Abstract
Diabetic neuropathy is a major complication of diabetes that affects the sensory and autonomic nervous systems and leads to significant morbidity and impact on quality of life of patients. Mitochondrial stress has been proposed as a major mediator of neurodegeneration in diabetes. This review briefly summarizes the nature of sensory and autonomic nerve dysfunction and presents these findings in the context of diabetes-induced nerve degeneration mediated by alterations in mitochondrial ultrastructure, physiology and trafficking. Diabetes-induced dysfunction in calcium homeostasis is discussed at length and causative associations with sub-optimal mitochondrial physiology are developed. It is clear that across a range of complications of diabetes that mitochondrial physiology is impaired, in general a reduction in electron transport chain capability is apparent. This abnormal activity may predispose mitochondria to generate elevated reactive oxygen species (ROS), although experimental proof remains lacking, but more importantly will deleteriously alter the bioenergetic status of neurons. It is proposed that the next five years of research should focus on identifying changes in mitochondrial phenotype and associated cellular impact, identifying sources of ROS in neurons and analyzing mitochondrial trafficking under diabetic conditions.
Keywords: calcium, dorsal root ganglia, electron transport chain, mitochondrial trafficking, reactive oxygen species, respiration, sensory polyneuropathy, sympathetic neuropathy
Clinical impact of diabetic neuropathy
The World Health Organization (WHO) predicts that by 2025 there will be 300 million people with diabetes. In North America 19 million people currently have diabetes that has an incidence of 6% and rising. Of those, about 90–95% have non-insulin-dependent diabetes mellitus (type 2 diabetes) and 5–10% have insulin-dependent diabetes mellitus (type 1 diabetes). In the USA approximately $25 billion per annum of health service costs is spent on treatment of diabetic complications that include retinopathy, nephropathy, heart disease and neuropathy. In 1998 approximately $15 billion of heath service expenditure was associated with the neurological complications (sensory and autonomic neuropathy and blindness). Incidence of sensory and autonomic neuropathy in diabetic patients can be as high as 50% and leads to incapacitating pain, digestive abnormalities, erectile dysfunction, heart arrhythmia, sensory loss, foot ulceration (up to 2 million Americans have this complaint), infection, gangrene and poor wound healing. The end result is often lower extremity amputation that accounts for approximately 80,000 cases each year in the USA and in rare cases sudden death, presumably on a cardiac basis. There is no effective therapy, only palliative treatment is available at the present time. These alarming figures are predicted to rise by approximately 5-fold over the next 10 years due to the epidemic in obesity and the associated increase in incidence and earlier time of onset of type 2 diabetes (from American Diabetes Association web site: http://www.diabetes.org).
Pathophysiological features of diabetic neuropathy
Diabetic neuropathy in type 1 and 2 diabetes in humans is comprised of symmetrical sensory polyneuropathy, autonomic neuropathy and a variety of rare forms. Sensory neuropathy is associated with reduction of motor and sensory nerve conduction velocity and structural changes in peripheral nerve including endoneurial microangiopathy, abnormal Schwann cell pathology, axonal degeneration, paranodal demyelination and loss of myelinated and unmyelinated fibers - the latter due to a dying-back of distal axons that presents clinically as reduced epidermal nerve fiber density [1–4]. Diabetic patients do not typically present with devastating autonomic failure involving the sympathetic and parasympathetic nervous systems, although the incidence of autonomic nervous system dysfunction is likely to reflect the diligence and sophisticated techniques with which it is sought. The morbidity contributed by complaints involving cardiovascular, genitourinary, sudomotor and alimentary symptoms is significant to individual patients lives, may result in subclinical disease diminishing the safety factor of autonomic function and, in a poorly understood manner, result in increased mortality [5]. The appreciation of the actual burden of autonomic neuropathy in diabetic patients has lagged well behind the characterization of somatic sensory neuropathy.
Neurodegeneration is most profound in the longest axons of neurons, and defective axon regeneration impedes tissue re-innervation [6]. There is no molecular signature or sign of apoptosis-dependent loss of sensory or sympathetic neuron perikarya in diabetic humans or animals [7–11] although loss of small neurons does occur in long-term animal models of diabetes [9,12]. The distal dying-back and formation of axonal dystrophy (with swellings) of axons are critical pathological features [6,13–17] and mimic axonal pruning and degeneration observed in the CNS and PNS in other pathological states [18].
Oxidative stress contributes to diabetic neuropathy
It is generally believed that oxidative stress is the key pathological process inducing nerve damage in diabetes [19,20]. Oxidative stress, possibly triggered by vascular abnormalities and associated microangiopathy in the nerve [1,21,22], is a key pathological process inducing nerve damage in diabetes in humans and experimental models [19,20,22]. Diabetes-induced oxidative stress in animal models of in type 1, type 2 and pre-diabetes in sensory neurons and peripheral nerve is demonstrated by increased production of reactive oxygen species (ROS) [23–27], lipid peroxidation [28–30] and protein nitrosylation [29–32], and diminished levels of reduced glutathione [28,33] and ascorbate [28]. Treatment with anti-oxidants such as α-lipoic acid, γ-linolenic acid and aldose reductase inhibitors prevent many indices of neuropathy in STZ-diabetic rats [19,20,25,27]. The neurons and Schwann cells do initiate protective mechanisms involving up-regulation of antioxidant pathways [34–36], however, the neurodegenerative outcome is energy failure in the nerve, observed as a decrease in high energy intermediates (e.g. phosphocreatine) [19,20], impaired axonal transport of proteins [37] and sub-optimal ion pumping [38–41].
Mitochondrial dysfunction and oxidative stress in diabetes
In cultured endothelial cells it has been proposed that high [glucose] drives excessive electron donation to the electron transport chain in mitochondria resulting in mitochondrial hyperpolarization and elevated production of ROS [23]. Brownlee et al. have proposed that this mitochondrial-dependent process is a central mediator of oxidative stress in complications of diabetes [23,42]. The theory suggests that high [glucose] in tissue targets for diabetic complications leads to increased supply of NADH in the mitochondria, and that this increased electron availability and/or saturation may cause partial reduction of oxygen to superoxide radicals in the proximal part of the electron transport chain [23,42]. Subsequent large elevations in ROS then induce degeneration of tissue. Studies in cultured embryonic sensory neurons have shown that high [glucose] induces toxicity through an apoptotic route involving a mitochondrial-dependent pathway [19]. While this work reveals some possible novel pathways for therapy, the interpretation of the results is difficult since in human diabetes and in animal models there is no evidence of apoptosis of neurons [8–10,43–45]. It is the case, however, that high [glucose] can induce neurotoxicity. For example, in STZ-diabetic rats the stress-activated kinase, p38, is enhanced in sensory neurons in lumbar dorsal root ganglia (DRG) and linked to nerve dysfunction but the apoptotic pathway is not triggered [7,46,47].
Studies on adult sensory neurons from normal and STZ-diabetic rats indicate that adult neurons have different responses to high [glucose] compared with endothelial cells and embryonic neurons
Our work and studies by Wiley et al. shows that in adult sensory neurons from STZ-diabetic rats the mitochondrial inner membrane potential is depolarized and not hyperpolarized as observed in endothelial cells exposed to high [glucose] [48–50]. Our work showed that mitochondrial depolarization in STZ-diabetes could be prevented by systemic treatment with low dose insulin, that did not impact on hyperglycemia, or neurotrophin-3 (NT-3) thus further questioning a central role for high [glucose] in mitochondrial dysfunction in diabetes [48,49]. Furthermore, insulin and other neurotrophic growth factors could directly modulate mitochondrial polarization through a phosphoinositide 3-kinase dependent pathway [51]. Adult sensory neurons cultured from normal rats and treated with high [glucose] did not develop any indices of oxidative stress (e.g. elevated caspase-3 activation or enhanced ROS) [35]. Furthermore, normal or diabetic neurons exposed to high [glucose], in fact as high as 50 mM, do not undergo apoptosis or any form of cell death [35,48,52]. In addition, embryonic neurons permitted to mature or adult mouse ganglia in vitro do not undergo apoptosis when exposed to high [glucose] [53,54]. Clearly, the phenotype of matured or adult sensory neurons and subsequent response of these cells to the diabetic state differs from that of endothelial cells and embryonic neurons where, for example, high [glucose] can induce apoptosis.
Impaired Ca2+ homeostasis and mitochondrial dysfunction
We and others have proposed that mitochondrial dysfunction in sensory neurons in diabetes could be associated with impaired Ca2+ homeostasis [38–40,48,55–61] and recently reviewed by Verkhratsky and Fernyhough [41]. This includes increased steady-state intracellular Ca2+ concentration ([Ca2+]i), increased frequency of high threshold Ca2+ currents and decreased depolarization-induced Ca2+ signals. Importantly, diabetes leads to a significant decrease of caffeine-induced Ca2+ release from intracellular stores [39,40,57,62], suggesting altered endoplasmic reticulum (ER) Ca2+ ([Ca2+]ER) homeostasis. In fact, we have shown that impaired Ca2+ ion homeostasis is most profound in lumbar DRG neurons, which have the longest axons [39] and are initially targeted in the human condition [2,3]. Sensory neurons of STZ-diabetic rats simultaneously develop depolarization of the mitochondrial inner membrane. Importantly, a rise in [Ca2+]i of 200 nM (which is seen in diabetes) can trigger elevated intramitochondrial Ca2+ concentration ([Ca2+]m) by entry of Ca2+ through the Ca2+ uniporter and other routes [63–67], which causes partial or complete inner mitochondrial membrane depolarization [68]. Plasma membrane depolarization-induced Ca2+ transients are prolonged in diabetic neurons and blockade of mitochondrial uptake of Ca2+ using the mitochondrial uncoupler, carbonyl cyanide m-chlorophenylhydrazone (CCCP), prevented these abnormalities [60]. This implies that mitochondrial buffering of Ca2+ plays a role in shaping Ca2+ transients in diabetic neurons. Finally, increased [Ca2+]m can stimulate ROS levels leading to oxidative stress [65,69–72].
Mitochondrial bioenergetics and ROS generation; role of [Ca2+]m
Extensive studies in a range of cell types have shown that a rise in [Ca2+]m can elevate the rate of NADH production by enzymes of the Krebs cycle including pyruvate dehydrogenase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase [73]. Further studies have shown that Ca2+ modulates the function of the F1F0 ATP synthase [74]. Furthermore, a rise in [Ca2+]m has been suggested to enhance electron transport and cytosolic Ca2+ to activate the adenine nucleotide translocase (ANT) [75]. The literature suggests that ATP production can be augmented quickly with a modest increase in [Ca2+]m. Rapid measurements of [Ca2+]m and NADH oxidation rate showed that Ca2+ uptake was rapid (less than 100 ms). The issue of how Ca2+ modulates mitochondrial ROS generation is at the heart of understanding how Ca2+ can be both a physiological and a pathological effector of mitochondrial function. Because the reduced ubiquinone (QH·) intermediate in the Q cycle is a significant source of ROS, two related parameters that can regulate ROS generation are 1) the effective concentration of QH·, which is increased when the distal respiratory chain is inhibited, and 2) the frequency of QH· occurrence, which is increased when the rate of electron transport is enhanced. Thus both stimulation and inhibition of mitochondria can result in enhanced ROS generation [65,72]. Stimulation of the tricarboxylic acid (TCA) cycle and oxidative phosphorylation by Ca2+ would enhance ROS output by making the mitochondrion work faster and consume more O2. Indeed, mitochondrial ROS generation correlates well with metabolic rate [65,76], suggesting that a faster metabolism simply results in more respiratory chain electron leakage (approximately 1–2% of transported electrons leak to generate ROS) [77,78]. It should be noted, however, that at this juncture there are no published studies demonstrating that abnormal Ca2+ buffering within the mitochondria acts as a source of ROS under diabetic conditions in adult neurons.
Impaired mitochondrial oxidative phosphorylation in diabetic complications
Studies on mitochondrial physiology in diabetic neuropathy lag behind those in other diabetic complications, such as cardiomyopathy and nephropathy. Table 1 summarizes findings in an array of animal models, and in humans, in type 1 and 2 diabetes with respect to analysis of functioning of the electron transport chain (ETC). In general, mitochondrial-based electron transport functions are diminished. The diabetes-induced factors causing reduced rates of ETC complex activities remain poorly understood, however, investigators have begun to use proteomic and gene array techniques to identify alterations in gene expression that presumably underpin such changes in mitochondrial physiology (for example, see Bugger et al (2009) [79]). Our preliminary results on mitochondrial function in DRG of age matched versus 22wk STZ-diabetic rats are shown in Table 2 and 3 and are in general agreement with the findings in heart, muscle and kidney. Mitochondrial respiration of the full ETC, coupled and uncoupled, and of complex IV (ascorbate/TMPD assay) are significantly decreased by 30–40% in STZ-diabetic rats (with correction by insulin therapy; Table 2). Table 3 shows that the complex I and IV enzyme activities are also significantly inhibited. This collection of results showing reduced complex I activity in diabetic tissues do appear to contrast with findings in epineurial arterioles serving the sciatic nerve in STZ-diabetic rats where elevated superoxide formation could be blocked using inhibitors of complex I [27], again highlighting the need to consider the role of mitochondrial function in diabetes on a per tissue and animal model basis.
Table 1.
Summary of alterations in mitochondrial physiology in heart, muscle and kidney in diabetes.
| Model, tissue | Mitochondrial characteristics | References |
|---|---|---|
| Type 1 diabetes | ||
| STZ-mice, myocardium | ↓ Respiration (Complex I, II, and IV) ↓ Enzymatic activity of Complexes I, III and V ↓ Mitochondrial content (mt-DNA) |
[110–112] |
| STZ-rat, heart, and gastrocnemius muscle | ↓ Respiration rate at state 3 with glutamate + malate, succinate and uncoupled respiration ↓Enzymatic activity of Complex I and II Protein level: ↑ UCP3, ↓ ANT1 |
[113,114] |
| Chronic OVE26 diabetic mice, heart | ↓ Respiratory rate at state 3 and respiratory ratio | [115] |
| Diabetic akita mouse, heart | ↓ Respiratory rate at state 3 with glutamate and pyruvate, ↓ATP synthesis with pyruvate and glutamate, ↓mRNA level (oxidative phosphorylation: Ndufa9, uqcrc1, COX4, ATPase6 antioxidant defense: SOD2, PRDX3), ↑ Protein level of UCP3 and ↑mRNA level of UCP2, UCP3 | [79,116] |
| STZ-rat, kidney | ↓ Enzymatic activity of Complex I, III, and IV, mitochondrial nitric oxide synthase, MnSOD ↑ Membrane potential, pyruvate content, Complex V activity |
[117,118] |
| Type 2 diabetes | ||
| Diabetic patients, skeletal muscle biopsy | ↓ Respiratory rate at state 3, uncoupled respiration, respiratory control index ↓ Enzymatic activity of Complex I, V and CS ↓ Size of skeletal mitochondria |
[119–123] |
| Diabetic patients, skeletal muscle biopsy | ↓Respiration rate with substrates for Complex I (pyruvate, malate, glutamate) and Complex II (succinate), no significant difference when normalized to CS activity | [124–126] |
| Diabetic Goto-Kakizaki rats, skeletal muscle | ↓ Enzymatic activity and protein expression of Complex I and II, mt-DNA | [127] |
| Zucker diabetic fatty rats, skeletal muscle | ↓ Enzymatic activity of Complex IV and CS, normal skeletal muscle mitochondrial oxidative capacity (31P magnetic resonance spectroscopy) | [128] |
Table 2.
Mitochondrial electron transport chain activity is impaired in freshly isolated mitochondria from lumbar DRG of 22 wks STZ-diabetic rats.
| Oxygen consumption (pmol O2/s/mg protein) | |||
|---|---|---|---|
| Substrates | Control | Diabetic | Diabetic + insulin |
| Pyruvate + Malate | 11.49 ± 6.64 | 10.38 ± 4.95 | 12.29 ± 4.71 |
| Pyruvate + Malate + ADP (coupled respiration) | 305.97 ± 53.93 | 210.18 ± 29.03 ** | 339.44 ± 98.03 |
| FCCP (uncoupled respiration) |
259.94 ± 43.21 | 183.65 ± 18.46 ** | 296.31 ± 87.30 |
| Ascorbate + TMPD (complex IV) | 704.55 ± 119.47 | 487.67 ± 64.55 * | 742.57 ± 133.44 |
Mitochondrial respiration rate was measured in freshly isolated mitochondria from lumbar dorsal root ganglia tissue using a Clarke-type oxygen electrode (OROBOROS Oxygraph 2K). Respiration rates were measured in presence of pyruvate (10 mM), malate (5 mM), ADP (2 mM), FCCP (0.5 μM), ascorbate (5 mM), TMPD (0.5 mM) and verified by their specific inhibitors [129]. Values are means ± SD, n = 5.
P<0.05 vs other groups;
P<0.05 vs Db + Ins (one-way ANOVA with Tukey’s posthoc comparison).
Table 3.
Enzymatic activities of mitochondrial respiratory chain complexes and Kreb’s cycle enzyme, citrate synthase are decreased in isolated mitochondria from lumbar DRG of STZ-diabetic rats.
| Enzymatic activity (nmol/min/mg protein) | ||
|---|---|---|
| Enzymes | Control | Diabetic |
| Complex I | 126.94 ± 26.43 | 88.23 ± 18.23 * |
| Complex IV | 1986.31 ± 122.25 | 1676.39 ± 117.67 * |
| Citrate synthase | 293.89 ± 16.00 | 172.29 ± 36.48* |
Enzymatic activity of complex I was assessed as rotenone-sensitive portion of NADH: cytochrome c reductase activity [130]. Complex IV activity was measured at 550 nm following the reduction of oxidized cytochrome c and the activity of citrate synthase by following the color of thionitrobezoic acid at 412 nm [131]. Values are means ± SD, n = 4–5.
P<0.05 vs control (Student’s t–test).
Ultrastructure of mitochondria in diabetic neuropathy
During sensory nerve degeneration in diabetes in humans and animal models subtle changes of mitochondrial number and size have been described in Schwann cells of myelinated and non-myelinated axons. Glycogen accumulation in the outer compartment of the mitochondrion were described in axons [43] but the ultrastructure of mitochondria in neuronal cell bodies is reportedly unremarkable [9]. Although mitochondria, vesicles, and 10–20 nm tubules have been described in intraepidermal axons, intraepidermal axonal swellings have not been characterized in purely diabetic patients [14,15]. The most detailed study to date [14] involved swellings in patients with HIV neuropathy (4 of which were also diabetic) and non-HIV idiopathic sensory neuropathy. Intraepidermal axonal swellings in that study contained numerous accumulated mitochondria, vesicles and neurofilaments and dermal nerves also contained intraxonal mildly enlarged mitochondria. Schwann cells with abnormal appearing mitochondria were also encountered [14] and confirmed previous work in sural nerve biopsies [43].
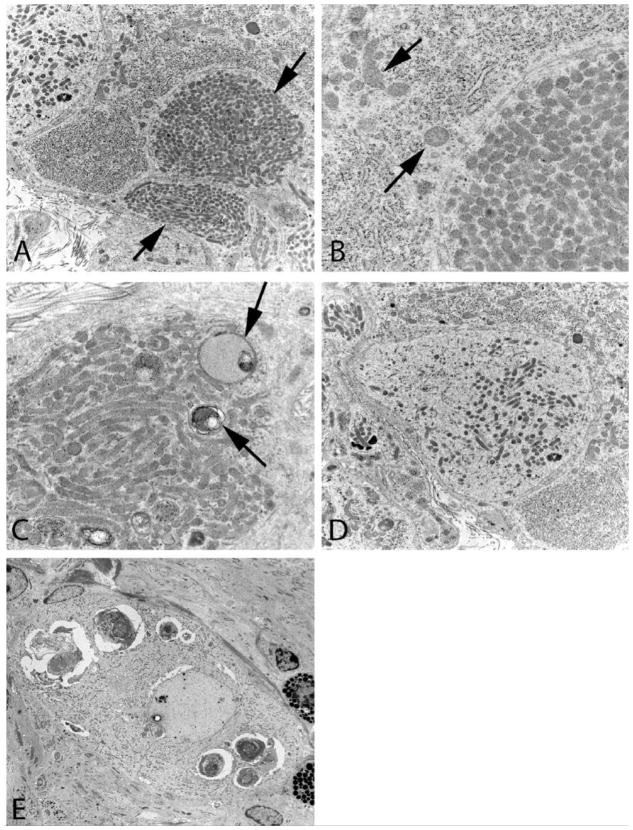
Abnormal mitochondrial ultrastructure is an important part of sympathetic autonomic neuropathology involving pre- and postsynaptic elements in prevertebral sympathetic ganglia in man and a variety of rodent models and appears to be part of an emerging story in type 1 and 2 diabetic mouse models [80–83]. Mitochondria are a normal part of presynaptic boutons, likely reflecting their metabolic activity, but have been recently found in increased numbers in autophagic vacuoles admixed with synaptic vesicles in murine diabetic sympathetic ganglia [83]. More impressive are the accumulated mitochondria, tightly aggregated without a significant amount of intervening cytoplasm, in post-synaptic dendrites in which they may produce nearly pure aggregates (arrows, Figure 1A) often composed of smaller mitochondria than those in adjacent cell bodies (arrows, Fig 1B). In some cases mitochondria are admixed with multivesicular bodies or autophagosomes (arrows, Figure 1C) or with delicate tubulovesicular elements (Figure 1D). It is not typical for dystrophic pre- and postsynaptic elements to occur together as part of single synapses, rather dystrophic pre- and postsynaptic elements are coupled with ultrastructurally unremarkable post- or presynaptic elements, respectively. Recent studies of prevertebral sympathetic ganglia of NOD, STZ-treated and STZ-treated NOD/SCID mice [80] and in the spontaneously genetically diabetic Akita mouse [82], have demonstrated the presence of striking abnormalities in mitochondrial ultrastructure. In particular, the accumulation of small, hyperchromatic dense mitochondria that were significantly smaller than those in nondiabetic control mouse ganglion or those in the superior cervical ganglia (SCG) in the same diabetic mice in which superior mesenteric ganglia (SMG) and celiac ganglia (CG) pathology routinely occurs. Rarely such changes accompany loss of ribosomes resulting in neuronal pallor or degenerative changes in neuronal nuclei not culminating in a classical apoptotic appearance (Figure 1E). These axonopathic and neuronopathic changes are accompanied by neuronal loss in the prevertebral SMG/CG of the Akita mouse. It should be remembered that early studies of human sympathetic ganglia in autopsied human subjects uncovered a mild, poorly characterized decrease in neuronal number expressed as decreased neuronal density/mm2 of ganglionic surface area [84]. Future studies of human ganglia using non-biased morphometric techniques may indeed identify both dystrophic terminals and neuronopathy underlying the development of autonomic neuropathy in diabetes, further complicating its pathology and possible pathogenesis. Nerve terminal damage is likely to dis- or misconnect ganglionic neurons and, particularly for prevertebral ganglia serving the viscera, contribute to the loss of integrated reflexes, perhaps complicated by the loss of selected subpopulations of neurons.
Figure 1.
Mitochondriopathy in diabetic mouse prevertebral sympathetic ganglia A–C) Dilated dendrites (arrows, A) containing large numbers of mitochondria which are smaller than those of an adjacent normal perikaryon (arrows, B). Mitochondria, which may form pure aggregates, are occasionally admixed with multivesicular/autophagic bodies (arrows, C) (Akita mouse celiac ganglion, original magnification: A - 10,000X; B,C - 25,000X). D) Some pale dendritic processes contain small mitochondria, tubulovesicular elements and little rough endoplasmic reticulum (Akita mouse celiac ganglion, original magnification: 10,000X). E) A degenerating neuron containing large membranous aggregates and minute hyperchromatic mitochondria represents a recent finding in murine models, particularly the Akita mouse (Akita mouse celiac ganglion, original magnification: 3000X)
Aberrant mitochondrial trafficking and impact on function in neurodegeneration
Determination of the functional significance of the alterations in the number and size of the mitochondria in diabetic sympathetic ganglia is difficult but insights have recently been provided by the analysis of a variety of human and experimental neuropathic conditions. Mitochondria are dynamic organelles which undergo fusion and fission which are regulated by a number of genes which have a role in control of normal energy supply and participate in the pathogenesis of a variety of neurodegenerative conditions [85–88]. Increased energy demand with accompanying changes in ATP/ADP gradients may induce fusion and division of mitochondria often resulting in the accumulation of small mitochondria in perikaryal and dendritic sites. Small mitochondria may more readily translocate from one dendritic and nerve terminal site of increased energy demand and help in the local regulation of energy demand and balance. Mitochondrial pathology is most prominent in dendrites, sites in which mitochondria are more highly charged and metabolically active than in axons [89] and, under normal conditions, they are more threadlike in shape than in axons and presynaptic terminals [90]. Significant functional differences (e.g., susceptibility to Ca2+ overload) have also been described between synaptic and nonsynaptic mitochondria [91]. Aggregates of mitochondria may also contribute to the development of local pathology as the result of increased production of ROS, loss of normal energy production or abnormality of Ca2+ handling ability.
There is a complex relationship between mitochondrial fragmentation and bioenergetics, as a decrease in ATP can also stimulate fragmentation [92]. Mitochondrial fission may initially help evenly distribute energy throughout the cell body and axon. Mitochondria also undergo fission or fragmentation in response to increased levels of nitric oxide, i.e. nitrosative stress, which may secondarily result in ATP decline, further synthesis of ROS, the overexpression of Drp1 and neuronal injury [93]. A local increase in NO resulting from the deposition of β-amyloid protein in patients with Alzheimer’s disease (AD) and in the CNS of mutant mouse models of AD has been proposed to produce S-nitrosylation of dynamin-related protein, mitochondrial fission, loss of dendritic spines and synaptic loss [94,95].
Genetic alterations in the fusion inducing protein mitofusin-2 (MFN2) of cultured DRG produced defects in axonal transport of mitochondria and their accumulation in abnormal clusters of small fragmented mitochondria in both neuronal cell bodies and proximal axons [85,96]. Proteotoxic stress in mitochondria resulting in the sumoylation of Drp1 also activates mitochondrial fission and induction of autophagy to eliminate oxidized proteins or protein quality control, preserving organelles [97]. In this setting, either inhibiting fission or promoting fusion prevents increase in ROS [98]. The impact of high glucose concentration on mitochondrial trafficking and associated functions remains to be fully elucidated. Studies of cultured rat liver cells or rat myoblasts demonstrated that mitochondrial fragmentation is necessary for high glucose-induced increase in respiration with concomitant overproduction of ROS [99]. Treatment of pancreatic beta cells, neurons and endothelial cells with high [glucose] results in fragmentation/fission of mitochondria [100–103]. In several settings mitochondrial fragmentation is eventually followed by cell death and/or apoptosis [103], an outcome which can be prevented or modulated by shifting the dynamic balance to favor mitochondrial fusion [104]. In cultured DRG initial mitochondrial fission has been proposed to result from up-regulation of the mitochondrial fission protein Drp1 resulting in protective or metabolic fission followed later by activation of Bim and Bax resulting in apoptosis, the latter phase resulting from regulation of Bim/Bax activation [100]. Although initial fragmentation and clumping of mitochondria has been demonstrated in vivo in diabetic animals, apoptosis of DRG neurons in diabetic animals remains a contentious arena. In cultured endothelial cells the balance between the metabolic stimulation of mitochondria by elevated glucose levels and free radical production has been proposed to represent the turning point of either adaptation for cell survival or initiation of fatal pathways resulting in cell death in diabetes [102].
Conclusion
Mitochondrial dysfunction occurs in a range of diabetic complications and given its central role in controlling the bioenergetic status of the cell must be considered a prime trigger of degeneration. It remains unclear how such impaired mitochondrial function triggers cell damage in the nervous system and enhanced ROS production derived from aberrant mitochondrial physiology remains an unproven causal factor. Failure to synthesize adequate ATP for high energy requiring axonal functions such as excitation, ion flux, axonal transport and growth cone motility would seem more attractive options, particularly in neurons where impaired bioenergetic status may enhance oxidative stress induced by high [glucose] or diminish ability to scavenge ROS. These issues are briefly outlined and discussed in the model displayed in Figure 2. In diabetic neuropathy studies have begun to outline the array of impairments in mitochondrial physiology, although studies still lag behind those in nephropathy and cardiomyopathy. Diabetes-induced changes in mitochondrial phenotype would seem key factors leading to altered activity of the electron transport chain and Krebs cycle components.
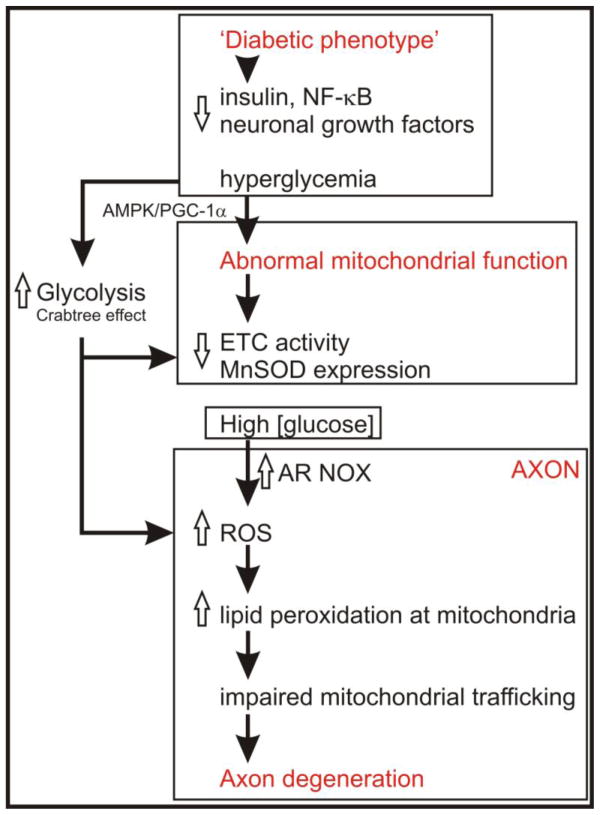
Figure 2.
Scheme outlining putative mechanisms whereby the diabetic state modulates mitochondrial function and axon degeneration. Reductions in growth factors or hyperglycemia combine to alter mitochondrial bioenergetics. High intracellular [glucose] in neurons may cause a general down-regulation of ETC components, possibly through the Crabtree effect, and involving the AMP-activated protein kinase (AMPK) and/or peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1a) pathways. Antioxidant pathways, such as manganese superoxide dismutase (MnSOD), are also impaired through lowered NF-κB activation. In neurons these processes appear to be more active within the axonal region resulting in raised ROS [35]. The source of ROS in axons remains elusive, however, impaired ETC capacity and altered mitochondrial bioenergetics could contribute. In addition, high [glucose] may enhance aldose reductase (AR) activity and/or NADPH oxidase (NOX) to generate ROS. Elevated generation of ROS at axonal sites causes a range of molecular changes in protein function, for example, lipid peroxidation-dependent amino acid adduct formation that impacts further on mitochondrial function and trafficking. Suboptimal mitochondrial bioenergetics and trafficking will lead to axonal maintenance breaking down through lack of available energy stores. The axon, and distal aspect in particular, is very sensitive to such energy failure due to its high demand for ATP for axon treadmilling and maintaining ion fluxes.
Expert commentary & five-year view
Aberrant growth factor-dependent signaling, especially through the insulin pathway could be involved, however, hyperglycemia and associated raising of intracellular glucose concentration maybe a central trigger of altered mitochondrial proteome expression. In many cell types high intracellular glucose causes inhibition of oxidative phosphorylation with enhancement of anaerobic glucose metabolism through glycolysis, known as the Crabtree effect [105]. Under such conditions the need for a fully functional mitochondrial proteome would be lessened and would explain recent findings and our preliminary data. It is critical in the next 5 years that the glucose-dependent signal transduction pathway that is proposed to become active in the diabetic state and that initiates altered cell bioenergetics in neurons is dissected and manipulated in vitro and in vivo. Inspection of the data from studies in non-neurons would suggest that the AMP-activated protein kinase (AMPK) and/or peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1a) pathways are worthy of investigation. There is a plausible role for the polyol pathway in such a paradigm, however, it must be remembered that neurons do not express aldose reductase and so the activity of such a pathway in neurons in diabetes remains unclear [106]. It should be noted, however, that polyol levels do accumulate in DRG, sympathetic ganglia and glomerular mesangial cells under hyperglycemic conditions [107–109] and treatment with aldose reductase inhibitors can modulate sensory and autonomic neuron phenotype in lumbar DRG of STZ-diabetic rats [46,109].
Key issues
Full description of mitochondrial physiology and proteome changes in dorsal root ganglia and sympathetic ganglia in animal models of type 1 and 2 diabetes.
Targeting of specific ETC components, using siRNA knockdown for example, to alter expression and mimic the diabetic state in normal neurons. Complementary studies using gene targeting to normalize mitochondrial function in diabetes.
Determine if mitochondria in neurons in the diabetic state are sources of ROS. Need for real time imaging approaches in vitro and in vivo, for example use of Mitosox red.
Establish if aberrant mitochondrial trafficking is present in neurons in diabetes and determine impact on mitochondrial physiology.
Acknowledgments
Dr. Chowdhury was supported by grants to PF from CIHR (grant # MOP-84214), to PF and RES from Juvenile Diabetes Research Foundation (grant # 1-2008-193) and to RES from the NIH (DK19645). This work was also funded by the St Boniface General Hospital and Research Foundation and the Manitoba Medical Services Foundation.
Contributor Information
Paul Fernyhough, Division of Neurodegenerative Disorders, St Boniface Hospital Research Centre, R4046 – 351 Taché Avenue, Winnipeg, MB R2H 2A6, Canada and Department of Pharmacology & Therapeutics, University of Manitoba, Winnipeg, MB, Canada, Tel: (204) 235 3692, Fax: (204) 237 4092.
Subir K. Roy Chowdhury, Division of Neurodegenerative Disorders, St Boniface Hospital Research Centre, R4046 – 351 Taché Avenue, Winnipeg, MB R2H 2A6, Canada, Tel: (204) 235 3692, Fax: (204) 237 4092.
Robert E. Schmidt, Dept of Pathology and Immunology, 3720 West Building, Washington University School of Medicine, St. Louis, MO 63110, USA, Tel: (314) 362-7429, Fax: (314) 362-4096.
References
- 1.Malik RA, Tesfaye S, Newrick PG, et al. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia. 2005;48(3):578–585. doi: 10.1007/s00125-004-1663-5. [DOI] [PubMed] [Google Scholar]
- 2.Sima AA. Diabetic neuropathy in type 1 and type 2 diabetes and the effects of C-peptide. J Neurol Sci. 2004;220(1–2):133–136. doi: 10.1016/j.jns.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Yagihashi S. Pathogenetic mechanisms of diabetic neuropathy: lessons from animal models. J Peripher Nerv Syst. 1997;2(2):113–132. [PubMed] [Google Scholar]
- 4.Toth C, Brussee V, Cheng C, Zochodne DW. Diabetes mellitus and the sensory neuron. J Neuropathol Exp Neurol. 2004;63(6):561–573. doi: 10.1093/jnen/63.6.561. [DOI] [PubMed] [Google Scholar]
- 5.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 6.Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127(Pt 7):1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- 7.Burnand RC, Price SA, McElhaney M, Barker D, Tomlinson DR. Expression of axotomy-inducible and apoptosis-related genes in sensory nerves of rats with experimental diabetes. Brain Res Mol Brain Res. 2004;132(2):235–240. doi: 10.1016/j.molbrainres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Cheng C, Zochodne DW. Sensory neurons with activated caspase-3 survive long-term experimental diabetes. Diabetes. 2003;52(9):2363–2371. doi: 10.2337/diabetes.52.9.2363. [DOI] [PubMed] [Google Scholar]
- 9.Kamiya H, Zhang W, Sima AA. Degeneration of the Golgi and neuronal loss in dorsal root ganglia in diabetic BioBreeding/Worcester rats. Diabetologia. 2006;49(11):2763–2774. doi: 10.1007/s00125-006-0379-0. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt RE, Dorsey D, Parvin CA, Beaudet LN, Plurad SB, Roth KA. Dystrophic axonal swellings develop as a function of age and diabetes in human dorsal root ganglia. J Neuropathol Exp Neurol. 1997;56(9):1028–1043. doi: 10.1097/00005072-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Sidenius P, Jakobsen J. Reduced perikaryal volume of lower motor and primary sensory neurons in early experimental diabetes. Diabetes. 1980;29(3):182–186. doi: 10.2337/diab.29.3.182. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy JM, Zochodne DW. Experimental diabetic neuropathy with spontaneous recovery: is there irreparable damage? Diabetes. 2005;54(3):830–837. doi: 10.2337/diabetes.54.3.830. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47(4):1042–1048. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- 14.Ebenezer GJ, McArthur JC, Thomas D, et al. Denervation of skin in neuropathies: the sequence of axonal and Schwann cell changes in skin biopsies. Brain. 2007;130(Pt 10):2703–2714. doi: 10.1093/brain/awm199. [DOI] [PubMed] [Google Scholar]
- 15.Lauria G, Morbin M, Lombardi R, et al. Axonal swellings predict the degeneration of epidermal nerve fibers in painful neuropathies. Neurology. 2003;61(5):631–636. doi: 10.1212/01.wnl.0000070781.92512.a4. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt RE. Neuropathology and pathogenesis of diabetic autonomic neuropathy. Int Rev Neurobiol. 2002;50:257–292. doi: 10.1016/s0074-7742(02)50080-5. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt RE, Nelson JS, Johnson EM., Jr Experimental diabetic autonomic neuropathy. Am J Pathol. 1981;103(2):210–225. [PMC free article] [PubMed] [Google Scholar]
- 18.Nja A, Purves D. The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J Physiol. 1978;277:53–75. [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25(4):612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 20.Obrosova IG. How does glucose generate oxidative stress in peripheral nerve? Int Rev Neurobiol. 2002;50:3–35. doi: 10.1016/s0074-7742(02)50071-4. [DOI] [PubMed] [Google Scholar]
- 21.Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44(11):1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- 22.Yorek MA. The role of oxidative stress in diabetic vascular and neural disease. Free Radic Res. 2003;37(5):471–480. doi: 10.1080/1071576031000083161. [DOI] [PubMed] [Google Scholar]
- 23.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 24.Russell JW, Golovoy D, Vincent AM, et al. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. Faseb J. 2002;16(13):1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 25.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes. 2001;50(8):1927–1937. doi: 10.2337/diabetes.50.8.1927. [DOI] [PubMed] [Google Scholar]
- 26.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Yorek MA. Changes in endoneurial blood flow, motor nerve conduction velocity and vascular relaxation of epineurial arterioles of the sciatic nerve in ZDF-obese diabetic rats. Diabetes Metab Res Rev. 2002;18(1):49–56. doi: 10.1002/dmrr.257. [DOI] [PubMed] [Google Scholar]
- 27.Coppey LJ, Gellett JS, Davidson EP, Yorek MA. Preventing superoxide formation in epineurial arterioles of the sciatic nerve from diabetic rats restores endothelium-dependent vasodilation. Free Radic Res. 2003;37(1):33–40. doi: 10.1080/1071576021000028442. [DOI] [PubMed] [Google Scholar]
- 28.Obrosova IG, Van Huysen C, Fathallah L, Cao XC, Greene DA, Stevens MJ. An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. FASEB J. 2002;16(1):123–125. doi: 10.1096/fj.01-0603fje. [DOI] [PubMed] [Google Scholar]
- 29.Drel VR, Mashtalir N, Ilnytska O, et al. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55(12):3335–3343. doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- 30.Obrosova IG, Ilnytska O, Lyzogubov VV, et al. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56(10):2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- 31.Obrosova IG, Drel VR, Pacher P, et al. Oxidative-Nitrosative Stress and Poly(ADP-Ribose) Polymerase (PARP) Activation in Experimental Diabetic Neuropathy: The Relation Is Revisited. Diabetes. 2005;54(12):3435–3441. doi: 10.2337/diabetes.54.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obrosova IG, Pacher P, Szabo C, et al. Aldose reductase inhibition counteracts oxidative-nitrosative stress and poly(ADP-ribose) polymerase activation in tissue sites for diabetes complications. Diabetes. 2005;54(1):234–242. doi: 10.2337/diabetes.54.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho EC, Lam KS, Chen YS, et al. Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes. 2006;55(7):1946–1953. doi: 10.2337/db05-1497. [DOI] [PubMed] [Google Scholar]
- 34.Price SA, Gardiner NJ, Duran-Jimenez B, Zeef LA, Obrosova IG, Tomlinson DR. Thioredoxin interacting protein is increased in sensory neurons in experimental diabetes. Brain Res. 2006;1116(1):206–214. doi: 10.1016/j.brainres.2006.07.109. [DOI] [PubMed] [Google Scholar]
- 35.Zherebitskaya E, Akude E, Smith DR, Fernyhough P. Development of selective axonopathy in adult sensory neurons isolated from diabetic rats: role of glucose-induced oxidative stress. Diabetes. 2009;58(6):1356–1364. doi: 10.2337/db09-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Fernyhough P, Schmidt RE. Neurofilaments in diabetic neuropathy. Int Rev Neurobiol. 2002;50:115–144. doi: 10.1016/s0074-7742(02)50075-1. [DOI] [PubMed] [Google Scholar]
- 38.Hall KE, Sima AA, Wiley JW. Voltage-dependent calcium currents are enhanced in dorsal root ganglion neurones from the Bio Bred/Worchester diabetic rat. J Physiol. 1995;486 ( Pt 2):313–322. doi: 10.1113/jphysiol.1995.sp020814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang TJ, Sayers NM, Fernyhough P, Verkhratsky A. Diabetes-induced alterations in calcium homeostasis in sensory neurones of streptozotocin-diabetic rats are restricted to lumbar ganglia and are prevented by neurotrophin-3. Diabetologia. 2002;45(4):560–570. doi: 10.1007/s00125-002-0785-x. [DOI] [PubMed] [Google Scholar]
- 40.Kruglikov I, Gryshchenko O, Shutov L, Kostyuk E, Kostyuk P, Voitenko N. Diabetes-induced abnormalities in ER calcium mobilization in primary and secondary nociceptive neurons. Pflugers Arch. 2004;448(4):395–401. doi: 10.1007/s00424-004-1263-8. [DOI] [PubMed] [Google Scholar]
- 41.Verkhratsky A, Fernyhough P. Mitochondrial malfunction and Ca2+ dyshomeostasis drive neuronal pathology in diabetes. Cell Calcium. 2008;44(1):112–122. doi: 10.1016/j.ceca.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa T, Edelstein D, Brownlee M. The missing link: A single unifying mechanism for diabetic complications. Kidney Int J1 - Kidney Int. 2000;58(Supp 77):S26–S30. doi: 10.1046/j.1523-1755.2000.07705.x. [DOI] [PubMed] [Google Scholar]
- 43.Kalichman MW, Powell HC, Mizisin AP. Reactive, degenerative, and proliferative Schwann cell responses in experimental galactose and human diabetic neuropathy. Acta Neuropathol. 1998;95(1):47–56. doi: 10.1007/s004010050764. [DOI] [PubMed] [Google Scholar]
- 44.Kamiya H, Zhangm W, Sima AA. Apoptotic stress is counterbalanced by survival elements preventing programmed cell death of dorsal root ganglions in subacute type 1 diabetic BB/Wor rats. Diabetes. 2005;54(11):3288–3295. doi: 10.2337/diabetes.54.11.3288. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt RE, Beaudet LN, Plurad SB, Dorsey DA. Axonal cytoskeletal pathology in aged and diabetic human sympathetic autonomic ganglia. Brain Res. 1997;769(2):375–383. doi: 10.1016/s0006-8993(97)00806-8. [DOI] [PubMed] [Google Scholar]
- 46.Price SA, Agthong S, Middlemas AB, Tomlinson DR. Mitogen-activated protein kinase p38 mediates reduced nerve conduction velocity in experimental diabetic neuropathy: interactions with aldose reductase. Diabetes. 2004;53(7):1851–1856. doi: 10.2337/diabetes.53.7.1851. [DOI] [PubMed] [Google Scholar]
- 47.Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9(1):36–45. doi: 10.1038/nrn2294. [DOI] [PubMed] [Google Scholar]
- 48.Huang TJ, Price SA, Chilton L, et al. Insulin prevents depolarization of the mitochondrial inner membrane in sensory neurons of type 1 diabetic rats in the presence of sustained hyperglycemia. Diabetes. 2003;52(8):2129–2136. doi: 10.2337/diabetes.52.8.2129. [DOI] [PubMed] [Google Scholar]
- 49.Huang TJ, Sayers NM, Verkhratsky A, Fernyhough P. Neurotrophin-3 prevents mitochondrial dysfunction in sensory neurons of streptozotocin-diabetic rats. Exp Neurol. 2005;194(1):279–283. doi: 10.1016/j.expneurol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Srinivasan S, Stevens M, Wiley JW. Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction. Diabetes. 2000;49(11):1932–1938. doi: 10.2337/diabetes.49.11.1932. [DOI] [PubMed] [Google Scholar]
- 51.Huang TJ, Verkhratsky A, Fernyhough P. Insulin enhances mitochondrial inner membrane potential and increases ATP levels through phosphoinositide 3-kinase in adult sensory neurons. Mol Cell Neurosci. 2005;28(1):42–54. doi: 10.1016/j.mcn.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Purves T, Middlemas A, Agthong S, et al. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. Faseb J. 2001;15(13):2508–2514. doi: 10.1096/fj.01-0253hyp. [DOI] [PubMed] [Google Scholar]
- 53.Gumy LF, Bampton ET, Tolkovsky AM. Hyperglycaemia inhibits Schwann cell proliferation and migration and restricts regeneration of axons and Schwann cells from adult murine DRG. Mol Cell Neurosci. 2008;37(2):298–311. doi: 10.1016/j.mcn.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Yu C, Rouen S, Dobrowsky RT. Hyperglycemia and downregulation of caveolin-1 enhance neuregulin-induced demyelination. Glia. 2008;56(8):877–887. doi: 10.1002/glia.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall KE, Liu J, Sima AA, Wiley JW. Impaired inhibitory G-protein function contributes to increased calcium currents in rats with diabetic neuropathy. J Neurophysiol. 2001;86(2):760–770. doi: 10.1152/jn.2001.86.2.760. [DOI] [PubMed] [Google Scholar]
- 56.Kostyuk E, Pronchuk N, Shmigol A. Calcium signal prolongation in sensory neurones of mice with experimental diabetes. Neuroreport. 1995;6(7):1010–1012. doi: 10.1097/00001756-199505090-00015. [DOI] [PubMed] [Google Scholar]
- 57.Kostyuk E, Voitenko N, Kruglikov I, et al. Diabetes-induced changes in calcium homeostasis and the effects of calcium channel blockers in rat and mice nociceptive neurons. Diabetologia. 2001;44(10):1302–1309. doi: 10.1007/s001250100642. [DOI] [PubMed] [Google Scholar]
- 58.Voitenko NV, Kostyuk EP, Kruglikov IA, Kostyuk PG. Changes in calcium signalling in dorsal horn neurons in rats with streptozotocin-induced diabetes. Neuroscience. 1999;94(3):887–890. doi: 10.1016/s0306-4522(99)00330-9. [DOI] [PubMed] [Google Scholar]
- 59.Voitenko NV, Kruglikov IA, Kostyuk EP, Kostyuk PG. Effect of streptozotocin-induced diabetes on the activity of calcium channels in rat dorsal horn neurons. Neuroscience. 2000;95(2):519–524. doi: 10.1016/s0306-4522(99)00453-4. [DOI] [PubMed] [Google Scholar]
- 60.Kostyuk E, Svichar N, Shishkin V, Kostyuk P. Role of mitochondrial dysfunction in calcium signalling alterations in dorsal root ganglion neurons of mice with experimentally-induced diabetes. Neuroscience. 1999;90(2):535–541. doi: 10.1016/s0306-4522(98)00471-0. [DOI] [PubMed] [Google Scholar]
- 61.Tahara M, Omatsu-Kanbe M, Sanada M, et al. Effect of protein kinase Cbeta inhibitor on Ca2+ homeostasis in diabetic sensory neurons. Neuroreport. 2006;17(6):683–688. doi: 10.1097/00001756-200604240-00026. [DOI] [PubMed] [Google Scholar]
- 62.Li F, Obrosova IG, Abatan O, et al. Taurine replacement attenuates hyperalgesia and abnormal calcium signaling in sensory neurons of STZ-D rats. Am J Physiol Endocrinol Metab. 2005;288(1):E29–36. doi: 10.1152/ajpendo.00168.2004. [DOI] [PubMed] [Google Scholar]
- 63.David G, Barrett EF. Stimulation-evoked increases in cytosolic [Ca(2+)] in mouse motor nerve terminals are limited by mitochondrial uptake and are temperature-dependent. J Neurosci. 2000;20(19):7290–7296. doi: 10.1523/JNEUROSCI.20-19-07290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.David G, Barrett JN, Barrett EF. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. J Physiol. 1998;509 ( Pt 1):59–65. doi: 10.1111/j.1469-7793.1998.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):C817–833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 66.Coatesworth W, Bolsover S. Spatially organised mitochondrial calcium uptake through a novel pathway in chick neurones. Cell Calcium. 2006;39(3):217–225. doi: 10.1016/j.ceca.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 67.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427(6972):360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 68.Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80(1):315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- 69.Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr Mol Med. 2004;4(2):149–177. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- 70.Nicholls DG. Mitochondria and calcium signaling. Cell Calcium. 2005;38(3–4):311–317. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 71.Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol. 2006;291(5):C1082–1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 72.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567(1):96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 73.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 74.Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVO2, NADH, AND light scattering. J Biol Chem. 2001;276(4):2586–2599. doi: 10.1074/jbc.M002923200. [DOI] [PubMed] [Google Scholar]
- 75.Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28(5–6):285–296. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- 76.Perez-Campo R, Lopez-Torres M, Cadenas S, Rojas C, Barja G. The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. J Comp Physiol [B] 1998;168(3):149–158. doi: 10.1007/s003600050131. [DOI] [PubMed] [Google Scholar]
- 77.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 78.Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol Sin. 2006;27(7):821–826. doi: 10.1111/j.1745-7254.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 79.Bugger H, Chen D, Riehle C, et al. Tissue-Specific Remodeling of the Mitochondrial Proteome in Type 1 Diabetic Akita Mice. Diabetes. 2009 doi: 10.2337/db09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmidt RE, Dorsey DA, Beaudet LN, et al. Non-obese diabetic mice rapidly develop dramatic sympathetic neuritic dystrophy: a new experimental model of diabetic autonomic neuropathy. Am J Pathol. 2003;163(5):2077–2091. doi: 10.1016/S0002-9440(10)63565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmidt RE, Dorsey DA, Beaudet LN, Peterson RG. Analysis of the Zucker Diabetic Fatty (ZDF) type 2 diabetic rat model suggests a neurotrophic role for insulin/IGF-I in diabetic autonomic neuropathy. Am J Pathol. 2003;163(1):21–28. doi: 10.1016/S0002-9440(10)63626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt RE, Green KG, Snipes LL, Feng D. Neuritic dystrophy and neuronopathy in Akita (Ins2(Akita)) diabetic mouse sympathetic ganglia. Exp Neurol. 2009;216(1):207–218. doi: 10.1016/j.expneurol.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmidt RE, Parvin CA, Green KG. Synaptic ultrastructural alterations anticipate the development of neuroaxonal dystrophy in sympathetic ganglia of aged and diabetic mice. J Neuropathol Exp Neurol. 2008;67(12):1166–1186. doi: 10.1097/NEN.0b013e318190d6db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schmidt RE, Plurad SB, Parvin CA, Roth KA. Effect of diabetes and aging on human sympathetic autonomic ganglia. Am J Pathol. 1993;143(1):143–153. [PMC free article] [PubMed] [Google Scholar]
- 85.Baloh RH. Mitochondrial dynamics and peripheral neuropathy. Neuroscientist. 2008;14(1):12–18. doi: 10.1177/1073858407307354. [DOI] [PubMed] [Google Scholar]
- 86.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125(7):1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 87.Frank S. Dysregulation of mitochondrial fusion and fission: an emerging concept in neurodegeneration. Acta Neuropathol. 2006;111(2):93–100. doi: 10.1007/s00401-005-0002-3. [DOI] [PubMed] [Google Scholar]
- 88.Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10(8):870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- 89.Overly CC, Rieff HI, Hollenbeck PJ. Organelle motility and metabolism in axons vs dendrites of cultured hippocampal neurons. J Cell Sci. 1996;109 ( Pt 5):971–980. doi: 10.1242/jcs.109.5.971. [DOI] [PubMed] [Google Scholar]
- 90.Muller M, Mironov SL, Ivannikov MV, Schmidt J, Richter DW. Mitochondrial organization and motility probed by two-photon microscopy in cultured mouse brainstem neurons. Exp Cell Res. 2005;303(1):114–127. doi: 10.1016/j.yexcr.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 91.Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem. 2006;281(17):11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 92.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9(7):505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barsoum MJ, Yuan H, Gerencser AA, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25(16):3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cho DH, Nakamura T, Fang J, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324(5923):102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baloh RH, Schmidt RE, Pestronk A, Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci. 2007;27(2):422–430. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Germain D. Ubiquitin-dependent and -independent mitochondrial protein quality controls: implications in ageing and neurodegenerative diseases. Mol Microbiol. 2008;70(6):1334–1341. doi: 10.1111/j.1365-2958.2008.06502.x. [DOI] [PubMed] [Google Scholar]
- 98.Westermann B. Nitric oxide links mitochondrial fission to Alzheimer’s disease. Sci Signal. 2009;2(69):pe29. doi: 10.1126/scisignal.269pe29. [DOI] [PubMed] [Google Scholar]
- 99.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103(8):2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leinninger GM, Backus C, Sastry AM, Yi YB, Wang CW, Feldman EL. Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiol Dis. 2006;23(1):11–22. doi: 10.1016/j.nbd.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 101.Molina AJ, Wikstrom JD, Stiles L, et al. Mitochondrial Networking Protects Beta Cells from Nutrient Induced Apoptosis. Diabetes. 2009 doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paltauf-Doburzynska J, Malli R, Graier WF. Hyperglycemic conditions affect shape and Ca2+ homeostasis of mitochondria in endothelial cells. J Cardiovasc Pharmacol. 2004;44(4):423–436. doi: 10.1097/01.fjc.0000139449.64337.1b. [DOI] [PubMed] [Google Scholar]
- 103.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79(2):341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park KS, Wiederkehr A, Kirkpatrick C, et al. Selective actions of mitochondrial fission/fusion genes on metabolism-secretion coupling in insulin-releasing cells. J Biol Chem. 2008;283(48):33347–33356. doi: 10.1074/jbc.M806251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ibsen HK. The Crabtree effect: a review. Cancer Research. 1961;21:829–841. [PubMed] [Google Scholar]
- 106.Jiang Y, Calcutt NA, Ramos KM, Mizisin AP. Novel sites of aldose reductase immunolocalization in normal and streptozotocin-diabetic rats. J Peripher Nerv Syst. 2006;11(4):274–285. doi: 10.1111/j.1529-8027.2006.00099.x. [DOI] [PubMed] [Google Scholar]
- 107.Kikkawa R, Umemura K, Haneda M, et al. Identification and characterization of aldose reductase in cultured rat mesangial cells. Diabetes. 1992;41(9):1165–1171. doi: 10.2337/diab.41.9.1165. [DOI] [PubMed] [Google Scholar]
- 108.Llewelyn JG, Thomas PK, Mirrlees DJ. Aldose reductase activity and myo-inositol levels in sciatic nerve and dorsal root ganglia of the diabetic mutant mouse [C57/BL/Ks (db/db)] Metabolism. 1991;40(10):1084–1087. doi: 10.1016/0026-0495(91)90134-i. [DOI] [PubMed] [Google Scholar]
- 109.Schmidt RE, Plurad SB, Sherman WR, Williamson JR, Tilton RG. Effects of aldose reductase inhibitor sorbinil on neuroaxonal dystrophy and levels of myo-inositol and sorbitol in sympathetic autonomic ganglia of streptozocin-induced diabetic rats. Diabetes. 1989;38(5):569–579. doi: 10.2337/diab.38.5.569. [DOI] [PubMed] [Google Scholar]
- 110.Dabkowski ER, Williamson CL, Bukowski VC, et al. Diabetic cardiomyopathy-associated dysfunction in spatially distinct mitochondrial subpopulations. Am J Physiol Heart Circ Physiol. 2009;296(2):H359–369. doi: 10.1152/ajpheart.00467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang JY, Yeh HY, Lin K, Wang PH. Insulin stimulates Akt translocation to mitochondria: implications on dysregulation of mitochondrial oxidative phosphorylation in diabetic myocardium. J Mol Cell Cardiol. 2009;46(6):919–926. doi: 10.1016/j.yjmcc.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu X, Tesiram YA, Towner RA, et al. Early myocardial dysfunction in streptozotocin-induced diabetic mice: a study using in vivo magnetic resonance imaging (MRI) Cardiovasc Diabetol. 2007;6:6. doi: 10.1186/1475-2840-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Herlein JA, Fink BD, O’Malley Y, Sivitz WI. Superoxide and respiratory coupling in mitochondria of insulin-deficient diabetic rats. Endocrinology. 2009;150(1):46–55. doi: 10.1210/en.2008-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lashin OM, Szweda PA, Szweda LI, Romani AM. Decreased complex II respiration and HNE-modified SDH subunit in diabetic heart. Free Radic Biol Med. 2006;40(5):886–896. doi: 10.1016/j.freeradbiomed.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 115.Shen X, Zheng S, Thongboonkerd V, et al. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol Metab. 2004;287(5):E896–905. doi: 10.1152/ajpendo.00047.2004. [DOI] [PubMed] [Google Scholar]
- 116.Bugger H, Boudina S, Hu XX, et al. Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes. 2008;57(11):2924–2932. doi: 10.2337/db08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Cavanagh EM, Ferder L, Toblli JE, et al. Renal mitochondrial impairment is attenuated by AT1 blockade in experimental Type I diabetes. Am J Physiol Heart Circ Physiol. 2008;294(1):H456–465. doi: 10.1152/ajpheart.00926.2007. [DOI] [PubMed] [Google Scholar]
- 118.Munusamy S, Saba H, Mitchell T, Megyesi JK, Brock RW, Macmillan-Crow LA. Alteration of renal respiratory Complex-III during experimental type-1 diabetes. BMC Endocr Disord. 2009;9:2. doi: 10.1186/1472-6823-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50(4):790–796. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mogensen M, Sahlin K, Fernstrom M, et al. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56(6):1592–1599. doi: 10.2337/db06-0981. [DOI] [PubMed] [Google Scholar]
- 121.Phielix E, Schrauwen-Hinderling VB, Mensink M, et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57(11):2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 123.Abdul-Ghani MA, Jani R, Chavez A, Molina-Carrion M, Tripathy D, Defronzo RA. Mitochondrial reactive oxygen species generation in obese non-diabetic and type 2 diabetic participants. Diabetologia. 2009;52(4):574–582. doi: 10.1007/s00125-009-1264-4. [DOI] [PubMed] [Google Scholar]
- 124.Rabol R, Hojberg PM, Almdal T, et al. Improved glycaemic control decreases inner mitochondrial membrane leak in type 2 diabetes. Diabetes Obes Metab. 2009;11(4):355–360. doi: 10.1111/j.1463-1326.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- 125.Rabol R, Hojberg PM, Almdal T, et al. Effect of hyperglycemia on mitochondrial respiration in type 2 diabetes. J Clin Endocrinol Metab. 2009;94(4):1372–1378. doi: 10.1210/jc.2008-1475. [DOI] [PubMed] [Google Scholar]
- 126.Rabol R, Svendsen PF, Skovbro M, et al. Reduced skeletal muscle mitochondrial respiration and improved glucose metabolism in nondiabetic obese women during a very low calorie dietary intervention leading to rapid weight loss. Metabolism. 2009;58:1145–1152. doi: 10.1016/j.metabol.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 127.Shen W, Hao J, Tian C, et al. A combination of nutriments improves mitochondrial biogenesis and function in skeletal muscle of type 2 diabetic Goto-Kakizaki rats. PLoS One. 2008;3(6):e2328. doi: 10.1371/journal.pone.0002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.De Feyter HM, Lenaers E, Houten SM, et al. Increased intramyocellular lipid content but normal skeletal muscle mitochondrial oxidative capacity throughout the pathogenesis of type 2 diabetes. Faseb J. 2008;22(11):3947–3955. doi: 10.1096/fj.08-112318. [DOI] [PubMed] [Google Scholar]
- 129.Chowdhury SK, Gemin A, Singh G. High activity of mitochondrial glycerophosphate dehydrogenase and glycerophosphate-dependent ROS production in prostate cancer cell lines. Biochem Biophys Res Commun. 2005;333(4):1139–1145. doi: 10.1016/j.bbrc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 130.Powers WJ, Haas RH, Le T, et al. Normal platelet mitochondrial complex I activity in Huntington’s disease. Neurobiol Dis. 2007;27(1):99–101. doi: 10.1016/j.nbd.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chowdhury SK, Raha S, Tarnopolsky MA, Singh G. Increased expression of mitochondrial glycerophosphate dehydrogenase and antioxidant enzymes in prostate cancer cell lines/cancer. Free Radic Res. 2007;41(10):1116–1124. doi: 10.1080/10715760701579314. [DOI] [PubMed] [Google Scholar]