Abstract
Background and Aims
Vascular epiphytes have to acquire nutrients from atmospheric wash out, stem-flow, canopy soils and trapped litter. Physiological studies on the adaptations to nutrient acquisition and plant utilization of nutrients have focused on phosphorus and nitrogen; potassium, as a third highly abundant nutrient element, has received minor attention. In the present study, potassium uptake kinetics by leaves, within-plant distribution and nutrient accumulation were analysed to gain an improved understanding of physiological adaptations to non-terrestrial nutrient supply of plants.
Methods
Radioactively labelled 86RbCl was used as an analogue to study uptake kinetics of potassium absorbed from tanks of epiphytes, its plant distribution and the correlation between uptake efficiency and abundance of trichomes, functioning as uptake organs of leaves. Potassium in leaves was additionally analysed by atomic absorption spectroscopy to assess plant responses to potassium deficiency.
Key Results
Labelled rubidium was taken up from tanks over a wide range of concentrations, 0·01–90 mm, which was achieved by two uptake systems. In four tank epiphytes, the high-affinity transporters had average Km values of 41·2 µm, and the low-affinity transporters average Km values of 44·8 mm. Further analysis in Vriesea splenriet showed that high-affinity uptake of rubidium was an ATP-dependent process, while low-affinity uptake was mediated by a K+-channel. The kinetic properties of both types of transporters are comparable with those of potassium transporters in roots of terrestrial plants. Specific differences in uptake velocities of epiphytes are correlated with the abundance of trichomes on their leaf surfaces. The main sinks for potassium were fully grown leaves. These leaves thus function as internal potassium sources, which allow growth to be maintained during periods of low external potassium availability.
Conclusions
Vascular epiphytes possess effective mechanisms to take up potassium from both highly diluted and highly concentrated solutions, enabling the plant to incorporate this nutrient element quickly and almost quantitatively from tank solutions. A surplus not needed for current metabolism is stored, i.e. plants show luxury consumption.
Keywords: Epiphytic bromeliads, potassium uptake, luxury consumption, plant nutrition, Vriesea splenriet
INTRODUCTION
Vascular plants that grow epiphytically in tree crowns cannot directly trap terrestrial nutrient pools, but have to acquire nutrients from atmospheric sources in precipitation, stem flow, throughfall, and from canopy soils and litter trapped in tanks. Nutrient supply from these sources is generally low and highly intermittent (Benzing and Renfrow, 1974; Clark et al., 1998), a situation that may be directly linked to morphological peculiarities such as the velamen radicum in orchids or bromeliad tanks with foliar uptake by absorbing trichomes (Benzing, 1990). Remarkably, more than a century after the early studies on epiphyte ecophysiology by Schimper (1888), the physiological properties of the uptake structures of bromeliads are still not well understood. For example, it was demonstrated only recently that bromeliad tanks possess membrane transporters for a number of different N-sources (Inselsbacher et al., 2007), which should enable plants to take advantage of the diversity of nutrient sources delivered by the metabolism of tank organisms (Benzing, 1970; Inselsbacher et al., 2007). Membrane transporters for N-sources (Inselsbacher et al., 2007), but also those for phosphate (Winkler and Zotz, 2009), are characterized by high substrate affinities and uptake velocities. Although there is indirect evidence for effective uptake of potassium as well (Richardson et al., 2000; Lin and Yeh, 2008), the physiological and biochemical properties of K+-transporters in trichomes have not yet been analysed, although next to N, potassium is usually the second most abundant nutrient element in plant tissues.
Unlike most other plant nutrients, K+ is not a constituent of biomolecules and is highly mobile in plants. In the cytoplasm, potassium acts as a counter ion to the large excess of negatively charged proteins and nucleic acids. Most of the cellular potassium is located in vacuoles, functioning as a principal vacuolar osmoticum. The potassium concentration (=[K+]) in this cell compartment can be highly variable, reflecting the potassium status of the plant – in times of sufficient supply the vacuolar pool is stocked up, while under limiting conditions the vacuolar potassium store is depleted to sustain metabolic functions in the cytoplasm. Such ‘functional storage’ periodically occurs in perennial trees (Rosecrance et al., 1998). In accordance with its significance for plant growth, uptake of potassium by roots is a well-regulated and efficient process. Membrane transporters for potassium were first characterized by their kinetic properties (Läuchli and Epstein, 1970). In roots of terrestrial plants there is a biphasic uptake system, consisting of high-affinity transporters with Km values of 10–50 µm and low-affinity transporters with Km values of 10–80 mm (Rodriguez-Navarro, 1999; Szczerba et al., 2009). With respect to the mechanism of transport, three types of membrane transporters have been identified in terrestrial plants. Transport can be mediated by secondary active K+ : H+ symport, K+ : H+ antiport or diffusion via K+ channels (Lebaudy et al., 2008; Szczerba et al., 2009). In contrast, based on numerous studies using plant roots, evidence for foliar uptake of potassium is restricted to carnivorous pitcher plants (Adlassnig et al., 2009).
Because of its experimental advantages, labelled 86Rb is often used as an analytical analogue for measuring acquisition and tissue-redistribution of potassium. Pioneering studies using different tissues have demonstrated that uptake kinetics for K+ and Rb+ are comparable and that both ions compete for the same membrane transporter (Läuchli and Epstein, 1970; Rodriguez-Navarro, 1999). Exceptions are skeletal muscles (Dørup and Clausen, 1994) or excised maize roots (Maas and Leggett, 1968), where the use of 86Rb+ as a reliable tracer for potassium was questioned. The properties of membrane transporters of foliar trichomes in bromeliads are not known, and therefore control experiments to compare uptake of potassium and 86Rb+ were included in the present experiments.
The aims here were three-fold. First, the hypothesis that leaves of epiphytic bromeliads show highly efficient uptake of potassium was tested with a detailed analysis of the uptake kinetics for Rb+. Second, uptake efficiency was studied in relation to trichome abundance of leaves of different epiphyte species as an additional step towards a more detailed understanding of trichome function. Third, the distribution of potassium within plants after a nutrient pulse was determined and the long-term effect of K+ supply on plant growth was studied to quantify the functional consequences of changing availability of this nutrient element for epiphytes.
MATERIAL AND METHODS
Plant material
Plant material was kindly supplied by a commercial nursery (Corn. Bak B.V., Asseldelft, Holland: Vriesea splenriet, Aechmea fasciata, Vriesea duvaliana), or was collected in the tropical lowland forest in Panama (all other species). Experiments to characterize rubidium uptake in tanks were mainly carried out with V. splenriet plants (Bromeliaceae). Kinetic experiments were also conducted with other tank bromeliads, namely A. fasciata, V. duvaliana and field-collected Werauhia sanguinolenta. All plants listed above had 12–16 leaves, were 12–16 cm tall and had a tank volume of approx. 2 mL. Other plants, which had also been collected from natural populations, were Guzmania monostachia, Tillandsia flexuosa, T. fasciculata and T. subulifera. These bromeliads differed in size as indicated in the figure legends. Additional plants of the three Tillandsia species were grown from seed in Oldenburg, which had also been collected in the field. These plants were <4 cm tall. Plants were kept in the greenhouse at 25 °C, at a relative humidity of approx. 60 % and a light–dark regime of 14/10 h, being illuminated by natural sunlight, supplemented with artificial light (Metal halid lamps, 400 W, master HPI-T plus; Philips, The Netherlands) when necessary to achieve a photosynthetic photon flux density of at least 150–180 µmol m−2 s−1 at the level of the plants.
A nutrient solution containing 3·2 mmol L−1 NH4NO3, 0·3 mmol L−1 KH2PO4, 0·65 mmol L−1 K2HPO4, 0·35 mmol L−1 K2SO4, 0·2 mmol L−1 MgSO4 and 0·34 mmol L−1 CaCl2 was applied by spraying the plants until tanks were completely filled. Potassium-free nutrient solution was obtained by omitting K2SO4, while K-phosphates were replaced by Na-phosphates. Plants were fertilized once a week, and were otherwise irrigated daily with water.
K+ deficiency experiment
Plants, which had been fertilized before the experiment, were grown under otherwise identical conditions in the greenhouse in complete nutrient solution and potassium-free nutrient solution as described above. As the water content of the plants remained constant during the 250 d of culture with and without potassium fertilization, relative growth rate (RGR) (Evans, 1972) could be calculated by the equation: RGR = (ln f. wtfinal – ln f. wtinitial)/(Δt), where f. wt is plant fresh weight and Δt is the duration of the experiment in days.
Rubidium uptake
During experiments in the radionuclide laboratories of the University of Oldenburg, plants were kept in Plexiglas boxes under conditions comparable with those in the greenhouse. Plant uptake was measured using aquatic solutions of 86RbCl (Hartmann Analytic, Braunschweig, Germany) with a specific activity of 720 MBq mg−1 Rb+. Radioactivity of aliquots used for plant incubation was measured by liquid scintillation counting, using lume gel save cocktail (Lumac, Rodgau, Germany) in a Wallac 1415 (Wallac, Uppsala, Sweden) liquid scintillation counter.
86Rb+ uptake from tanks
Before starting experiments, tanks were repeatedly thoroughly rinsed with 1 mL of incubation solution, containing 1 mm Mes-buffer, pH 6·1, and 1 mm CaCl2. Experiments were started by adding 1–1·5 mL of incubation solution, 5–10 µL labelled 86RbCl to obtain an initial activity of 2·5 × 107 d.p.m. mL−1, and 10–90 mm RbCl to tanks. Before sampling, plants were weighed, and weight loss during experiments was compensated for by adding water. After standardizing the fluid volume in this way, tank fluids were mixed with Pasteur pipettes and radioactivity of 5-μL aliquots was measured by liquid scintillation counting. For kinetic measurements, samples were taken every 30 min up to 6 h and uptake rates of unlabelled Rb+ were calculated from the initial linear phase of 86Rb+ depletion. Tank depletion obtained for Rb+ at an initial concentration of 0·5 and 50 mm was compared in separate experiments with the decrease of potassium in tank solutions using the same starting concentrations and plants of similar size. Potassium concentrations were measured by atomic absorption spectroscopy. Comparable rates of tank-depletion of Rb+ and K+ (Table 1) indicated that Rb+ may indeed be used as tracer for the study of the acquisition and tissue-redistribution of potassium in these epiphytic plants.
Table 1.
Depletion of potassium and rubidium from tanks of Vriesea splenriet (n = 4)
| Depletion from tanks (μmol h−1 g−1 d. wt mL−1 tank volume) |
||
|---|---|---|
| Nutrient concentration (mm) | Potassium | Rubidium |
| 0·5 | 0·25 ± 0·019 | 0·23 ± 0·015 |
| 50 | 4·03 ± 0·44 | 4·25 ± 0·32 |
Means were not significantly different (t-tests, t > 0·83, P > 0·05).
Uptake of 86Rb+ into plant tissue
Tanks of Vriesea splenriet were supplied with 0·5 mm RbCl and 86Rb+ to obtain an initial activity of 2·5 × 107 d.p.m. mL−1. Radioactivity in tissue was measured from crushed plant material (approx. 0·25 g f. wt) by liquid scintillation counting after 1 and 9 d. After the initial Rb+ pulse, plants were watered only.
Uptake of 86Rb+ by whole plants
Small plants of 1–5 cm were transferred to screw cap tubes and submerged in incubation solutions containing 0·5 mm unlabelled RbCl, and 0·5–1·0 µL mL−1 labelled 86RbCl to obtain an initial activity of 2·5 × 106 d.p.m. mL−1. Depending on plant size, aliquots of 10–40 µL were sampled after 6–12 h to measure radioactivity by liquid scintillation counting. The concentration of Rb+ used is regarded as an average value of naturally occurring [K+] available to bromeliads from rain water, throughfall and stem flow waters ranging from 0·1 to 0·5 mm (Benzing, 2000; Richardson et al., 2000).
Uptake of 86Rb+ by leaf discs
Discs of 30 mm diameter were cut from large leaves and tightened in Plexiglas tubes sealed with O-rings to prevent uptake by cut tissue. Leaf discs were overlaid with 2 mL of incubation solution as described above for uptake by whole plants. Samples of 50 µL were taken after 6–12 h to measure radioactivity.
Inhibitor experiments
In another set of experiments, concentration dependencies of inhibition of Rb+ uptake of V. splenriet by 1 mm carbonyl cyanide m-chlorophenylhydrazone (CCCP) and 1 mm tetraethylammonium chloride (TEA) were analysed. CCCP is a well-known ATPase inhibitor that is used to separate active from passive uptake (Dahlmann et al., 2004), whereas TEA acts as inhibitor of K+-channels. Experiments were started by filling tanks with 1 mL of incubation solutions as described under 86Rb uptake from tanks containing additionally 1 mm CCCP or 1 mm TEA.
All other experimental details regarding nutrient uptake, measurement of radioactivity in plant tissue and calculation of uptake rates were as described by Winkler and Zotz (2009).
Other measurements
Trichome numbers for whole plants and leaf discs were counted under a microscope after tissue-staining with 1 % methyl blue. Trichome numbers of whole plants were averaged from counting basal, middle and distal parts of the upper and lower side of 4–8 leaves. Leaf areas were estimated from scanned leaf pictures by using the measuring tools in Adobe Photoshop CS 3 extended. Potassium was determined from oven-dried (36 h, 85 °C) and milled plant samples. Briefly, 10–20 mg was digested in screw-cap tubes in a 10 : 1 : 4 mixture of nitric, sulfuric and perchloric acid for 8 h at 80 °C (Jackson, 1958), and digests were analysed in a Perkin-Elmer atomic absorption photometer.
Data analysis
All statistical analyses were performed with R 2.6.0 (R Development Core Team, 2007). Error terms are standard deviations. The Michaelis constant (Km) and maximal enzyme velocities (Vmax) were determined via non-linear regression of the Michaelis–Menten equation for four replicate runs.
RESULTS
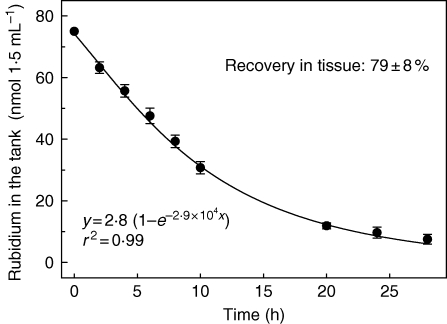
In an initial experiment, 1·5 mL of labelled 86Rb+ and 75 nmol unlabelled Rb+ were filled into tanks of V. splenriet. From the decrease in radioactivity, it was calculated that tank depletion decreased with time according to the decreasing concentration in the tank. About 70 nmol of Rb+ was removed from the tanks after 28 h (Fig. 1). Even at this low initial Rb+ concentration, probably favouring surface adsorption, about 79 ± 8 % (mean ± s.d., n = 4) of unlabelled Rb+ was recovered in plant tissue, as calculated from the radioactivity estimated in the plant material. This recovery is regarded as being sufficient to study uptake kinetics and mechanisms for 86Rb+ by its tank depletion, especially if initial uptake rates are measured.
Fig. 1.
Time course of rubidium depletion from tanks of Vriesea splenriet. Tanks were filled with a mixture of 2·5 × 107 d.p.m. 86Rb+ and 75 nmol unlabelled RbCl in 1·5 mL Mes-buffer, 1 mm, pH 6·1, containing 1 mm CaCl2. Tissue recovery was calculated from the label reanalysed in plant tissue. Data are means ± s.d.; n = 4. The regression equation is indicated.
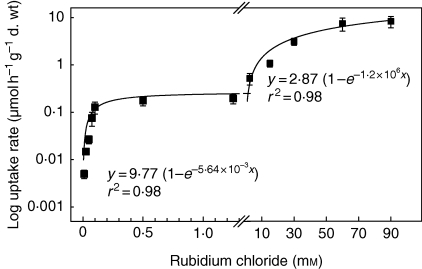
The concentration dependence of Rb+ uptake, determined based on the decrease of radioactivity in the tanks at concentrations ranging from 0·01 to 90 mm RbCl, indicated a biphasic uptake system (Fig. 2). A first transporter system was saturated at Rb+ concentrations of approx. 0·2 mm, and a second one at Rb+ concentrations that were about two orders of magnitude higher. Both uptake systems also differed in maximal uptake rates (Table 2). The high-affinity transport system was characterized by a low maximal uptake velocity, whereas the low-affinity system was able to accumulate high amounts of Rb+ very rapidly. Applying the Michaelis–Menten plot, Km values of 41·3 ± 8·7 µm and 56·5 ± 13·7 mm were calculated for the two types of transporters, respectively. Kinetic properties obtained for Rb+ uptake in three other tank bromeliads were comparable with those of V. splenriet (Table 2).
Fig. 2.
Biphasic kinetics of rubidium uptake from tanks of Vriesea splenriet in the presence of 0·01–90 mm substrate. Experimental detail as in Fig. 1 except that different concentrations of unlabelled RbCl were used as indicated in the diagram. Uptake rates were calculated from the decrease of 86Rb+ radioactivity during the first 1–2 h of uptake. Data are means ± s.d.; n = 4. The regression equations for the low and high concentration ranges are indicated.
Table 2.
Kinetic properties of rubidium uptake in tanks of different tank bromeliads in low and high concentration ranges
| <1 mm rubidium |
>10 mm rubidium |
|||
|---|---|---|---|---|
| Species | Km (μm) | Vmax (nmol h−1 g−1 d. wt mL−1) | Km (mM) | Vmax (μmol h−1 g−1 d. wt mL−1) |
| Vriesea splenriet | 41·3 ± 8·7 | 14·9 ± 1·3 | 56·5 ± 13·7 | 5·1 ± 0·9 |
| Vriesea duvaliana | 32·4 ± 14·2 | 21·8 ± 5·1 | 25·0 ± 12·1 | 18·2 ± 2·2 |
| Werauhia sanguinolenta | 38·3 ± 5·2 | 40·2 ± 14·9 | – | – |
| Aechmea fasciata | 52·4 ± 10·6 | 24·8 ± 4·8 | 52·8 ± 12·1 | 18·7 ± 3·8 |
Data are means ± s.d. (n = 4); –, not determined.
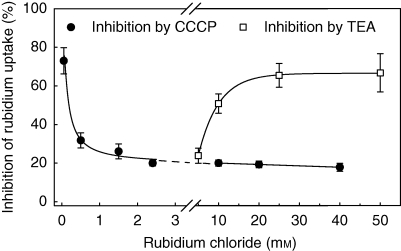
The physiological mechanisms behind low- and high-affinity Rb+ uptake were studied with well-known inhibitors in V. splenriet. Rates for Rb+ uptake in the low molecular range of substrate decreased in the presence of the ATPase inhibitor CCCP (Fig. 3). At concentrations above approx. 10 mm, uptake of RbCl was inhibited by the K+-channel inhibitor TEA. Saturation of inhibition with TEA was obtained above approx. 30 mm RbCl.
Fig. 3.
Inhibition of rubidium uptake from tanks of Vriesea splenriet by carbonyl cyanide m-chlorophenylhydrazone (CCCP) and tetraethylammonium chloride (TEA) at different Rb+ concentrations. Uptake rates are expressed as percentage inhibition by CCCP or TEA compared with a control without inhibitors. Tanks were supplied with 2·5 × 107 d.p.m. 86Rb+ and 1·5 mL Mes-buffer, 1 mm, pH 6·1, containing 1 mm CaCl2, rubidium chloride as indicated and either 1 mm CCCP or 1 mm TEA. Other experimental details are as described in Fig. 1. Data are means ± s.d.; n = 4. The regression equations for inhibition by TEA and by CCCP are: y=2·87 (1–e−1·2×106x), r2=0·98; y=6·07 (1–e−0·13x), r2=0·97.
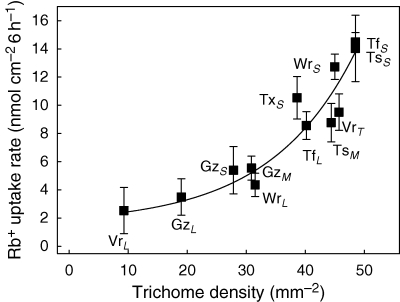
The relationship between nutrient uptake rates and trichome density (trichomes mm−2) was analysed for a range of plant species covering different plant habits, i.e. tanks and atmospherics, and different plant sizes. All juvenile plants were of the atmospheric habit, and larger plants were either atmospherics (e.g. Tillandsia subulifera, T. flexuosa), or had a tank (e.g. V. splenriet, Werauhia sanguinolenta). Figure 4 shows the positive, albeit non-linear, relationship between trichome density and uptake rate for Rb+ (r2 = 0·86).
Fig. 4.
Relationship between rubidium uptake and trichome density in different bromeliads. All plants except large plants of Guzmania monostachia and Werauhia sanguinolenta were measured completely submerged in label solution containing 0·5 mm rubidium chloride and 1 × 107 d.p.m. mL−1 86Rb+. Trichome densities were averaged by counting basal, middle and distal parts of the upper and lower side of 4–8 leaves. Uptake rates for large plants from Guzmania and Werauhia were measured in the same label solution using discs prepared from basal, middle and distal sections of upper and lower sides of older and younger leaves. Trichome density is given as number per mm−2. Abbreviations: VrL = Vriesea splenriet (plants of the same size as used in other experiments); VrT = Vriesea splenriet, basal segments of tank leaves (plants of the same size as used in other experiments); GzS = Guzmania monostachia, plant size 2–4 cm; GzM = Guzmania monostachia, plant size 8–12 cm; GzL = Guzmania monostachia, plant size 22–25 cm; WrS = Werauhia sanguinolenta, plant size 2–4 cm; WrL = Werauhia sanguinolenta, plant size 16–18 cm; TfS = Tillandsia fasciculata, plant size 1–3 cm; TfL = Tillandsia fasciculata, plant size 14–18 cm; TsS = Tillandsia subulifera, plant size 1–3 cm; TsM = Tillandsia subulifera, plant size 8–12 cm; TxS = Tillandsia flexuosa, plant size 2–4 cm. The regression equation is: y=7·45x−103 (1–e−2·8×10−4x), r2=0·99.
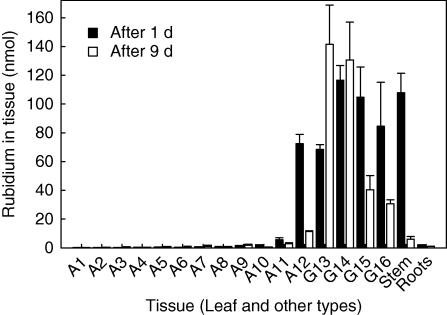
The subsequent fate of acquired Rb+ within a plant was analysed by subjecting V. splenriet to a radioactivity pulse, followed by supply with distilled water only (Fig. 5). Irrespective of incubation time, most leaves and roots received only a small portion of the incorporated Rb+. One day after labelling, 86Rb+ was only found in tissue that had direct contact to the tank solution or was adjacent to these leaves, i.e. in several actively growing leaves and the stem. Nine days after the pulse, more than 70 % of the 86Rb+ was found in just two actively growing leaves (G13 and G14 in Fig. 5), while 86Rb+ had decreased substantially in all other leaves and the stem.
Fig. 5.
Distribution of labelled rubidium in leaves, stems and roots of Vriesea splenriet. Experimental details for the uptake from tanks as described in Fig. 1, except that tanks were supplied with 0·5 mm unlabelled RbCl. After complete uptake of Rb+, tanks were watered only. After 1 and 9 d, plants were harvested and cut into small sections. Amounts of rubidium were calculated from the label in these sections. Leaves are numbered according to their age (A1 = oldest, non-senescent leaf). During ontogenetic development, the length of successive leaves is increasing in Vriesea. Thereby, fully grown adult leaves (A1–A12) can be distinguished from growing leaves (G13–G16) by comparing the length of leaves. Data are means ± s.d.; n = 4.
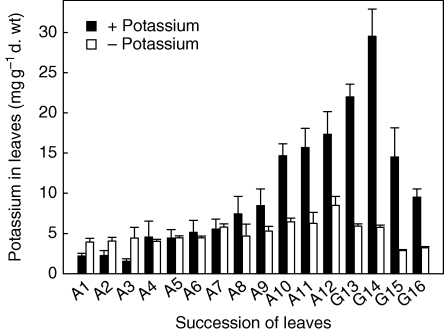
Radioactive Rb+ may be used as a potassium analogue in uptake processes but does not necessarily replace potassium functionally. Therefore, foliar [K+] was analysed directly (Fig. 6). Plants of V. splenriet had been cultivated in the greenhouse under different K+ fertilization treatments, but otherwise identical conditions, for 250 d. In plants receiving fertilizer including potassium, [K+] increased only in the younger, fully developed leaves (A8–A12) and the younger, actively growing leaves (G13–G16). The highest [K+] was found in the same leaves that had accumulated most of the labelled Rb+ in the short-term experiment (G13 and G14; Fig. 5). Long-term K starvation hardly affected [K+] in mature leaves (A1–A7). Arguably, the observed [K+] of 4·2 ± 1·2 mg K+ g−1 d. wt in older adult leaves (A1–A4) of starved and fertilized plants represents the minimum [K+] necessary to sustain metabolic functions of this leaf type. In younger adult and growing leaves, a culture time of 250 d may still have been too short to reach this minimum, explaining why K+ deficiency had no significant effect on RGR.
Fig. 6.
Distribution of potassium in leaves of Vriesea splenriet after 250 d of growth with and without potassium. Plants, which had been fertilized before the experiment, were grown under otherwise identical conditions in the greenhouse. Leaves are numbered according to their age. A1 was the oldest adult and A12 the youngest fully developed leaf. G13–G16 are still growing leaves. Data are means ± s.d.; n = 4.
As the potassium content of the entire plant did not significantly decrease during the 250 d without fertilization with potassium (7·7 ± 1·4 mg K+ at the start, and 8·1 ± 0·7 after 250 d of fertilization, and 7·9 ± 1·1 mg K+ after 250 d of potassium starvation; n = 4), decreasing [K+] in the growing leaves must be related to an increasing biomass of these leaves, and potassium must have been transferred from the younger mature leaves to the growing leaves to maintain growth. Notably, potassium starvation over a period of 250 d did not reduce growth rates of Vriesea plants compared with fertilized plants. Indeed, cumulative RGRs were even slightly higher: 4·0 ± 0·3 × 10−3 d−1 versus 3·6 ± 0·3 × 10−3 d−1 (t-test, t = –2·3, P = 0·04).
DISCUSSION
In many epiphytic bromeliads, roots serve only as hold-fast, their original functions in water and nutrient uptake being adopted by leaves with absorbing trichomes (Benzing, 2000). Together with the findings of earlier studies on N (Inselsbacher et al., 2007) and P uptake (Winkler and Zotz, 2009), the present results provide compelling evidence that these trichomes and the fine roots of terrestrial plants, although very different in morphology and anatomy, share comparable biochemical properties and uptake mechanisms. Highly efficient and biphasic uptake systems for potassium in all studied species of tank bromeliads are characterized by high-affinity transporters with Km values ranging from 32 to 52 µm and by low-affinity transporters with Km values between 25 and 57 mm. These values compare favourably with those obtained from fine roots of terrestrial plants, in which Km values of high-affinity transporters range from 20 to 50 µm and those of low-affinity transporters from 10 to 80 mm (Rodriguez-Navarro, 1999; Szczerba et al., 2009). Inhibitor studies with V. splenriet suggest that the high-affinity uptake is ATP dependent, indicating a secondary active transporter. The low-affinity uptake of K+ in this bromeliad was not affected by the ATPase inhibitor, but uptake rates were reduced by inhibitors of K+-channels. Vmax also differed in these biphasic uptake systems: active uptake allowed only low uptake velocities, whereas diffusion through membrane channels was a much faster process, as long as a sufficiently large concentration gradient existed. All these characteristics fully agree with the uptake systems for K+ found in roots.
In agreement with findings for K+, uptake of P (as phosphate) and N (in various nitrogen compounds) in bromeliads is similarly mediated by secondary active transporters (Inselsbacher et al., 2007; Winkler and Zotz, 2009) and this type of transport is also common in roots (Szczerba et al., 2009). Taken together, the current evidence suggests that fine roots and trichomes share transporters with comparable substrate affinities and transport mechanisms. In situ, nutrient solutions are believed to be highly diluted (Benzing, 1990), and nutrients can – in general – only be taken up by the high-affinity transporters, while K+ channels may allow the plants to take advantage of occasional, high external concentrations of K+, for example when larger plant or animal parts decompose in a tank. Unfortunately, our understanding of the nutrient fluxes in the canopy is too rudimentary to put these speculations to the test.
Nutrient transfer into leaf tissue not only depends on the biochemical properties of membrane transporters, but also on their abundance. This abundance, in turn, should correlate with the density of trichomes, which are composed of dead wing cells, covering the leaf surface to suck in water and nutrients, and of central stalk cells. These stalk cells are alive and their membranes constitute the supposed metabolically active site in nutrient transfer into the plant (Benzing, 1970; Benzing and Pridgeon, 1983). Trichome density alone explains a surprisingly large proportion of the interspecific and intraspecific variation in the uptake rates of K+. The non-linear relationship between trichome number and the uptake rates of K+ suggests that the density and/or the specific type of active transporter in the cell membranes of the stalk cells may vary among species and or ontogenetic stage.
Are epiphytes limited by the supply of K under natural conditions? A survey of published data of the foliar [K+] in field-collected epiphytic bromeliads revealed an average [K+] of 1·1 ± 0·6 % g−1 d. wt (Table 3), which is close to the general minimum requirement for eutrophic vegetation (1 % K; Epstein, 1972). In epiphytes K+ is recycled before leaf abscission to a considerable degree (approx. 40 % of green leaf concentrations), comparable with N (35 %) and P (62 %) (Zotz, 2004). This may indicate that K+ is indeed in short supply. In contrast, at least in the time frame of weeks or months, increased supply of K does not stimulate growth.
Table 3.
Published potassium concentrations of 16 field-grown species of epiphytic bromeliads (1·16 ± 0·63 % d. wt, mean ± s.d.)
| Species | % K | Variation (% K) | Site description | Source |
|---|---|---|---|---|
| Aechmea nudicaulis | 1·94 | n.a. | Jamaica | Benzing and Renfrow (1974) |
| Aechmea dactylina | 1·57 | 0·42 (s.d., n = 5) | Panama, wet forest | Pierce et al. (2002) |
| Guzmania lingulata | 1·59 | n.a. | Jamaica | Benzing and Renfrow (1974) |
| Racinaea spiculosa | 0·75 | 0·08 (s.d., n = 3) | Panama, wet forest | Elias et al. (2008) |
| Tillandsia balbisiana | 0·83 | n.a. | Florida | Benzing and Renfrow (1974) |
| Tillandsia circinnata | 0·85 | (0·3–1·4; n = 2) | Florida | Benzing and Renfrow (1971, 1974) |
| Tillandsia compacta | 2·80 | n.a. | Colombia, upper montane forest | Caballero-Rueda et al. (1997) |
| Tillandsia pohliana | 1·60 | 0·9 (s.d.; n = 12) | Brazil, lowland forest | Pagano and Sartori (1980) |
| Tillandsia recurvata | 1·50 | 1·1 (s.d.; n = 12) | Brazil, lowland forest | Pagano and Sartori (1980) |
| Tillandsia usneoides | 0·48 | (0·26–0·67; n = 3) | Florida | Benzing and Renfrow (1974), Schlesinger and Marks (1977), Husk et al. (2004) |
| Vriesea altodaserrae | 0·62 | 0·29 (s.d., n = 3) | Brazil, restinga forest | Elias et al. (2008) |
| Vriesea atra | 0·63 | 0·10 (s.d., n = 3) | Brazil, restinga forest | Elias et al. (2008) |
| Vriesea carinata | 1·02 | 0·11 (s.d., n = 3) | Brazil, restinga forest | Elias et al. (2008) |
| Vriesea rodigasiana | 0·59 | 0·03 (s.d., n = 3) | Brazil, restinga forest | Elias et al. (2008) |
| Werauhia sanguinolenta | 0·95 | 0·16 (s.d.; n = 8) | Panama, moist lowland forest | Zotz and Richter (2006) |
| Werauhia capitata | 0·78 | 0·12 (s.d., n = 5) | Panama, wet forest | Pierce et al. (2002) |
Percentage K represents mean or single values. Variation is given as s.d. for n replications within a study, or as range when values are from several studies (n studies),
n.a. = not available.
This finding of the present study is consistent with the results of a greenhouse study with the tank-bromeliad Guzmania lingulata, in which potassium fertilization did not lead to higher final plant dry weight either (Lin and Yeh, 2008). The most likely explanation for these surprising results is an accumulation of K+ in the vacuoles beyond the immediate metabolic needs for this element in protein synthesis, enzyme reactions or stomatal regulations, during times when availability of K+ is high. Because the most prominent role of K+ is its osmotic function, where K+ can be (partly) replaced by other cations such as Mg2+, Na+ or Ca2+, internal transfer to growing organs during periods of insufficient supply may allow these bromeliads to keep up growth for a considerable amount of time. In turn, efficient uptake when K+ is highly available can be considered a type of luxury consumption (Rosecrance et al., 1998; Gierth and Mäser, 2008), similar to, for example, the situation in P, where storage forms (phytin) exist (Winkler and Zotz, 2009).
There is further evidence that the laboratory results are of ecological relevance. In seven species of tank bromeliads, collected in the Sao Paulo state forest, [K+] of old, mature and young leaves was not significantly different in all but one species (Elias et al., 2008). The distribution of leaf-potassium in most of these naturally grown bromeliads resembled the within-plant distribution in starved glasshouse cultures of V. splenriet and thereby probably reflects the low and intermittent nutrient supply in situ. In contrast to actively growing leaves, [K+] in old leaves of V. splenriet did not decrease after potassium starvation. As these leaves showed no sign of senescence, [K+] of approx. 4 mg K+ g−1 d. wt possibly reflects the minimum value necessary to sustain metabolic functions in this species. In the field-grown bromeliads analysed by Elias et al. (2008), [K+] varied from 0·7 to 3·9 g−1 mg d. wt in old leaves, possibly indicating species-specific minimal potassium demand. Different minimal concentrations and functional storage could explain much of the interspecific variation in [K+] in bromeliads. These values therefore are not suitable parameters to predict the potassium status of these plants. To determine a possible potassium limitation in naturally growing tank bromeliads, comparison of potassium contents between old and growing leaves seems to be a more appropriate method.
In conclusion, the results of the present experiments shed new light on the physiological basis that allows tank epiphytes to cope with the low and intermittent nutrient supply in situ. Characterization of the uptake kinetics of the major nutrient elements, nitrogen (Inselsbacher et al., 2007), phosphorus (Winkler and Zotz, 2009) and potassium (present study), clearly demonstrate that leaf trichomes are as efficient as fine roots in their function as uptake organs. Finally, the results should also be relevant for other plant groups that use similar strategies for nutrient acquisition via leaf glands, such as carnivorous plants (Ellison, 2006).
ACKNOWLEDGEMENTS
We thank the Republic of Panama for making its natural resources available for study (SEX/AP-1-09) and Corn. Bak B.V., Asseldelft, the Netherlands, for the supply of V. splenriet plants. We acknowledge the skilful help and excellent assistance of Peter Kanje and Brigitte Rieger in performing the experiments.
LITERATURE CITED
- Adlassnig W, Steinhauser, Peroutka M, et al. Uptake of potassium, iron and manganese by carnivorous pitcher plants. Applied Radiation and Isotopes. 2009;67:2117–2122. doi: 10.1016/j.apradiso.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Benzing DH. Availability of exogenously supplied nitrogen to seedlings of Bromeliaceae. Bulletin of the Torrey Botanical Club. 1970;97:154–159. [Google Scholar]
- Benzing DH. Vascular epiphytes. General biology and related biota. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- Benzing DH. Bromeliaceae. Profile of an adaptive radiation. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Benzing DH, Pridgeon AM. Foliar trichomes of Pleurothallidinae (Orchidaceae): functional significance. American Journal of Botany. 1983;70:173–180. [Google Scholar]
- Benzing DH, Renfrow A. Significance of the patterns of CO2 exchange to the ecology and phylogeny of the Tillandsioideae (Bromeliaceae) Bulletin of the Torrey Botanical Club. 1971;98:322–327. [Google Scholar]
- Benzing DH, Renfrow A. The mineral nutrition of Bromeliaceae. Botanical Gazette. 1974;135:281–288. [Google Scholar]
- Caballero-Rueda LM, Rodríguez N, Martin C. Dinámica de elementos en epífitos de un bosque altoandino de la Cordillera Oriental de Colombia. Caldasia. 1997;19:311–322. [Google Scholar]
- Clark KL, Nadkarni NM, Gholz HL, Schaefer DS. Atmospheric deposition and net canopy in a tropical montane forest. Journal of Tropical Ecology. 1998;14:27–45. [Google Scholar]
- Dahlmann L, Persson J, Palmquist K, Näsholm T. Organic and inorganic nitrogen uptake in lichens. Planta. 2004;129:459–467. doi: 10.1007/s00425-004-1247-0. [DOI] [PubMed] [Google Scholar]
- Dørup I, Clausen T. 86Rb is not a reliable tracer for potassium in skeletal muscle. Biochemical Journal. 1994;302:745–751. doi: 10.1042/bj3020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias C, Fernandes EAN, Franca EJ. Chemical changes in bromeliad leaves at vegetative stages. Journal of Radioanalytical Nuclear Chemistry. 2008;282:111–115. [Google Scholar]
- Ellison AM. Nutrient limitation and stoichiometry of carnivorous plants. Plant Biology. 2006;8:740–747. doi: 10.1055/s-2006-923956. [DOI] [PubMed] [Google Scholar]
- Epstein E. Mineral nutrition of plants: principles and perspectives. New York: John Wiley and Sons; 1972. [Google Scholar]
- Evans GC. The quantitative analysis of growth. Oxford: Blackwell Scientific Publications; 1972. [Google Scholar]
- Gierth M, Mäser P. Potassium transporters in plants–Involvement in K+ acquisition, redistribution and homeostasis. FEBS Letters. 2008;581:2348–2356. doi: 10.1016/j.febslet.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Husk GJ, Weishampel E, Schlesinger WH. Mineral dynamics in Spanish moss, Tillandsia usneoides L. (Bromeliaceae), from Central Florida, USA. Science of the Total Environment. 2004;321:165–172. doi: 10.1016/j.scitotenv.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Inselsbacher E, Cambui CA, Richter A, Stange CF, Mercier H, Wanek W. Microbial activities and foliar uptake of nitrogen in the epiphytic bromeliad Vriesea gigantea. New Phytologist. 2007;175:311–320. doi: 10.1111/j.1469-8137.2007.02098.x. [DOI] [PubMed] [Google Scholar]
- Jackson ML. Soil chemical analysis. Englewood Cliffs, NJ: Prentice-Hall; 1958. [Google Scholar]
- Läuchli A, Epstein E. Transport of potassium and rubidium in plant roots: the significance of calcium. Plant Physiology. 1970;45:639–641. doi: 10.1104/pp.45.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaudy A, Very A-A, Sentenac H. K+ channel activity in plants: genes, regulations and functions. FEBS Letters. 2008;581:2357–2366. doi: 10.1016/j.febslet.2007.03.058. [DOI] [PubMed] [Google Scholar]
- Lin C-Y, Yeh D-M. Potassium nutrition affects leaf growth, anatomy and macroelements of Guzmania. Hort Science. 2008;43:146–148. [Google Scholar]
- Maas EV, Leggett JE. Uptake of 86Rb and K by excised maize roots. Plant Physiology. 1968;43:2054–2056. doi: 10.1104/pp.43.12.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano SN, Sartori AA. Annual variation of nitrogen phosphorous and potassium in the leaves of 2 epiphytic Bromeliaceae. Revista Brasileira de Biología. 1980;40:25–30. [Google Scholar]
- Pierce S, Winter K, Griffiths H. The role of CAM in high rainfall cloud forests: an in situ comparison of photosynthetic pathways in Bromeliaceae. Plant, Cell and Environment. 2002;25:1181–1189. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2007. [Google Scholar]
- Richardson BA, Rogers C, Richardson MJ. Nutrients, diversity, and community structure of two phytotelm systems in a lower montane forest, Puerto Rico. Ecological Entomology. 2000;25:348–356. [Google Scholar]
- Rodriguez-Navarro A. Potassium transport in fungi and plants. Biochimica et Biophysica Acta. 1999;1469:1–30. doi: 10.1016/s0304-4157(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Rosecrance RC, Weinbaum SA, Brown PA. Alternate bearing affects nitrogen, phosphorus, potassium, and starch storage pools in mature pistachio trees. Annals of Botany. 1998;82:463–470. [Google Scholar]
- Schimper AFW. Die epiphytische Vegetation Amerikas. Jena: Gustav Fischer Verlag; 1888. [Google Scholar]
- Schlesinger WJ, Marks L. Mineral cycling and the niche of Spanish moss, Tillandsia usneoides L. American Journal of Botany. 1977;64:1254–1262. [Google Scholar]
- Szczerba MW, Britto DT, Kronzucker HJ. K+ transport in plants: physiology and molecular biology. Journal of Plant Physiology. 2009;166:447–466. doi: 10.1016/j.jplph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Winkler U, Zotz G. Highly efficient uptake of phosphorus in epiphytic bromeliads. Annals of Botany. 2009;103:477–484. doi: 10.1093/aob/mcn231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotz G. The resorption of phosphorus is greater than that of nitrogen in senescing leaves of vascular epiphytes from lowland Panama. Journal of Tropical Ecology. 2004;20:639–696. [Google Scholar]
- Zotz G, Richter A. Changes in carbohydrate and nutrient contents throughout a reproductive cycle indicate that phosphorus is a limiting nutrient in the epiphytic bromeliad, Werauhia sanguinolenta. Annals of Botany. 2006;97:745–754. doi: 10.1093/aob/mcl026. [DOI] [PMC free article] [PubMed] [Google Scholar]