Abstract
The chemokine CCL27 has chemoattractant properties for memory T cells and has been implicated in skin allergic reactions. The present study reports the expression in the brain of two CCL27 splice variants localized in the cerebral cortex and limbic regions. CCL27-like immunoreactivity was identified mainly in neurons. Variant 1 was found elevated in the olfactory bulbs during allergic inflammation induced by intranasal challenge with allergen. This was accompanied by the presence of T cells in the olfactory bulbs. Intranasal administration of neutralizing antibodies against CCL27 reduced the presence of T cells in the olfactory bulbs suggesting a function in T cell activity in the brain.
Keywords: CTACK, PESKY, alternative splicing, in situ hybridization, neuroimmune, RT-PCR
1. Introduction
The chemokine CCL27 is a member of the beta family of chemokines and has been shown to display specific homing properties for memory CD4+ T cells expressing the cutaneous lymphocyte antigen (CLA) (Morales et al., 1999). Initial studies found that CCL27 was constitutively and selectively expressed by keratinocytes in the skin and because of its homing properties for memory T cells was named cutaneous T cell attracting chemokine (CTACK) (Morales et al., 1999). This chemokine has important functions in skin lymphocyte trafficking and inflammation (Morales et al., 1999; Homey et al., 2002) and plays a pivotal role on skin allergic processes including atopic dermatitis and delayed type hypersensitivity reactions in both human and experimental animal models (Homey et al., Huang et al., 2008; Kunkel and Butcher, 2002; Vastergaard et al, 2004a,b; Reiss et al., 2001). Increased production of CCL27 and expression of its cognate receptor CCR10 has been reported in the skin of atopic dermatitis patients (Homey et al., 2002). Further, the role of CCL27 in skin allergy is supported by studies showing that overexpression of CCL27 in transgenic mice promotes the development of skin contact hypersensitivity and the production of interleukin-4 (Kagami et al., 2008) and CCL27 was shown essential for the development of atopic skin inflammatory processes in an IL-4 transgenic mice model of atopic dermatitis (Chen et al., 2006). Lastly, CCL27 may present a viable target for the treatment of skin cancer (Gao et al., 2009, 2003; Pivarcsi et al., 2007). CCR10 is the only receptor known to bind CCL27 and mediate its effects (Homey et al., 2000; Jarmin et al, 2000). The mRNA and genomic sequence for CCL27 were contemporarily obtained by several groups resulting in different names including ESkine (stem cell derived chemokine) (Baird et al., 1999), ILC ( interleukin-11 receptor alpha-locus chemokine) (Ishikawa-Mochizuki et al., 1999) and ALP (amino terminal peptide sequence) (Hromas et al., 1999). The genomic sequence is located in chromosome 4 in mice and chromosome 9 in human and partially overlaps, in the opposite direction, with that of interleukin-11 receptor alpha (Baird et al., 1999; Ishikawa-Mochizuki et al., 1999). Interestingly, CCL27, which is encoded in 3 exons, has 2 different isoforms resulting from alternative splicing of exon 1 which gives the canonical secreted mature form of CCL27 expressed in the skin and placenta and a second protein with a different exon 1 called PESKY, which is highly expressed in the brain and testis (Morales et al., 1999; Baird et al., 1999; Gortz et al., 2002; Nibbs and Graham, 2003). The current nomenclature for these transcripts assigned the name CCL27 variant 1 for PESKY and CCL27 variant 2 for CTACK. While variant 2 (CTACK) is the main isoform and mediates interferon-γ negative CD4+ memory T cell activity in the skin, variant 1 (PESKY) has been shown to produce cytoskeletal actin re-arrangement promoting cell motility (Baird et al., 1999; Gortz et al., 2002; Nibbs and Graham, 2003). Previous studies have assessed the role of interferon-γ negative CD4+ T cells in neurodegenerative (Brochard et al., 2009; Seksenyan et al., 2009) and neuroprotective (Lewitus and Schwartz, 2009; Lewitus et al., 2008; Ziv et al., 2006; Cohen et al., 2006) mechanisms in the brain and reported the expression of CCR10 receptors in astrocytes (Dorf et al., 2000) and neurons (Meucci et al., 1998; Cartier et al., 2005), however there are no studies on the expression and/or function in the brain of CCL27 variants.
We had previously shown that in the eye, both under normal conditions and during inflammatory processes, there are mRNA transcripts for variant 2 (CTACK) and variant 1 (PESKY), as well as 2 other transcripts of variant 2 that retain intronic sequences (Ledee et al., 2004). These precursor splice variants of canonical CCL27 were not detected in the skin suggesting a different transcriptional regulation of CCl27 in the eye. Further, localization studies showed that the mRNAs splice variants for CCL27 were produced specifically by the retinal cell layer (Ledee et al., 2004). The cells in this layer are mostly of neuronal origin raising the possibility that these mRNA species may be also produced by other cells of the nervous system. The present study examined the expression and localization of splice variants for CCL27 in the murine brain. Because CCL27 has been implicated in allergic inflammatory processes, we also studied the regulation of these transcripts in a model of allergic inflammation using sensitization with albumin from chicken egg (OVA) and later challenge with OVA via intranasal instillations (Tonelli et al, 2009). This model results in an inflammatory process in the nasal cavities which mimics allergic rhinitis (KleinJan et al., 2006) and is paralleled by increased transcription in the olfactory bulbs (OB) of the TH2 cytokines IL-4, IL-5 and IL-13 (Tonelli et al., 2009). We also analyzed the presence of mature T cells in the OB and the effects of treating allergic mice with intranasal administrations of neutralizing antibodies against CCL27. The results of the present study show a wide expression of CCL27 variants mostly in neurons of the cerebral cortex and limbic regions and suggest a function in neuroimmune communication with T cells during peripheral allergic inflammation.
2. Materials and methods
2.1 Animals
Male BALB/c mice (n = 36 total) were obtained from Taconic farms at 8 weeks of age. They were housed in groups of four in microisolator cages with standard food pellets and water available ad libitum in a room at a constant temperature of 23° C. All animals were maintained on a 12:12 L:D cycle (lights on at 07:00 hr). The animals were left undisturbed for one week before starting with the experimental procedures.
2.2 Sensitization and challenge with ovoalbumin (OVA)
Allergic rhinitis to OVA was induced as previously described (KleinJan et al., 2006; Tonelli et al., 2009). Briefly, albumin from chicken egg (OVA) was purchased from Sigma (St Louis MO) and absorbed to aluminum hydroxide (Alum) (Pierce) in a 1:1 mixture for 30 minutes under constant shaking at room temperature. Mice were immunized by intraperitoneal injections of Alum alone or with 100 μg/mice OVA/Alum in a volume of 200 μl on day 1 and boosted with the same reagents on day 14. Mice were challenged on day 21, 22 and 23 by intranasal instillations with a 1% OVA solution in PBS (allergic mice) or PBS alone (control mice). For this procedure, mice were slightly anesthetized with isofluorane in induction chambers and at the moment of awakening 25 μl of OVA/PBS or PBS alone was delivered with a micropipette on each nostril. Mice were euthanized one day later after the last intranasal challenge and a fluid lavage procedure was performed followed by differential staining using cytospin Diff-Quick (Dade Behring, Newark, DE) as described in Kelly-Welch et al, 2004. Brains were removed and frozen in isopentane until further processing. A total of 1 ml of fluid lavage was centrifuged and the cells re-suspended in 100 μl of buffer, stained and counted under microscopic examination. Supernatants were used for cytokine determination using commercially available ELISA kits. Additional groups of mice were sensitized and challenged with OVA as described above and administered with an additional intranasal dose of neutralizing antibody against CCL27 (R&D Systems cat#AF725; 100 μg/ml in 25 μl/nostril) 30 minutes after the OVA challenge.
All the procedures were approved by the University of Maryland Institutional Animal Care and Use Committee.
2.3 RT-PCR and Real-time RT-PCR
Brain tissue was processed for mRNA extraction using the Trizol reagent (Invitrogen, USA) as described previously (Tonelli et al., 2009; 2008). Total RNA was treated with DNAse I (Invitrogen, USA) for 15 minutes at room temperature according to manufacturer's instruction. For sequencing studies, 1 microgram of total RNA per sample were reverse transcribed into cDNA in a 20 μl reaction using a high fidelity reverse transcriptase kit (Invitrogen). For quantitative real time PCR studies, 500 ng of total RNA were reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to manufacturer's instructions.
Primers targeting specific regions of CCL27 transcripts that were used for sequencing or real-time RT-PCR were listed earlier (Ledee et al., 2004). Primers used to quantify cytokines and chemokines were listed in Tonelli et al, 2009 and Pedras-Vasconcelos et al, 2006. Analysis of sequencing and primer design was done using the web based tools from NCBI and the Accelrys Gene 2.5v software. The PCR products derived from all sets of primers were run on 0.9% agarose gel and analyzed under UV exposure using a gel imaging system (Alpha Innotech, Santa Clara, CA). The amplified products were directly cloned into the pCRII-Topo vector (Invitrogen, Paisley, Scotland, UK) and sequenced by the NIH DNA Sequencing core facility. Real-time RT-PCR was conducted using the iQ SYBR Green Supermix (Bio-Rad) in a 25 μl reaction and run on a MyiQ instrument (Bio-Rad) with a three step cycling program as described earlier (Tonelli et al., 2009; 2008). Efficiency and consistency of the cDNA synthesis was determined by amplification of the 18S gene as a control. For each sample of a specific transcript, each cycle threshold was normalized with respect to the average of 3 control genes including 18S, GAPDH and Actin-β. Relative expression was determined using the 2-ΔΔCt method as described (Livak and Schmittgen, 2001).
2.4 In situ hybridization histochemistry
Specific sequences were produced to target exon 1 corresponding to mouse variant 1 (PESKY; Genebank accession number NM_001048179), or intron 1 corresponding to the mouse variant 2 retaining intron 1 (Genebank accession number AY744155), or the common sequence corresponding to exon 3 as described earlier (Ledee et al., 2004). The specific fragments were produced by PCR amplification using the PCR supermix high fidelity enzyme mixture (Invitrogen). Anti-sense and sense (control) riboprobes were labeled by in vitro transcription in the presence of 10 μM [35S]UTPαS (Amersham Pharmacia; > 1000 Ci/mmol), 1 μg of linearized plasmid, and 20 units of T3 or T7 RNA polymerase using the RNA labeling kit (Amersham Pharmacia) according to manufacturer's protocol. After transcription, the template DNA was digested with Dnase I for 15 minutes at 37° C. Unincorporated [35S]UTPαS was removed by centrifugation through ProbeQuant G-50 micro columns (Amersham Pharmacia).
The in situ hybridization procedure was carried out as described earlier (Tonelli et al., 2003). Serial consecutive brain sections were cut in a cryostat at 20 mm of thickness through the entire murine brain and collected onto silanated slides and stored at −20° C until further processing. Sections were fixed for 10 minutes with a 4% paraformaldehyde solution in phosphate buffered saline (PBS), rinsed twice in PBS, acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine-HCl ph 8.0 for 15 minutes and dehydrated through graded ethanol. Each slide was treated with 150 μl of hybridization buffer containing 40,000 cpm/μl of labeled sense or anti-sense riboprobe, 50% formamide, 0.3 M NaCl, 2 mM EDTA, 20 mM Tris ph 8.00, 1 X Denhardt's solution, 10% dextran sulfate, 100 μg/ml salmon sperm DNA, 250 μg/ml yeast RNA, 250 μg/ml yeast tRNA, 100 mM dithiothreitol and 0.1% sodium dodecyl sulfate. After hybridization for 16-18 h at 54° C, sections were rinsed 4 times in 4 X saline citrate buffer (SSC) to remove coverslips and excess of riboprobes. Non-hybridized riboprobes were digested by incubation with 40 μg/ml Rnase A (Sigma) for 30 minutes at room temperature. After a final high stringency wash in 0.1 SSC at 65° C for 60 minutes, sections were dehydrated in graded ethanol containing 0.3 M ammonium acetate and air-dried.
Sections were exposed to BioMax film (Eastman Kodak, Rochester, NY) along with [14]C standards (Amersham Pharmacia) for 4 days and developed in an automatic film developer X-OMAT (Eastman Kodak). The mRNA expression was analyzed by measuring optical film densities using the public domain NIH Image 1.62 program. Values were transformed to nCi/g of gray matter after calibration with standards. Background signal from sense control slides was subtracted to obtain the specific signal with antisense riboprobe. Anatomical localization of the signals was determined by Nissl staining of adjacent sections. For evaluation at the cellular level of mRNA expression, slides were dipped in NTB2 photo emulsion (Eastman Kodak), exposed for 3 weeks and developed in D-19 developer (Kodak) for 4 minutes at 15° C, fixed for 4 minutes and counterstained with toluidine blue. Positive hybridization signal was evaluated with the use of a Nikon Microphot-FX microscope (Nikon, Japan). Sections were analyzed at 100 times magnification with a Nikon Plan-Apo lens of 0.45 numerical aperture and a dark field condenser of 0.95 to 0.8 numerical aperture. Positive hybridization signals were detected by using a macro-program that identifies light peaks of rounded shape. Identified light peaks were then analyzed at 600 times magnification to confirm that silver grains were deposited over cell bodies.
2.5 Immunohistochemistry
Two protocols were employed using either fresh frozen or paraformaldehyde fixation. Fresh frozen sections of 20 μm of thickness were cut with a cryostat and mounted on gelatinized slides. The sections were fixed in a 1:1 acetone-methanol solution at −20° C for 15 minutes. Sections were then washed with PBS and incubated for 20 minutes in 10% bovine serum albumin (BSA). Sections were incubated overnight with a polyclonal goat anti-mouse CCL27 antibody (R&D Systems cat#AF725) (1:3,000 dilution). After washing with PBS the sections were incubated for 2 hours with donkey anti-goat antibody conjugated with Cy3 fluoroprobe (Jackson Immunoresearch Laboratories). Sections were counterstained with Hoechst solution. For colocalization studies with cellular markers, BALB/c mice were transcardially perfused with 30 ml of saline followed by 4% paraformaldehyde. The brains were removed and post-fixed for 4 hours by immersion in the same solution and cryoprotected. Sections were cut in a cryostat at 50 μm of thickness and collected in phosphate buffered saline. Free floating sections were incubated with 5% triton x-100 in PBS followed with 10% BSA for 1 hour at room temperature. Sections were incubated with the same goat anti-mouse CCL27 antibody used in the fresh frozen protocol for 24 hours at 4° C (1:1,000 dilution) in combination with mouse monoclonal anti-NeuN (1:10,000 dilution) or rabbit polyclonal anti-glial fibrillary acidic protein (GFAP) (1:5000 dilution) or anti-CD3 antibody (Serotec; MCA 1477at 1:1000 dilution). Sections were then washed and incubated for 2 hours with donkey anti-goat antibody conjugated with Cy3 and rabbit-anti mouse antibody conjugated with FITC or rat anti-rabbit antibody conjugated with FITC or rabbit anti-rat antibody conjugated with FITC. Sections were analyzed with a Zeiss LSM510 confocal microscope with ZEN software. Intrinsic resultion was 1024×1024 with a 1 μm step between z-stack optical sections. Sections were analyzed at 4 times magnification for anatomical localization followed by analysis at 100, 200 and 630 times magnification.
For stereology of CD3 positive cells in the olfactory bulbs, identified cells were counted in 6 sections per mice in a total of 6 mice per condition.
2.6 Statistics
Real-time RT-PCR data and total number of CD3 positive cells were analyzed with ANOVA with treatment as factors. Tukey post doc tests were used to compare pair wise. Numbers of cells in the FL were compared by t-test. Significance level was set a p < 0.05.
3. Results
3.1 The brain expresses two splice variants for CCL27
Gel electrophoresis of amplified products using specific primers showed a single band migrating to the expected size for variant 1 (PESKY) and a band of higher molecular weight than the expected for variant 2 (CTACK) (Figure 1 A). Using skin tissue as positive control, it was observed that no product co-migrated with the fully spliced mature form of variant 2 (Figure 1 A). Analysis of sequences of PCR amplified products from brain tissue using the NCBI BLAST function showed that the normal brain constitutively expresses the full mature mRNA corresponding to CCL27 variant 1 (NM_001048179; PESKY) and the precursor AY744155 corresponding to the variant 2 (CTACK) retaining intron 1 (Figure 1 A). These data are in agreement with initial studies reporting that the expression of full spliced variant 2 (NM_011336; CTACK) was not detected in the brain (Morales et al., 1999; Baird et al., 1999). Analysis of the sequence of the AY744155 mRNA precursor showed that the retained intronic sequence introduces a stop codon that will truncate translation at amino acid 38. Analysis of the open reading frame downstream to this stop sequence indicates a potential initiation site which will result in a predicted truncated peptide of 67 amino acids corresponding to the c-terminus of the mature form of variant 2 (CTACK) lacking the first 53 amino acids which includes the –CC-motif (supplemental figure 1). Real-time RT-PCR using specific primers against variant 1 exon 1, and variant 2 exon 1 showed that variant 1 is the most abundant isoform and it is expressed about 5 times more than AY744155 (variant 2 retaining intron 1) (Figure 1 B).
FIGURE 1.
Gel electrophoresis (A) of RT-PCR amplified products from skin (positive control) or brain RNA using primers encompassing the entire mRNA of canonical CCL27 variant 2 (CTACK) or variant 1 (PESKY) in the absence (RT−) or presence (RT+) of reverse transcriptase. AY744155 correspond to the partially spliced mRNA of variant 2 retaining intron1. No product co-migrated with the fully spliced form of CCL27 variant 2 (CTAK) in the brain. Real-time RT-PCR (B) quantification using β-acting as a reference gene showed that PESKY is expressed at higher levels with respect to AY744155. (***) Significant differences by t-test (p<0.00001).
3.2 Neuroanatomical localization of CCL 27 transcripts
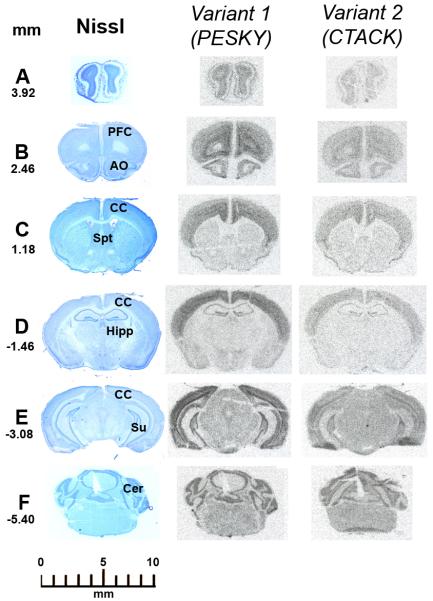
Specific riboprobes directed against exon 1 of variant 1 and intron 1 of variant 2 or to the common sequence corresponding to exon 3 (Ledee et al., 2004) produced the same hybridization signal indicating that the same regions of the brain express these transcripts (Figure 2 and supplemental figure 2). Positive hybridization signal for both variant 1 and 2 was found throughout the cerebral cortex (Figure 2). Sense control riboprobes gave no signal also indicating that there was no cross-hybridization with potential mRNAs from the interleukin receptor 11 (Supplemental figure 1). Specific hybridization signal detected by film autoradiography was also found in the olfactory bulbs and dentate gyrus of the hippocampal formation and the external layers of the cerebellum. Additional signals were found in the dorsal hypothalamus and central and lateral nucleus of the amygdala (Figure 2). Quantification by film density autoradiography showed that variant 1 is expressed with higher intensity than variant 2 confirming the real-time results. Variant 1 expressed through the cortical mantle an average of 192 ± 32 nCi/g of gray matter compared to 46 ± 11 nCi/g of gray matter for variant 2. The hybridization signal produced by the riboprobe covering the sequence corresponding to exon 3 common to both variant 1 and 2 saturated the film at the same exposure time used for quantification of variant 1 and 2 specific signals (supplemental figure 1). Further quantification showed that the cortex expressed the highest levels followed by the dentate gyrus, olfactory bulbs, dorsal hypothalamus and amygdala with the lowest levels in the cerebellum. Analysis of emulsion coated slides by dark field microscopy showed that specific signals were of round shape corresponding to cells and mostly expressed in discrete layers of the cortex (Figure 3). Most densely expressed hybridization signals were found in layers III, V and VI and less dense in layer IV with few sparse cells in layers II and no signal in layer I. Analysis of emulsion coated slides by bright field microscopy confirmed that silver grains were deposited over Nissl stained cells (supplemental figure 3). Specific hybridization signal was also confirmed by emulsion autoradiography in the dentate gyrus, dorsal hypothalamus, amygdala and cerebellum (data not shown).
FIGURE 2.
Film autoradiographic images of the hybridization signal produced by radioactive riboprobes directed against the exon 1 of variant 1 or intron 1 of variant 2 of CCL27 in coronal serial consecutive sections of the murine brain. The left panel correspond to sections stained with Nissl for anatomical localization. The numbers represents the coronal levels in mm from Bregma. Section A is at the levels of the olfactory bulbs. Section B is at the level of the prefrontal cortex (PFC) and anterior olfactory nucleus (AO). Section C is at the level of the septum (Spt). Section D is at the level of the anterior hippocampus (Hipp). Section E is at the level of the posterior hippocampus and Subiculum (Su). Section F is at the level of the cerebellum (Cer). Specific signals for both variants are distributed trough the cerebral cortex (CC). For more detail see the text.
FIGURE 3.
Representative digital image of a section of emulsion coated slides under bright field illumination showing the neuroanatomical localization in the cerebral cortex at the level of the anterior hippocampus of the signal shown under dark field illumination of the areas marked in A and B. Note the round shape of cumulated silver grains produced by riboprobes directed against intron 1 of variant 2 (CTACK). Arrowheads indicate the surface of the cortex. CA1: Pyramidal cell layer of the CA1 region of the hippocampus; Ctx: Cerebral cortex; DG: Dentate gyrus.
3.3 CCL27-like immunoreactivity
The antibody utilized recognizes the peptides 26 to 120 of the mature variant 2 form of CCL27 (CTACK). This antibody also identifies the protein produced by variant 1 (PESKY) since variant 1 and 2 have a common sequence starting with amino acid 23. CCL27-like immunoreactivity displayed the same distribution than the hybridization signals. In particular, positive signals were observed in the cerebral cortex with a similar pattern to that of emulsion dipped slides from in situ hybridization. Figure 4A shows the distribution of positive cells in the cortex close to the cerebral midline and figure 4B shows the approximate boundaries of cortical layers in the same sections counter-stained with Hoechst. Analysis of the immunoreactivity at higher magnification revealed that the protein identified by the CCL27 antibody has a perinuclear localization (Figure 4D, E and F). This was confirmed in additional brain areas including the dentate gyrus (supplemental figure 4), olfactory bulbs, central and basolateral nucleus of the amygdala and dorsal hypothalamus. To determine the types of cells expressing CCL27-like immunoreactivity, co-localization studies with neuronal or glial specific markers was performed in 50 μm thickness sections and analyzed with confocal microscopy. Analysis of the signal in sections co-incubated with the nuclear neuronal marker NeuN revealed that almost all the signal was co-localized in neurons and confirmed the same perinuclear expression as observed earlier (Figure 5). Confocal images further revealed that CCL27-like immunoreactivity consisted from 2 to 5 densely stained immunoreactive elements per cells around the nucleus with few elements of nuclear localization (Figure 5). Few CCL27-like immuoreactive cells were determined of non-neuronal phenotype (Figure 5) and corresponding to GFAP positive cells (data not shown).
FIGURE 4.

CCL27-like immunoreactivity (red) in the cerebral cortex at the level of the anterior hippocampus close to the cerebral midline. A: Digital image at 100 X magnification of the fluorescent signal of CCL27 visualized with Cy3. B: Hoechst staining of the same section shown in A. I to VI are the approximate boundaries of the layers of the cerebral cortex. B: Merged image of the signals shown in A and B. D: 200 X magnification of the signal shown in A. E: Hoechst staining of the same section in D. F: Merged images of D and E showing association of CCL27-like immunoreactivity with nuclear DNA stained with Hoechst. Note the perinuclear localization of the immuoreactivity. Arrowheads indicate the surface of the cortex.
FIGURE 5.

Digital images obtained from a Z-stack originated with a Zeiss LSM 510 confocal microscope at 630 times magnification in layer 5 of the cerebral cortex. Consecutive images (A, C, E, G) at 1μm step between images revealing CCL27 immunostaining (red) in combination with Hoechst nuclear staining (blue). Corresponding same consecutive images are shown in B, D, F, H, revealing CCL27 immunostaining (red) and the nuclear neuronal marker NeuN (green). The arrows indicate the same position between stacks of selected neurons showing perinuclear CCL27 immunoreactivity. The image show that almost all the CCL27 signal is co-localized with neurons with the exception of one cell indicated with an arrowhead. Scale bar in G and H = 10 μm applied to all panels.
3.5 Transcriptional regulation of CCL27 variants in the olfactory bulbs during allergic inflammation in the nasal cavities
Allergen challenge (OVA) in sensitized mice resulted in increases in total cell content in the fluid lavage (FL) (Table 1). Increases were observed for all cell type studied including macrophages, lymphocytes, eosinophils and neutrophils (Table 1). These results are similar to those reported previously for sensitization and challenge with OVA in BALB/c mice (Kelly-Welch et al, 2004) confirming inflammation in the respiratory tract. We have previously shown that intranasal challenge with OVA in sensitized mice results in elevations of mRNA expression of the cytokines IL-4, IL-5 and IL-13 in the olfactory bulbs (Tonelli et al, 2009). We therefore studied in parallel the expression of these cytokines and CCL27 splice variants to gain insight on possible functional relationships. We compared further in the same model the expression of CCL27 with that of the pro-inflammatory cytokines TNF-α and IL-1β and the chemokines CCL5 (RANTES) and CX3CL1 (fractalkine). Analysis with ANOVA showed significant differences when comparing the expression of these cytokines and chemokines in PBS vs OVA challenged mice (F = 72.05; p < 0.0001). In accordance with our previous results (Tonelli et al, 2009), Tukey's pos doc comparisons showed that sensitization and challenge with OVA via intranasal administration resulted in significant elevations of mRNA expression of IL-4, IL-5 and IL-13 in the olfactory bulbs (Figure 6). This was paralleled by significant increases in the expression of CCL27 variant 1 (PESKY) (Figure 6). No significant increases were observed for variant 2 mRNA corresponding to AY744155. No significant changes were detected in the expression levels of the cytokines TNF-α and IL-1β and the chemokines CCL5 and CX3CL1 (Figure 6). These results show that CCL27 variant 1 (PESKY) in the olfactory bulbs is responsive to peripheral allergic inflammation in the nasal cavities and that the mRNA is increased in parallel with that of the TH2 cytokines IL-4, IL-5 and IL-13.
TABLE 1.
Cell composition in the fluid lavage (FL) of sensitized mice challenged with PBS or albumin from chicken egg (OVA). Significant differences were observed using t-test for total number of cells (t = 11.5; p < 0.0003), macrophages (t = 4.07; p < 0.015) and lymphocytes (t = 18.9; p < 0.002). Significant numbers of eosinophils and neutrophils were also observed that were not detected in control conditions.
| PBS | OVA | |
|---|---|---|
| Total Cells | 155,000 ± 18,929 | 500,000 ± 23,529 |
| Macrophages | 152,812 ± 18,726 | 264,633 ± 20,500 |
| Lymphocytes | 1,837 ± 142 | 75,608 ± 3,963 |
| Eosinopohils | ND | 102,208 ± 4,105 |
| Neutrophils | ND | 48,736 ± 20,429 |
FIGURE 6.
Real-time RT-PCR quantification of mRNA expression for cytokines and chemokines in the olfactory bulbs of sensitized mice challenged intranasally with albumin from chicken egg (OVA) with respect to control sensitized and challenged with PBS. Scale in the Y axis is fold increase with respect to control treated mice represented by the number 1. Significant increases were observed for interleukin-4 (IL-4) (*** p < 0.001); interleukin-5 and 13 (IL-5; IL-13) (** p < 0.01) and CCL27 variant 1 (CCL27 v1) (* p < 0.05). No significant differences were detected for CCL27 variant 2 (CCL27 v2), CCL5 (RANTES), CX3CL1 (fractalkine) and the cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β).
3.6 Effect of CCL27 on lymphocyte infiltration of in the olfactory bulbs during allergic inflammation
Lymphocytes play a pivotal role in directing allergic inflammatory processes and invade the nasal epithelium during allergic rhinitis (KleinJan et al, 2006). Because neurons of the olfactory bulbs are in direct contact with the nasal epithelium and some cells have been shown to migrate from the nasal mucosa into the brain through the cribriform plate along the olfactory neural pathway (Danielyan et al, 2009), we studied if T lymphocytes invade the olfactory bulbs during allergic rhinitis and if this process is mediated, at least in part, by CCL27. To block CCL27 we used a dose of 100 μg/ml of neutralizing antibody which has been shown to provide maximum inhibition of the chemokine activity. The biological activity and neutralizing properties of the antibody (the same used in immunohistochemical studies) have been determined by in vitro assays by R&D systems (see: http://www.rndsystems.com/pdf/af725.pdf).
Positive cells for the surface marker CD3 corresponding to mature T lymphocytes were found in the olfactory bulbs of allergic mice (Figures 7, 8). Most of the cells were found in meningeal spaces (Figure 7) and less frequent in the parenchyma of the olfactory bulbs at the level of the glomerular cell layer (Figure 8). CD3 positive signals were confirmed to co-localize with nuclear DNA as revealed by Hoechst staining (Figure 7, 8). Double fluorescent immunohistochemistry showed that CD3 positive cells in the parenchyma of the OB were in close proximity of CCL27 positive cells (Figure 9). Few CD3 positive cells per mice were found in the meningeal spaces of control sensitized mice challenged intranasally with PBS and no cells were found in the parenchyma of control mice. Cell counting of total CD3 positive cells in 6 sections per mice in 6 mice per condition indicated an average of 12.33 ± 7.8 (SD) cells in OVA treated mice with respect to 1.33 ± 1.21 (SD) cells of control PBS treated mice. Intranasal administration of neutralizing antibody against CCL27 reduced the number of CD3 cells to 2.16 ± 1.17 (SD) in OVA treated mice. Analysis with ANOVA detected significant differences when comparing treatments (F = 13.96; p < 0.0004). Tukey's comparisons test indicated significant differences when comparing OVA treated mice with control PBS (p < 0.001) and with mice treated with OVA plus neutralizing antibodies (p < 0.01).
FIGURE 7.
Digital images at 200 times magnification showing FITC immunofluorescent signals corresponding to CD3 positive cells (A) in the meningeal spaces of the olfactory bulbs of mice sensitized and challenged with albumin from chicken egg (OVA). Images obtained from Hoechst staining (B) were merged with those of CD3 to confirm co-localization of the CD3 signal with nuclear DNA (C).
FIGURE 8.
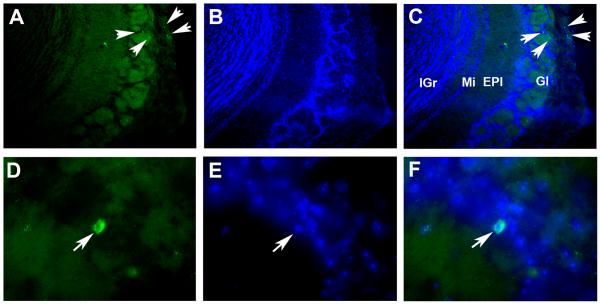
Digital images at 100 times magnification (A, B, C) or 400 times magnification (D, E, F) of FITC immunofluorescent signals corresponding to CD3 positive cells (A, D) in the olfactory bulbs of mice sensitized and challenged with albumin from chicken egg (OVA). Images obtained from Hoechst staining (B, E) were merged with those of CD3 to confirm co-localization of the CD3 signal with nuclear DNA (C, F). Arrows indicate some identified CD3 positive cells in the glomerular cell layer (Gl). EPl: external plexiform cell layer; Mi: mitral cell layer; IGr: internal granular cell layer.
FIGURE 9.

Digital image of double fluorescent immunohistochemistry of a CD3 positive cell (FITC, green) in close proximity of CCL27 positive (Cy3, red) cells in the glomerular cell layer of the olfactory bulbs of mice sensitized and challenged with albumin from chicken egg (OVA). Sections were counter stained with Hoechst to reveal nuclear DNA (blue). Scale bar 10 μm.
4. Discussion
The present study showed alternative splicing of the CCL27 gene locus in neurons. These mRNAs are localized predominantly in the cerebral cortex and limbic structures suggesting specific functions in discrete neuronal populations. It also suggests that the expression of variant 1, PESKY, is regulated during allergic inflammation and that this variant may be involved in lymphocyte trafficking into the brain at least during allergic inflammatory conditions. Together, the present studies provide evidence of a role for CCL27-related genes in neuroimmune functions.
An early study using NIH3T3 cells transfected with variant 1 (PESKY) showed that stable expression of this protein reduces the amount of actin stress fibers resulting in relaxation of the cytoskeleton and increased migratory capacities (Gortz et al., 2002). Responsiveness of the PESKY mRNA to allergic inflammation suggests that this gene may be also related with TH2 processes as it has been shown for the skin specific isoform CTACK (Huang et al., 2008; Kagami et al., 2008). Migration into the brain of CD4+ lymphocytes of the TH1 type is known to occur in models of autoimmune diseases, viral infections and tissue damage. A number of chemokines and their receptors widely expressed in the brain have been shown to participate in these mechanisms (Prendergast and Anderton, 2009; O'Connor et al, 2008; Aloisi et al., 2008; 2000). On the other side, much less is known about migration and molecular mechanisms mediating TH2 lymphocyte function in the central nervous system. The present study suggest that CCL27 variants may play specific functions in the migration of T cells from the nasal epithelium into the olfactory bulbs during allergic inflammation as evidenced by the reduced number of CD3 positive cells of animals treated with neutralizing antibodies. Trafficking of T cells into the olfactory bulbs from the nasal epithelium may be favored by the lack of blood brain barrier (Danielyan et al, 2009) and this process may be mediated, at least in part, by neuronal forms of CCL27. However, additional studies are required to confirm specific chemoattractant properties on T cells for the brain splice variants of CCL27. This can be approached by employing cell culture systems which can measure migration into a gradient of CCL27 isoforms and also culture systems using nerve cells from the CCL27 transgenic mice. However, this is matter of futures studies.
It has been proposed during recent years that T lymphocytes migrate into the brain in the absence of inflammation. It has been recently shown that CD4+ T cells migrate into specific brain regions in response to psychogenic stressors and that this mechanism is mediated by the actions of glucocorticoids (Lewitus and Schwartz, 2009; Lewitus et al., 2008; 2009). Importantly, activated CD4+ lymphocytes have been shown to contribute to neurogenesis in the hippocampus and improve learning and cognitive function (Ziv et al., 2006). These studies have shown that CD4+ T cells provide resistance to the deleterious effects of stress on the brain and to prevent the development of anxiety (Cohen et al., 2006) and depression (Lewitus et al., 2009) after exposure to several psychogenic stressors. The mechanisms that have been proposed on how peripheral T cells confers neurobehavioral protection and promote neurogenesis are by trafficking into the brain after stress where they stimulate the release of neurotrophic factors (Lewitus et al., 2009; Schwartz and Ziv, 2008). Interestingly, canonical variant 2 isoform CTACK is a chemokine that has been shown elevated after exposure to acute stress and to participate in enhanced skin immune function (Dhabhar et al., 2010). It has been proposed, yet not demonstrated, that the phenotype of T cells that migrate into the brain after stress is of the TH2 type (Schwartz and Ziv, 2008). The expression of CCL27 splice variants and CCR10 in the dentate gyrus of the hippocampal formation where neurogenesis occurs may provide molecular basis about some of the neuroimmune mechanisms involving neurons, astrocytes and TH2 lymphocytes.
The neuronal splice variants reported in the present study may have other functions in addition to lymphocyte trafficking during allergic inflammation. In this regard, the expression in the same regions and likely the same cells of the mRNA specie AY744155 corresponding to variant 2 (CTACK) retaining intron 1 is of relevance. As discussed earlier, this may potentially result in two short proteins fragments of canonical variant 2 of CCL27 separated close to the CC motif. Regardless whether these fragments are translated or AY744155 is a long non-coding RNA, the expression of this transcript has important implications for the regulation of CCL27 splice variants (Wilusz et al., 2009). In support of this view is the unique pattern of immunoreactivity found in neurons. The predominant perinuclear localization of small densely packed elements has not been previously described in other tissues or in the transfection systems previously employed (Baird et al., 2009; Gortz et al., 2002; Nibbs and Graham, 2003). Early studies showed that the PESKY exon 1 contains a nuclear localization signal and that this protein is expressed in the nucleus (Gortz et al., 2002). It is possible that the expression of AY744155 results in sub-cellular re-arrangements and that these proteins localize in other compartments in addition to the nucleus. The CCR10 receptor found in astrocytes and neurons of the hippocampal formation suggest a functional role for CCL27-related peptides with the capacity to bind the CCR10 receptor. However, it is not known if these protein products are released outside the cell and therefore act in autocrine or paracrine manner. Also, while the present study did not detected fully spliced canonical CCL27 in the brain, it is possible that under certain conditions local production of fully spliced CCL27 may occur.
In summary, the present study shows that neurons express CCL27 splice variants which are regulated upon allergic inflammatory processes and related to the number of T cells found in some regions of the brain. These results suggest a functional role for neuronal CCL27 variants on neuroimmune function and provide molecular basis on potential interactions with lymphocytes.
Supplementary Material
Schematic protein alignment of the predicted peptide products produced by translation of the mRNA sequence of CCL27 variant 2 retaining intron 1 (AY744155).
Film autoradiographic images of the hybridization signal produced by radioactive riboprobes directed against the exon 1 of variant 1 or intron 1 of variant 2 of CCL27 or to the shared exon 3 of both variants. Bottom panels are consecutive sections hybridized with sense control riboprobes labeled at the same activity than antisense specific riboprobes (top panels). The sections have been exposed for 4 days. Note that the images corresponding for the common sequence saturated the film indicating the additive effect of hybridization of both variants in the same regions.
Bright field digital images at 40 times magnification of emulsion coated slides hybridized with riboprobes against variant 1 of CCL27 counterstained with toluidine blue (Nissl staining). Note the deposition of silver grains over blue stained cells.
Digital images at 200 X magnification of the fluorescent signal produced by CCL27 in the dentate gyrus (A). B: Image of the same section counterstained with Hoechst. C: Merged image showing localization of red signals corresponding to CCL27 around nuclear DNA stained with Hoechst.
Acknowledgements
The authors whish to thank Dr. Esther M. Sternberg from the National Institute of Mental Health for her logistic support and Dr. Steven Bernstein from University of Maryland for his help with antibodies used in this study. This study was supported by an Intramural Award from the Integrative Neural Immune Program, NHI, to PZ and LHT and by funds from the Chairman of the Department of Psychiatry, University of Maryland School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aloisi F, Columba-Cabezas S, Franciotta D, Rosicarelli B, Magliozzi R, Reynolds R, Ambrosini E, Coccia E, Salvetti M, Serafini B. Lymphoid chemokines in chronic neuroinflammation. J Neuroimmunol. 2008;198:106–12. doi: 10.1016/j.jneuroim.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi F, Serafini B, Adorini L. Glia-T cell dialogue. J Neuroimmunol. 2000;107:111–7. doi: 10.1016/s0165-5728(00)00231-9. [DOI] [PubMed] [Google Scholar]
- Baird JW, Nibbs RJ, Komai-Koma M, Connolly JA, Ottersbach K, Clark-Lewis I, Liew FY, Graham GJ. ESkine, a novel beta-chemokine, is differentially spliced to produce secretable and nuclear targeted isoforms. J Biol Chem. 1999;274:33496–503. doi: 10.1074/jbc.274.47.33496. [DOI] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–92. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Chen L, Lin SX, Agha-Majzoub R, Overbergh L, Mathieu C, Chan LS. CCL27 is a critical factor for the development of atopic dermatitis in the keratin-14 IL-4 transgenic mouse model. Int Immunol. 2006;18:1233–42. doi: 10.1093/intimm/dxl054. [DOI] [PubMed] [Google Scholar]
- Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, Schwartz M, Kipnis J. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol. 2006;66:552–63. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- Danielyan L, Schäfer R, von Ameln-Mayerhofer A, Buadze M, Geisler J, Klopfer T, Burkhardt U, Proksch B, Verleysdonk S, Ayturan M, Buniatian GH, Gleiter CH, Frey WH., 2nd Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88(6):315–24. doi: 10.1016/j.ejcb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Saul AN, Daugherty C, Holmes TH, Bouley DM, Oberyszyn TM. Short-term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain Behav Immun. 2010;24(1):127–37. doi: 10.1016/j.bbi.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorf ME, Berman MA, Tanabe S, Heesen M, Luo Y. Astrocytes express functional chemokine receptors. J Neuroimmunol. 2000;111:109–21. doi: 10.1016/s0165-5728(00)00371-4. [DOI] [PubMed] [Google Scholar]
- Gao JQ, Tsuda Y, Han M, Xu DH, Kanagawa N, Hatanaka Y, Tani Y, Mizuguchi H, Tsutsumi Y, Mayumi T, Okada N, Nakagawa S. NK cells are migrated and indispensable in the anti-tumor activity induced by CCL27 gene therapy. Cancer Immunol Immunother. 2009;58:291–9. doi: 10.1007/s00262-008-0554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JQ, Tsuda Y, Katayama K, Nakayama T, Hatanaka Y, Tani Y, Mizuguchi H, Hayakawa T, Yoshie O, Tsutsumi Y, Mayumi T, Nakagawa S. Antitumor effect by interleukin-11 receptor alpha-locus chemokine/CCL27, introduced into tumor cells through a recombinant adenovirus vector. Cancer Res. 2003;63:4420–5. [PubMed] [Google Scholar]
- Gortz A, Nibbs RJ, McLean P, Jarmin D, Lambie W, Baird JW, Graham GJ. The chemokine ESkine/CCL27 displays novel modes of intracrine and paracrine function. J Immunol. 2002;169:1387–94. doi: 10.4049/jimmunol.169.3.1387. [DOI] [PubMed] [Google Scholar]
- Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, Lehto M, Wolff H, Yen D, Marxhausen H, To W, Sedgwick J, Ruzicka T, Lehmann P, Zlotnik A. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–65. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, Muller A, McClanahan TK, Dieu-Nosjean MC, Orozco R, Ruzicka T, Lehmann P, Oldham E, Zlotnik A. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC) J Immunol. 2000;164:3465–70. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- Hromas R, Broxmeyer HE, Kim C, Christopherson K, 2nd, Hou YH. Isolation of ALP, a novel divergent murine CC chemokine with a unique carboxy terminal extension. Biochem Biophys Res Commun. 1999;258:737–40. doi: 10.1006/bbrc.1999.0507. [DOI] [PubMed] [Google Scholar]
- Huang V, Lonsdorf AS, Fang L, Kakinuma T, Lee VC, Cha E, Zhang H, Nagao K, Zaleska M, Olszewski WL, Hwang ST. Cutting edge: rapid accumulation of epidermal CCL27 in skin-draining lymph nodes following topical application of a contact sensitizer recruits CCR10-expressing T cells. J Immunol. 2008;180:6462–6. doi: 10.4049/jimmunol.180.10.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa-Mochizuki I, Kitaura M, Baba M, Nakayama T, Izawa D, Imai T, Yamada H, Hieshima K, Suzuki R, Nomiyama H, Yoshie O. Molecular cloning of a novel CC chemokine, interleukin-11 receptor alpha-locus chemokine (ILC), which is located on chromosome 9p13 and a potential homologue of a CC chemokine encoded by molluscum contagiosum virus. FEBS Lett. 1999;460:544–8. doi: 10.1016/s0014-5793(99)01406-4. [DOI] [PubMed] [Google Scholar]
- Jarmin DI, Rits M, Bota D, Gerard NP, Graham GJ, Clark-Lewis I, Gerard C. Cutting edge: identification of the orphan receptor G-protein-coupled receptor 2 as CCR10, a specific receptor for the chemokine ESkine. J Immunol. 2000;164:3460–4. doi: 10.4049/jimmunol.164.7.3460. [DOI] [PubMed] [Google Scholar]
- Kagami S, Saeki H, Tsunemi Y, Nakamura K, Kuwano Y, Komine M, Nakayama T, Yoshie O, Tamaki K. CCL27-transgenic mice show enhanced contact hypersensitivity to Th2, but not Th1 stimuli. Eur J Immunol. 2008;38:647–57. doi: 10.1002/eji.200737685. [DOI] [PubMed] [Google Scholar]
- Kaneko YS, Mori K, Nakashima A, Sawada M, Nagatsu I, Ota A. Peripheral injection of lipopolysaccharide enhances expression of inflammatory cytokines in murine locus coeruleus: possible role of increased norepinephrine turnover. J Neurochem. 2005;94:393–404. doi: 10.1111/j.1471-4159.2005.03209.x. [DOI] [PubMed] [Google Scholar]
- Kelly-Welch AE, Melo ME, Smith E, Ford AQ, Haudenschild C, Noben-Trauth N, Keegan AD. Complex role of the IL-4 receptor alpha in a murine model of airway inflammation: expression of the IL-4 receptor alpha on nonlymphoid cells of bone marrow origin contributes to severity of inflammation. J Immunol. 2004;172:4545–55. doi: 10.4049/jimmunol.172.7.4545. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101:8180–5. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KleinJan A, Willart M, van Rijt LS, Braunstahl GJ, Leman K, Jung S, Hoogsteden HC, Lambrecht BN. An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol. 2006;118:1117–25. doi: 10.1016/j.jaci.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- Ledee DR, Chen J, Tonelli LH, Takase H, Gery I, Zelenka PS. Differential expression of splice variants of chemokine CCL27 mRNA in lens, cornea, and retina of the normal mouse eye. Mol Vis. 2004;10:663–7. [PubMed] [Google Scholar]
- Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain Behav Immun. 2008;22:1108–14. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Schwartz M. Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Mol Psychiatry. 2009;14:532–6. doi: 10.1038/mp.2008.103. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, Schwartz M. Vaccination as a novel approach for treating depressive behavior. Biol Psychiatry. 2009;65:283–8. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–5. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, Zlotnik A. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci U S A. 1999;96:14470–5. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbs RJ, Graham GJ. CCL27/PESKY: a novel paradigm for chemokine function. Expert Opin Biol Ther. 2003;3:15–22. doi: 10.1517/14712598.3.1.15. [DOI] [PubMed] [Google Scholar]
- Nilsberth C, Hamzic N, Norell M, Blomqvist A. Peripheral lipopolysaccharide administration induces cytokine mRNA expression in the viscera and brain of fever-refractory mice lacking microsomal prostaglandin E synthase-1. J Neuroendocrinol. 2009;21:715–21. doi: 10.1111/j.1365-2826.2009.01888.x. [DOI] [PubMed] [Google Scholar]
- O'Connor RA, Prendergast CT, Sabatos CA, Lau CW, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181:3750–4. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedras-Vasconcelos JA, Goucher D, Puig M, Tonelli LH, Wang V, Ito S, Verthelyi D. CpG oligodeoxynucleotides protect newborn mice from a lethal challenge with the neurotropic Tacaribe arenavirus. J Immunol. 2006;176:4940–9. doi: 10.4049/jimmunol.176.8.4940. [DOI] [PubMed] [Google Scholar]
- Pedras-Vasconcelos JA, Puig M, Sauder C, Wolbert C, Ovanesov M, Goucher D, Verthelyi D. Immunotherapy with CpG oligonucleotides and antibodies to TNF-alpha rescues neonatal mice from lethal arenavirus-induced meningoencephalitis. J Immunol. 2008;180:8231–40. doi: 10.4049/jimmunol.180.12.8231. [DOI] [PubMed] [Google Scholar]
- Pivarcsi A, Muller A, Hippe A, Rieker J, van Lierop A, Steinhoff M, Seeliger S, Kubitza R, Pippirs U, Meller S, Gerber PA, Liersch R, Buenemann E, Sonkoly E, Wiesner U, Hoffmann TK, Schneider L, Piekorz R, Enderlein E, Reifenberger J, Rohr UP, Haas R, Boukamp P, Haase I, Nurnberg B, Ruzicka T, Zlotnik A, Homey B. Tumor immune escape by the loss of homeostatic chemokine expression. Proc Natl Acad Sci U S A. 2007;104:19055–60. doi: 10.1073/pnas.0705673104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast CT, Anderton SM. Immune Cell Entry to Central Nervous System-Current Understanding and Prospective Therapeutic Targets. Endocr Metab Immune Disord Drug Targets. 2009 doi: 10.2174/187153009789839219. [DOI] [PubMed] [Google Scholar]
- Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–7. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, London A, Shechter R. Boosting T-cell immunity as a therapeutic approach for neurodegenerative conditions: the role of innate immunity. Neuroscience. 2009;158:1133–42. doi: 10.1016/j.neuroscience.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Ziv Y. Immunity to self and self-maintenance: a unified theory of brain pathologies. Trends Immunol. 2008;29:211–9. doi: 10.1016/j.it.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Ziv Y. Immunity to self and self-maintenance: what can tumor immunology teach us about ALS and Alzheimer's disease? Trends Pharmacol Sci. 2008;29:287–93. doi: 10.1016/j.tips.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Seksenyan A, Ron-Harel N, Azoulay D, Cahalon L, Cardon M, Rogeri P, Ko MK, Weil M, Bulvik S, Rechavi G, Amariglio N, Konen E, Koronyo-Hamaoui M, Somech R, Schwartz M. Thymic Involution in Amyotrophic Lateral Sclerosis. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli LH, Holmes A, Postolache TT. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 2008;33:1038–48. doi: 10.1038/sj.npp.1301488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli LH, Katz M, Kovacsics CE, Gould TD, Joppy B, Hoshino A, Hoffman G, Komarow H, Postolache TT. Allergic rhinitis induces anxiety-like behavior and altered social interaction in rodents. Brain Behav Immun. 2009;23:784–93. doi: 10.1016/j.bbi.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli LH, Maeda S, Rapp KL, Sternberg EM. Differential induction of interleukin-I beta mRNA in the brain parenchyma of Lewis and Fischer rats after peripheral injection of lipopolysaccharides. J Neuroimmunol. 2003;140:126–36. doi: 10.1016/s0165-5728(03)00171-1. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Postolache TT. Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurol Res. 2005;27:679–84. doi: 10.1179/016164105X49463. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Postolache TT, Sternberg EM. Inflammatory genes and neural activity: involvement of immune genes in synaptic function and behavior. Front Biosci. 2005;10:675–80. doi: 10.2741/1562. [DOI] [PubMed] [Google Scholar]
- Vestergaard C, Deleuran M, Gesser B, Larsen CG. Thymus- and activation-regulated chemokine (TARC/CCL17) induces a Th2-dominated inflammatory reaction on intradermal injection in mice. Exp Dermatol. 2004;13:265–71. doi: 10.1111/j.0906-6705.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- Vestergaard C, Johansen C, Christensen U, Just H, Hohwy T, Deleuran M. TARC augments TNF-alpha-induced CTACK production in keratinocytes. Exp Dermatol. 2004;13:551–7. doi: 10.1111/j.0906-6705.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–75. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic protein alignment of the predicted peptide products produced by translation of the mRNA sequence of CCL27 variant 2 retaining intron 1 (AY744155).
Film autoradiographic images of the hybridization signal produced by radioactive riboprobes directed against the exon 1 of variant 1 or intron 1 of variant 2 of CCL27 or to the shared exon 3 of both variants. Bottom panels are consecutive sections hybridized with sense control riboprobes labeled at the same activity than antisense specific riboprobes (top panels). The sections have been exposed for 4 days. Note that the images corresponding for the common sequence saturated the film indicating the additive effect of hybridization of both variants in the same regions.
Bright field digital images at 40 times magnification of emulsion coated slides hybridized with riboprobes against variant 1 of CCL27 counterstained with toluidine blue (Nissl staining). Note the deposition of silver grains over blue stained cells.
Digital images at 200 X magnification of the fluorescent signal produced by CCL27 in the dentate gyrus (A). B: Image of the same section counterstained with Hoechst. C: Merged image showing localization of red signals corresponding to CCL27 around nuclear DNA stained with Hoechst.