Abstract
We have previously reported that 14-days of chronic intermittent cold (CIC) stress induced a cognitive deficit in reversal learning on the rat attentional set-shifting test. This effect may be related to dysregulation of 5-HT function in orbitofrontal cortex, as a model of cognitive dysfunction in depression. To test the ability of chronic antidepressant drug treatment to reverse the cognitive deficit induced by CIC, it was first necessary to assess the temporal characteristics of the CIC-induced cognitive deficit. Thus, in the first study, we assessed the duration of the cognitive deficit following 2-weeks CIC stress. Replicating previous experiments, CIC induced a reversal learning deficit tested 3 days after the last cold exposure. However, cognitive performance of CIC-stressed rats was no different from unstressed controls when tested 7, 14 or 21 days after termination of the stress treatment. We next compared behavior 3 days after 2-weeks CIC to that seen 3 days after 5-weeks CIC, and found similar deficits in reversal learning. Thus, in the final study, antidepressant drug treatment was initiated after 2-weeks of CIC stress, and was maintained for 3 weeks, concurrent with the continuation of CIC stress. Both chronic and acute treatment with the selective serotonin reuptake inhibitor, citalopram, but not the norepinephrine reuptake blocker, desipramine, reversed the cognitive deficit induced by CIC stress. Thus, this stress-induced cognitive deficit may be a useful model for cognitive deficits related to prefrontal cortical hypoactivity in depression, and for investigating neurobiological mechanisms underlying the beneficial effects of chronic antidepressant drug treatment.
Keywords: antidepressants, chronic stress, cognition, depression, prefrontal cortex
Introduction
Chronic stress is a risk factor for depression and dysregulation of brain monoaminergic neurotransmitters (Anisman and Zacharko, 1986). Monoamines play a role in state-related modulation of executive function, and disturbances of executive function have been implicated in depression (Beck, 1976; Beck et al., 1987). Deficits in executive function, such as impaired cognitive set-shifting, behavioral inflexibility and perseveration (see Fossati et al., 1999), are consistent with the perseverative negative ruminations and emotional biases present in depression (Murphy et al., 2003; Murphy et al., 1999), and are associated with prefrontal cortical hypoactivity (Rogers et al., 2004; Sheline, 2003), which resolves with successful antidepressant treatment (Goldapple et al., 2004; Kennedy et al., 2001; Prasko et al., 2004).
In previous studies, we have used an attentional set-shifting test (AST) for rats (Birrell and Brown, 2000), to address modulatory effects of monoamines on cognitive function in prefrontal cortex, and possible mechanisms underlying chronic stress-induced cognitive dysfunction. In the AST, adapted from tests of cognitive flexibility in non-human primates (Roberts et al., 1992), and humans (Beats et al., 1996), rats learn a series of contingencies in which cues within one of two stimulus dimension (odor or texture) signal the location of a food reward. Once they master the contingency on each successive task, the rules are changed, and they must suppress the previously successful strategy to learn the new rule and proceed through the next task. The manner in which the rules are changed allows an assessment of different forms of cognitive flexibility, each dependent to varying degrees on the integrity of different sub-regions of prefrontal cortex (see Birrell and Brown, 2000; Bondi et al., 2008; Lapiz et al., 2007; Lapiz and Morilak, 2006; Lapiz-Bluhm et al., 2009; McAlonan and Brown, 2003). Lesions of medial prefrontal cortex (mPFC) caused selective impairment of extra-dimensional (ED) cognitive set-shifting (Birrell and Brown, 2000), whereas lesions of orbitofrontal cortex (OFC) resulted in selective impairment of reversal learning (McAlonan and Brown, 2003). Previous studies also indicate that these cognitive processes are modulated by monoaminergic neurotransmission. Increasing noradrenergic transmission in mPFC facilitated ED set-shifting (Lapiz et al., 2007; Lapiz and Morilak, 2006), and serotonergic transmission in OFC selectively facilitated reversal learning (Clarke et al., 2004; Clarke et al., 2005; Clarke et al., 2007).
We have used the AST previously to study chronic stress-induced changes in cognitive flexibility relevant to depression. Chronic unpredictable stress (CUS), a psychogenic stressor, impaired ED set-shifting and reversal learning, and these impairments were prevented by concurrent treatment with desipramine (DMI), a selective norepinephrine (NE) reuptake blocker (Bondi et al., 2008). By contrast, chronic intermittent cold (CIC) stress, a metabolic stressor, induced a reproducible and selective impairment in reversal learning that was mimicked by central serotonin depletion (Lapiz-Bluhm et al., 2009). Extracellular levels of 5-HT, measured by microdialysis in the OFC during testing, were reduced in CIC-stressed rats, and the CIC stress-induced reversal learning deficit was attenuated by elevating serotonergic neurotransmission with an acute administration of citalopram, a selective serotonin (5-HT) reuptake inhibitor (Lapiz-Bluhm et al., 2009). The effect of chronic SSRI treatment has not yet been tested.
In these previous studies, reuptake blockers were administered either acutely, immediately prior to testing, or chronically by osmotic minipump using a preventive strategy, in which drug treatment was initiated prior to the beginning of stress, and was maintained during the course of chronic stress treatment until testing. However, it would be more relevant to the human clinical situation, and would allow a more informative investigation of adaptive mechanisms underlying chronic antidepressant drug effects, if treatment could be initiated after the targeted behavioral effect of stress had been established, to test its ability to reverse rather than prevent the stress-induced cognitive deficit. Toward this end, the purpose of the first study in this paper was to characterize the duration of the reversal learning deficit induced after two-weeks of CIC stress. This study replicated the selective deficit in reversal learning shown previously when tested 3 days, but not 7, 14 or 21 days after the final cold stress exposure, indicating that the duration of effect was insufficient for testing the efficacy of 3-week antidepressant drug treatment initiated after the termination of stress. Thus, in the second experiment, an alternate approach was tested, extending CIC stress treatment to 5 weeks, which would allow a 3-week drug treatment to be initiated after the cognitive deficit had been established at 2 weeks. In this experiment, a selective reversal learning deficit was seen after 5 weeks of CIC stress, similar to that after 2 weeks. Thus, the 5-week CIC treatment protocol was used in the third experiment to test the ability of chronic treatment with an SSRI, citalopram, or a selective NE reuptake inhibitor, DMI, initiated after 2-weeks of CIC stress and maintained while stress treatment was continued for an additional 3 weeks, to reverse the cognitive deficit induced by CIC stress. Portions of this work have been presented in abstract form (Lapiz-Bluhm et al., 2008).
Methods
Animals
A total of 221 adult male Sprague-Dawley rats, weighing 220–240g on arrival, were initially group-housed (3/cage) in 25×45×15 cm plastic cages, on a 12/12-hr light/dark cycle (lights on at 0:700 hr), with ad libitum access to food and water. After acclimatizing for at least 7 days, rats were housed individually prior to any experimental manipulation. Experiments were conducted during the light portion of the cycle, between 09:00–17:00h. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio, and were consistent with NIH guidelines for the care and use of laboratory animals. All efforts were made to minimize pain, discomfort, suffering, and the number of rats used.
Chronic Intermittent Cold (CIC) Stress
The procedure for CIC stress exposure was as described previously (Lapiz-Bluhm et al., 2009; Ma and Morilak, 2005). Rats were randomly assigned to control or CIC stress conditions. Rats in the CIC stress group were transported in their home cages, with food, water and bedding, into a cold room (4 °C) for 6 hr, then returned to the housing room, every day for 14 consecutive days (experiments 1 and 2), or for 5 weeks (experiments 2 and 3). Control rats remained undisturbed in the housing facility during this time. One week before testing, rats were restricted to 14 g of food per day, with water freely available. After the CIC treatment period, rats were taken through the 3-day AST protocol.
Attentional Set-shifting Test
The AST was conducted as described previously (Lapiz and Morilak, 2006), in a rectangular wooden arena (length × width × height: 71×40×20 cm) painted white on all surfaces. A removable divider separated one-third of the arena, forming a start box which also served as a holding area following each trial. To begin a trial, a rat was placed in the start box, and the divider lifted. A white plexiglas panel divided the opposite third of the arena into two sections. At testing, a terracotta digging bowl (internal rim diameter 7 cm; depth 6 cm) was placed in each section. Each bowl was defined by a pair of cues along two stimulus dimensions, the material with which the pot was filled and an odor (see Table 1). To mark each pot with a distinct odor, two drops (20 µl) of scented aromatic oil (Frontier Natural Brands, Boulder, CO, USA) was applied to the inner rim 5 days before use, and 3–5 µl reapplied the day before use. The bait, a 1/4 Honey Nut Cheerio (General Mills Cereals, Minneapolis, MN, USA), was buried 2 cm below the surface of the digging medium in the “positive” pot. At the beginning of each task, a small quantity of powdered Cheerio was sprinkled onto the medium in both pots to prevent rats from locating the bait by smell rather than learning the discrimination.
Table 1. Behavioral protocol for the attentional set-shifting test.
Representative example of stimulus pairs, and the progression through stages of the attentional set-shifting test protocol. In this example, odor was the initial discriminative stimulus dimension, shifting to digging medium in the ED stage. For each stage, the positive stimulus is in bold, and is paired randomly across trials with the two stimuli from the irrelevant dimension.
| Discrimination Stage |
Dimensions | Example Combinations | ||
|---|---|---|---|---|
| Relevant | Irrelevant | (+) | (−) | |
| Simple (SD) | Odor | Clove/Sawdust | Nutmeg/Sawdust | |
| Compound (CD) | Odor | Medium | Clove/Raffia | Nutmeg/Metallic Filler |
| Clove/Metallic Filler | Nutmeg/Raffia | |||
| Reversal 1 (R1) | Odor | Medium | Nutmeg/Raffia | Clove/Metallic Filler |
| Nutmeg/Metallic Filler | Clove/Raffia | |||
| Intradimensional Shift (ID) |
Odor | Medium | Rosemary/Wood balls | Cinnamon/Plastic beads |
| Rosemary/Plastic beads | Cinnamon/Wood balls | |||
| Reversal 2 (R2) | Odor | Medium | Cinnamon/Plastic beads | Rosemary/Wood balls |
| Cinnamon/Wood balls | Rosemary/Plastic beads | |||
| Extradimensional shift (ED) |
Medium | Odor | Velvet/Citronella | Crepe/Thyme |
| Velvet/Thyme | Crepe/Citronella | |||
| Reversal 3 (R3) | Medium | Odor | Crepe/Thyme | Velvet/Citronella |
| Crepe/Citronella | Velvet/Thyme | |||
Digging was defined as vigorous displacement of the medium to retrieve the reward. Simply investigating the rim or surface of the medium with paws or snout without displacing material was not scored as a “dig”. Thus, rats could access tactile, visual and olfactory cues.
The procedure entailed three days:
Day 1, Habituation
Rats were trained to dig in the pots for food reward. Two unscented baited pots were placed in the home cage for a series of three exposures, 5 min each, in which the bait was covered with increasing amounts of sawdust. Once the rat was digging reliably, it was placed in the test arena for 3 trials to retrieve reward from both sawdust-filled pots.
Day 2, Training
Rats were trained on a series of simple discriminations, to a criterion of 6 consecutive correct trials. They first had to learn to associate the food reward with an odor cue (lemon vs. rosewood, both pots filled with sawdust). After reaching criterion, they then had to discriminate by the digging media (felt strips vs. shredded paper, no odor). All rats were trained using the same stimuli in the same order. Training stimuli were not used again in testing trials.
Day 3, Testing
Rats were tested on a series of discrimination tasks (see Table 1). When the rat reached criterion of 6 consecutive correct trials, testing proceeded to the next stage. The first stage was a simple discrimination (SD), involving only one stimulus dimension. Half the rats in each group were required at this stage to discriminate between 2 odors, only one of which was associated with reward, with both pots filled with sawdust. The other half began by discriminating the digging media, with no odors applied to the pots (for the sake of clarity, the remainder of this description considers only the example beginning with odor discrimination). The second task was a compound discrimination (CD), where the second, irrelevant stimulus was introduced. Only one odor was associated with reward, as in the SD, but two different digging media were paired randomly with the odors. The third stage was a reversal of this discrimination (R1), in which the same odors and media were used, and odor was still the relevant dimension, but the negative odor from the previous stage was now positive (i.e., associated with the reward), and the positive odor from the previous stage was now negative. The fourth stage was an intradimensional (ID) shift, wherein odor was still the relevant dimension but all new stimuli were introduced. The fifth stage was a reversal of this discrimination (R2), in which the previously negative odor was now positive, as in R1. The sixth stage required an extra-dimensional (ED) set-shift, in which new stimuli were again introduced, but this time the relevant dimension was also changed, e.g., the digging medium became the relevant dimension and odor was now irrelevant. Finally, the seventh stage was another reversal (R3), where the previously negative medium was now positive. Table 1 outlines the progression through these stages, and provides examples of the cue combinations used. The dependent measure was Trials to Criterion (TTC), the number of trials to reach criterion of 6 consecutive correct responses at each test stage.
Elevated Plus Maze
In experiment 2, the elevated plus maze (EPM) test was conducted as described previously (Bondi et al., 2008), the day after the AST. Rats were transported to the testing room and allowed to acclimate for 15 min. The EPM had four white plastic arms, 10 × 50 cm, oriented in the shape of a cross, intersecting at a 10 × 10 cm platform. Two closed arms situated opposite each other were enclosed by 48 cm walls, and the remaining two open arms had no walls. The open arms were fitted with a 0.5 cm clear plastic rim around the edges to prevent animals from falling off. The maze was elevated 100 cm from the floor, and testing took place under normal ambient overhead lighting (200 lux in the open arms) with 60 dB background white noise. To begin the 5 min test, a rat was placed in the center platform facing the junction of a closed and open arm. Activity was tracked using ANYmaze software (Stoelting Co., Wood Dale, IL, USA). Measures included time, number of entries, and distance traveled in each area of the maze. Open to total ratios (OTR) for time and entries (open/open+closed) were calculated as indices of open-arm exploration. Total distance traveled was analyzed as a measure of non-specific changes in locomotion independent of OTR. The maze was cleaned with 70% ethanol, then water, and dried completely before the next test.
Experiment 1: Duration of the cognitive effect induced by 14-day CIC stress exposure
Eighty-three rats were randomly assigned to 2 groups: control or 14-day CIC stress. Seven days before testing, food was restricted to 14 g/day. Separate groups of control and CIC-stressed rats underwent habituation, training and testing as above, at 3 days, 7 days, 14 days or 21 days after the last cold exposure (n=8–12 per group at each time point).
Experiment 2: Effects of 2-week vs 5-week CIC stress exposure
To compare the effects of 2-week and 5-week CIC stress, 23 rats were randomly assigned to 2 groups: CIC stress or control, then further subdivided into 2- or 5-week treatment groups. CIC stress was conducted as above. Seven days before testing, food was restricted to 14 g/day. Following the last day of cold exposure, rats were habituated and trained as above, and testing was conducted 3 days after the last cold exposure. The beginning of chronic stress treatment was staggered such that animals from all groups were tested together. As there were no differences between the two control groups, these were pooled into a single unstressed control group, resulting in n=7–8 rats in each of 3 groups (unstressed controls, 2-week CIC, 5-week CIC). The day following the AST, rats were tested on the EPM.
Experiment 3: Effects of chronic antidepressant treatment on the CIC stress-induced reversal learning deficit
Fifty-nine rats in two independent cohorts (n=30 and n=29, respectively) were assigned to 2 treatment groups, control or 5-week CIC stress. These were each further subdivided into 2 chronic drug treatments, either citalopram (CIT) and its vehicle control group (n=7–9/group), or desipramine (DMI) and its vehicle control group (n=7–8/group). Rats were exposed to 2-week CIC stress as above. On day 15, they were anesthetized (ketamine 43 mg/ml, acepromazine 1.4 mg/ml, and xylazine 8.6 mg/ml, in 1.0 ml/kg i.m.). Osmotic minipumps (model 2ML4, Alzet Corp., Palo Alto, CA), containing citalopram hydrobromide (Shanco International), desipramine hydrochloride (Sigma, St. Louis, MO), or vehicle (10% ethanol in saline), were implanted intraperitoneally. CIT was delivered at a dose of 20 mg/kg/day free base, and DMI at 7.5 mg/kg/day free base. Rats were given antibiotic (penicillin G, 300,000 IU/ml/kg). Three days after surgery, CIC stress treatment resumed for the remainder of the 5-week period. Seven days before testing, food was restricted to 14 g/day. Rats underwent habituation and training as above, with testing conducted 3 days after the last cold exposure. Chronic stress and drug treatments were staggered such that rats from all groups were tested together. Each drug group was compared to its respective vehicle control. Because the high drug concentrations required for chronic delivery by minipump exceeded their solubility in saline, 10% ethanol was used as vehicle. A pilot comparison showed no significant difference in performance on the AST of rats treated chronically by minipump with saline vs 10% ethanol (F1, 5 = 0.82, p = 0.41).
A separate set of 56 rats were used to test acute drug effects. Two independent cohorts (n=32 and n=24, respectively) were assigned to two treatment groups, control or CIC stress, then subdivided into 2 acute drug treatments, either CIT and its vehicle control (n=8/group), or DMI and its vehicle control (n=6/group). Because the cognitive deficits after 2- and 5-week CIC stress were comparable, acute drug treatments were tested only in rats exposed to 2-week CIC. After stress or control treatments, rats were habituated and trained as above. On the test day, they were taken through the SD and CD test stages, then received an i.p. injection of either CIT (5.0 mg/kg free base) or saline vehicle (1 ml/kg). Our previous study showed that this dose of citalopram acutely improved reversal learning performance of CIC-stressed rats (Lapiz-Bluhm et al., 2009). Testing resumed on the reversal stage 30 min after injection. Other rats, treated and tested in the same manner, received either DMI (5.0 mg/kg) or saline vehicle (1 ml/kg, i.p.), 30 min before reversal testing. This acute dose of DMI, slightly lower than doses we have used previously, was selected based on pilot results indicating that doses of 7.5 mg/kg and above hindered performance of CIC-stressed rats on the AST. Control rats given 7.5 mg/kg DMI were mildly sedated and took longer to perform, but generally completed all stages of the test, while a number of CIC-stressed rats did not, sometimes refusing to dig for >4 hrs.
Data analysis
Investigators were blind to the treatment conditions of rats being tested. Mean trials to criterion on the SD task on the training day were first compared by ANOVA, to ensure that acquisition and general performance capability were equivalent between groups.
For Experiment 1, data collected at each time point following termination of CIC stress were analyzed by two-way ANOVA (Stress × Task), with repeated measures over Task. For Experiment 2, data were analyzed by two-way ANOVA (Stress × Task), with repeated measures. Data from the EPM were analyzed by one-way ANOVA. In Experiment 3, effects of CIT- and DMI-treatment were analyzed separately by three-way ANOVA (Stress × Drug × Task), comparing each drug group to their respective vehicle-controls. For acute injections, data collected for SD and CD tasks prior to the injections were analyzed by two-way ANOVA (Stress × Task). Performance on the reversal task (R1) following acute injections was then analyzed using two-way ANOVA (Stress × Drug). For all analyses, where significant main effects or interactions were indicated, post hoc comparisons were performed using the Newman-Keuls test. Significance was determined at p < 0.05.
In all experiments, rats were weighed before beginning CIC stress or the corresponding control period, and again on the day of testing. Mean body weight gain was analyzed by one-way ANOVA in experiments with Stress as the between-group factor, or by two-way ANOVA with Stress and Drug as between-group factors.
Only rats completing all stages of the AST were included in the final analyses. A rat was eliminated if it stopped responding, or failed to complete all test stages within 5.5 hr of testing, as continuing to test beyond that time approached the transition into the dark phase of the light cycle. This resulted in elimination of 3 rats from experiment 1; 3 rats from experiment 2; 7 rats from the chronic drug treatment study in experiment 3, and 3 rats from the acute drug treatment study. Animals so excluded were not included in the reported number of rats used.
Results
Table 2 shows mean body weight gain in each experiment. In general, CIC stress slowed body weight gain moderately, but this was significant in only a few cases: in experiment 1, the 3-day post-CIC group; and in experiment 3, the acute citalopram study.
Table 2.
Mean body weight gain at testing (weight at testing – starting weight)
| Experiment | Group | Body weight gain (Mean ± SEM, g) |
F-Value (*p<0.05) |
|---|---|---|---|
| Experiment 1 | |||
| 3 days post-CIC | Control | 18.67 ± 5.80 | |
| CIC | 14.33 ± 3.77 | F 1,22 =4.70* | |
| 7 days post-CIC | Control | 22.36 ± 6.55 | |
| CIC | 18.33 ± 9.02 | F 1,21 =1.48 | |
| 14 days post-CIC | Control | 26.25 ± 2.92 | |
| CIC | 22.80 ± 2.81 | F 1,16 =0.80 | |
| 21 days post-CIC | Control | 36.63 ± 2.72 | |
| CIC | 31.50 ± 1.63 | F 1,16 =3.22 | |
| Experiment 2 | |||
| 2-week CIC | Control | 18.75 ± 14.48 | |
| CIC | 11.63 ± 10.51 | F 1,10 =0.18 | |
| 5-week CIC | Control | 91.50 ± 10.74 | |
| CIC | 88.86 ± 9.75 | F 1,9 =0.04 | |
| Experiment 3 | |||
| Chronic CIT | Control-Veh | 65.33 ± 4.27 | |
| Control-Cit | 59.71 ± 8.56 | F 1,26 =0.02 (Drug) | |
| CIC-Veh | 52.71 ± 7.58 | F 1,26 =1.74 (Stress) | |
| CIC-Cit | 56.86 ± 4.57 | ||
| Chronic DMI | Control-Veh | 61.25 ± 5.45 | |
| Control-Cit | 64.86 ± 4.48 | F 1,25 =0.04 (Drug) | |
| CIC-Veh | 57.00 ± 6.58 | F 1,25 =1.93 (Stress) | |
| CIC-Cit | 55.29 ± 4.48 | ||
| Acute CIT | Control | 14.63 ± 3.43 | |
| CIC | 5.63 ± 3.86 | F 1,30=4.33* | |
| Acute DMI | Control | 6.00 ± 5.21 | |
| CIC | 3.33 ± 3.47 | F 1,22 =0.20 | |
p<0.05 compared to the corresponding control group
Experiment 1: Duration of the cognitive effect induced by 14-day CIC stress exposure
CIC stress had no significant effect on training (F1,22=0.21; p=0.65), indicating that chronic stress did not impair acquisition or the ability to perform on the test in general. This was true for all groups, regardless of the time at which testing was conducted after the last cold exposure.
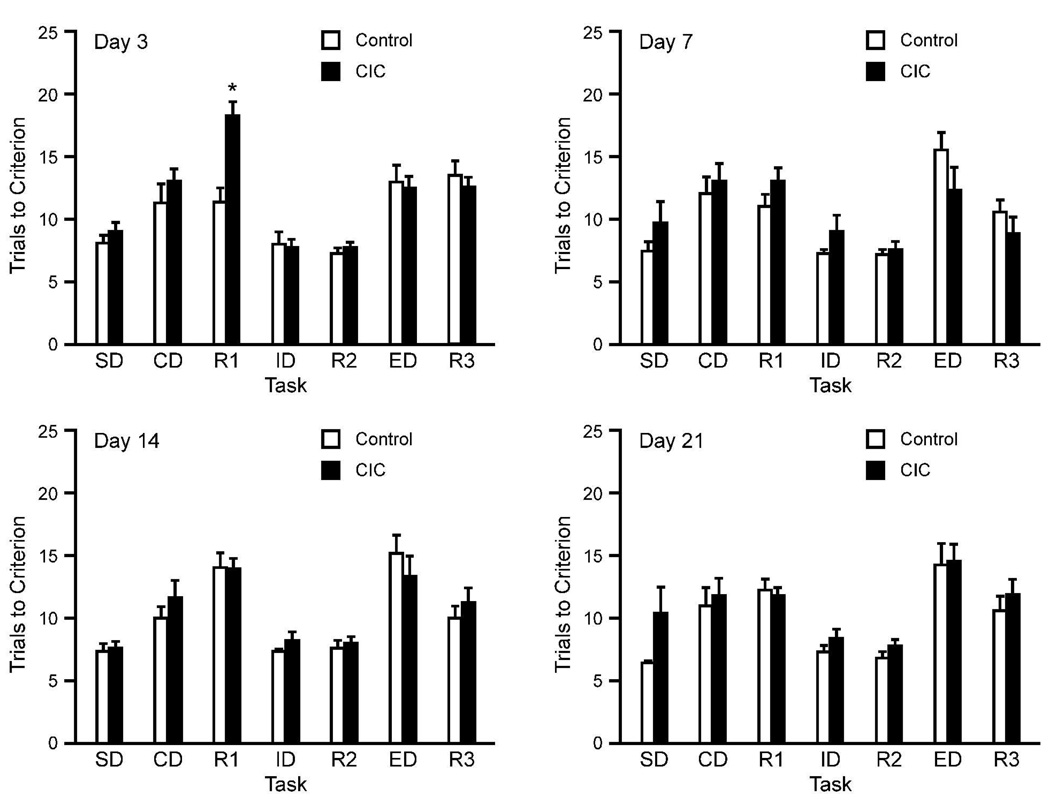
Replicating previous results (Lapiz-Bluhm et al., 2009), 14-days CIC stress significantly impaired reversal learning 3 days following the last cold exposure (Figure 1). ANOVA indicated no overall main effect of Stress (F1,22=2.99; p=0.09), but a significant effect of Task (F1,22=11.31; p<0.001), and a significant Stress × Task interaction (F1,22=2.38; p=0.03). Post hoc analysis at day 3 showed that CIC-stressed rats required significantly more trials to reach criterion selectively on the R1 reversal stage compared to unstressed controls (p<0.01). Similar analyses performed on data collected at days 7, 14 and 21 following the last cold exposure, however, showed no significant differences in performance between controls and CIC-stressed rats on any task (Figure 1).
Figure 1.
Cognitive effects of 14-day CIC stress, tested at 3, 7, 14 and 21 days after termination of cold stress exposure. CIC induced a reversal learning deficit tested 3 days after the last stress exposure (A). However, this effect dissipated at 7, 14, and 21 days after cold exposure (B–D). At day 3, CIC–stressed rats required significantly more trials to reach criterion on the R1 reversal task compared to non-stressed controls (*p < 0.01 compared to unstressed control rats on the same task; mean ± SEM; n = 8–12 per group).
Experiment 2: Effects of 2-week vs 5-week CIC stress exposure
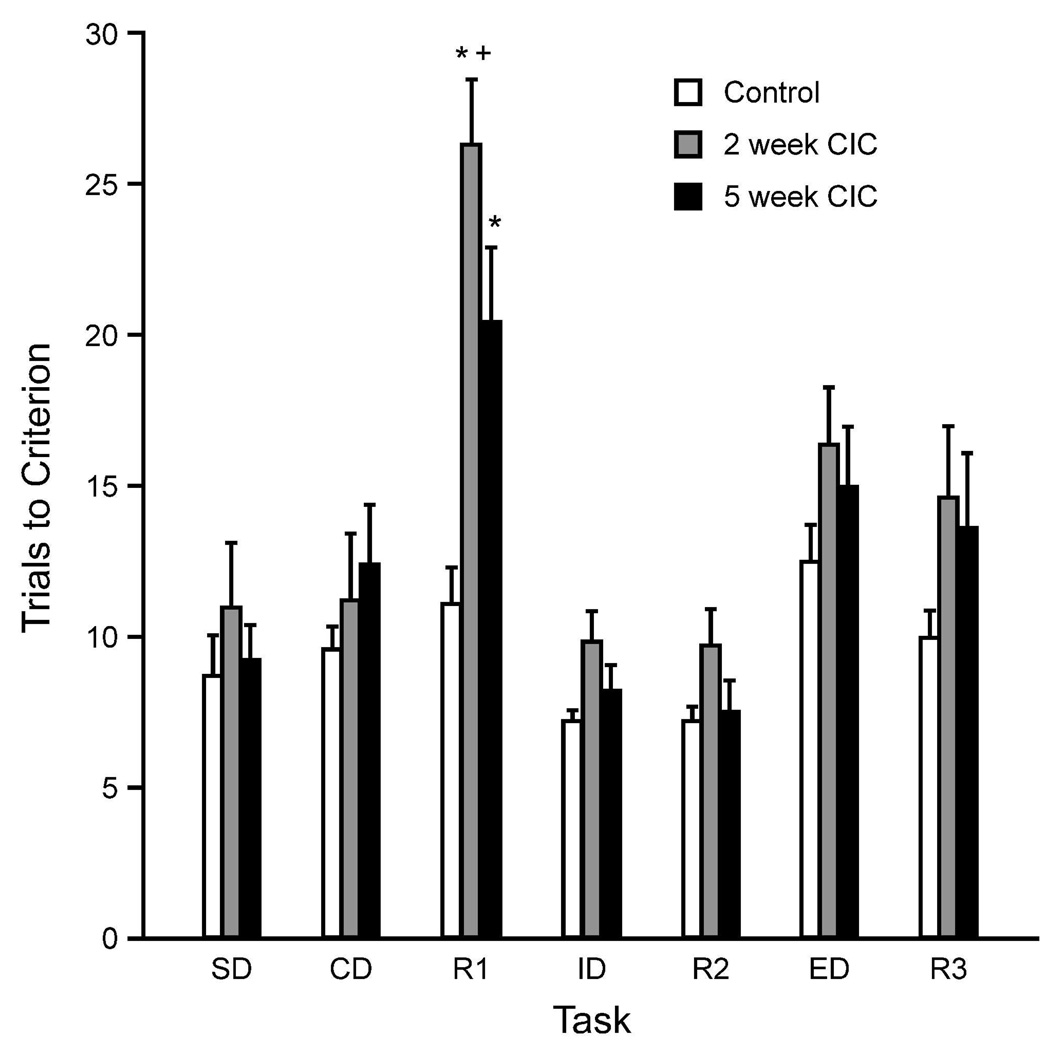
There was no difference in training of CIC-stressed rats and controls (F3,19 =1.92; p=0.16). Figure 2 shows the test-day performance of 2-week and 5-week CIC-stressed rats compared to unstressed controls. ANOVA revealed significant main effects of Stress (F2,20=6.49; p<0.01) and Task (F6,120=18.22; p<0.001), and a Stress × Task interaction (F12,120=2.45; p<0.01). Post hoc analyses again showed a significant deficit on the R1 reversal task, in both 2-week (p<0.001), and 5-week CIC-stressed rats (p<0.01). Although in this experiment, the trials to criterion on the R1 task were significantly higher in the 2-week stress group than in the 5-week stress group (p<0.05), the reversal deficit at 5-weeks was comparable to that after 2-weeks in experiment 1, and in previous studies (Lapiz-Bluhm et al., 2009).
Figure 2.
Exposure to CIC stress for either 2- or 5-weeks induced a selective and significant impairment in reversal learning compared to unstressed controls, tested 3 days after the termination of chronic cold stress exposure (*p < 0.01 compared to unstressed control rats on the same task; +p < 0.05 two-week compared to 5-week CIC stressed rats on the same task; mean ± SEM; n= 7–8 per group).
On the EPM, CIC stress induced no anxiety-like changes in open-arm exploration (Figure 3), analyzed as OTR for Time (F2,20=1.42; p=0.27), or Entries (F2,20=0.77; p=0.48). There were also no non-specific locomotor effects (F2,20=1.29; p=0.30 for total distance traveled, not shown).
Figure 3.
There were no anxiety-like changes in open-arm exploratory behavior on the elevated plus-maze after CIC stress exposure for 2- or 5-weeks, measured either as OTR for Time (panel A) or OTR for Entries (panel B; mean ± SEM; n= 7–8 per group).
Experiment 3: Effects of chronic and acute antidepressant treatment on the CIC stress-induced reversal learning deficit
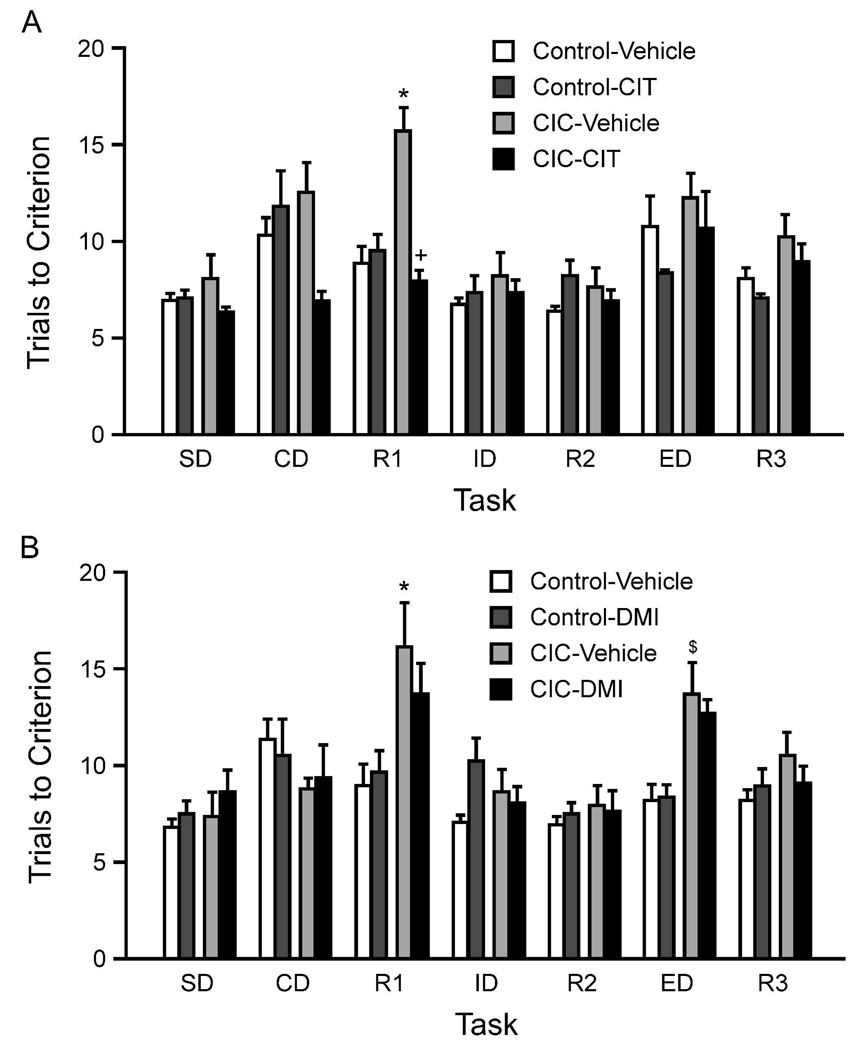
Neither stress (F1,53=0.42, p=0.51) nor drug treatment (F2,53=0.70, p=0.50) affected training. Figure 4 shows performance on the AST following 5-weeks of CIC stress exposure, with concomitant drug treatment during the final 3 weeks. In the citalopram experiment, ANOVA revealed significant effects of Stress (F1,26=4.42, p=0.045), Drug (F1,26=9.82, p<0.01) and Task (F6,156=10.43, p<0.001), with significant interactions between Stress × Drug (F1,26=12.84, p<0.01), and Stress × Drug × Task (F6,156=3.05, p<0.01). As in the preceding experiments, post hoc analyses again revealed a CIC stress-induced deficit in reversal learning, with a significant increase in trials to criterion on the R1 task compared to vehicle-treated unstressed controls (p<0.001). Chronic CIT treatment significantly reversed the CIC-induced impairment on the R1 task (p<0.001, Figure 4A).
Figure 4.
Effects of chronic antidepressant drug treatment, initiated after 2-weeks of CIC stress and maintained during continuation of CIC stress exposure for 3 more weeks. Panel A: Chronic treatment with CIT (20 mg/kg/day) reversed the CIC stress-induced cognitive deficit (*p < 0.001 compared to non-stressed control rats on the same task; +p < 0.001 compared to vehicle-treated CIC-stressed rats on the same task; mean ± SEM; n = 7–9 per group). Panel B: Chronic treatment with DMI (7.5 mg/kg/day) had no beneficial effect on the elevated number of trials to criterion on the R1 task after CIC stress, comparable to vehicle-treated CIC-stressed rats. In this experiment (but not in any others), CIC stress also increased trials to criterion on the ED cognitive set-shifting task, and DMI also did not reverse this effect. *p < 0.001 compared to vehicle-treated, non-stressed control rats on the same task; $ p < 0.05 compared to vehicle-treated, non-stressed control rats on the same task; mean ± SEM; n = 7–8 per group).
In the chronic DMI experiment, ANOVA revealed significant main effects of Stress (F1,25=8.71, p<0.01) and Task (F6,150=9.21, p<0.01), but no effect of Drug (F1,25=0.03, p=0.86). There was a significant Stress × Task interaction (F6,150=5.90, p<0.01). As above, post hoc analysis showed that CIC stress significantly increased trials to criterion on the R1 reversal task (p<0.001; Figure 4B). In this experiment (but not in any others), there was a significant effect of CIC stress on the ED set-shifting task (p<0.05). Neither of the CIC stress-induced deficits were reversed by chronic DMI treatment (Figure 4B).
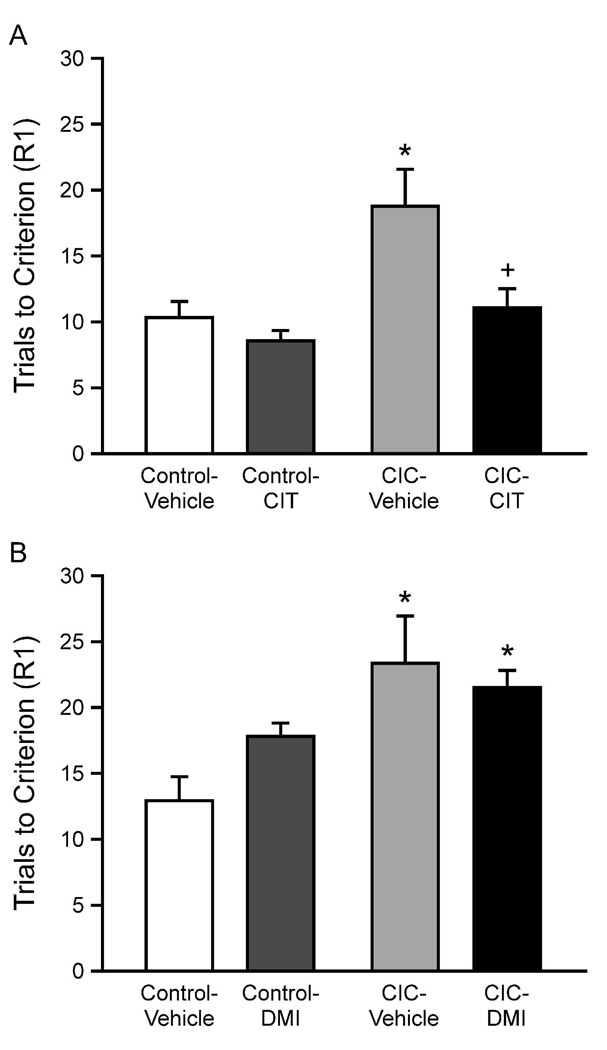
In the acute drug study, there were no pre-existing differences in performance on the training day between rats in the drug and vehicle groups (CIT: F2,29=0.96, p=0.59); DMI: F2,21=0.81, p=0.11). On the test day, there were also no differences between either of the drug groups and their respective vehicle control groups in performance on the SD and CD tasks before drug administration (CIT: F1,30=0.005, p=0.99; for DMI: F1,22=0.24, p=0.62). Analysis of the R1 reversal task after acute CIT treatment showed significant main effects of Stress (F1,28=8.93, p<0.01) and Drug (F1,28=6.64, p<0.05), but no interaction (F1,28=2.60, p=0.12). Post hoc comparisons showed that CIC stress increased trials to criterion on the R1 task, and acute citalopram significantly reduced trials to criterion on the reversal task in CIC-stressed rats, to a level comparable to that of unstressed controls (Figure 5A), replicating our previous results (Lapiz-Bluhm et al., 2009). By contrast, acute DMI treatment had no such effect (Figure 5B). ANOVA showed significant effects of stress (F1,20 =9.91, p<0.01), but no effect of drug (F1,20 =0.46, p=0.51), nor a Stress × Drug interaction (F1,20 =2.27, p=0.15)
Figure 5.
Effects of acute antidepressant drug treatment, given 30 min prior to the R1 reversal task. Panel A: Acute treatment with CIT (5 mg/kg, i.p.) improved performance of CIC stress-stressed rats on the R1 reversal task (*p < 0.01 compared to non-stressed control rats on the same task; +p < 0.01 compared to vehicle-treated CIC-stressed rats on the same task; mean ± SEM; n = 8 per group). Panel B: Acute treatment with DMI (5 mg/kg, i.p.) had no beneficial effect on the increased number of trials required to reach criterion on the R1 task after CIC stress, comparable to vehicle-treated CIC-stressed rats. (*p < 0.05 compared to vehicle-treated, non-stressed control rats; mean ± SEM; n = 6 per group).
Discussion
Executive dysfunction related to prefrontal cortical dysregulation, specifically perseveration, or the failure to alter behavior in response to environmental feedback, is prominent in depression (Channon, 1996). Cognitive-behavioral approaches (Beck, 1976; Beck, 2005) presume that depression is associated with ruminative perseverations within a set of negative perceptions and beliefs about the self, the world and the future. The intent is to help patients challenge such biases, establishing a more flexible, realistic and adaptive cognitive schema based on hypothesis-testing and use of objective evidence to modify thoughts and behaviors. An animal model that reflects cognitive functioning of prefrontal cortex, is sensitive to stress, and is modulated by monoaminergic neurotransmitters and antidepressants, would be a valuable tool for investigating mechanisms involved in the dysregulation and restoration of cognitive flexibility in depression.
Results of the present study replicated our previous finding that CIC-stress induces a selective impairment in reversal learning in rats, without interfering with acquisition of new contingencies. There were no differences in training, simple discrimination, compound discrimination or intra-dimensional set-shift tasks. Also as reported previously, CIC stress did not generally affect ED cognitive set-shifting (except in one experiment, see below), despite the rats having greater difficulty mastering the reversal task earlier in the test sequence, further demonstrating the relative selectivity of the CIC-stress effect.
The deficit in reversal learning after 2-weeks of CIC treatment was transient. It was evident when tested 3 days after the last cold exposure, but had dissipated at time points further past the last cold exposure, i.e., at 7, 14, or 21 days. This was not of sufficient duration to investigate the effects of chronic antidepressant drug treatment initiated after the stress-induced cognitive deficit had been established, as in the clinical treatment of depression. Thus, in experiment 2, the feasibility of continuing chronic stress treatment for a time that would be sufficient to allow chronic drug intervention was investigated. Five-week CIC stress induced a reversal learning deficit comparable to that after 2-weeks. Thus, the 5-week stress protocol was used in experiment 3, with chronic antidepressant drug treatment beginning after 2 weeks, when the reversal deficit had been established, and maintained for 3 weeks thereafter, while CIC stress continued. This design is more relevant to the clinical treatment of depression, allowing chronic drug treatment to be initiated after the pathological behavior has been established, but also because treatment is maintained in the presence of continuing stress, as depressed patients undergoing antidepressant therapy will undoubtedly continue to experience many of the same life stressors that existed prior to the initiation of treatment. In this experiment, chronic treatment with the SSRI, citalopram, but not the selective NE reuptake blocker, desipramine, effectively reversed the CIC-stress induced cognitive deficit.
Reversal learning, wherein a previously positive discriminative stimulus becomes negative, and a previously negative stimulus becomes positive, has been studied as a measure of cognitive flexibility in humans (Fellows and Farah, 2003; Hornak et al., 2004; Murphy et al., 2002; Rogers et al., 2000; Rolls et al., 1994), nonhuman primates (Clarke et al., 2004; Clarke et al., 2005; Clarke et al., 2007; Dias et al., 1996; Lee et al., 2007), and rats (Birrell and Brown, 2000; Boulougouris et al., 2007; Boulougouris et al., 2008; Idris et al., 2005; McAlonan and Brown, 2003; van der Meulen et al., 2007). Reversal learning requires integrity of the orbitofrontal cortex (McAlonan and Brown, 2003). Thus, the present results suggesting that reversal learning is sensitive to chronic stress may provide insight into how chronic stress alters prefrontal cortical function in ways that are relevant to the cognitive deficits in depression. Human studies have shown that acute cold exposure degrades cognitive performance, including vigilance, reaction time, reasoning and short-term memory (e.g., see Coleshaw et al., 1983; Mahoney et al., 2007; Patil et al., 1995; Shurtleff et al., 1993). Cold stress impaired performance on 4-choice reaction time and delayed match-to-sample tasks, and increased measures of tension, confusion and depression, as well as “total mood disturbance” scores (Mahoney et al., 2007). Limited studies of chronic cold exposure also suggest adverse effects on both cognition and mood (Makinen, 2007).
Food deprivation and changes in body weight alone have been reported to influence cognition in rats, although the evidence is equivocal. Food restriction for 3 months promoted long-term recovery of learning and memory following global ischemia (Roberge et al., 2008a; Roberge et al., 2008b). However, one-year food restriction had negative effects (Yanai et al., 2004), and 40% caloric restriction for six month had no effects on cognition (Martin et al., 2007). In the present study, the relatively mild CIC-stress treatment only moderately slowed body weight gain. CIC-stressed rats and controls had comparable performance during training, and on all cognitive tasks except the reversal task, and there were no differences in the effect of CIC-stress on weight gain that could explain the beneficial effects of SSRI treatment. Thus, it is unlikely that differences in body weight alone could account for either the detrimental effects of CIC stress on reversal learning, or the beneficial effects of SSRI treatment.
We and others have also shown that reversal learning is specifically modulated by 5-HT neurotransmission in orbitofontal cortex (Clarke et al., 2004; Clarke et al., 2005; Clarke et al., 2007; Lapiz-Bluhm et al., 2009). In our previous study, the CIC stress-induced impairment in reversal learning was mimicked by 5-HT depletion, and was attenuated by acute administration of citalopram, as in the present study. Together with a decrease in extracellular 5-HT levels measured by microdialysis in OFC during testing, these findings indicated a possible dysregulation of serotonergic modulatory function in the OFC of rats exposed to CIC stress (Lapiz-Bluhm et al., 2009). The present study provides further evidence that 5-HT facilitates reversal learning, in that chronic citalopram treatment reversed the detrimental effect of CIC stress, even though stress exposure continued during drug treatment.
By contrast with citalopram, the selective NE reuptake blocker, DMI, had no effect on the CIC stress-induced reversal learning impairment, acutely or chronically. Anecdotally, as noted in the Methods, CIC-stressed rats seemed more sensitive to the non-cognitive, locomotor-inhibitory effects of acute DMI. At doses that did not hinder performance of controls in completing the AST, CIC-stressed rats remained inactive, although seemingly alert, for several hours post-injection. Further experimentation would be required to investigate mechanisms of any possible increase in sensitivity to certain effects of DMI, but these observations may be consistent with previous observations indicating that CIC stress can sensitize the response of sub-cortical noradrenergic receptors to NE (Ma and Morilak, 2005).
In the present experiments, only the first reversal task (R1) was consistently affected by CIC stress, whereas the second (R2) and third (R3) reversals were generally unaffected. Similarly inconsistent effects on the later reversal tasks have been reported in other contexts (e.g., Bondi et al., 2008; Boulougouris et al., 2008; Hatcher et al., 2005; Tait and Brown, 2008). We have speculated that R1 may be more vulnerable to manipulation because of differences in the difficulty involved in reversing the contingencies established in the stages immediately preceding each of the reversals. R2 is similar to R1, as it follows a new acquisition within the same stimulus dimension, but it differs in that the animal has just had prior experience performing a reversal in R1. The ease with which rats typically master R2 may reflect a “learning-to-learn” phenomenon, that may make R2 more resistant to disruption. By contrast, R3 is preceded by the ED set-shift, in which rats must abandon a cognitive set that had been reinforced repeatedly in all stages up to that point. Thus, the new contingency acquired in the ED task may not be as “strong” as those established in earlier acquisitions, and less “flexibility” may be required to achieve subsequent reversal in R3, making R3 less prone to disruption.
The selective reversal deficit after the chronic metabolic stressor in the present study was also different from cognitive effects reported previously after a more psychogenic stress treatment, chronic unpredictable stress (CUS), which consistently induced extra-dimensional setshifting deficits, with less consistent effects on reversal learning (Bondi et al., 2008). CIC stress affected ED set-shifting in only one experiment in the present study. Moreover, chronic DMI prevented the deficit in ED set-shifting induced by CUS (Bondi et al., 2008), but did not alter the effect of CIC stress on ED set-shifting in the present study, consistent with its lack of effect on the reversal deficit induced by CIC stress. Thus, it would seem that the prefrontal substrates affected by CIC stress are subtly different than those affected by chronic unpredictable stress, and different types of stress may impact different forms of cognitive flexibility, dependent on different sub-regions of prefrontal cortex. CUS might produce effects that reflect functional changes in mPFC (see Bondi et al., 2008), while reversal learning, affected selectively by CIC stress, may reflect changes more specifically in functioning of OFC.
Further, CIC stress may be even more broadly useful for modeling other dimensions of depression as well. We have shown that CIC stress induced a dysregulation of the HPA axis, as well as changes in NE release and post-synaptic adrenergic receptor sensitivity in stressresponsive sub-cortical brain regions, including the paraventricular nucleus of the hypothalamus (Ma and Morilak, 2005; Pardon et al., 2003). However, in the present experiment there was no anxiety-like reduction in open-arm exploration on the EPM after CIC stress, as was seen previously after chronic unpredictable stress (Bondi et al., 2008). Moreover, some the effects of CIC stress in previous studies were more pronounced in WKY rats, a genetically stressvulnerable strain, compared to the more resilient Sprague-Dawley rats used in the present experiments. Thus, CIC as a model of metabolic stress might be useful specifically in addressing mechanisms underlying vulnerability to stress-induced behavioral and physiological pathology. Further research is required to understand how the behavioral, cognitive, physiologic and affective dimensions affected by CIC stress might be related to each other, to the mechanisms underlying long-term consequences of chronic stress, or to differences in the efficacy of therapeutic interventions.
Acknowledgements
We thank Mr. Gus Rodriguez, Ms. Julianne Jett and Ms. Ashley Furr for their expert technical assistance. Support was provided by a Young Investigator Award from NARSAD: The Mental Health Association (to MDSLB), and by research grants from the National Institute of Mental Health (MH083669 and MH072672 to DAM).
Footnotes
Statement of Interest
The authors have no conflicts of interest to report, nor any involvement to disclose, financial or otherwise, that may bias the conduct, interpretation or presentation of this work.
References
- Anisman H, Zacharko RM. Behavioral and neurochemical consequences associated with stressors. Annals of the New York Academy of Science. 1986;467:205–225. doi: 10.1111/j.1749-6632.1986.tb14630.x. [DOI] [PubMed] [Google Scholar]
- Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychological Medicine. 1996;26:591–604. doi: 10.1017/s0033291700035662. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy and the emotional disorders. New York: Int. Univ. Press; 1976. [Google Scholar]
- Beck AT. The current state of cognitive therapy: A 40-year retrospective. Archives of General Psychiatry. 2005;62:953–959. doi: 10.1001/archpsyc.62.9.953. [DOI] [PubMed] [Google Scholar]
- Beck AT, Brown G, Steer RA, Eidelson JI, et al. Differentiating anxiety and depression: A test of the cognitive content-specificity hypothesis. Journal of Abnormal Psychology. 1987;96:179–183. doi: 10.1037//0021-843x.96.3.179. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. Journal of Neuroscience. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, et al. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behavioural Brain Research. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2008;33:2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- Channon S. Executive dysfunction in depression: The Wisconsin Card Sorting Test. Journal of Affective Disorders. 1996;39:107–114. doi: 10.1016/0165-0327(96)00027-4. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, et al. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, et al. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. Journal of Neuroscience. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, et al. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cerebral Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Coleshaw SRK, Van Someren RNM, Wolff AH, Davis HM, et al. Impaired memory registration and speed of reasoning caused by low body temperature. Journal of Applied Physiology. 1983;55:27–31. doi: 10.1152/jappl.1983.55.1.27. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: Effects of excitotoxic lesions of the prefrontal cortex of the marmoset. Behavioral Neuroscience. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: Evidence from reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fossati P, Amar G, Raoux N, Ergis AM, et al. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Research. 1999;89:171–187. doi: 10.1016/s0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, et al. Modulation of cortico-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Archives of General Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Hatcher PD, Brown VJ, Tait DS, Bate S, et al. 5-HT6 receptor antagonists improve performance in an attentional set shifting task in rats. Psychopharmacology. 2005;181:253–259. doi: 10.1007/s00213-005-2261-z. [DOI] [PubMed] [Google Scholar]
- Hornak J, O'Doherty J, Bramham J, Rolls ET, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Idris NF, Repeto P, Neill JC, Large CH. Investigation of the effects of lamotrigmine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and D-amphetamine in the rat. Psychopharmacology (Berl) 2005;179:336–348. doi: 10.1007/s00213-004-2058-5. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Bondi CO, Morilak DA. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set shifting test. Neuropsychopharmacology. 2007;32:1000–1010. doi: 10.1038/sj.npp.1301235. [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MDS, Jett JD, Rodriguez G, Morilak DA. Temporal characterization of the cognitive deficit induced by chronic intermittent cold stress on an attentional set shifting test in rats. Society for Neuroscience Abstracts. 2008;34 Online, Program number 196.6. [Google Scholar]
- Lapiz-Bluhm MDS, Soto-Piña AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology. 2009;202:329–341. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Chronic intermittent cold stress sensitizes the HPA response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. Journal of Neuroendocrinology. 2005;17:761–769. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- Mahoney CR, Castellani J, Kramer FM, Young A, et al. Tyrosine supplementation mitigates working memory decrements during cold exposure. Physiology and Behavior. 2007;92:575–582. doi: 10.1016/j.physbeh.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Makinen TM. Human cold exposure, adaptation, and performance in high latitude environments. American Journal of Human Biology. 2007;19:155–164. doi: 10.1002/ajhb.20627. [DOI] [PubMed] [Google Scholar]
- Martin B, Pearson M, Kebejian L, Golden E, et al. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148:4318–4333. doi: 10.1210/en.2007-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairments in patients with major depressive disorder: The effects of feedback on task performance. Psychological Medicine. 2003;33:455–467. doi: 10.1017/s0033291702007018. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, et al. Emotional bias and inhibitory control processes in mania and depression. Psychological Medicine. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Smith KA, Cowen PJ, Robbins TW, et al. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 2002;163:42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- Pardon M-C, Ma S, Morilak DA. Chronic cold stress sensitizes brain noradrenergic reactivity and noradrenergic facilitation of the HPA stress response in Wistar Kyoto rats. Brain Research. 2003;971:55–65. doi: 10.1016/s0006-8993(03)02355-2. [DOI] [PubMed] [Google Scholar]
- Patil PG, Apfelbaum JL, Zacny JP. Effects of a cold-water stressor on psychomotor and cognitive functioning in humans. Physiology and Behavior. 1995;58:1281–1286. doi: 10.1016/0031-9384(95)02071-3. [DOI] [PubMed] [Google Scholar]
- Prasko J, Hornacek J, Zalesky R, Kopecek M, et al. The change of regional brain metabolism (18FDG PET) in panic disorder during the treatment with cognitive behavioral therapy or antidepressants. Neuro Endocrinology Letters. 2004;25:340–348. [PubMed] [Google Scholar]
- Roberge MC, Hotte-Bernard J, Messier C, Plamondon H. Food restriction attenuates ischemia-induced spatial learning and memory deficits despite extensive CA1 ischemic injury. Behavioural Brain Research. 2008a;187:123–132. doi: 10.1016/j.bbr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Roberge MC, Messier C, Staines WA, Plamondon H. Food restriction induces longlasting recovery of spatial memory deficits following global ischemia in delayed matching and non-matching-to-sample radial arm maze tasks. Neuroscience. 2008b;156:11–29. doi: 10.1016/j.neuroscience.2008.05.062. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, Everitt BJ, Muir JL. A specific form of cognitive rigidity following excitotoxic lesions of the basal forebrain in marmosets. Neuroscience. 1992;47:251–264. doi: 10.1016/0306-4522(92)90241-s. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, et al. Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neuroscience Research. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, et al. Contrasting cortical and subcortical activations produced by attentional set shifting and reversal learning in humans. Journal of Cognitive Neuroscience. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biological Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Shurtleff D, Thomas JR, Ahlers ST, Schrot J. Tyrosine ameliorates a cold-induced delayed matching-to-sample performance decrement in rats. Psychopharmacology (Berl) 1993;112:228–232. doi: 10.1007/BF02244915. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ. Lesions of the basal forebrain impair reversal learning but not shifting of attentional set in rats. Behavioural Brain Research. 2008;187:100–108. doi: 10.1016/j.bbr.2007.08.035. [DOI] [PubMed] [Google Scholar]
- van der Meulen JAJ, Joosten RNJMA, de Bruin JPC, Feenstra MGP. Dopamine and noradrenaline efflux in the medial prefrontal cortex during serial reversals and extinction of instrumental goal-directed behavior. Cerebral Cortex. 2007;17:1444–1453. doi: 10.1093/cercor/bhl057. [DOI] [PubMed] [Google Scholar]
- Yanai S, Okaichi Y, Okaichi H. Long-term dietary restriction causes negative effects on cognitive functions in rats. Neurobiology of Aging. 2004;25:325–332. doi: 10.1016/S0197-4580(03)00115-5. [DOI] [PubMed] [Google Scholar]