Abstract
Objectives
In a univentricular Fontan circulation, modest augmentation of existing cavopulmonary pressure head (2–5 mmHg) would reduce systemic venous pressure, increase ventricular filling, and thus, substantially improve circulatory status. An ideal means of providing mechanical cavopulmonary support does not exist. We hypothesized that a viscous impeller pump, based on the von Kármán viscous pump principle, is optimal for this role.
Methods
A 3-dimensional computational model of the total cavopulmonary connection was created. The impeller was represented as a smooth 2-sided conical actuator disk with rotation in the vena caval axis. Flow was modeled under 3 conditions: 1) passive flow with no disc; 2) passive flow with a non-rotating disk, and 3) induced flow with disc rotation (0–5K rpm). Flow patterns and hydraulic performance were examined for each case. Hydraulic performance for a vaned impeller was assessed by measuring pressure rise and induced flow over 0–7K rpm in a laboratory mock loop.
Results
A nonrotating actuator disc stabilizes cavopulmonary flow, reducing power loss by 88%. Disk rotation (from baseline dynamic flow of 4.4 L/min) resulted in a pressure rise of 0.03 mmHg. A further increase of pressure of 5–20 mmHg and 0–5 L/min flow were obtained with a vaned impeller at 0–7K rpm in a laboratory mock loop.
Conclusions
A single viscous impeller pump stabilizes and augments cavopulmonary flow in 4 directions, in the desired pressure range, without venous pathway obstruction. It applies to the existing staged protocol as a temporary bridge-to-recovery or –transplant in established univentricular Fontan circulations. It may also enable compressed palliation of single ventricle without need for intermediary surgical staging or use of a systemic-to-pulmonary arterial shunt.
In Fontan repair of functional single ventricle, a staged surgical approach is necessary to achieve the end goal of a series connection of the systemic venous return to the pulmonary arteries. Despite medical and surgical advances, however, this approach remains problematic [1]. At the expense of providing a reliable source of neonatal pulmonary blood flow, interim use of a systemic-to-pulmonary arterial shunt creates serious pathophysiologic consequences. Shunt physiology may impair timing for Fontan conversion and detrimentally impact late Fontan status [2]. Once Fontan palliation is complete, the absence of a subpulmonary ventricle results in chronic elevation in systemic venous, capillary, and interstitial pressure, and reduction in ventricular preload and subnormal cardiac output for the duration of life. These problems can be addressed by pump augmentation of existing Fontan cavopulmonary flow [3,4].
A pump to accomplish this does not currently exist. If a safe and reliable device were developed, it may be possible to stabilize patients with “failing Fontan” physiology, early or late after repair, by temporarily reversing the Fontan paradox of elevated venous pressure and impaired ventricular filling [5]. We hypothesized that a viscous impeller pump (VIP), based on the von Kármán viscous pump principle, possesses qualities which are ideal to serve in this capacity [6,7]. In this report, we present an initial analysis, and background and rationale, for a viscous impeller pump to provide cavopulmonary assist.
Methods
Computational fluid dynamic (CFD) analysis of an actuator disk within a total cavopulmonary connection (TCPC)
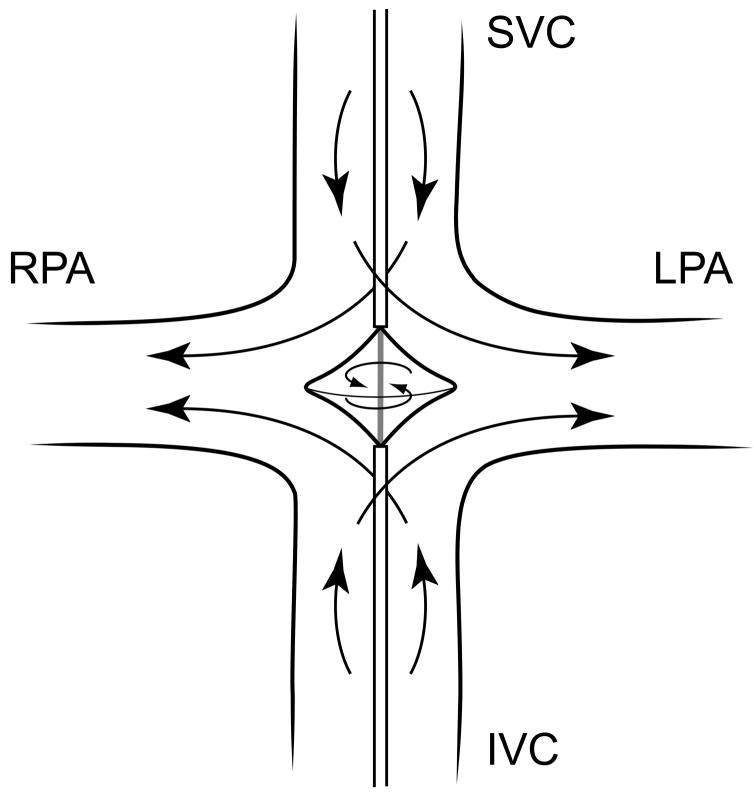
CFD (Fluent, Ansys Inc, Canonsburg, PA) was used to examine the hydraulic performance and flow conditions of a viscous impeller pump (tip diameter 20 mm; hub diameter 6 mm) in an idealized adult TCPC model of semi-physiologic flow (22 mm diameter IVC/SVC, 19 mm diameter LPA/RPA, slightly expanded at junction resulting in tip gap clearance of 8 mm, 4.4L/min baseline flow, 50/50 inlet-outlet split) (Figure 1). These are reasonable estimates of adult TCPC dimensions and flow, and provide a reasonable clinical safety margin against impeller strike and vessel injury.
Figure 1.
Concept of a viscous impeller pump to provide cavopulmonary assist (SVC, superior vena cava; IVC, inferior vena cava, LPA, left pulmonary artery; RPA, right pulmonary artery).
The following conditions were considered: 1) absence of the pump; 2) presence of a stationary pump; and 3) presence of a rotating pump (0–5000 rpm). Impeller geometry for the CFD studies was patterned as a smooth surfaced, two-sided, centrifugal pump. Tetrahedral meshes were produced using Gambit (preprocessor for Fluent) with 900,000 elements. Laminar flow was assumed for the case without the pump and with the stationary pump. The unsteady Reynolds-Averaged Navier-Stokes equations employing the realizable k-ε turbulence model, no-slip boundary conditions, and y+ grid requirements were integrated within Fluent only for the case of the rotating pump. The inlet turbulence intensity was selected as 3% and variation of this value did not significantly affect the results. The fluid medium was assigned a physiologic density of 1060 kg/m3 and viscosity of 3.5 cP; both were assumed to be constant.
In vitro hydraulic studies
The hydraulic performance of a vaned impeller was assessed in an in vitro mock circulatory loop with a 4-way TCPC conduit. The mock loop utilized compliance and resistance elements to mimic the physiologic parameters of an adult Fontan circulation [8]. The impeller tested was constructed as an SLA rapid-prototype from CAD drawings using optically transparent Accura 60 resin. Impeller and conduit dimensions used in the mock circulatory loop were identical to the CFD model, with the exception that surface vane structure was added to the impeller surface. This adult scale rapid-prototype impeller simulates the expanded device and is 18 mm dia. and 6 mm length. The impeller is intended to be collapsed to 2.86 mm dia to fit an 8 French catheter. The operational range is 0–7000 rpm. There are 4 vanes on the version tested; each 2 mm height at the center and blended at inflow hub and outflow tip. An external brushless DC motor was used to drive the impeller in the Fontan mock circuit.
Results
CFD modeling and flow patterns
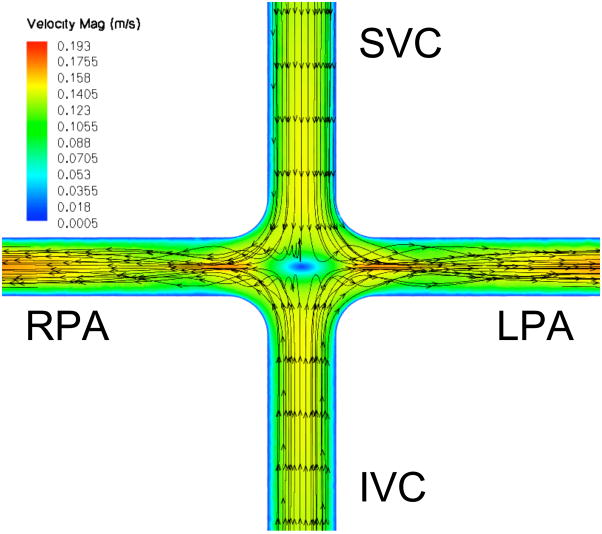
A mid-plane contour plot of instantaneous velocity magnitude with streamlines, with and without a stationary pump present, is shown in Figure 2. TCPC flow patterns in the absence of a central stabilizing body (actuator disk/pump) match previously reported studies utilizing CFD and magnetic resonance imaging, where irregular flow in the central stagnation region accounts for energy loss and inefficiency [9,10]. In the presence of the stationary pump, TCPC flow patterns are improved with 88% reduction in power loss (Table 1). Thus, the presence of the pump, rotating or not, has a marked stabilizing effect in this 4-way flow configuration.
Figure 2.
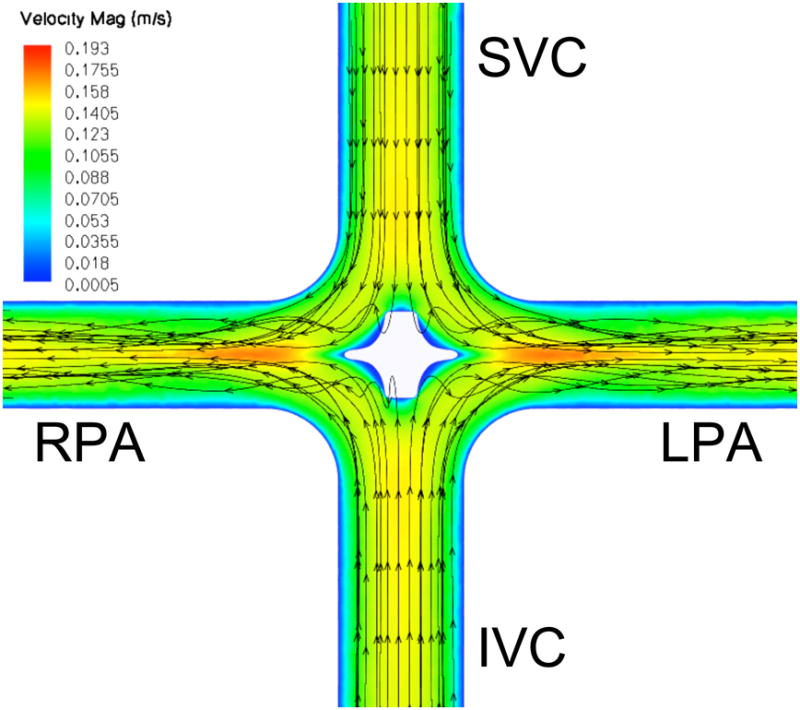
Velocity magnitude (m/s) contour plot within the TCPC at flow 4.4 liters per minute. A: No impeller. Incoming flow is irregular at the intersection. B: Stationary impeller. Flow pattern is stabilized, reducing power loss by 88%. (SVC, superior vena cava; IVC, inferior vena cava, LPA, left pulmonary artery; RPA, right pulmonary artery)
Table 1.
Transition from flow stabilizer to pump with increased rotation.
| RPM | Pressure rise (mmHg) | Maximum Wall Shear (Pa)* | Power Loss (Watts) |
|---|---|---|---|
| No impeller | −0.43 | 1.4 | 0.25 |
| 0 | −0.05 | 7.6 | 0.03 |
| 500 | −0.05 | 15.6 | 0.03 |
| 1000 | −0.04 | 29.3 | 0.02 |
| 2500 | 0.02 | 98.7 | −0.01 |
| 5000 | 0.03 | 130.8 | −0.02 |
RPM: revolutions per minute; Pa: pascal,
location of maximum wall shear stress is on the TCPC vessel wall for case with no impeller and on impeller surface for cases with impeller
Predicted hydraulic performance and Wall Shear Stress
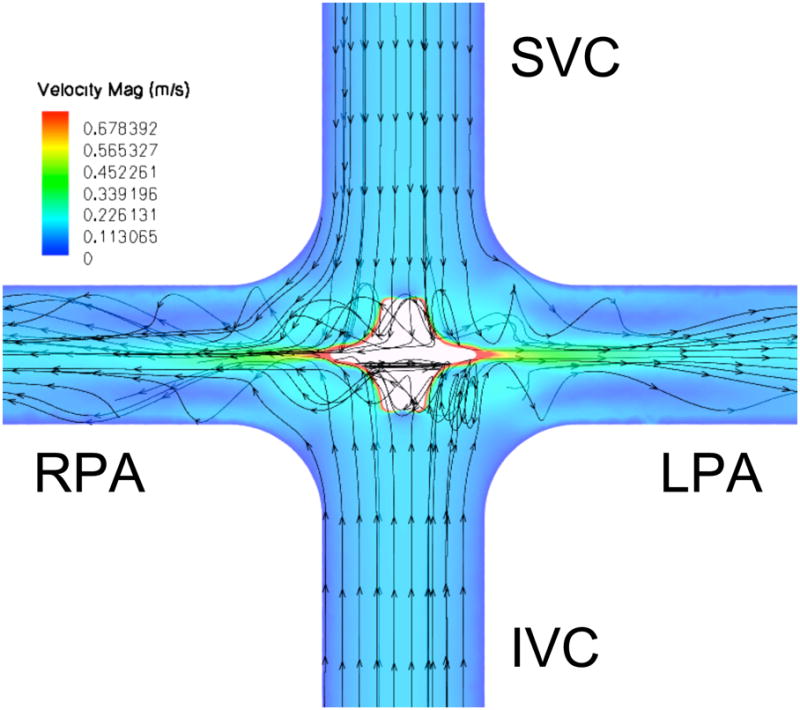
CFD predictions for hydraulic performance of a smooth-surfaced impeller within the TCPC, and comparison to a TCPC without a pump, are shown in Table 1. According to the boundary conditions used as constraints in the computations, the flow rate remained constant for each case (4.4 L/min), while the effect of the pump was demonstrated in generating a pressure increase, hence reducing power loss (ΔP (Pa) × 4.4L/min and then converted to m3/s) within the fluid of the model. Although the pressure increase in each case is comparatively small, viscous flow losses are negated by the actuator disk between 1000 and 2500 rpm, whereupon the function of the disk makes a transition from the role of flow stabilizer to that of a pump. Axial (vena caval) inflow increased consistent with pressure generated by higher rates of impeller rotation.
We further utilized CFD to identify regions of both high stress (hemolysis and platelet activation) and low stress (flow stasis and thrombogenicity) within the impeller. Scalar stress estimations for the smooth impeller at 5000 RPM are shown in Figure 3A [11]. Relatively low scalar stress is found on the surface of the pump and rotation of the fluid as it transitions from axial to radial flow. All stress values were above zero. Scalar stress values on the impeller surface were lower than 100 Pa, with the highest levels at the outflow edge of the impeller (Figure 3B). Efficiency can be optimized by modifying pump geometry to distribute flow from the pump periphery over the entire cross sectional area of the LPA and RPA. As spatial distribution is optimized and magnitude of turbulence is minimized, the risk of shear induced blood damage is reduced [11–13]. We expect scalar stress with the viscous impeller pump will remain less than 300 Pa, within the clinically acceptable range.
Figure 3.
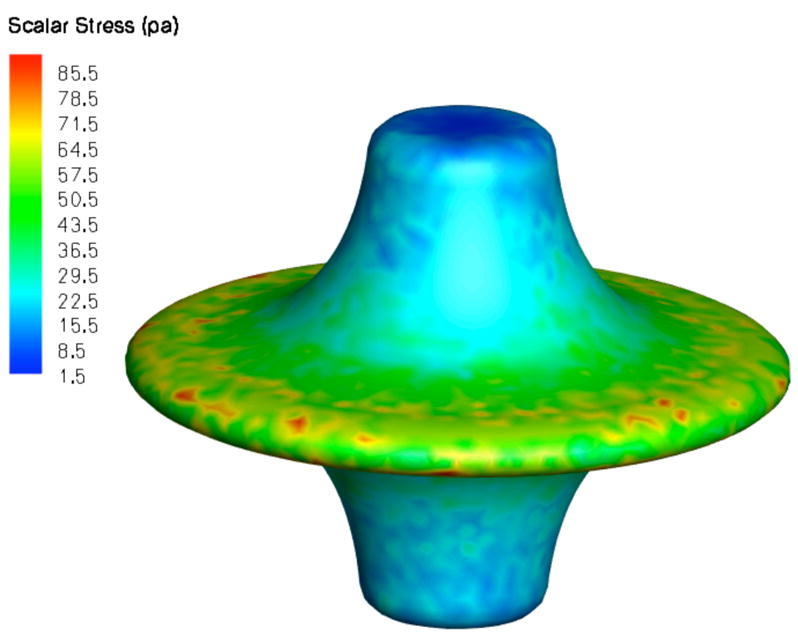
Predicted scalar stress (Pa) (A) and fluid streamlines as colored by total pressure (Pa) (B) for a smooth surface viscous impeller pump rotating at 5000 rpm. (SVC, superior vena cava; IVC, inferior vena cava, LPA, left pulmonary artery; RPA, right pulmonary artery)
In vitro hydraulic performance
Hydraulic performance of a surface modified viscous impeller pump (2 mm surface vanes, and with cage present) was tested in the mock circulatory loop shown in Figure 4A. The impeller induced a pressure rise of 0–20 mmHg and flow rates of 0–5 L/min at rotational speeds of 0–7K rpm (Figure 4B). No reduction in flow rate was observed at 0 rpm and no gross cavitation was observed at 7K rpm. Performance curves are characteristically flat, signifying consistent low-pressure performance over a wide range of flow conditions. At high pump speeds, the device is capable of generating pressure rise that is above the 2–5 mmHg optimum range due to the presence of surface vanes. Lower pressure rise values can be obtained at lower pump speeds or by using a more smoothly surfaced impeller (lower profile surface vanes). The operational speed of the pump and extent of surface vane expression may vary depending upon the clinical conditions in which the pump is applied. The use of surface vanes to modify impeller performance must be balanced with acceptable shear rates at defined operational specifications.
Figure 4.
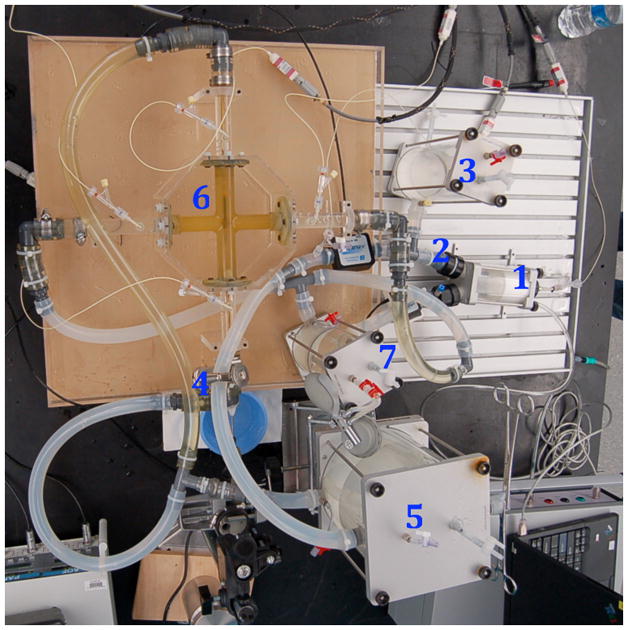
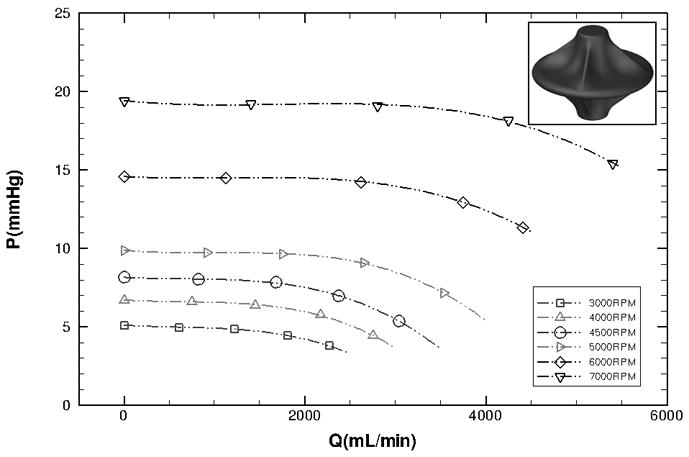
Prototype hydraulic performance. Left: In vitro mock circulatory loop consisting of the following elements: 1) single ventricle, 2) aorta, 3) arterial compliance, 4) systemic vascular resistance, 5) venous compliance, 6) Fontan junction with viscous impeller pump, 7) pulmonary compliance. Right: Experimental hydraulic performance for a vaned impeller with cage present (P, pressure mmHg; Q, flow mL/min).
Discussion
“Fontan failure”-not the same as heart failure
Fontan patients are at high risk of regression from compensated to uncompensated Fontan physiology not only at the time of repair, but also as they age [5]. This risk is, in part, a reflection of their initial staging operations in which a shunt was utilized [2]. The increasing number of survivors with Fontan physiology constitutes an emerging public health concern [14]. Although children and adults with “Fontan failure” exhibit classic features of congestive heart failure (increased tissue/organ water, decreased tissue/organ perfusion), the underlying cause is not primary myocardial dysfunction. The ventricular (diastolic) dysfunction observed in Fontan patients may be to a large extent attributable to chronically reduced filling [15–17].
Treatment options for this group are limited and less than ideal. For example, diuretic therapy may alleviate symptoms of increased tissue water, but at the expense of circulating blood volume; ideal therapy would be to simply reduce systemic venous pressure alone. Similarly, inotrope therapy will improve cardiac output inefficiently in an under filled ventricle; ideal therapy would be to simply increase ventricular filling. Cavopulmonary assist can ideally and concurrently address both of these circumstances.
A pump “primer” rather than a primary pump
In a reversal of assumptions, it can be stated that other segments of the circulation are failing the Fontan heart. Thus (assuming normal systolic function), the target for therapeutic support of the Fontan circulation is the cavopulmonary circulation, rather than the systemic circulation. A cavopulmonary assist device would serve as a low input “primer” for the primary pump (systemic single ventricle), rather than as a primary pump per se. A modest increase in downstream ventricular filling (2–5 mmHg) will improve myocardial performance and cardiac output. Simultaneously, reduction in upstream systemic venous pressure will markedly benefit the systemic and mesenteric venous circulation and interstitium. This is identical to the essential function of the right ventricle in a normal biventricular circulation [18].
Unique pump requirements
Mechanical cavopulmonary assist within the TCPC presents unique anatomic and physiologic challenges which are markedly dissimilar to any other mechanical circulatory support application. Flow must be augmented in a highly complex 3- or 4-axis geometry in which incoming and outgoing flow is perpendicularly opposed. The pump will provide partial support in a location where no ventricle will recover to assume function of the pump; it is not a ventricular assist device. Ambient hydrostatic pressure in the TCPC is low (10–12 mmHg), increasing concern for flow stasis and thrombogenicity. Additionally, the desired flow is high-volume, low-pressure flow. A gentle pressure step-up of 2–5 mmHg may be ideal. In certain cases, higher pressure may be undesirable or detrimental. In other instances, higher pressure may be necessary against increased pressure head (for example, pulmonary hypertension). Because there is no volume reservoir for the pump inlet to draw from, risk of vein collapse and cavitation due to inlet suction is high. Thus, a high degree of fluid slip (low preload dependence) is preferable. Similarly, downstream flow must remain low-pressure to avoid perfusion lung injury (similarly, a high degree of fluid slip is preferable). Importantly, there is no natural barrier (such as a valve) present within the TCPC pathway to prevent recirculation around the pump body. For a fixed-diameter small percutaneous device positioned within the large diameter central veins, recirculation must be addressed. Finally, and perhaps most importantly, it is critical that the cavopulmonary pathways remain unobstructed during pump deployment, as pump support is weaned to no net contribution prior to removal, when the pump is shut off, in the event of rotation failure, and after the pump is withdrawn.
Limitations of microaxial flow pumps
At first thought, adapting traditional axial flow design is an appealing solution to this problem. A computational model of an axial flow pump intended for use in the IVC has, in fact, been reported in which blade geometry and rotational speed were simply modified to operate in the low pressure venous circulation [19]. On the contrary, in our experience, microaxial pumps have critical limitations. Most importantly, the pump itself, or combination of pump, housing, and/or barrier to recirculation, is obstructive to flow. Once in place, the pump cannot be simply shut off. In the event of failure or shut-down, the venous pressure necessary to maintain cardiac output would be untenable in an environment where only 2–5 mmHg obstruction has grave consequences. For cavopulmonary assist, the ability to wean support to very low levels - without any degree of obstruction – is critical. Second, the inlet pressure of a microaxial pump is in an unacceptable negative range, portending suction collapse of the vena cava. The smaller the diameter of a fixed-radius microaxial pump, the greater is the rotational speed required to transfer equivalent volume and the greater is the risk for inefficient recirculation. A truly percutaneous microaxial device, at perhaps less than 10% of vena caval cross-sectional area, must greatly accelerate flow to deliver equivalent effective flow. This increases risk for suction, cavitation, and blood component activation. Third, due to size (anything > 4 mm dia. in adults), placement and removal requires open-heart surgery and cardiopulmonary bypass. Fourth, a microaxial device requires a contained housing, inducer, and diffuser, which increase complexity, synthetic material burden, and thrombogenicity risk. Fifth, microaxial pump flow is unidirectional: This mandates either the use of 2 separate pumps or undesirable surgical revision of the systemic venous pathways to prevent retrograde pressure elevation and backflow into the opposing vena cava [20,21].
An expandable, multi-directional rotary pump
Due to these limitations, we abandoned existing microaxial designs and shifted to consider a rotary pump which is both expandable and multi-directional. The ability to expand the radius of a peripherally inserted, centrally deployed intravascular pump would reduce rotational rates, and provide a functional barrier to recirculation [22]. The native, endothelial-lined vessel walls would serve as the conduit for flow. Advanced numerical modeling has shown that a fixed central diverting body at the TCPC intersection will beneficially split incoming TCPC flow toward the outlets, minimizing turbulent energy loss [23]. By combining passive flow optimization with the need for an expandable impeller, we had the insight to apply rotational energy to an expandable, central impeller suspended in the midst of the TCPC, independent of the vessel walls.
von Kármán viscous pump [6]
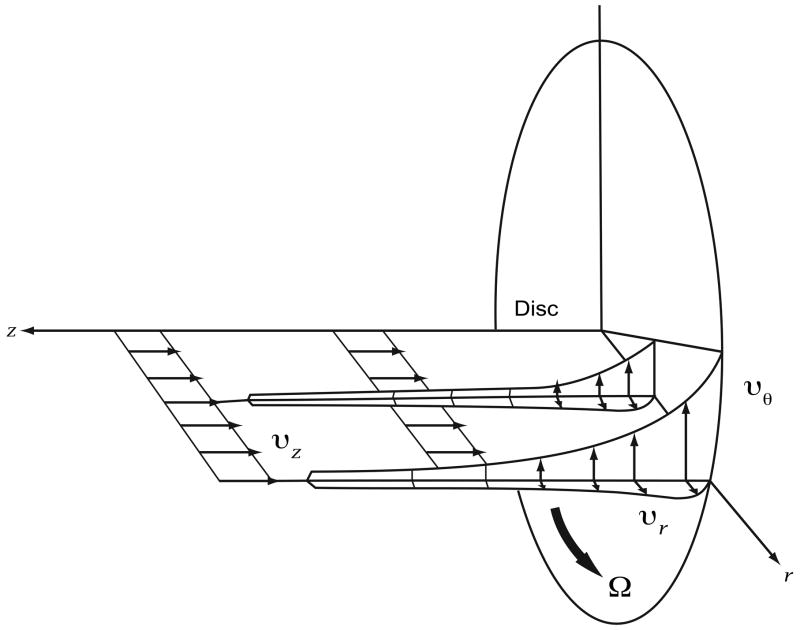
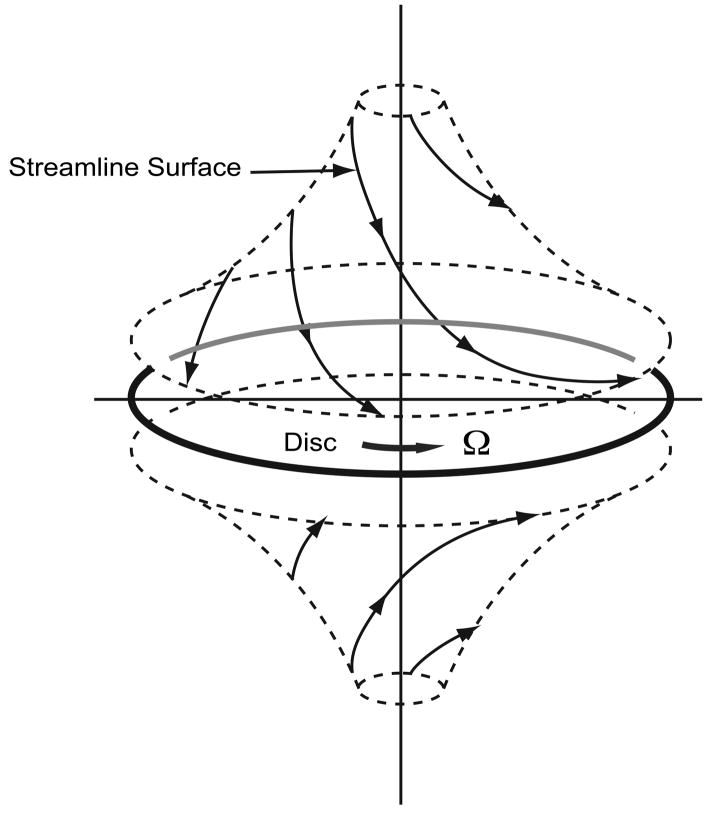
Consider a large, flat disk rotated in fluid. The no-slip condition requires that fluid at the surface of the disk rotate with the same velocity (Figure 5A). Viscous effects diffuse away from the disk and induce rotation. However, there is no pressure gradient in the radial direction to balance the centrifugal force. Once fluid has been accelerated by the plate, it is also flung out in a radial direction. Conservation requires that the outwardly displaced fluid is replaced by axial inflow from quiescent fluid far away from disk. Thus, fluid is pumped from the far axial stream (SVC above; IVC below) toward the disk, where viscous forces induce a swirl and the resulting centrifugal effect produces radial outflow (Figure 5B) [7]. Based on this fundamental fluid dynamic principal, a single impeller will stabilize and augment TCPC flow in 4 axes.
Figure 5.
Von Kármán viscous pump: A) Flow patterns induced by actuator disk rotation. B) Flow streamlines depicted on two sides of an actuator disk. (P, pressure rise mmHg; Q, flow rate ml/min).
A viscous impeller pump is an exciting conceptual advance. The problem is dramatically simplified from a complex 2-pump solution with competing and colliding flows to a much more advantageous, single impeller solution with multidirectional flow capability (3-way inverted “T”; 4-way “t”). The preferred TCPC pathway can be maintained, avoiding unnecessary surgical venous pathway revision that might be necessary with unidirectional devices [20,21]. An impeller and protective centering cage, collapsed for percutaneous insertion, advanced to the intersection of the TCPC, opened and rotated, will draw venous flow inward from opposed directions, and propel blood radially outward in the pulmonary arteries, regardless of orientation (Figure 6).
Figure 6.
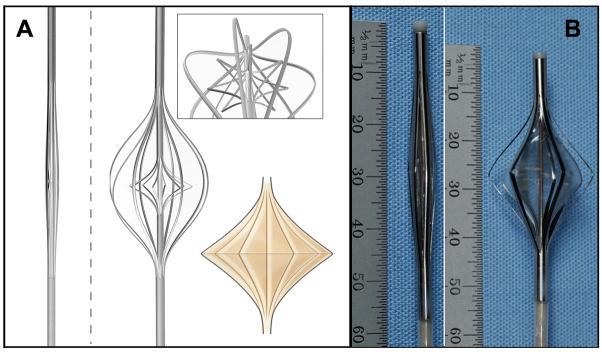
Expandable viscous impeller pump using “cage within a cage” concept. A: Large scale model in collapsed configuration. B: same model expanded by axial compression. The outer cage serves to center the impeller and protect the vessel wall. The inner cage, covered with an elastomer, forms the rotating impeller surface. The expanded to collapsed ratio is 500%. C: Adult scale functional prototype: closed diameter 8 French, 2.86 mm; open diameter 22 mm outer cage.
Broad applicability of cavopulmonary assist
Cavopulmonary assist is fully compatible with the existing staged paradigm. It can be applied for temporary perioperative support after stage-2 or -3 repair alone. It can also be applied late after repair for support of “failing Fontan” physiology in older children or adults. It can be implemented non-invasively in the catheterization lab or intensive care unit setting via percutaneous venous access and Seldinger technique. Cavopulmonary assist may also enable a new paradigm of compressed or combined surgical staging: 1) Stage-2 and -3 combined, or one-stage Fontan conversion; 2) Stage-1 and -2 combined (avoidance of shunt physiology) [24]; 3) Stages 1,2,3 combined (one-stage neonatal Fontan repair). This technology applies to both a 3-way “T” (Stage-2 Glenn; hemi-Fontan) and 4-way “t” (Stage-3; TCPC). As a platform device, asymmetric impeller or surface (vane) modification can address differential inflow and/or outflow rates. In adults, the majority of complications of failing Fontan physiology arise from the IVC distribution, which accounts for 70% of total systemic venous return. Conversely, in neonates, upper body venous return comprises 60–70% total venous flow, which is largely a reflection of cerebral blood flow (~90% SVC flow) [25].
This report describes a de novo pump concept, and its anatomic and physiologic limitations and constraints. Certainly, much work remains to carry this concept to clinical implementation. A limitation of this preliminary analysis is that the impeller tested was a rigid rather than expandable prototype. Furthermore, the device was scaled to adult dimensions; performance at dimensions suitable for infants and neonates remains to be determined. Future investigation will include reduced scale analyses, hydraulic optimization, and hemolysis and thrombogenicity assessment. Shear stress and hemolysis are not expected to be problems given the lower rotation requirements of this device in comparison to commercially available microaxial devices. A viscous impeller pump makes mechanical circulatory support of a univentricular Fontan circulation a much more realistic consideration. Proven safe and effective, a viscous impeller pump will greatly improve therapy for individuals with single ventricle heart disease.
Acknowledgments
This work was supported in part by National Institutes of Health Grant HL080089 (MDR); Collaboration in Bioengineering Grant (MDR, SHF), and Research Support Funds Grant (MDR), Indiana University Purdue University Indianapolis.
Footnotes
Presented at the 89th Annual Meeting of the American Association for Thoracic Surgery, May 6-13, 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashburn DA, McCrindle BW, Tchervenkov CI, Jacobs ML, Lofland GK, Bove EL, et al. Outcomes after the Norwood operation in neonates with critical aortic valve stenosis or aortic valve atresia. J Thorac Cardiovasc Surg. 2003;125:1070–82. doi: 10.1067/mtc.2003.183. [DOI] [PubMed] [Google Scholar]
- 2.Khambadkone S, Li J, de Leval MR, Cullen S, Deanfield JE, Redington AN. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation. 2003;107:3204–8. doi: 10.1161/01.CIR.0000074210.49434.40. [DOI] [PubMed] [Google Scholar]
- 3.Rodefeld MD, Boyd JH, LaLone BJ, Bezrucko AJ, Potter AW, Brown JW. Cavopulmonary assist: circulatory support for the univentricular Fontan circulation. Ann Thorac Surg. 2003;76:1911–6. doi: 10.1016/s0003-4975(03)01014-2. [DOI] [PubMed] [Google Scholar]
- 4.Rodefeld MD, Boyd JH, Myers CD, Presson RG, Wagner WW, Brown JW. Cavopulmonary assist in the neonate: an alternative strategy for single-ventricle palliation. J Thorac Cardiovasc Surg. 2004;127:705–11. doi: 10.1016/j.jtcvs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 5.deLeval M. The Fontan circulation: what have we learned? What to expect? Pediatric Cardiol. 1998;19:316–20. doi: 10.1007/s002469900315. [DOI] [PubMed] [Google Scholar]
- 6.von Kármán T, Agnew Z. Math Mech. 1921;1:233. [Google Scholar]
- 7.Panton RL. Incompressible Flow. 2. Wiley Interscience; 1996. p. 294. [Google Scholar]
- 8.Pantalos G. Use of computer and in vitro modeling techniques during the development of pediatric circulatory support devices. NHLBI Pediatric Assist Device Contractor’s Meeting: Pediatric modeling techniques working group. ASAIO J. 2009;55:3–5. doi: 10.1097/MAT.0b013e318198dd88. [DOI] [PubMed] [Google Scholar]
- 9.Migliavacca F, Dubini G, Bove EL, de Leval MR. Computational Fluid Dynamics Simulations in Realistic 3-D Geometries of the Total Cavopulmonary Anastomosis: The Influence of the Inferior Caval Anastomosis. J Biomech Eng. 2003;125:805–13. doi: 10.1115/1.1632523. [DOI] [PubMed] [Google Scholar]
- 10.Pekkan K, Zelicourt DD, Ge L, Sotiropoulos F, Frakes D, Fogel MA, Yoganathan AP. Physics-driven CFD modeling of complex anatomical cardiovascular flows – a TCPC case study. Ann Biomed Eng. 2005;33:284–300. doi: 10.1007/s10439-005-1731-0. [DOI] [PubMed] [Google Scholar]
- 11.Bludszuweit C. Three-dimensional numerical prediction of stress loading of blood particles in a centrifugal pump. Artif Organs. 1995;19:590–6. doi: 10.1111/j.1525-1594.1995.tb02386.x. [DOI] [PubMed] [Google Scholar]
- 12.Apel J, Paul R, Klaus S, Siess T, Reul H. Assessment of hemolysis related quantities in a microaxial blood pump by computational fluid dynamics. Artif Organs. 2001;25:341–7. doi: 10.1046/j.1525-1594.2001.025005341.x. [DOI] [PubMed] [Google Scholar]
- 13.Bludszuweit C. Model for general mechanical blood damage prediction. Artif Organs. 1995;19:583–9. doi: 10.1111/j.1525-1594.1995.tb02385.x. [DOI] [PubMed] [Google Scholar]
- 14.Williams R, Pearson GD, Barst RJ, Child JS, del Nido P, Gersony WM, et al. Report of the National Heart, Lung, and Blood Institute Working Group on Research in Adult Congenital Heart Disease. J Am Coll Cardiol. 2006;47:701–7. doi: 10.1016/j.jacc.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 15.Eicken A, Petzuch K, Marek J, Vogel M, Hagler A, Vogt M, et al. Characteristics of Doppler myocardial echocardiography in patients with tricuspid atresia after total cavopulmonary connection with preserved systolic ventricular function. Int J Cardiol. 2007;116:212–8. doi: 10.1016/j.ijcard.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, McCrindle BW, Paridon S, Schwartz M, Stylianou M, Williams RV, Clark BJ. Contemporary outcomes after the Fontan procedure. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backer CL. The Fontan procedure. Our odyssey continues (editorial) J Am Coll Cardiol. 2008;52:114–6. doi: 10.1016/j.jacc.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 18.Furey SA, Zieske HA, Levy MN. The essential function of the right ventricle. Am Heart J. 1984;107:404–10. doi: 10.1016/0002-8703(84)90402-2. [DOI] [PubMed] [Google Scholar]
- 19.Wang R, Lacour-Gayet F, Lanning CJ, Rech BA, Kilfoil PJ, Hertzberg J, Shandas R. Initial experience with the development and numerical and in vitro studies of a novel low-pressure artificial right ventricle for pediatric Fontan patients. ASAIO J. 2006;52:682–92. doi: 10.1097/01.mat.0000249038.69048.3c. [DOI] [PubMed] [Google Scholar]
- 20.Lacour-Gayet FG, Wang R, Lanning CJ, Goldberg SP, Rech BA, Shandas R. Experimental study of an artificial right ventricle for failing Fontan: in vitro and computational evaluation (abstract). The Society of Thoracic Surgeons, 45th Annual Meeting; Jan 27–30, 2009; [DOI] [PubMed] [Google Scholar]
- 21.Marsden AL, Bernstein AJ, Reddy VM, Shadden SC, Spilker RL, Chan FP, et al. Evaluation of a novel Y-shaped extracardiac Fontan Baffle using computational fluid dynamics. J Thorac Cardiovasc Surg. 2009;137:394–403. doi: 10.1016/j.jtcvs.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 22.Throckmorton AL, Ballman KK, Myers CD, Frankel SH, Brown JW, Rodefeld MD. Performance of a 3-bladed propeller pump to provide cavopulmonary assist in the failing Fontan circulation. Ann Thorac Surg. 2008;86:1343–7. doi: 10.1016/j.athoracsur.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Soerensen DD, Pekkan K, de Zelicourt D, Sharma S, Kanter K, Fogel M, Yoganathan AP. Introduction of a new optimized total cavopulmonary connection. Ann Thorac Surg. 2007;83:2182–80. doi: 10.1016/j.athoracsur.2006.12.079. [DOI] [PubMed] [Google Scholar]
- 24.Honjo O, Merklinger SL, Poe JB, Guerguerian AM, Alghamdi AA, Takatani S, Van Arsdell GS. Mechanical cavopulmonary assist maintains pulmonary and cerebral blood flow in a piglet model of a bidirectional cavopulmonary shunt with high pulmonary vascular resistance. J Thorac Cardiovasc Surg. 2009;137:355–61. doi: 10.1016/j.jtcvs.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Fogel M, Durning S, Wernovsky G, Pollock AN, Gaynor JW, Nicolson S. Brain versus lung: hierarchy of feedback loops in single-ventricle patients with superior cavopulmonary connection. Circulation. 2004;(suppl II):II-147–52. doi: 10.1161/01.CIR.0000138346.34596.99. [DOI] [PubMed] [Google Scholar]