Abstract
Background
We evaluated the role of renal tubular Nox-2 in the pathogenesis of epithelial-to-mesenchymal transition (EMT) in kidney allografts.
Methods
We examined this question in human kidney allografts with tubulointerstitial fibrosis not otherwise specified (IFTANOS), in the Fisher to Lewis rat transplant model and in the in vitro model of TGF-β1-induced EMT in normal rat kidney epithelial cells (NRK52E).
Results
We first demonstrated that Nox-2 and α-SMA were increased in renal tubules from kidney transplant recipients on calcineurin inhibitors, mycophenolic acid and prednisone with IFTANOS, suggestive of EMT (n=6). Next we examined Nox-2 expression and fibrogenesis in syngeneic transplants; allogeneic transplants treated with MPA 40 mg/kg/24h and untreated allogeneic transplants for 6 months (n=14 in each group). Immunofluorescent and immunohistochemical studies for Nox-2, α-SMA and E-cadherin showed that similar to patients with IFTANOS, rat allografts had greater tubulointerstitial staining for Nox-2 and α-SMA. MPA therapy prevented these changes. Immunoblot analyses examining Nox-2 signaling (phospho-NF-κB), redox signaling (phospho-smad2) and fibrosis (α-SMA and fibronectin) demonstrated that MPA treatment prevented the upregulation of Nox-2, inhibited p-NF-κB and p-smad2 and downregulated α-SMA and fibronectin levels. Last, we examined Nox-2 signaling in vitro and confirmed that MPA inhibited phospho-NF-κB, Nox-2, phospho-smad2 and α-SMA during TGF-β1-induced EMT of NRK52E cells while reducing Nox-2, vimentin and Fibronectin mRNA levels.
Conclusions
MPA may downregulate Nox-2 activation and EMT via the NF-κB pathway in tubular epithelial cells suggesting a novel role for this drug independent of its immunosuppressive properties.
Introduction
Chronic interstitial fibrosis and tubular atrophy (IFTA) is a progressive injury that limits the long-term survival of kidney transplants. It results from both immunological and non-immunological insults. A better understanding of the cellular and molecular mechanisms that regulate IFTA may result in the development of treatment approaches that will enhance allograft survival.
Oxidative stress is a common injury pathway activated by the immune response (1). We believe that Nox-2 plays an important role in the regulation of allograft fibrosis. Nox-2 is the classical phagocytic NADPH oxidase enzyme responsible for the generation of superoxide anion and hydrogen peroxide and the “oxidative burst” (2). However, emerging evidence suggests that Nox-2 is also induced in nonphagocytic cells including neurons, hepatocytes, fibroblasts, cardiomyocytes and endothelial cells, where it plays an important role in cell signaling (2, 3). In support of these data, we recently demonstrated that Nox-2 is involved in the pathogenesis of tubulointerstitial fibrosis in the kidney allograft (4).
We hypothesized that inhibition of reactive oxygen species (ROS) generation via Nox-2 delays allograft fibrosis. We tested this hypothesis by examining the effects of mycophenolic acid (MPA) on Nox-2 activation and fibrosis in vitro and in the rat model of kidney allograft fibrosis. MPA (Cellcept or Myfortic) is a key antimetabolite drug used as part of the maintenance immunosuppressive regimens in the vast majority of kidney transplant recipients (5). It reduces the incidence of chronic allograft nephropathy independently of its effect on acute rejection (6). In addition, in vitro studies suggest that MPA inhibits epithelial-to-mesenchymal transition (EMT) in renal tubular epithelial cells (7). However, the molecular mechanisms that regulate the effects of MPA are largely unknown. We hypothesized that MPA inhibits Nox-2-induced fibrogenesis. We evaluated Nox-2 expression in kidney transplant recipients receiving standard immunosuppression with calcineurin inhibitors, MPA, prednisone and undergoing IFTA. Next, we assessed the effects of MPA on Nox-2 expression and fibrogenesis in vitro using NRK52E proximal tubular epithelial cells and the Fisher 344 to Lewis rat model of chronic kidney allograft fibrosis.
Results
Allografts with IFTA and treated with calcineurin inhibitors, MPA and prednisone had greater expression of Nox-2 and α-SMA
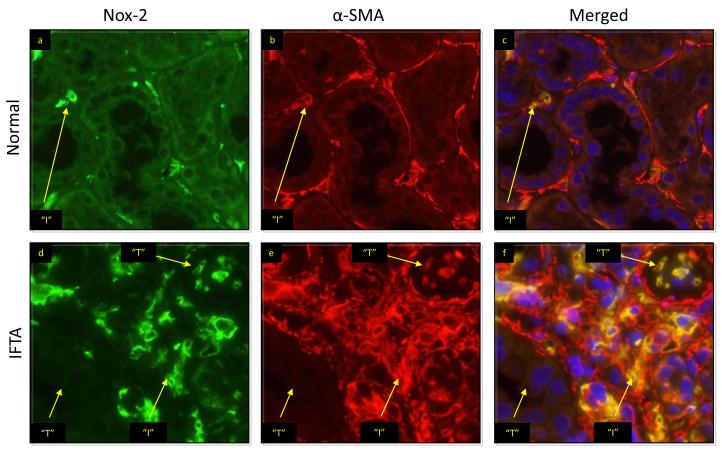
There were 6 patients in the study (Table 1). All were Caucasian, 5 were female and half had diabetes as the cause of kidney failure. At the time of biopsy, all patients were receiving prednisone, MPA (Cellcept) and calcineurin inhibitors. Most patients had moderate fibrosis (grade 2), moderate interstitial fibrosis (ci=1.5) and tubular atrophy (ct=2). Median serum creatinine and eGFR levels were 2.3 mg/dL and 29.5 ml/min/1.73m2 respectively. Biopsies from these allografts showed significantly increased Nox-2 and α-SMA staining in the tubulointerstitium compared to normal controls (Figure 1). The mean Nox-2 tubular staining score was 2.1±0.5 compared to 0.2±0.4, p<0.001. Importantly, Nox-2 and α-SMA costained in the tubules suggestive of EMT (Figure 1-f).
Table 1.
Characteristics of patients with IFTANOS and treated with MPA, calcineurin inhibitors and steroids
| Number of patients | 6 |
| Female | 5 |
| Caucasian | 6 |
| Age (years) | 49 (28-61) |
| Transplant to Biopsy Interval (years) | 9 (2-13) |
| Cause of ESRD (DM/GD/ADPKD) | 3/2/1 |
| Transplant Type (DD/LD/KP) | 1/3/2 |
| Banff Grade | 2 (1-3) |
| ci score | 1.5 (1-3) |
| ct score | 2 (1-3) |
| cv score | 1.5 (0-2) |
| cg score | 1 (0-2) |
| Serum creatinine (mg/dL) | 2.3 (1.4-3.2) |
| eGFR (ml/min/1.73m2) | 29.5 (17-41) |
Data provided as median and range
Figure 1.
Representative kidney sections from normal control and a transplanted human allograft with IFTA. Nox-2 was stained in green and α-SMA was stained in red using fluorescent probes with anti-Nox-2 and anti-α-SMA monoclonal antibodies. In normal kidneys Nox-2 was found in the interstitium (“I”) but not the tubules (“T”). Merged images (c and f) demonstrated that Nox-2 expression was increased in kidney allografts with IFTA and that Nox-2 was expressed not only in the interstitium but also in renal tubules.
MPA reduced Nox-2 in rat kidney allografts undergoing fibrogenesis
To confirm these findings in the Fisher 344 to Lewis rat model of chronic IFTA we first performed dose-response studies. These analyses evaluated the most effective dose and mode of delivery of MPA. MPA (Cellcept, Roche) was thus delivered to 4 different groups (by gavage 20 mg/kg/24h or 40 mg/kg/24h, in drinking water at 40 mg/kg/24h or in diet at 40 mg/kg/24h, n=5 in each group) and results compared to no treatment. After three weeks, animals receiving MPA 40 mg/kg/24h in drinking water or by oral gavage had similar and significantly higher MPA levels (2±0.2 mcg/mL and 2.1±0.3 mcg/mL, p<0.05) compared to other groups (0.8±0.2, 0.2±0.3 and 0 mcg/mL in diet 40 mg/kg/24h, gavage 20 mg/kg/24h and no treatment respectively). Since the study had to be carried out for 6 months and the risk of injury was significantly greater with daily oral gavage, prospective animals were treated with 40 mg/kg/24h MPA in their drinking water. We therefore examined Nox-2 expression and outcomes in syngeneic transplants (Lewis to Lewis), allogeneic transplants (Fisher to Lewis) and allogeneic transplants with daily MPA (40 mg/kg/24 in drinking water) for 6 months. There were 14 animals in each group. At the end of the study, Scr levels were significantly lower in the syngeneic (0.5±0.1 mg/dL) and allogeneic transplants treated with MPA (0.7 ± 0.1 mg/dL) compared to no treatment (2.2 ± 0.2 mg/dL, p<0.05).
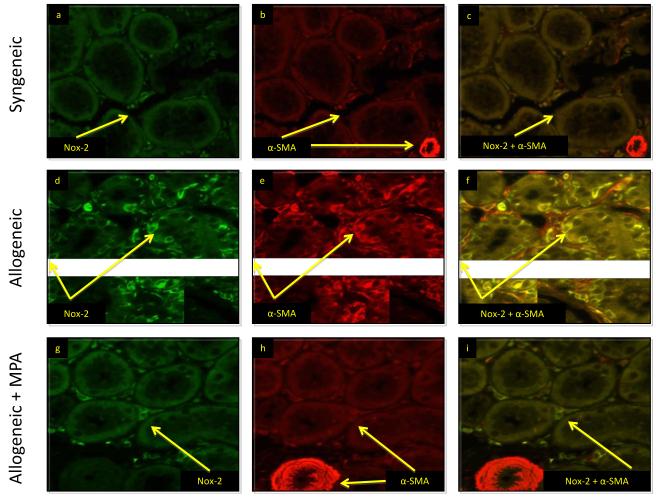
We then posed the question whether Nox-2 was associated with EMT in this transplant model. We performed immunofluorescent studies for Nox-2 and α-SMA and showed that similar to control human kidneys, Nox-2 was confined to the interstitium in syngeneic transplants and there was no costaining of Nox-2 and α-SMA in the tubules (Figure 2 a-c). However, as in patients with IFTA, rat allografts showed greater Nox-2 and α-SMA staining in the tubulointerstitium suggesting a role for Nox-2 in EMT (Figure 2 d-f). Treatment with MPA downregulated tubulointerstial staining of Nox-2 and α-SMA (Figure 2 g-i). Nox-2 tubular staining scores were similar in syngeneic (0.09±0.3) and MPA treated animals (0.14±0.35) and significantly lower compared to untreated rats (2.74±0.4, p < 0.001).
Figure 2.
MPA inhibited tubulointerstitial Nox-2 and α-SMA staining in rat kidney allografts. Nox-2 (green) and α-SMA (red) in all panels. Merged images shown in panels c, f, i. These studies showed that Nox-2 was confined to the interstitium in syngeneic transplants. There was no costaining of Nox-2 and α-SMA in the tubules (a-c). Nox-2 and α-SMA increased in the tubulointerstitium of untreated rat allografts with IFTA (d-f). Treatment with MPA downregulated tubulointerstial staining of Nox-2 and α-SMA (g-i).
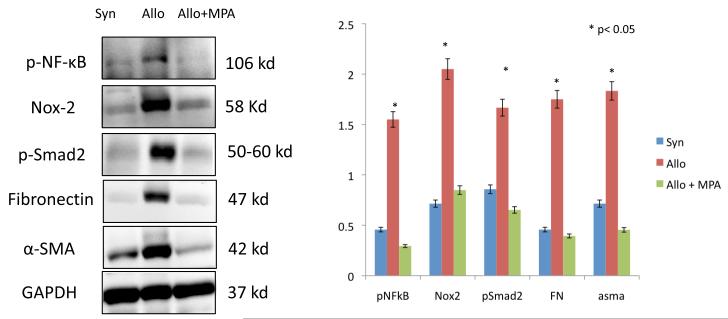
To confirm and to quantitate the above findings, we performed immunoblot analyses examining Nox-2 signaling and biomarkers of fibrogenesis in whole kidney samples. These results are presented in Figure 3. We assessed active (phosphorylated) NF-κB levels since this transcription factor regulates the expression of Nox-2 in human phagocytes (13). We also examined active (phosphorylated) Smad-2 since this redox sensitive transcription factor is involved in the pathogenesis of fibrosis and EMT (14). These studies demonstrated that MPA treatment prevented the upregulation of Nox-2 and fibrosis (α-SMA and fibronectin levels) in kidney allografts and indicated that the NF-κB and smad pathways may be involved in Nox-2 signaling (Figure 3).
Figure 3.
MPA (40 mg/kg/d × 6 months) prevented EMT in rat kidney allografts. Immunoblot analyses for syngeneic (S), allogeneic (A) and allogeneic transplants treated with MPA (Allo + MPA) demonstrated that MPA therapy was associated with significant downregulation of Nox-2 and fibrogenesis as evidenced by decreased amounts of phospho-NF-κB, phospho-smad2, fibronectin and α-SMA. All experiments were performed three times and the bar graph represents fold changes (arbitrary units using the NIH image J software) compared to GAPDH internal control from three independent experiments.
MPA inhibited Nox-2 and EMT in renal proximal tubular epithelial cells
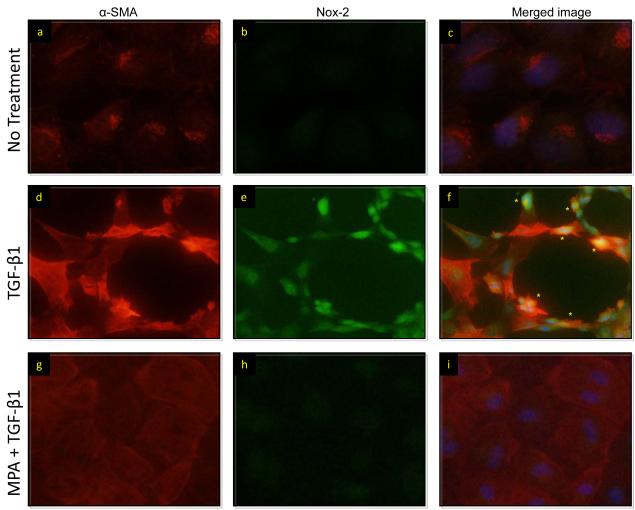
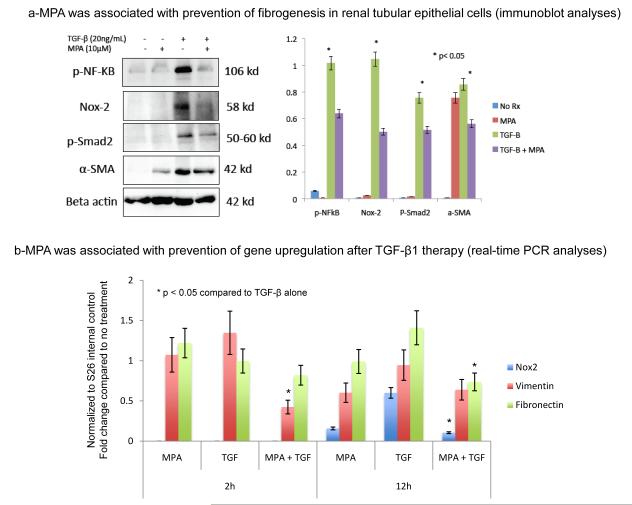
The effects of MPA on Nox-2 and fibrosis in whole kidney samples may be related to the drug’s immunosuppressive properties. To address this question and to determine the pure effects of MPA on EMT we examined Nox-2 signaling in normal rat tubular epithelial cells in vitro (Figures 4 and 5). These studies were critical as they eliminated the potential immunomodulatory effects of the drug on phagocytes. Normal rat proximal tubular epithelial cells (NRK52E) were cultured for two days in the presence of TGF-β1 (20 ng/mL) to induce EMT. Some were pretreated (30 minutes) with MPA (10 μM) for two days. Immunofluorescent staining showed that MPA treatment prevented EMT and down-regulated TGF-β induced expression of Nox2 and α-SMA (Figure 4 g-i). We confirmed these studies using western blot analyses and demonstrated that MPA treatment inhibited phospho-NF-κB, Nox-2, phospho-smad2 and α-SMA (Figure 5a). To determine if MPA therapy was associated with transcriptional regulation of Nox-2, we performed RT-PCR analyses for Nox-2, vimentin and fibronectin at baseline, 2 and 12 h after treatment and demonstrated that the addition of MPA to TGF-β1 was associated with downregulation of vimentin mRNA at 2h and Nox-2 and fibronectin mRNA at 12h (Figure 5-b). Nox-2 mRNA levels were undetectable at 2h.
Figure 4.
MPA was associated with the prevention of EMT in vitro. NRK52E cells were treated with TGF-β1 (20 ng/mL), MPA (10 μM) or TGF-β1 + MPA for 48 hours. Nox-2 and α-SMA were stained in green and red in all panels and merged images are shown in panels c, f and i. TGF-β1 (d,e,f) induced Nox-2 and α-SMA costaining (*, yellow staining) in cells with pronounced features of EMT. MPA therapy was associated with prevention of typical EMT features and Nox-2 and α-SMA costaining.
Figure 5.
(a) MPA prevented TGF-β1-induced expression of Nox-2 and fibrogenesis. NRK52E cells were treated with TGF-β1 to induce EMT. Some cells were pretreated with MPA (10μM) for 30 minutes prior to TGF-β1 (20 ng/mL) addition or treated with MPA alone. We harvested the cells after 48 hours, extracted proteins and performed immunoblot analyses for phospho-NF-κB, Nox-2, phospho-smad2, α-SMA and β-actin internal control protein. The bar graph on the right shows the average of three independent studies and reflects the fold changes (arbitrary units using the NIH image J software) of protein expression compared to β-actin the internal control. (b) Bar graph representing real time PCR data from NRK52E cells treated with MPA, TGF-β1 or MPA plus TGF-β1 for 2 to 12h. Data were normalized to S26 internal control and presented as fold change compared to no treatment. All studies were repeated three times and the bar graphs represent the average fold change compared to no treatment.
Discussion
We examined the effects of MPA on Nox-2 and EMT in renal tubular epithelial cells. First we evaluated Nox-2 and EMT in human and rat kidney allografts undergoing tubulointerstitial fibrosis. Next, we assessed fibrogenesis and its related signaling pathways in vitro, using rat tubular epithelial cells. We demonstrate that Nox-2 was upregulated in tubular epithelial cells undergoing EMT in vivo and in vitro. Furthermore, we suggest that MPA has pleiotropic antioxidant and antifibrotic effects in addition to its traditional immunosuppressive properties. More specifically, MPA was associated with inhibition of Nox-2 and fibrogenesis in vivo and in vitro. Since NF-κB regulates Nox-2 expression in human phagocytes (13), our studies suggest that in addition to its immunosuppressive properties, MPA may delay allograft fibrosis by inhibiting TGF-β1-induced activation of Nox-2 via the NF-κB pathway.
As expected, MPA was not sufficient to fully prevent renal allograft fibrosis in human kidney allografts. However, our studies are in agreement with previous observations suggesting that MPA inhibits EMT and fibroblast proliferation (7, 15). Copeland et al studied the effects of standard immunosuppressive drugs EMT of renal tubular epithelial cells by examining cell morphology and α-SMA expression. They demonstrated that Rapamycin and Mycophenolate Mofetil (MMF) prevented EMT compared to Cyclosporine A, Azathioprine and methylprednisolone and concluded that these drugs have a greater inhibitory effect on EMT in vitro than older immunosuppressive agents and may result in less fibrosis and a better long-term allograft survival (7). Since fibroblasts rely on the de novo synthesis of guanosine nucleotides, Morath et al examined the effects of MPA on fibroblast proliferation and migration (15). They showed a downregulation of the cytoskeletal proteins vinculin, actin and tubulin in fibroblasts exposed to pharmacological doses of MPA using microarray technology, real-time PCR and Western blot analyses. The reduction in RNA and protein content was accompanied by a substantial rearrangement of the cytoskeleton in MPA-treated fibroblasts. The authors concluded that MPA-induced cytoskeletal dysfunction may have beneficial effects on renal allografts with respect to scarring (15). Our findings complement these observations and provide novel molecular mechanisms that may regulate tubular epithelial cell activation and EMT.
We believe that oxidative stress and more specifically the Nox-2 enzyme is a mediator of fibrogenesis in kidney allografts (1, 4). Nox-2 is the prototype NADPH oxidase, typically involved in immune responses related to phagocytes including the “oxidative burst” (2, 3). Nox-2 is constitutively associated with the transmembrane Nox stabilizing protein p22phox and recruitment of the activating cytosolic components p47phox, p67phox, and p40phox are needed for function. Nox-2 has been considered to have a limited phagocyte-specific expression and role. However, emerging evidence suggests that Nox-2 can be induced in nonphagocytic cells including neurons, hepatocytes, fibroblasts, cardiomyocytes and endothelial cells and play an important role in cell signaling (2, 3). In support of these data, we demonstrate that Nox-2 is inducible in renal tubular epithelial cells and that this molecule is involved in the pathogenesis of EMT. Since short-term inhibition of Nox-2 with apocynin and diphenyleneiodonium prevented fibrogenesis in rat kidney allografts (4) it is possible that specific Nox-2 inhibition strategies may play a role in the prevention of chronic kidney allograft fibrosis. Our study is limited since we did not measure MPA levels in patients who underwent indication biopsies to determine MPA exposure. In addition, further studies are needed to determine the molecular mechanisms that modulate MPA-induced regulation of NF-κB, Nox-2 and smad2 in renal tubular epithelial cells. These findings may have important implications for the use of MPA in patients with native and transplant kidney disease.
Material and Methods
Patients
Adult kidney transplant recipients undergoing indication biopsies for a rise in serum creatinine between November 2006 and January 2007 were invited to participate in our study. Patients were selected from a previously reported study (4) and were included if (1) they were on MPA, calcineurin inhibitors (cyclosporine A or tacrolimus) and prednisone at the time of biopsy and (2) if histopathological findings were consistent with interstitial fibrosis and tubular atrophy not otherwise specified (IFTANOS) (8, 9). Chronic injury was assessed and graded according to the modified Banff 1997 classification scheme and the proportion of cortical area affected: grade 0 < 6% of the cortical area affected; grade 1: 6-25%; grade 2: 26-50%; and grade 3 > 50% affected (8, 9). Interstitial, tubular, vascular and glomerular chronic injury scores were reported as ct, ci, cv and cg, respectively. Control human kidney sections were prepared from tumor-free areas following resection surgery for malignancy. Pathologists blinded to the study read the biopsies after H&E, PAS, PAMM, Trichrome and immunohistochemical studies. Leftover tissue was then examined for Nox-2 and α-smooth muscle actin (α-SMA, biomarker for myofibroblasts) using standard immunostaining protocols. The Human Subjects Committee and the Institutional Review Board at the University of Wisconsin Madison School of Medicine and Public Health approved this study.
Animals
Adult (9-11 week-old) male Fisher 344 and Lewis rats (250-300 g) were purchased from Harlan Sprague-Dawley (Madison, WI). Animals were housed in the animal care facility at the William Middleton VA Hospital in Madison, WI and the procedures were performed in accordance with the Animal Care Policies at the VA Hospital and the UW. Kidney transplants were performed as previously described (4, 10). Briefly, the left kidney of the Fisher or Lewis donor rat was isolated, perfused (with 10 ml cold University of Wisconsin preservation solution), excised and transplanted orthotopically into weight-matched Lewis recipients following the excision of the native kidney. Total cold ischemic time was less than 30 minutes. End-to-end anastomosis of donor and recipient renal artery, vein, and ureter was performed with 10-0 prolene sutures. The contralateral native kidney was excised 10 days later and the graft was checked macroscopically to exclude hydronephrosis. These studies were performed in two steps. Because our goal was to examine the long-term effects of MPA on Nox-2 and fibrogenesis we first performed dose-response studies to evaluate the most effective dose and mode of delivery of Cellcept (Roche, MPA) to allograft recipients. We thus defined five groups (n=5 in each group) (a) No MPA (b) MPA gavage 20 mg/kg/24h (c) MPA gavage 40 mg/kg/24h (d) MPA mixed with diet at 40 mg/kg/24h and (e) MPA in drinking water at 40 mg/kg24h. All animals received CsA 1.5 mg/kg/d for 10 days to prevent allograft rejection due to acute rejection. After three weeks, blood MPA and serum creatinine were determined. All animals had their blood drawn at the same time of the day. The best MPA delivery method was defined as a process that resulted in therapeutic blood MPA levels (1.6 to 2.6 mcg/mL) (11), low serum creatinine levels and an acceptable safety profile for animals to be treated over a course of 6 months. Animals treated by this method were euthanized by exsanguination under general anesthesia.
Cell culture experiments
Normal rat kidney proximal epithelial cells (NRK52E) were obtained from the American Type Culture Collection (ATCC, Rockwell, MD) and maintained at 37 °C in a humidified atmosphere containing 5% CO2. Cells were seeded at 2.5×105 cells per well into six-well culture plates in DMEM (high glucose) containing 5% heat inactivated FBS, 44mM NaHCO3, 5000 IU Penicillin and 5000μg/ml Streptomycin (Cellgro, VA). At 80% confluency, media was changed to serum free DMEM supplemented with 0.1% BSA for 12 hours to arrest growth and synchronize cell activity. Cells were then cultured for two days in the presence of TGF-β1 (20 ng/mL) to induce EMT. Some were pretreated (30 minutes) with MPA (10 μM) in addition to TGF-β1 or treated with MPA (10μM) alone. All studies were performed repeated three times.
Antibodies
Primary antibodies against α-SMA (Clone 1A4, A2547, Sigma), GAPDH (ab8245, 6C5, Abcam, Cambridge, MA), E-cadherin (Clone 36, 610181, BD Pharmigen, San Diego, CA), Fibronectin (ab6328, Abcam, Cambridge, MA), Nox-2 (611414, BD Pharmigen), phospho-smad2 (05-953, Upstate, Billerica, MA) and phospho-NFκB (ab28849, Abcam, Cambridge, MA), were used for immunoblot, immunofluorescence and immunohistochemical analyses.
RNA Extraction, Purification, and Real-Time PCR (RT-PCR) Analyses
Total RNA extraction was performed as previously described (10,12). Briefly, RNA was extracted from cell lysates using the TRIzol protocol (GIBCO BRL, Life Technologies, Rockville, MD). We utilized rat Nox-2 (Unigene ID: Rn.98491, exon boundary 3-4), Vimentin (Unigene ID: Rn.2710, exon boundary 2–3), Fibronectin (Unigene ID: Rn.1604, exon boundary 23–24) and S26 internal control (Refseq: XM 001066146.1 exon boundary 1–1) primers. Mean Ct, SD, and ΔCt (compared with S26 internal control) were evaluated at 2h and 12h post treatment. Experiments were performed in triplicate and data were presented as bar graphs representing fold changes compared to no treatment.
Immunoblotting
Western blotting was performed on 20 μg protein lysates obtained from whole kidney tissue or cell lysates as described earlier (10, 12). Membranes were incubated the next day with antibodies against Nox-2 (1:250), α-SMA (1:2000), Fibronectin (1:400), phospho-smad2 (1:5000) and phospho-NF-κB (1:250), GAPDH (1:5000), β-actin (1:7500). Binding of primary antibodies was followed by incubation for 1 hour at room temperature with a secondary HRP-conjugated IgG in 10% nonfat milk. Signals were visualized by enhanced chemiluminescence signals. Data was normalized to GAPDH or β-actin internal control. Results were displayed as representative gels of three sets of independent experiments (three separate rats from each treatment group or three separate in vitro studies) and bar graphs displaying mean densitometric units of the three experiments using the NIH Image J software, downloaded from http://rsb.info.nih.gov/ij.
Immunofluorescence in vivo (human and rat)
Formalin fixed paraffin embedded sections were cut into 4-5 micron sections. Slides were deparaffinized and rehydrated from xylene through a graded ethanol series to dH2O. Antigen retrieval was performed at 25 psi for 2 min. Nonspecific background staining was blocked using Sniper (Biocare Medical). Double-staining studies were performed with Nox-2 (1:15) and α-SMA (1:50000) antibodies. Coverslips were secured using the ProLong Gold Antifade reagent with DAPI. All slides were viewed on a Nikon Eclipse E600 microscope with an Olympus DP70 camera. Images were analyzed using the DP70 imaging software. Quantification of the percentage of tubular cells expressing Nox-2 was performed according to a semi-quantitative scoring system: 0 (no staining), 1 (10-25% of tubules), 2 (25-50% of tubules), 3 (greater than 50% of tubules) per 40X power field. Ten fields per slide were scored and data were presented as mean ± SD.
Immunofluorescence in vitro
NRK cells were cultured on 0.1% gelatin coated coverslips in a 24-well plate. Cells were fixed with 4% paraformaldehyde for 10 min at room temperature, washed, and permeabilized with 1% Triton X-100 for 3 min. Anti-Nox-2 (1:15) staining was done overnight at 4°C followed by an anti-mouse Alexa 488 secondary antibody (1:1,000, Invitrogen, Carlsbad, CA). Coverslips were then incubated with the second primary antibody (α-SMA, 1:50000) at room temperature for 1 hour followed by an anti-mouse Alexa 594 secondary antibody (1:1000, Invitrogen, Carlsbad, CA). ProLong Gold Antifade reagent with DAPI (Invitrogen) was used to mount the coverslips to slides.
Statistical Analysis
Students’ t test and the non-parametric Mann-Whitney rank sum test (Sigma Stat Software, Jandel Scientific) were utilized when appropriate to compare differences in MPA, Scr and protein expression between groups. P values ≤ 0.05 were considered significant.
Acknowledgements
AD was supported by grants from the NIH grants DK 067981-05, DK 070243-04, American Society of Nephrology-American Society of Transplantation John Merrill Award 2008-2010, Roche investigator initiated award 2004-7.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Djamali A. Oxidative Stress as a Common Pathway to Chronic Tubulointerstitial Injury in Kidney Allografts. Am J Physiol Renal Physiol. 2007;293(2):F445. doi: 10.1152/ajprenal.00037.2007. [DOI] [PubMed] [Google Scholar]
- 2.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 3.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid.Redox.Signal. 2006;8(9-10):1597. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 4.Djamali A, Vidyasagar A, Adulla M, Hullett D, Reese S. Nox-2 Is a Modulator of Fibrogenesis in Kidney Allografts. Am J Transplant. 2009;9(1):74. doi: 10.1111/j.1600-6143.2008.02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samaniego M, Becker BN, Djamali A. Drug insight: maintenance immunosuppression in kidney transplant recipients. Nat.Clin.Pract.Nephrol. 2006;2(12):688. doi: 10.1038/ncpneph0343. [DOI] [PubMed] [Google Scholar]
- 6.Knight SR, Russell NK, Barcena L, Morris PJ. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation. 2009;87(6):785. doi: 10.1097/TP.0b013e3181952623. [DOI] [PubMed] [Google Scholar]
- 7.Copeland JW, Beaumont BW, Merrilees MJ, Pilmore HL. Epithelial-to-mesenchymal transition of human proximal tubular epithelial cells: effects of rapamycin, mycophenolate, cyclosporin, azathioprine, and methylprednisolone. Transplantation. 2007;83(6):809. doi: 10.1097/01.tp.0000255680.71816.aa. [DOI] [PubMed] [Google Scholar]
- 8.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 9.Solez K, Colvin RB, Racusen LC, et al. Banff ’05 Meeting Report: Differential Diagnosis of Chronic Allograft Injury and Elimination of Chronic Allograft Nephropathy (‘CAN’) Am J Transplant. 2007;7(3):518. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 10.Djamali A, Reese S, Yracheta J, Oberley T, Hullett D, Becker B. Epithelial-to-Mesenchymal Transition and Oxidative Stress in Chronic Allograft Nephropathy. Am.J.Transplant. 2005;5(3):500. doi: 10.1111/j.1600-6143.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 11.Borrows R, Chusney G, Loucaidou M, et al. Mycophenolic acid 12-h trough level monitoring in renal transplantation: association with acute rejection and toxicity. Am J Transplant. 2006;6(1):121. doi: 10.1111/j.1600-6143.2005.01151.x. [DOI] [PubMed] [Google Scholar]
- 12.Djamali A, Reese S, Oberley T, Hullett D, Becker B. Heat shock protein 27 in chronic allograft nephropathy: a local stress response. Transplantation. 2005;79(12):1645. doi: 10.1097/01.tp.0000164319.83159.a7. [DOI] [PubMed] [Google Scholar]
- 13.Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281(9):5657. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- 14.Rhyu DY, Yang Y, Ha H, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc.Nephrol. 2005;16(3):667. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 15.Morath C, Reuter H, Simon V, et al. Effects of mycophenolic acid on human fibroblast proliferation, migration and adhesion in vitro and in vivo. Am J Transplant. 2008;8(9):1786. doi: 10.1111/j.1600-6143.2008.02322.x. [DOI] [PubMed] [Google Scholar]