Abstract
Purpose
To determine the safety of oropharyngeal administration of own mother’s colostrum to ELBW infants in first days of life. A secondary purpose was to investigate the feasibility of (1) delivering this intervention to ELBW infants in the first days of life, and (2) measuring concentrations of secretory immunoglobulin A (sIgA) and lactoferrin in tracheal aspirate secretions and urine of these infants.
Subjects
Five ELBW infants (mean BW and gestational age = 657 grams and 25.5 weeks, respectively).
Design
Quasi experimental, one group, pretest-posttest design.
Methods
Subjects received 0.2 mL of OMC administered oropharyngeally every two hours for 48 consecutive hours, beginning at 48 hours of life. Concentrations of sIgA and lactoferrin were measured in tracheal aspirates and urine of each subject at baseline, at the completion of the intervention and again 2 weeks later.
Results
All infants completed the entire treatment protocol, each receiving 24 treatments. A total of 15 urine specimens were collected and 14 were sufficient in volume for analysis. A total of 15 tracheal aspirates were collected, but only 7 specimens (47%) were sufficient in volume for analysis. There was wide variation in concentrations of sIgA and lactoferrin in urine and tracheal aspirates among the five infants; however several results were outside the limits of assay detection. All infants began to suck on the endotracheal tube during the administration of colostrum drops. Oxygen saturation measures remained stable or increased slightly during each of the treatment sessions. There were no episodes of apnea, bradycardia, hypotension or other adverse effects associated with the administration of colostrum.
Conclusions
Oropharyngeal administration of OMC is easy, inexpensive, and well-tolerated by even the smallest and sickest ELBW infants. Future research should continue to examine the optimal procedure for measuring the direct immune effects of this therapy, as well as the clinical outcomes such as infections, particularly ventilator-associated pneumonia (VAP).
Keywords: extremely low birth weight, human milk, oropharyngeal administration of colostrum, immunotherapy
Dramatic advances in neonatal medicine over recent decades have resulted in decreased mortality for extremely low birth weight (ELBW; BW <1000g) infants. Approximately 80% of infants born at 24 weeks of gestation will survive to discharge, compared to 50% just 15 years ago.1 However, the survival of these infants is associated with significant short and long-term morbidity, including nosocomial infection, 2, 3 necrotizing enterocolitis, 4 intraventricular hemorrhage, 4,5 periventricular leukomalacia 6 and adverse long-term neurodevelopmental sequelae including cognitive and motor disabilities, visual and/or hearing impairment, and cerebral palsy. 7–9
Nosocomial infection, an acquired infection after 48 hours of hospital admission, is particularly important because of its high prevalence and its association with other morbidities including poor growth, 10, 11 adverse long-term neurological sequelae, 10 increased length of hospital stay, 12 and a substantial cost 12 to families, hospitals, and society. The risk of acquiring a nosocomial infection is inversely related to birth weight and gestational age, and directly related to the severity of illness at birth.13 The ELBW infant is therefore at highest risk, with recent data showing that up to 65% of ELBW infants will have at least one infection during their neonatal intensive care unit (NICU) stay. 10
Nosocomial infection of the blood-stream (bacteremia) is especially common in ELBW infants, primarily as a result of long-term vascular access for parenteral nutrition. A single blood-stream infection extends the length of hospital stay by 7 days for ELBW infants, and costs an additional $5875 for infants weighing 401 to 750 grams and $12,480 for infant weighing 751 to 1000 grams.12 Using recent birth data, 14 and assuming a 30% rate of bacteremia 10, 15 per ELBW infant born in the United States, the estimated annual cost would be $56,400,000 to $119, 808,000 per year. However, many ELBW infants have several cases of bacteremia during their 3 to 4 month hospitalization, thereby increasing these baseline cost estimates.
The risks of acquiring a nosocomial infection are modifiable through the use of own mother’s milk feedings (OMM) for the ELBW infant in the NICU. Research has linked OMM feedings with a lower incidence of nosocomial infection 16–20 in very low birth weight (VLBW; BW < 1500g) infants compared to formula-fed infants. Although these studies have not focused specifically on the ELBW population, evidence from biochemical and immunological studies suggest that OMM feedings, especially colostrum, may provide the highest level of protection from nosocomial infection for the least mature ELBW infants. 21–25 Unfortunately, clinical instability typically precludes enteral feedings in the first days of life for ELBW infants and alternative methods for administering colostrum have not been investigated.
The purpose of this study was to determine the safety of oropharyngeal administration of own mother’s colostrum to ELBW infants in first days of life. A secondary purpose was to investigate the feasibility of (1) delivering this intervention to ELBW infants in the first days of life, and (2) measuring concentrations of sIgA and lactoferrin in tracheal aspirate secretions and urine of these infants.
Background
Immune Protection in Own Mother’s Colostrum
The comprehensive immune protection derived from OMM is attributed to a multitude of immunologically-derived factors that provide antimicrobial and anti-inflammatory protection against infection and modulate the recipient infant’s immune system. 26 Recent studies suggest an inverse relationship between the concentration of protective immune factors in colostrum and the duration of pregnancy. 21–25 Thus, many of these protective factors are more highly concentrated in the colostrum of women who deliver ELBW infants, which suggests that these factors have an important biological role in protecting the recipient infant during the first days of life and that the first days of life may be a critical exposure period for the ELBW infant.
Based on these findings, preterm colostrum may be especially protective during the first days of life when ELBW infants are the sickest and at highest risk for acquiring an infection. However, the underdeveloped gastrointestinal tract and the presence of prematurity-associated morbidities that compromise gastrointestinal perfusion typically preclude enteral feedings during this period. The prolonged inability to feed enterally can lead to intestinal atrophy 27 which increases the risk of localized inflammation, feeding intolerance, necrotizing enterocolitis (NEC) and nosocomial infections. Thus, there is a critical need to identify safe and efficacious alternatives for administering preterm colostrum as a potential “immune therapy” to ELBW infants in the first days of life when they cannot be fed enterally.
Oropharyngeal Administration of Colostrum
An alternative method of administering colostrum is by the oropharyngeal route. This involves placing small amounts of the colostrum directly onto the oral mucosa with the expectation that the colostrum or selected components are absorbed by the mucous membranes. 28 Additionally, cytokines present in colostrum (colostral cytokines) may interact with lymphoid cells within the recipient infant’s oropharyngeal associated mucosal tissues, 29, 30 while human milk oligosaccharides (HMOs) may provide local barrier protection, based on their ability to inhibit the adhesion of pathogens to epithelial cell surface receptors. 31, 32 The oropharyngeal route has been used to administer immunomodulating agents under other circumstances. For example, interferon-alpha (IFN-alpha), an immune cell-derived cytokine, has been administered safely and effectively to adult human subjects 33–37 using the oropharyngeal route.
A recent review 28 supports the concept that own mother’s colostrum, when administered oropharyngeally, may serve as a potential immune therapy for the ELBW infant. Based on this evidence, it is plausible that oropharyngeally administered colostrum may evoke systemic immunostimulatory effects and potentially protect against nosocomial infection, including pneumonia.
Most of the research pertaining to colostral cytokines has focused on their role within the gastrointestinal tract. However, during feeding at the breast, the exogenous cytokines in mother’s milk may stimulate the infant’s oropharyngeal-associated lymphoid tissue (OFALT) and gut-associated lymphoid tissue (GALT), in a synergistic manner, with a combined response potentially greater than that for a single site of stimulation. Separate work has suggested that the HMOs interfere with adhesion of bacteria in the oral cavity, 38 which translates into reduction in risk for otitis media and lower respiratory tract infections. The term, breastfed infant would be the recipient of this combined effect. However, for the ELBW infant in the NICU who receives enteral feedings, such as minimal volume feedings, via a nasogastric tube, colostral cytokines and HMOs would not have contact with OFALT or mucous membranes in the oral cavity. This deficit could be corrected with the oropharyngeal administration of colostrum, and as such, it would be considered a complement rather than a substitute for minimal volume (trophic) feedings.
Theoretically, oropharyngeal administration of colostrum would provide local barrier protection and change levels of immunologically-derived factors, such as secretory immunoglobulin A and lactoferrin, in bodily fluids. The potential influence of these factors on immune system integrity in ELBW infants is described below.
Immunologically-derived Factors
Secretory immunoglobulin A (sIgA) is an antibody that inhibits attachment of pathogens to the respiratory and intestinal mucosal epithelial barrier, 39 maintains mucosal intestinal integrity, and provides specific barrier protection against pathogens that cause respiratory and gastrointestinal infections. 24, 39–41 Interleukin- 6 (IL-6), cytokine in mother’s milk, stimulates the growth and differentiation of B lymphocytes into IgA-secreting plasma cells. 42 Theoretically, oropharyngeal administration of colostral IL-6 may stimulate sIgA production, possibly resulting in higher concentrations of sIgA in tracheal secretions and urine.
Lactoferrin is a glycosylated, iron-binding protein with potent bacteriocidal, bacteriostatic, antiviral, anti-inflammatory, and immunomodulatory properties. 25, 43–46 Like sIgA, lactoferrin is highly concentrated in colostrum, 45, 47 resistant to digestive enzymes, 44 and excreted at higher levels in urine and stool of mother’s milk-fed infants, as compared to those of formula-fed cohorts.44, 48, 49 The appearance of lactoferrin in the urine suggests that it is systemically absorbed and may influence the infant’s systemic response to infection. 50, 51 Lactoferrin may also protect against infection by inhibiting the attachment of pathogenic bacteria to cells lining the oropharyngeal mucosa, and if swallowed may provide similar barrier protection in the gastrointestinal tract. 52
In combination sIgA, lactoferrin, and HMOs provide barrier protection against respiratory pathogens that may penetrate the mucosa of the upper respiratory tract and cause ventilator-associated pneumonia (VAP) in ELBW infants. As the second most common nosocomial infection in the U.S., VAP is associated with a significantly prolonged length of stay, considerable costs, and high mortality rate for extremely premature infants. 53 Prevention of VAP in ELBW infants is therefore a clinical priority; however the majority of commercially available oral care products contain chemicals that may be unsafe for ELBW infants. Even those that contain antibacterial components naturally occurring in human milk have not been well-tested for safety and efficacy in the ELBW population. However, the use of own mother’s colostrum via the nasogastric route for ELBW infants is safe, inexpensive, readily available, and not associated with any adverse effects to the recipient infant. This study was designed to determine the safety and feasibility of administering colostrum via the oropharyngeal route.
Methods
Design
This pilot study used a quasi-experimental, one group, pretest- posttest design. The independent variable was the oropharyngeal administration of own mother’s colostrum. The dependent variables were sIgA and lactoferrin concentrations in the infant’s tracheal aspirates and urine before and after treatment with colostrum.
Subjects and Setting
Data were collected from infants cared for in a 44-bed tertiary NICU, who met the following inclusion criteria: BW<1000 grams; <28 week’s gestation; and appropriate for gestational age (AGA). Exclusion criteria were infants with congenital anomalies; gastrointestinal disorders; renal disorders; receipt of vasopressor medications > 10 mcg/kg/minute; maternal history of substance abuse; or positive maternal HIV status. The study was approved by the Institutional Review Board (IRB) of the institutions where data were collected and where the principal investigator was a doctoral student.
This pilot study employed a labor-intensive protocol and necessitated the antenatal recruitment of ELBW infants who would serve as subjects. Maternal informed consent was obtained prior to the infant’s birth. Seventeen mothers were approached and fifteen agreed to participate. However, of these 15 women, 10 (67%) sustained their pregnancies beyond 28 weeks and were no longer eligible to participate. The remaining five mothers served as subjects for this study.
Five ELBW infants participated in this study. The mean birth weight was 657 grams (Range; 585–725g). The mean gestational age was 25.5 weeks (Range; 24 3/7 to 28 0/7 weeks). Two infants were Caucasian, two were African American, and one was Middle-Eastern. There were three males and two females.
Measures
SIgA and lactoferrin were measured in the infant’s tracheal aspirates and urine, before and after the oropharyngeal administration of colostrum.
A higher concentration of these immune products in tracheal aspirate secretions would suggest enhanced local mucosal immune protection, whereas elevated concentrations in urine would be consistent with enhanced systemic protection against infection.
Commercially available enzyme linked immunoassay (ELISA) kits were used to measure sIgA (ALPCO Diagnostics; Windham, NH, U.S.A.) and lactoferrin (Calbiochem-EMD Biosciences Inc; San Diego, CA, U.S.A.) concentrations. Results were determined using a standard curve as the point of reference. Prior to analysis, the specimens were thawed, volumes measured; and they were then centrifuged at 1500 rpm for 30 minutes.
Procedures
Maternal informed consent for study participation was secured prior to the infant’s birth. A baseline tracheal aspirate specimen was obtained in the delivery room prior to the administration of surfactant.
Collection of colostrum
Within 24 hours after delivery, the principal investigator met with each mother enrolled in the study and explained the standardized protocol for milk removal utilizing a hospital-grade electric breast pump (Symphony, Medela, Inc. McHenry, IL, U.S.A) and demonstrated hand-expression of colostrum. Mothers were encouraged to pump using a double pump collection kit every two to three hours for a total of eight times per 24 hour period. Mothers received these instructions both verbally and in written form.
During this meeting, the principal investigator gave each mother a supply of six sterile, pre-labeled specimen cups. Mothers were instructed to manually express 1.0 mL of colostrum into each cup as soon as possible after delivery. Mothers were reminded to wash their hands prior to expressing milk, and especially before handling the small cups that would be utilized for colostrum storage. They were instructed to send all colostrum specimens to the NICU for immediate refrigeration.
Preparation of syringes
The syringes that would be used to oropharyngeally administer the colostrum were prepared by the principal investigator when at least 2.4 mL of own mother’s colostrum was available in the NICU. While a total of 4.8 mL of colostrum was necessary to provide colostrum drops to the infant for the entire 48-hour treatment period, 2.4 mL was sufficient to initiate the protocol and provide drops for the first 24 hours of the treatment period. Using sterile gloves and sterile technique, the principal investigator filled each of 12 sterile, needle-less, tuberculin syringes with 0.2 mL of own mother’s colostrum. The syringes were labeled with the infant’s pre-printed hospital label, which included the medical record number and patient name, and with the date and time of sample preparation. The labeled syringes were placed in a pre-labeled plastic container and refrigerated in the infant’s room. An additional 12 syringes, for the second 24 hours of the treatment protocol, were prepared by the principal investigator in the same manner when another 2.4 mL of own mother’s colostrum were available.
Delivery of intervention during treatment protocol
Once the first 12 syringes were prepared, the infant’s nurse obtained a baseline urine specimen. Then two nurses checked the infant’s name against the label on the pre-filled syringe. The syringe was placed in warm water for five minutes to bring the colostrum to room temperature. The infant’s nurse then followed a standardized “oropharyngeal administration of colostrum” protocol to administer the drops as depicted in Figure 1.
Figure 1.
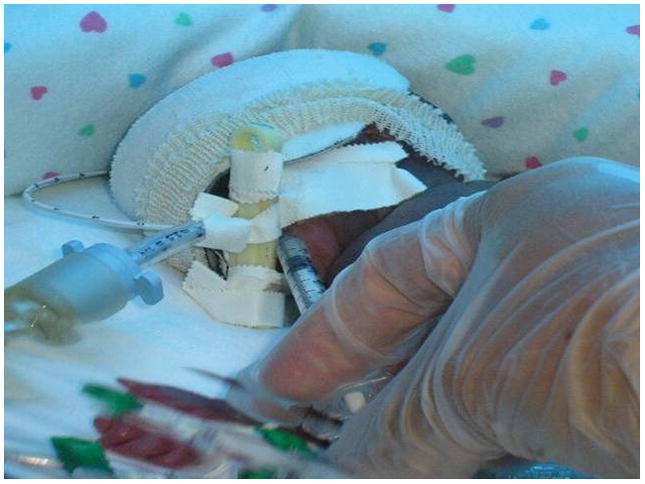
During administration, the nurse placed the syringe tip into the infant’s mouth, along the right buccal mucosal tissue (mucous membrane that lines the inside of the cheek), and directed it posteriorly towards the oropharynx (area of the throat that is at the back of the mouth). The nurse administered 0.1mL (approximately seven drops) of the colostrum over a period of at least two minutes. Without removing the syringe from within the infant’s mouth, the nurse then carefully re-directed the syringe to the left buccal mucosal tissue, with the tip directed posteriorly towards the oropharynx. The nurse then slowly administered an additional 0.1 mL of the colostrum over a period of at least two minutes. Thus, a total of 0.2 mL, or approximately 14 drops, were administered per treatment session. This procedure was carried out every two hours over a period of 48 hours, beginning within 48 hours of life.
Throughout the treatment protocol, the infant’s heart rate, respiratory rate, oxygen saturation, and blood pressure were monitored. The nurse was instructed to immediately stop the procedure if the infant demonstrated signs of agitation, had a significant desaturation episode with oxygen saturation less than 88%, or experienced a change in vital signs. Upon completion of the treatment protocol, the nurse recorded the date and time of colostrum administration on the intake flow sheet in the infant’s electronic medical record. Tracheal aspirate and urine specimens were collected within 6 hours of completion of the treatment protocol and again two weeks later. All specimens were centrifuged, and immediately frozen at −80°C until they were transported to a collaborating laboratory (University of Tennessee) for subsequent biochemical analysis.
Results
All infants in this study completed the entire treatment protocol, each receiving a total of 24 treatments during a period of 48 consecutive hours. The first treatment was given within 48 hours of life. Urine and tracheal aspirates were collected from each infant prior to, and at the completion of, the treatment protocol and again 2 weeks later. A total of 15 urine specimens were collected for the 5 infants, however 1 specimen (.07%) was insufficient in volume for analysis. A total of 15 tracheal aspirates were also collected, however 8 specimens (53%) were insufficient in volume for analysis.
For all infants in this study, oxygen saturation measures remained stable or increased slightly during each of the treatment sessions. There were no episodes of apnea, bradycardia, hypotension or other adverse effects. All infants began to suck on the endotracheal tube during the administration of drops.
There was wide variation in concentrations of sIgA and lactoferrin in urine and tracheal aspirates among the five infants. The ranges of sIgA and lactoferrin concentrations for urine and tracheal aspirates are described in Table 1. Data from some samples could not be obtained because results fell above or below the limits of assay detection, based on sample dilution.
Table 1.
| Sample Source | Usable ‘n’ | median | minimum | maximum |
|---|---|---|---|---|
| sIgA Urine ng/mL | 14 | 83.8 | 0.8 | 167 |
| sIgA Tracheal Aspirate ng/mL | 7 | 91,470 | 5.8 | 183,000 |
| Lactoferrin Urine ng/mL | 14 | 278.6 | 1.34 | 556 |
| Lactoferrin Tracheal Aspirate mcg/mL | 7 | 90.0 | 16 | 164 |
Discussion
This study is the first to investigate the safety and feasibility of oropharyngeal administration of own mother’s colostrum drops to ELBW infants in a clinical setting. Using mother’s colostrum in this manner requires a change in thinking, to view colostrum as a potential immune-therapy and not simply as a feeding. As such, the oropharyngeal administration of colostrum can be an alternative to nil per os (NPO) status and/or a complement to trophic feeds in the first days of life for the ELBW infants.
Mothers were willing to participate in this study; 88% of the women who were approached agreed to participate in this study. In fact, many of the mothers wanted their babies to participate and receive colostrum drops because they felt it would be beneficial. They realized that, as per standard care in the NICU, an ELBW infant would not “taste” mother’s milk until at least 32 weeks corrected gestational age when per oral feeds are introduced. Mothers in this study knew their infants would taste their colostrum as early as 48 hours after birth, as opposed to 8 weeks later. This protocol was also well-received by the nurses and physicians at the research site.
The pilot study demonstrated that this protocol for administering colostrum oropharyngeally was well-tolerated by even the smallest and sickest ELBW infants. Oxygen saturations remained stable and no adverse events occurred. An observation of interest during the oropharyngeal administration of colostrum was that infants appeared to “taste” the colostrum, as noted by sucking on the breathing tube. This observation may have developmental implications. Mothers reacted positively to their infant’s responses when “tasting” the colostrum, an observation that may have psychological benefit for these women.
The data from this study were limited for the following reasons. First, over 50% of the tracheal aspirate samples were insufficient in quantity for analysis. This raises the question of the feasibility of using tracheal aspirates in future studies. Another limitation of this study was the lack of reference values for immune markers in ELBW infants in the first days of life, because the only existing reference values are for larger preterm or term neonates. Therefore, without a reference range for sIgA and lactoferrin, it was difficult to determine how samples should be diluted to allow data to fall within a quantifiable range. Thus, data from some specimens fell above or below the limits of the assay. These issues with sampling and measurement informed the subsequent study.
In summary, results from this pilot study showed that oropharyngeal administration of own mother’s colostrum is easy, inexpensive, and well-tolerated by even the smallest and sickest ELBW infants. Despite limitations, including a small sample and missing data, the information from this study, particularly in terms of subject recruitment and sample collection and analysis, provided guidance for future studies. Future research should investigate normal range references for these immune markers in ELBW infants, and address the immune effects and the developmental implications of ELBW infants who receive this intervention. Of particular interest is whether this easy and inexpensive intervention may provide protection against VAP for ELBW infants in the NICU.
Current evidence suggests that many of the 130 known HMOs inhibit the adherence of bacteria to mucosal surfaces. 54 They may be especially important in protecting the oropharyngeal area and this may be a mechanism for protection from pneumonia in ventilator-dependent infants, when own mother’s colostrum is administered oropharyngeally. Further research in this area is warranted.
Acknowledgments
The authors thank Dr. Velio Bocci (Emeritus Professor of Physiology, University of Sienna, Italy) for his invaluable assistance in this research. This study was supported by the National Institute of Health (Funded by NIH (F31NR007584 and NR010009) and Medela, Inc. (McHenry, IL, USA).
Funded by NIH (F31NR007584 and NR010009) and Medela, Inc. (McHenry, IL, USA)
References
- 1.Hoekstra RE, Ferrera TB, Couser RJ, Payne NR, Connett JE. Survival and long-term neurodevelopmental outcome of extremely premature infants born at 23–26 weeks gestation age at a tertiary center. Pediatrics. 2004;(113):el–6. doi: 10.1542/peds.113.1.e1. [DOI] [PubMed] [Google Scholar]
- 2.Mcguire W, Clerihew L, Fowlie PW. Infection in the preterm infant. British Medical Journal. 2004;329(7477):1277–1280. doi: 10.1136/bmj.329.7477.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strodbeck F. The role of early enteral nutrition in protecting premature infants from sepsis. Critical Care Nursing Clinics of North America. 2003;15 (1):79–87. doi: 10.1016/s0899-5885(02)00043-6. [DOI] [PubMed] [Google Scholar]
- 4.Fanaroff AA, Hack M, Walsh MC. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Seminars in Perinatology. 2003;27 (4):281–7. doi: 10.1016/s0146-0005(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 5.Tommiska V, Heinonen K, Lehtonen L, Renlund M, Saarela T, Tammela O, et al. No improvement in outcome of nationwide extremely low birth weight infant populations between 1996–1997 and 1999–2000. Pediatrics. 2007;119 (1):29–36. doi: 10.1542/peds.2006-1472. [DOI] [PubMed] [Google Scholar]
- 6.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115 (4):997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 7.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. The New England Journal of Medicine. 2005;352 (1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 8.Vohr B, Marilee A. Extreme prematurity-The continuing dilemma. The New England Journal of Medicine. 2005;352 (1):71–72. doi: 10.1056/NEJMe048323. [DOI] [PubMed] [Google Scholar]
- 9.Vohr B, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 10.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low birth weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 11.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: growth and associated problems in children born at 25 weeks of gestation or less. Archives of Disease in Childhood Fetal & Neonatal Edition. 2003;88 (6):F492–500. doi: 10.1136/fn.88.6.F492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics. 2004;114 (2 part 1):348–355. doi: 10.1542/peds.114.2.348. [DOI] [PubMed] [Google Scholar]
- 13.Mcguire W, Clerihew L, Fowlie PW. Infection in the preterm infant. British Medical Journal. 2004;329 (7477):1277–1280. doi: 10.1136/bmj.329.7477.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary Data for 2006. National Vital Statistics Reports. 2007;56(7) [PubMed] [Google Scholar]
- 15.Fanaroff AA, Korones SB, Wright LL, Verter J, Poland RL, Bauer CR, et al. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. The Pediatric Infectious Disease Journal. 1998;17 (7):593–598. doi: 10.1097/00006454-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Furman L, Taylor G, Minich N, Hack M. The effect of maternal milk on neonatal morbidity of very-low-birth-weight infants. Arch Pediatr Adolesc Med. 2003;157:66–71. doi: 10.1001/archpedi.157.1.66. [DOI] [PubMed] [Google Scholar]
- 17.Hylander MA, Strobino DM, Dhanireddy R. Human milk feedings and infection among very low birth weight infants. Pediatrics. 1998;102:E38. doi: 10.1542/peds.102.3.e38. [DOI] [PubMed] [Google Scholar]
- 18.Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infant’s risk of necrotizing enterocolitis or death. Journal of Perinatology. 2009;29 (1):57–62. doi: 10.1038/jp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schanler RJ, Schulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics. 1999;103:1150–6. doi: 10.1542/peds.103.6.1150. [DOI] [PubMed] [Google Scholar]
- 20.Uraizee F, Gross SJ. Improved feeding tolerance and reduced incidence of sepsis in sick very low birth weight (VLBW) infants fed maternal milk. Pediatric Research. 1989;25:298A. [Google Scholar]
- 21.Araujo ED, Goncalves AK, Cornetta M, Cunha H, Cardoso ML, Morais SS, et al. Evaluation of the secretory immunologlobulin A levels in the colostrum and milk of mothers of term and preterm infants. Braz J Infect Dis. 2005;9:357–62. doi: 10.1590/S1413-86702005000500002. [DOI] [PubMed] [Google Scholar]
- 22.Dvorak B, Fituch CC, Williams CS, Hurst NM, Schanler RJ. Increased epidermal growth factor levels in human milk of mothers with extremely premature infants. Pediatr Res. 2003;54:15–19. doi: 10.1203/01.PDR.0000065729.74325.71. [DOI] [PubMed] [Google Scholar]
- 23.Koenig A, de Albuquerque Diniz EM, Barbosa SF, Vaz FA. Immunologic factors in human milk: the effects of gestational age and pasteurization. J Hum Lact. 2005;21:439–43. doi: 10.1177/0890334405280652. [DOI] [PubMed] [Google Scholar]
- 24.Montagne P, Cuilliere ML, Mole C, Bene MC, Faure G. Immunological and nutritional composition of human milk in relation to prematurity and mothers’ parity during the first 2 weeks of lactation. J Pediatr Gastroenterol Nutr. 1999;29:75–80. doi: 10.1097/00005176-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Ronayne de Ferrer PA, Baroni A, Sambucetti ME, Lopez NE, Cernadas JMC. Lactoferrin levels in term and preterm milk. J Am Coll Nutr. 2000;19:370–373. doi: 10.1080/07315724.2000.10718933. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez NA, Miracle DJ, Meier PP. Sharing the science on human milk feedings with mothers of very low birth weight infants. JOGNN. 2005;34:109–119. doi: 10.1177/0884217504272807. [DOI] [PubMed] [Google Scholar]
- 27.LaGamma EF, Brown LE. Feeding practices for infants weighing less than 1500 g at birth and the pathogenesis of necrotizing enterocolitis. Clin Perinatol. 1994;21 (2):271–306. [PubMed] [Google Scholar]
- 28.Rodriguez NA, Meier PP, Groer MW, Zeller JM. Oropharyngeal administration of colostrum to extremely low birth weight infants: theoretical perspectives. Journal of Perinatology. 2009;29:1–7. doi: 10.1038/jp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bocci V. Absorption of cytokines via oropharyngeal-associated lymphoid tissue. Does an unorthodox route improve the therapeutic index of interferon? Clinical Pharmacokinetics. 1991;21 (6):411–417. doi: 10.2165/00003088-199121060-00002. [DOI] [PubMed] [Google Scholar]
- 30.Bocci V, von Bremen K, Corradeschi F, Luzzi E, Paulesu L. What is the role of cytokines in human colostrum? Journal of Biological Regulators & Homeostatic Agents. 1991;5:121–4. [PubMed] [Google Scholar]
- 31.Boehm G, Stahl B. Oligosaccharides from milk. Journal of Nutrition. 2007;137(3):847S–9S. doi: 10.1093/jn/137.3.847S. [DOI] [PubMed] [Google Scholar]
- 32.Coppa GV, Zampini L, Galeazzi T, Facinelli B, Ferrante L, Capretti R, Orazio Human milk oligosaccharides inhibit the adhesion of caco-2 cells of diarrheal pathogens: escherichia coli, vibrio cholerae and salmonella fyris. Pediatric Research. 2006;59 (3):377–382. doi: 10.1203/01.pdr.0000200805.45593.17. [DOI] [PubMed] [Google Scholar]
- 33.Hutchinson V, Cummins JM. Low-dose oral interferon in patients with AIDs. Lancet. 1987;2:1530–31. doi: 10.1016/s0140-6736(87)92671-7. [DOI] [PubMed] [Google Scholar]
- 34.Koech DK, Obel AO, Minowada J, Hutchinson VA, Cummins JM. Low dose oral alpha-interferon therapy for patients seropositive for human immunodeficiency virus type-1 (HIV-1) Mol Biother. 1990;2:91–95. [PubMed] [Google Scholar]
- 35.Caban J, Mossor-Ostrowska J, Zyrkowska-Bieda T, Zejc M, Janas-Skulina U, Ciesla A, et al. Treatment of chronic viral hepatitis B with oral mucosal administration of natural human interferon alpha lozenges. Arch Immunol Ther Exp. 1993;41:229–35. [PubMed] [Google Scholar]
- 36.Zielinska W, Paszkiewicz J, Korczak A, Wlasiuk M, Zoltowska A, Szutowicz A, et al. Treatment of fourteen chronic active HBsAg+, HBeAg+ hepatitis patients with low dose natural human interferon alpha administered orally. Arch Immunol Ther Exp. 1993;41:241–51. [PubMed] [Google Scholar]
- 37.Bocci V. The oropharyngeal delivery of interferons: where are we and where do we need to go? J Interferon Cytokine Res. 1999;19:859–61. doi: 10.1089/107999099313361. [DOI] [PubMed] [Google Scholar]
- 38.Andersson B, Porras O, Hanson LA, Lagergard T, Svanborg-Eden C. Inhibition of attachment of streptococcus pneumoniae and haemophilus influenzae by human milk and receptor oligosaccharides. The Journal of Infectious Diseases. 1986;153 (2):232–237. doi: 10.1093/infdis/153.2.232. [DOI] [PubMed] [Google Scholar]
- 39.Brandtzaeg P. The secretory immunoglobulin system: Regulation and biological significance: Focusing on human mammary glands. In: Davis MK, editor. Integrating Population Outcomes, Biological Mechanisms and Research Methods in the Study of Human Milk and Lactation. New York: Plenum Press; 2002. pp. 1–16. [DOI] [PubMed] [Google Scholar]
- 40.Mathur B, Dwarkadas AM, Sharma VK, Saha K, Jain K. Anti-infective factors in preterm colostrum. Acta Paediatr Scand. 1990;79:1039–44. doi: 10.1111/j.1651-2227.1990.tb11380.x. [DOI] [PubMed] [Google Scholar]
- 41.Schanler RJ, Atkinson SA. Effects of nutrients in human milk on the recipient premature infant. Journal of Mammary Gland Biology & Neoplasia. 1999;4(3):297–307. doi: 10.1023/a:1018754014330. [DOI] [PubMed] [Google Scholar]
- 42.Garofolo RP, Goldman AS. Cytokines, chemokines and colony- stimulating factors in human milk: The 1997 update. Biology of the Neonate. 1998;74:134–142. doi: 10.1159/000014019. [DOI] [PubMed] [Google Scholar]
- 43.Chheda S, Keeney SE, Goldman AS. Immunology of Human Milk and Host Immunity. In: Polin RA, Fox WW, editors. Fetal and Neonatal Physiology. Philadelphia, PA: W.B. Saunders; 1998. pp. 2022–2032. [Google Scholar]
- 44.Hamosh M. Protective function of protein and lipids in human milk. Biology of the Neonate. 1998;74:163–176. doi: 10.1159/000014021. [DOI] [PubMed] [Google Scholar]
- 45.Montagne P, Cuilliere ML, Mole C, Bene MD, Faure G. Changes in lactoferrin and lysozyme levels in human milk during the first twelve weeks of lactation. Advances in Experimental Medicine and Biology. 2001;501:241–247. doi: 10.1007/978-1-4615-1371-1_30. [DOI] [PubMed] [Google Scholar]
- 46.Xanthou M. Immune protection of human milk. Biology of the Neonate. 1998;74:121–133. doi: 10.1159/000014018. [DOI] [PubMed] [Google Scholar]
- 47.Neville MC. Anatomy and Physiology of Lactation. Breastfeeding 2001: The Evidence. In: Schanler RJ, editor. Pediatric Clinics of North America. 1. Vol. 48. 2001. pp. 13–34. [DOI] [PubMed] [Google Scholar]
- 48.Goldblum RM, Schanler RJ, Garza C, Goldman AS. Human milk feedings enhances the urinary excretion of immunologic factors in low birth weight infants. Pediatric Research. 1989;25:184–188. doi: 10.1203/00006450-198902000-00021. [DOI] [PubMed] [Google Scholar]
- 49.Schanler RJ, Goldblum RM, Garza C, Goldman AS. Enhanced fecal excretion of selected immune factors in very low birth weight infants fed fortified human milk. Pediatric Research. 1986;20:711–715. doi: 10.1203/00006450-198608000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Hutchens TW, Henry JF, Yip T. Origin of intact lactoferrin and its DNA-binding fragments found in the urine of human milk-fed preterm Infants: Evaluation by stable isotope enrichment. Pediatric Research. 1991;29:243–250. doi: 10.1203/00006450-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Schanler R. The use of human milk for premature infants. Pediatric Clinics of North America. 2001;48 (1):207–219. doi: 10.1016/s0031-3955(05)70295-9. [DOI] [PubMed] [Google Scholar]
- 52.Edde L, Hipolito RB, Hwang FF, Headon DR, Shalwitz RA, Sherman MP. Lactoferrin protects neonatal rats from gut-related systemic infection. American Journal of Physiology Gastrointestinal and Physiology. 2001;281:G1140–50. doi: 10.1152/ajpgi.2001.281.5.G1140. [DOI] [PubMed] [Google Scholar]
- 53.Apisarnthanarak A, Holzmann-Pazgal G, Hamvas A, Olsen MA, Fraser VJ. Ventilator-associated pneumonia in extremely preterm neonates in a neonatal intensive care unit: Characteristics, risk factors and outcomes. Pediatrics. 2003;112 (6):1283–1289. doi: 10.1542/peds.112.6.1283. [DOI] [PubMed] [Google Scholar]
- 54.Brand Miller JC, McVeagh P. Human milk oligosaccharides: 130 reasons to breast-feed. British Journal of Nutrition. 1999;82:333–335. doi: 10.1017/s0007114599001567. [DOI] [PubMed] [Google Scholar]


