Abstract
Background:
Our objective was to determine whether antibodies against the Epstein-Barr virus (EBV) nuclear antigen-1 (EBNA-1), early antigen (EA), and EBV neutralizing antibodies (NeutAb) are altered in multiple sclerosis (MS).
Methods:
We measured EBNA-1 IgG, EA IgG, and EA IgA using quantitative ELISA. We measured NeutAb using a quantitative competitive ELISA. We studied 80 MS patients and 80 matched controls, and 19 MS patients with samples collected both while stable and in relapse.
Results:
EBNA-1 IgG and EA IgA were increased in MS compared to controls. The EBNA-1 index value was 23.3 ± 18.3 in the MS patients (mean ± standard deviation) and 16.3 ± 17.4 in the controls (p=0.007, paired t-test). EA IgA had a median value of 1.964 in the MS patients and 1.248 in the controls (p=0.029, Wilcoxon signed rank test). EA IgG and NeutAb were not significantly different. None of the antibody levels were altered in relapse. The correlation between concentrations of different antibodies was minimal.
Conclusions:
IgG antibodies to EBNA-1 are significantly increased in MS. IgA antibodies against EBV early antigen are also increased. The EBV neutralizing antibody response is similar in MS and controls.
Keywords: Epstein-Barr virus, EBV, multiple sclerosis, neutralizing antibodies, early antigen
Introduction
Epstein-Barr virus is considered as a possible causative agent of MS [1, 2]. The experimental evidence consists of a higher prevalence of antibodies against EBV in both adults and children [3-5], increased risk of MS following delayed primary infection with EBV [6], and increased antibodies against EBV in subjects who later develop MS [7-9]. Consistent increases are found in antibodies to the EBV nuclear antigen (EBNA), one of the few EBV proteins expressed in latent infection. EBNA IgG antibodies appear during convalescence from primary infection, remain present long term, and are used as a marker for prior infection [10].
There are multiple other EBV antigens which elicit measurable antibody responses. Early antigens (EA) are expressed early in lytic infection, and EA antibodies appear early in primary infection and may increase in active infection [10-12]. Results with EA antibodies in MS have been mixed. Some investigators have found increased prevalence of EA antibodies in MS [13-16] while others have not [17-19]. There is some suggestion that high levels of anti-EA IgG correlate with disease activity [15, 18]. One study with longitudinal samples over 1 year suggested that EA IgA increased preceding clinical relapse [18], while a different longitudinal study found no change in EA IgG with relapse [20].
EBV neutralizing antibodies are defined by their ability to block infectivity of EBV in vitro. They may play an important role in controlling the persistent EBV infection. All known neutralizing antibodies bind to gp350, the major EBV envelope glycoprotein [21]. The traditional method of testing sera or monoclonal antibodies for neutralizing activity is labor intensive and time consuming, and is impractical for large numbers of samples. Wilson and Morgan have developed an ELISA which gives equivalent results to the traditional assays [22]. This assay takes advantage of the fact that the majority of known neutralizing antibodies bind the same epitope on gp350 [23] and tests the ability of unknown samples to compete for binding to gp350 with the 72A1 mouse monoclonal, a well characterized neutralizing antibody [24]. EBV NeutAb have never been tested in MS.
We undertook this study to further investigate the anti-EBV humoral response in MS. Our initial hypothesis was that EBV infection is poorly controlled in MS. We predicted that EA antibodies would be increased in MS compared to controls, that EA antibodies should increase in relapse, and that protective NeutAb would be decreased in MS.
Materials and Methods
Specimen collection
Blood samples were collected from patients with multiple sclerosis and controls, and serum was stored frozen at −70°C. We selected serum samples from 80 MS patients and 80 controls matched for gender, ethnicity, and age within 5 years. Each group included 51 females and 29 males, 51 caucasians, 19 African-Americans, 8 hispanics, and 2 asians. The mean±sd age was 35.7±9.8 years for the MS patients and 34.2±11.7 for the controls. The MS patients included 73 relapsing-remitting, 5 secondary progressive, and 2 primary progressive. We also tested sera from 19 patients with relapsing-remitting MS with samples collected both during an acute relapse and while clinically stable. The relapse specimens were collected during an urgent clinic visit for new symptoms before any treatment with corticosteroids. We defined a relapse as new neurologic symptoms or worsening of previous neurologic symptoms lasting more than 24 hours and occurring after at least 30 days of clinically stable disease. Sample collection was approved by the University of Texas-Houston Committee for the Protection of Human Subjects, and all subjects signed an informed consent prior to sample collection.
EBNA-1 IgG and EA IgG ELISA
IgG antibodies for EBNA-1 and EA were measured using commercially available ELISA kits with slight modifications to the manufacturer's protocol (Wampole, Princeton, NJ). The EA kit detects both the diffuse and restricted forms of this antigen. These kits are used in clinical testing to determine an index value relative to a calibrator sample with known antibody concentration. We modified the procedure to include a standard curve with the calibrator at 0, 1, 2, and 4 times the usual concentration to permit more accurate quantification. Sera were diluted as needed to fall within the range of the standard curve, usually 1:4, and were run in duplicate. The paired MS and control specimens or the paired relapse and stable specimens were always run adjacent on the same plate.
EA IgA ELISA
To measure EA IgA, we replaced the anti-human IgG secondary antibody in the EA kits with a goat anti-human IgA-horseradish peroxidase at a 1:5000 dilution (Southern Biotech, Birmingham, AL). We used a standard curve with different concentrations of the calibrator for comparison, and the sera values are reported relative to the calibrator raw values. Different batches of calibrator are not standardized for IgA content, so we used a single sample of calibrator for all IgA experiments.
72A1 monoclonal antibody
The 72A1 hybridoma (ATCC, Manassas, VA) was grown in tissue culture under the recommended conditions, and the antibody was purified from culture supernatant with a protein G column (GE Healthcare, Uppsala, Sweden). The concentration of the purified antibody was measured using a Bradford assay.
Preparation of gp350 antigen
B95.8 cells (kind gift of Dr. Cliona Rooney) were grown at 106/ml in RPMI media supplemented with 10% fetal calf serum and stimulated for 5 days with 40 ng/ml phorbol 12-myristate 13-acetate (Sigma-Aldrich, St. Louis, MO) to increase virus production. Cells were collected, resuspended at 5×107/ml, and homogenized in 0.32 M sucrose/Tris 10 mM/pH 7.5 using a motor driven teflon/glass homogenizer. Nuclei were removed by centrifugation for 3 minutes at 1000g, the supernatant was centrifuged one hour at 50,000g, and the cell membrane fraction in the high-speed pellet was resuspended in 9 volumes of water. Triton X100 was added to a final concentration of 1%, and 500 μl of the detergent solubilized fraction were passed through a 0.45 μ filter and separated on a Superose 1.2×30 cm gel filtration column (Pharmacia, Piscataway, NJ) with collection of 1.25 ml fractions. The column fractions were tested for reactivity with the 72A1 monoclonal antibody in an ELISA. This procedure was performed twice, and in both cases the second fraction had the highest reactivity with the 72A1 antibody. Neither the control monoclonal mouse IgG1 nor the secondary antibody alone bound to this fraction, nor did the 72A1 bind later column fractions with much higher protein concentrations.
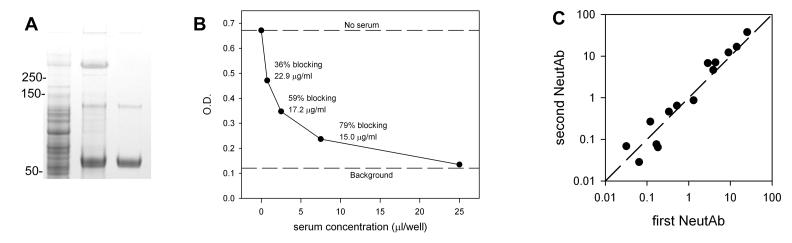
On polyacrylamide gels both the crude cell membrane fraction and the second column fractions contained a protein with molecular weight >250 kilodaltons which stained with Alcian. This protein was immunoprecipitated using 72A1 antibody and protein G beads to confirm its identity as gp350 (Figure 1A).
Figure 1. A. Immunoprecipitation of gp350 with 72A1 antibody.
The first lane is 5 μl of the crude membrane fraction, the second lane is the immunoprecipitate of the membrane fraction with 5 μg 72A1, and the third lane is 72A1 antibody alone. The crude membrane fraction has a protein at >250 kilodaltons which is immunoprecipitated with 72A1. Numbers indicate positions of the molecular weight markers. Gradient gel 4-12% polyacrylamide stained with Coomassie and Alcian. B. Example of neutralizing antibody competitive ELISA. See text for details. C. Reproducibility of the NeutAb ELISA. Results of repeat measurements of the same serum sample at different times. Both axes are on a logarithmic scale.
Neutralizing antibody ELISA
We used a modification of the ELISA method of Wilson and Morgan [22]. This assay measures the ability of neutralizing antibodies in human serum to compete for binding to gp350 with the 72A1 mouse monoclonal neutralizing antibody. The gp350 was diluted in carbonate coating buffer, pH 9.2, and 50 μl/well was added to a flexible microassay plate (BD Falcon, Durham, NC) and incubated at 4°C overnight. The amount of gp350 per well was sufficient to bind 2 ng of 72A1 antibody. Wells were washed five times with PBS/Tween 0.05%, and then blocked with 100 μl/well 10% fetal calf serum in wash buffer. This was followed by incubation with 50 μl/well of mixtures of serum and 72A1. Serum was tested at concentrations of 25, 7.5, 2.5, and 0.75 μl/well, while 72A1 was kept at a constant concentration of 3 ng/well. Sera and 72A1 were mixed together before addition to the ELISA plate. Controls needed for the calculations included 72A1 with no serum and a negative control with neither antibody nor serum. The serum/72A1 mixtures were done in triplicate, and the controls were done in quadruplicate. Sera with high concentrations of NeutAb were diluted further to bring them within the range of the assay. After incubation with serum/72A1 mixture, the plate was washed and incubated with 50 μl of a 1:5000 dilution of goat anti-mouse IgG1 conjugated to horseradish peroxidase (Southern Biotech). The plate was then washed, developed with o-phenylenediamine in citrate buffer, and optical densities were read on an ELISA reader. All incubations were for one hour at room temperature.
Calculations and Statistics
The % blocking of 72A1 binding by serum is calculated from the OD values, and the neutralizing activity of the serum is calculated using the equations:
Solving the equations and then dividing by the volume of serum used yields neutralizing activity in 72A1 μg equivalents/ml. This procedure is illustrated in Figure 1B, which gives the results from an individual with a high neutralizing antibody level. As expected, the OD decreases and the % blocking increases as increasing amounts of serum compete with a fixed amount of 72A1. The three middle points on the curve, representing 0.75, 2.5, and 7.5 μl serum per well, are labeled with their calculated % blocking and their calculated neutralizing activity in 72A1 equivalents. Theoretically, the calculated neutralizing activity should be the same regardless of which serum concentration is used, but there is some variation. We used values which fall in the range of 20 to 80% blocking, and chose the value closest to 50% blocking when multiple values fell in this range. The lower limit of the assay was 0.03 72A1 μg/ml equivalents.
Statistical analysis was performed with SigmaPlot 11.0 using paired parametric or non-parametric tests as appropriate for the observed data distribution.
Results
Comparison of MS and controls
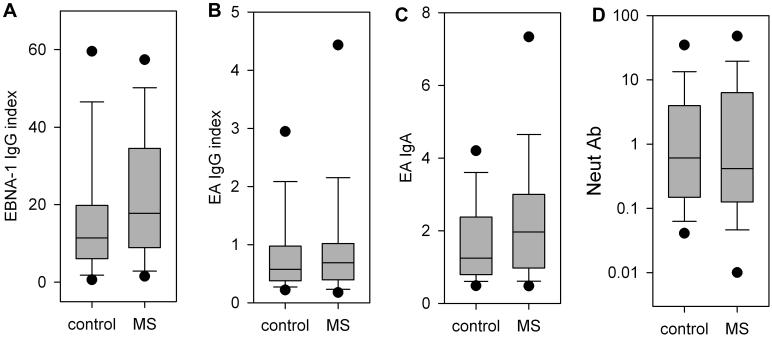
EBNA-1 IgG was significantly higher in the MS patients (Figure 2A). The majority of the subjects, 78 of the MS patients and 74 of the controls, had an EBNA-1 index value greater than 1.1, indicating prior infection with EBV, and the values were significantly higher in the MS group. The EBNA-1 IgG index values were normally distributed with a mean ± standard deviation of 23.3 ± 18.3 in the MS patients and 16.3 ± 17.4 in the controls (p=0.007, paired t-test).
Figure 2. Antibody responses in MS and controls.
The box encloses the values for the 25th to 75th percentiles, the whiskers indicate the 10th and 90th percentiles, and the dots indicate the 5th and 95th percentiles. The median is indicated by the line across the box. A. EBNA-1 IgG, values are given as index values relative to a calibrator sample, the difference between groups is significant, p=0.007, paired t test. B. EA IgG, values are given as index values relative to a calibrator sample. C. EA IgA, values given are the sample OD divided by the OD of a single comparison sample used for all experiments. The difference between groups is significant, p=0.029, Wilcoxon signed rank test. D. NeutAb IgG, values measured in microgram equivalents of the monoclonal neutralizing antibody 72A1, y-axis is logarithmic and values below the limit of the assay are plotted at 0.01.
The EA IgG was not significantly different between the two groups (Figure 2B). Only a minority of the EA IgG index values were in the positive range, with 16 of the MS patients and 18 controls having an index value greater than 1.1. The EA IgG index values had a skewed distribution, with a median of 0.689 in the MS patients and 0.574 in the controls (p=0.50, Wilcoxon signed rank test).
Similarly, the distribution of EA IgA was skewed towards lower values, but EA IgA was higher in the MS group (Figure 2C). The median value was 1.964 in the MS patients and 1.248 in the controls (p=0.029, Wilcoxon signed rank test).
The NeutAb ELISA had good sensitivity and detected measurable NeutAb in the majority of subjects. Only 6 of the MS patients and 3 of the controls had a level less than the assay lower limit of 0.03 μg/ml. The reproducibility of the assay was good. To assess the interassay variability, we repeated measurements on 14 samples spanning the range of concentrations. The values were generally quite close with a correlation coefficient of 0.99 (Figure 1C).
For both MS and controls, the concentrations of NeutAb varied widely with a skewed distribution (Figure 2D). The median was 0.415 in MS and 0.608 in controls, but the difference between groups was not significant (p=0.742, Wilcoxon signed rank test).
Comparison of relapse and stable
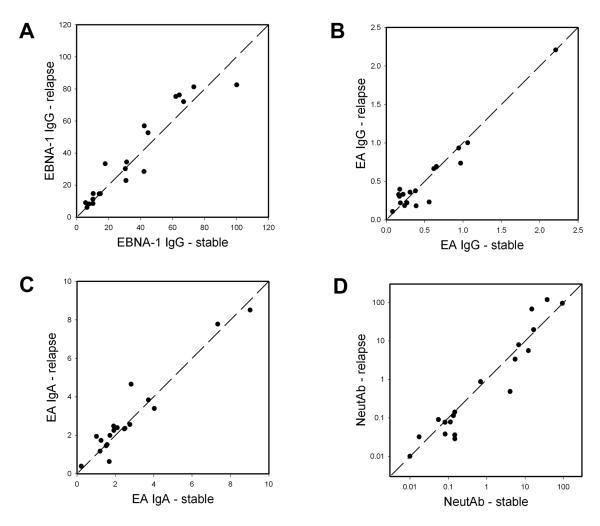
Because of previous findings that EA IgG or IgA might change with disease activity, we tested MS patients who had samples collected while stable and early in a clinical relapse. Neither EA IgG nor IgA increased in relapse (Figure 3). On the contrary, EA antibody levels tended to remain stable over time, as reported by others [15, 20]. Patients with high values tended to stay high, and patients with low values tended to stay low. This same pattern was seen with EBNA-1 IgG and NeutAb.
Figure 3. Changes in antibody titers with relapse.
Each point represents a single MS patient, the value while stable is on the x-axis and the value during relapse is on the y-axis. Subjects with higher values in relapse will be plotted to the upper left of the diagonal line, lower values in relapse will be below and to the right of the diagonal. The units are the same as in Figure 2.
Correlation of antibody responses
The correlation between concentrations of antibodies with different specificities was minimal in both MS and controls (Table). EBNA-1 IgG correlated modestly with EA IgG, but correlations between other antibodies was minimal.
Table.
Correlation coefficients for antibody responses in MS and controls
| Controls | |||
|---|---|---|---|
| NeutAb | EBNA-1 IgG | EA IgG | |
| EBNA-1 IgG | 0.101 | ||
| EA IgG | −0.040 | 0.212 | |
| EA IgA | 0.091 | −0.022 | −0.020 |
| MS | |||
| NeutAb | EBNA-1 IgG | EA IgG | |
| EBNA-1 IgG | −0.002 | ||
| EA IgG | 0.019 | 0.332 | |
| EA IgA | 0.005 | 0.113 | 0.139 |
Discussion
Multiple other investigators have reported an increase in the prevalence or concentration of antibodies to EBNA in MS [13, 14, 16, 18, 19, 25]. We robustly confirmed this finding in our sample of 80 patients and 80 controls. Reported results with antibodies to EA have been variable. Some investigators report an increase in anti-EA [13-16] while others do not [17-19], and the reported prevalence of elevated anti-EA in MS patients ranges from 13% to 69%. We found 20% of our MS group had increased EA IgG, but this was not different from controls. We did find a modest increase in EA IgA in MS, whereas the only previous study that measured this antibody found no difference [17].
Antibodies against EA are of interest since they may indicate active EBV replication. If EBV infection is driving the immune response in MS, then we would expect increased anti-EA in relapse compared to stable disease and in MS compared to controls. There is suggestive experimental evidence that this may be the case. Buljevac et al. [15] found that patients with increased EA IgG were more likely to have enhancing lesions on MRI, but there was no difference in number of clinical relapses. Like the present study, they noted EA IgG was stable over time. Wandinger et al. [18] followed 19 patients with monthly blood samples for a year. Patients with elevated EA IgG were more likely to have relapses, and 8 of their 11 patients with active disease had an increase in EA IgA in association with the relapse. None of their 8 stable patients had an increase in EA IgA. We did find an increase in EA IgA in MS compared to controls, but we did not find an increase in EA IgA with relapse.
Neutralizing antibodies are also of interest in MS. These antibodies may play a role in control of EBV in vivo, and low levels of antibodies might permit more active infection. Neutralizing antibodies have never been measured in MS. We did not find any difference in the levels of NeutAb.
We expected that subjects with high concentrations of antibodies against one EBV antigen would also have increased antibodies to other EBV antigens. This was not the case. There was a modest correlation between the IgG for EBNA-1 and EA, but the other responses were independent. The responses to all the measured antigens in the MS patients tended to remain the same over time and did not change with clinical disease activity. This suggests that each subject forms a unique pattern of anti-EBV antibody reactivity which remains stable, at least over the interval tested in this study. Our observation that the most significant difference between MS and control is in the anti-EBNA antibodies is in agreement with multiple recent studies of humoral and cellular immune responses which suggest a special relevance of EBNA for MS [7-9, 26, 27].
We did not find any evidence to support the idea that EBV infection is poorly controlled in MS. NeutAb are not deficient, and EA IgG is not increased as might be expected in more active infection. The relevance of EA IgA in MS requires further investigation.
Acknowledgements
We thank the nurses of the Clinical Research Unit at the University of Texas-Houston for assistance with sample collection (supported by NIH grant UL1 RR024148). This work supported in part by the Clayton Foundation for Research and the National Multiple Sclerosis Society pilot grant PP1470.
References
- 1.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 2.Lunemann JD, Munz C. EBV in MS: guilty by association? Trends Immunol. 2009;30:243–248. doi: 10.1016/j.it.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A, Munch M. Epstein-Barr virus and multiple sclerosis. Epidemiology. 2000;11:220–224. doi: 10.1097/00001648-200003000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Pohl D, Krone B, Rostasy K, et al. High seroprevalence of Epstein-Barr virus in children with multiple sclerosis. Neurology. 2006;67:2063–2065. doi: 10.1212/01.wnl.0000247665.94088.8d. [DOI] [PubMed] [Google Scholar]
- 5.Banwell B, Krupp L, Kennedy J, et al. Clinical features and viral serologies in children with multiple sclerosis: a multinational observational study. Lancet Neurol. 2007;6:773–781. doi: 10.1016/S1474-4422(07)70196-5. [DOI] [PubMed] [Google Scholar]
- 6.Thacker EL, Mirzaei F, Ascherio A. Infectious mononucleosis and risk for multiple sclerosis: A meta-analysis. Ann Neurol. 2006;59:499–503. doi: 10.1002/ana.20820. [DOI] [PubMed] [Google Scholar]
- 7.DeLorenze GN, Munger KL, Lennette ET, Orentreich N, Vogelman JH, Ascherio A. Epstein-Barr virus and multiple sclerosis: evidence of association from a prospective study with long-term follow-up. Arch Neurol. 2006;63:839–844. doi: 10.1001/archneur.63.6.noc50328. [DOI] [PubMed] [Google Scholar]
- 8.Levin LI, Munger KL, Rubertone MV, et al. Temporal relationship between elevation of Epstein-Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. Jama. 2005;293:2496–2500. doi: 10.1001/jama.293.20.2496. [DOI] [PubMed] [Google Scholar]
- 9.Sundstrom P, Juto P, Wadell G, et al. An altered immune response to Epstein-Barr virus in multiple sclerosis: a prospective study. Neurology. 2004;62:2277–2282. doi: 10.1212/01.wnl.0000130496.51156.d7. [DOI] [PubMed] [Google Scholar]
- 10.Rickinson AB, Kieff E. Epstein-Barr Virus. In: Knipe DM, Howley PM, editors. Fields Virology. 4th edn. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 2575–2627. [Google Scholar]
- 11.Henle W, Henle G, Zajac BA, Pearson G, Waubke R, Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970;169:188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- 12.Buisson M, Fleurent B, Mak M, et al. Novel immunoblot assay using four recombinant antigens for diagnosis of Epstein-Barr virus primary infection and reactivation. J Clin Microbiol. 1999;37:2709–2714. doi: 10.1128/jcm.37.8.2709-2714.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumaya CV, Myers LW, Ellison GW, Ench Y. Increased prevalence and titer of Epstein-Barr virus antibodies in patients with multiple sclerosis. Ann Neurol. 1985;17:371–377. doi: 10.1002/ana.410170412. [DOI] [PubMed] [Google Scholar]
- 14.Myhr KM, Riise T, Barrett-Connor E, et al. Altered antibody pattern to Epstein-Barr virus but not to other herpesviruses in multiple sclerosis: a population based case-control study from western Norway. J Neurol Neurosurg Psychiatry. 1998;64:539–542. doi: 10.1136/jnnp.64.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buljevac D, van Doornum GJ, Flach HZ, et al. Epstein-Barr virus and disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76:1377–1381. doi: 10.1136/jnnp.2004.048504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riverol M, Sepulcre J, Fernandez-Alonso M, et al. Antibodies against Epstein-Barr virus and herpesvirus type 6 are associated with the early phases of Multiple Sclerosis. J Neuroimmunol. 2007;192:184–185. doi: 10.1016/j.jneuroim.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Wagner HJ, Hennig H, Jabs WJ, Siekhaus A, Wessel K, Wandinger KP. Altered prevalence and reactivity of anti-Epstein-Barr virus antibodies in patients with multiple sclerosis. Viral Immunol. 2000;13:497–502. doi: 10.1089/vim.2000.13.497. [DOI] [PubMed] [Google Scholar]
- 18.Wandinger K, Jabs W, Siekhaus A, et al. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology. 2000;55:178–184. doi: 10.1212/wnl.55.2.178. [DOI] [PubMed] [Google Scholar]
- 19.Munch M, Riisom K, Christensen T, Moller-Larsen A, Haahr S. The significance of Epstein-Barr virus seropositivity in multiple sclerosis patients? Acta Neurol Scand. 1998;97:171–174. doi: 10.1111/j.1600-0404.1998.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 20.Torkildsen O, Nyland H, Myrmel H, Myhr KM. Epstein-Barr virus reactivation and multiple sclerosis. Eur J Neurol. 2008;15:106–108. doi: 10.1111/j.1468-1331.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- 21.Thorley-Lawson DA, Poodry CA. Identification and isolation of the main component (gp350-gp220) of Epstein-Barr virus responsible for generating neutralizing antibodies in vivo. J Virol. 1982;43:730–736. doi: 10.1128/jvi.43.2.730-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson AD, Morgan AJ. Indirect measurement of Epstein-Barr virus neutralising antibodies by ELISA. J Virol Methods. 1998;73:11–19. doi: 10.1016/s0166-0934(98)00054-8. [DOI] [PubMed] [Google Scholar]
- 23.Qualtiere LF, Decoteau JF, Hassan Nasr-el-Din M. Epitope mapping of the major Epstein-Barr virus outer envelope glycoprotein gp350/220. J Gen Virol. 1987;68(Pt 2):535–543. doi: 10.1099/0022-1317-68-2-535. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman GJ, Lazarowitz SG, Hayward SD. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc Natl Acad Sci U S A. 1980;77:2979–2983. doi: 10.1073/pnas.77.5.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen PD, Bloomer LC, Bray PF. Epstein-Barr nuclear antigen and viral capsid antigen antibody titers in multiple sclerosis. Neurology. 1985;35:435–438. doi: 10.1212/wnl.35.3.435. [DOI] [PubMed] [Google Scholar]
- 26.Lunemann JD, Edwards N, Muraro PA, et al. Increased frequency and broadened specificity of latent EBV nuclear antigen-1-specific T cells in multiple sclerosis. Brain. 2006;129:1493–1506. doi: 10.1093/brain/awl067. [DOI] [PubMed] [Google Scholar]
- 27.Lunemann JD, Huppke P, Roberts S, Bruck W, Gartner J, Munz C. Broadened and elevated humoral immune response to EBNA1 in pediatric multiple sclerosis. Neurology. 2008;71:1033–1035. doi: 10.1212/01.wnl.0000326576.91097.87. [DOI] [PMC free article] [PubMed] [Google Scholar]