Abstract
Blockade of Toll-like receptor (TLR)-mediated inflammatory responses represents a new approach in the development of anti-inflammation therapeutics. In the present study, we have screened for TLR2-mediated inflammation inhibitors from a small molecule compound library using a sensitive cell line stably expressing TLR2, CD14, and an NF-κB-driven-luciferase reporter gene. Lymphocytic choriomeningitis virus (LCMV) was used as a virus model. This arenavirus activates a TLR2/CD14-dependent NF-κB signaling pathway. We have identified 10 potential anti-inflammatory compounds out of 101306 compounds. We further evaluated 1 of these positive compounds, E567. We demonstrated that compound E567 efficiently inhibits both LCMV and Herpes simplex virus 1 (HSV-1) induced cytokine responses in both human and mouse cell cultures. We also demonstrated that E567 inhibits cytokine responses in the mouse. Remarkably, E567 is also capable of inhibiting LCMV replication in mice. This is a new model for developing drugs for use in treating viral illnesses.
Keywords: Toll-like receptor (TLR), Lymphocytic choriomeningitis virus (LCMV), compound screening
1. Introduction
Toll-like receptors (TLRs), a family of important innate immune molecules, are expressed both on the cell surface and in intracellular compartments of many immune and non-immune cells (Medzhitov and Janeway, 2000; Takeda and Akira, 2005). TLRs are transmembrane proteins which function as pattern recognition receptors (PRRs) for the detection and response to microbial ligands (Akira, Uematsu, and Takeuchi, 2006; Finberg and Kurt-Jones, 2004). To date, 10 TLRs have been identified in humans and 11 in mice(Takeuchi and Akira, 2009). Natural or synthetic ligands for at least 9 TLRs have been identified (Takeuchi and Akira, 2009). Activation of TLRs results in the recruitment of adaptor proteins including MyD88 (all known TLRs except TLR3) to the TIR domain (Takeda and Akira, 2004). A series of phosphorylation/recruitment/activation events leads to the activation and translocation of the transcription factors nuclear factor-κB (NF-κB) to the nucleus and the transcription of inflammatory and anti-inflammatory cytokine genes. While TLR induced innate immune responses help control viral infections, TLRs have also been implicated in the immunopathology of virus infection. The immunopathological role of TLRs in viral infections has been noticed using different virus models. So far, it has been demonstrated that TLR2 is associated with herpes encephalitis in mice(Kurt-Jones et al., 2004) and TLR3 is associated with herpes encephalitis in humans (Zhang et al., 2007). TLR3 contributes to diseases caused by both West Nile virus (WNV) (Wang et al., 2004) and influenza virus(Le Goffic et al., 2006) infections. Very recently, it has been found that sustained stimulation of the TLR7 signaling pathway with the TLR7/TLR8 agonist R-848 results in a HIV disease-like characterized by progressive lymphoid destruction (Baenziger et al., 2008). Therefore, in some circumstances, manipulating the activation of TLR signaling may be a useful strategy for controlling virus induced disease (Czarniecki, 2008; Nakamura et al., 2007; Seyberth et al., 2006)
Although originally described as receptors for bacteria and fungi, it has now become clear that TLRs mediate the production of cytokines in response to a variety of viruses and viral ligands (Kurt-Jones et al., 2000; Takeuchi and Akira, 2009; Wang, Kurt-Jones, and Finberg, 2007). A role for the Toll-like receptors, TLR2, TLR3, TLR4, TLR7 and TLR9, in the response to viruses has been established (Takeuchi and Akira, 2009). Our previous experiments have demonstrated that the cytokine response to human cytomegalovirus (CMV) (Compton et al., 2003), lymphocytic choriomeningitis virus (LCMV) (Zhou et al., 2008; Zhou et al., 2005), and Herpes simplex virus-1 (HSV) is controlled by TLR2 (Kurt-Jones et al., 2004), while the response to respiratory syncytial virus (RSV) is dependent on both TLR4 (Kurt-Jones et al., 2000) and TLR2 (Murawski et al., 2009). Our previous studies have demonstrated that LCMV infection induces the activation of transcription factor NF-κB and inflammatory responses through a TLR2/TLR6/CD14-MyD88/Mal-dependent signaling pathway (Zhou et al., 2008; Zhou et al., 2005). LCMV is the prototypic virus of the family Arenaviridae. Several members in the Arenaviridae family, including Lassa hemorrhagic fever (HF) virus and Argentine HF virus, cause severe, often lethal, viral hemorrhagic fevers in humans (Geisbert and Jahrling, 2004). Moreover, HF viruses have recently received considerable scrutiny because of the potential use of arenaviruses as biological weapons for bioterrorism (http://www.bt.cdc.gov/agent/vhf/). The strong pro-inflammatory cytokine response following arenavirus infection is thought to be responsible for the initial phase of hemorrhagic fever symptoms, such as fever, malaise, anorexia, muscle aches, headache, nausea, and vomiting (Geisbert and Jahrling, 2004).
Inhibiting TLR signaling in LCMV infected cells could have great therapeutic potential, not only in the treatment of arenavirus infection, but also in the treatment of other viral diseases involving TLR activation, including herpes encephalitis (HSV-1), genital herpes (HSV-2) and cytomegalovirus infections.
Ribavirin, a member of the nucleoside antimetabolite drugs that interfere with duplication of viral genetic material, is active against a number of DNA and RNA viruses, including hemorrhagic fever viruses (Gowen et al., 2008); however, due to its side-effects, such as dose-dependent inhibitory effect on DNA synthesis, hemolytic anemia, and significant teratogenic effects, its clinical use is limited (Dove et al., 2009).
The aim of this work was to use LCMV as a virus model to screen small molecule compounds that could be used to block LCMV induced TLR2-mediated inflammatory responses. To screen such compounds, we have established a human embryonic kidney (HEK) cell line stably expressing human TLR2, CD14, and NF-κB-driven firefly luciferase and developed an effective small molecule compound screening protocol. We have screened over 100000 small molecule compounds from several compound libraries and identified 10 candidate compounds that are able to specifically inhibit LCMV-induced cytokine production, and we have further characterized 1 of the 10 candidate compounds, E567. Our studies demonstrated that E567 strongly inhibits LCMV-induced NF-κB activation and cytokine responses.
2. Materials and methods
2.1. Virus
The LCMV-Arm was kindly provided by Dr. Liisa K. Selin (University of Massachusetts Medical School, Worcester, MA. USA) and was propagated on BHK-21 cells. Viral titers were determined with an immunological focus assay using antibody against LCMV-NP (Battegay et al., 1991).
2.2. Establishment of protocol for primary screening of compounds
Primary screening of small molecule compounds was carried out at the NERCE / NSRB screening facility at Harvard Medical School. A 384-well plate format screening assay was employed throughout the primary screening. To develop the screening protocol, SZ10 cells were seeded into duplicated 384-well tissue culture white styrene plates (Corning) at 0.5–1×103 cells/30µl/well and incubated overnight at 37°C in 5% CO2 humidified incubator. The next day, cells were treated with compounds at 0.1µl/well (5 mg/ml in DMSO) through an automated pin-based compound transfer robot. After incubation for 1 h at 37°C in 5% CO2 humidified incubator, cells were then challenged with either LCMV-Arm or control TNF-α for counterscreen (10µl/well) using liquid handling robot. Plates were briefly spun and cells were incubated for additional 16–18 h. Thereafter, 20µl/well of 1:1 pre-diluted/PBS buffered Steady-Glo luciferase buffer were added and luciferase activity was read using Envision II plate reader. Results were recorded as luminescence. TNF-α and TLR2 agonist (e.g. LCMV) utilize different adaptors to engage the innate immune signaling pathway, however both lead to the activation of the common down-stream transcriptional factor NF-κB targets. Thus TNF-α is a useful control for specific blockade of virus-induced responses. TNF-α was run side-by-side each time on separate plates in our full screen. Only those compounds that significantly reduced LCMV-induced NF-κB luciferase activity by 50% or greater than positive controls (DMSO only), with no change of TNF-α-induced NF-κB luciferase activity were cherry-picked for secondary screening.
For primary screening, compounds were reconstituted with dimethyl sulfoxide (DMSO) to 5 mg/ml stocks. Compound libraries screened included those from ChemDiv, Maybridge, ChemBridge, Biomol-TimTec, and some Fungal Extracts.
2.3. Determination of the impact of compound on TLR2 expression
SZ10 cells were plated into 24-well plate at 1×105/well and incubated overnight at 37°C in 5% CO2 humidified incubator. Cells were treated with compound or DMSO (compound carrier control). After incubation for 1 h at 37°C in 5% CO2 humidified incubator, cells were then challenged with either LCMV-Arm or control TNF-α and were incubated for additional 16–18h. The expression of TLR2 was determined using flow cytometry staining with anti-human TLR2 (11G7 clone). Samples were acquired on a BD-LSR-II flow cytometer (Becton Dickinson). Data was analyzed with Flowjo software (Tree Star Inc.)
2.4. Determination of the effect of compound on LCMV replication
Two quantitative methods were utilized to determine the effect of compound on LCMV replication. Flow cytometry approach was used in 24-well plate format. Vero cells were seeded at density of 1.5 ×105/well and incubated for overnight until cells were confluent. Cells were cultured in media containing 3.3µM compound E567 for 60 min. Control cells were cultured in media containing equal amount of DMSO. Then the cells were challenged with LCMV-Arm at different MOI. After adsorption for 1 h at 37°C in 5% CO2 humidified incubator, free virus was removed and replaced with fresh medium. Cells were incubated for 16–18 h. LCMV replication was determined by flow cytometry staining using anti-LCMV-NP antibody VL4 (Battegay et al., 1991). Samples were acquired on a BD-LSR-II flow cytometer (BD). Data was analyzed with Flowjo software (Tree Star Inc.).
Plaque assay was performed in a 6-well plate format. Vero cells were seeded at density of 5×105/well and incubated for overnight until cells were 100% confluent. Cells were similarly treated with compound (3.3 µM). Control cells were treated DMSO alone. After treatment, cells were challenged with LCMV-Arm at 50 pfu/well. After adsorption for 1 h at 37°C in 5% CO2 humidified incubator, free virus was removed and replaced with a mixture of complete 2×M199/10% FCS and 1% agarose. 3–4 days after incubation, the secondary overlay mixture of 2×M199/10% FCS and 1% agarose containing neutral red was added. After incubation for additional 4–6 h or overnight at 37°C in 5% CO2 humidified incubator, plaques were counted. Results were shown as number of plaques.
To evaluate if compound E567 inhibits LCMV replication in BHK-21 cells, BHK-21 cells in 24-well plates were infected with LCMV-Arm (MOI:0.1) for one hour and were washed to remove free virus. 1 ml of MEM-5%FCS was added to each well. 48 hr post-infection, supernatants were collected and LCMV titers were determined with an immunological focus assay with antibody against LCMV NP (VL4) (Battegay et al., 1991). Results were shown as log10 pfu/ml.
2.5. Evaluation of compound in mouse primary macrophages
Mice were injected intraperitoneally with 1 ml 3% thioglycollate. Peritoneal exudates macrophages were harvested 4 days later by peritoneal lavage with ice-cold Hanks' balanced salt solution. Cells (1×105 cells/150µl/well in 96-well plates) were treated with E567 or DMSO at various concentrations. 1 h post-compound treatment, cells were challenged with medium (negative control), LCMV-Arm, HSV-1 (7134R isolate, WT HSV-1), or other TLR ligands. After incubation for additional 16–18 h, culture supernatants were collected and the levels of mouse MCP-1 and RANTES were determined by ELISA (BD), following the manufacturer's instructions.
2.6. Evaluation of compound in human peripheral blood monocytes
Human peripheral blood mononuclear cells (PBMC) were prepared from buffy coats by lymphocytes separation medium gradient centrifugation. Monocytes were isolated by depletion of non-monocytes (negative selection) using the monocyte isolation kit II (Miltenyi Biotech Inc. Order number: 130-091-153). The purity was approximately 80% by flow cytometry staining with antibody against CD14. Cells were seeded into 96-well plate (triplicate) at the density of 1×104/100µl/well in DMEM-10%FCS and treated with E567 or DMSO at various concentrations. 1 h post-compound treatment, cells were challenged with medium (negative control), LCMV-Arm, or human TNF-α. After incubation for additional 16–18 h, culture supernatants were collected and IL-8 levels were determined by ELISA (BD) following the manufacturer's instruction.
2.7. In vivo evaluation of compounds
C57BL/6J mice (female, 5–6 weeks old) were injected i.p. with DMSO carrier or compound E567 at 400 µg/mouse, followed by infection i.p. with LCMV-Arm at 2×105pfu/mouse. 18 hours p.i., serum and spleen were collected. The levels of MCP-1 in serum were determined by ELISA (BD). The level of type I IFN was measured by type I IFN bioassay. To evaluate if E567 inhibits LCMV replication in vivo, group of 8 C57BL/6J mice were treated i.p. with compound E567 (400 µg/mouse), or DMSO, or left untreated, followed by challenge i.p. with LCMV-Arm. Mice received additional 400 µg doses of compound at 6 hr, 18 hr and 24 hr p.i. At 48 h p.i., mice were sacrificed and spleens were collected. Virus titers in spleens were determined using an immunofocus assay with antibody against LCMV NP (VL4) (Battegay et al., 1991). To evaluate whether E567 blocks inflammatory responses induced by other TLR ligands, mice were treated with compound E567 or DMSO as described above, followed by injection i.p. with either TLR2 ligand Pam2CSK4 (0.8 mg/kg of mouse) or TLR4 ligand LPS (0.8 mg/kg of mouse). 6 hours post-injection, mice were sacrificed and serum samples were collected. The levels of IL-6, MCP-1, and RANTES were determined by ELISA (BD).
2.8. Statistical analysis
For the in vitro and in vivo studies comparing the effect of E567 on cytokine responses, the differences between E567 and DMSO were evaluated using the two-tailed Student`s t test. For the in vivo study examining the effects of E567 on virus replication in individual mice (Fig 10C), means, medians and percentiles were estimated to describe the distribution of titer values. An F-test was used to compare variances between groups (Pagano)(Gauvreau and Pagano, 1993). Two sample t-tests using unequal variances were used to test means between groups. Quantile regression with bootstrapped standard errors was used to compare 25 percentiles (Gould). Results were expressed as means+/-standard deviation. Values of p <0.05 were regarded as significant.
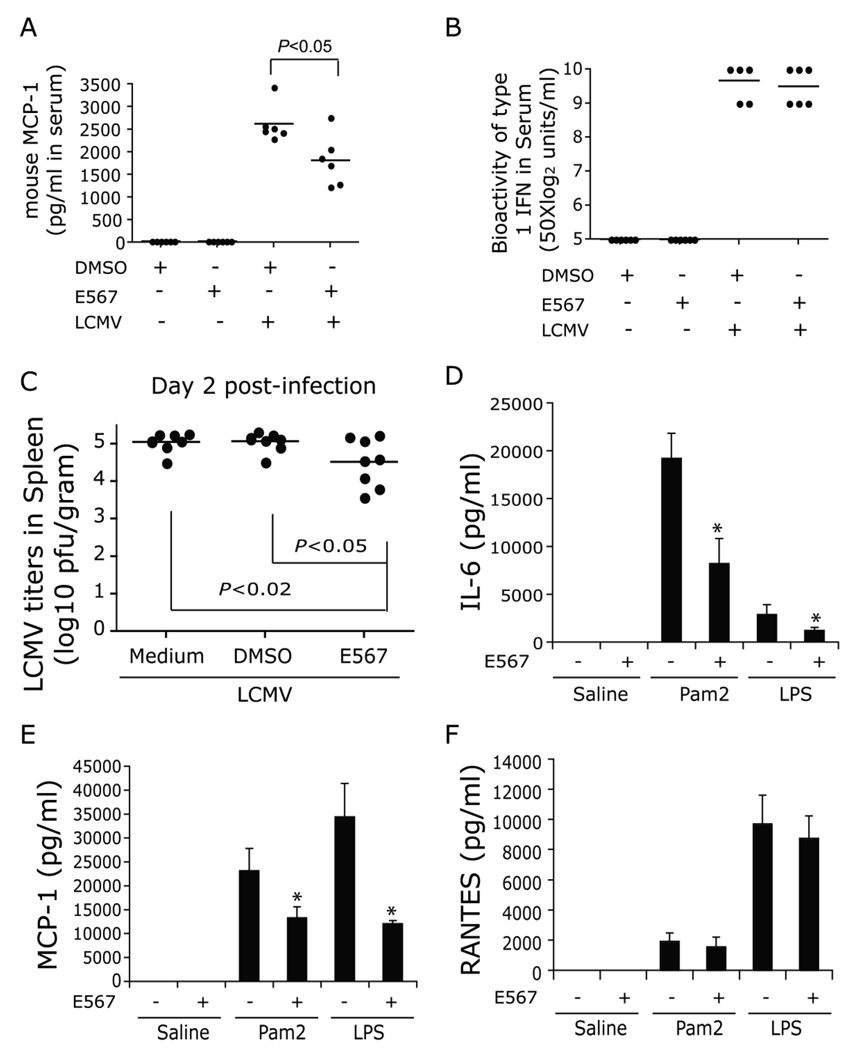
Fig 10. Compound E567 inhibits both LCMV and other TLR ligands induced cytokine production and viral replication in vivo.
C57BL/6 mice were injected i.p. with compound E567 at 400 µg/mouse or DMSO. Within 20 min post-treatment, mice were challenged with LCMV-Arm (2×105 pfu). 18 h post-infection, levels of MCP-1 in serum were determined by ELISA (A) and the bioactivity of type I IFN was measured by type I IFN bioassay (B). To determine if compound E567 inhibits LCMV replication in vivo, groups of 8 C57BL/6 mice were treated as described in the Materials and Methods. Mice were sacrificed 48 h post-infection and spleens were collected. Virus titers in spleens were determined using an immunofocus assay with antibody against LCMV NP (VL4). Spleen viral titers in individual mice were shown (n=8) (C). C57BL/6 mice were intravenously injected with compound E567 at 400 µg/mouse or DMSO. Within 10 min post-treatment, mice were challenged with either pam2CSK (0.8 mg/kg) or LPS (0.8 mg/kg). Six hrs post-infection, levels of IL-6 (D), MCP-1 (E), or RANTES (F) in serum were determined by ELISA. * p< 0.05.
3. Results
3.1. Establishment and characterization of SZ10 cell line, an HEK cell line stably expressing TLR2, CD14, and NF- κB-driven firefly luciferase
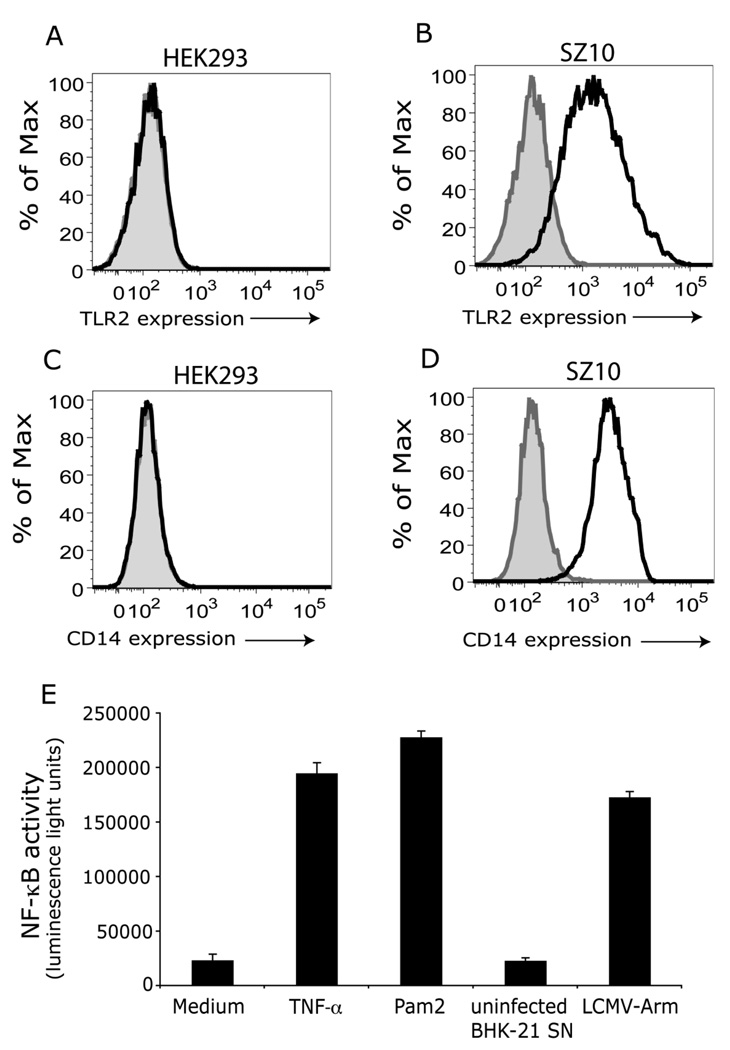
Our previous studies have shown LCMV infection induced NF-κB activation and cytokine and chemokine release through a TLR2 and CD14-dependent signaling pathway (Zhou et al., 2005). Luciferase is a commonly used reporter because of the high sensitivity of detection and the absence of endogenous luciferase activity in mammalian cells, thus making it suitable for large-scale screening of compounds. To generate a stable HEK cell line expressing TLR2, CD14, and luciferase reporter, expression plasmids for human TLR2, CD14 and a hygromycin resistance gene, and an NF-κB-driven firefly luciferase report gene were co-transfected into HEK293 cells using GeneJuice transfection reagent followed by selection with hygromycin. After 3 weeks selection, individual clones of cells were isolated and expanded. The luciferase activity and expression of both TLR2 and CD14 were used to characterize and select positive clones. Cells were plated into 96-well plates and stimulated with medium, recombinant human IL-1β or TNF-α, Pam2CSK4 (TLR2 ligand), and LCMV-Arm. We obtained several positive clones. Clone number 10 (hereafter referred as SZ10 cells) is a representative of the positive clones. Untransfected HEK293 cells did not express either TLR2 (Fig 1A) or CD14 (Fig 1C). In contrast, SZ10 cells expressed both TLR2 (Fig 1B) and CD14 (Fig 1D), and stimulation with either LCMV, or Pam2CSK4 (TLR2 ligand), or TNF-α (TLR independent stimuli) induced the activation of NF-κB activity (Fig 1E).
Fig 1. A and B: Establishment and characterization of HEK cells stably expressing TLR2, CD14 and NF-κB-driven firefly luciferase.
HEK293 cells were co-transfected with plasmids expressing human CD14 -hygromycin and NF-κB-luciferase (NF-κB-driven firefly luciferase), and followed by selection with hygromycin (200 µg/ml) for 3 – 4 weeks. Individual clones were then expanded and characterized. Clone SZ10 is a representative from 12 individual clones. The expression of both TLR2 and CD14 in untransfected HEK293 cells (panels A and C) and SZ10 cells (panels B and D) was confirmed by flow cytometry staining with anti-human TLR2 antibody (clone 11G7) (black line) (A and B) and CD14 (black line) (C and D). Grey and tinted area, isotype antibody controls. Panel E: SZ10 cells in 96-well plates were challenged with the following stimuli (triplicate wells per stimulant): medium, LCMV-Arm, uninfected BHK-21 cell supernatant, TNF-α (non-TLR stimulant), and Pam2CSK4 (TLR2 ligand). Cells were incubated additional 16 hr at 37°C, 5% CO2. NF-κB activity (luminescence light unit) was determined by luciferase assay. Data are means and standard errors of triplicate wells per stimulant.
3.2. Pilot screening to validate SZ10 cells with known activity compounds
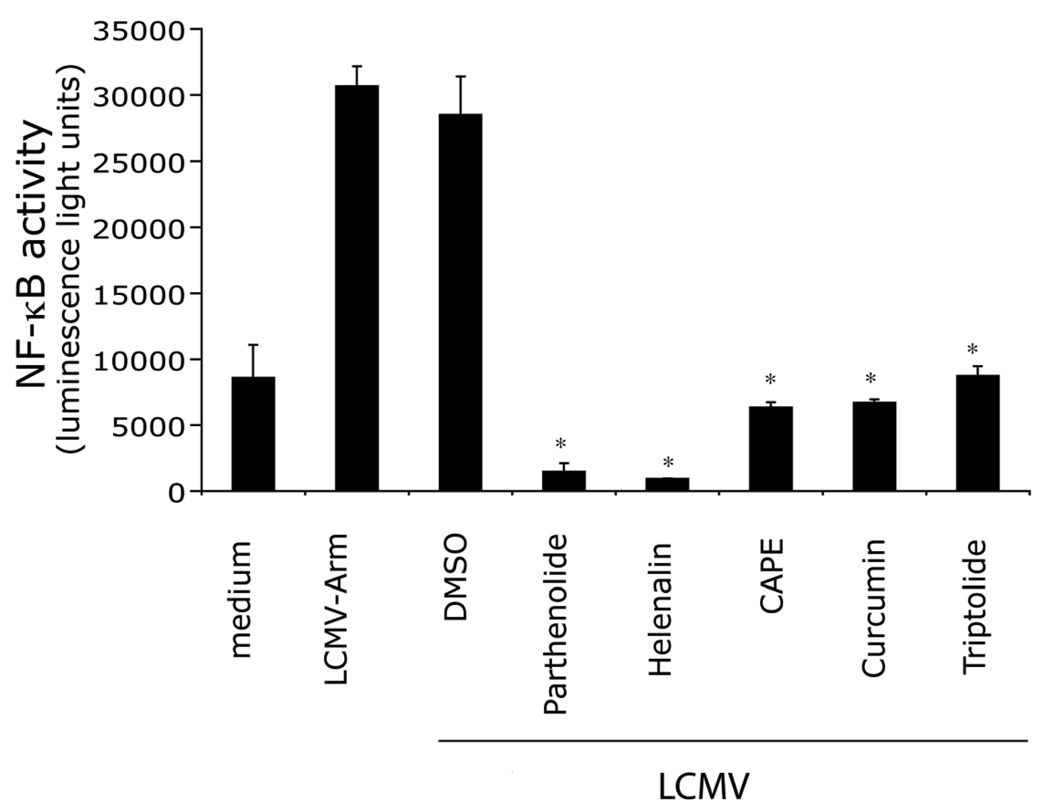
To validate the specificity and sensitivity of SZ10 cells, we performed a pilot screening with known bioactives collection. The collection contains many classes of compounds including kinase inhibitors, protease inhibitors, and ion channel blockers. Cells were plated into 384-well plate and treated with compounds for 30 min, then challenged with LCMV. Cells were cultured for additional 5 hrs, and luciferase activity was measured in cell lysates. As expected, compared with DMSO (compound carrier), all 5 known NF-κB inhibitory compounds, including known inhibitors for IκB (Parthenolide) and NF-κB (Helenalin, CAPE, Curcumin, Triptolide), effectively blocked LCMV-induced NF-κB activation (Fig 2). Therefore, these results demonstrate that SZ10 cells and the screen protocol are suitable for screening compounds targeting LCMV-induced NF-κB activation.
Fig 2. Validation of SZ10 cells and the screen protocol using “known bioactives” compounds.
SZ10 cells in 384-well plates (duplicate plates) were treated with “known bioactives” compounds (at levels demonstrating not to be toxic by the screening facility) or DMSO and incubated for 60 min. Cells were challenged with LCMV-Arm and incubated for additional 16–18 hr at 37°C, 5% CO2. NF-κB activity (luminescence light unit) was determined by luciferase assay. Data are means and standard errors of duplicate wells per stimulant.
3.3. Screening for small molecule compounds that inhibit LCMV-induced TLR2-mediated signaling pathway
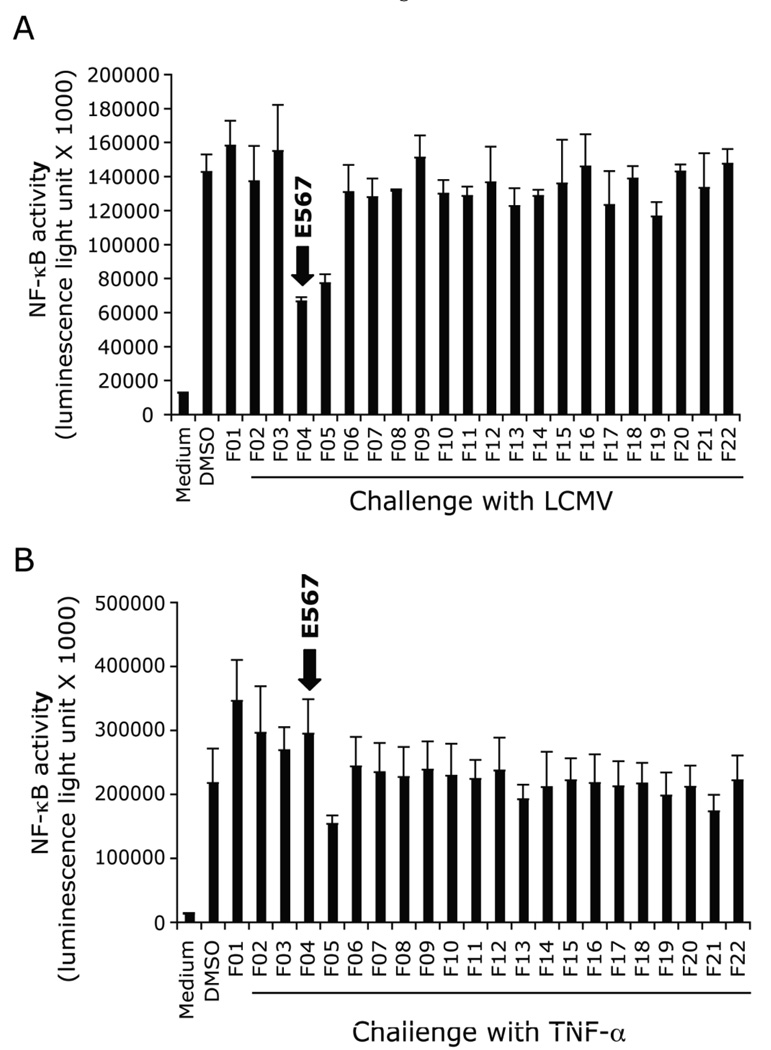
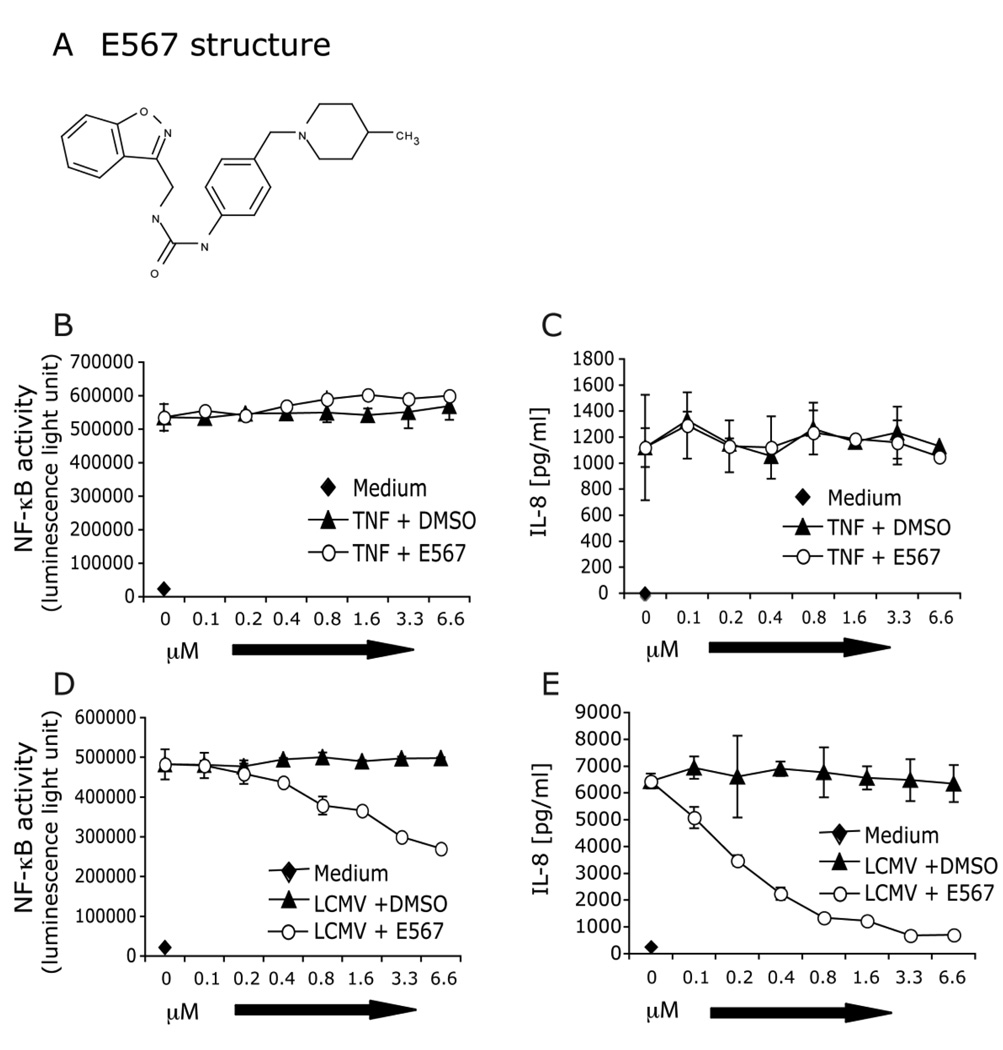
In our primary screening, we screened 101306 compounds and cherry-picked 217 compounds. The positive hits were defined as those that inhibited luciferase activity by ≥ 50% as compared to DMSO-treated cells challenged with LCMV for 18 hrs (optimal incubation time established in pilot studies). A counterscreen with TNF-α stimulation was included in each of the screening assay to distinguish compounds that had a specific effect on LCMV stimulation from compounds that were toxic or which targeted NF-κB itself (in either case, both LCMV and TNF-α responses would be blocked). From the 217 hits, 10 compounds, including compound E567 (Fig 3, 4A), were discovered in the primary screen and confirmed in the secondary screen. All of these compounds could specifically inhibit LCMV-induced NF-κB activity and cytokine response but not TNF-α induced responses. Based on the ability of this compound to inhibit both cytokine production and LCMV replication, in present report, we further characterized compound E567 (Fig 4A).
Fig 3. Discovery of compound E567 in the primary compound screen.
SZ10 cells were plated into 384 well plates in duplicate and incubated overnight at 37°C, 5% CO2 incubator. The next day, cells were treated with small molecule candidates (F1-F22: representative compounds) or compound carrier DMSO at 0.1 µl/well using pin transfer robot. Cells were incubated for one hour followed by challenge with either LCMV-Arm (A) or control stimulant TNF-a for 18–22 hours. Steady-Glo luciferase buffer was added into each well and the plates were read using an Envision II plate reader. Raw data were collected and shown as luminescence light unit. The intensity of luminescence is an indicator of the level of NF-κB-luc activation. Data are means and standard errors of duplicated wells.
Fig 4. E567 blocks LCMV-induced TLR2-mediated NF-κB activity and production of IL-8.
SZ10 cells were plated into 96-well plates and incubated overnight at 37°C, 5% CO2. Cells were treated with compound E567 (A) or compound carrier DMSO at various concentrations and incubated for one hour followed by challenge with either control stimulant TNF-a (B and C) or LCMV-Arm (D and E). Cells were incubated for additional 16–18 hr at 37°C, 5% CO2. Levels of IL-8 (C and E) in the culture supernatants were measured by ELISA. Cell lysates were used to determine NF-κB activity (luciferase assay) (B and D). Data are means and standard errors of triplicate wells. Results are representative of more than five separate experiments.
We examined the effect of E567 on LCMV-induced NF-κB activity as well as IL-8 production. HEK cells stably expressing TLR2 and CD14 predominantly produce IL-8 in response to LCMV challenge and its induction is NF-κB-dependent. Consistent with the results from primary screening and cherry-pick, E567 dramatically inhibited LCMV-induced NF-κB activity (Fig 4D) and IL-8 production (Fig 4E) in a dose-dependent manner. In contrast, E567 did not affect TNF-α-induced NF-κB activity (Fig 4B) or IL-8 production (Fig 4C).
3.4. Compound E567 down-regulates TLR2 expression
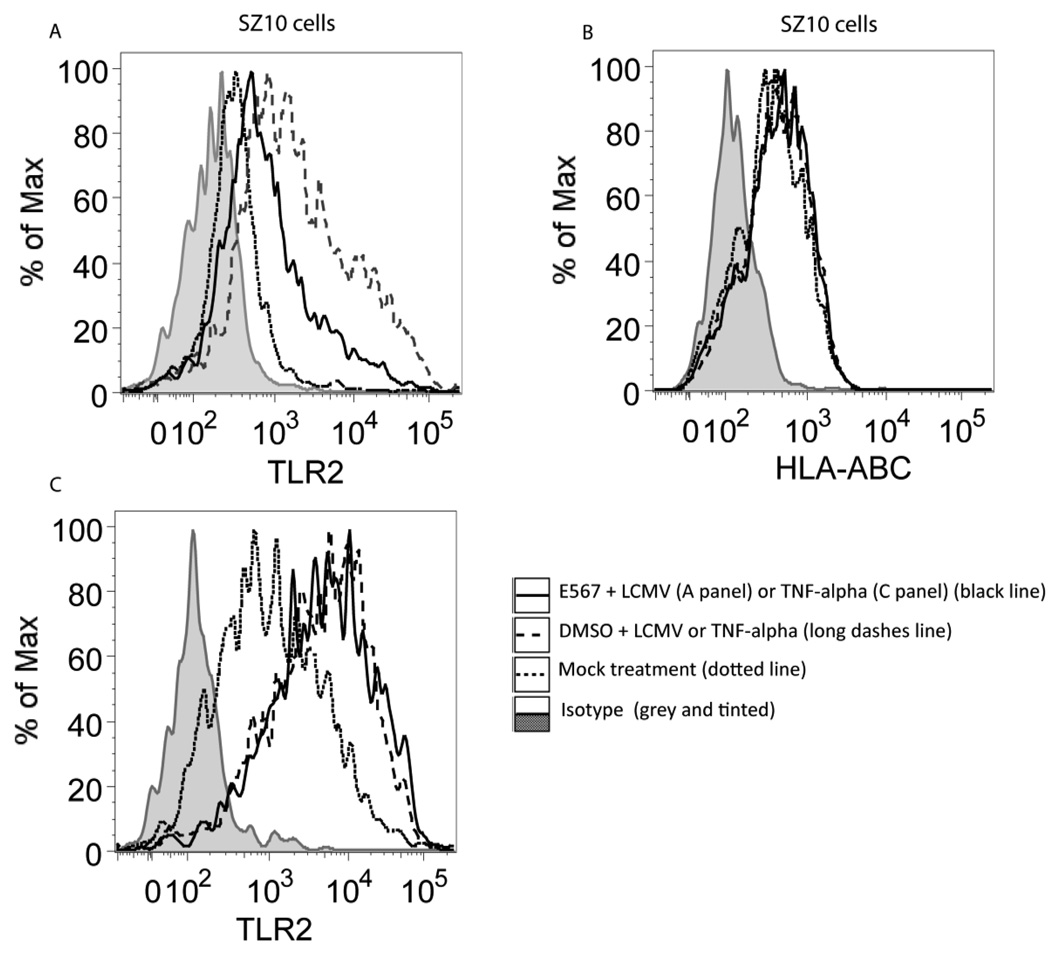
We have previously demonstrated that expression of TLR2 is required for the LCMV-induced cytokine response (Zhou et al., 2008; Zhou et al., 2005). To further define the mechanism by which compound E567 inhibits LCMV-induced cytokine response, we examined if compound E567 affects TLR2 expression. SZ10 cells were treated with various doses of E567 or DMSO followed by challenge with LCMV or control TNF-α. The expression of TLR2 was examined by flow cytometry staining. The expression of HLA-ABC was utilized as a control. As expected, LCMV challenge enhanced expression of TLR2 and DMSO did not affect LCMV induced up-expression of TLR2 (Fig 5A. long dashes line). In contrast, incubation with E567 prevented LCMV-induced enhanced expression of TLR2 (Fig 5A. black line), but did not affect the expression of HLA-ABC (Fig 5B). Treatment with E567 did not affect TNF-α-induced expression of TLR2 (Fig 5C).
Fig 5. Compound E567 inhibits LCMV-induced TLR2 expression in HEK293 cells.
SZ10 cells were plated in 24-well plate and were mock-treated (dotted line), or treated with compound E567 (3.3 µM) (black line), or DMSO (long dashes line) for one hour followed by challenge with LCMV (A) or TNF-α (B) in the presence of E567 or DMSO. Twenty hours post-infection, the expression of TLR2 in SZ10 cells was determined by flow cytometry staining with anti-TLR2 antibody (clone 11G7) (A). The grey and tinted area represents the isotype for anti-TLR2 antibody. The expression of HLA-ABC in the same treated SZ10 cells was determined by flow cytometry staining with antibody against human HLA-ABC (B). Grey and tinted area, isotype control; dotted line, mock-treated cells; black line, E567-treated cells; lone dashes line, DMSO-treated cells. The MFI data for 5A (which shows the effect of the compound on TLR2 expression) are: isotype: 174; basal TLR2 <dotted line>: 353; DMSO + LCMV <long dashes line>: 1978; E567 + LCMV <black line>: 804. The MFI for 5C (TNF-α control for 5A) are: isotype: 132; basal TLR2 <dotted line>: 1077; DMSO + TNF-a <long dashes line>: 4224; E567 + TNF-a <black line>: 4711.
3.5. Compound E567 inhibits LCMV replication
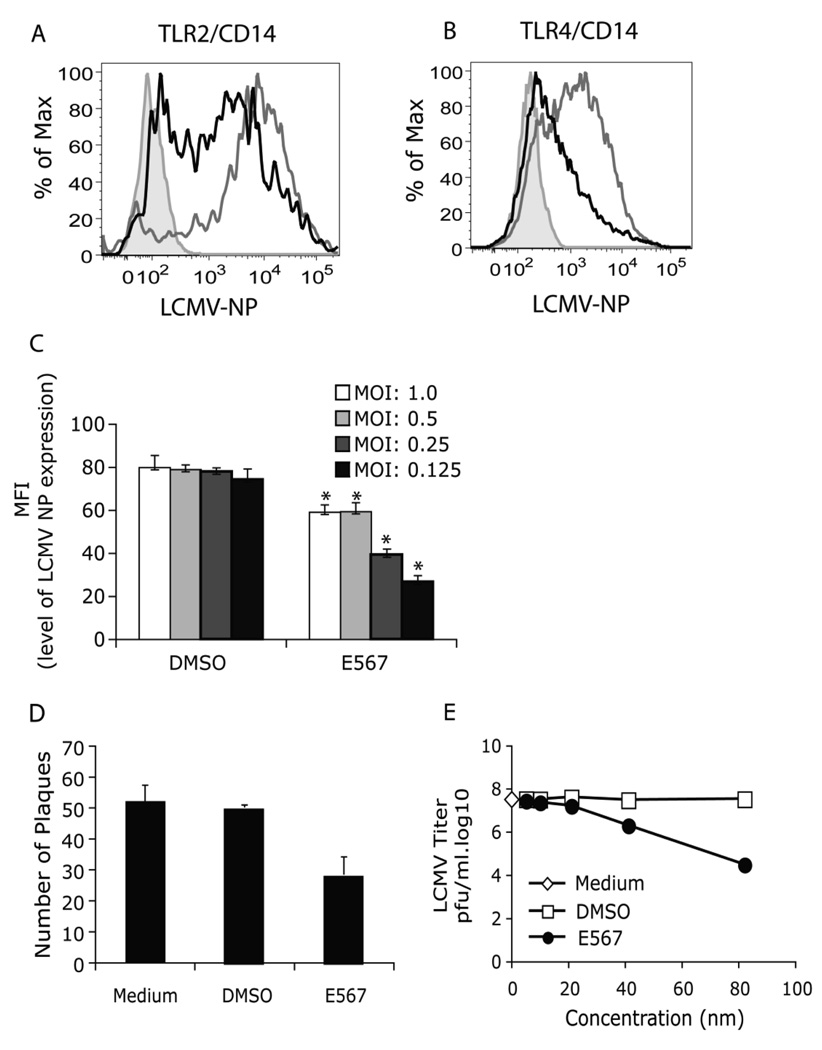
Our previously studies have demonstrated that LCMV replication is critical for a cytokine response but LCMV replication is independent of TLR2 (Zhou et al., 2005). To determine whether compound E567 may also affect LCMV replication, the effect of compound E567 on LCMV replication was evaluated using anti-LCMV NP antibody staining and flow cytometry to monitor the infection. E567 dramatically inhibited expression of LCMV-NP (Fig 6A) and this effect was independent on the expression of TLR2, because E567 could also inhibit LCMV replication in HEK cells that did not express TLR2 (Fig 6B). DMSO had no effect on LCMV replication (Fig 6A–B).
Fig 6. Compound E567 blocks LCMV replication.
LCMV replication was determined by flow cytometry staining of LCMV-NP expression. (A-B) HEK293 cells stably expressing either TLR2/CD14 (SZ10 cells) (A) or TLR4/CD14 (B) were plated in 24-well plate and treated with compound E567 (black lines) or DMSO (grey lines) followed by challenge with LCMV as described in Fig 5. 20 h post-infection, the expression of LCMV NP was determined by flow cytometry staining with VL4 antibody. Grey and tinted area, isotype control. Results are representative of two to three separate experiments. (C) Vero cells were plated in 24-well plates. The next day, cells were treated with compound E567 (3.3 µM) or equal amount of DMSO for 60 min followed by challenge with LCMV at different MOI. After incubation for 1 h, free virus was removed and replaced with fresh medium. Cells were incubated for additional 16–18 h. The expression of LCMV-NP was determined by flow cytometry staining with VL4 antibody and results were shown as the mean fluorescence intensity (MFI) (C). In addition, the replication of LCMV was also determined using the classical plaque assay in Vero cells as described in Materials and Methods, and results were shown as number of plaques (D). (E) BHK-21 cells in 24-well plates were treated with E567 or control DMSO as described in Materials and Methods. The virus yield in the supernatants of BHK-21 cells was determined using an immunological focus assay. * p<0.05.
To further define the effect of E567 on LCMV replication, Vero cells were utilized, because Vero cells are sensitive to LCMV infection and are commonly used to titrate LCMV. Vero cells were treated with E567 (3.3 µM) or DMSO alone, followed by challenge with different amounts of LCMV. Compound E567 significantly inhibited LCMV replication in Vero cells evaluated by either flow cytometry staining (expression of LCMV NP) (Fig 6C) or classic plaque assay (Fig 6D).
To extend the investigation into the anti-LCMV activity of E567, we carried out another experiment in BHK-21 cells. BHK-21 cells are extremely sensitive to LCMV-Arm replication and have been commonly used to propagate LCMV-Arm. The effect of E567 on viral replication in BHK-21 cells was determined by plaque assay as described above. Our results demonstrated that E567 inhibited LCMV replication in BHK-21 cells in a dose-dependent manner (Fig 6E).
3.6. E567 inhibits both LCMV and HSV induced cytokine responses in primary mouse macrophages
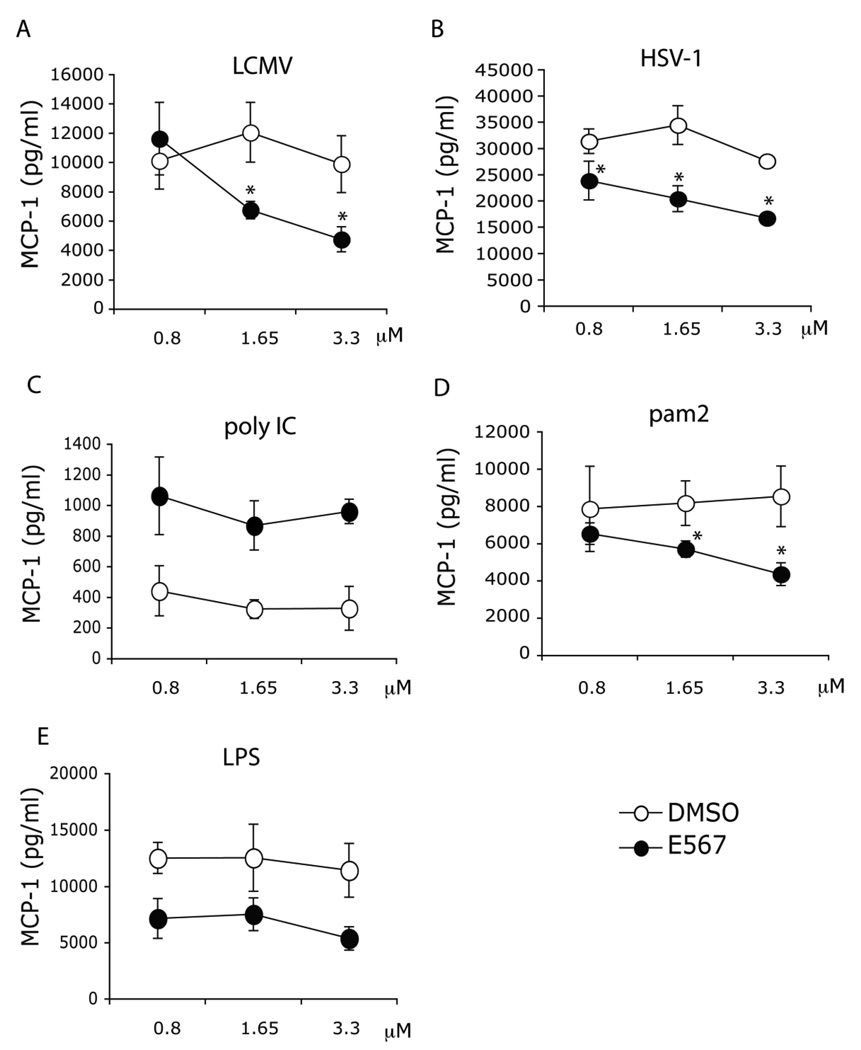
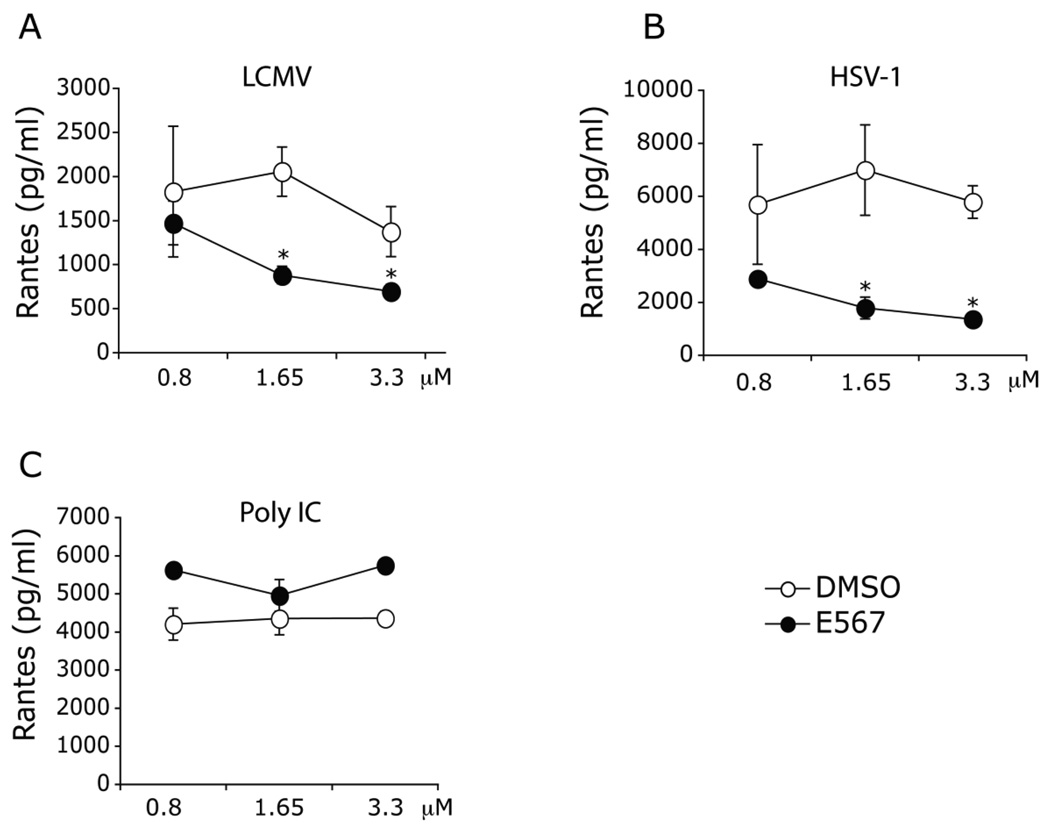
Macrophages are important inflammatory cells in the host response to virus. To evaluate the effect of compound E567 in modulating cytokine responses in mouse primary macrophages, thioglycollate-elicited peritoneal exudate cells (PECs) isolated from mice and were treated with various doses of E567 or DMSO control followed by infection with LCMV. Treatment with compound E567 significantly inhibited LCMV induced production of both MCP-1 (Fig 7A) and RANTES (Fig 8A) from mouse macrophages.
Fig 7. Compound E567 inhibits both LCMV and HSV-1-induced MCP-1 production in mouse primary macrophages.
Thioglycollate-elicited peritoneal exudate cells in 96-well plates were treated with compound E567 or DMSO for 60 min followed by challenge with LCMV (A), HSV-1 7134R isolate (WT HSV-1) (B), poly IC (C), pam2 (D), or LPS (E). Cells were incubated for additional 16–18 hr. Levels of MCP-1 in the culture supernatants were measured by ELISA. A representative of three separate experiments. * p< 0.05.
Fig 8. Compound E567 inhibits both LCMV and HSV-1-induced RANTES production in mouse primary macrophages.
Thioglycollate-elicited peritoneal exudate cells in 96-well plates were treated with compound E567 or DMSO for 60 min followed by challenge with LCMV (A), HSV-1 7134R isolate (WT HSV-1) (B), or poly IC (C). Cells were incubated for additional 16–18 hr. Levels of RANTES in the culture supernatants were measured by ELISA. A representative of three separate experiments. * p< 0.05.
Herpes simplex virus 1 (HSV-1) causes a wide array of human diseases from the common herpes labialis or “cold sores” to the more severe, sometimes, lethal herpes encephalitis. We have previously demonstrated that TLR2 is important in the host response to HSV-1 infection (Kurt-Jones et al., 2004). To determine whether compound E567 could modulate HSV-1 induced cytokine / chemokine responses in mouse primary macrophages, PECs were treated with compound E567 or DMSO followed by challenge with HSV-1 isolate, 7134R (WT). Interestingly, compound E567 efficiently blocked both HSV-1 induced MCP-1 (Fig 7B) and RANTES (Fig 8B) production in mouse macrophages.
Next, we evaluated whether compound E567 could also affect the cytokine responses elucidated by other TLR ligands. Both the TLR2 ligand Pam2CSK4, a synthetic diacylated lipopeptide commonly found in Gram-positive bacteria, and the TLR4 ligand LPS, a cell wall component of Gram-negative bacteria, are able to efficiently induce MCP-1 production (Fig 7E) in macrophages but induced little RANTES under our experimental conditions. Interestingly, treatment with compound E567 inhibited both Pam2CSK4 and LPS induced MCP-1 production (Fig 7D–E). In contrast, treatment with compound E567 did not affect poly IC, a synthetic dsRNA and a TLR3 ligand, induced production of either MCP-1 or RANTES (Fig 7C, 8C).
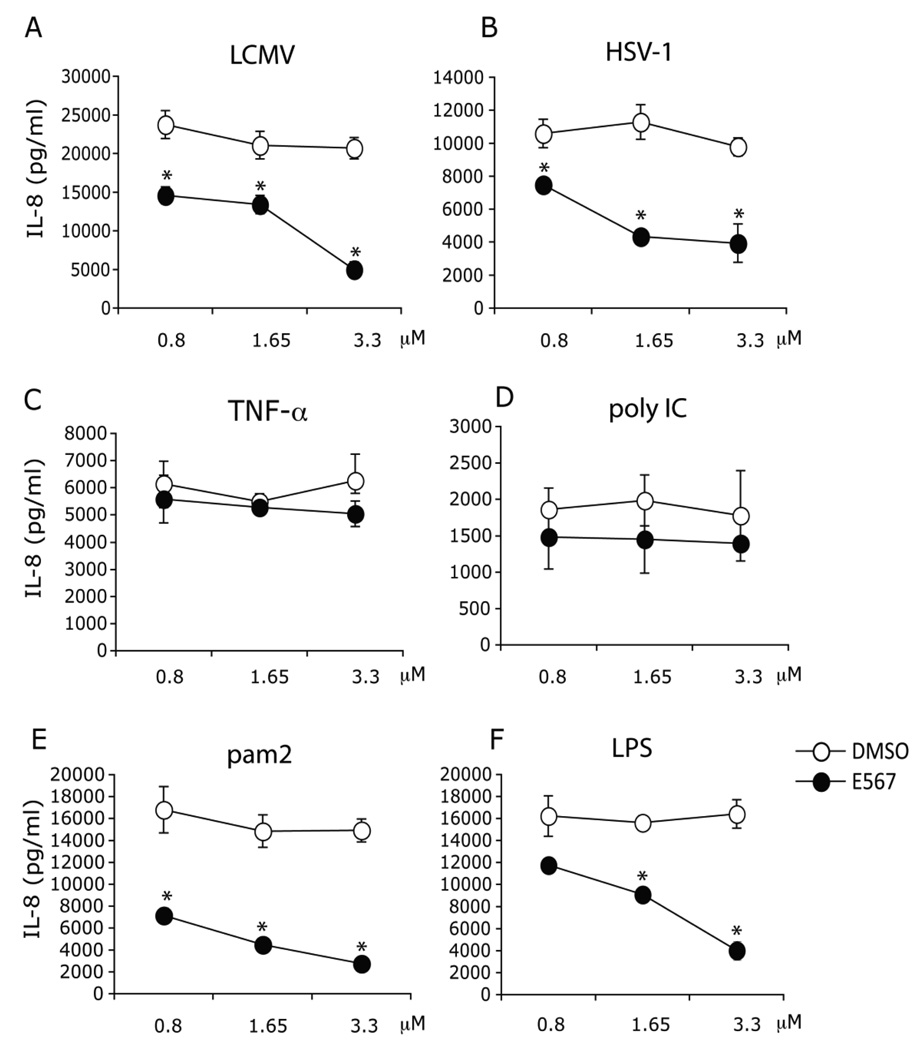
3.7. E567 inhibits both LCMV and HSV-1 induced IL-8 production in primary human monocytes
Human monocytes, circulating in the blood stream and sensing the presence of invading microbials, are important immune cells in both innate and adaptive immune responses. Human monocytes express a variety of TLRs, including high levels of TLR2 Hornung et al., 2005). Treatment with compound E567 resulted in a significant reduction of LCMV induced IL-8 production from monocytes compared to DMSO control Fig 9A), and this effect was dose-dependent. A similar effect of compound E567 was observed on monocyte production of IL-8 in response to HSV-1 (Fig 9B), Pam2CSK4 Fig 9E), and LPS (Fig 9F). Interestingly, treatment with compound E567 did not affect either recombinant human TNF-α or poly IC induced production of IL-8 (Fig 9C–D), demonstrating that the impact of compound E567 on LCMV and HSV-1 induced monocyte IL-8 production is not due to the toxicity of compound.
Fig 9. Compound E567 inhibits LCMV induced IL-8 production in primary human monocytes.
Purified primary human monocytes were plated in 96-well plates and treated with compound E567 or DMSO. 60 min post-compound treatment, cells were challenged with LCMV (A), HSV-1 7134R isolate (WT HSV-1) (B), or the following stimuli: recombinant human TNF-α (C), poly IC (D), pam2 (E), or LPS (F). Cells were incubated for additional 16–18 hr. Levels of IL-8 in the culture supernatants were measured by ELISA. A representative of three individual experiments. * p< 0.05.
3.8. E567 blocks LCMV and other TLR ligands induced inflammation in vivo
To evaluate the effect of compound E567 on inhibition of LCMV induced inflammation in vivo, C57BL/6J mice were pre-treated with DMSO or compound E567 once via intravenous route at 400 µg/mouse (16 mg/kg) followed by challenge with LCMV-Arm via intraperitoneal administration. Treatment with a single dose of E567 resulted in a significant decrease in LCMV induced MCP-1 production as compared with levels in DMSO treated mice (Fig 10A). In contrast, E567 treatment did not affect LCMV induced type I IFN production (Fig 10B). E567-treated mice had significantly lower levels of virus p<0.05) in the representative organ, the spleen, compared to DMSO-treated or untreated mice (Fig 10C). Interestingly, E567 treatment also significantly blocked both TLR2 ligand Pam2CSK4 and TLR4 ligand LPS induced IL-6 and MCP-1 production (Fig 10D–E). In contrast, E567 did not affect either Pam2CSK4 or LPS-induced RANTES production (Fig 10F).
4. Discussion
We have previously demonstrated that the expression of TLR2 and CD14 is required for LCMV induced activation of the innate immune signaling pathway (Zhou et al., 2008; Zhou et al., 2005). Based on these findings, we hypothesized that blockade of the TLR2-mediated signaling pathway could be a useful strategy to block virus induced inflammatory responses. In the present study, we have conducted small molecule screening to identify TLR2 signaling inhibitors that could be used to treat TLR-mediated inflammation. Using a HEK293 based cell line stably expressing TLR2, CD14 and NF-κB-driven-luciferase, we have identified 10 positive compounds from over 100 000 small molecules. This is the first report to use such a cell line to identify TLR2 signaling inhibitors with antiviral properties. There are several advantages of using this cell line in the screening of TLR2 signaling inhibitors. First, the luciferase reporter is a commonly used sensitive reporter in the study of signaling pathway. Second, the results obtained from using this stable cell line are more consistent than results obtained from transient transfection. Third, HEK cells are sensitive to most virus infections. Thus the effect of compound on NF-κB driven gene expression and virus replication could be characterized using the same cell line.
In present report, we further characterized one of the 10 candidate compounds, named E567. We demonstrated that E567 is able to modulate TLR2, and to a lesser extent, TLR4 signaling. Remarkably, compound E567 has the ability to inhibit LCMV replication both in vitro in cell culture and in vivo in the mouse.
While TLRs are important in initiating the protective innate immune response, TLRs have also been demonstrated to cause disease by virtue of their ability to stimulate inflammatory responses (Baenziger et al., 2008; Kurt-Jones et al., 2004; Wang et al., 2004). TLR-mediated responses to the invading microbial pathogens can lead to the production of excessive chemokines and cytokines and cause immunopathology (Finberg and Wang, 2009; O'Neill, 2006). Thus TLR signaling inhibitors might have potential therapeutic beneficial effects in treating viral infection-associated diseases (O'Neill, 2006). TLR antagonists to TLR2, TLR4, nd TLR9 have been developed for clinical use (Czarniecki, 2008; Nakamura et al., 2007; Yamada et al., 2005). TLR4 antagonists have been reported to be able to inhibit TLR4 agonist LPS-induced inflammation in vitro as well as suppress LPS-induced disease in a mouse model (Nakamura et al., 2007). Lipolanthionine peptides have been developed based on the structural characteristics of TLR2 agonist Pam3CSK4. These peptides have been shown to suppress Pam3CSK4-induced inflammation in cell culture, however, the effect of these TLR2 antagonists in vivo has not been reported (Seyberth et al., 2006). The role of TLR inhibitors in inhibition of viral infection-associated inflammation had not been previously explored.
Macrophages and monocytes play critical roles in the host response to infection with various microbial pathogens. Both cell types express various TLRs, including high levels of TLR2 (Hornung et al., 2005). Expression of TLR2 is involved in both LCMV and HSV-1-induced production of cytokines / chemokines in these two cell types (Kurt-Jones et al., 2004; Zhou et al., 2005). Our studies demonstrated that compound E567 is able to block both LCMV and HSV-1-induced inflammatory responses in these cell types. Our results revealed that treatment with compound E567 also blocked both Pam2CSK4 (TLR2 ligand) and LPS (TLR4 ligand) induced inflammatory responses in these cell types, but it did not affect the production of cytokines / chemokines induced by either recombinant human TNF-α or poly IC in human monocytes (Fig 9C–D) and mouse primary macrophages (Fig 7C, 8C). Moreover, we demonstrated that compound E567 blocks both the TLR2 agonist Pam2CSK4 and the TLR4 agonist LPS induced cytokine responses in mice (Fig 10D–F). This is consistent with other published results indicating that a TLR2 signaling inhibitor could affect both TLR2 and TLR4 signaling pathways (Czarniecki, 2008; Nakamura et al., 2007; Yamada et al., 2005). Although the exact mechanisms involved in the effect of E567 on cytokine/chemokine responses in both mouse and human primary cells are not yet fully defined, certain findings are likely to be relevant. Since the adapter proteins MyD88 and MyD88 adapter-like (MAL) (Fitzgerald et al., 2001) are involved in both the TLR2 and TLR4 signaling pathways, it is possible that compound E567 might target these adapter proteins. Alternatively, E567 could act on other molecules downstream of TLR2 but shared by TLR4 signaling pathways.
Compound E567 blocks LCMV-induced cytokine response in a mouse model. The compound E567 inhibited LCMV replication (Fig 10C) and modulated TLR2 signaling. Either or both of the effects could be responsible for the ability of the compound to decrease the cytokine response in vivo in response to LCMV infection. We have previously demonstrated that LCMV induced a potent dose dependent cytokine response that requires live virus (Zhou et al., 2005). Although TLR2 is required for LCMV-induced inflammation, expression of TLR2 is not required for LCMV infectivity (Zhou et al., 2005). So far, although a few publications have described TLR inhibitor small molecule compounds, none of these compounds have been reported to be able to inhibit viral replication both in vitro and in vivo (Czarniecki, 2008; Nakamura et al., 2007; Yamada et al., 2005). To our knowledge, this is the first report that a small molecule compound identified using a TLR2 expressing model that has the ability to inhibit both viral replication and TLR ligand-induced inflammation.
In summary, using a cell-based screen system, we have identified small molecule compounds capable of modulating TLR2 signaling. In particular, we have further characterized compound E567 and demonstrated that E567 inhibits inflammatory responses induced by both viruses and other TLR2 and TLR4 agonists. Remarkably, this agent has effect on virus replication. Further structure-activity relationship (SAR) studies and the pharmacokinetics (PK) evaluation of these candidates are under way to define the therapeutic potential of this agent. We believe that this cell line and these positive compounds will enhance not only the understanding of TLR2-mediated signaling pathway, but will also shed light on the development of new types of drugs that target TLR-mediated inflammation, including viral and bacterial infections.
Acknowledgments
We are grateful to Sean Johnston, Ruchir Shah, Stewart Rudnicki, Katrina Schulberg, Brian C Kraybill, David Wrobel, Kyungae Lee, Greg Cuny, Su Chiang, and Caroline Shamu at the NERCE / NSRB screening facility at Harvard Medical School for their help and assistance with the compound library screening. We would like to thank Dr. George Reed (University of Massachusetts Medical School, Worcester, MA. USA ) for his advice on the statistical analysis. Thanks to Jayashree Paranjape for editing the figures and reading the manuscript. This work was supported by NIAID Regional Center of Excellence Grant AI 057159, NIH grants R01 AI 49309, P01 AI 0577484, and JDRF 24-2008-950 (R.W.F.) and NIH grant AI 51405 (E.A.K.-J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Baenziger S, Heikenwalder M, Johansen P, Schlaepfer E, Hofer U, Miller RC, Diemand S, Honda K, Kundig TM, Aguzzi A, Speck RF. Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood. 2008 doi: 10.1182/blood-2008-04-151712. [DOI] [PubMed] [Google Scholar]
- Battegay M, Cooper S, Althage A, Banziger J, Hengartner H, Zinkernagel RM. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J Virol Methods. 1991;33(1–2):191–198. doi: 10.1016/0166-0934(91)90018-u. [published errata appears in J Virol Methods 1991 Nov;35(1):115 and 1992 Aug;38(2):263] [DOI] [PubMed] [Google Scholar]
- Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77(8):4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarniecki M. Small molecule modulators of toll-like receptors. J Med Chem. 2008;51(21):6621–6626. doi: 10.1021/jm800957k. [DOI] [PubMed] [Google Scholar]
- Dove LM, Rosen RC, Ramcharran D, Wahed AS, Belle SH, Brown RS, Hoofnagle JH. Decline in male sexual desire, function, and satisfaction during and after antiviral therapy for chronic hepatitis C. Gastroenterology. 2009;137(3):873–884. doi: 10.1053/j.gastro.2009.05.060. 884 e1. [DOI] [PubMed] [Google Scholar]
- Finberg RW, Kurt-Jones EA. Viruses and Toll-like receptors. Microbes Infect. 2004;6(15):1356–1360. doi: 10.1016/j.micinf.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Finberg RW, Wang JP. Antiviral responses: different roles for different tolls. Immunity. 2009;30(2):173–175. doi: 10.1016/j.immuni.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O'Neill LA. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413(6851):78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- Gauvreau K, Pagano M. Hypothesis tests. Nutrition. 1993;9(2):186–187. [PubMed] [Google Scholar]
- Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10(12 Suppl):S110–S121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- Gowen BB, Smee DF, Wong MH, Hall JO, Jung KH, Bailey KW, Stevens JR, Furuta Y, Morrey JD. Treatment of late stage disease in a model of arenaviral hemorrhagic fever: T-705 efficacy and reduced toxicity suggests an alternative to ribavirin. PLoS One. 2008;3(11):e3725. doi: 10.1371/journal.pone.0003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, Endres S, Hartmann G. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11(3):263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101(5):1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1(5):398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- Le Goffic R, Balloy V, Lagranderie M, Alexopoulou L, Escriou N, Flavell R, Chignard M, Si-Tahar M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2(6):e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C., Jr The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8(10):452–456. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- Murawski MR, Bowen GN, Cerny AM, Anderson LJ, Haynes LM, Tripp RA, Kurt-Jones EA, Finberg RW. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83(3):1492–1500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Shimizu Y, Sato Y, Miyazaki Y, Satoh T, Mizuno M, Kato Y, Hosaka Y, Furusako S. Toll-like receptor 4 signal transduction inhibitor, M62812, suppresses endothelial cell and leukocyte activation and prevents lethal septic shock in mice. Eur J Pharmacol. 2007;569(3):237–243. doi: 10.1016/j.ejphar.2007.05.013. [DOI] [PubMed] [Google Scholar]
- O'Neill LA. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov. 2006;5(7):549–563. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
- Seyberth T, Voss S, Brock R, Wiesmuller KH, Jung G. Lipolanthionine peptides act as inhibitors of TLR2-mediated IL-8 secretion. Synthesis and structure-activity relationships. J Med Chem. 2006;49(5):1754–1765. doi: 10.1021/jm050585d. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227(1):75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP, Kurt-Jones EA, Finberg RW. Innate immunity to respiratory viruses. Cell Microbiol. 2007;9(7):1641–1646. doi: 10.1111/j.1462-5822.2007.00961.x. [DOI] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10(12):1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ichikawa T, Ii M, Sunamoto M, Itoh K, Tamura N, Kitazaki T. Discovery of novel and potent small-molecule inhibitors of NO and cytokine production as antisepsis agents: synthesis and biological activity of alkyl 6-(N-substituted sulfamoyl)cyclohex-1-ene-1-carboxylate. J Med Chem. 2005;48(23):7457–7467. doi: 10.1021/jm050623t. [DOI] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317(5844):1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- Zhou S, Halle A, Kurt-Jones EA, Cerny AM, Porpiglia E, Rogers M, Golenbock DT, Finberg RW. Lymphocytic choriomeningitis virus (LCMV) infection of CNS glial cells results in TLR2-MyD88/Mal-dependent inflammatory responses. J Neuroimmunol. 2008;194(1–2):70–82. doi: 10.1016/j.jneuroim.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Kurt-Jones EA, Mandell L, Cerny A, Chan M, Golenbock DT, Finberg RW. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur J Immunol. 2005;35(3):822–830. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]