Abstract
Nearly a century after the significance of the human complement system was recognized we have come to realize that its versatile functions extend far beyond the elimination of microbes. Indeed, complement acts as a rapid and efficient immune surveillance system that has distinct effects on healthy and altered host cells and foreign intruders. By eliminating cellular debris and infectious microbes, orchestrating immune responses, and sending `danger' signals, complement contributes substantially to homeostasis, but it may also take action against healthy cells if not properly controlled. This review describes our updated view of the function, structure, and dynamics of the complement network, highlights its interconnection with immunity at large and with other endogenous pathways, and illustrates its dual role in homeostasis and disease.
`A hidden connection is stronger than an obvious one.'
- Heraclitus of Ephesus (535-475 B.C.)
Traditionally, complement has been primarily viewed as a supportive `first line of defense' against microbial intruders, quickly tagging and eliminating them and buying the adaptive immune response time to pick up momentum. Today, we know that complement truly lives up to its name and `complements', or even orchestrates, immunological and inflammatory processes, extending far beyond simple `danger elimination'. The past decade has drawn a picture of how complement acts as an intricate immune surveillance system to discriminate between healthy host tissue, cellular debris, apoptotic cells, and foreign intruders and tune its response accordingly (Fig. 1a–c). Indeed, besides its obvious involvement in eliminating microbes, complement is recognized as participating in such diverse processes as synapse maturation, clearance of immune complexes, angiogenesis, mobilization of hematopoietic stem/progenitor cells (HSPC), tissue regeneration, and lipid metabolism. This versatility appears less surprising when one considers that complement represents one of the most ancient cornerstones of immunity and has tightly co-evolved with phylogenetically younger pathways1. The intricate network of effectors, receptors, and regulators at the heart of complement has been continuously extended as new `players' have been discovered or experienced a resurrection, and novel initiation pathways have been defined (Table 1). Structural and functional studies have provided unprecedented insight into the finely balanced machinery that undergirds these versatile complement functions. However, it also emerged that any trigger that tips this delicate balance between complement activation and regulation can induce self-attack (Fig. 1d). Indeed, complement may contribute to various immune, inflammatory, neurodegenerative, ischemic, and age-related diseases, and complement-targeted therapeutics have recently re-entered the spotlight of drug discovery efforts. Our review will highlight recent and emerging trends that better define complement's role in physiology and pathophysiology.
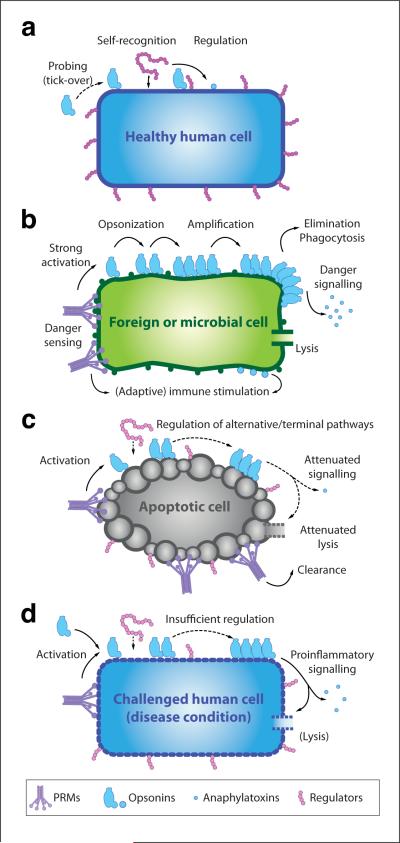
Figure 1. Immune surveillance functions of complement.
A constant low level of complement activation (i.e., tick-over) ensures occasional probing of healthy human cells (a), but the presence of surface-bound regulators and self-recognition by solution-based regulators prevents any amplification of the response. In the case of microbial intruders (b), however, a strong complement response is actively induced by various pattern recognition proteins and subsequently amplified in the absence of regulators. Opsonization by complement fragments and proinflammatory signaling via anaphylatoxins recruit macrophages and enable phagocytosis, and the formation of a lytic membrane attack complex on certain cells such as Gram-negative bacteria leads to cell death. Finally, complement degradation products stimulate downstream immune responses. Although pattern recognition proteins also recognize surfaces of apoptotic cells (c), residual and recruited complement regulators hold amplification and terminal pathways in check. Thus, opsonization facilitates elimination of the cell without triggering danger signals and further immune responses. While this fine-tuned interplay between complement recognition, activation, and regulation usually enables the differential reaction to healthy, apoptotic, and foreign cells, any imbalance between these events may lead to an attack on self-cells (d) and trigger a series of immune and inflammatory diseases. Although proinflammatory and immune signaling appears to be the driving force in most complement-mediated diseases, other processes like TCC-mediated lysis may be involved (e.g., of erythrocytes in the case of paroxysmal nocturnal hemoglobinuria).
Table 1.
Proteins involved in the core complement cascade
| Pattern-Recognition | Alternative Names | Function |
|---|---|---|
| C1q | Part of the C1 complex; recognizes surface-bound IgG, IgM, CRP, and PAMP.; initiates CP | |
| MBL (mannose-binding lectin) | Recognizes carbohydrate patterns; initiates LP | |
| Ficolin-1 | M-Ficolin | Recognizes carbohydrate patterns; initiates LP |
| Ficolin-2 | L-Ficolin; Hucolin | Recognizes carbohydrate patterns; initiates LP |
| Ficolin-3 | H-Ficolin; HAKA1 | Recognizes carbohydrate patterns; initiates LP |
| Properdin | Factor P | Recognizes PAMP and DAMP; initiates AP; stabilizes AP convertases |
| CRP (C-reactive protein)1 | Recognizes DAMP/PAMP on apoptotic and microbial cell cells; binds C1q | |
| CFHR-4 (Factor H-related protein 4) | FHR-4 | Recruits monomeric CRP to necrotic cells; facilitates activation of CP via C1q |
| Proteases | Alternative Names | Function |
|---|---|---|
| C1r | Part of the C1 complex; cleaves C1s | |
| C1s | Part of the C1 complex; cleaves C2 and C4 | |
| MASP-1 (MBL-associated serine protease 1) | Binds to MBL/Ficolins; cleaves C2 (but not C4); may cleave MASP-2 and C3; activates pro-FD; potential role in coagulation cascade? | |
| MASP-2 (MBL-associated serine protease 2) | Binds to MBL/Ficolins; cleaves C2 and C4 | |
| MASP-3 (MBL-associated serine protease 3) | Unknown (binds to MBL/Ficolins, does not cleave C2 or C4) | |
| C2 | Part of the CP/LP convertases; cleaves C3/C5 | |
| fB (Factor B) | CFB | Part of the AP C3/C5 convertases; cleaves C3/C5 |
| fD (Factor D) | CFD | Cleaves C3b-bound FB to form the AP C3/C5 convertases |
| fI (Factor I) | CFI | Degrades C3b and C4b |
| Complement Components | Alternative Names | Function |
|---|---|---|
| C3 | Progenitor for anaphylatoxin C3a, opsonin C3b and signaling fragments (iC3b, C3c, C3d); part of the AP C3 and all C5 convertases | |
| C4 | Progenitor for opsonin C4b; part of the CP/LP convertases | |
| C5 | Progenitor for anaphylatoxin C5a and C5b/TCC | |
| C6 | Part of TCC (membrane-insertion) | |
| C7 | Part of TCC (membrane-insertion) | |
| C8 | Part of TCC (induction of pore formation) | |
| C9 | Part of TCC (forms lytic pore) |
| Receptors2 | Alternative Names | Function |
|---|---|---|
| CR1 (complement receptor 1) | CD35; C3b/C4b-receptor | Binds C3b/iC3b; Induces phagocytosis: accelerates decay of convertases; cofactor for fI |
| CR2 (complement receptor 2) | CD21; C3d-receptor | Binds iC3b/C3d; lowers threshold for B-cell stimulation |
| CR3 (complement receptor 3) | CD11b/CD18; Mac-1 (Macrophage-1 antigen); integrin aMb2 | Induces phagocytosis via interaction with iC3b; modulates IL-12 family in APC |
| CR4 (complement receptor 4) | CD11c/CD18; p150/95; integrin aXb2 | Induces phagocytosis via interaction with iC3b |
| C3aR (C3a receptor) | Binds C3a; triggers proinflammatory signaling | |
| C5aR (C5a receptor) | CD88 | Binds C5a; triggers proinflammatory signaling |
| C5L2 (C5a receptor 2) | GPR77 | Binds C5a / C5adesArg (may bind C3a/C3adesArg); function controversial |
| CRIg (Complement receptor of the immunoglobulin family) | Z93Ig, VSIG4 | Induces phagocytosis via interaction with iC3b/C3c; regulatory effect on C5 convertases |
| cC1qR (C1q receptor for collagen region) | Calreticulin; collectin receptor | Recognizes bound C1q; induces phagocytic signaling via CD91 |
| gC1qR (C1q receptor for globular heads) | C1qbp (C1q-binding protein); p33 | Recognizes C1q; potential role in phagocytosis and signaling; modulates IL-12 on APC |
| C1qRp | (CD93 + unknown mediator) | Part of receptor complex that binds C1q and mediates phagocytosis |
| Regulators | Alternative Names | Function |
|---|---|---|
| C1-INH (C1 esterase inhibitor) | SERPIN1 (Serine protease inhibitor 1) | Inhibits C1r/s and MASPs |
| sMAP (small MBL-associated protein) | MAp19 (MBL-associated protein 19) | Binds to MBL, competes with MASPs |
| MAP-1 (MBL/ficolin-associated protein 1) | MAp44 ((MBL-associated protein 44) | Binds to MBL/ficolins; inhibits C4 deposition |
| C4BP (C4b-binding protein) | C4-binding protein | Accelerates decay of LC/CP convertases; cofactor for fI |
| fH (Factor H) | CFH | Recognizes self-surfaces; accelerates convertase decay; cofactor for fI |
| FHL-1 (Factor H-like protein 1) | Reconectin, CFHL1 | Accelerates convertase decay; cofactor for fI |
| MCP (Membrane Cofactor Protein) | CD46 | Cofactor for fI |
| DAF (Decay Accelerating Factor) | CD55 | Accelerates decay of convertases |
| CFHR-1 (Factor H-related protein 1) | FHR-1 | Recognizes self-surfaces and C5; inhibits C5 cleavage and TCC formation |
| CD59 | Protectin | Binds to C8 and C9; prevents assembly of TCC |
| VTN (Vitronectin) | S-protein; S40 | Binds to C5b-9; prevents assembly of TCC |
| CLU (Clusterin) | Apolipoprotein J; SP-40,40 | Binds to C7–C9; prevents assembly of TCC |
| CPN (Carboxypeptidase-N) | Degrades C3a and C5a to their desArg forms | |
| CRIT (C2 receptor inhibitor trispanning) | Binds to C2; prevents cleavage by C1s |
Although CRP is the best-described representative mediator of this category, other pentraxins may exert similar functions
Additional receptor for C1q (e.g. CR1, integrin α2β1, murine SIGN-R1) have been proposed but not yet been confirmed.
Complement initiation and amplification
Although complement is commonly depicted as a linear cascade of separate pathways, it is essentially a hub-like network tightly connected to other systems (Supplementary Fig. 1). Depending on the trigger, several initiation and regulatory mechanisms act together to produce an anticipated result in immune surveillance (Fig. 1 and 2).
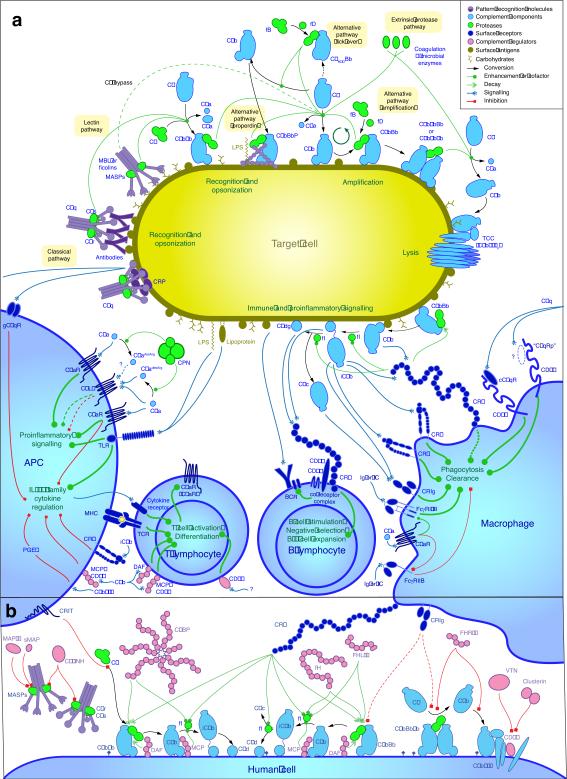
Figure 2. Detailed view of complement activation, amplification, signaling, and regulation.
(a) An intricate network of soluble and cell-surface- bound proteins enables the recognition, tagging, and elimination of microbial intruders and foreign cells and stimulates downstream immune responses. In the classical pathway (CP), C1q recognizes pathogen- or damage-associated patterns (such as IgG, IgM, or CRP) on foreign or apoptotic cells, inducing the formation of the CP C3 convertase (C4b2b) via cleavage of C2 and C4 by C1s. Detection of carbohydrate patches by MBL or ficolins associated with MASP via the lectin pathway enables the formation of the same convertase, which subsequently activates the abundant plasma protein C3, generating its active fragments C3a and C3b. Covalent deposition of C3b on nearby surfaces (i.e. opsonization) leads to the binding of factor B and conversion into the alternative pathway (AP) C3 convertase (C3bBb), which cleaves more C3 into C3b and thereby amplifies the complement response. In addition, a low level of complement activation is maintained in solution (i.e., tick-over), and resulting C3b or C3 convertases can be recruited to foreign surfaces and stabilized by properdin (P). Increasing densities of C3b on the surface lead to a gradual substrate shift of the convertases from C3 to C5. Cleavage of C5 into C5a and C5b initiates the assembly of the lytic terminal complement complex (TCC) on susceptible cells. Opsonization by C1q, C3b, and its degradation products iC3b, C3c, and C3d induces phagocytosis via a panel of complement receptors. In addition, the anaphylatoxins C3a and C5a cause strong proinflammatory signaling via their respective GPCR. C5a also co-regulates immunoglobulin (Ig)-mediated phagocytosis of immune complexes (IC) by modulating the differential expression of activating (FcγRI/III) and deactivating (FcγRIIB) Fc-gamma receptors. On B cells, binding of C3dg to the CR2/CD19 co-receptor complex lowers the threshold of activation by several orders of magnitude and plays an important role in their maturation. Close crosstalk between Toll-like receptors (TLR), complement receptors, and regulators modulates IL-12 in antigen-presenting cells (APC) and thereby influences activation and differentiation of T cells. (b) On healthy human cells, any complement activation or amplification is immediately attenuated by a panel of surface-bound regulators that either accelerate decay of the convertases (CR1, DAF), act as a cofactor for the factor I-mediated degradation of C3b and C4b (CR1, MCP), or prevent the formation of the TCC (CD59). In addition, solution-based regulators such as C4BP, FH, or FHL-1 recognize self-surface pattern-like glycosaminoglycans and further impair activation. Finally, a diverse set of regulators enables control at the level of initiation (C1-INH, MAP-1, sMAP, CRIT, FHR-4), the C5 convertases (FHR-1, CRIg) or TCC (FHR-1, VTN, clusterin).
The classical pathway (CP) is often referred to as `antibody-dependent' because of its strong initiation by IgM/IgG clusters. However, the versatile pattern recognition molecule (PRM) C1q activates complement by recognizing distinct structures directly on microbial and apoptotic cells or via endogenous PRMs like immunoglobulins and pentraxins (e.g., C-reactive protein; CRP). As part of the C1 complex (i.e., C1qr2s2), the proteases C1r and C1s are consecutively activated upon surface binding of C1q2,3. C1s subsequently cleaves C4 into C4a and C4b, exposing a previously hidden thioester and leading to covalent deposition of C4b on surfaces in the immediate vicinity of the activation sites (i.e., opsonization). By cleaving C4b-bound C2 into C2aa and C2ba, C1s also mediates the generation of the CP C3 convertase (C4b2ba), which is capable of cleaving C3 and initiating amplification and downstream effector functions.
In the functionally similar lectin pathway (LP), mannose-binding lectin (MBL) and ficolins act as PRM that predominantly recognize carbohydrate patterns. Each PRM assembles with MBL-associated serine proteases (MASPs) that share structural similarity with C1r/C1s. Of these, only MASP-2 cleaves both C4 and C2, generating the same C3 convertase of the CP. In contrast, MASP-1 cleaves C2 but not C4 and may supplement the LP response once initiated4, thereby increasing the efficiency of convertase formation5,6.
In contrast to the CP and LP, the `alternative' pathway (AP) represents three distinct but partially overlapping processes. The `tick-over' segment keeps complement alert allowing for constant probing of cells7,8. In its native form, C3, the central molecule of the AP, has few ligands and is relatively inert. However, a small fraction of the C3 molecules are hydrolyzed to C3H2O, exposing new binding sites. The factor B (fB) protease binds C3H2O and is cleaved by factor D (fD), generating an initial, mainly solvent-based C3 convertase (C3H2OBb) that activates complement by cleaving C3 into its active fragments, C3a and C3b. Cleavage of C3 exposes a reactive short-lived thioester moiety in C3b. This initial tagging is quickly amplified on foreign cells but is immediately regulated on human cells. Moreover, the reactivity of the thioester moiety to specific carbohydrates may lead to preferential opsonization of foreign particles and represent a basic pattern recognition mechanism9,10. The AP also includes a PRM-based initiation mechanism that resembles those found in the LP/CP and involves properdin. The latter recognizes several pathogen- or damage-associated molecular patterns (PAMP and DAMP, respectively) on foreign and apoptotic cells. Once bound, it initiates and propagates the complement response by attracting fluid-phase C3b to recognized surfaces11 and allowing de novo convertase assembly, and by stabilizing C3 convertase complexes (i.e., C3bBbP)12.
All surface-bound C3 convertases, regardless of origin, can induce the amplification branch of the AP via activation of C3. The resulting C3b is rapidly deposited in the immediate vicinity of the activation and forms the major AP C3 convertase in the presence of fB and fD (i.e., C3bBb), thereby creating an efficient cycle of C3 cleavage and convertase assembly that dramatically amplifies the response. Despite its name, the AP may account for up to 80–90% of total complement activation, even when initially triggered by the CP or LP13. Complement amplification may also occur in plasma when C3b forms dimeric complexes with IgG, which are partially protected from degradation and further stabilized by binding properdin14.
AP amplification increases the density of deposited C3b and gradually leads to formation of convertases that contain an additional C3b molecule (i.e., C4b2b3b or C3bBb3b) and shift the substrate specificity from C3 to C5. These C5 convertases cleave C5 into the anaphylatoxin C5a and fragment C5b. When C5b associates with C6 and C7, the complex becomes inserted into cell membranes and interacts with C8, inducing the binding of several units of C9 to form a lytic pore, the terminal complement complex (TCC; C5b-9n; also known as membrane attack complex)15.
Substitute routes of complement activation have emerged in recent years. Although it has long been known that proteases such as plasmin, thrombin, and plasma kallikrein can cleave and activate C3, this `extrinsic protease' pathway (Fig. 2a) has gained more attention in the context of a potential crosstalk between complement and coagulation pathways16. Recently, thrombin was shown to induce C5a generation in the absence of C317, indicating that the extrinsic protease pathway extends to C5. Furthermore, target-bound MBL can activate C3 independently of MASP-2, C2, and C4 in vitro18 (`C2 bypass'; Fig. 2a), yet the physiological implications of this bypass require further investigation19.
Effector functions of complement
Although TCC-mediated lysis is considered a hallmark of complement attack, there are surprisingly few supportive examples. In fact, many pathogens are protected from lysis through their cell wall architecture (e.g., Gram-positive bacteria) or by employing evasive strategies that interfere with TCC assembly20. However, even sublytic amounts of TCC or partial complexes like C5b-8 play important roles in `non-lethal' signaling events21.
Proinflammatory signaling and phagocytosis are critical for complement-mediated defense against most foreign cells. During activation and amplification, C3a and C5a are constantly released and trigger proinflammatory signaling via their corresponding G-protein-coupled receptors, i.e., C3a receptor (C3aR) and C5a receptor (C5aR; CD88). A third but G-protein-independent anaphylatoxin receptor, C5L2 (GPR77), has recently been discovered; its functions are still matter of debate22, ranging from decoy to regulatory or even proinflammatory23,24. Whereas C3aR binds only C3a, C5aR recognizes both C5a and (more weakly) its degradation product C5adesArg. Interestingly, C5L2 interacts with C5adesArg and C5a with comparable affinities25. Although a functional link between C3a/C3adesArg and C5L2 is suggested, proof of a direct interaction remains controversial. Anaphylatoxins are highly potent effectors and play a number of critical roles in immune responses and inflammation, which are discussed below and reviewed elsewhere26,27.
C3a, and especially C5a, are powerful chemoattractants that guide neutrophils, monocytes, and macrophages towards sites of complement activation. Thereby, they promote phagocytosis via the interaction of opsonins with complement receptors (CR)28. Among these, CR1 (CD35) is particularly significant, since it interacts not only with C3b and C4b to promote neutrophil-mediated phagocytosis but also contributes to regulatory degradation of its ligands by factor I (fI)29. Thus, CR1 attenuates complement amplification by removing C3b/C4b, while simultaneously rendering the opsonins accessible to other CRs and promoting downstream immune responses. The integrin receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) both bind to iC3b and contribute to phagocytosis, while CR3 also regulates cytokine responses, leukocyte trafficking, and synapse formation.
Recently, another phagocytic CR, the complement receptor of the immunoglobulin family (CRIg), has been identified. CRIg is exclusively expressed on certain tissue-resident macrophages and enhances phagocytosis by recognizing cell-bound C3b and iC3b30. It is unique in that it also binds C3c, although whether this binding serves to clear C3c or induces signaling events has yet to be determined31.
Despite their structural similarity, CR1 and CR2 (CD21) have distinct yet highly synergistic functions: CR1 is a major contributor to surface-bound iC3b and the only cofactor that enables further cleavage to C3dg, both of which serve as CR2 ligands for B cell activation and differentiation32,33. Besides initiating the complement cascade, PRM also act as opsonins and participate in phagocytic and inflammatory signaling. Though several receptors for C1q have been described, their exact roles are not well defined. Originally designated cC1qR because of its ability to bind the collagenous part of C1q (and collectins such as MBL), cC1qR was later found to be identical to the secreted protein calreticulin and to require a transmembrane mediator, most likely CD91, to induce phagocytosis34. The C1q receptor of phagocytosis (C1qRp/CD93) appears to behave similarly, since it apparently does not bind C1q directly and may require an unidentified bridging molecule to form a phagocytic receptor complex35. Finally, gC1qR, which interacts with the globular head domains of C1q, is involved in cytokine regulation (Fig. 2a). Importantly, genetic deletion of either CD91 or CD93 in murine macrophages did not significantly alter C1q-mediated phagocytosis36,37, thereby underscoring the complexity and potential redundancy in C1q interactions.
Complement regulation
Soluble and cell-bound complement regulators help control complement attack and adjust its severity, propagation, and endpoints to the cellular target (Fig. 1a–c; Fig. 2b)38. In addition to C1 esterase inhibitor (C1-INH), a secreted glycoprotein of the serpin family that inhibits several CP/LP proteases, two other LP modulators have been identified: sMAP and MAP-1 are non-proteolytic splice products of the MASP2 and MASP1/3 genes, respectively, that apparently compete with MASPs for binding to MBL and ficolins. The `C2 receptor inhibitor trispanning' also binds to C2 and inhibits its activation by C1s39. In the AP, activation in solution is primarily controlled by the abundant factor H (fH) and its truncated homolog `factor H-like protein 1' (FHL-1). fH mainly acts on AP C3 convertases, either competitively removing Bb from the C3bBb complex (i.e., decay acceleration) or serving as a cofactor for the fI-mediated degradation of C3b. Another fluid-phase regulator, C4b-binding protein (C4BP), has similar effects on CP/LP convertases. Most importantly, fH, FHL-1, and C4BP also support complement regulation on human cells by engaging host-specific surface patterns (e.g., sialic acid or glycosaminoglycans), thereby contributing to self-recognition and prevention of host attack. In addition, most human cells expose convertase regulators that act as decay accelerators, like CR1 or the shorter decay accelerating protein (DAF/CD55), or as cofactors for fI, like CR1 and membrane cofactor protein (MCP/CD46)38,40. In contrast to the relative abundance of C3 convertase regulators, C5-specific regulators are rare and only recently described: Whereas `fH-related protein 1' directly binds C5 and inhibits C5 convertase activity, CRIg regulates the C3b-containing C3 and C5 convertases, although the physiological implications of the latter mechanism are still unknown. A cell-based regulator, CD59, acts on TCC by preventing the formation of both sublytic and lytic complexes. In addition, the TCC is controlled by soluble regulators such as vitronectin and clusterin. Finally, carboxypeptidase-N quickly converts anaphylatoxins to their desarginated forms; while this cleavage impairs signaling via the primary receptors C3aR and C5aR, it shifts the signaling pattern, since C3adesArg and C5adesArg themselves may trigger important functions, e.g., during HSPC mobilization41,42 or lipid metabolism43.
Structure and dynamics in complement
Complement must react quickly to potential danger, yet be selective enough to avoid wreaking havoc on the host. Molecular studies provided a fascinating insight into how time, location, concentration, and dynamics are used to achieve selectivity and orchestrate cascading events. For example, the highly abundant C3 can act as an omnipresent sentinel that has few endogenous ligands44, but becomes transformed into one of the most versatile binding partners in circulation upon activation to C3b. Whereas the exposure of a highly reactive but short-lived acyl-imidazole moiety restricts deposition of C3b to immediate sites of activation, additional control mechanisms are responsible for keeping amplification through C3 convertases in check. Structural studies of the C3 convertase suggested a mechanism whereby C3b bridges and orients the proteolytic Bb fragment of fB with C3 to allow its cleavage45. Moreover, the recent report of a C3b-fH complex46 provided a molecular base for major regulatory mechanisms; bound fH not only sterically interferes with contact areas of fB and Bb on C3b to accelerate convertase decay, but also forms a joined binding pocket with C3b that allows fI to bind and cleave C3b, while stabilizing the domain arrangement of C3b during cleavage46. This conversion disrupts domains involved in amplification yet exposes CR-specific binding sites, thereby facilitating downstream signaling steps. These and similar studies on other components have complemented our molecular picture of complement initiation, amplification, regulation, and signaling and revealed that many of these proteins rely on dynamic rearrangements to fulfill their roles in these processes.
Interaction with microorganisms
Once PAMP are detected on invading microorganisms, one or several complement initiation pathways are triggered aiming to eliminate microbial intruders. As microbes normally lack complement regulators, the response is rapidly amplified by the AP and results in opsonization, proinflammatory signaling, mobilization of immune cells, phagocytosis, and, on certain pathogens such as Gram-negative bacteria (mostly Neisseria species) or parasites, formation of TCC and subsequent cell lysis (Fig. 1b). Given its central and early role in antimicrobial attack and the millennia of co-evolution between microorganisms and this ancient component of immunity, it is not surprising that many pathogens evolved counterstrategies to evade complement. Indeed, elaborate complement evasion mechanisms are found in bacteria, viruses, fungi, and parasites20. These strategies most commonly involve the non-activating capture of complement initiators (e.g., immunoglobulins), inactivation or depletion of complement components by secreted proteases, recruitment of complement regulators to the pathogen surface or secretion of regulator mimics, molecular inhibition of convertase activity, interference with TCC formation, or competitive and antagonistic prevention of immune signaling (Supplementary Fig. 2)20. Even more intriguingly, some pathogens take advantage of the complement system to enter cells by binding to cell-bound complement receptors and regulators, either via pathogen-expressed surface proteins or `voluntary opsonization' with complement fragments20,47,48.
Removal of apoptotic cells
The role of complement is not limited to inductive and effector phases of immunity, since it contributes to the resolution of inflammation by promoting safe clearance of apoptotic cells and immune complexes through the cooperative action of its soluble PRM, opsonins, and receptors49,50. Importantly, complement can differentiate between real threats and challenges that normally pose minimal danger: in contrast to a vigorous response against foreign intruders, removal of endogenous debris requires a much more `tactful' approach, in which complement avoids mechanisms downstream of C3 like the inflammatory C5a-C5aR axis or TCC. Alterations in cell surface molecules during apoptosis allow dying cells to bind complement-related PRM and become opsonized. The modified surfaces of apoptotic cells, which rapidly shed MCP/CD46 and CD5951, permit complement activation and their opsonization with C3b and C4b, followed by phagocytic cell uptake38,49. However, residual regulators on the cell surface and in circulation tame subsequent complement amplification, thereby largely preventing the generation of danger signals (Fig. 1c). This activity is highly dependent on the CP since macrophages display reduced ability to take up apoptotic cells in individuals deficient in C1q, C4, C2, or C352. Early-stage effectors are preferentially utilized because the modified apoptotic cell surface acquires CRP and C1q together with fH38,53. Specifically, CRP promotes the binding of C1q to apoptotic cells, amplifies CP activation, and recruits fH, which inhibits AP amplification and C5 convertase formation, thereby protecting cells from necrotic lysis and the host from unwarranted inflammation53. As a further safety mechanism, the clearance of apoptotic cells through iC3b opsonization and CR3 phagocytosis is accompanied by IL-12 downregulation and a lack of oxidative burst in macrophages54–56 or by reduced co-stimulatory molecule expression and impaired maturation of DC57,58. Furthermore, even when the host sustains injury during an assault, complement may contribute to homeostasis by promoting tissue repair59,60(Fig. 3).
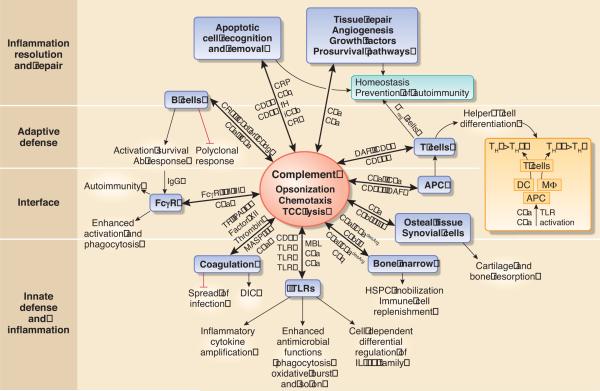
Figure 3. Integrative role of complement in host defense and homeostasis.
The traditional functions of complement (within the oval shape) and its orchestrating role in immunity and homeostasis are shown (key participating molecules are indicated next to the arrows): Complement regulates TLR signaling to coordinate innate defenses and potentiates coagulation to additionally provide a mechanical barrier against the spread of invading bacteria. It also facilitates IgG-mediated phagocytic killing of microbes by reducing the threshold for FcγR activation and promotes specific IgG antibody responses through B-cell activation. Complement regulation of APC TLRs impacts on the differentiation of T cells, which can also be affected directly by complement. Regulation of helper T cell differentiation by complement is a complex process; however, in the context of complement-TLR crosstalk, the anaphylatoxins may either promote or inhibit TH1 or TH17 development in vivo, depending on whether they act through DC or macrophages (MΦ) as APC. To replenish the immune system during infection or injury, complement regulates the mobilization of HSPC from the bone marrow. Most, if not all, mechanisms regulated by complement may also be involved in immunopathology, such as FcγR-mediated autoimmunity, disseminated intravascular coagulation (DIC), or inflammatory bone resorption (e.g., through sublytic C5b-9 signaling). The functional repertoire of complement includes a major role in the resolution of inflammation through induction of regulatory T cells (Treg), non-inflammatory clearance of apoptotic cells (which permit complement activation through loss of complement regulatory proteins), and promotion of tissue repair. Most complement interactions are bidirectional, in that the production and activation of complement proteins and receptors is regulated by other receptors or system components (e.g., FcγRs, TLRs, thrombin).
Complement crosstalk with TLRs
To efficiently respond to `danger', complement uses both `pattern recognition' and `missing-self recognition' strategies61, deploys its rapid cascade, and, importantly, crosstalks with other biological systems (Fig. 3). For example, it coordinates innate immunity in cooperation with Toll-like receptors (TLRs)61, helps block the spread of infection by potentiating coagulation16, links the innate response to both humoral and cellular adaptive immunity62, and regulates bone marrow mobilization of HSPC to replenish the immune system41. Both complement and TLRs are swiftly activated in response to infection or common microbial structures, such as lipopolysaccharides (LPS) or microbial CpG DNA. An emerging body of evidence indicates extensive and bidirectional cooperation between the two systems, shaping the innate response through both synergistic and antagonistic crosstalk61. Concomitant detection of infection by distinct innate defense systems validates the presence of genuine danger, justifying and necessitating the amplification of the host's antimicrobial and inflammatory response. For instance, complement synergistically enhances TLR-induced production of proinflammatory cytokines (TNF, IL-1β, and IL-6) in vitro and in vivo through C3aR, and especially C5aR signaling63. At least three TLRs (TLR2, TLR4, and TLR9) are involved in complement crosstalk, and their pathways converge with anaphylatoxin signaling at the level of mitogen-activated protein kinases (MAPKs), specifically ERK1/2 and JNK63. This crosstalk partially explains earlier observations that inhibiting C5a signaling protects against sepsis induced by high doses of LPS or by cecal ligation and puncture (CLP) peritonitis64. Reciprocally, TLR activation induces expression of complement components or receptors65.
Intriguingly, C5a-induced TLR crosstalk might not only involve C5aR but also the still-enigmatic G protein-independent C5L2, which may have both regulatory and proinflammatory roles23,61,66(Fig. 2a). Thus, C5L2 is essential for the induction of high-mobility group box-1 (HMGB1), through which C5L2 contributes synergistically with C5aR to inflammatory lethality in CLP sepsis23. At least in vitro, HMGB1 induction by C5a or LPS (or both) is diminished in C5l2−/− but not C5ar−/− macrophages23. These findings suggest possible C5L2-TLR4 cooperation in HMGB1 induction, perhaps through signaling crosstalk involving the MAPK and phosphatidylinositol-3 pathways23. Alternatively, C5L2 might act as a co-receptor for TLR4 activation.
Whether complement receptors co-associate with TLRs in functional multi-receptor complexes is currently being investigated: Confocal microscopy and fluorescent resonance energy transfer experiments suggest C5aR and TLR2 co-association in activated macrophages67. Furthermore, in the apparent absence of C5a, blockade or genetic ablation of C5aR inhibits TLR2-induced cytokine production in dendritic cells (DC), suggesting a C5a-independent C5aR effect on TLR2 signaling68. Moreover, MBL forms a functional complex with TLR2 in the phagosome and potentiates protective TLR2 signaling, at least in response to Staphylococcus aureus69. However, the observation that CD14 blockade inhibits complement inflammatory activities in a human whole blood model70 could reflect either inhibition of complement-TLR crosstalk (CD14 lacks a transmembrane signaling domain and `signals' through TLR4 or TLR2) or extracellular interactions of CD14 with complement receptors. CD14 can physically co-associate with CR371, although direct interactions with anaphylatoxin receptors have not been reported.
Complement's ability to cooperate with TLRs may even undermine host immunity, if these crosstalk interactions are induced by microbial cells. For example, the oral pathogen Porphyromonas gingivalis induces C5aR-TLR2 crosstalk that impairs nitric oxide-dependent killing in macrophages67. Because this crosstalk inhibits only a subset of TLR2 signaling events67, C5aR was characterized as a `TLR modulatory receptor' to distinguish it from `TLR inhibitory receptors' (e.g., IL-10R or TGF-βR), which inhibit most, if not all, inflammatory responses72. Moreover, although TLR2-induced transactivation of CR3 contributes to leukocyte trafficking73, certain pathogens activate this TLR2 signaling pathway to exploit transactivated CR347,48. Reciprocally, CR3 facilitates TLR2 or TLR4 signaling by promoting the recruitment of their sorting adaptor TIRAP74.
In summary, by cooperating with other innate receptors, complement receptors can diversify their pattern-recognition and signaling or regulatory capacities, and these same properties can be exploited by pathogens to undermine immune defense.
Complement-coagulation interplay
Another immediate early crosstalk event during infection occurs between complement and the coagulation system, ostensibly aiming to enhance local clotting and prevent microbial spread through the circulation16. Complement amplifies coagulation and inhibits fibrinolysis, mainly through C5a, which induces the expression of tissue factor (TF)75 and plasminogen-activator inhibitor 1 (PA-I1)16. Moreover, MASP-2 can simultaneously activate complement and coagulation, the latter through generation of thrombin from prothrombin76. Reciprocally, components of the coagulation cascade amplify complement activation (see also the `extrinsic pathway' above and Fig. 2a); for instance, activated clotting factor XII can activate the CP through C1 cleavage77, whereas thrombin directly cleaves C5 and generates biologically active C5a17.
These pro-coagulatory activities, however, are counteracted by certain pathogens, which subvert the fibrinolytic system and cause disseminating infections78. On the other hand, when the complement-coagulation crosstalk is activated systemically in an uncontrolled manner, as in sepsis, it can lead to life-threatening conditions such as disseminated intravascular coagulation79. Additional aspects of this important crosstalk, such as the interplay between complement and platelet activation, have been covered elsewhere16.
Complementing humoral immunity
As alluded to above, C3dg functions as a natural adjuvant, since binding of C3dg-opsonized antigen to CR2 on the B cell co-receptor complex (CR2-CD19-CD81) has a strong co-stimulatory effect on B cells32,80(Fig. 2a). Moreover, CR2 mediates antigen-independent signals that are necessary for B cell survival within germinal centers81. On follicular DC, CR1 and CR2 are the main receptors for uptake and long-term retention of antigen, thereby contributing to B cell memory maintenance32. However, antibody responses to T-dependent antigens are impaired in mice with a B cell-specific CR1/CR2 deficiency (a single gene, Cr1/2, encodes both molecules), despite normal follicular DC expression of both receptors82. Therefore, the interaction of B cell-expressed CR2 and/or CR1 with C3 cleavage products is essential for antigen-specific antibody responses, though not polyclonal antibody responses83. Experiments in CR1/CR2-deficient mice have confirmed the importance of complement for antibody responses to both T-dependent and T-independent antigens, including protective humoral immunity to certain bacteria and viruses84–86. The anaphylatoxins also have regulatory effects on B cells, including suppression of B cell polyclonal responses (C3a)87 and promotion of the migratory activity of naïve and memory B cells (C5a)88.
Complement promotes the effector function of the antibody response in ways that far exceed its originally identified role as a heat-sensitive activity in serum that complements that of antibody in causing bacterial lysis. At the interface of innate and humoral immunity, C5aR signaling lowers the threshold for Fcγ receptor activation by upregulating the expression of activating Fcγ receptors (FcγR I and III) and downregulating the expression of the inhibitory FcγRIIB89,90. Conversely, FcγR activation enhances the synthesis of C5 for C5a generation90. This mutually reinforcing C5a-FcγR crosstalk (Fig. 2a) is important in infection as it promotes clearance of microbial intruders by combining phagocytosis with the specificity of IgG antibody. However, since IgG immune complexes are implicated in autoimmune disorders (e.g., rheumatoid arthritis), complement-FcγR crosstalk may actually exacerbate autoimmune pathology, as demonstrated in a mouse model of autoimmune hemolytic anemia90.
Regulatory role of complement in T cell immunity
Early reports that anti-CR1/CR2 monoclonal antibody (mAb)-treated mice exhibit impaired antibody but intact T helper responses91 could be interpreted as a lack of C3 involvement in T cell immunity. Similarly, CR1/CR2-deficient mice develop normal CD4+ and CD8+ T-cell immunity and readily clear infection with influenza virus92; however, pathogen-specific T cell responses are impaired in C3-deficient mice in a CR1/CR2-independent manner92,93. C3 deficiency also results in impaired T cell responses in models of autoimmune disease and transplantation rejection94,95, at least in part because of lack of C3a and altered antigen-presenting cell (APC) function. For instance, C3aR-deficient DCs lose their ability to induce potent alloreactive CD4+ T cell responses96. Moreover, C3a and C5a can be produced locally at the APC-T cell interface and may effectively determine the outcome of APC-T cell interactions62,97–99(Fig. 3).
Experiments in mice lacking complement inhibitory proteins (DAF, CD59) highlight an important regulatory role for complement in the development of T cell immunity62,100,101. Interestingly, complement regulatory proteins exert both indirect (via APC) and direct effects on T cells (Fig. 2a). For instance, DAF-deficient T cells display heightened cytokine responses and CD59 ligation of CD4+ T cells downregulates their activation, whereas activation of CD46-ligated CD4+ T cells promotes their differentiation to a T regulatory 1 (TR1) phenotype62,100,102. CD46- and CD59-mediated signaling in T cells may contribute to the resolution of the immune response, thereby preventing immunopathology100,102. Conversely, bacterial or viral pathogens capable of directly engaging these complement regulators may undermine T cell immunity as a survival tactic62,103. For example, Streptococcus pyogenes interacts via its M protein with CD46 on human CD4+ T cells to induce a TR1-like phenotype that suppresses bystander T cell activation103.
It should be noted that the effects of complement on TH responses are influenced by tissue-specific microenvironmental conditions and timing variables. For instance, C5aR signaling protects the lungs against allergic asthma during the allergen-sensitization phase (C5a signaling at the DC-T cell interface leads to induction of TGF-β and IL-10), whereas it drives a TH2-mediated eosinophil/mast cell destructive response once allergic inflammation is established104; Intriguingly, however, the protective role of C5aR signaling during the allergen-sensitization phase is countered by C3aR signaling105, which, moreover, contributes to TH2-dependent airway hyperreactivity106. The emerging roles of IL-17 and TH17 in asthma107 and the ability of anaphylatoxins to regulate these responses in cooperation with TLRs68,108,109 suggest that complement's influence on this disease may be more complex than originally thought.
Impact of complement-TLR crosstalk on T-cell responses
Recent work has focused on how complement-TLR crosstalk in macrophages and DC regulates T cell immunity. Induction of C5aR (and, to a lesser extent, C3aR) signaling in TLR-activated macrophages selectively inhibits the transcription of genes encoding IL-12 family cytokines63,110,111(Fig. 2a). These cytokines (IL-12, IL-23, IL-27) play a major role in the activation and differentiation of distinct T cell subsets. For example, IL-12 (a p35/p40 heterodimer) induces the differentiation of the TH1 lineage from naïve CD4+ T cells, whereas IL-23 (p19/p40) drives the expansion of the TH17 subset112. IL-27 (p28/EBI3) regulates the TH1/T 17 balance by limiting TH17 development and favoring TH1112. These regulatory effects might be relevant to the attenuation of T cell-mediated inflammatory tissue damage (e.g., inhibition of TH1-mediated pathology by C5aR-TLR4 crosstalk). However, the significance of this crosstalk becomes quite evident in microbial immune evasion: Specifically, Leishmania major, a macrophage intracellular pathogen, appears to benefit from C5aR-induced inhibition of TH1 immunity110.
Similar inhibitory effects on TLR-induced IL-12 production are seen when other complement receptors (gC1qR, MCP/CD46, and CR3) are activated concomitantly with TLR4/TLR2 in mouse macrophages or human monocytes113–115(Fig. 2a). These crosstalk pathways are likewise exploited by several pathogens to suppress TH1 immunity and IL-12/IFN-γ-dependent clearance, including hepatitis C virus, measles virus, and P. gingivalis, which interact with gC1qR113, MCP/CD46114, and CR3116, respectively. However, when the same receptors are instead activated by their natural ligands, their crosstalk with TLR pathways can play important physiological roles. For instance, interactions between macrophage CR3 and iC3b-coated apoptotic cells inhibit IL-12, preventing unwarranted inflammation during apoptotic cell phagocytosis55,61. Moreover, C1q binding by gC1qR may represent a homeostatic mechanism for regulating T cell immunity; if so, this role would be consistent with observations that C1q deficiency in humans and mice causes inflammatory autoimmune pathology117.
The complement-TLR crosstalk related to IL-12 regulation is dynamic and contextual, depending at least in part on the cell type involved. For example, activation of MCP/CD46 signaling in LPS-stimulated human DC does not inhibit IL-12 production as seen in monocytes, but rather promotes the expression of IL-12p35, IL-12/IL-23p40, and IL-23p19118. Moreover, in contrast to their effects on monocytes/macrophages, C5a and C3a do not inhibit TLR-induced IL-12 production in human or mouse DC98,99,119. Instead, these anaphylatoxins promote IL-12 production in LPS-stimulated DC and favor the development of TH1 responses, at least in a mouse model of allostimulation96,98,99. These effects reflect the ability of both anaphylatoxins to inhibit immunosuppressive cAMP-dependent protein kinase A signaling, thereby releasing inhibitory constraints in DC98,99. In contrast, this pathway is not inhibited by the anaphylatoxins in macrophages or neutrophils67,79. The findings from the allostimulation model are consistent with observations that C3ar−/− and C5ar−/− mice fail to mount TH1 immune responses and succumb to Toxoplasma gondii infection97. C3a and C5a are likely generated locally by both partners at the DC-T cell interface, leading to functional co-stimulation and differentiation of naïve CD4+ T cells to TH196,97. Conversely, blockade or absence of C5aR in DC prevents TH1 differentiation, leading instead to enhanced TGF-β, IL-6, and IL-23 responses that promote T cell differentiation to CD25+ Foxp3+ regulatory T cells and TH17 in vitro and in vivo68.
In contrast to DC, TLR-activated macrophages require intact C5aR signaling to promote TH17 differentiation of CD4+ T cells109. In vivo, this TH17-promoting crosstalk can induce experimental autoimmune encephalomyelitis or autoimmune arthritis and is dependent on synergistic IL-6 induction by C5aR and several TLRs (TLR-2, -4, or -9)109,120. Thus, in the context of TLR crosstalk, complement could conceivably either promote or inhibit TH1 or TH17 development, depending on the type of APC involved. By acting through DC, complement favors TH1 and inhibits TH17, whereas by acting through macrophages, it favors TH17 and inhibits TH1 (Fig. 3). In general, complement can reduce the threshold for T cell polarization to either TH1 or TH17, depending on other concomitant environmental stimuli. Indeed, Daf1−/− (but not Daf1−/−C3ar−/− or Daf1−/−C5ar−/−) T cells exhibit considerably higher production of IFN-γ or IL-17 than wild-type controls in response to IL-12 or IL-23, respectively108. In summary, complement exerts profound and complex effects on the initiation, differentiation, and even the replenishment of immune responses (through HSPC mobilization41; Supplementary Fig. 3), necessitating a precise and contextual understanding of the signaling pathways involved.
Complement-related diseases
While complement's involvement in pathophysiology was historically defined by observations in patients with complement deficiencies or dysfunctions121,122, our knowledge has been boosted by improved animal models and genome-wide association studies (GWAS). The growing list of diseases with complement connection has fueled an interest in developing complement-targeted therapeutics123–125. Despite challenges like high plasma concentrations of targets and a prevalence of protein-protein interactions, the first complement-directed drugs, including recombinant C1-INH (e.g., Cinryze™, ViroPharma) for the treatment of hereditary angioedema and a therapeutic anti-C5 antibody (Soliris®, Alexion) for paroxysmal nocturnal hemoglobulinuria, are already being marketed, with more candidates in clinical trials or pre-clinical development. Here we address some emerging aspects of complement in health and disease, while referring to specialized reviews for detailed coverage of its role in rheumatoid arthritis126, asthma127, and other diseases128,129, or in complications arising from biomaterial-induced complement activation130 (e.g., during hemodialysis131 or cardiopulmonary bypass surgery132).
Inflammatory diseases
Accumulation and unsuccessful removal of cellular debris may not only contribute to autoimmune disorders like SLE (often associated with C1q, C4, or C2 deficiencies)50,122 but also various age-related and neurodegenerative diseases with a chronic inflammatory component. Age-related macular degeneration (AMD) has shown particularly strong ties to complement: this leading cause of blindness in elderly people has a complex etiology and produces geographic atrophy and neovascularization of the subretinal tissue that gradually leads to loss of central vision. After several complement components were detected in subretinal lipoprotein deposits (drusen)133,134, AMD research rapidly gained momentum when GWAS identified polymorphisms in the fH gene as major risk factors for AMD135–138. Meanwhile, additional polymorphisms and deletions that mostly affect the AP (e.g., C3, fB, or FHL-1) have been discovered, suggesting that disruption of the delicate balance between complement activation and regulation in the subretinal tissue may contribute to AMD progression. Although its exact pathogenesis is not fully understood, a slow but vicious cycle of tissue damage (e.g., by oxidative stress), accumulation of debris, chronic complement activation, and inflammation that perpetuates tissue damage appears to be at the heart of the disease134. Given the strong association between AMD and complement and the high prevalence of the disease, it is not surprising that considerable complement-targeted drug development efforts are being directed toward AMD. Actually, complement inhibitors are among the few promising options for treating the early dry form of AMD and potentially preventing vision loss139.
Excessive complement activation is also involved in two rare but severe kidney diseases that often culminate in end-stage renal failure. Both membranoproliferative glomerulonephritis type II (MPGN II; dense-deposit disease) and atypical hemolytic uremic syndrome (aHUS) progress aggressively and often manifest at a very young age. In both diseases, deficiencies and polymorphisms in components of the AP (e.g., fH, C3, fB, fI, MCP) or auto-antibodies that either neutralize regulators or stabilize the C3 convertase (i.e., nephritic factor) lead to excessive complement activation140,141. Whereas plasma infusion or plasma exchange therapy and renal transplantation are therapeutic options for aHUS and MPGN II, clinical trials involving complement inhibitors such as Soliris® are being conducted in aHUS patients to help identify better therapeutic options125,141.
Recent studies also tie Alzheimer's disease (AD) to complement since both C1q and C3 recognize accumulating amyloid fibrils and induce persistent complement activation. The release of anaphylatoxins may attract microglia and astrocytes that contribute to phagocytosis but can also be activated to release cytokines, proteases, and reactive oxygen species (ROS), thereby contributing to inflammation and accelerating neuronal dysfunction142. Importantly, administration of a C5aR antagonist in a mouse model of AD has significantly improved memory performance and reduced pathologic markers such as amyloid deposits143. However, complement may also exert protective effects during the early stages of AD; indeed, certain complement regulators are upregulated in early AD, and opsonization with C1q may facilitate clearance of damaged neurons. With increasing damage to the brain, however, the deleterious effects of complement seem to prevail.
Acute-phase disorders
In contrast to the diseases mentioned above, acute-phase disorders like sepsis or ischemia/reperfusion (I/R) injury trigger a more aggressive and highly distinctive complement response that can critically contribute to tissue damage. In sepsis, severe infection with microorganisms can trigger acute inflammatory reactions associated with a storm of cytokines and other mediators, producing hypotension, multi-organ failure, and death; this overwhelming immune response causes organ damage long after the triggers have been cleared. Given complement's role in first-line defense against microbial intruders, complement activity that contributes to control of the infection is clearly beneficial in the early stages of sepsis. However, complement, and especially C5a, can contribute to organ damage in concert with the cytokine storm in the later stages of sepsis79,144. Thus, both the direct impact of C5a on immune cells and the induction of uncontrolled coagulation by C5a-mediated TF expression contribute to the devastating effects seen in sepsis (Fig. 4a). Therapeutic regimens focusing on early antimicrobial intervention and anti-inflammatory treatment during later stages therefore appear most appropriate, and complement inhibition at the C3 level in a primate model of late-stage sepsis dramatically improved organ preservation and other clinical parameters145.
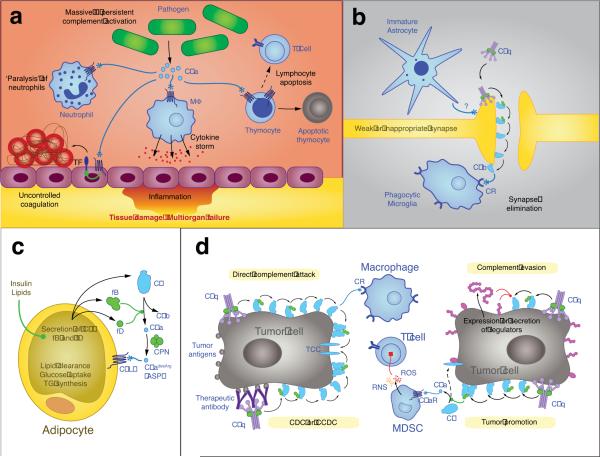
Figure 4. Emerging roles of complement in health and disease.
Besides the more `classical' roles of complement in the elimination of microbial intruders and clearance of apoptotic debris (as depicted in Fig. 1b,c), complement has important roles in cell homeostasis and several disease states. In the case of sepsis (a), high levels of infectious microorganisms in the blood cause excessive complement activation with release of C5a that contributes to a series of devastating effects ranging from immune depletion (e.g., by paralyzing neutrophils and inducing apoptosis in lymphocytes) to severe inflammation (by triggering a cytokine storm) and disseminated coagulation (partly via inducing the expression of tissue factor, TF), all of which may culminate in tissue damage, multi-organ failure, and death. Complement has also been shown to be important in synaptogenesis (b), eliminating weak or immature synapses. An unknown signal derived from immature astrocytes promotes recognition by C1q, which in turn leads to opsonization with C3b and iC3b and facilitates complement receptor (CR)-mediated phagocytosis by activated microglia. Although the role of the C5L2 receptor in the immune response is still a matter of debate, it appears to have an important role in lipid metabolism (c). Adipocytes are known to secrete C3, fB, and fD, and their expression may be promoted by stimuli such as insulin or lipids, leading to a higher turnover of the AP and generation of C5a, which is rapidly transformed into C5adesArg. Also known as acetylation stimulating protein (ASP), this anaphylatoxin fragment has been reported to induce lipid clearance, glucose uptake, and triglyceride (TG) synthesis in adipocytes via C5L2 signaling. In cancer (d), complement is likely to have a dual role. On the one hand, it contributes to protection via direct activation of complement or as part of the complement-dependent cytotoxicity (CDC) of tumor-directed therapeutic antibodies. On the other hand, many tumors escape complement attack by expressing and secreting complement inhibitors that largely prevent amplification, TCC formation, or complement-mediated phagocytosis. Furthermore, recent research has shown that the generation of C5a in the tumor microenvironment may attract myeloid-derived suppressor cells (MDSC) and induce the generation of reactive oxygen and nitrogen species (ROS and RNS, respectively) via the C5a receptor (C5aR), which impairs the tumor-directed effect of T cells.
Complement-associated tissue damage is also seen in I/R-associated injuries related to myocardial infarction and stroke, or induced during procedures such as vascular surgery or organ transplantation. Reperfusion of tissue following temporary occlusion of the blood supply triggers inflammatory and immune responses, including complement activation, that can lead to self-attack, in which the contribution of individual pathways appears to be dependent on the affected organ146. Complement-mediated recognition of damaged cells further activates the cascade and enhances anaphylatoxin release, fueling inflammation and recruiting immune cells. The ensuing release of ROS and other signals generates a vicious cycle of damage and clinical manifestations. Several complement-directed therapies have achieved only limited success in I/R injury, especially in myocardial infarction, but recent insights into the role of complement have suggested novel and promising therapeutic strategies146.
Immune connections to development and metabolism
In analogy to studies in amphibians147, evidence is growing that complement contributes to the development and repair of mammalian tissue (e.g., in the resolution phase of inflammation). Both its role in the disposal of dead cells and the induction of growth factors that promote restoration of tissue homeostasis are considered important: C5a and C3a, for instance, induce expression of vascular endothelial growth factor, which is required for angiogenesis and tissue repair after injury60. The pro-repair role of complement is apparent in liver regeneration; indeed, anaphylatoxin-induced IL-6 and TNF prosurvival signaling promotes hepatocyte growth and proliferation148, which is dramatically impaired in C3−/−, C3ar−/−, and C5ar−/− mice59. However, C5 and C5a are implicated as profibrotic factors in liver, lung, and renal fibrosis148–150, and a C5 SNP is associated with liver fibrosis149. Therefore, as in immunity, complement's role in tissue repair involves a complex and delicate process that can even produce undesirable outcomes.
Complement-mediated remodeling of synaptic connections in the developing nervous system is similarly a two-edged sword: Whereas immature astrocytes may induce elimination of improper synaptic connections via recognition by C1q and induction of C3b/iC3b-mediated phagocytosis (Fig. 4b)142,151, the same process can be triggered by reactive astrocytes in the damaged or diseased brain, thereby contributing to the pathogenesis of neurological and neurodegenerative diseases. Indeed, complement has been linked not only to AD (see above) but also to glaucoma152, Parkinson's disease153, multiple sclerosis154, and schizophrenia155,156. Conversely, impaired complement activity early in development may be detrimental, given the surprising role for C3a-C3aR signaling in the control of neural crest migration during early embryogenesis157. Complement, and specifically C3a, also fulfills important regulatory effects on the differentiation and migration of neural progenitor cells158, thereby affecting neurogenesis.
Furthermore, complement is involved in prostaglandin (PG) synthesis in bone, which is considered important for its metabolism and physiologic remodeling159–161. In particular, sublytic signaling by C5b-9/C5b-8 activates phospholipase A2, releases arachidonic acid, and induces synthesis of PG-E2 in synovial cells or macrophages160,162. However, certain autoimmune and inflammatory diseases involve complement-associated and PG-dependent bone immunopathology124,126,163–165; both rheumatoid arthritis and periodontitis have been associated with a C5 SNP that is linked to increased serum C5 levels166,167. While these findings do not necessarily indicate an involvement of the C5b-9 pathway, it is striking that CD59 deficiency causes enhanced cartilage and bone erosion in animal models whereas it is reversed by C6 deficiency168,169.
Although the immune and metabolic systems have evolved from common ancestors, functional links between these two central pillars of survival have only recently been drawn170. Metabolic surplus may trigger inflammatory pathways and mediators (i.e., `metaflammation')170, which has been associated with obesity and type II diabetes171. The secretion of regulatory adipokines and crosstalk between adipocytes and macrophages play a crucial role in this context43 and complement appears to be an important mediator. Adipocytes are the major source of fD, and its mouse homolog, adipsin, is important in the differentiation of preadipocytes. They also produce C3 and fB, the former of which is significantly increased after exposure to insulin or dietary lipid (Fig. 4c). In fact, C3 was described as a strong marker of insulin resistance in an elderly population172. The local secretion of AP components triggers a higher turnover of C3 into C3b and C3a, which is readily processed to C3adesArg. Intriguingly, C3adesArg was identified as the acetylation stimulating protein (ASP) that has lipogenic activity and increases glucose uptake and triglyceride synthesis43. Whereas recent studies suggest an involvement of C5L2 (Fig. 4c), which is expressed on adipocytes and preadipocytes, a direct interaction with ASP/C3adesArg remains controversial22,26,43. Importantly, C3−/− and C5l2−/− mice share a phenotype that is characterized by hypophagy, delayed lipid clearance, and reduced triglyceride synthesis and similar alterations in lipid metabolism were achieved after treatment of wild-type mice with neutralizing anti-ASP and anti-C5L2 mAbs. Interestingly, anaphylatoxins may also have a direct effect on food intake regulation via the CNS: In mice, C3a promotes anorexia by influencing the PG-E pathway, whereas C5a stimulates food intake mediated by PG-D and neuropeptide Y173. Although additional studies are certainly warranted, the observed effects on food intake and lipid metabolism make complement a highly interesting target for future basic and translational studies of metabolic diseases.
Dual role in cancer
Based on its involvement in immune surveillance and microbial defense, complement has long been assumed to play an active and beneficial role in the fight against malignant cells. Indeed, studies have found PRM, opsonins, and effectors on the surface of various tumor cells, suggesting that complement is substantially activated in the tumor microenvironment (Fig. 4d). Furthermore, complement-dependent cytotoxicity synergizes with tumor-directed antibody therapy174. However, the majority of persisting tumors express high amounts of membrane-bound complement regulators, mainly TCC, DAF, and CD59, which prevent amplification and TCC formation (Fig. 4d). In addition, soluble regulators such as fH, FHL-1, and C4BP can be recruited to or secreted by tumors, thereby contributing to the tumors' complement evasion strategy174. Unexpectedly, the dogma of the protective role of complement against tumor attack was challenged when a recent study demonstrated that CP-induced generation of C5a in the tumor microenvironment leads to significant progression in tumor growth in a mouse model of cervical cancer175. This mechanism is mediated via distinct C5aR signaling effects on two subpopulations of myeloid-derived suppressor cells (MDSC) representing immature monocytes and neutrophils175. In the cervical cancer model, C5a attracted neutrophil-like MDSC to the tumor and induced the generation of ROS and reactive nitrogen species in monocyte-like MDSC (Fig. 4d)175, which interfere with the responsiveness of T cells against tumor antigens176. Importantly, treatment of tumor-bearing mice with a C5aR antagonist slowed tumor progression to a similar extent as the conventional drug paclitaxel175. These findings suggest that C5aR-directed therapy has potential for use in certain cancers with a chronic inflammatory component.
Conclusions and outlook
Our perspective on the human complement system has experienced a remarkable transformation during the course of a century: After its discovery as a critical system in microbial defense at the dawn of the 20th century, the following decades provided a wealth of information about its specific function in infection and immunity. The turn of the current century appears to be similarly significant, as we now begin to envision complement as a global player and mediator in immune surveillance, cell homeostasis, and tissue development and repair (Fig. 3), and we eagerly await even deeper insights into these and other novel roles. Ongoing improvements in animal models, systems biology, GWAS, molecular and cellular techniques, and clinical diagnostics represent key factors that will direct the field into new endeavors. At the same time, our deepening understanding of complement's involvement in various diseases is likely to yield promising novel treatment options. Complement has truly come out of hiding and exhibited fascinating connections we had never before imagined, and these hidden connections may indeed be stronger than the original obvious ones.
Supplementary Material
Acknowledgments
The plethora of novel discoveries and exciting findings that the field of complement research has experienced in the past decade makes it impossible to cover every important aspect in the scope of a review; regrettably, we have had to restrict our focus on certain key areas, and we acknowledge all the brilliant research that we were unable to mention specifically. We thank Dr. Deborah McClellan for excellent editorial assistance and Dr. Andrea Tenner for her constructive comments. Studies performed in the authors' laboratories and cited in this paper were supported by U.S. Public Health Service Grants CA112162, AI68730, AI30040, AI72106, EB3968, GM62134 (to JDL), and DE015254 and DE018292 (to GH).
Footnotes
Based on recent discussions in the field and in order to streamline fragment nomenclature for all the complement pathways, we designate the small, non-proteolytic C2 fragment as C2a and the protease segment as C2b. As a consequence, the CP/LP C3 convertase is referred to as C4b2b (by analogy to the AP C3 convertase, C3bBb).
References
- 1.Sunyer JO, Zarkadis IK, Lambris JD. Complement diversity: a mechanism for generating immune diversity? Immunol. Today. 1998;19:519–523. doi: 10.1016/s0167-5699(98)01341-3. [DOI] [PubMed] [Google Scholar]
- 2.Gaboriaud C, et al. Structure and activation of the C1 complex of complement: unraveling the puzzle. Trends Immunol. 2004;25:368–373. doi: 10.1016/j.it.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Wallis R, Mitchell DA, Schmid R, Schwaeble WJ, Keeble AH. Paths reunited: Initiation of the classical and lectin pathways of complement activation. Immunobiology. 2010;215:1–11. doi: 10.1016/j.imbio.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CB, Wallis R. Two mechanisms for mannose-binding protein modulation of the activity of its associated serine proteases. J. Biol. Chem. 2004;279:26058–26065. doi: 10.1074/jbc.M401318200. [DOI] [PubMed] [Google Scholar]
- 5.Dobo J, et al. MASP-1, a promiscuous complement protease: structure of its catalytic region reveals the basis of its broad specificity. J. Immunol. 2009;183:1207–1214. doi: 10.4049/jimmunol.0901141. [DOI] [PubMed] [Google Scholar]
- 6.Rawal N, Rajagopalan R, Salvi VP. Activation of complement component C5: comparison of C5 convertases of the lectin pathway and the classical pathway of complement. J. Biol. Chem. 2008;283:7853–7863. doi: 10.1074/jbc.M707591200. [DOI] [PubMed] [Google Scholar]
- 7.Bexborn F, Andersson PO, Chen H, Nilsson B, Ekdahl KN. The tick-over theory revisited: formation and regulation of the soluble alternative complement C3 convertase (C3(H2O)Bb) Mol. Immunol. 2008;45:2370–2379. doi: 10.1016/j.molimm.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J. Exp. Med. 1981;154:856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pangburn MK, Ferreira VP, Cortes C. Discrimination between host and pathogens by the complement system. Vaccine. 2008;26(Suppl 8):I15–21. doi: 10.1016/j.vaccine.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahu A, Kozel TR, Pangburn MK. Specificity of the thioester-containing reactive site of human C3 and its significance to complement activation. Biochem. J. 1994;302(Pt 2):429–436. doi: 10.1042/bj3020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J. Immunol. 2007;179:2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- 12.Fearon DT, Austen KF. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J. Exp. Med. 1975;142:856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harboe M, Mollnes TE. The alternative complement pathway revisited. J. Cell. Mol. Med. 2008;12:1074–1084. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutz HU, Jelezarova E. Complement amplification revisited. Mol. Immunol. 2006;43:2–12. doi: 10.1016/j.molimm.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Muller-Eberhard HJ. The killer molecule of complement. J. Invest. Dermatol. 1985;85:47s–52s. doi: 10.1111/1523-1747.ep12275445. [DOI] [PubMed] [Google Scholar]
- 16.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Huber-Lang M, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 18.Selander B, et al. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J. Clin. Invest. 2006;116:1425–1434. doi: 10.1172/JCI25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkinson JP, Frank MM. Bypassing complement: evolutionary lessons and future implications. J. Clin. Invest. 2006;116:1215–1218. doi: 10.1172/JCI28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat. Rev. Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole DS, Morgan BP. Beyond lysis: how complement influences cell fate. Clin. Sci. (Lond.) 2003;104:455–466. doi: 10.1042/CS20020362. [DOI] [PubMed] [Google Scholar]
- 22.Ward PA. Functions of C5a receptors. J. Mol. Med. 2009;87:375–378. doi: 10.1007/s00109-009-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rittirsch D, et al. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bamberg CE, et al. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2009;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scola AM, et al. The role of the N-terminal domain of the complement fragment receptor C5L2 in ligand binding. J. Biol. Chem. 2007;282:3664–3671. doi: 10.1074/jbc.M609178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klos A, et al. The role of the anaphylatoxins in health and disease. Mol. Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas PJ, van Strijp J. Anaphylatoxins: their role in bacterial infection and inflammation. Immunol. Res. 2007;37:161–175. doi: 10.1007/BF02697367. [DOI] [PubMed] [Google Scholar]
- 28.van Lookeren Campagne M, Wiesmann C, Brown EJ. Macrophage complement receptors and pathogen clearance. Cell. Microbiol. 2007;9:2095–2102. doi: 10.1111/j.1462-5822.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- 29.Krych-Goldberg M, Atkinson JP. Structure-function relationships of complement receptor type 1. Immunol. Rev. 2001;180:112–122. doi: 10.1034/j.1600-065x.2001.1800110.x. [DOI] [PubMed] [Google Scholar]
- 30.Helmy KY, et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 31.He JQ, Wiesmann C, van Lookeren Campagne M. A role of macrophage complement receptor CRIg in immune clearance and inflammation. Mol. Immunol. 2008;45:4041–4047. doi: 10.1016/j.molimm.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Roozendaal R, Carroll MC. Complement receptors CD21 and CD35 in humoral immunity. Immunol Rev. 2007;219:157–166. doi: 10.1111/j.1600-065X.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 33.Carroll MC. Complement and humoral immunity. Vaccine. 2008;26(Suppl 8):I28–33. doi: 10.1016/j.vaccine.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogden CA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarr J, Eggleton P. Immune function of C1q and its modulators CD91 and CD93. Crit. Rev. Immunol. 2005;25:305–330. doi: 10.1615/critrevimmunol.v25.i4.40. [DOI] [PubMed] [Google Scholar]
- 36.Lillis AP, et al. Murine low-density lipoprotein receptor-related protein 1 (LRP) is required for phagocytosis of targets bearing LRP ligands but is not required for C1q-triggered enhancement of phagocytosis. J. Immunol. 2008;181:364–373. doi: 10.4049/jimmunol.181.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norsworthy PJ, et al. Murine CD93 (C1qRp) contributes to the removal of apoptotic cells in vivo but is not required for C1q-mediated enhancement of phagocytosis. J. Immunol. 2004;172:3406–3414. doi: 10.4049/jimmunol.172.6.3406. [DOI] [PubMed] [Google Scholar]
- 38.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 39.Inal JM, et al. Complement C2 receptor inhibitor trispanning: a novel human complement inhibitory receptor. J. Immunol. 2005;174:356–366. doi: 10.4049/jimmunol.174.1.356. [DOI] [PubMed] [Google Scholar]
- 40.Kim DD, Song WC. Membrane complement regulatory proteins. Clin. Immunol. 2006;118:127–136. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Jalili A, et al. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: Further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010;38:321–332. doi: 10.1016/j.exphem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the SDF-1-CXCR4 axis by the third complement component (C3)--implications for trafficking of CXCR4+ stem cells. Exp. Hematol. 2006;34:986–995. doi: 10.1016/j.exphem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 43.MacLaren R, Cui W, Cianflone K. Adipokines and the immune system: an adipocentric view. Adv. Exp. Med. Biol. 2008;632:1–21. doi: 10.1007/978-0-387-78952-1_1. [DOI] [PubMed] [Google Scholar]
- 44.Sahu A, Lambris JD. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol. Rev. 2001;180:35–48. doi: 10.1034/j.1600-065x.2001.1800103.x. [DOI] [PubMed] [Google Scholar]
- 45.Rooijakkers SH, et al. Structural and functional implications of the alternative complement pathway C3 convertase stabilized by a staphylococcal inhibitor. Nat. Immunol. 2009;10:721–727. doi: 10.1038/ni.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J, et al. Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat. Immunol. 2009;10:728–733. doi: 10.1038/ni.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliva C, Turnbough CL, Jr., Kearney JF. CD14-Mac-1 interactions in Bacillus anthracis spore internalization by macrophages. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13957–13962. doi: 10.1073/pnas.0902392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang M, et al. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J. Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- 49.Flierman R, Daha MR. The clearance of apoptotic cells by complement. Immunobiology. 2007;212:363–370. doi: 10.1016/j.imbio.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Trouw LA, Blom AM, Gasque P. Role of complement and complement regulators in the removal of apoptotic cells. Mol Immunol. 2008;45:1199–1207. doi: 10.1016/j.molimm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Cole DS, Hughes TR, Gasque P, Morgan BP. Complement regulator loss on apoptotic neuronal cells causes increased complement activation and promotes both phagocytosis and cell lysis. Mol Immunol. 2006;43:1953–1964. doi: 10.1016/j.molimm.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 52.Gullstrand B, Martensson U, Sturfelt G, Bengtsson AA, Truedsson L. Complement classical pathway components are all important in clearance of apoptotic and secondary necrotic cells. Clin Exp Immunol. 2009;156:303–311. doi: 10.1111/j.1365-2249.2009.03896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lowell CA. Rewiring phagocytic signal transduction. Immunity. 2006;24:243–245. doi: 10.1016/j.immuni.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21:643–653. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morelli AE, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–620. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 58.Verbovetski I, et al. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J Exp Med. 2002;196:1553–1561. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Markiewski MM, et al. The regulation of liver cell survival by complement. J Immunol. 2009;182:5412–5418. doi: 10.4049/jimmunol.0804179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nozaki M, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo RF, Riedemann NC, Ward PA. Role of C5a-C5aR interaction in sepsis. Shock. 2004;21:1–7. doi: 10.1097/01.shk.0000105502.75189.5e. [DOI] [PubMed] [Google Scholar]
- 65.Kaczorowski DJ, et al. Pivotal Advance: The pattern recognition receptor ligands lipopolysaccharide and polyinosine-polycytidylic acid stimulate factor B synthesis by the macrophage through distinct but overlapping mechanisms. J Leukoc Biol. 2010 doi: 10.1189/jlb.0809588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen NJ, et al. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446:203–207. doi: 10.1038/nature05559. [DOI] [PubMed] [Google Scholar]
- 67.Wang M, et al. Microbial hijacking of complement-Toll-like receptor crosstalk. Sci. Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weaver DJ, Jr., et al. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur J Immunol. 2010;40:710–721. doi: 10.1002/eji.200939333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ip WKE, Takahashi K, Moore KJ, Stuart LM, Ezekowitz RAB. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. The Journal of Experimental Medicine. 2008;205:169–181. doi: 10.1084/jem.20071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lappegård KT, et al. Human genetic deficiencies reveal the roles of complement in the inflammatory network: lessons from nature. Proc Natl Acad Sci U S A. 2009;106:15861–15866. doi: 10.1073/pnas.0903613106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zarewych DM, Kindzelskii AL, Todd RF, Petty HR. LPS induces CD14 association with complement receptor 3, which is reversed by neutrophil adhesion. J. Immunol. 1996;156:430–433. [PubMed] [Google Scholar]
- 72.Kagan JC. “Complementing” toll signaling. Sci Signal. 2010;3:pe15. doi: 10.1126/scisignal.3120pe15. [DOI] [PubMed] [Google Scholar]
- 73.Harokopakis E, Albzreh MH, Martin MH, Hajishengallis G. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J. Immunol. 2006;176:7645–7656. doi: 10.4049/jimmunol.176.12.7645. [DOI] [PubMed] [Google Scholar]
- 74.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 75.Ritis K, et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 76.Krarup A, Wallis R, Presanis JS, Gal P, Sim RB. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One. 2007;2:e623. doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghebrehiwet B, Silverberg M, Kaplan AP. Activation of the classical pathway of complement by Hageman factor fragment. J Exp Med. 1981;153:665–676. doi: 10.1084/jem.153.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bergmann S, Hammerschmidt S. Fibrinolysis and host response in bacterial infections. Thromb Haemost. 2007;98:512–520. [PubMed] [Google Scholar]
- 79.Ward PA. The dark side of C5a in sepsis. Nat. Rev. Immunol. 2004;4:133–142. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 80.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 81.Fischer MB, et al. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science. 1998;280:582–585. doi: 10.1126/science.280.5363.582. [DOI] [PubMed] [Google Scholar]
- 82.Croix DA, et al. Antibody response to a T-dependent antigen requires B cell expression of complement receptors. J Exp Med. 1996;183:1857–1864. doi: 10.1084/jem.183.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thornton BP, Vetvicka V, Ross GD. Function of C3 in a humoral response: iC3b/C3dg bound to an immune complex generated with natural antibody and a primary antigen promotes antigen uptake and the expression of co-stimulatory molecules by all B cells, but only stimulates immunoglobulin synthesis by antigen-specific B cells. Clin Exp Immunol. 1996;104:531–537. doi: 10.1046/j.1365-2249.1996.57761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 85.Haas KM, et al. Complement receptors CD21/35 link innate and protective immunity during Streptococcus pneumoniae infection by regulating IgG3 antibody responses. Immunity. 2002;17:713–723. doi: 10.1016/s1074-7613(02)00483-1. [DOI] [PubMed] [Google Scholar]
- 86.Da Costa XJ, et al. Humoral response to herpes simplex virus is complement-dependent. Proc Natl Acad Sci U S A. 1999;96:12708–12712. doi: 10.1073/pnas.96.22.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fischer WH, Hugli TE. Regulation of B cell functions by C3a and C3a(desArg): suppression of TNF-alpha, IL-6, and the polyclonal immune response. J Immunol. 1997;159:4279–4286. [PubMed] [Google Scholar]
- 88.Ottonello L, et al. rC5a directs the in vitro migration of human memory and naive tonsillar B lymphocytes: implications for B cell trafficking in secondary lymphoid tissues. J Immunol. 1999;162:6510–6517. [PubMed] [Google Scholar]
- 89.Shushakova N, et al. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgammaRs in immune complex-induced lung disease. J Clin Invest. 2002;110:1823–1830. doi: 10.1172/JCI200216577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar V, et al. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest. 2006;116:512–520. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gustavsson S, Kinoshita T, Heyman B. Antibodies to murine complement receptor 1 and 2 can inhibit the antibody response in vivo without inhibiting T helper cell induction. J Immunol. 1995;154:6524–6528. [PubMed] [Google Scholar]
- 92.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8:373–378. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 93.Nakayama Y, et al. C3 promotes expansion of CD8+ and CD4+ T cells in a Listeria monocytogenes infection. J Immunol. 2009;183:2921–2931. doi: 10.4049/jimmunol.0801191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaya Z, et al. Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nat Immunol. 2001;2:739–745. doi: 10.1038/90686. [DOI] [PubMed] [Google Scholar]
- 95.Marsh JE, et al. The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. Transplantation. 2001;72:1310–1318. doi: 10.1097/00007890-200110150-00022. [DOI] [PubMed] [Google Scholar]
- 96.Sacks SH. Complement fragments C3a and C5a: The salt and pepper of the immune response. European Journal of Immunology. 2010;40:668–670. doi: 10.1002/eji.201040355. [DOI] [PubMed] [Google Scholar]
- 97.Strainic MG, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li K, et al. Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood. 2008;112:5084–5094. doi: 10.1182/blood-2008-05-156646. [DOI] [PubMed] [Google Scholar]
- 99.Peng Q, et al. Dendritic cell function in allostimulation is modulated by C5aR signaling. J Immunol. 2009;183:6058–6068. doi: 10.4049/jimmunol.0804186. [DOI] [PubMed] [Google Scholar]
- 100.Longhi MP, Harris CL, Morgan BP, Gallimore A. Holding T cells in check--a new role for complement regulators? Trends Immunol. 2006;27:102–108. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 101.Wagner C, et al. The complement receptor 1, CR1 (CD35), mediates inhibitory signals in human T-lymphocytes. Mol Immunol. 2006;43:643–651. doi: 10.1016/j.molimm.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 102.Friec GL, Kemper C. Complement: coming full circle. Arch Immunol Ther Exp (Warsz) 2009;57:393–407. doi: 10.1007/s00005-009-0047-4. [DOI] [PubMed] [Google Scholar]
- 103.Price JD, et al. Induction of a regulatory phenotype in human CD4+ T cells by streptococcal M protein. J Immunol. 2005;175:677–684. doi: 10.4049/jimmunol.175.2.677. [DOI] [PubMed] [Google Scholar]
- 104.Kohl J, et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest. 2006;116:783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang X, et al. A protective role for C5a in the development of allergic asthma associated with altered levels of B7-H1 and B7-DC on plasmacytoid dendritic cells. J Immunol. 2009;182:5123–5130. doi: 10.4049/jimmunol.0804276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Drouin SM, Corry DB, Hollman TJ, Kildsgaard J, Wetsel RA. Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2002;169:5926–5933. doi: 10.4049/jimmunol.169.10.5926. [DOI] [PubMed] [Google Scholar]
- 107.Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol. 2010;184:1663–1674. doi: 10.4049/jimmunol.0902185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu J, et al. IFN-γ and IL-17 production in experimental autoimmune encephalomyelitis depends on local APC-T cell complement production. J Immunol. 2008;180:5882–5889. doi: 10.4049/jimmunol.180.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fang C, Zhang X, Miwa T, Song WC. Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with Toll-like receptor signaling and interleukin-6 production. Blood. 2009;114:1005–1015. doi: 10.1182/blood-2009-01-198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hawlisch H, et al. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 111.la Sala A, Gadina M, Kelsall BL. G(i)-protein-dependent inhibition of IL-12 production is mediated by activation of the phosphatidylinositol 3-kinase-protein 3 kinase B/Akt pathway and JNK. J Immunol. 2005;175:2994–2999. doi: 10.4049/jimmunol.175.5.2994. [DOI] [PubMed] [Google Scholar]
- 112.Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nat. Rev. Immunol. 2008;8:81–86. doi: 10.1038/nri2225. [DOI] [PubMed] [Google Scholar]
- 113.Waggoner SN, Cruise MW, Kassel R, Hahn YS. gC1q receptor ligation selectively down-regulates human IL-12 production through activation of the phosphoinositide 3-kinase pathway. J Immunol. 2005;175:4706–4714. doi: 10.4049/jimmunol.175.7.4706. [DOI] [PubMed] [Google Scholar]
- 114.Karp CL, et al. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 115.Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. J. Exp. Med. 1997;185:1987–1995. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hajishengallis G, Shakhatreh M-AK, Wang M, Liang S. Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J. Immunol. 2007;179:2359–2367. doi: 10.4049/jimmunol.179.4.2359. [DOI] [PubMed] [Google Scholar]
- 117.Manderson AP, Botto M, Walport MJ. The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol. 2004;22:431–456. doi: 10.1146/annurev.immunol.22.012703.104549. [DOI] [PubMed] [Google Scholar]
- 118.Vaknin-Dembinsky A, Murugaiyan G, Hafler DA, Astier AL, Weiner HL. Increased IL-23 secretion and altered chemokine production by dendritic cells upon CD46 activation in patients with multiple sclerosis. Journal of Neuroimmunology. 2008;195:140–145. doi: 10.1016/j.jneuroim.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Braun MC, Lahey E, Kelsall BL. Selective suppression of IL-12 production by chemoattractants. J Immunol. 2000;164:3009–3017. doi: 10.4049/jimmunol.164.6.3009. [DOI] [PubMed] [Google Scholar]
- 120.Hashimoto M, et al. Complement drives Th17 cell differentiation and triggers autoimmune arthritis. J. Exp. Med. 2010;207:1135–43. doi: 10.1084/jem.20092301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Botto M, et al. Complement in human diseases: Lessons from complement deficiencies. Mol. Immunol. 2009;46:2774–2783. doi: 10.1016/j.molimm.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 122.Pettigrew HD, Teuber SS, Gershwin ME. Clinical significance of complement deficiencies. Ann N Y Acad Sci. 2009;1173:108–123. doi: 10.1111/j.1749-6632.2009.04633.x. [DOI] [PubMed] [Google Scholar]
- 123.Lachmann PJ, Smith RA. Taking complement to the clinic--has the time finally come? Scand. J. Immunol. 2009;69:471–478. doi: 10.1111/j.1365-3083.2009.02258.x. [DOI] [PubMed] [Google Scholar]
- 124.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat. Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wagner E, Frank MM. Therapeutic potential of complement modulation. Nat. Rev. Drug Discov. 2010;9:43–56. doi: 10.1038/nrd3011. [DOI] [PubMed] [Google Scholar]
- 126.Okroj M, Heinegard D, Holmdahl R, Blom AM. Rheumatoid arthritis and the complement system. Ann. Med. 2007;39:517–530. doi: 10.1080/07853890701477546. [DOI] [PubMed] [Google Scholar]
- 127.Zhang X, Kohl J. A complex role for complement in allergic asthma. Expert Rev. Clin. Immunol. 2010;6:269–277. doi: 10.1586/eci.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol. Rev. 2008;223:300–316. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 129.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nilsson B, Korsgren O, Lambris JD, Ekdahl KN. Can cells and biomaterials in therapeutic medicine be shielded from innate immune recognition? Trends Immunol. 31:32–38. doi: 10.1016/j.it.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kourtzelis I, et al. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood. doi: 10.1182/blood-2010-01-264051. prepublished online April 27, 2010; DOI 10.1182/blood-2010-01-264051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Warren OJ, et al. The inflammatory response to cardiopulmonary bypass: part 1--mechanisms of pathogenesis. J. Cardiothorac. Vasc. Anesth. 2009;23:223–231. doi: 10.1053/j.jvca.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 133.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 134.Anderson DH, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog. Retin. Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Edwards AO, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 136.Hageman GS, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haines JL, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 138.Klein RJ, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gehrs KM, Jackson JR, Brown EN, Allikmets R, Hageman GS. Complement, age-related macular degeneration and a vision of the future. Arch. Ophthalmol. 2010;128:349–358. doi: 10.1001/archophthalmol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Skerka C, et al. Autoimmune forms of thrombotic microangiopathy and membranoproliferative glomerulonephritis: Indications for a disease spectrum and common pathogenic principles. Mol. Immunol. 2009;46:2801–2807. doi: 10.1016/j.molimm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 141.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 142.Alexander JJ, Anderson AJ, Barnum SR, Stevens B, Tenner AJ. The complement cascade: Yin-Yang in neuroinflammation--neuro-protection and -degeneration. J. Neurochem. 2008;107:1169–1187. doi: 10.1111/j.1471-4159.2008.05668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fonseca MI, et al. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer's disease. J. Immunol. 2009;183:1375–1383. doi: 10.4049/jimmunol.0901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Markiewski MM, DeAngelis RA, Lambris JD. Complexity of complement activation in sepsis. J. Cell. Mol. Med. 2008;12:2245–2254. doi: 10.1111/j.1582-4934.2008.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Silasi-Mansat R, et al. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of E. coli sepsis. Blood. doi: 10.1182/blood-2010-02-269746. prepublished online May 13, 2010; DOI 10.1182/blood-2010-02-269746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Diepenhorst GM, van Gulik TM, Hack CE. Complement-mediated ischemia-reperfusion injury: lessons learned from animal and clinical studies. Ann. Surg. 2009;249:889–899. doi: 10.1097/SLA.0b013e3181a38f45. [DOI] [PubMed] [Google Scholar]
- 147.Tsonis PA, Lambris JD, Del Rio-Tsonis K. To regeneration…with complement. Adv. Exp. Med. Biol. 2006;586:63–70. doi: 10.1007/0-387-34134-X_5. [DOI] [PubMed] [Google Scholar]
- 148.Markiewski MM, DeAngelis RA, Lambris JD. Liver inflammation and regeneration: two distinct biological phenomena or parallel pathophysiologic processes? Mol Immunol. 2006;43:45–56. doi: 10.1016/j.molimm.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 149.Hillebrandt S, et al. Complement factor 5 is a quantitative trait gene that modifies liver fibrogenesis in mice and humans. Nat Genet. 2005;37:835–843. doi: 10.1038/ng1599. [DOI] [PubMed] [Google Scholar]
- 150.Addis-Lieser E, Kohl J, Chiaramonte MG. Opposing regulatory roles of complement factor 5 in the development of bleomycin-induced pulmonary fibrosis. J Immunol. 2005;175:1894–1902. doi: 10.4049/jimmunol.175.3.1894. [DOI] [PubMed] [Google Scholar]
- 151.Schafer DP, Stevens B. Synapse elimination during development and disease: immune molecules take centre stage. Biochem. Soc. Trans. 38:476–481. doi: 10.1042/BST0380476. [DOI] [PubMed] [Google Scholar]
- 152.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 153.Loeffler DA, Camp DM, Conant SB. Complement activation in the Parkinson's disease substantia nigra: an immunocytochemical study. J. Neuroinflammation. 2006;3:29. doi: 10.1186/1742-2094-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ingram G, Hakobyan S, Robertson NP, Morgan BP. Complement in multiple sclerosis: its role in disease and potential as a biomarker. Clin. Exp. Immunol. 2009;155:128–139. doi: 10.1111/j.1365-2249.2008.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Boyajyan A, Khoyetsyan A, Chavushyan A. Alternative Complement Pathway in Schizophrenia. Neurochem. Res. doi: 10.1007/s11064-010-0126-2. [DOI] [PubMed] [Google Scholar]
- 156.Mayilyan KR, Weinberger DR, Sim RB. The complement system in schizophrenia. Drug News Perspect. 2008;21:200–210. doi: 10.1358/dnp.2008.21.4.1213349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Carmona-Fontaine C, et al. C3 controls neural crest migration during embryo development. 6th International Conference in Innate Immunity.2009. p. 36. [Google Scholar]
- 158.Shinjyo N, Stahlberg A, Dragunow M, Pekny M, Pekna M. Complement-derived anaphylatoxin C3a regulates in vitro differentiation and migration of neural progenitor cells. Stem Cells. 2009;27:2824–2832. doi: 10.1002/stem.225. [DOI] [PubMed] [Google Scholar]
- 159.Raisz LG. Potential impact of selective cyclooxygenase-2 inhibitors on bone metabolism in health and disease. Am J Med. 2001;110(Suppl 3A):43S–45S. doi: 10.1016/s0002-9343(00)00684-7. [DOI] [PubMed] [Google Scholar]
- 160.Niculescu F, Rus H. Mechanisms of signal transduction activated by sublytic assembly of terminal complement complexes on nucleated cells. Immunol Res. 2001;24:191–199. doi: 10.1385/ir:24:2:191. [DOI] [PubMed] [Google Scholar]
- 161.Raisz LG, Sandberg AL, Goodson JM, Simmons HA, Mergenhagen SE. Complement-dependent stimulation of prostaglandin synthesis and bone resorption. Science. 1974;185:789–791. doi: 10.1126/science.185.4153.789. [DOI] [PubMed] [Google Scholar]
- 162.Nicholson-Weller A, Halperin JA. Membrane signaling by complement C5b-9, the membrane attack complex. Immunol Res. 1993;12:244–257. doi: 10.1007/BF02918256. [DOI] [PubMed] [Google Scholar]
- 163.de Pablo P, Chapple IL, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218–224. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- 164.Krauss JL, Potempa J, Lambris JD, Hajishengallis G. Complementary Tolls in the periodontium: how periodontal bacteria modify complement and Toll-like receptor responses to prevail in the host. Periodontol 2000. 2010;52:141–162. doi: 10.1111/j.1600-0757.2009.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110:651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chai L, Song Y-Q, Zee K-Y, Leung WK. Single nucleotide polymorphisms of complement component 5 and periodontitis. Journal of Periodontal Research. 2010;45:301–308. doi: 10.1111/j.1600-0765.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- 167.Chang M, et al. A large-scale rheumatoid arthritis genetic study identifies association at chromosome 9q33.2. PLoS Genet. 2008;4:e1000107. doi: 10.1371/journal.pgen.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fischetti F, et al. Selective therapeutic control of C5a and the terminal complement complex by anti-C5 single-chain Fv in an experimental model of antigen-induced arthritis in rats. Arthritis Rheum. 2007;56:1187–1197. doi: 10.1002/art.22492. [DOI] [PubMed] [Google Scholar]
- 169.Williams AS, Mizuno M, Richards PJ, Holt DS, Morgan BP. Deletion of the gene encoding CD59a in mice increases disease severity in a murine model of rheumatoid arthritis. Arthritis Rheum. 2004;50:3035–3044. doi: 10.1002/art.20478. [DOI] [PubMed] [Google Scholar]
- 170.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 171.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J. Clin. Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Muscari A, et al. Serum C3 is a stronger inflammatory marker of insulin resistance than C-reactive protein, leukocyte count, and erythrocyte sedimentation rate: comparison study in an elderly population. Diabetes Care. 2007;30:2362–2368. doi: 10.2337/dc07-0637. [DOI] [PubMed] [Google Scholar]
- 173.Ohinata K, Yoshikawa M. Food intake regulation by central complement system. Adv. Exp. Med. Biol. 2008;632:35–46. [PubMed] [Google Scholar]
- 174.Markiewski MM, Lambris JD. Is complement good or bad for cancer patients? A new perspective on an old dilemma. Trends Immunol. 2009;30:286–292. doi: 10.1016/j.it.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Markiewski MM, et al. Modulation of the antitumor immune response by complement. Nat. Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.