2 cytokine responses. How TSLP promotes basophil responses and whether TSLP-induced TH2 cytokine responses are dependent on eliciting antigen-presenting functions in basophil populations remains to be examined. In addition, how basophils and DCs interact to promote TH2 cell differentiation and whether this interaction is regulated by TSLP in the lymph node, as well as its consequences on promoting optimal TH2 cytokine responses, are unclear at present.
In addition to its effects on the differentiation of CD4+ TH2 cells potentially via DCs and/or basophils, TSLP is able to directly promote the TH2 cell differentiation of naive T cells. The combination of TCR stimulation and TSLP treatment can induce IL-4 transcription and further TH2 differentiation28. The induction of IL-4 transcription is accompanied by partial remodeling of the Il4 locus (M. Omori and S.F.Z., unpublished data).
In addition to DCs, basophils and CD4+ T cells, several other cell types are able to respond to TSLP (Fig. 1). For example, TSLP can costimulate the activation of both mast cells and natural killer T cells, which results in increased cytokine production29–31. Eosinophils respond to TSLP by upregulating the common myeloid marker CD11b and the integrin αLβ2 ligand ICAM-1, which suggests that TSLP may recruit eosinophils to sites of TH2 cytokine–associated inflammation32. Together, these data suggest a model in which TSLP, acting through DCs, granulocytes, natural killer T cells or directly on CD4+ T cells, can promote TH2 cell differentiation and TH2 cytokine–associated inflammation.
TSLP and normal barrier homeostasis
Despite the initial identification of TSLP in the culture supernatant of a thymic stromal cell line, this cytokine is expressed mainly by epithelial cells at barrier surfaces (skin, gut and lung)4. There is mounting evidence that epithelial cell–derived factors are critical for the generation and maintenance of noninflammatory, tissue-resident DCs in the gut, an important aspect of gut immune homeostasis33–36. TSLP is expressed constitutively in intestinal epithelial cells, with its highest expression in colonic epithelial cells19. DCs licensed by those cells can drive the generation of induced Foxp3+ T regulatory cells, commonly secrete less IL-12p40 and generally drive TH2 cytokine responses34,37–39. TSLP produced by the colonic epithelium has a key role in this tonic signal to DCs18,19,34,40. One of the signals that induces TSLP expression by these cells may be interactions with commensal bacteria, thus providing a mechanistic rationale for tolerance to commensals41. Consistent with that, TSLPR-deficient mice show a more rapid onset and severity of disease in a commensal-dependent mouse model of inflammatory bowel disease19. Further evidence for the involvement of TSLP in maintaining gut homeostasis is the finding that colonic epithelial cells from patients with Crohn’s disease have lower expression of the TSLP gene40.
TSLP promotes immunity to helminth parasites
In addition to maintaining intestinal immune cell homeostasis in the steady state, TSLP-TSLPR interactions have a profound influence on the function of cells of the immune response, tissue inflammation and/or host protective immunity after exposure to helminth parasites. In the Trichuris model, treatment with monoclonal antibody to TSLP or deletion of TSLPR in normally resistant wild-type mice is associated with lower expression of pathogen-specific TH2 cytokine responses, which results in a failure to control infection. Disruption of the TSLP-TSLPR pathway also increases expression of the IL-12p40 subunit, interferon-γ and IL-17A and the development of severe infection- induced inflammation19. Notably, blocking p40 or interferon-γ in Trichuris-infected TSLPR-deficient mice restores expression of TH2 cytokines and host protective immunity19,27, which suggests that TSLP-independent TH2 responses can develop if endogenous proinflammatory responses are blocked. Consistent with that, TSLP-TSLPR interactions are dispensable for immunity to infection with Nippostrongylus and Heligmosomoides, two other intestinal nematodes. Unlike antigens from Trichuris, antigens derived from these pathogens can directly limit expression of the IL-12p40 subunit independently of TSLP27. The existence of TSLP-independent TH2 cytokine responses in vivo has been confirmed in the Schistosoma mansoni model. Experiments with TSLPR-deficient mice have shown that although the TSLP-TSLPR pathway contributes to the development of S. mansoni egg–induced CD4+ TH2 cell responses, its influence on the development of TH2 cytokine–dependent inflammation is transient42. Specifically, although expression of IL-5 and IL-13 is lower in egg-injected TSLPR-deficient mice, the effect on infection-induced TH2 cytokine–dependent inflammation is minimal. Although TSLP expression seems to have a critical role in the development of optimal TH2 cytokine responses, these experimental findings suggest that the requirements for this pathway in the development of type 2 inflammation are dependent on the antigenic stimulus, route of exposure and site of the inflammatory lesion.
TSLP and allergic inflammation
The atopic diseases consist of the triad of asthma, allergic rhinitis and atopic dermatitis43,44. These share a common pathogenesis and, importantly, frequently present together in the same person and family, which suggests that common factors and mechanisms could be involved45. Although common effectors such as TH2 cytokines, IgE, mast cells and eosinophils are involved, the mechanisms underlying the ‘preferential’ activation of TH2 cells by environmental allergens in atopic patients are still obscure. Higher expression of TSLP in the inflamed tissue is another common feature of these diseases11,46–48. Genetic analysis of atopic populations has shown an association of polymorphisms in TSLP with aspects of atopic allergic disease, including asthma and airway hyper-responsiveness, IgE concentrations and eosinophilia49–52.
The first indication of a connection between TSLP and allergic inflammatory responses came from patients with atopic dermatitis. The epidermis of lesional skin in patients with allergic forms of dermatitis has higher TSLP expression than that of epidermis in uninvolved skin or skin from people with nonallergic dermatitis19. Interestingly, skin-resident DCs in patients with atopic dermatitis have an activated phenotype and seem to migrate toward the draining lymph node, consistent with a role for TSLP in the regulation of tissue-resident DC responses.
In mouse models, more TSLP in the skin leads to a TH2 cytokine–dominant inflammatory disease. Inducible epidermal TSLP expression in mice induces a spontaneous atopic dermatitis–like disease53. The disease in these mice has all the cardinal features of human atopic dermatitis53. Epidermis-specific ablation of the steroid hormone receptors RXRα and RXRβ leads to spontaneous dermatitis54, which is associated with higher epidermal TSLP expression. Finally, keratinocyte-specific ablation of Notch signaling, through deletion of the Notch transcriptional effector RBP-j, leads to higher TSLP concentrations and dermatitis55. These experimental models demonstrate that increasing TSLP concentrations in the epidermis induces the onset of TH2 cytokine–associated inflammation, which suggests that the higher expression of TSLP in the lesional skin of patients with atopic dermatitis could be causative and is not a consequence of disease.
Perhaps the most direct causal link between higher expression of TSLP in the skin and human disease is Netherton syndrome, a genetic skin disease with severe atopic manifestations (recurrent atopic dermatitis, higher IgE concentrations, asthma and multiple food allergies)56. Netherton syndrome is caused by mutations in SPINK5, which encodes the protease inhibitor LEKTI57. LEKTI deficiency leads to dysregulation of kallekrein 5, which in turn activates the protease-activated receptor PAR-2. Activated PAR-2 induces the expression of TSLP from either keratinocytes or airway epithelial cells57,58. Thus, a mutation that increases TSLP expression in the skin has direct consequences on the development of a severe atopic disease.
Higher TSLP expression in the skin can increase airway inflammatory responses in mouse models, providing a potential link between atopic dermatitis and asthma58,59 (H. Han and S.F.Z., unpublished data). However, an important caveat for these animal models is the very high concentrations of circulating TSLP, which is not seen in people with atopic dermatitis. Thus, although it is a likely hypothesis, it is not yet clear what role, if any, TSLP has in the ‘atopic march’, the term used to describe the phenomenon in which people with one atopic disease (such as atopic dermatitis) are more likely to develop a second or third43–45. In general, people tend to develop atopic dermatitis first, followed by asthma, then allergic rhinitis44.
TSLP is both necessary and sufficient for the development of TH2 cytokine–associated inflammation of the airways in rodents. Mice expressing a TSLP transgene in the airway epithelium develop a spontaneous, progressive inflammatory disease with all the characteristics of human asthma12, whereas direct intranasal delivery of TSLP (in the presence of antigen) leads to rapid onset of severe disease60. The disease in these mice develops slowly over a 3-month period, as determined on the basis of adaptive responses to environmental antigens60. Challenge at an early age with antigen leads to the immediate onset of disease, which suggests that TSLP is functioning to condition the local environment to respond to aerosolized antigens60. In addition, human asthmatics have higher concentrations of TSLP in their lungs46,61.
The most compelling evidence for the importance of TSLP in the development of airway inflammation has been provided by genetic studies of mice. TSLPR-deficient mice are resistant to the development of inflammation in the classical ovalbumin-plus-alum priming model in mice12,62. It has been suggested that this is due to the inability of CD4+ T cells to respond to TSLP, as reconstitution with TSLPR- sufficient T cells restores aspects of the inflammatory disease62.
Consistent with the above mentioned link between TSLP and airway inflammation, factors known to be involved in either the development of asthma or the exacerbation of existing disease can induce TSLP expression in airway epithelial cells. These factors include inflammatory cytokines present in asthmatic lungs (IL-1β, tumor necrosis factor, IL-4, IL-13 and IL-25) and respiratory viruses63,64 (M.B. Headley, H.-C. Lee and S.F.Z., unpublished data). The finding that infection with respiratory syncytial virus can induce TSLP expression is especially interesting, as it has been linked to both the development of wheezing and subsequent asthma in infants and asthma exacerbations in affected people65–68. Respiratory syncytial virus can induce both protective TH1 cytokine responses and nonproductive TH2 cytokine responses in humans and susceptible mouse strains65. The TH2 cytokine response, which is unique to this virus, is thought to be a form of an immune-evasion process of the virus, involving dampening of the TH1 response, as well as affecting memory generation69. It is also possible that TSLP induced by infection of airway epithelial cells with respiratory syncytial virus is critical for the TH2 cytokine responses after infection.
Concluding comments
Building on original studies that identified a function for TSLP in activation of B cells and DCs70, subsequent studies have indicated that TSLP coordinates the effector functions of many myeloid and lymphoid populations (Fig. 1). These findings suggest a model in which TSLP promotes TH2 cytokine responses that can be either host protective or pathological. Epithelial cells and other cells, including keratinocytes and granulocytes, produce TSLP as a result of a real insult (Fig. 2a) or a perceived insult (Fig. 2b). TSLP then acts to create an environment that is permissive to the development of TH2 cytokine responses. This environment can either function to maintain normal barrier homeostasis or lead to the development of type 2 inflammation. In the latter case, TSLP seems to coordinately inhibit p40 expression in DC populations and activate basophils that can then migrate into lymph node that drains the site of infection or insult. DCs conditioned by TSLP at the tissue site may be simultaneously or subsequently recruited to lymph node and participate in T cell activation11 (R.P. Larson and S.F.Z., unpublished data). In the lymph node, DCs and basophils may act cooperatively to promote optimal TH2 cell differentiation.
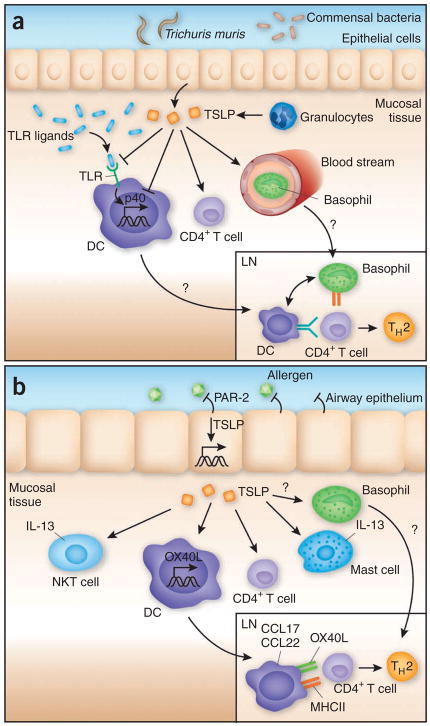
Figure 2.
TSLP regulates TH2 cytokine responses after helminth infection and exposure to allergens. (a) After exposure to helminth parasites, infection and/or disruption of colonic epithelium elicits responses to Toll-like receptor (TLR) and Nod-like receptor agonists that are able to induce IL-12p40 expression and subsequent TH1 cytokine responses. TSLP, which is also induced during infection, acts to suppress p40 expression by DCs, which inhibits the development of TH1 responses, while also inducing OX40L to promote TH2 responses. TSLP may also act to recruit IL-4-producing basophils to draining lymph nodes (LN) that act cooperatively with DCs to prime TH2 cytokine responses. (b) After allergen exposure, proteases present in the allergen complex activate PAR-2, which in turn induces TSLP expression. TSLP induces resident DCs to upregulate OX40L and to produce chemokines (CCL17 and CCL22) to promote TH2 responses. TSLP acts on resident mast cells and natural killer T cells to increase cytokine production, which further promotes the TH2 inflammatory cascade. MHCII, major histocompatibility complex class II.
In circumstances in which infection with a helminth parasite may induce epithelial damage, subsequent exposure to Toll-like receptor agonists from the pathogen or resident microbial communities would be expected to elicit a potent TH1 cytokine response. Microbial products can also elicit TSLP expression41,71, and the ability of TSLP to modulate or inhibit TH1 responses may be its chief function in these circumstances (Fig. 2a). However, after exposure to an innocuous allergen, TSLP expression may be induced through the protease-activated receptor pathway58. In this scenario, as no TH1 responses will be induced, TSLP can actively drive a TH2 cytokine response, potentially through effects on DCs, granulocytes, natural killer cells and CD4+ T cells (Fig. 2b). It is this last aspect of TSLP function that makes it a likely therapeutic target for the treatment of allergic diseases.
Acknowledgments
We thank D. Campbell, M. Siracusa and G. Sonnenberg for reviewing the manuscript. Supported by the National Institutes of Health (AI068731, AI044259, AR055695 and AR56113 to S.F.Z., and AI061570, AI074878 and AI083480 to D.A.), the Burroughs Wellcome Fund (D.A.), the Crohn’s and Colitis Foundation of America (D.A.) and the University of Pennsylvania (D.A.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Friend SL, et al. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 2.Sims JE, et al. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med. 2000;192:671–680. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray RJ, Furlonger C, Williams DE, Paige CJ. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol. 1996;26:10–16. doi: 10.1002/eji.1830260103. [DOI] [PubMed] [Google Scholar]
- 4.Reche PA, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 5.Quentmeier H, et al. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. 2001;15:1286–1292. doi: 10.1038/sj.leu.2402175. [DOI] [PubMed] [Google Scholar]
- 6.Park LS, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey A, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 8.Levin SD, et al. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol. 1999;162:677–683. [PubMed] [Google Scholar]
- 9.Isaksen DE, et al. Requirement for STAT5 in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 1999;163:5971–5977. [PubMed] [Google Scholar]
- 10.Al Shami A, et al. A role for thymic stromal lymphopoietin in CD4+ T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 12.Zhou B, et al. Thymic stromal lymphopoietin (TSLP) as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe N, et al. Human thymic stromal lymphopoietin promotes dendritic cell–mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5:426–434. doi: 10.1038/ni1048. [DOI] [PubMed] [Google Scholar]
- 14.Ito T, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YH, et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Rimoldi M, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 17.Rimoldi M, et al. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 2005;106:2818–2826. doi: 10.1182/blood-2004-11-4321. [DOI] [PubMed] [Google Scholar]
- 18.Zaph C, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 19.Taylor BC, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambrecht BN, et al. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106:551–559. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 22.van Rijt LS, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearce EJ, Kane CM, Sun J. Regulation of dendritic cell function by pathogen-derived molecules plays a key role in dictating the outcome of the adaptive immune response. Chem Immunol Allergy. 2006;90:82–90. doi: 10.1159/000088882. [DOI] [PubMed] [Google Scholar]
- 24.Perrigoue JG, et al. MHC class II–dependent basophil-CD4+ T cell interactions promote TH2 cytokine–dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokol CL, et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimoto T, et al. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide–MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 27.Massacand JC, et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci USA. 2009;106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omori M, Ziegler S. Induction of IL-4 expression in CD4+ T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 29.Allakhverdi Z, Comeau MR, Jessup HK, Delespesse G. Thymic stromal lymphopoietin as a mediator of crosstalk between bronchial smooth muscles and mast cells. J Allergy Clin Immunol. 2009;123:958–960. doi: 10.1016/j.jaci.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 30.Allakhverdi Z, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagata Y, Kamijuku H, Taniguchi M, Ziegler S, Seino K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int Arch Allergy Immunol. 2007;144:305–314. doi: 10.1159/000106319. [DOI] [PubMed] [Google Scholar]
- 32.Wong CK, Hu S, Cheung PF, Lam CW. TSLP induces chemotactic and pro-survival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2009 October 20; doi: 10.1165/rcmb.2009-0168OC. published online. [DOI] [PubMed] [Google Scholar]
- 33.Iliev ID, Matteoli G, Rescigno M. The yin and yang of intestinal epithelial cells in controlling dendritic cell function. J Exp Med. 2007;204:2253–2257. doi: 10.1084/jem.20062535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iliev ID, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 35.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 36.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rimoldi M, et al. Intestinal immune homeostasis is regulated by the corsstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 41.Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramalingam TR, et al. Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin. J Immunol. 2009;182:6452–6459. doi: 10.4049/jimmunol.0900181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ker J, Hartert TV. The atopic march: what’s the evidence? Ann Allergy Asthma Immunol. 2009;103:282–289. doi: 10.1016/S1081-1206(10)60526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahn EL, Bacharier LB. The atopic march: the pattern of allergic disease development in childhood. Immunol Allergy Clin North Am. 2005;25:231–246. doi: 10.1016/j.iac.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 46.Ying S, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 47.Miyata M, et al. Mast cell regulation of epithelial TSLP expression plays an important role in the development of allergic rhinitis. Eur J Immunol. 2008;38:1487–1492. doi: 10.1002/eji.200737809. [DOI] [PubMed] [Google Scholar]
- 48.Mou Z, et al. Overexpression of thymic stromal lymphopoietin in allergic rhinitis. Acta Otolaryngol (Stockh) 2008;8:1–5. doi: 10.1080/00016480802225884. [DOI] [PubMed] [Google Scholar]
- 49.Gudbjartsson DF, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41:342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 50.He HQ, et al. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol. 2009;124:222–229. doi: 10.1016/j.jaci.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 51.Hunninghake GM, et al. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. Am J Respir Crit Care Med. 2008;177:830–836. doi: 10.1164/rccm.200711-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harada M, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40:368–374. doi: 10.1165/rcmb.2008-0041OC. [DOI] [PubMed] [Google Scholar]
- 53.Yoo J, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li M, et al. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc Natl Acad Sci USA. 2005;102:14795–14800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Demehri S, et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6:e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Judge MR, Morgan G, Harper JI. A clinical and immunological study of Netherton’s syndrome. Br J Dermatol. 1994;131:615–621. doi: 10.1111/j.1365-2133.1994.tb04971.x. [DOI] [PubMed] [Google Scholar]
- 57.Chavanas S, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141–142. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 58.Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067. doi: 10.1371/journal.pbio.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z, et al. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc Natl Acad Sci USA. 2009;106:1536–1541. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Headley MB, et al. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182:1641–1647. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ying S, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 62.Al Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFκB. Proc Natl Acad Sci USA. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holt PG, Sly PD. Interactions between RSV infection, asthma, and atopy: unraveling the complexities. J Exp Med. 2002;196:1271–1275. doi: 10.1084/jem.20021572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 67.Martinez FD. Respiratory syncytial virus bronchiolotis and the pathogenesis of childhood asthma. Pediatr Infect Dis J. 2003;22:S76–S82. doi: 10.1097/01.inf.0000053889.39392.a7. [DOI] [PubMed] [Google Scholar]
- 68.Stein RT, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 69.Yoon JS, Kim HH, Lee Y, Lee JS. Cytokine induction by respiratory syncytial virus and adenovirus in bronchial epethelial cells. Pediatr Pulmonol. 2007;42:277–282. doi: 10.1002/ppul.20574. [DOI] [PubMed] [Google Scholar]
- 70.Leonard WJ. TSLP: finally in the limelight. Nat Immunol. 2002;3:605–607. doi: 10.1038/ni0702-605. [DOI] [PubMed] [Google Scholar]
- 71.Kido M, et al. Helicobacter pylori promotes the production of thymic stromal lymphopoietin by gastric epithelial cells and induces dendritic cell-mediated inflammatory Th2 responses. Infect Immun. 2010;78:108–114. doi: 10.1128/IAI.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]