Abstract
Rationale
The orphan nuclear receptor NOR1 is a member of the evolutionary highly conserved and ligand-independent NR4A subfamily of the nuclear hormone receptor superfamily. Members of this subfamily have been characterized as early response genes regulating essential biological processes including inflammation and proliferation; however the role of NOR1 in atherosclerosis remains unknown.
Objective
The goal of the present study was to determine the causal contribution of NOR1 to atherosclerosis development and to identify the mechanism by which this nuclear receptor participates in the disease process.
Methods and Results
In the present study, we demonstrate expression of NOR1 in endothelial cells of human atherosclerotic lesions. In response to inflammatory stimuli, NOR1 expression is rapidly induced in endothelial cells through a NF-κB-dependent transactivation of the NOR1 promoter. Overexpression of NOR1 in human endothelial cells increased the expression of VCAM-1 and ICAM-1 while NOR1 deficiency altered adhesion molecule expression in response to inflammatory stimuli. Transient transfection experiments and chromatin immunoprecipitation assays revealed that NOR1 induces VCAM-1 promoter activity by binding to a canonical response element for NR4A receptors in the VCAM-1 promoter. Further functional studies confirmed that NOR1 mediates monocyte adhesion by inducing VCAM-1 and ICAM-1 expression in endothelial cells. Finally, we demonstrate that NOR1-deficiency reduces hypercholesterolemia-induced atherosclerosis formation in apoE−/− mice by decreasing the macrophage content of the lesion.
Conclusion
In concert, these studies identify a novel pathway underlying monocyte adhesion and establish that NOR1 serves a previously unrecognized atherogenic role in mice by positively regulating monocyte recruitment to the vascular wall.
Keywords: nuclear receptor, endothelial cell, adhesion, atherosclerosis
INTRODUCTION
The transcription factor NOR1 (NR4A3) belongs to the highly conserved NR4A subfamily of orphan nuclear hormone receptors. 1, 2 Members of this subgroup are classified as early response genes, which are induced by a pleiotropy of stimuli, including mitogens and inflammatory signals. 3, 4 In contrast to other members of the nuclear receptor superfamily, NR4A receptors function as constitutively-active and ligand-independent transcription factors. 5, 6 Therefore, the transcriptional activity of NR4A receptors is determined by the expression level and by posttranslational modifications of the receptor. 7 NR4A receptors positively regulate target gene expression by binding as monomer or homodimer to different variations of the canonical 5′-A/TAAAGGTCA NGFI-B response element (NBRE). 8 In addition, (deleted “the NOR1 siblings”) Nur77 (NR4A1) and Nurr1 (NR4A2) exhibit transcriptionally distinct mechanisms, as both are able to transactivate target genes as a heterodimer with RXR. 9 Consistent with the pleiotropic stimuli that induce the expression of NR4A receptors, these transcription factors have been implicated in regulating key cellular functions, including inflammation, proliferation, and cell survival. 1, 2
NOR1 was first cloned from neuronal cells, and its deletion in mice results in hippocampal dysgenesis and inner ear defects. 10, 11 In addition to neurons, NOR1 is highly expressed in atherosclerotic lesions. 3, 12-14 NOR1 is induced by mitogens in smooth muscle cells (SMC) and required for proliferative remodeling following vascular injury. 3, 14, 15 In macrophages, NOR1 is induced during inflammation and represses cytokine secretion. 4, 13 Finally, NOR1 expression is increased by vascular endothelial growth factor (VEGF) in endothelial cells. 16 However, the functional role of NOR1 in endothelial cells and its contribution to atherosclerosis remain to be investigated.
In the present study, we provide first evidence that NOR1 plays an essential role in the regulation of monocyte adhesion to the endothelium by positively regulating VCAM-1 and ICAM-1 expression. In vivo, loss of NOR1 function in apoE-deficient mice reduces atherosclerosis formation and macrophage recruitment to the arterial wall. These studies identify NOR1 as a previously unrecognized key component of a transcriptional cascade regulating monocyte adhesion during atherogenesis.
METHODS
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Mice
Littermate NOR1−/− and NOR1+/+ mice on a mixed C57BL/6J/129Sv background were used as previously described. 14, 15 ApoE−/− mice on a C57BL/6J background (N10) were obtained from The Jackson Laboratory (stock #002052) and interbred with NOR1−/− mice to obtain NOR1−/−apoE−/− mice. At 8 to 10 weeks of age, littermate female NOR1+/+apoE−/−, NOR1+/−apoE−/− and NOR1−/−apoE−/− mice were fed a saturated fat enriched diet (TD88137; Harlan Teklad) for 12 weeks for atherosclerosis analysis or for 2 weeks to analyze NR4A gene expression.
Isolation of murine endothelial cells
Mouse aortic endothelial cells (MAEC) were isolated from aortae of littermate NOR1+/+ and NOR−/− mice using an explant technique. Aortic segments were placed on Cultrex Basement Membrane Extract gel (R & D Systems) and incubated in low-glucose DMEM supplemented with 15 % FBS, 180 μg/ml heparin and 20 μg/ml endothelial cell growth supplement. Migrated endothelial cells were passaged using dispase (BD Sciences) and cultured for 2 days in media containing D-valine to limit fibroblast contamination. Once confluent, MAEC were incubated with TNFα (R & D Systems) as indicated.
Adhesion assay
HUVEC were infected with 50 PFU Ad-CMV-Null or Ad-CMV-NOR1 for 6 hours and recovered for 48 hours. HUVEC treated with TNFα were employed as a positive control. THP-1 monocytes were pre-stained with 5 μM calcein-AM (Sigma-Aldrich) at 37 °C for 30 minutes. After washing in PBS, fluorescently labeled THP-1 monocytes were added onto the HUVEC monolayers at the density of 106 cells/ml. To block VCAM-1 and ICAM-1 function, HUVEC monolayers were incubated with blocking antibodies against VCAM-1 (25 μg/ml, BBA5, R & D Systems) and ICAM-1 (10 μg/ml, BBA3, R & D Systems) for 1 hour prior to the addition of THP-1 monocytes. Non-adherent monocytes were washed off after 30 minutes. For mouse monocyte adhesion assays the thoracic aortae of littermate NOR1+/+ or NOR1−/− mice were cut into segments, pinned on dental wax, and maintained in MAEC growth media supplemented with TNFα (1 ng/ml) or vehicle (PBS). After 6 hours, aortae were washed with PBS, and 700 μl of fluorescently labeled WEHI-274.1 cells were added at the density of 106 cells/ml. Non-adherent monocytes were removed after 30 minutes by washing with PBS. For both experiments, six pictures were taken for each condition, and adhesion was quantified by counting fluorescent monocytes attached to the endothelium.
RESULTS
NOR1 is expressed in endothelial cells of human coronary atherosclerotic lesions
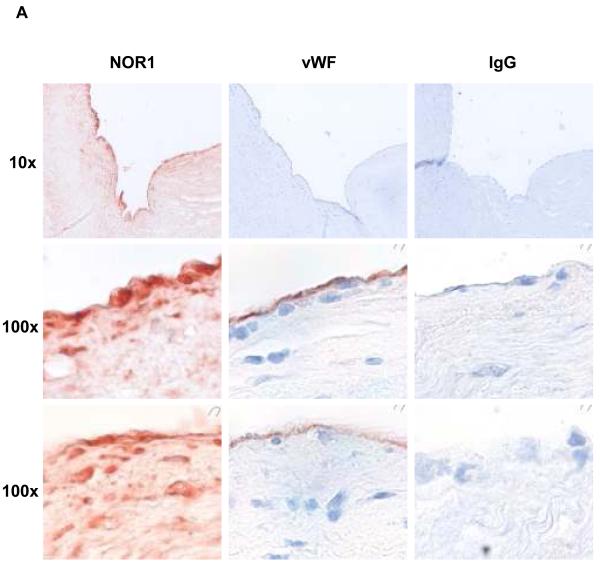
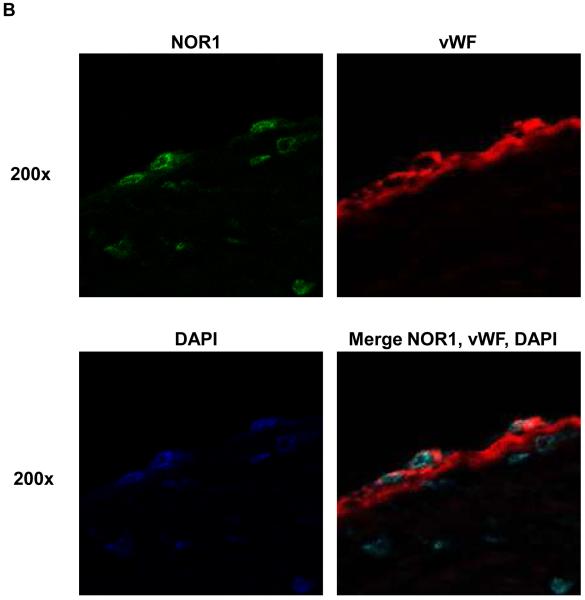
To characterize the distribution of NOR1 expression in atherosclerosis, human coronary arteries were immunostained for NOR1. NOR1 protein was readily detectable in atherosclerotic lesions (Figure 1A) but negligible in normal arteries (data not shown). In these atherosclerotic sections, high levels of NOR1 immunostaining were observed in the endothelial cell layer and in subendothelial cells of advanced atherosclerotic lesions. Colocalization experiments using confocal microscopy confirmed a typical nuclear expression pattern of NOR1 in endothelial cells staining positive for the endothelial cell marker vWF (Figure 1B).
Figure 1. NOR1 is present in endothelial cells of human coronary atherosclerotic lesions.
Sections of atherosclerotic human coronary arteries were immunostained for NOR1 and von Willebrand Factor (vWF) to identify endothelial cells. Species-matched normal IgG served as negative control. Images were acquired using (A) light microscopy and (B) confocal microscopy. In (B) stainings for NOR1, vWF and DAPI were merged into one image as indicated. Objective magnifications are indicated.
NOR1 is induced by inflammatory stimuli in endothelial cells
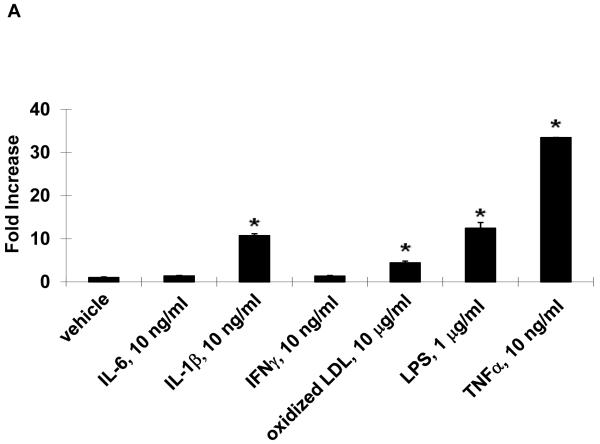
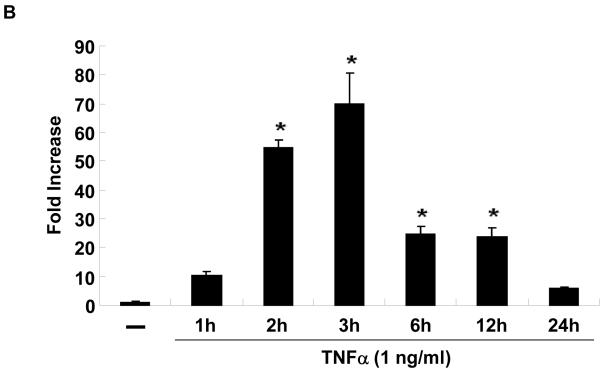
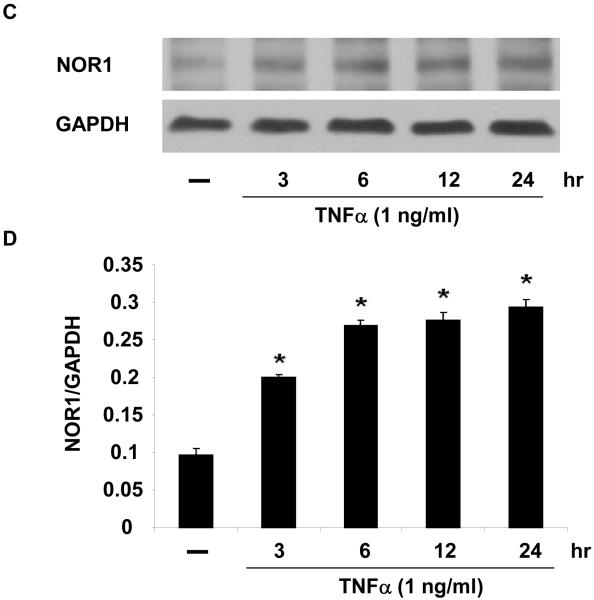
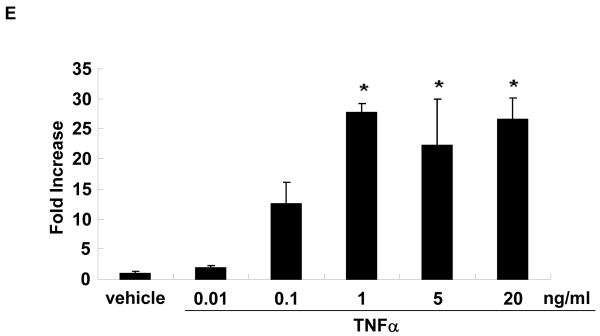
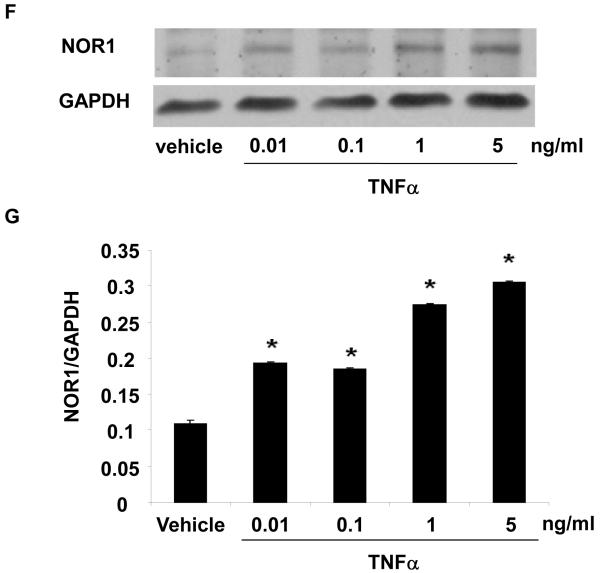
In vitro, stimulation of HUVEC with the inflammatory mediators IL-1β, oxidized LDL, LPS, or TNFα highly induced NOR1 mRNA expression (Figure 2A). In contrast, IL-6 or IFN-γ did not significantly induce NOR1 transcript levels in HUVEC. Considering that TNFα elicited a maximal increase in NOR1 expression, subsequent experiments focused on the regulation of NOR1 by TNFα. Consistent with previous reports characterizing NOR1 as an early response gene, 3, 4 time-course experiments confirmed a maximal induction of NOR1 mRNA after 3 h of TNFα stimulation (Figure 2B). This increase in NOR1 transcript levels was followed by a maximal induction of NOR1 protein expression after 6 h (Figure 2C-D). The observed regulation of NOR1 mRNA and protein expression by TNFα was dose-dependent revealing a maximal increase of NOR1 expression with 1 ng/ml TNFα (Figure 2E-G, respectively). Finally, TNFα induced a similar induction of NOR1 mRNA expression in primary MAEC isolated from NOR1 wildtype mice (Supplemental Figure I). In contrast, the other two members of the NR4A subfamily Nur77 and Nurr1 were only modestly induced in NOR1 wildtype cells, and this induction was lost in NOR1−/− MAEC.
Figure 2. NOR1 is induced by inflammatory stimuli in endothelial cells.
(A) HUVEC were stimulated as indicated for 2 hours and NOR1 mRNA expression was analyzed. (B-D) HUVEC were stimulated with TNF⟨ (1 ng/ml) and NOR1 mRNA (B) and protein expression (C) were analyzed at the indicated time points. (D) Densitometric analysis of NOR1 protein expression in (C). (E-G) HUVEC were stimulated with vehicle (PBS) or the indicated dose of TNF⟨. NOR1 mRNA (E) and protein (F) expression were analyzed after 2 hours or 6 hours, respectively. (G) Densitometric analysis of NOR1 protein expression in (F). NOR1 mRNA expression levels were normalized to hTBP and presented as mean ± SEM fold increase over vehicle-treated cells (*P < 0.05 vs. vehicle). Densitometric analysis was performed on three independent experiments. Cohybridization for GAPDH was performed to assess equal loading.
NOR1 expression in endothelial cells is mediated through an NF-κB-dependent transactivation of the NOR1 promoter
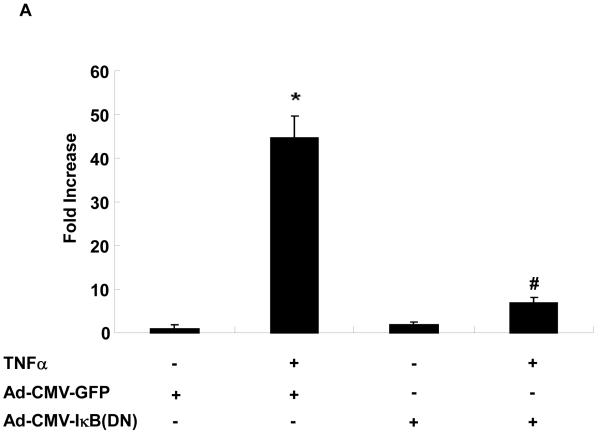
NOR1 is primarily regulated through transcriptional mechanisms, and sequence analysis of the NOR1 promoter identified several putative NF-κB binding sites. To address the functional relevance of these NF-κB sites, endothelial cells were infected with an adenoviral construct overexpressing a dominant-negative IκBα mutant. In cells overexpressing GFP as control, TNFα treatment resulted in a significant increase of NOR1 mRNA expression (Figure 3A). In contrast, overexpression of the dominant-negative IκBα mutant resulted in an almost complete inhibition of TNFα-induced NOR1 mRNA expression. These experiments indicate that TNFα-induced NOR1 expression is primarily mediated via NF-κB signaling in endothelial cells.
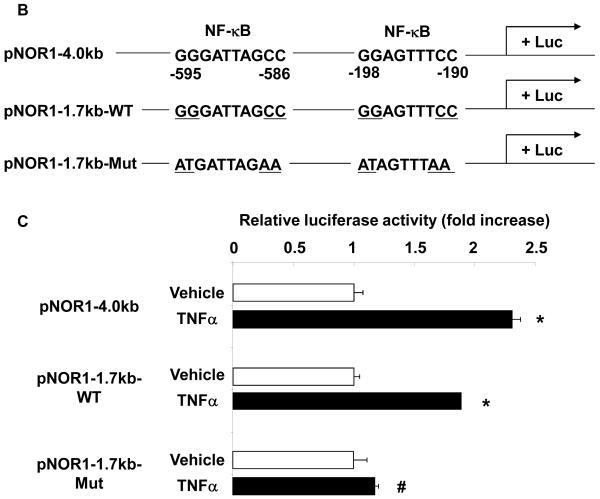
Figure 3. NOR1 expression in endothelial cells is mediated through NF-| B-dependent signaling pathways.
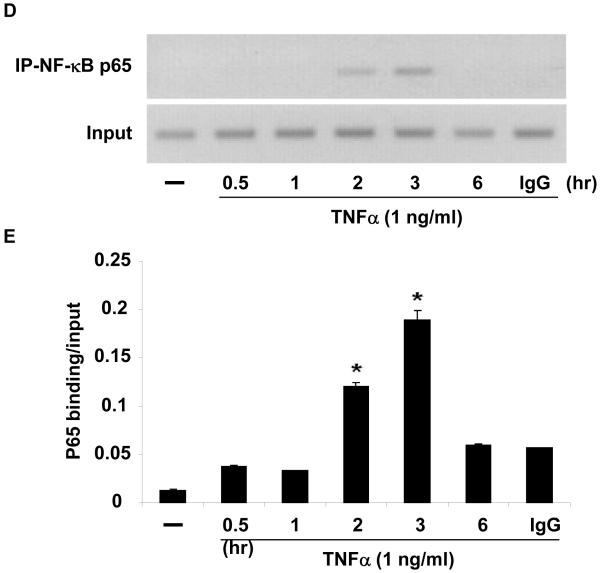
(A) HUVEC were infected with 25 PFU Ad-CMV-I| B(DN) or Ad-CMVGFP and stimulated with vehicle (PBS) or TNF⟨ (1 ng/ml) for 2 hours. NOR1 mRNA expression was analyzed and normalized to hTBP. Data are presented as mean ± SEM fold increase over Ad-CMV-GFP-infected cells treated with vehicle (*p < 0.05 vs. vehicle; #p < 0.05 vs. Ad-CMV-GFP). (B) Schematic structure of the human 4.0 kb and 1.7 kb NOR1 promoter constructs. (C) HAEC were transfected with a luciferase reporter construct driven by the indicated NOR1 promoter, stimulated with vehicle (PBS) or TNF⟨ (10 ng/ml), and analyzed for luciferase activities. Data are presented as mean ± SEM from three independently performed experiments (*p < 0.05 vs. vehicle; #p < 0.05 vs. pNOR1-1.7kb-WT). (D-E) HUVEC were stimulated with TNF⟨ (1 ng/ml) and harvested at the indicated time point for ChIP assays. Chromatin complexes were immunoprecipitated with an antibody against NF-| B p65 or species-matched IgG. PCR products were amplified using primers covering the −595 bp to −586 bp NF-| B binding site. (D) The autoradiograms are representative of three independently performed experiments. (E) Densitometric analysis of NF-| B p65 binding normalized to Input from three independently performed experiments.
To further confirm an NF-κB-dependent transcriptional regulation of NOR1 expression in endothelial cells, we performed transient transfection assays using a NOR1 promoter construct. This 4.0 kb full-length human NOR1 promoter construct includes two putative NF-κB binding sites at −595 to −586 and −198 to −190 from the transcription initiation site (Figure 3B). Stimulation of endothelial cells with TNFα resulted in a significant activation of the full-length NOR1 promoter (Figure 3C). This induction of the NOR1 promoter was maintained upon 5′-deletion to 1.7 kb but completely abolished following mutation of the two NF-κB binding sites, confirming that TNFα induces NOR1 promoter activity via an NF-κB-dependent transactivation of the promoter. As depicted in Figure 3D and E, ChIP assays revealed the recruitment of p65 to the NF-κB site in the endogenous NOR1 promoter in response to TNFα. Maximal promoter occupancy was observed after 3 h, which is consistent with the kinetics of NOR1 mRNA expression in response to inflammatory stimuli. In concert, these data demonstrate that TNFα induces NOR1 expression through a NF-κB-dependent signaling pathway resulting in the subsequent transactivation of the proximal NOR1 promoter.
NOR1 positively regulates VCAM-1 and ICAM-1 in endothelial cells
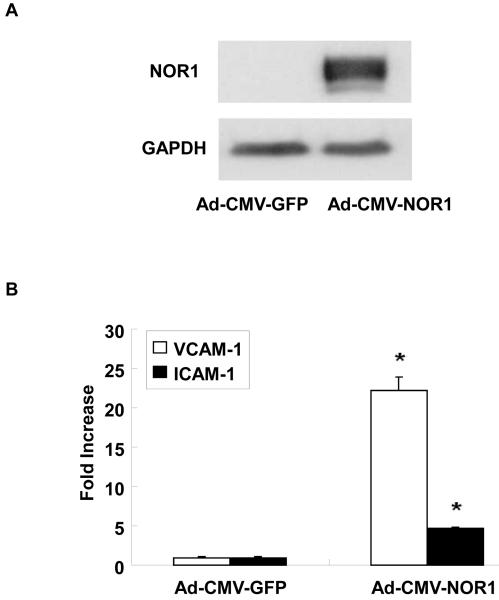
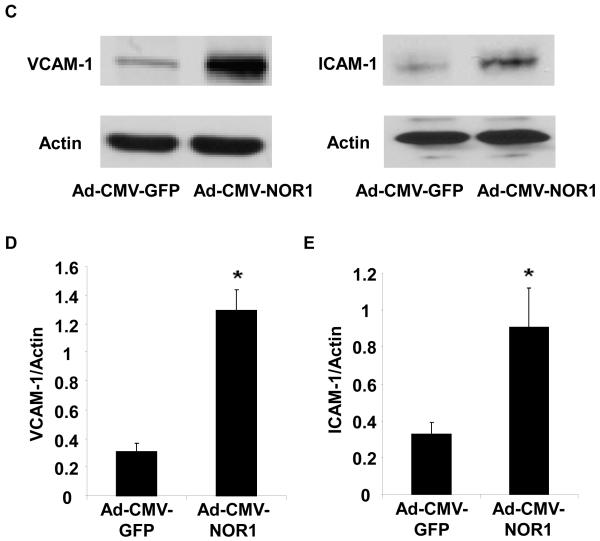
To explore whether NOR1 is involved in the transcriptional control of endothelial cell responses, we next infected HUVEC with an adenovirus overexpressing human NOR1 (Figure 4A). As depicted in Figure 4B-E, NOR1 overexpression resulted in a prominent induction of VCAM-1 and ICAM-1 mRNA and protein expression levels, respectively.
Figure 4. NOR1 induces VCAM-1 and ICAM-1 expression in endothelial cells.
(A-E) HUVEC were infected with 50 PFU Ad-CMV- NOR1 or Ad-CMV-GFP. (A) Western Blotting confirmed NOR1 overexpression after transduction. (B) VCAM-1 and ICAM-1 mRNA expression was determined by real-time RT-PCR. Experiments were performed at least three times in triplicate. Data were calculated by normalizing NOR1 values to hTBP values and presented as mean ± SEM fold induction over Ad-CMV-GFP infected cells (*p < 0.05 vs. Ad-CMV-GFP). (C-E) VCAM-1 and ICAM-1 protein expression was analyzed by Western blotting. Cohybridization for actin was performed to assess equal loading. (C) Representative autoradiograms from densitometric analysis (D and E) of three independently performed experiments.
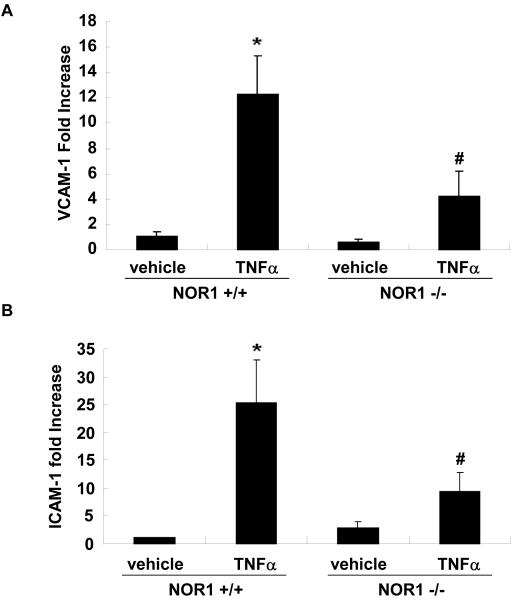
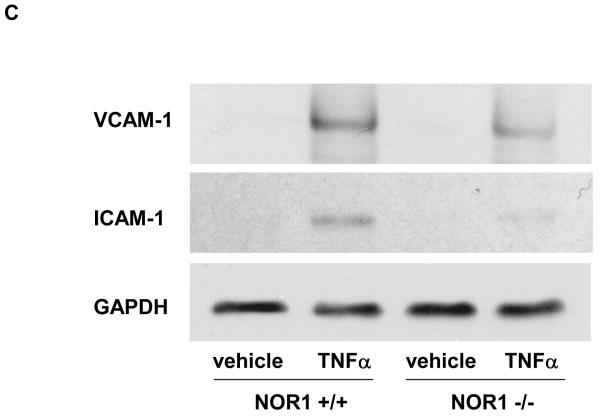
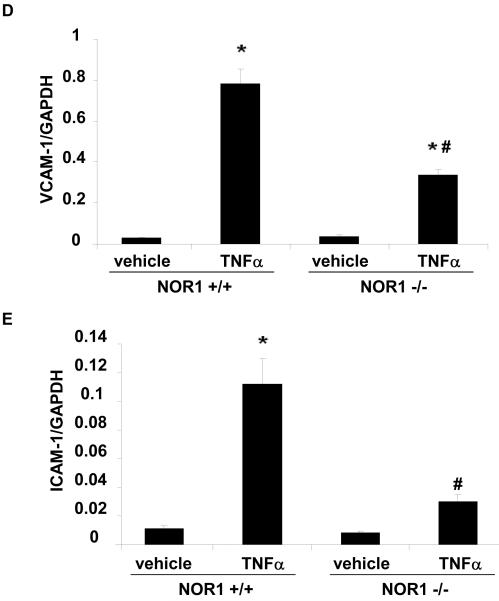
We next employed a murine model to address whether NOR1 is also required for VCAM-1 and ICAM-1 expression in endothelial cells. In these experiments, MAEC were isolated from littermate NOR1 wildtype and NOR1-deficient mice. 14, 15 As depicted in Figure 5A-E, TNFα stimulation profoundly increased VCAM-1 and ICAM-1 mRNA and protein expression in NOR1 wildtype MAEC. In contrast, the induction of both adhesion molecules was markedly reduced in MAEC isolated from NOR1-deficient mice. Similar data were obtained when cells were stimulated with IL-1β or LPS (Supplemental Figure IIA-C).
Figure 5. NOR1 is required for VCAM-1 and ICAM-1 expression in endothelial cells.
(A-E) NOR1+/+ and NOR1−/− MAEC were stimulated with vehicle or TNF⟨ (1 ng/ml) for 6 hours. VCAM-1 and ICAM-1 mRNA (A and B) and protein (C-E) expression were analyzed by real-time RT-PCR and Western blotting, respectively. (C) The autoradiograms are representative of three independent experiments. (D and E) Densitometric analysis of VCAM-1 and ICAM-1 protein expression after Western blotting. All experiments were repeated at least three times in duplicates with different cell preparations. Results are presented as mean ± SEM fold increase over vehicle-treated wildtype cells (*P < 0.05 vs. vehicle, # P < 0.05 vs. NOR1+/+ cells).
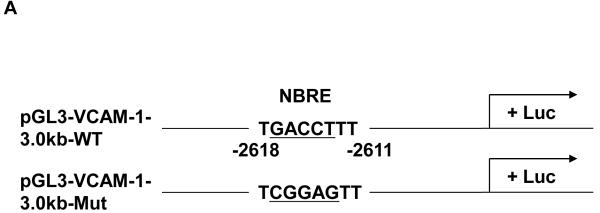
NOR1 transactivates the VCAM-1 promoter by binding to a canonical NBRE consensus site
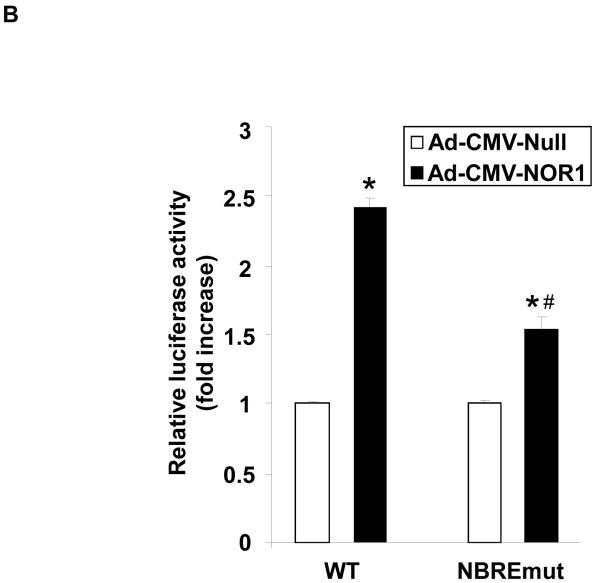
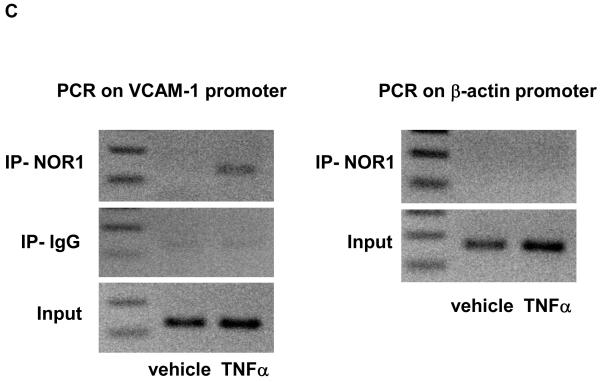
We next performed transient transfection assays to investigate the underlying mechanism by which NOR1 regulates VCAM-1 expression in endothelial cells. NOR1 induces transcription by binding to NBRE consensus sites in target gene promoters. 8 Interestingly, sequence analysis of the human VCAM-1 promoter identified a canonical NBRE site at −2618 bp from the transcription initiation site (Figure 6A). Transient transfection of HUVEC with a luciferase reporter construct driven by the human 3.0 kb VCAM-1 promoter revealed that overexpression of NOR1 increases VCAM-1 promoter activity (Figure 6B). However, this transcriptional induction was significantly altered upon site-directed mutagenesis of the canonical NBRE motif. ChIP assays subsequently confirmed that TNFα induced the recruitment of NOR1 to this NBRE site in the VCAM-1 promoter (Figure 6C). These experiments demonstrate that NOR1 transactivates the VCAM-1 promoter by binding to a NBRE consensus site in the promoter and characterize VCAM-1 as NOR1-regulated target gene.
Figure 6. NOR1 transactivates the VCAM-1 promoter by binding to an NBRE consensus site.
(A) Schematic structure of the human 3.0 kb VCAM-1 promoter and the NBRE consensus site at −2618 bp. (B) HUVEC were infected with 50 PFU Ad-CMV-Null or Ad-CMV-NOR1 for 6 hours and recovered for 24 hours. Infected cells were transiently transfected with luciferase reporter constructs driven by the VCAM-1 wildtype promoter or the similar promoter bearing a mutation in the NBRE site. Luciferase activity was analyzed after 48 h and data are presented as mean ± SEM fold induction from three independently performed experiments (*p < 0.05 vs. empty vector; #p < 0.05 vs. pVCAM-1-WT). (C) HUVEC were stimulated with vehicle (PBS) or TNF⟨ (1 ng/ml) for ChIP assays. PCR for an unrelated promoter fragment in the ®-actin promoter served as control for specificity. The autoradiograms are representative of three independently performed experiments.
NOR1 mediates monocyte adhesion by regulating VCAM-1 and ICAM-1 expression
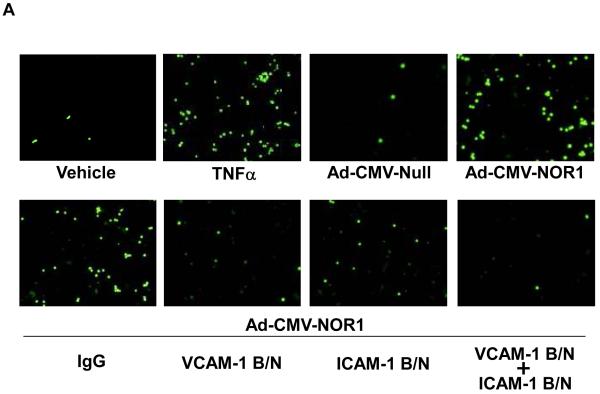
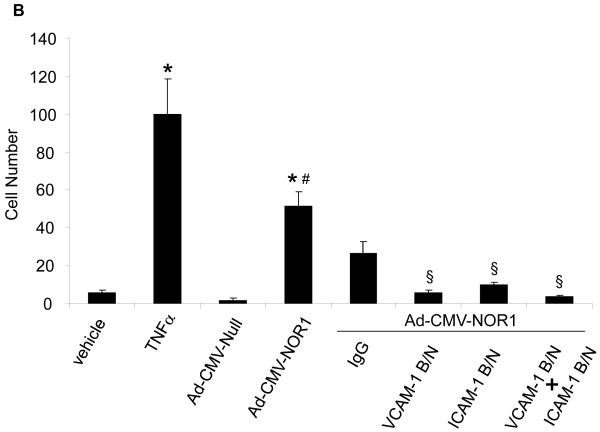
VCAM-1 and ICAM-1 have both been well characterized to mediate monocyte adhesion to the endothelium leading to the infiltration of monocytes into the sub-endothelial area and atherosclerosis development. 17 To investigate whether the transcriptional induction of VCAM-1 and ICAM-1 by NOR1 is sufficient to promote monocyte adhesion, we analyzed THP-1 monocyte adhesion to HUVEC overexpressing NOR1. Stimulation of a HUVEC monolayer with TNFα profoundly increased monocyte adhesion, confirming the validity of the assay (Figure 7A, upper panel). Compared to HUVEC infected with an adenovirus overexpressing an empty vector as control, overexpression of NOR1 significantly increased monocyte adhesion in the absence of TNFα stimulation (Figure 7A and B). Furthermore, monocyte adhesion induced by NOR1 overexpression was almost completely abolished by pre-incubation of HUVEC with VCAM-1 and ICAM-1 blocking antibodies (Figure 7A and 7B). Conversely, acute knock-down of NOR1 in HUVEC using siRNA significantly decreased monocyte adhesion (Supplemental Figure IIIA-C). Collectively, these studies indicate that NOR1 is necessary and sufficient for monocyte adhesion and that this activity is mediated primarily by inducing the expression of VCAM-1 and ICAM-1.
Figure 7. NOR1 mediates monocyte adhesion by regulating VCAM-1 and ICAM-1 expression.
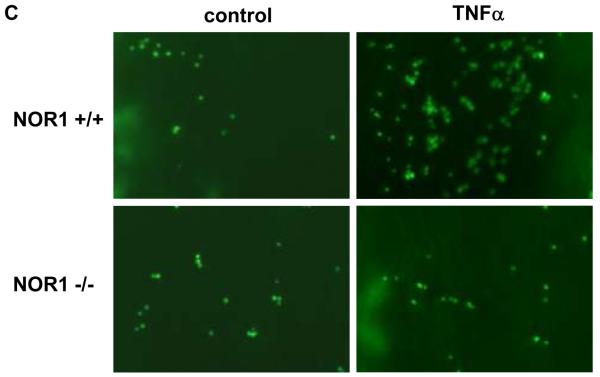
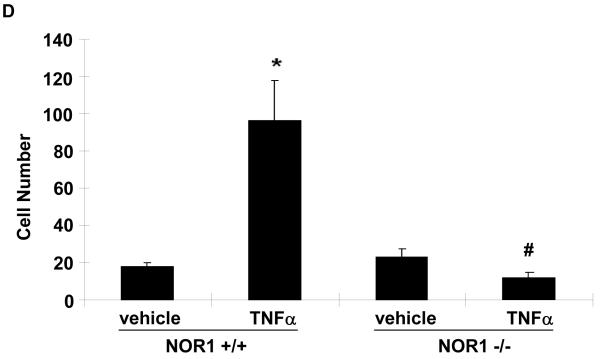
(A) Upper panel: HUVEC were infected with 50 PFU Ad-CMV-NOR1 or Ad-CMV-Null, and fluorescently labeled THP-1 monocytes were added onto HUVEC monolayers. TNF⟨-treated (1 ng/ml) HUVEC were employed as a positive control. Lower panel: Ad-CMV-NOR1-infected cells were pre-incubated with VCAM-1 and ICAM-1 blocking antibodies (B/N) or control IgG. After 1 hour, fluorescently labeled THP-1 monocytes were added onto HUVEC monolayers. (B) Quantification is presented as mean ± SEM from three independently performed experiments in duplicates (*p < 0.05 vs. vehicle or Ad-CMV-Null, # p < 0.05 vs. TNF⟨, § p < 0.05 vs. IgG). (C and D) Aortae were isolated from NOR1+/+ and NOR1−/− mice and TNF⟨-induced monocyte adhesion was analyzed. (C) Representative sections demonstrating adhesion of monocytes. (D) Quantification of adhesion from three independent experiments performed in duplicates using different aortic preparations. Data are expressed as mean ± SEM (*P < 0.05 vs. vehicle, # P < 0.05 vs. NOR1+/+ cells).
To further confirm a causal contribution of NOR1-dependent VCAM-1 and ICAM-1 expression for monocyte adhesion ex vivo, we performed adhesion assays with aortae isolated from NOR1+/+ and NOR1−/− mice. Incubation with TNFα significantly increased the adhesion of monocytes to the aortic endothelial cell layer of NOR1 wildtype mice (Figures 7C-D). However, this inducible adhesion was completely abolished on aortae of NOR1-deficient mice. From these findings we infer that NOR1 expression in resident endothelial cells is necessary for mediating monocyte adhesion.
NOR1 deficiency decreases atherosclerosis formation and reduces macrophage recruitment in apoE−/− mice
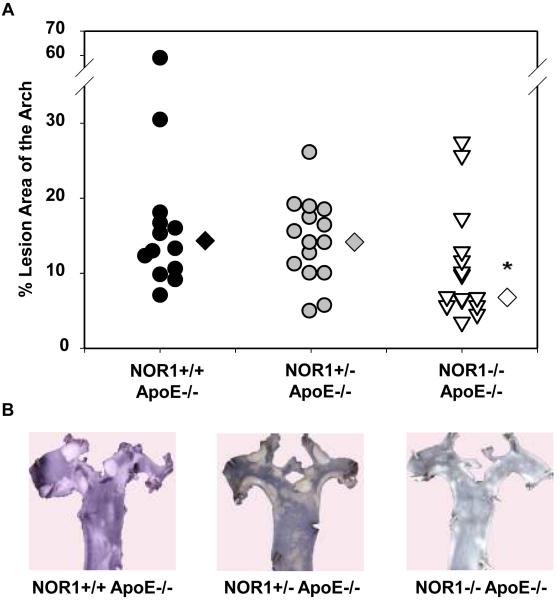
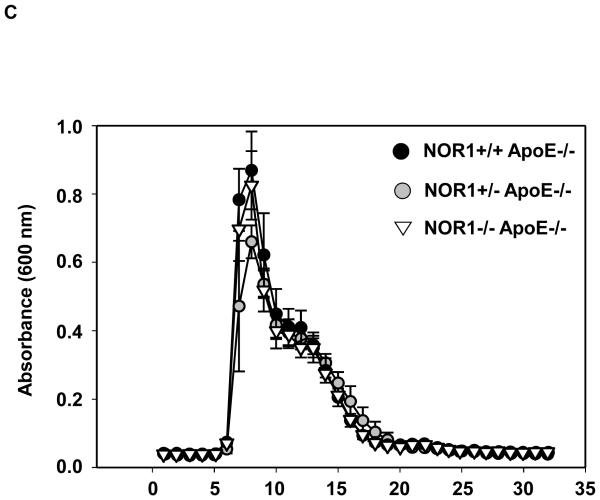
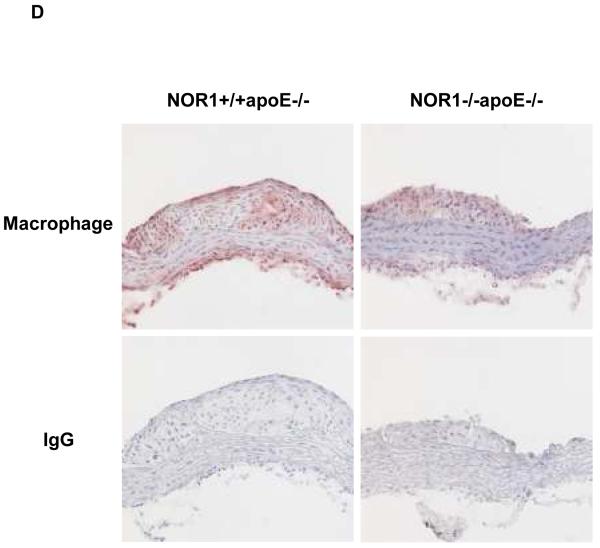
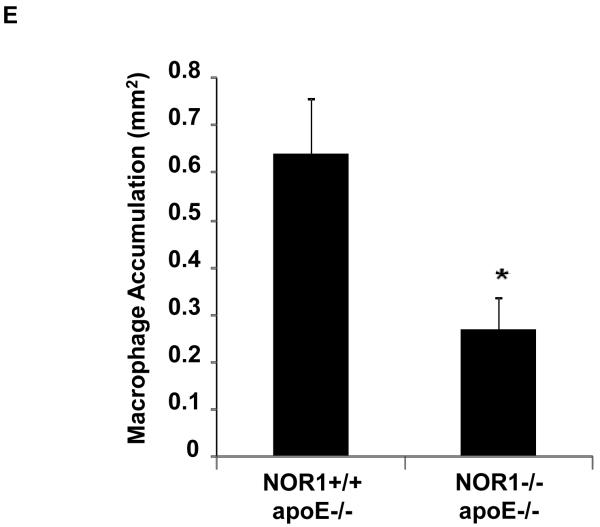
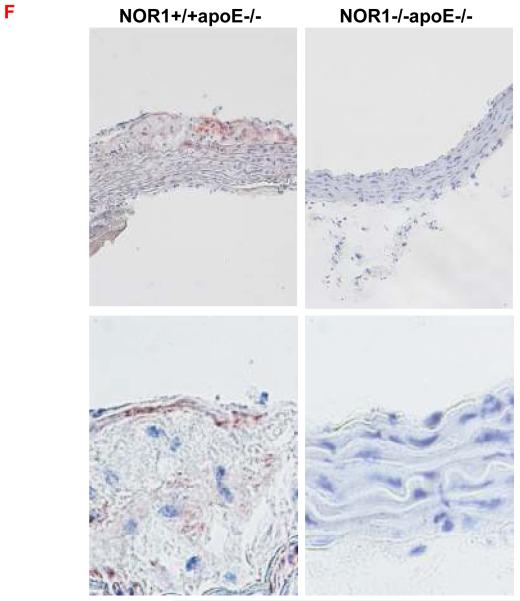
Considering that monocyte adhesion constitutes a critical initial step for atherogenesis, 17 we next investigated whether NOR1-deficiency decreases atherosclerotic lesion development. Consistent with the important function of NOR1 to regulate monocyte adhesion ex vivo, homozygous deletion of NOR1 in apoE−/− mice resulted in a 52 % reduction of atherosclerosis compared to their wildtype littermates (NOR1+/+apoE−/− 14.3 % (n=14), NOR1+/−apoE−/− 14.0 % (n=15), and NOR1−/−apoE−/− 6.8 % (n=15) median atherosclerotic lesion area of aortic arches; P < 0.05; Figure 8A-B). NOR1-deficiency revealed no overt effect on cholesterol distribution confirming a direct effect of NOR1 on lesion formation (Figure 8C). Immunostaining of atherosclerotic tissues and quantification of the macrophage content confirmed macrophage-enriched lesions in NOR1+/+apoE−/− mice that were considerably less with decreased macrophage content in NOR1−/−apoE−/− mice (Figure 8D-E and Supplemental Figure IV). Finally, while VCAM-1 was readily detectable in atherosclerotic lesions from NOR1+/+apoE−/− mice, there was a paucity of immunoreactivity for this adhesion molecule noted in the vascular wall of NOR1−/−apoE−/− mice (Fig. 8F).
Figure 8. NOR1 deficiency decreases atherosclerosis in apoE−/− mice.
(A) Atherosclerotic lesion size was measured on aortic arches from female NOR1+/+apoE−/− (n=14), NOR1+/−apoE−/− (n=15) and NOR1−/−apoE−/− (n=15) mice. Circles and triangles represent individual mice; diamonds represent medians (P < 0.05 between group, *P < 0.05 vs. NOR1+/+apoE−/−). (B) Representative aortic arches from each genotype. (C) Lipoprotein cholesterol distributions. Values represent the mean cholesterol content of each fraction (± SEM). (D-F) Sections of atherosclerotic lesions were immunostained using antisera against macrophages or VCAM-1. (D) Representative sections for macrophage staining (top) or IgG (bottom) (objective magnification ×20). (E) Macrophage accumulation was quantified in the aortic arch from NOR1+/+apoE−/− and NOR1−/−apoE−/− mice (*P < 0.05 vs. NOR1+/+apoE−/−). (F) Representative sections for VCAM-1 staining (objective magnification ×20 and ×100).
DISCUSSION
Monocyte adhesion constitutes a critical event for the initiation of atherosclerosis; 17 however, the molecular mechanisms that orchestrate monocyte-endothelial cell interactions are incompletely understood. In the present study, we report a previously unrecognized role for the nuclear receptor NOR1 to serve as a transcriptional regulator of adhesion molecule expression and monocyte recruitment during atherosclerosis. In endothelial cells, NOR1 is rapidly induced by inflammatory stimuli via an NF-κB-dependent transactivation of the NOR1 promoter. Loss and gain-of-function studies establish that NOR1 positively regulates VCAM-1 and ICAM-1 expression in endothelial cells leading to increased monocyte adhesion. Consistent with the key role of NOR1 to promote monocyte adhesion to the endothelium, our studies further demonstrate that NOR1 deficiency results in decreased atherosclerosis development and macrophage recruitment in apoE-deficient mice.
Consistent with recent studies, we identified abundant NOR1 expression in endothelial cells12 as well as in cells of the subendothelial space likely representing macrophages and SMC. 14, 18-20 However, the transcriptional mechanisms governing inducible NOR1 expression in endothelial cells remain elusive. Our data provides evidence for an NF-κB-dependent induction of NOR1 transcription during endothelial cell inflammation. Inhibition of NF-κB signaling in endothelial cells repressed inducible NOR1 expression in response to inflammatory stimulation. Using site-directed mutagenesis and ChIP assays, we identified two functional NF-κB sites in the NOR1 promoter, to which p65 is recruited during inflammatory activation of endothelial cells. Earlier studies have demonstrated that NOR1 expression in endothelial cells is highly induced by mitogens through a cAMP response element binding protein (CREB)-dependent activation of the proximal region of NOR1 promoter. 16 Furthermore, hypoxia has recently been reported to induce NOR1 in endothelial cells through a mechanism involving the hypoxia-inducible factor (HIF) family of transcription factors. 21 These studies, in concert with our data characterizing NOR1 as a NF-κB target gene in endothelial cells, point to distinct transcriptional mechanisms regulating the rapid NOR1 induction in response to various environmental cues.
Compared with the well-studied early-response genes encoding proteins of the AP-1 complex, little is known about the physiological function of NOR1 and its regulated target genes, yet the high degree of conservation points to an important role in the control of gene expression. A previous study has provided initial evidence to support a functional role of NOR1 in endothelial cells by demonstrating that NOR1 regulates growth of this cell type. 16 The data presented here extends these findings and points to an unsuspected function of NOR1 to serve as a positive regulator of monocyte adhesion. In experiments using adenoviral-mediated overexpression, NOR1 induced VCAM-1 and ICAM-1 expression resulting in increased monocyte adhesion. The observation that this inducible adhesion was abolished when VCAM-1 and ICAM-1 function were blocked, suggests that the induction of both adhesion molecules by NOR1 constitutes a primary mechanism by which NOR1 induces monocyte adhesion. Conversely, the inducible expression of both adhesion molecules in response to inflammatory activation was attenuated in NOR1-deficient endothelial cells. Furthermore, consistent with these findings, TNFα-induced monocyte adhesion to HUVEC transfected with NOR1 siRNA or to the endothelium of NOR-deficient mice was altered ex vivo, suggesting that vascular NOR1 expression is not only sufficient but also required for monocyte adhesion.
An intriguing question that arises from the observation that NOR1 induces the expression of VCAM-1 and ICAM-1 relates to the mechanisms by which NOR1 positively regulates these genes. Initial sequence analysis identified putative NBRE consensus sites in both the VCAM-1 and ICAM-1 promoters. Exemplified by the VCAM-1 promoter, our studies demonstrate that the molecular mechanisms underlying this novel function of NOR1 involve at least in part a direct transactivation of the VCAM-1 promoter by NOR1. In response to inflammatory activation NOR1 is recruited to a canonical NBRE site in the VCAM-1 promoter. Moreover, the observation that NOR1-dependent VCAM-1 promoter transactivation was attenuated upon mutation of this NBRE site confirms the functionality of this NBRE motif. However, the residual induction of the VCAM-1 promoter bearing a mutation of this NBRE site suggests that additional transcriptional mechanisms may regulate NOR1 expression. NOR1 has recently been shown to transactivate the inducible IκB kinase (IKKi/IKKepsilon) promoter, which phosphorylates IκBα and induces NF-κB activation. 22 Therefore, in addition to a direct transactivation, NOR1 may function as a positive upstream regulator of NF-κB signaling and indirectly activate the VCAM-1 and ICAM-1 promoters. Alternatively, NOR1 deficiency may affect other transcriptional networks acting on these promoters, including for example the AP-1 complex. We have recently demonstrated that the combined deficiency of NOR1 and its sibling Nur77 decreases the expression of AP-1 transcription factors, 23 which may regulate adhesion molecule expression. 24 Clearly, the findings presented here provide justification for further investigating the transcriptional mechanisms by which NOR1 regulates endothelial cell gene expression and promotes monocyte adhesion.
The protein products of the VCAM-1 and ICAM-1 genes are well established to participate in atherogenesis by promoting macrophage accumulation in the arterial intima. 17 Consistent with this evidence and with the observed regulation of endothelial cell adhesion molecule expression by NOR1, we provide the first evidence that NOR1 deficiency decreases atherosclerosis in apoE-deficient mice. Considering that all three members of the NR4A receptor subfamily bind to an NBRE site, functional redundancy in certain cell types between Nur77 and NOR1 has been suggested. 25 However, NOR1-deficiency did not result in a compensatory upregulation of the siblings Nur77 and Nurr1 in endothelial cells (Supplemental Figure 1) or in the aortae of NOR1−/−apoE−/− mice (Supplemental Figure V). Therefore, the previously reported phenotypes in NOR1-deficient mice 10, 11, 15 in concert with the decreased atherosclerosis in NOR1−/−apoE−/− mice presented in this study, point to a function of NOR1 that is distinct and not compensated by Nur77 or Nurr1. As evidenced by the decreased accumulation of macrophages in the vascular wall of NOR1−/−apoE−/− mice, monocyte recruitment represents at least one plausible mechanism by which NOR1 acts atherogenic. However, it is possible if not likely that there are additional mechanisms by which NOR1 promotes atherosclerosis development. NOR1 induces neointimal proliferation of SMC 15 and inflammatory gene expression in macrophages 22, which both could affect lesion development. Therefore, characterization of the cell-specific role of NOR1 in atherosclerosis will be necessary and will have to rely on tissue-specific gene targeting strategies. In conclusion, data presented here characterize the orphan nuclear receptor NOR1 as a novel positive regulator of monocyte adhesion by inducing VCAM-1 and ICAM-1 transcription. Continued investigation of the transcriptional networks regulated by NOR1 will provide new insights into how this orphan nuclear receptor participates in the development of vascular diseases.
NOVELTY AND SIGNIFICANCE.
What is known?
NR4A orphan nuclear receptors constitute a group of conserved transcription factors that function as early response genes.
Members of the NR4A orphan nuclear receptor subfamily, including Nur77, NOR1, and Nurr1, are highly expressed in atherosclerotic lesions.
NOR1 expression is inducible in endothelial cells.
What New Information Does This Article Contribute?
NOR1 expression in endothelial cells is mediated via an NF-κB-dependent activation of its promoter.
NOR1 induces monocyte adhesion through a transcriptional induction of the adhesion molecules VCAM-1 and ICAM-1 in endothelial cells
NOR1 transactivates the VCAM-1 promoter in endothelial cells by binding to a canonical response element for NR4A receptors.
Deficiency of NOR1 in apoE−/− mice reduces atherosclerosis formation and macrophage content of the lesion.
Monocyte adhesion and recruitment into the subendothelial space constitute critical first events for the initiation of atherosclerosis. In this study, we introduce a novel transcriptional mechanism mediating monocyte adhesion to the endothelium and subsequent atherosclerosis development. Although NR4A orphan nuclear receptors are highly conserved transcription factors expressed in atherosclerosis, surprisingly little is known about their function, and whether these receptors play a causal role in atherosclerosis has not been investigated. Expression of the NR4A receptor NOR1 was induced in endothelial cells by proatherogenic stimuli and mediated through an NF-κB-dependent transcriptional mechanism. Using loss- and gain-of-function approaches, we establish that NOR1 induces expression of the key adhesion molecules VCAM-1 and ICAM-1 resulting in increased monocyte adhesion. VCAM-1 constitutes a bona fide target gene for NOR1, which is activated by binding to a canonical response element. Consistent with this novel activity of NOR1, we demonstrate for the first time that deletion of this NR4A receptor reduces atherosclerotic lesion formation and monocyte recruitment into the arterial wall. Collectively, these experiments characterize a novel transcriptional cascade underlying atherogenesis and have important implications for our understanding of this disease.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
These studies were supported by the NIH (RO1HL084611 and RO1HL084611-04S1 to D. B. and RO1 CA111411 to O.M.C.). F. Gizard and Y. Zhao were supported by Fellowship Grants from the American Heart Association (0725313B and 0815514D, respectively).
Non-standard Abbreviations and Acronyms
- IKKi/IKKepsilon
inducible IκB kinase
- FPLC
fast protein liquid chromatography
- HAEC
human aortic endothelial cells
- MAEC
mouse aortic endothelial cells
- NBRE
NGFI-B response element
- TFIIB
transcription factor IIB
- TBP
TATA-binding protein
Footnotes
DISCLOSURES
None.
Subject Codes: [96] Mechanism of atherosclerosis/growth factors; [134] Pathophysiology; [95] Endothelium/vascular type/nitric oxide
In May 2010, the average time from submission to first decision for all original research papers submitted to Circulation Research was 14.6 days.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal. 2006;4:e002. doi: 10.1621/nrs.04002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pols TW, Bonta PI, de Vries CJ. NR4A nuclear orphan receptors: protective in vascular disease? Curr Opin Lipidol. 2007;18:515–520. doi: 10.1097/MOL.0b013e3282ef77d1. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Gonzalez J, Rius J, Castello A, Cases-Langhoff C, Badimon L. Neuron-Derived Orphan Receptor-1 (NOR-1) Modulates Vascular Smooth Muscle Cell Proliferation. Circ Res. 2003;92:96–103. doi: 10.1161/01.es.0000050921.53008.47. [DOI] [PubMed] [Google Scholar]
- 4.Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A Orphan Nuclear Receptor Expression in Macrophages in Response to Inflammatory Stimuli. J. Biol. Chem. 2005;280:29256–29262. doi: 10.1074/jbc.M502606200. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, Xu H, Walker NPC, Perlmann T. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature. 2003;423:555–560. doi: 10.1038/nature01645. [DOI] [PubMed] [Google Scholar]
- 6.Paulsen RE, Weaver CA, Fahrner TJ, Milbrandt J. Domains regulating transcriptional activity of the inducible orphan receptor NGFI-B. J Biol Chem. 1992;267:16491–16496. [PubMed] [Google Scholar]
- 7.Davis I, Hazel T, Chen R, Blenis J, Lau L. Functional domains and phosphorylation of the orphan receptor Nur77. Mol Endocrinol. 1993;7:953–964. doi: 10.1210/mend.7.8.8232315. [DOI] [PubMed] [Google Scholar]
- 8.Wilson TE, Fahrner TJ, Johnston M, Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252:1296–1300. doi: 10.1126/science.1925541. [DOI] [PubMed] [Google Scholar]
- 9.Zetterstrom R, Solomin L, Mitsiadis T, Olson L, Perlmann T. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol Endocrinol. 1996;10:1656–1666. doi: 10.1210/mend.10.12.8961274. [DOI] [PubMed] [Google Scholar]
- 10.Ponnio T, Burton Q, Pereira FA, Wu DK, Conneely OM. The nuclear receptor Nor-1 is essential for proliferation of the semicircular canals of the mouse inner ear. Mol. Cell. Biol. 2002;22:935–945. doi: 10.1128/MCB.22.3.935-945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponnio T, Conneely OM. nor-1 Regulates Hippocampal Axon Guidance, Pyramidal Cell Survival, and Seizure Susceptibility. Mol. Cell. Biol. 2004;24:9070–9078. doi: 10.1128/MCB.24.20.9070-9078.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arkenbout EK, van Bragt M, Eldering E, van Bree C, Grimbergen JM, Quax PHA, Pannekoek H, de Vries CJM. TR3 Orphan Receptor Is Expressed in Vascular Endothelial Cells and Mediates Cell Cycle Arrest. Arterioscler Thromb Vasc Biol. 2003;23:1535–1540. doi: 10.1161/01.ATV.0000084639.16462.7A. [DOI] [PubMed] [Google Scholar]
- 13.Bonta PI, van Tiel CM, Vos M, van Thienen JV, Ferreira V, Arkenbout EK, Seppen J, Spek CA, van der Poll T, Pannekoek H, de Vries CJM. Nuclear Receptors Nur77, Nurr1, and NOR-1 Expressed in Atherosclerotic Lesion Macrophages Reduce Lipid Loading and Inflammatory Responses. Arterioscler Thromb Vasc Biol. 2006;26:2288–94. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- 14.Nomiyama T, Nakamachi T, Gizard F, Heywood EB, Jones KL, Ohkura N, Kawamori R, Conneely OM, Bruemmer D. The NR4A orphan nuclear receptor NOR1 is induced by platelet-derived growth factor and mediates vascular smooth muscle cell proliferation. J Biol Chem. 2006;281:33467–33476. doi: 10.1074/jbc.M603436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nomiyama T, Zhao Y, Gizard F, Findeisen HM, Heywood EB, Jones KL, Conneely OM, Bruemmer D. Deficiency of the NR4A neuron-derived orphan receptor-1 attenuates neointima formation after vascular injury. Circulation. 2009;119:577–586. doi: 10.1161/CIRCULATIONAHA.108.822056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rius J, Martinez-Gonzalez J, Crespo J, Badimon L. NOR-1 is involved in VEGF-induced endothelial cell growth. Atherosclerosis. 2006;184:276–282. doi: 10.1016/j.atherosclerosis.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Galkina E, Ley K. Vascular Adhesion Molecules in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 18.Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem. 2005;280:29256–29262. doi: 10.1074/jbc.M502606200. [DOI] [PubMed] [Google Scholar]
- 19.Bonta PI, van Tiel CM, Vos M, Pols TW, van Thienen JV, Ferreira V, Arkenbout EK, Seppen J, Spek CA, van der Poll T, Pannekoek H, de Vries CJ. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006;26:2288–2294. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- 20.Arkenbout EK, de Waard V, van Bragt M, van Achterberg TA, Grimbergen JM, Pichon B, Pannekoek H, de Vries CJ. Protective function of transcription factor TR3 orphan receptor in atherogenesis: decreased lesion formation in carotid artery ligation model in TR3 transgenic mice. Circulation. 2002;106:1530–1535. doi: 10.1161/01.cir.0000028811.03056.bf. [DOI] [PubMed] [Google Scholar]
- 21.Martorell L, Gentile M, Rius J, Rodriguez C, Crespo J, Badimon L, Martinez-Gonzalez J. The Hypoxia-Inducible Factor 1/NOR-1 Axis Regulates the Survival Response of Endothelial Cells to Hypoxia. Mol. Cell. Biol. 2009;29:5828–5842. doi: 10.1128/MCB.00945-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pei L, Castrillo A, Tontonoz P. Regulation of Macrophage Inflammatory Gene Expression by the Orphan Nuclear Receptor Nur77. Mol Endocrinol. 2006;20:786–794. doi: 10.1210/me.2005-0331. [DOI] [PubMed] [Google Scholar]
- 23.Mullican SE, Zhang S, Konopleva M, Ruvolo V, Andreeff M, Milbrandt J, Conneely OM. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med. 2007;13:730–735. doi: 10.1038/nm1579. [DOI] [PubMed] [Google Scholar]
- 24.Lin JHC, Zhu Y, Ling Liao H, Kobari Y, Groszek L, Stemerman MB. Induction of vascular cell adhesion molecule-1 by low-density lipoprotein. Atherosclerosis. 1996;127:185–194. doi: 10.1016/s0021-9150(96)05951-5. [DOI] [PubMed] [Google Scholar]
- 25.Cheng LE, Chan FK, Cado D, Winoto A. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. Embo J. 1997;16:1865–1875. doi: 10.1093/emboj/16.8.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.