Abstract
The quest to understand why we age has given rise to numerous lines of investigation that have gradually converged to include metabolic control by mitochondrial activity as a major player. That is, the ideal balance between nutrient uptake, its transduction into usable energy, and the mitigation of damaging byproducts can be regulated by mitochondrial respiration and output (ATP, reactive oxygen species (ROS), and heat). Mitochondrial inefficiency through proton leak, which uncouples substrate oxidation from ADP phosphorylation, can comprise as much as 30% of the basal metabolic rate. This uncoupling is hypothesized to protect cells from conditions that favor ROS production. Uncoupling can also occur through pharmacological induction of proton leak and activity of the uncoupling proteins. Mitochondrial uncoupling is implicated in lifespan extension through its effects on metabolic rate and ROS production. However, evidence to date does not suggest a consistent role for uncoupling in lifespan. The purpose of this review is to discuss recent work examining how mitochondrial uncoupling impacts lifespan.
Keywords: mitochondria, lifespan, uncoupling, UCP, ROS
1. Metabolism, ROS, and aging
Early attempts to understand senescence and aging framed lifespan in terms of metabolic rate, proposing that an organism has a finite metabolic capacity (i.e., number of chemical transformations), and once it is reached, mortality occurs (Pearl, 1928). This “rate of living” hypothesis predicts that increased metabolic rate per gram tissue correlates with shorter lifespan and vice versa. However, multiple examples of size-matched and metabolic rate-matched animals with significantly different lifespans, both ectothermic and endothermic, have contradicted rate-of-living as the sole determinant of aging (e.g., rats/pigeons (Barja, 1998), snakes (Robert et al., 2007), bats/mice (Jürgens and Prothero, 1987), deer mice/lab mice (Ungvari et al., 2008), naked mole rats (O'Connor et al., 2002).
The free radical theory of aging first arose as a mechanistic explanation for the rate of living model, by which reactive oxygen species generated during respiration lead directly to aging (Harman, 1956). Targets of ROS that show accumulated damage or dysfunction over time include macromolecules, e.g., nuclear and mitochondrial DNA, lipids, and proteins, which in turn affect mechanisms such as apoptosis, protein turnover, and multiple mitochondrial functions (rev. in Kowaltowski et al., 2009). As discussed below, numerous studies have shown that ROS production and oxidative damage can directly modulate lifespan.
The free radical theory of aging also separates aging from metabolic rate by proposing that it is the products of metabolism, not metabolic activity, that causes aging. That is, if ROS production and consequent oxidative damage cause aging, then metabolic rate should be independent of lifespan as long as intracellular ROS can be mitigated. Therefore, organisms with long lifespans should display lower ROS levels, through increased antioxidant activity, decreased ROS production, or both, or have increased resistance to ROS damage (e.g.,Lambert et al., 2007).
Recently, the importance of metabolic control has also become a major focus of aging research, beginning with the demonstration that single-gene disruptions of the insulin/IGF pathways in Caenorhabditis elegans could drastically affect lifespan (Kenyon et al., 1993). However, although deletion of Daf-2 (part of the insulin signaling pathway) confers resistance to oxidative stress, concurrent with an increase in lifespan, recent work has shown that oxidative stress in a Daf2-/- worm can be attenuated without any change to lifespan, arguing against a strictly causal link (Honda et al., 2008). This example and others have renewed interest in the rate-of living model, defined not simply by basal metabolic rate (measured by oxygen consumption) but by the homeostasis of energy demand and expenditure during periods of activity and rest, as regulated by various signals, including nutrients, hormones, and ROS themselves. This framework imposes an additional facet onto the rate-of-living and mitochondrial free-radical theories of aging, which is that metabolism must respond effectively and correctly to fluctuations in nutrient supply and demand. Metabolic rate, ROS production, and metabolic homeostasis are interconnected factors that are all likely to contribute to the accumulation of damage and dysfunction, suggesting that a synthesis of these models may be required to understand the aging process.
2. ROS production during mitochondrial respiration
During oxidative phosphorylation, metabolites are oxidized, donating reducing equivalents to the carriers NAD+ and ubiquinone (Q) to generate NADH and QH2. The electrons then enter the electron transport chain complexes in the mitochondrial inner membrane and pass down a decreasing energy potential gradient. The resulting energy release drives proton pumping across the mitochondrial inner membrane, from the matrix to the intermembrane space, by complexes I, III, and IV. The electrochemical gradient, or proton motive force (Δp), that is established by proton pumping then drives protons back into the mitochondrial matrix through the ATP synthase to generate ATP. Electrons are ultimately collected by complex IV, cytochrome c oxidase, and then donated to molecular oxygen in the coordinated reaction 4H+ + 4e- + O2 → 2H2O. Electrons can also escape from the electron transport chain at other sites, singly reducing O2 to form superoxide, O2• (HO2•/O2•-). Superoxide itself is relatively unreactive; however, it can directly damage proteins containing Fe-S centers, which includes Krebs cycle and electron transport chain components, and lead to the formation of highly reactive derivatives, such as HO•, that cause widespread oxidative damage.
ROS production in the electron transport chain occurs primarily at complexes I and III. While a precise understanding of in vivo ROS generation is incomplete, experiments with isolated mitochondria have established the major sites and their topology, and that a high Δp is conducive to high rates of ROS production from particular sites. Experiments with isolated enzymes have yielded additional information on the sites and mechanisms of ROS production.
Complex I can produce superoxide during both forward (NADH-oxidizing) and reverse (NAD+-reducing) electron transport (Lambert and Brand, 2004b, 2009). ROS generated by complex I are released to the matrix. During forward electron transport, two electrons from NADH are passed to a flavin mononucleotide (FMN) cofactor and, via a series of iron-sulfur centers, ultimately reduce Q (Walker, 1992). The principal ROS-generating site during forward electron transport when reduction of Q is prevented is the fully reduced FMNH2 (Kussmaul and Hirst, 2006). In isolated mitochondria, complex I also catalyses rotenone-sensitive superoxide production when Q is reduced and Δp is high. This state of reverse electron transport leads to an observable reduction of the NAD+ pool. The pathway of reverse electron flow can be interrogated with complex I inhibitors to determine where superoxide originates; superoxide production during reverse electron transport is sensitive to the complex I inhibitor rotenone, which inhibits the Q-binding site. This finding is consistent with superoxide production from the Q site during reverse electron transport. However, it remains contentious whether the dominant site of superoxide from complex I in energized mitochondria is the flavin, the Q binding site, or elsewhere (Andreyev et al., 2005; Lambert and Brand, 2009; Murphy, 2009).
ROS are also generated by complex III, which oxidizes QH2 to reduce cytochrome c (Zhang et al., 1998; Darrouzet et al., 2001). Complex III is capable of producing significant amounts of superoxide to both sides of the inner membrane in the presence of the Qi site inhibitor antimycin, but this rate is low in the absence of inhibitors. Additionally, α-ketoglutarate dehydrogenase, α-glycerophosphate dehydrogenase, and electron transferring flavoprotein quinone oxidoreductase may also contribute to mitochondrial ROS production under various conditions (Lambert and Brand, 2009).
3. Mitochondrial uncoupling modulates ROS production
Mitochondrial uncoupling is any process by which electron transport is not used to drive ATP synthesis or to do other useful work such as net ion translocation. Mechanisms that allow protons to bypass the ATP synthase while entering the matrix essentially “short-circuit” the coupling of substrate oxidation to ADP phosphorylation. Why might this be beneficial? One reason is that a high Δp promotes ROS production. In the “uncoupling to survive” hypothesis, the attenuation of ROS by partial uncoupling while maintaining sufficient ATP production is a potential mechanism for delaying cellular senescence (Papa and Skulachev, 1997; Brand, 2000). This model contrasts with the idea first put forth to explain first the rate-of-living model (and currently sometimes used to explain the effects of dietary restriction (DR)) that a low metabolic rate should confer a long lifespan. Instead, it argues that mild uncoupling will decrease ROS production and thereby extend lifespan even if it increases the “rate of living”. To test this, Speakman et al. (2004) separated mice into quartiles of metabolic intensity (kJ/g) and then investigated longevity. They found that individual mice in the highest quartile lived 36% longer than those in the lowest. They also displayed higher resting oxygen consumption rate and a higher rate of proton leak in skeletal muscle. In a different study, a tightly-coupled muscle group showed greater deterioration with age than a relatively uncoupled one (Amara et al., 2007). The following sections will discuss in greater detail the proposed role of uncoupling in direct ROS mitigation, and how this may extend lifespan.
4. Mitochondrial uncoupling lowers ROS by decreasing Δp
Mitochondrial ROS production can be highly sensitive to a decrease in Δp (Korshunov et al., 1997; Liu, 1997; Papa and Skulachev, 1997; Miwa and Brand, 2003). During forward electron transport, the dependence of ROS production on Δp is due to the flow of electrons through the respiratory chain. A high Δp slows electron transfer at specific sites, increasing the concentration of one-electron species which can react with O2 (Brand, 2000). For example, the membrane potential opposes the oxidation of the transient semiquinone radical at the Qo site of complex III. Lowering the membrane potential by mild uncoupling can therefore promote the forward flow of electrons through the respiratory chain, decreasing the lifetime and the steady-state concentration of the semiquinone, and thereby lower ROS production.
The highest ROS production by isolated mitochondria occurs during reverse electron transport. This process is critically dependent on Δp, which is needed to drive the electrons from Q thermodynamically uphill into complex I. ROS generation from complex I during reverse electron transport is therefore exquisitely sensitive to Δp. This is demonstrated in isolated mitochondria, where mild uncoupling during reverse electron transport dramatically lowers superoxide production by complex I. In addition, for undefined reasons linked to the proton-pumping mechanism of complex I, ROS generation is more dependent on changes in the transmembrane pH gradient (ΔpH) than on the membrane potential (Δψm) (Lambert and Brand, 2004a). As mild uncoupling decreases Δp by lowering both ψpH and Δψm, it is an effective means to lower mitochondrial superoxide production at the cost of efficient ATP synthesis (Brand et al., 2004).
Uncoupling also increases respiration, which decreases the local concentration of O2 and therefore decreases the rate of ROS production (Papa and Skulachev, 1997). Importantly, generation of ROS is not directly related to the rate of electron transfer, as inhibitors of the respiratory complexes can cause either increases or decreases in ROS production depending on how they modulate the redox state of Q and other ROS-producing sites in the electron transport chain (Brand, 2000).
5. Distribution and putative functions of the uncoupling proteins (UCPs)
The uncoupling proteins are members of the mitochondrial anion carrier family, which transport substrates across the mitochondrial inner membrane (Pedersen, 1993; Krauss et al., 2005). They share a basic tripartite structure with six membrane-spanning α-helices divided by short helical domains in the matrix and loops in the intermembrane space. This family includes the adenine nucleotide translocase (ANT), an ATP/ADP antiporter, and multiple other metabolite and ion transporters.
Proteins in this family, particularly the ANT, mediate the majority of basal proton leak, which accounts for up to 30% of respiratory O2 consumption at rest in a rat (Brand et al., 1994). In contrast to basal leak, which is unregulated, uncoupling proteins can catalyze inducible proton leak that is sensitive to inhibitors. UCP1 was the first identified uncoupling protein, and mediates non-shivering thermogenesis by brown adipose tissue (BAT) (Ricquier and Bouillaud, 2000; Nicholls, 2001). UCP1 is activated by free fatty acids and inhibited by purine nucleoside di- and triphosphates (Klingenberg, 2008). It is also present in thymus (Adams et al., 2008), though its function in this tissue is unclear.
Four other members of this family share the name “uncoupling protein”. (Golozoubova et al., 2001) . UCP2 (Fleury et al., 1997) and UCP3 (Boss et al., 1997b; Gong et al., 1997; Vidal-Puig et al., 1997), which are closest in amino acid identity to UCP1 (57% and 59%, respectively), both display uncoupling activity when activated (Echtay et al., 2002b; Echtay et al., 2003). Due to their relatively low abundance, the degree of uncoupling by UCP2 and UCP3 in cells is much lower than UCP1 (Harper et al., 2002). The “mild uncoupling” that they catalyze has been proposed to function as an evolutionarily conserved mechanism to attenuate ROS production, explaining the presence of uncoupling protein homologs in homeothermic organisms. Alternatively, UCP2 and UCP3 have been speculated to function as ROS transporters, substrate sensors or fatty acid transporters, (Himms-Hagen and Harper, 2001; Jaburek et al., 2004; Schrauwen and Hesselink, 2004; Bouillaud, 2009), but these hypotheses await experimental confirmation.
UCP2, identified through its homology to UCP1, is broadly expressed. Ucp2 mRNA can be found throughout the body, while detectable protein is restricted to specific tissues, including pancreatic α- and β-cells, kidney, liver, spleen, macrophages, and central nervous system (Fleury et al., 1997; Pecqueur, 2001). At its highest levels, UCP2 protein is 102-103-fold less abundant than UCP1 in brown adipose tissue (Pecqueur, 2001). Post-transcriptional regulation plays an important role in determining UCP2 protein levels. Translational control occurs through inhibition by an upstream, untranslated open reading frame; this inhibition is lifted by physiological concentrations of glutamine (Hurtaud et al., 2006; Hurtaud et al., 2007). Additionally, UCP2 is rapidly turned over with a half-life of about one hour (Rousset et al., 2007; Azzu et al., 2008b). Its activity in pancreatic β-cells dampens glucose-stimulated insulin secretion and ROS production, making it a well-studied target for diabetes intervention and treatment. Other proposed roles for UCP2 include the integration of glucose and fatty acid sensing (Brand and Esteves, 2005; Bouillaud, 2009).
Like UCP2, UCP3 mRNA expression has been reported in many tissues (Bosset al., 1997b; Vidal-Puig et al., 1997), but protein is prevalent mainly in skeletal muscle and brown adipose tissue, where its concentration is again very low compared to UCP1 in brown adipose tissue (Harper et al., 2001). UCP3 overexpression in mice confers increased glucose tolerance, with lowered glucose and insulin levels (Clapham et al., 2000). Unlike UCP1, UCP3 expression is upregulated during fasting by fatty acid stimulation of transcription (Boss et al., 1997a; Fleury et al., 1997) Other modulators of UCP3 expression include thyroid hormone, leptin, and β-adrenergic signaling (Gong et al., 1997; Barbe et al., 2001). UCP3 activity does not contribute to adaptive thermogenesis, as it cannot complement ablation of UCP1 (Golozoubova et al., 2001) and UCP3 gene expression is not upregulated in skeletal muscle by cold (Boss et al., 1997b). However, UCP3 does appear to partially mediate the pharmacological hyperthermia induced by 3,4-methylenedioxymethamphetamine (MDMA), or ecstasy (Mills et al., 2003). Uncoupling by UCP3 likely occurs indirectly through β-adrenergic activation, as MDMA does not stimulate uncoupling in isolated mitochondria (Rusyniak et al., 2005).
Competing interpretations of UCP3 activity are that it is not a direct uncoupler, but rather a fatty acid transporter, coordinating fatty acid and glucose catabolism (Schrauwen et al., 2006). However, export of fatty acid anion from the mitochondrial matrix was recently shown to be independent of UCP3 (Seifert et al., 2008). Surprisingly, both overexpression and ablation of UCP3 result in decreased insulin resistance, though it is unlikely to be through the same mechanism and may also be complicated by compensatory effects (Costford et al., 2006)
The neuronal “UCPs”, UCP4 and BMCP1/UCP5 were identified by sequence similarity to UCPs 1-3 (Sanchis et al., 1998; Mao et al., 1999), but they share less amino acid sequence identity with UCP1 (<30%) than do the dicarboxylate and 2-oxoglutarate carriers, and are not obviously members of the UCP family. Drosophila melanogaster UCP5 had uncoupling activity that responded to free fatty acids (laurate) and GDP when overexpressed in yeast (Fridell et al., 2004). However, DmUCP5 ablation in flies did not alter mitochondrial uncoupling (Sánchez-Blanco et al., 2006). In neuronal cell culture, UCP4 overexpression led to a surprising increase in ATP levels and resistance to ROS-generating agents, but whether it represents regulated mitochondrial uncoupling or occurs in vivo is unknown (Chu et al., 2009; Wei et al., 2009).
6. UCP-mediated life extension through decreased ROS
Multiple recent reviews discuss the putative biochemical and physiological functions of the uncoupling proteins (Brand and Esteves, 2005; Cannon et al., 2006; Echtay, 2007; Affourtit and Brand, 2008; Cioffi et al., 2009). Here, we discuss the application of these potential functions to how they may modulate lifespan.
Uncoupling by UCPs can be activated by superoxide (Echtay et al., 2002a; Considine et al., 2003; Talbot et al., 2004). The ROS-activated proton leak catalyzed by UCPs is inhibited by GDP and is absent in mitochondria which either essentially lack UCPs or in which endogenous UCP has been ablated. The lipid peroxidation product 4-hydroxy-2-nonenal (HNE) can also stimulate inhibitor-sensitive proton conductance through the UCPs and adenine nucleotide translocase (ANT) (Echtay et al., 2003). Furthermore, UCP activation by superoxide is blunted by the spin trap antioxidant phenyl-N-tert-butylnitrone, when targeted to the mitochondrial matrix (mitoPBN). Importantly, mitoPBN, which reacts with carbon-centered radicals but not with superoxide itself or lipid peroxidation products, does not affect HNE-induced uncoupling (Murphy et al., 2003). Taken together, these data suggest that superoxide may activate uncoupling proteins indirectly by attacking n-6 polyunsaturated acyl groups in the phospholipid membrane, initiating a chemical cascade to produce reactive alkenals (Brand and Esteves, 2005).
In this way, HNE may transduce high ROS production into a UCP-activating signal (Parola et al., 2001). HNE is most reactive with cysteine, lysine or histidine residues via Schiff bases and Michael adducts (Schaur, 2003). As alkenal-stimulated uncoupling requires either an acyl or carbonyl functional group and a double bond between the C2 and C3 carbons, HNE may induce uncoupling by covalent modification of UCPs and the ANT (Echtay et al., 2005). In fact, HNE has been demonstrated to covalently modify other mitochondrial proteins in vivo (Musatov et al., 2002; Isom et al., 2004). Furthermore, the retinoic acid analog TTNPB, which contains the same obligatory functional groups, has been shown to stimulate UCP-mediated proton leak in intact thymocytes (Krauss et al., 2002).
Based on the observations above and other experiments in the literature, a putative function for all UCPs, including the plant and avian UCPs, is to protect mitochondria from oxidative damage by lowering Δp through induced proton leak (Brand et al., 2004). A putative mechanism for UCP activation can be described: high Δp leads to high levels of matrix superoxide, which peroxidize lipids to form reactive species such as HNE, which then covalently modify UCP family members, activating mild uncoupling. This lowers Δp, attenuates ROS production and limits oxidative damage (Brand et al., 2004). Recently, it was demonstrated that high membrane potential is required for HNE-induced uncoupling and that the HNE-stimulated proton conductance through ANT is not readily reversible by the potent inhibitor carboxyatractylate (CAT) (Azzu et al., 2008a; Parker et al., 2008). This suggests that a sustained high Δp maybe required for mild uncoupling, perhaps forcing ANT (and possibly UCPs) into a conformation accessible to modification by alkenals.
Some genetic manipulation studies support this model, consistent with ROS as a determinant of aging and with a role for mild uncoupling in attenuating ROS. Fridell et al. (2005) showed that neuronal-specific UCP2 expression in Drosophila led to increased rates of oligomycin-insensitive (i.e., non-ATP generating) respiration and lower rates of ROS production in isolated mitochondria, a decrease in sensitivity of flies to the radical-generator paraquat, and a lifespan increase of 11-28%. The increased mitochondrial respiration was GDP-sensitive, although whether it was solely attributable to UCP2 is unclear. Humphrey et. al. (2009) extended this work by expressing human UCP3 in Drosophila either ubiquitously or targeted to adult neurons. They found that moderate levels of pan-neuronal expression (but not ubiquitous expression) conferred a slight increase in median lifespan in male flies. However, in contrast to Fridell et al. (2005), when UCP3 was expressed in neurons at sufficient levels to increase proton conductance, lifespan was significantly shortened. Restricting UCP3 expression to the median neurosecretory cells, a subpopulation of neurons that secrete several insulin-like peptides (DILPs), also shortened lifespan, and increased DILP2 protein levels. This suggested that the neurosecretory cells could be mediating the lifespan shortening effect of high UCP3 overexpression through a mechanism involving DILP2.
UCP ablation in mice also provides supporting evidence for a ROS-limiting function for UCP2 and UCP3. Pancreatic islets from UCP2-/- mice display increased ROS production relative to wild-type (Krauss, 2003), and skeletal muscle mitochondria from Ucp3-/- mice have greater oxidative damage than controls (Vidal-Puig et al., 2000; Brand et al., 2002). In Ucp2-/- mice, resistance to Toxoplasma gondii infection (Arsenijevic et al., 2000) and atherosclerosis (Blanc et al., 2003) appears to result from higher ROS levels than their wild-type littermates.
7. Lifespan and ROS production
Is ROS attenuation a credible means for uncoupling to mediate lifespan extension? Recent findings by Mcdonald, et al. describe no difference in lifespan in either UCP2- or UCP3-ablated mice relative to wild-type controls (Mcdonald et al., 2008). Moreover, low ROS production and long lifespan are separable. In Drosophila, Miwa et al. (2004) demonstrated increased lifespan by calorie restriction without a corresponding decrease in ROS production. Conversely, overexpressing the adenine nucleotide translocase (ANT) in flies resulted in greater mitochondrial uncoupling and significantly decreased ROS, but conferred no lifespan increase.
These data warrant careful interpretation before dismissing the idea entirely, however. First, mitochondrial uncoupling may control lifespan under metabolic conditions other than those examined. Second, the interplay of positive and negative effects of UCP ablation may result in no net change in lifespan, even though the bioenergetics are affected as predicted. Third, mitochondrial uncoupling may attenuate aging phenotypes without conferring a lifespan extension.
It is useful also to consider that attempts to mitigate ROS in other ways, namely though manipulation of antioxidant pathways, have not yielded a clear understanding of the role of ROS in lifespan determination. ROS production has been extensively linked to cell and tissue deterioration with age, including a recent investigation in skeletal muscle (Jang et al., 2009a). But is it a controlling factor in lifespan? In C. elegans, deletion of individual superoxide dismutase (SOD) isoforms did not affect mean lifespan (Yen et al., 2009). In mice, overexpression of the mitochondrial matrix MnSOD (Sod2) decreased ROS without a corresponding lifespan increase (Jang et al., 2009b). Conversely, previous work showed that mitochondrially-targeted catalase did significantly increase lifespan (Schriner et al., 2005), suggesting that H2O2 production may more directly related to lifespan determination than O2•- . Additionally, mitochondrially-targeted catalase reportedly mitigates multiple age-related pathologies, including cardiac tissue pathology (Treuting et al., 2008; Dai et al., 2009), hearing loss (Someya et al., 2009), and comorbidity factors including tumor burden (Treuting et al., 2008).
In a companion study to Jang, et al. 2009, Perez et al. (2009) collected lifespan data from multiple studies using mice either under- or overexpressing different antioxidant system components, including Mn-SOD, CuZn-SOD (Sod1), and glutathione peroxidases 1 and 4. Of these, only Sod1-/- mice had a significantly shorter lifespan. However, these mice also displayed levels of oxidative damage 4-5-fold higher than in aged wild type mice, and had a high incidence of hepatocellular carcinoma, suggesting that their lifespan deficit may not represent normal mechanisms of aging. Likewise, the Sod2-/- mouse, with its embryonic lethality, is unlikely to appropriately model aging.
Two major considerations emerge from these studies; in addition to the amount of ROS, both the species of ROS and the subcellular location of antioxidant activity, and not simply ROS levels, can strongly affect whether increased ROS production correlates with decreased lifespan.
8. Can uncoupling mimic dietary restriction as a means of lifespan extension?
Dietary restriction without malnutrition (DR) is a well-tested intervention that prolongs lifespan in almost all models used to test it (Masoro, 2009). Because mild uncoupling increases metabolic inefficiency, effectively “restricting” caloric conversion into biological work, it is sometimes proposed as a mechanism for DR-mediated lifespan extension. If true, then chemical uncouplers and biological uncoupling proteins may represent effective DR mimetics.
The protonophore 2,4-dinitrophenol (DNP), which enjoyed extensive use as an obesity treatment in the 1930s prior to its discontinuation due to toxic side effects (Colman, 2007), has been increasingly utilized as a putative DR mimetic. Promisingly, in flies and mice, DNP has recently been shown to increase lifespan, accompanied by decreases in oxidative damage (Padalko, 2005; Caldeira da Silva et al., 2008). In mice, DNP particularly affected respiration in the brain, though whether this occurred through increased concentration or enhanced activity is unclear (Caldeira da Silva et al., 2008). However, DNP equilibrates proton concentration and membrane potential across not only mitochondrial, but also endosome and plasma membranes, and therefore does more than simply uncouple mitochondria. Additionally, the small therapeutic range, sub-lethal side effects, high variability of optimal dose, and non-specific distribution of DNP in the body are important caveats when considering its application toward lifespan extension.
Does uncoupling effectively impose DR? In multiple studies, dietary restriction was found to upregulate UCP2 and UCP3 mRNA expression, often interpreted as support for mitochondrial uncoupling during DR (Bevilacqua et al., 2004; Mcdonald et al., 2008). In contrast, SIRT1, a histone deacetylase that is also upregulated during DR, has been implicated in the direct repression of UCP2 transcription (Bordone et al., 2006) by binding to the UCP2 promoter. SIRT1 also inhibits glucocorticoid-dependent UCP3 transcriptional activation (Amat et al., 2007). Moreover, mice with targeted SIRT1 overexpression in the pancreas display a ~2-fold reduction in pancreatic UCP2 protein levels, and enhanced insulin secretion, consistent with repression of Ucp2 (Moynihan et al., 2005). These data suggest that SIRT-dependent repression of UCP2 and UCP3 expression may occur during DR, arguing against a role for DR in inducing uncoupling through these proteins. However, fasting upregulates UCP2 and 3, making the degree of dietary restriction, among other factors, a potentially key determinant in whether UCPs are up- or down-regulated (Boss et al., 1997a; Cadenas et al., 1999)
In addition, transcriptional upregulation of uncoupling proteins, though relatively easy to measure, is a poor indicator of protein uncoupling activity. Multiple examples indicate that UCP mRNA and even protein can be increased without increased proton conductance. In one example, UCP2 and UCP3 transcript levels rose several-fold following 24-hour starvation, accompanied by a doubling in UCP3 protein levels, but with no change in the proton conductance in skeletal muscle mitochondria (Cadenas et al., 1999). UCP3 protein was also found to be increased after DR, concurrent with an unexpected decrease in proton leak in skeletal muscle and liver (Bevilacqua et al., 2004, 2005; Hagopian et al., 2005).
Studies that directly manipulate UCP levels and measure lifespan are limited. Recently, Andrews and Horvath (2009) showed that while Ucp2-/- mice showed significantly reduced survival relative to wild type, transgenic overexpression of UCP2 had no effect on lifespan. Because UCP2 overexpression partially rescued mice with a lethal Sod2-/- genotype, with small but significant reductions in ROS production, these results are consistent with a role for UCP2 in attenuating ROS production. That UCP2 can be responsible for lifespan extension, however, cannot be concluded. Other studies found no change in lifespan of Ucp1-/-, Ucp2-/-, Ucp3-/-, or transgenic UCP3-overexpressing mice relative to wild type (Kontani et al., 2005; Mcdonald et al., 2008), despite an increased incidence of obesity in the Ucp1-/- mice at thermoneutrality (Kontani et al., 2005; Feldmann et al., 2009). However, unlike UCP3, targeted expression of UCP1 to skeletal muscle confers increased median lifespan, reduced adiposity and increased energy expenditure (Li et al., 2000; Gateset al., 2007; Katterle et al., 2008). Moreover, Gates et al. (2007) observed reduced incidence of lymphoma and atherosclerosis in UCP1-overexpressing mice, suggesting that despite a lack of lifespan extension, an increase in “healthspan” could be attributed to UCP1-dependent uncoupling. The recent finding of active brown adipose tissue expressing UCP1 in adult humans may make UCP1 a relevant therapeutic target (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanenet al., 2009; Zingaretti et al., 2009).
Taken together, these results fail to show that uncoupling proteins can extend maximum lifespan under “optimal” (i.e., ad libitum, low-fat diet , thermoneutral, non gene-disrupted) conditions in experimental models, and are therefore unlikely to mediate DR-dependent lifespan extension. It is possible that uncoupling protein activity in conjunction with other factors induced by DR may lead to lifespan extension, but existing evidence for this is weak. Direct investigation of the interaction between uncoupling and DR, for example, by studying UCP-ablated animals undergoing DR, or determining whether manipulating uncoupling affects the signaling transduction pathways upregulated in DR, may shed light on this issue.
When considering potential mechanisms of lifespan extension, it is important to distinguish between factors that slow what we understand as the aging process and those that decrease the risk of mortality. Since it is hard to conclude that any human population is living under “optimal” conditions for maximizing lifespan, any interventions that extend lifespan could be the result of either, or both. Moreover, the controlled contexts (housing and ad libitum feeding conditions, low risk of infection, highly inbred strains) of most animal studies may not accurately reflect human populations. The evidence suggesting that uncoupling both contributes to and can ameliorate metabolic dysfunction is considerable. Metabolic dysregulation, in turn, can both increase the accumulation of damage that broadly defines aging, and confer chronic and debilitating disease (Fontana, 2009). As described above, DR is believed to be one of the few interventions that consistently leads to lifespan extension, though whether this is through reducing mortality or by slowing the accumulation of age-related damage is unclear and varies across different animal models. In addition to ROS attenuation, multiple other mechanisms are implicated in the lifespan-altering effects of DR, including reduced core body temperature and altered insulin signaling. The following sections will discuss how uncoupling may play a role in these mechanisms.
9. Uncoupling modulates glucose-stimulated insulin secretion (GSIS)
One particular area of interest in the metabolic control of lifespan is the role that UCP2 plays in insulin secretion from pancreatic β-cells, where it modulates glucose-stimulated insulin secretion (GSIS). In GSIS, abundant blood glucose drives respiration to generate ATP, triggering a cascade that stimulates exocytotic release of insulin-containing granules from β-cells. Recent evidence suggests that signaling by ROS comprises part of the GSIS response (Pi et al., 2007).
UCP2 is expressed in pancreatic β-cells, where its activity attenuates insulin secretion (Chan et al., 2004; Chan and Kashemsant, 2006; Affourtit and Brand, 2008). Accordingly, Ucp2 deletion or knockdown enhances insulin release (Zhang et al., 2001; Affourtit and Brand, 2008). UCP2 overexpression diminishes GSIS (Chan et al., 1999; Hong et al., 2001), but conflicting reports exist (Produit-Zengaffinen et al., 2007). Work by Krauss et al. (2003) suggests that endogenous ROS is sufficient to activate UCP2-mediated uncoupling, consistent with a role for UCP2 in mild uncoupling.
UCP2 has been studied in relation to type II diabetes, as mitochondrial function controls how pancreatic β-cells transduce nutrient signals into an insulin response. One important diabetogenic event is the loss of an insulin response by β-cells following chronic exposure to circulating free fatty acids, concomitant with increased proton conductance. Early findings of free fatty acid-stimulated uncoupling in β-cells of diabetes models led to investigation of UCP2 as the mediator of this uncoupling. Although upregulation of UCP2 transcription (Medvedev et al., 2002) and protein levels (Lameloise et al., 2001) by long-term free fatty acid exposure were observed, consistent with a role for UCP2 in free fatty acid-mediated uncoupling, direct evidence for free fatty-acid stimulation of UCP2 activity in β-cells is weak. There is indirect evidence for oleate-induced mitochondrial uncoupling that was attributed to UCP2 and the dicarboxylate carrier (Lameloise et al., 2001). However, later work has found no stimulation of UCP2-mediated uncoupling in β-cells (Galetti et al., 2009).
As described above, stronger evidence exists for superoxide-mediated activation of UCP2. Endogenous superoxide activated proton conductance in islet cells that was GDP-sensitive and dependent on UCP2, directly implicating UCP2 in islet cell uncoupling. Moreover, it also suggested that UCP2 mediated the phenomenon that, following chronic hyperglycemia, β-cells are unresponsive to glucose stimulation. Islets isolated from Ucp2-/- mice retained similar GSIS to wild-type controls following a 72-hour hyperglycemia treatment (Krauss, 2003). Further work found that Ucp2-/- mice were resistant to GSIS attenuation caused by a high fat diet (Joseph, 2004).
Under high-fat and high-glucose conditions, therefore, the presence of functional UCP2 consistently exacerbates dysregulation of insulin and glucose homeostasis, leading to the development of insulin resistance and diabetes. However, UCP2-mediated uncoupling in β (and other) cells is also predicted to prevent chronic oxidative stress by attenuating ROS generation. Following backcrossing of the Ucp2-/- mouse, this genotype conferred unchanged (Parker et al., 2009) or decreased, not increased, GSIS, which was attributed to β-cell dysfunction as a result of persistent oxidative stress (Pi et al., 2009).
Interestingly, a recent report proposes that superoxide production is an upstream event in insulin resistance. By decreasing ROS production via FCCP or rotenone treatment, and by decreasing ROS damage via transgenic MnSOD overexpression, Hoehn et al. (2009) showed reversal of insulin resistance in mice fed a high fat diet. Also, Costford et al. (2009) showed increased oxidative damage upon glucose challenge in satellite cells from previously diabetic individuals. This was at least partially attributable to an inability of these cells to lower Δψm following glucose influx, which normally mitigates ROS production. The lack of a decrease in Δψm correlated with a three-fold drop in UCP3 protein levels.
Taken together, these data suggest that, given the correct balance and supply of nutrient intake, uncoupling proteins can properly regulate lipid and glucose catabolism and maintain ROS homeostasis to allow its proper function as a signaling agent while minimizing its ability to cause cellular damage. However, given the excess fats and sugars that occur in the so-called “western diet”, it may be that deactivation of UCP activity may have health benefits that positively affect lifespan under these conditions.
10. Uncoupling-mediated body temperature modulation
The observation that reduced body temperature results in lifespan extension in invertebrates and vertebrate ectotherms is several decades old (Lamb, 1968; Liu and Walford, 1972). Importantly, this effect is not dependent on a slower metabolic rate (rev. in Yen et al., 2004), and, of all the interventions known to increase lifespan, it is hypothesized that only temperature reduction (Mair et al., 2003) or only DR and temperature reduction actually decrease the rate of aging, rather than the incidence of mortality (Yen and Mobbs, 2009). How may this be usefully applied to endotherms, where manipulation of core body temperature is not as simple as changing ambient temperature (Vaanholt et al., 2009)?
One way to manipulate mammalian core body temperature is through DR itself (Ferguson et al., 2007). Another way is through targeted mitochondrial uncoupling. Conti et al. (2006) targeted transgenic UCP2 expression to hypothalamic neurons in mice. This resulted in an average 0.65°C temperature increase in the lateral hypothalamus, concurrent with a compensatory reduction in core body temperature of approximately 0.3°C during the active phase. These mice displayed a 12%-20% increase in median lifespan. Although UCP2 is endogenously expressed in the mouse hypothalamus (Richard et al., 2001), it is unlikely that this finding has physiological relevance or gives insight into the native function of UCP2. However, along with the overexpression of UCP1 by Gates et al. (2007), it does illustrate the utility of ectopic UCP expression for manipulating metabolism. While these studies involved different tissues and did not both measure the same parameters of uncoupling, they demonstrate how targeted uncoupling can be used to test hypotheses about aging mechanisms. It should be emphasized that ectopic UCP expression that results in proton conductance should not be interpreted as native protein function without also determining that it is properly regulated.
11. Perspective and Conclusion
Aging is correlated with changes in many different processes, including DNA replication and repair, apoptotic signaling, metabolic signaling and sensing, proteasomal and lysosomal activity, ATP production, and mitochondrial coupling efficiency. The inconsistent effects of uncoupling interventions on lifespan may reflect the limitations of current experimental approaches, or that we have not adequately considered the contexts in which mild uncoupling may be most effective at modulating lifespan. For example, under optimal maintenance conditions, as in Perez et al. (2009), oxidative damage may not be the determining factor in mortality; rather, it may be the failure of other mechanisms that triggers senescence.
Ultimately, is mitochondrial uncoupling beneficial or detrimental to health and longevity? The answer to this question is hampered by an incomplete understanding of both the functions of uncoupling in maintaining energy homeostasis, and the biological mechanisms of aging. However, most evidence supports a role for mild uncoupling in ROS attenuation, and also in maintaining nutrient and energy homeostasis, both of which are likely important for optimizing lifespan. Conversely, when homeostasis is disrupted, as in insulin insensitivity, uncoupling may exacerbate dysfunction. An increased understanding of aging, the functions and physiological roles of the uncoupling proteins, as well as the development of targetable chemical uncouplers, could further the use of uncoupling as a therapeutic strategy to maintain healthy aging.
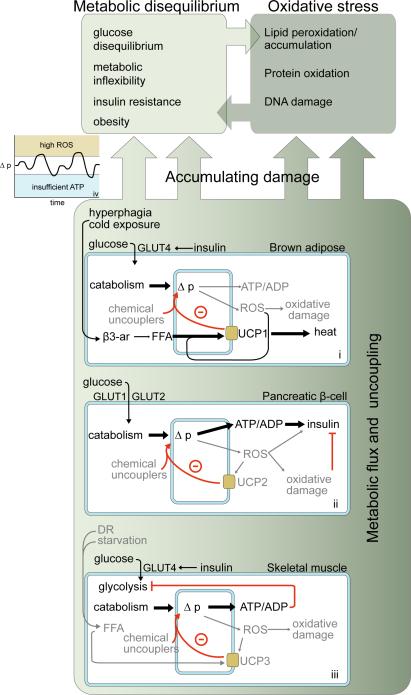
Figure 1.
Examples of different metabolic systems in which biological uncoupling may function. Outer and inner blue shapes represent plasma and mitochondrial membranes, respectively. Black arrows and text denote the primary metabolic mechanisms in each system. (i): In brown adipose tissue, activation of UCP1 by free fatty acids (FFA) dissipates Δp as heat. Loss of UCP1 leads to cold sensitivity and (at thermoneutrality) obesity. (ii): In the pancreatic β-cell, Δp and ATP/ADP fluctuate in response to glucose, allowing glucose-stimulated insulin secretion (GSIS). Uncoupling by UCP2 attenuates GSIS and lowers ROS. UCP2 depletion may therefore lead to short-term increases in GSIS sensitivity, with long-term dysfunction due to oxidative damage. (iii) In skeletal muscle, glucose catabolism is tightly regulated to maintain constant ATP/ADP. Uncoupling by UCP3 may decrease ROS production when ATP demand and therefore Δp is high (e.g., during exercise). (iv): In all cases, a general hypothesis for uncoupling is that it may blunt Δp fluctuation to minimize ROS production. Dysfunction may accumulate faster if the Δp strays from an “optimal” range, while its maintenance within this range may slow cellular damage over time. Whether uncoupling has an ultimately positive or negative impact on lifespan therefore depends on the bioenergetic context in which uncoupling occurs, whether uncoupling acts to maintain or further destabilize metabolic equilibrium, and the control that metabolic equilibrium has over lifespan in an organism.
Acknowledgements
Supported by grants from the National Institutes of Health (P01 AG025901, PL1 AG032118, P30 AG025708 and R01 AG033542), the W.M. Keck foundation, The Ellison Medical Foundation (AG-SS-2288-09), the Deutsche Forschungsgemeinschaft (JA 1884/2-1) and a British Marshall Scholarship and National Science Foundation Graduate Research Fellowship to ASD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams AE, Hanrahan O, Nolan DN, Voorheis HP, Fallon P, Porter RK. Images of mitochondrial UCP 1 in mouse thymocytes using confocal microscopy. Biochim Biophys Acta. 2008;1777:115–117. doi: 10.1016/j.bbabio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Affourtit C, Brand MD. On the role of uncoupling protein-2 in pancreatic beta cells. Biochim Biophys Acta. 2008;1777:973–979. doi: 10.1016/j.bbabio.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA. 2007;104:1057–1062. doi: 10.1073/pnas.0610131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat R, Solanes G, Giralt M, Villarroya F. SIRT1 Is Involved in Glucocorticoid-mediated Control of Uncoupling Protein-3 Gene Transcription. J Biol Chem. 2007;282:34066–34076. doi: 10.1074/jbc.M707114200. [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Horvath TL. Uncoupling protein-2 regulates lifespan in mice. Am J Physiol Endocrinol Metab. 2009;296:E621–627. doi: 10.1152/ajpendo.90903.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry Mosc. 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- Azzu V, Parker N, Brand MD. High membrane potential promotes alkenal-induced mitochondrial uncoupling and influences adenine nucleotide translocase conformation. Biochem J. 2008a;413:323. doi: 10.1042/BJ20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzu V, Affourtit C, Breen E, Parker N, Brand MD. Dynamic regulation of uncoupling protein 2 content in INS-1E insulinoma cells. Biochim Biophys Acta. 2008b;1777:1378–1383. doi: 10.1016/j.bbabio.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe P, Larrouy D, Boulanger C, Chevillotte E, Viguerie N, Thalamas C, Oliva Trastoy M, Roques M, Vidal H, Langin D. Triiodothyronine-mediated up-regulation of UCP2 and UCP3 mRNA expression in human skeletal muscle without coordinated induction of mitochondrial respiratory chain genes. FASEB J. 2001;15:13–15. doi: 10.1096/fj.00-0502fje. [DOI] [PubMed] [Google Scholar]
- Barja G. Mitochondrial free radical production and aging in mammals and birds. Ann N Y Acad Sci. 1998;854:224–238. doi: 10.1111/j.1749-6632.1998.tb09905.x. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper M-E. Effects of short- and medium-term calorie restriction on muscle mitochondrial proton leak and reactive oxygen species production. Am J Physiol Endocrinol Metab. 2004;286:E852–861. doi: 10.1152/ajpendo.00367.2003. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper M-E. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol Endocrinol Metab. 2005;289:E429–438. doi: 10.1152/ajpendo.00435.2004. [DOI] [PubMed] [Google Scholar]
- Blanc J, Alves-Guerra MC, Esposito B, Rousset S, Gourdy P, Ricquier D, Tedgui A, Miroux B, Mallat Z. Protective role of uncoupling protein 2 in atherosclerosis. Circulation. 2003;107:388–390. doi: 10.1161/01.cir.0000051722.66074.60. [DOI] [PubMed] [Google Scholar]
- Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, Mcdonagh T, Lemieux M, Mcburney M, Szilvasi A, Easlon EJ, Lin S-J, Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. Plos Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss O, Samec S, Dulloo A, Seydoux J, Muzzin P, Giacobino JP. Tissue-dependent upregulation of rat uncoupling protein-2 expression in response to fasting or cold. FEBS Lett. 1997a;412:111–114. doi: 10.1016/s0014-5793(97)00755-2. [DOI] [PubMed] [Google Scholar]
- Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, Muzzin P, Giacobino JP. Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997b;408:39–42. doi: 10.1016/s0014-5793(97)00384-0. [DOI] [PubMed] [Google Scholar]
- Bouillaud F. UCP2, not a physiologically relevant uncoupler but a glucose sparing switch impacting ROS production and glucose sensing. Biochim Biophys Acta. 2009:377–383. doi: 10.1016/j.bbabio.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2. [DOI] [PubMed] [Google Scholar]
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Brand MD, Chien LF, Ainscow EK, Rolfe DF, Porter RK. The causes and functions of mitochondrial proton leak. Biochim Biophys Acta. 1994;1187:132–139. doi: 10.1016/0005-2728(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Brand MD, Pamplona R, Portero-OtiN M, Requena JR, Roebuck SJ, Buckingham JA, Clapham JC, Cadenas S. Oxidative damage and phospholipid fatty acyl composition in skeletal muscle mitochondria from mice underexpressing or overexpressing uncoupling protein 3. Biochem J. 2002;368:597. doi: 10.1042/BJ20021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Cadenas S, Buckingham JA, Samec S, Seydoux J, Din N, Dulloo A, Brand MD. UCP2 and UCP3 rise in starved rat skeletal muscle but mitochondrial proton conductance is unchanged. FEBS Lett. 1999;462:257–260. doi: 10.1016/s0014-5793(99)01540-9. [DOI] [PubMed] [Google Scholar]
- Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MHG, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7:552–560. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- Cannon B, Shabalina IG, Kramarova TV, Petrovic N, Nedergaard J. Uncoupling proteins: a role in protection against reactive oxygen species--or not? Biochim Biophys Acta. 2006;1757:449–458. doi: 10.1016/j.bbabio.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Chan CB, Kashemsant N. Regulation of insulin secretion by uncoupling protein. Biochem Soc Trans. 2006;34:802–805. doi: 10.1042/BST0340802. [DOI] [PubMed] [Google Scholar]
- Chan CB, Saleh MC, Koshkin V, Wheeler MB. Uncoupling protein 2 and islet function. Diabetes. 2004;53(Suppl 1):S136–142. doi: 10.2337/diabetes.53.2007.s136. [DOI] [PubMed] [Google Scholar]
- Chan CB, MacDonald PE, Saleh MC, Johns DC, Marbàn E, Wheeler MB. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes. 1999;48:1482–1486. doi: 10.2337/diabetes.48.7.1482. [DOI] [PubMed] [Google Scholar]
- Chu AC-Y, Ho PW-L, Kwok KH-H, Ho JW-M, Chan K-H, Liu H-F, Kung MH-W, Ramsden DB, Ho S-L. Mitochondrial UCP4 attenuates MPP+ - and dopamine-induced oxidative stress, mitochondrial depolarization, and ATP deficiency in neurons and is interlinked with UCP2 expression. Free Radic Biol Med. 2009;46:810–820. doi: 10.1016/j.freeradbiomed.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Cioffi F, Senese R, de Lange P, Goglia F, Lanni A, Lombardi A. Uncoupling proteins: A complex journey to function discovery. BioFactors. 2009;35:417–428. doi: 10.1002/biof.54. [DOI] [PubMed] [Google Scholar]
- Clapham JC, et al. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature. 2000;406:415–418. doi: 10.1038/35019082. [DOI] [PubMed] [Google Scholar]
- Colman E. Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regul Toxicol Pharmacol. 2007;48:115–117. doi: 10.1016/j.yrtph.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Considine MJ, Goodman M, Echtay KS, Laloi M, Whelan J, Brand MD, Sweetlove LJ. Superoxide stimulates a proton leak in potato mitochondria that is related to the activity of uncoupling protein. J Biol Chem. 2003;278:22298–22302. doi: 10.1074/jbc.M301075200. [DOI] [PubMed] [Google Scholar]
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- Costford SR, Chaudhry SN, Salkhordeh M, Harper M-E. Effects of the presence, absence, and overexpression of uncoupling protein-3 on adiposity and fuel metabolism in congenic mice. Am J Physiol Endocrinol Metab. 2006;290:E1304–1312. doi: 10.1152/ajpendo.00401.2005. [DOI] [PubMed] [Google Scholar]
- Costford SR, Crawford SA, Dent R, McPherson R, Harper M-E. Increased susceptibility to oxidative damage in post-diabetic human myotubes. Diabetologia. 2009;52:2405–2415. doi: 10.1007/s00125-009-1480-y. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng Y-H, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D-F, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrouzet E, Moser CC, Dutton PL, Daldal F. Large scale domain movement in cytochrome bc1: a new device for electron transfer in proteins. Trends Biochem Sci. 2001;26:445–451. doi: 10.1016/s0968-0004(01)01897-7. [DOI] [PubMed] [Google Scholar]
- Echtay K. Mitochondrial uncoupling proteins—What is their physiological role? Free Radical Biol Med. 2007;43:1351–1371. doi: 10.1016/j.freeradbiomed.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Pakay JL, Esteves TC, Brand MD. Hydroxynonenal and uncoupling proteins: a model for protection against oxidative damage. BioFactors. 2005;24:119–130. doi: 10.1002/biof.5520240114. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Murphy MP, Smith RAJ, Talbot DA, Brand MD. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. J Biol Chem. 2002a;277:47129–47135. doi: 10.1074/jbc.M208262200. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otín M, Pamplona R, Vidal-Puig AJ, Wang S, Roebuck SJ, Brand MD. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002b;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Ferguson M, Sohal BH, Forster MJ, Sohal RS. Effect of long-term caloric restriction on oxygen consumption and body temperature in two different strains of mice. Mech Ageing Dev. 2007;128:539–545. doi: 10.1016/j.mad.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D, Warden CH. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- Fontana L. Modulating human aging and age-associated diseases. Biochim Biophys Acta. 2009;1790:1133–1138. doi: 10.1016/j.bbagen.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridell Y-WC, Sánchez-Blanco A, Silvia BA, Helfand SL. Functional characterization of a Drosophila mitochondrial uncoupling protein. J Bioenerg Biomembr. 2004;36:219–228. doi: 10.1023/b:jobb.0000031973.20153.c6. [DOI] [PubMed] [Google Scholar]
- Fridell Y-WC, Sánchez-Blanco A, Silvia BA, Helfand SL. Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends life span in the fly. Cell Metab. 2005;1:145–152. doi: 10.1016/j.cmet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Galetti S, Sarre A, Perreten H, Produit-Zengaffinen N, Muzzin P, Assimacopoulos-Jeannet F. Fatty acids do not activate UCP2 in pancreatic beta cells: comparison with UCP1. Pflugers Arch - Eur J Physiol. 2009;457:931–940. doi: 10.1007/s00424-008-0548-8. [DOI] [PubMed] [Google Scholar]
- Gates AC, Bernal-Mizrachi C, Chinault SL, Feng C, Schneider JG, Coleman T, Malone JP, Townsend RR, Chakravarthy MV, Semenkovich CF. Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab. 2007;6:497–505. doi: 10.1016/j.cmet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J. Only UCP1 can mediate adaptive thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- Gong DW, He Y, Karas M, Retiman M. Uncoupling protein-3 is a mediator of thermogenesis regulated by thyroid hormone, beta3-adenergic agonists, and leptin. J Biol Chem. 1997;272:24129–24132. doi: 10.1074/jbc.272.39.24129. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Harper M-E, Ram JJ, Humble SJ, Weindruch R, Ramsey JJ. Long-term calorie restriction reduces proton leak and hydrogen peroxide production in liver mitochondria. Am J Physiol Endocrinol Metab. 2005;288:E674–684. doi: 10.1152/ajpendo.00382.2004. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11:298–200. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harper JA, Dickinson K, Brand MD. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obesity Rev. 2001;2:255–265. doi: 10.1046/j.1467-789x.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- Harper JA, Stuart JA, Jekabsons MB, Roussel D, Brindle KM, Dickinson K, Jones RB, Brand MD. Artifactual uncoupling by uncoupling protein 3 in yeast mitochondria at the concentrations found in mouse and rat skeletal-muscle mitochondria. Biochem J. 2002;361:49–56. doi: 10.1042/0264-6021:3610049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J, Harper ME. Physiological role of UCP3 may be export of fatty acids from mitochondrial when fatty acid oxidation predominates: an hypothesis. Exp Biol Med. 2001;226:78–84. doi: 10.1177/153537020122600204. [DOI] [PubMed] [Google Scholar]
- Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW, Cooney GJ, Richardson AR, James DE. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA. 2009;106:17787–17792. doi: 10.1073/pnas.0902380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Tanaka M, Honda S. Modulation of longevity and diapause by redox regulation mechanisms under the insulin-like signaling control in Caenorhabditis elegans. Exp Gerontol. 2008;43:520–529. doi: 10.1016/j.exger.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Hong Y, Fink BD, Dillon JS, Sivitz WI. Effects of adenoviral overexpression of uncoupling protein-2 and -3 on mitochondrial respiration in insulinoma cells. Endocrinology. 2001;142:249–256. doi: 10.1210/endo.142.1.7889. [DOI] [PubMed] [Google Scholar]
- Humphrey DM, Toivonen JM, Giannakou M, Partridge L, Brand MD. Expression of human uncoupling protein-3 in Drosophila insulin-producing cells increases insulin-like peptide (DILP) levels and shortens lifespan. Exp Gerontol. 2009;44:316–327. doi: 10.1016/j.exger.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtaud C, Gelly C, Bouillaud F, Lévi-Meyrueis C. Translation control of UCP2 synthesis by the upstream open reading frame. Cell Mol Life Sci. 2006;63:1780–1789. doi: 10.1007/s00018-006-6129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtaud C, Gelly C, Chen Z, Lévi-Meyrueis C, Bouillaud F. Glutamine stimulates translation of uncoupling protein-2 mRNA. Cell Mol Life Sci. 2007;64:1853–1860. doi: 10.1007/s00018-007-7039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom AL, Barnes S, Wilson L, Kirk M, Coward L, Darley-Usmar V. Modification of Cytochrome c by 4-hydroxy- 2-nonenal: evidence for histidine, lysine, and arginine-aldehyde adducts. J Am Soc Mass Spectrom. 2004;15:1136–1147. doi: 10.1016/j.jasms.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Jaburek M, Miyamoto S, Di Mascio P, Garlid KD, Jezek P. Hydroperoxy fatty acid cycling mediated by mitochondrial uncoupling protein UCP2. J Biol Chem. 2004;279:53097–53102. doi: 10.1074/jbc.M405339200. [DOI] [PubMed] [Google Scholar]
- Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, Salmon AB, Brooks SV, Larkin L, Hayworth CR, Richardson A, Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2009a doi: 10.1096/fj.09-146308. E-pub 29 Dec 2009 DOI: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YC, Pérez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, Ikeno Y, Epstein CJ, Van Remmen H, Richardson A. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci. 2009b;64:1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J. Free Fatty Acid-induced β-Cell Defects Are Dependent on Uncoupling Protein 2 Expression. J Biol Chem. 2004;279:51049–51056. doi: 10.1074/jbc.M409189200. [DOI] [PubMed] [Google Scholar]
- Jürgens KD, Prothero J. Scaling of maximal lifespan in bats. Comp Biochem Physiol A Comp Physiol. 1987;88:361–367. doi: 10.1016/0300-9629(87)90498-1. [DOI] [PubMed] [Google Scholar]
- Katterle Y, Keipert S, Hof J, Klaus S. Dissociation of obesity and insulin resistance in transgenic mice with skeletal muscle expression of uncoupling protein 1. Physiol Genomics. 2008;32:352–359. doi: 10.1152/physiolgenomics.00194.2007. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. The ADP and ATP transport in mitochondria and its carrier. Biochim Biophys Acta. 2008;1778:1978–2021. doi: 10.1016/j.bbamem.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Kontani Y, Wang Y, Kimura K, Inokuma K-I, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell. 2005;4:147–155. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Krauss S. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic β-cell dysfunction. J Clin Invest. 2003;112:1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Zhang C-Y, Lowell BB. A significant portion of mitochondrial proton leak in intact thymocytes depends on expression of UCP2. Proc Natl Acad Sci USA. 2002;99:118–122. doi: 10.1073/pnas.012410699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci USA. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb MJ. Temperature and lifespan in Drosophila. Nature. 1968;220:808–809. doi: 10.1038/220808a0. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J. 2004a;382:511–517. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J Biol Chem. 2004b;279:39414–39420. doi: 10.1074/jbc.M406576200. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD. Reactive oxygen species production by mitochondria. Methods Mol Biol. 2009;554:165–181. doi: 10.1007/978-1-59745-521-3_11. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R, Brand MD. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum lifespan in vertebrate homeotherms. Aging Cell. 2007;6:607–618. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Lameloise N, Muzzin P, Prentki M, Assimacopoulos-Jeannet F. Uncoupling protein 2: a possible link between fatty acid excess and impaired glucose-induced insulin secretion? Diabetes. 2001;50:803–809. doi: 10.2337/diabetes.50.4.803. [DOI] [PubMed] [Google Scholar]
- Li B, Nolte LA, Ju JS, Han DH, Coleman T, Holloszy JO, Semenkovich CF. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat Med. 2000;6:1115–1120. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- Liu RK, Walford RL. The effect of lowered body temperature on lifespan and immune and non-immune processes. Gerontologia. 1972;18:363–388. doi: 10.1159/000211944. [DOI] [PubMed] [Google Scholar]
- Liu SS. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci Rep. 1997;17:259–272. doi: 10.1023/a:1027328510931. [DOI] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Mao W, Yu XX, Zhong A, Li W, Brush J, Sherwood SW, Adams SH, Pan G. UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett. 1999;443:326–330. doi: 10.1016/s0014-5793(98)01713-x. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction-induced life extension of rats and mice: A critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790:1040–1048. doi: 10.1016/j.bbagen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Mcdonald RB, Walker KM, Warman DB, Griffey SM, Warden CH, Ramsey JJ, Horwitz BA. Characterization of survival and phenotype throughout the life span in UCP2/UCP3 genetically altered mice. Exp Gerontol. 2008;43:1061–1068. doi: 10.1016/j.exger.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Medvedev AV, Robidoux J, Bai X, Cao W, Floering LM, Daniel KW, Collins S. Regulation of the uncoupling protein-2 gene in INS-1 beta-cells by oleic acid. J Biol Chem. 2002;277:42639–42644. doi: 10.1074/jbc.M208645200. [DOI] [PubMed] [Google Scholar]
- Mills EM, Banks ML, Sprague JE, Finkel T. Pharmacology: uncoupling the agony from ecstasy. Nature. 2003;426:403–404. doi: 10.1038/426403a. [DOI] [PubMed] [Google Scholar]
- Miwa S, Brand MD. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem Soc Trans. 2003;31:1300–1301. doi: 10.1042/bst0311300. [DOI] [PubMed] [Google Scholar]
- Miwa S, Riyahi K, Partridge L, Brand MD. Lack of correlation between mitochondrial reactive oxygen species production and life span in Drosophila. Ann N Y Acad Sci. 2004;1019:388–391. doi: 10.1196/annals.1297.069. [DOI] [PubMed] [Google Scholar]
- Moynihan K, Grimm A, Plueger M, Bernalmizrachi E, Ford E, Crasmeneur C, Permutt M, Imai S. Increased dosage of mammalian Sir2 in pancreatic cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Echtay KS, Blaikie FH, Asin-Cayuela J, Cocheme HM, Green K, Buckingham JA, Taylor ER, Hurrell F, Hughes G, Miwa S, Cooper CE, Svistunenko DA, Smith RAJ, Brand MD. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation: studies using a mitochondria-targeted spin trap derived from alpha-phenyl-N-tert-butylnitrone. J Biol Chem. 2003;278:48534–48545. doi: 10.1074/jbc.M308529200. [DOI] [PubMed] [Google Scholar]
- Musatov A, Carroll CA, Liu Y-C, Henderson GI, Weintraub ST, Robinson NC. Identification of Bovine Heart Cytochrome c Oxidase Subunits Modified by the Lipid Peroxidation Product 4-Hydroxy-2-nonenal. Biochemistry. 2002;41:8212–8220. doi: 10.1021/bi025896u. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. A history of UCP1. Biochem Soc Trans. 2001;29:751–755. doi: 10.1042/bst0290751. [DOI] [PubMed] [Google Scholar]
- O'Connor TP, Lee A, Jarvis JUM, Buffenstein R. Prolonged longevity in naked mole-rats: age-related changes in metabolism, body composition and gastrointestinal function. Comp Biochem Physiol, Part A Mol Integr Physiol. 2002;133:835–842. doi: 10.1016/s1095-6433(02)00198-8. [DOI] [PubMed] [Google Scholar]
- Padalko VI. Uncoupler of oxidative phosphorylation prolongs the lifespan of Drosophila. Biochemistry Mosc. 2005;70:986–989. doi: 10.1007/s10541-005-0213-1. [DOI] [PubMed] [Google Scholar]
- Papa S, Skulachev VP. Reactive oxygen species, mitochondria, apoptosis and aging. Mol Cell Biochem. 1997;174:305–319. [PubMed] [Google Scholar]
- Parker N, Vidal-Puig AJ, Brand MD. Stimulation of mitochondrial proton conductance by hydroxynonenal requires a high membrane potential. Biosci Rep. 2008;28:83. doi: 10.1042/BSR20080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker N, Vidal-Puig AJ, Azzu V, Brand MD. Dysregulation of glucose homeostasis in nicotinamide nucleotide transhydrogenase knockout mice is independent of uncoupling protein 2. Biochim Biophys Acta. 2009;1787:1451–1457. doi: 10.1016/j.bbabio.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola M, Bellomo G, Robino G, Barrera G, Dianzani MU. 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid Redox Signal. 2001;1:255–284. doi: 10.1089/ars.1999.1.3-255. [DOI] [PubMed] [Google Scholar]
- Pearl R. The Rate of Living. University of London Press; London: 1928. [Google Scholar]
- Pecqueur C. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem. 2001;276:8705–8712. doi: 10.1074/jbc.M006938200. [DOI] [PubMed] [Google Scholar]
- Pedersen PL. An introduction to the mitochondrial anion carrier family. J Bioenerg Biomembr. 1993;25:431–434. doi: 10.1007/BF01108400. [DOI] [PubMed] [Google Scholar]
- Pérez VI, Bokov A, Remmen HV, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Bai Y, Daniel KW, Liu D, Lyght O, Edelstein D, Brownlee M, Corkey BE, Collins S. Persistent oxidative stress due to absence of uncoupling protein 2 associated with impaired pancreatic beta-cell function. Endocrinology. 2009;150:3040–3048. doi: 10.1210/en.2008-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- Produit-Zengaffinen N, Davis-Lameloise N, Perreten H, Bécard D, Gjinovci A, Keller PA, Wollheim CB, Herrera P, Muzzin P, Assimacopoulos-Jeannet F. Increasing uncoupling protein-2 in pancreatic beta cells does not alter glucose-induced insulin secretion but decreases production of reactive oxygen species. Diabetologia. 2007;50:84–93. doi: 10.1007/s00125-006-0499-6. [DOI] [PubMed] [Google Scholar]
- Richard D, Clavel S, Huang Q, Sanchis D, Ricquier D. Uncoupling protein 2 in the brain: distribution and function. Biochem Soc Trans. 2001;29:812–817. doi: 10.1042/0300-5127:0290812. [DOI] [PubMed] [Google Scholar]
- Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J. 2000;345:161–179. [PMC free article] [PubMed] [Google Scholar]
- Robert KA, Brunet-Rossinni A, Bronikowski AM. Testing the ‘free radical theory of aging’ hypothesis: physiological differences in long-lived and short-lived colubrid snakes. Aging Cell. 2007;6:395–404. doi: 10.1111/j.1474-9726.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- Rousset S, Mozo J, Dujardin G, Emre Y, Masscheleyn S, Ricquier D, Cassarddoulcier A. UCP2 is a mitochondrial transporter with an unusual very short half-life. FEBS Lett. 2007;581:479–482. doi: 10.1016/j.febslet.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE, Tandy SL, Hekmatyar SK, Mills E, Smith DJ, Bansal N, MacLellan D, Harper M-E, Sprague JE. The role of mitochondrial uncoupling in 3,4-methylenedioxymethamphetamine-mediated skeletal muscle hyperthermia and rhabdomyolysis. J Pharmacol Exp Ther. 2005;313:629–639. doi: 10.1124/jpet.104.079236. [DOI] [PubMed] [Google Scholar]
- Sánchez-Blanco A, Fridell Y-WC, Helfand SL. Involvement of Drosophila uncoupling protein 5 in metabolism and aging. Genetics. 2006;172:1699–1710. doi: 10.1534/genetics.105.053389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis D, Fleury C, Chomiki N, Goubern M, Huang Q, Neverova M, Grégoire F, Easlick J, Raimbault S, Lévi-Meyrueis C, Miroux B, Collins S, Seldin M, Richard D, Warden C, Bouillaud F, Ricquier D. BMCP1, a novel mitochondrial carrier with high expression in the central nervous system of humans and rodents, and respiration uncoupling activity in recombinant yeast. J Biol Chem. 1998;273:34611–34615. doi: 10.1074/jbc.273.51.34611. [DOI] [PubMed] [Google Scholar]
- Schaur RJ. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol Aspects Med. 2003;24:149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Hesselink MKC. The role of uncoupling protein 3 in fatty acid metabolism: protection against lipotoxicity? Proc Nutr Soc. 2004;63:287–292. doi: 10.1079/PNS2003336. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Hoeks J, Hesselink MKC. Putative function and physiological relevance of the mitochondrial uncoupling protein-3: involvement in fatty acid metabolism? Prog Lipid Res. 2006;45:17–41. doi: 10.1016/j.plipres.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Seifert E, Bezaire V, Estey C, Harper ME. Essential Role for Uncoupling Protein-3 in Mitochondrial Adaptation to Fasting but Not in Fatty Acid Oxidation or Fatty Acid Anion Export. J Biol Chem. 2008;283:25124–25131. doi: 10.1074/jbc.M803871200. [DOI] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M, Prolla TA. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci USA. 2009;106:19432–19437. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Talbot DA, Selman C, Snart S, Mclaren JS, Redman P, Krol E, Jackson DM, Johnson MS, Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- Talbot DA, Lambert AJ, Brand MD. Production of endogenous matrix superoxide from mitochondrial complex I leads to activation of uncoupling protein 3. FEBS Lett. 2004;556:111–115. doi: 10.1016/s0014-5793(03)01386-3. [DOI] [PubMed] [Google Scholar]
- Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Martin GM, Rabinovitch PS, Ladiges WC. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol A Biol Sci Med Sci. 2008;63:813–822. doi: 10.1093/gerona/63.8.813. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Krasnikov BF, Csiszar A, Labinskyy N, Mukhopadhyay P, Pacher P, Cooper AJL, Podlutskaya N, Austad SN, Podlutsky A. Testing hypotheses of aging in long-lived mice of the genus Peromyscus: association between longevity and mitochondrial stress resistance, ROS detoxification pathways, and DNA repair efficiency. Age (Dordrecht, Netherlands) 2008;30:121–133. doi: 10.1007/s11357-008-9059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaanholt LM, Daan S, Schubert KA, Visser GH. Metabolism and aging: effects of cold exposure on metabolic rate, body composition, and longevity in mice. Physiol Biochem Zool. 2009;82:314–324. doi: 10.1086/589727. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig AJ, Solanes G, Grujic D, Flier JS, Lowell BB. UCP3: an uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochem Biophys Res Commun. 1997;235:79–82. doi: 10.1006/bbrc.1997.6740. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, Szczepanik A, Wade J, Mootha V, Cortright R, Muoio DM, Lowell BB. Energy metabolism in uncoupling protein 3 gene knockout mice. J Biol Chem. 2000;275:16258–16266. doi: 10.1074/jbc.M910179199. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Walker JE. The NADH:ubiquinone oxidoreductase (complex I) of respiratory chains. Q Rev Biophys. 1992;25:253–324. doi: 10.1017/s003358350000425x. [DOI] [PubMed] [Google Scholar]
- Wei Z, Chigurupati S, Bagsiyao P, Henriquez A, Chan SL. The Brain Uncoupling Protein UCP4 Attenuates Mitochondrial Toxin-Induced Cell Death: Role of Extracellular Signal-Regulated Kinases in Bioenergetics Adaptation and Cell Survival. Neurotoc Res. 2009;16:14–29. doi: 10.1007/s12640-009-9039-8. [DOI] [PubMed] [Google Scholar]
- Yen K, Mobbs C. Evidence for only two independent pathways for decreasing senescence in Caenorhabditis elegans. Age (Dordrecht, Netherlands) 2009 doi: 10.1007/s11357-009-9110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K, Mastitis JW, Mobbs CV. Lifespan is not determined by metabolic rate: evidence from fishes and C. elegans. Exp Gerontol. 2004;39:947–949. doi: 10.1016/j.exger.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Yen K, Patel HB, Lublin AL, Mobbs CV. SOD isoforms play no role in lifespan in ad lib or dietary restricted conditions, but mutational inactivation of SOD-1 reduces life extension by cold. Mech Age Dev. 2009;130:173–178. doi: 10.1016/j.mad.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Huang L, Shulmeister VM, Chi YI, Kim KK, Hung LW, Crofts AR, Berry EA, Kim SH. Electron transfer by domain movement in cytochrome bc1. Nature. 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]