Abstract
Mouse DC-SIGN CD209a is a type II transmembrane protein, one of a family of C-type lectin genes syntenic and homologous to human DC-SIGN. Current anti-mouse DC-SIGN monoclonal antibodies (MAbs) are unable to react with DC-SIGN in acetone fixed cells, limiting the chance to visualize DC-SIGN in tissue sections. We first produced rabbit polyclonal PAb-DSCYT14 against a 14-aa peptide in the cytosolic domain of mouse DC-SIGN, and it specifically detected DC-SIGN and not the related lectins, SIGN-R1 and SIGN-R3 expressed in transfected CHO cells. MAbs were generated by immunizing rats and DC-SIGN knockout mice with the extracellular region of mouse DC-SIGN.. Five rat IgG2a or IgM MAbs, named BMD10, 11, 24, 25, and 30, were selected and each MAb specifically detected DC-SIGN by FACS and Western blots, although BMD25 was cross-reactive to SIGN-R1. Two mouse IgG2c MAbs MMD2 and MMD3 interestingly bound mouse DC-SIGN but at 10 fold higher levels than the rat MAbs. When the binding epitopes of the new BMD and two other commercial rat anti-DC-SIGN MAbs, 5H10 and LWC06, were examined by competition assays, the epitopes of BMD11, 24, and LWC06 were identical or closely overlapping while BMD10, 30, and 5H10 were shown to bind different epitopes. MMD2 and MMD3 epitopes were on a 3rd noncompeting region of mouse DC-SIGN. DC-SIGN expressed on the cell surface was sensitive to collagenase treatment, as monitored by polyclonal and MAb. These new reagents should be helpful to probe the biology of DC-SIGN in vivo.
Keywords: Monoclonal Antibody, Polyclonal Antibody, DC-SIGN, CD209a, Dendritic Cells
1. Introduction
Dendritic cells (DCs) are potent antigen-presenting cells. DCs are found at several interfaces between the organism and its environment, where they function as sentinels, efficiently capturing and responding to foreign antigens, and transporting them to draining lymph nodes for presentation of antigenic peptides to naïve T cells (Banchereau and Steinman, 1998). DCs enhance their recognition of antigens through several surface receptors including C-type lectins that bind carbohydrates in a calcium-dependent manner via conserved carbohydrate recognition domains (CRD) (Figdor et al., 2002; Geijtenbeek et al., 2004). C-type lectins, which are pattern recognition receptors for glycosylated molecules, function in DCs and macrophages in clearance and presentation of glycosylated antigens and microbes in vivo. To study these receptors, it is crucial to have good antibodies, e.g., to visualize the receptors in cell suspensions and tissue sections.
DC-SIGN was originally discovered in human placenta as a C-type lectin receptor for HIV gp120 (Curtis et al., 1992). Later DC-SIGN was identified on the surface of human monocyte-derived DCs, to bind ICAM-3 on T cells (Geijtenbeek et al., 2000c) and ICAM-2 on endothelial cells (Geijtenbeek et al., 2000a) as well as HIV to transmit HIV to susceptible cells (Geijtenbeek et al., 2000b). A series of studies also demonstrated that the CRD of human DC-SIGN is able to bind other pathogens, such as Ebola virus (Alvarez et al., 2002), Dengue virus (Navarro-Sanchez et al., 2003; Tassaneetrithep et al., 2003), mycobacteria (Geijtenbeek et al., 2003; Tailleux et al., 2003), Yersinia (Zhang et al., 2008a), Leishmania (Colmenares et al., 2002), and the eggs of Schistosoma mansoni (van Die et al., 2003). It has been reported that human DC-SIGN in vivo is expressed in subpopulations of DCs and macrophages in spleen, lymph nodes, tonsil, skin, intestine, and cervix (Geijtenbeek et al., 2000a; Geijtenbeek et al., 2000b; Geijtenbeek et al., 2000c; Soilleux et al., 2001; Jameson et al., 2002; Soilleux et al., 2002; Ebner et al., 2004; Granelli-Piperno et al., 2005; Pack et al., 2008).
In the mouse, 5 genes with close sequence similarity to one another are located in a genetic locus and are homologous to human DC-SIGN (Caminschi et al., 2001; Park et al., 2001). One of the five was named mouse DC-SIGN because of its syntenic localization to human DC-SIGN close to the CD23 gene (Park et al., 2001). Three members (mouse DC-SIGN, SIGN-R1, and SIGN-R3) show significant expression in various mouse tissues and have the structure of type II transmembrane proteins with a single CRD domain at the COOH-terminus (Park et al., 2001). However, unlike human DC-SIGN, which is one of the most studied C-type lectins, neither the expression nor function of mouse DC-SIGN has been examined in detail because of a lack of good antibodies. So far two monoclonal antibodies (MAbs) against mouse DC-SIGN, i.e. 5H10 (Caminschi et al., 2006) and LWC06 (eBioscience, San Diego, CA), are available, but neither are able to detect DC-SIGN in mouse tissues.
In this report, we have generated a polyclonal antibody (PAb) against a unique 14-aa peptide in the cytosolic domain of mouse DC-SIGN (PAb-DSCYT14) and a series of MAbs against the CRD domain of mouse DC-SIGN. We will demonstrate that PAb-DSCYT14 selectively detects the expression of mouse DC-SIGN and not the related lectins SIGN-R1 and SIGN-R3 by Western blot. Also, we prepared new rat and mouse MAbs that help identify 3 immunogenic regions in the extracellular region of mouse DC-SIGN, and bind to the lectin in acetone fixed cells.
2. Materials and methods
2.1. Animals
Female Wistar Furth rats were purchased from Charles River Laboratories (Wilmington, MA). DC-SIGN knockout (KO) mice were generously provided by the Consortium for Functional Glycomics (CFG, The Scripps Research Institute, La Jolla, CA). All animals were maintained under specific pathogen-free conditions. Animal care and experiments were conducted according to institutional guidelines of the Rockefeller University and Memorial Sloan-Kettering Cancer Center.
2.2. Cells
Hybridoma, Chinese hamster ovary (CHO), and 293TAg cells were cultured in DMEM (GIBCO Invitrogen, catalog number 11995) with 7 % FBS (Sigma) or 5 % Ultra-Low IgG FBS (GIBCO Invitrogen) supplemented with 1× solutions of 2-mercaptoethanol (GIBCO Invitrogen), Antibiotic-Antimycotic (GIBCO Invitrogen), and Non-Essential Amino Acids (GIBCO Invitrogen).
2.3. Antibodies
We purchased anti-rat IgG isotypes, anti-mouse IgG isotypes, and anti-rat IgM conjugated with HRP, PE, or PE/Cy5.5 from Southern Biotech (Birmingham, AL), and streptavidin conjugated with PE, APC, or Alexa fluorochromes from Invitrogen (Carlsbad, CA) and BD Biosciences (San Jose, CA). PE- or biotin-conjugated anti-mouse DC-SIGN MAbs, 5H10 and LWC06, were purchased or kindly provided by eBioscience (San Diego, CA). Rabbit polyclonal antibodies against the C-terminal 13-aa peptide of mouse SIGN-R1 (PAb-R1C13) and the16-aa peptide in the carbohydrate recognition domain (CRD) of mouse SIGN-R3 (PAb-R3CRD16) were described previously (Kang et al., 2003; Kang et al., 2004). Similarly, a rabbit polyclonal antibody against the 14-aa peptide (NH2–GKRQLRPLDEELLT-COOH) in the cytosolic domain of mouse DC-SIGN (PAb-DSCYT14) were generated by Invitrogen, as previously described (Kang et al., 2003; Kang et al., 2004).
2.4. Construction of vectors and expression of proteins
CHO cells (CHO-S cells, GIBCO Invitrogen) stably expressing DC-SIGN, SIGN-R1, and SIGN-R3 were described previously (Kang et al., 2003; Zhang et al., 2008b). CHO/DCIR2 cells were generated as follows. The cDNAs encoding the open reading frames (ORFs) of mouse DCIR2 were cloned from a mouse splenic cDNA library (Park et al., 1996) by PCR, sequenced, and inserted into the pCMV mammalian expression vector (Clontech). Then, the stable CHO cells were generated by transfection with pCMV-DCIR2 by Lipofectamine™ 2000 reagent (Invitrogen) followed by selection of G418 (1.5 mg/ml) in DMEM culture medium.
Soluble human IgG1 Fc (S.hIgG1Fc) fusion protein of mouse DC-SIGN extracellular domain (ECD) was generated similarly to a method described previously (Galustian et al., 2004). In brief, the synthetic DNA encoding mouse DC-SIGN ECD (GenBank accession number FJ168685) was generated by PCR, sequenced, and inserted into the pCMV mammalian expression vector carrying a soluble hIgG1 Fc fusion cassette. Stable CHO cells (CHO/ S.hIgG1Fc.DC-SIGN.ECD) were generated as described above. Then, soluble Fc-tagged DC-SIGN ECD protein was purified from culture supernatants of stable CHO cells by Protein A affinity column as described (Galustian et al., 2004).
The His-tagged C-type lectin domains of mouse DC-SIGN (His.DC-SIGN.CRD; residues 98 to 239) and SIGN-R3 (His.SIGN-R3.CRD; residues 95 to 238) were cloned into pET22b(+) (Novagen). The identities of the cloned genes were verified by plasmid sequencing. The proteins were expressed in E. coli strain BL21(DE3) by inducing with 0.6–0.8 mM IPTG at 37 °C for 3 to 4 hrs. Both proteins were expressed in insoluble inclusion bodies. The cells were lysed by sonication and the inclusion bodies were washed for 3 times in a buffer containing 50 mM Tris, 100 mM NaCl, 5 mM EDTA, and 0.5% Triton-X100 at pH 8.0. Purified inclusion bodies were resuspended in water (~5 ml per 4 liter of culture) and dissolved in 7 M guanidine hydrochloride. The proteins were refolded by quick dilution into a refolding buffer containing 0.1 M Tris, 0.4 M arginine hydrochloride, 5 mM reduced glutathione, and 1 mM of oxidized glutathione at pH 8.3. About 100 to 200 mgs of denatured protein were diluted into 1 liter of refolding buffer. Refolded proteins were concentrated and purified by gel filtration chromatography on a Superdex200 (2.6×60) column (GE Healthcare) following elution with 20 mM Tris, 150 mM NaCl buffer at pH 7.5. The proteins were further purified by Ni affinity chromatography followed by gel filtration chromatography to remove the imidazole from the purified proteins. All the proteins purified were monomeric as analyzed by gel filtration chromatography.
2.5. Animal immunization and monoclonal antibody production
Wistar Furth rats or DC-SIGN KO mice at 4~6 wks of age were immunized subcutaneously (s.c.) with 50 µg of a protein mixture composed of S.hIgG1Fc.DC-SIGN. ECD and His.DC-SIGN.CRD in equal amounts following emulsification with TiterMax® adjuvant (TiterMax USA, Inc., Norcross, GA) according to the manufacturer’s instruction. Rats were immunized 11 times and DC-SIGN KO mice were 6 times at monthly intervals on average. In addition, rats were boosted s.c. once with a cell lysate of 1×106 splenic low density cells enriched for DCs by floating in 30 % BSA (Inaba et al., 1998), prepared by repeated freezing (−80 °C) and thawing (37 °C) in PBS, between the 5th and the 6th immunizations. Seven days after each immunization, sera were collected and screened by Western blot and ELISA as described below to determine which animal had the highest anti-DC-SIGN specific responses.
Rats, four months after the last round of immunization, and DC-SIGN KO mice, two months after the last round of immunization, were selected to receive intraperitoneally a final boost of 50 µg S.hIgG1Fc.DC-SIGN.ECD in the absence of adjuvant. After 5 days, spleen cells were used for hybridoma fusion at the Monoclonal Antibody Core Facility of the Rockefeller University and Memorial Sloan-Kettering Cancer Center. In brief, murine myelomas P3X63Ag8.653 (for rats) or SP2/0-Ag14 (for mice) cells (ATCC, Manassas, VA) were fused with spleen cells from the donor animal at a 1:4 ratio in 50 % polyethylene glycol (PEG; EM Science; Germany). Stable hybrids were selected by growth in Hybridoma-SFM (GIBCO Invitrogen) medium containing 15 % FBS, hypoxanthine, aminopterin and thymidine, according to standard protocols, and distributed into 96-well plates. Supernatants from the hybridoma cultures were screened by ELISA as described below.
Hybridoma cultures with supernatants showing antibody activity against mouse DC-SIGN were expanded and cloned twice by limiting dilution. Some hybridomas were grown in DMEM media containing 5 % ultra low IgG FBS. Culture supernatants were collected and the MAbs were purified with protein A/G (Pierce, Rockford, IL) or Protein G (GE Healthcare, Piscataway, NJ), according to manufacturer’s instructions. Purified MAbs were labeled with Alexa 488, Alexa 647 (Molecular Probes, Invitrogen) or EZ-Link Biotin (Pierce, Thermo Scientific) reagents following manufacturer’s instructions.
2.6. Detection of rat and mouse anti-mouse DC-SIGN MAb by ELISA
For the screening of hybridomas, the purified His.DC-SIGN.CRD protein was used for ELISA as described (Park et al., 2000; Cheong et al., 2007). In brief, ELISA plates (Microtest™ 96-Well ELISA Plate, BD Falcon, Bedford, MA) were coated overnight with 50 µl of 2 µg/ml of His.DC-SIGN.CRD (or His.SIGN-R3.CRD for a control) in PBS. Plates were then washed with PBS with 0.1 % Tween 20 (PBS-T) and blocked with 5 % normal goat serum (Sigma-Aldrich) in PBS-T (blocking solution) for 1 hr at 37 °C. Serial dilutions of the sera in blocking solution or undiluted hybridoma supernatants were incubated for 1 hr at 37 °C and were visualized with anti-rat or mouse IgG heavy (γ) chain conjugated to HRP (Southern Biotech), followed by colorimetric assay using OPD (o-phenylenediamine dihydrochloride; Sigma-Aldrich) in CPB solution, i.e. 10 mg OPD tablet in 25 ml CPB buffer (25 mM citric acid, 50 mM Na2HPO4) with 10 µl of 30 % H2O2 solution, or TMB One Component HRP microwell substrate (BioFX Laboratories, Owings Mills, MD). Optical density (OD) at 450 nm was measured using an Opsys MR microplate reader (Thermo Labsystems, Chantilly, VA). The rat and mouse Ig isotypes of MAb heavy and light chains were determined by the ELISA using MAb supernatant as primary Ab and rat or mouse isotype specific HRP-conjugated Abs (Southern Biotech) as secondary Ab.
2.7. SDS-PAGE and Western blot analysis
Cell-lines expressing various C-type lectins were lysed with RIPA lysis buffer (150 mM NaCl, 50 mM Tris-HCl pH 8.0, 1 % NP-40, 0.5 % desoxycholate, 0.1 % SDS) including protease inhibitor cocktail (Sigma-Aldrich) or directly in sample buffers (Laemmli sample buffer without β-mercaptoethanol, Bio-Rad Laboratories; protein loading buffer blue with β-mercaptoethanol, National Diagnostics). Cell lysates, mixed with sample buffers and boiled for 5 min, were separated on 10 %, 12 %, or 15 % SDS-PAGE and blotted onto Hybond™-P polyvinylidine difluoride (PVDF) membrane (GE Healthcare, Piscataway, NJ). Blotted membranes were blocked with 10 % non-fat dry milk in PBS-T, incubated with serum/supernatant/antibody samples, detected by anti-rat, anti-mouse, or anti-rabbit IgG secondary antibodies conjugated with HRP (Southern Biotech, Birmingham, AL), and visualized with ECL Plus™ reagents (GE Healthcare).
2.8. Immunolabeling by flow cytometry and immunocytochemistry
After detaching with 1 mM EDTA in PBS for 10 min, stable CHO cells were incubated with primary Abs for 30 min at 4 °C. Cells were washed, detected by fluorochrome-conjugated secondary Abs or streptavidin for 30 min at 4 °C, and analyzed with FACSCalibur™ flow cytometer or LSR-II (BD Biosciences) at the Rockefeller University Flow Cytometry Resource Center. For competition assays, an excess amount of unlabeled, competing MAb was incubated with CHO/DC-SIGN cells for 10 min at 4 °C prior to adding the fluorochrome-conjugated MAb for additional 30 min incubation at 4 °C, followed by flow cytometric analysis.
For immunocytochemistry, stable transfectant CHO cells were grown on cover slides for 24 hrs. The slides were fixed with acetone for 10 min and then stained as above, followed by microscopic examination by deconvolution microscopy (Olympus, Melville, NY) or a Zeiss LSM 510 system (Carl Zeiss MicroImaging, Thornwood, NY) at the Rockefeller University Bio-Imaging Resource Center.
2.9. Collagenase treatment
CHO cells expressing mouse DC-SIGN, DCIR2, or control Neomycin were incubated in Hanks’ Balanced Salt Solution (HBSS) supplemented without or with 400 units/ml collagenase D (Roche Applied Science, Indianapolis, IN) at 37 °C for 30 or 60 min. EDTA (10 mM final concentration) was added to stop the reaction before the samples were divided into halves to be analyzed by FACS and Western blots.
3. Results
3.1. Polyclonal antibodies specifically recognize mouse DC-SIGN and related lectins
We first produced polyclonal antibody (PAb) to the extracellular domain (ECD) of DC-SIGN, using the C-terminal 13-aa peptide of DC-SIGN to immunize rabbits, similarly to our previous generation of a sensitive and specific PAb against the C-terminal 13-aa peptide of SIGN-R1 (PAb-R1C13) in rabbits (Kang et al., 2003; Kang et al., 2004). However, unlike PAb-R1C13, which selectively reacted with SIGN-R1 and not SIGN-R3 or DC-SIGN (Fig. 1), the PAb generated against the C-terminal 13-aa peptide of DC-SIGN was completely non-reactive to the DC-SIGN protein expressed in mammalian cells although the ELISA titer of the PAb against the peptide was high (data not shown). It appears that the C-terminal peptide of DC-SIGN protein expressed in mammalian cells is inaccessible to the PAb specific to the peptide.
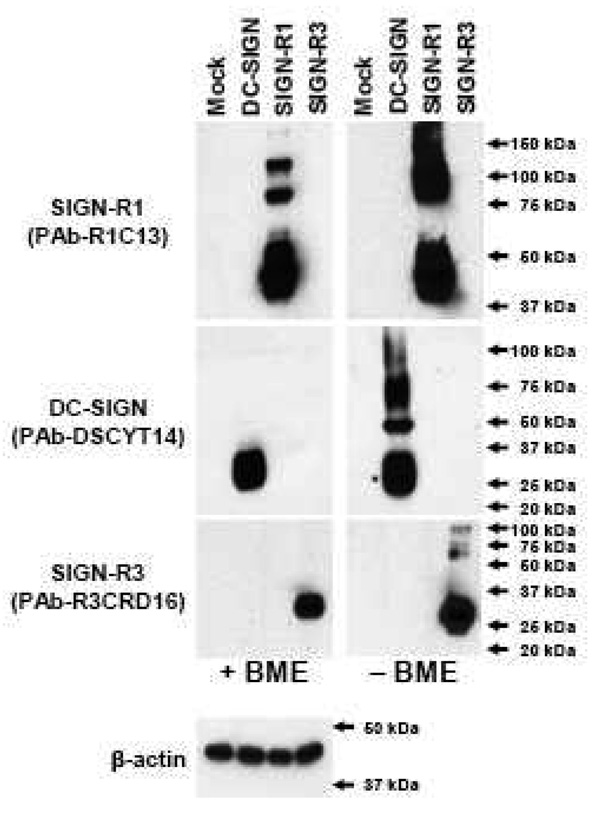
Figure 1. Specific detection of DC-SIGN by rabbit polyclonal antibody DSCYT14.
CMV mammalian expression vectors containing no insert (Mock), mouse DC-SIGN, SIGN-R1, SIGN-R3 cDNA were transfected into 293TAg cells and cell lysates were prepared 1 day later. Equivalent amounts of cell lysates were boiled in sample buffers with (+) or without (−) β-mercaptoethanol (BME) prior to Western blot analyses with rabbit polyclonal antibodies against each mouse SIGN molecule or anti-β-actin antibody.
Therefore, we generated another rabbit PAb against the 14-aa peptide (NH2–GKRQLRPLDEELLT-COOH) in the cytosolic domain of DC-SIGN (PAb-DSCYT14). This cytosolic sequence is unique not only amongst the homologous mouse SIGN family genes but also in the entire proteome of the mouse. When the different but highly homologous mouse SIGN proteins, i.e. DC-SIGN, SIGN-R1, and SIGN-R3, were expressed in mammalian cells, the 30 kDa DC-SIGN protein was specifically detected by PAb-DSCYT14 (Fig. 1). The formation of complexes (dimer, trimer, or tetramer) of DC-SIGN and other mouse SIGN molecules was observed in the Western blots when the samples were boiled without β-mercaptoethanol (Fig. 1). However, only SIGN-R1 could form multimers following the treatment of samples with β-mercaptoethanol in cell-lines (Fig. 1) as well as in tissues (Kang et al., 2003). Thus a PAb to the cytosolic domain of DC-SIGN can be used to distinguish this lectin from other mouse SIGN family members.
3.2. Production of rat monoclonal antibodies against DC-SIGN extracellular domain
To immunize rats, 2 forms of mouse DC-SIGN protein were produced. One contained the extracellular domain (ECD) of mouse DC-SIGN, consisting of a short neck and single carbohydrate recognition (CRD) domains. The ECD of mDC-SIGN was fused to a human IgG1 Fc cassette and was stably transfected into CHO cells. The fusion protein (S.hIgG1Fc.DC-SIGN.ECD) was purified from cultured supernatant using Protein A Sepharose. To screen immune sera by ELISA, a second form of DC-SIGN containing only the CRD domain was expressed in bacteria as a His-tagged fusion protein (His.DC-SIGN. CRD) and purified.
The rats were immunized at monthly intervals with 50 µg of a 1:1 mixture of S.hIgG1Fc.DC-SIGN.ECD and His.DC-SIGN.CRD proteins. Each rat’s serum was screened for antibody responses to His.DC-SIGN.CRD protein relative to non-specific SIGN-R3 protein, His.SIGN-R3.CRD at 7 days after injection. The Western blot results showed strong blotting signals in both specific (DC-SIGN) and non-specific (SIGN-R3) targets. However, by ELISA assays, the optical density (OD) values specific to the CRD of DC-SIGN were larger than to the CRD of SIGN-R3. After 5 immunizations the absolute OD values were lower than those detected in the immunized rats during our previous production of MAb’s (Cheong et al., 2007; Park et al., 2008). Therefore, the rats were further immunized with DC-SIGN protein for a total of 11 times. In addition, the rats were inoculated once with a lysate of splenic low density cells enriched for DCs. This immunization protocol is similar to the one used in our previous generation of anti-Langerin MAb hybridomas in rats (Cheong et al., 2007).
One rat was selected based on the highest ELISA titer, boosted with S.hIgG1Fc.DC-SIGN.ECD, and subjected to hybridoma formation. Almost 800 hybridomas were obtained, and the supernatants were screened by ELISA using anti-rat IgG heavy (γ) chain as secondary Ab. Five hybridomas (named BMD10, 11, 24, 25, 30) were strongly reactive to His.DC-SIGN.CRD by ELISA assay. BMD25 secreted rat IgM/lambda, while the remaining 4 hybridomas secreted rat IgG2a/kappa.
By FACS analysis BMD25 was cross-reactive to SIGN-R1, but BMD10, 11, 24, and 30 only reacted with DC-SIGN (Fig. 2A). Similarly when the lysates of cells transfected with different lectins were blotted as in Figure 1, only the DC-SIGN sample was detected by BMD10, 11, 24, and 30 (data not shown). However, the sensitivity of BMD10, 11, 24, and 30 for Western blot detection was much weaker than that of PAb-DSCYT14, i.e. BMD10, 11, 24, and 30 could readily detect DC-SIGN in transfected 293TAg cells but hardly recognized DC-SIGN in mouse tissue samples (data not shown).
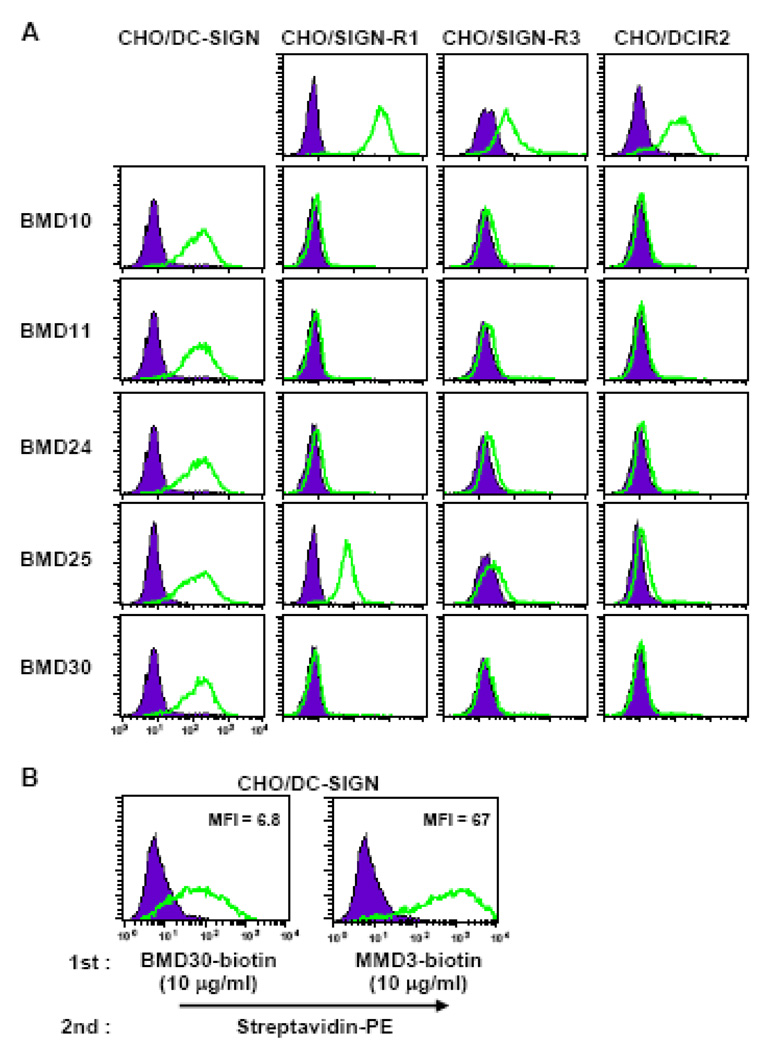
Figure 2. Specific binding of anti-DC-SIGN MAbs to stably transfected CHO cells.
(A) CHO cells expressing three mouse SIGN molecules (DC-SIGN, SIGN-R1, and SIGN-R3) or DCIR2 were surface stained with the new anti-DC-SIGN MAbs BMD10, 11, 24, 25 and 30. BMD10, 11, 24, and 30 were detected by anti-rat IgG-PE/Cy5.5, while BMD25 was detected by anti-rat IgM-PE. The control CHO cells in the top row were stained by MAb 22D1 (anti-SIGN-R1), PAb-R3CRD16 (anti-SIGN-R3), and MAb 33D1 (anti-DCIR2) respectively. (B) CHO cells expressing mouse DC-SIGN were stained with an equal amount of biotinylated rat BMD30 or mouse MMD3 MAb to DC-SIGN followed by the detection of streptavidin-PE. Relative binding of BMD30 and MMD3 are shown as mean fluorescent index (MFI).
3.3. Production of mouse monoclonal antibodies against DC-SIGN extracellular domain
To generate additional DC-SIGN MAbs we immunized DC-SIGN knockout mice 6 times with the mixture of mouse DC-SIGN proteins similarly to the immunization of rats described above. One mouse was selected based on the highest ELISA titer, boosted with S.hIgG1Fc.DC-SIGN.ECD, and subjected to hybridoma formation. Almost 500 hybridomas were obtained, and the supernatants were screened by ELISA using antimouse IgG heavy (γ) chain as secondary Ab. Two hybridomas (named MMD2 and 3) were detected reactive to His.DC-SIGN.CRD by ELISA assay, and both MMD2 and MMD3 secreted mouse IgG2c/kappa. From the result of the screen, supernatants of more than 50 ELISA-negative hybridomas were selected randomly and analyzed for mouse Ig isotypes. A hybridoma was identified to secrete mouse IgG2c/kappa MAb, named MIC-G2c, of unknown antigen specificity. MIC-G2c is useful as an isotype control for the MMD2 and MMD3 anti-DC-SIGN mAbs.
By FACS analysis and Western blot of transfectant cells as in Figures 1 and 2A, both MMD2 and MMD3 were not cross-reactive to SIGN-R1, SIGN-R3, or DCIR2 (data not shown). However, the binding of MMD2 and MMD3 by FACS (Fig. 2B) and Western blot (not shown) was 10 times stronger.
3.4. Three different immunogenic regions in the extracellular domain of DC-SIGN
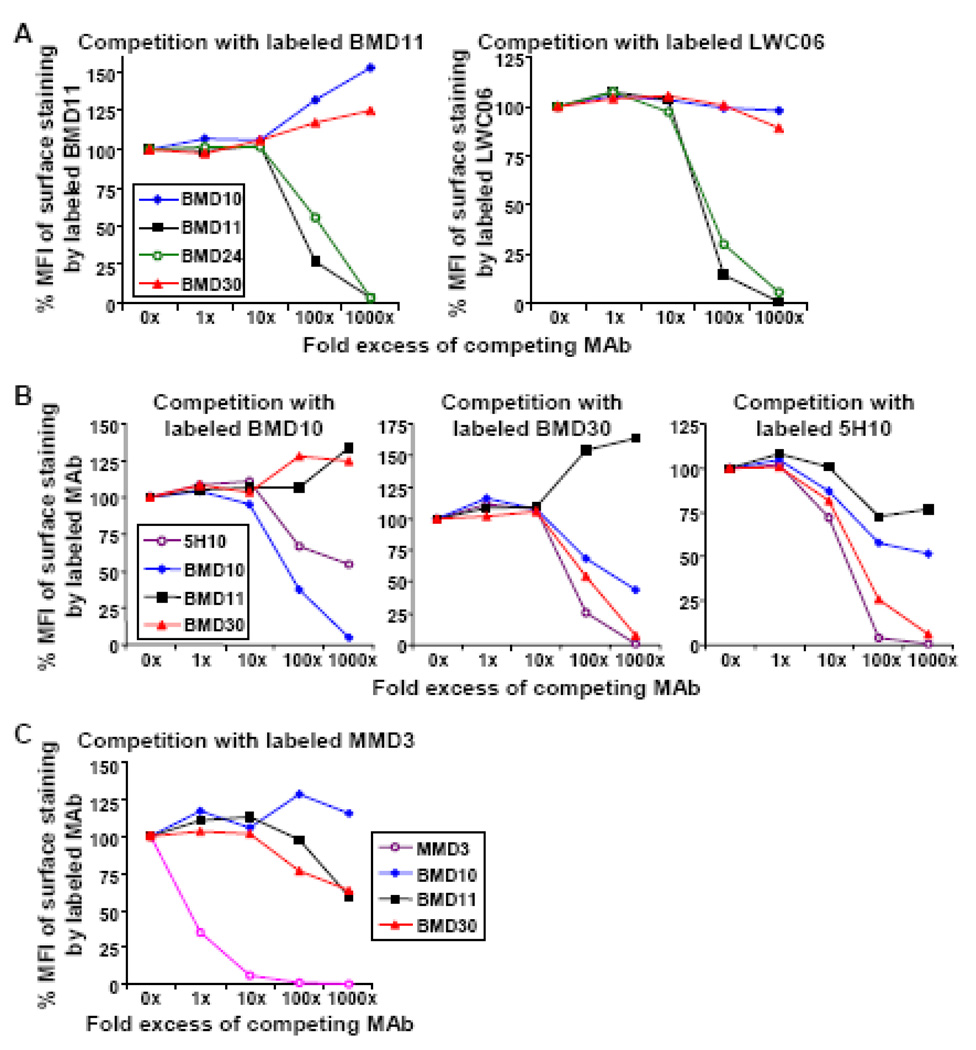
Currently two rat IgG2a/kappa MAbs against mouse DC-SIGN are commercially available, which are 5H10 (Caminschi et al., 2006) and LWC06 (eBioscience). To test whether the binding epitopes of newly generated BMD MAbs overlapp with one another or other anti-DC-SIGN MAbs, we labeled BMD10, BMD11, and BMD30 MAbs with Alexa647 and also obtained unlabeled and PE-labeled 5H10 and LWC06 from eBioscience. Each fluorescent-labeled MAb was used to stain CHO/DC-SIGN cells with/without the excessive amounts of unlabeled, competing MAbs, and then assessed for the inhibition of surface staining on CHO/DC-SIGN cells by FACS analysis. As shown in Figure 3A, BMD11 and BMD24 can compete efficiently for each other: in addition, both BMD11 and BMD24 blocked effectively the staining of LWC06-PE (Fig. 3A). Therefore, the binding epitopes in mouse DC-SIGN for BMD11, BMD24, and LWC06 are either identical or closely overlapping.
Figure 3. Competition assays for the binding of different anti-DC-SIGN MAbs.
(A, B, C) CHO/DC-SIGN cells were pre-incubated for 10 min with indicated amounts of unlabeled competing MAbs prior to the incubation of each fluorescent-labeled anti-DC-SIGN MAb for 30 min. The binding of fluorescent-labeled MAb to CHO/DC-SIGN cells was calculated as the percent (%) value of each MFI (Median Fluorescence Intensity) compared to the MFI of CHO/DC-SIGN cells stained without competing MAbs.
When we examined the competition of labeled BMD10, BMD30, or 5H10 MAbs with unlabeled, excessive MAbs, the results were more complex (Fig. 3B). BMD30 and 5H10 were able to block each other’s binding efficiently, but only 5H10 could partially inhibit the binding of BMD10 while BMD30 could not interfere with the binding of BMD10. Meanwhile, BMD10 partially blocked the binding of both BMD30 and 5H10. Based on these observations, we speculate that the binding epitopes in DC-SIGN for BMD10, BMD30, and 5H10 are partially overlapping or closely located sterically.
When we examined the competition of labeled MMD3 with unlabeled, excessive MAbs, all BMD MAbs were not able to block the binding of MMD3 (Fig. 3C). Meanwhile, MMD2 could block the binding of MMD3 completely and vice versa (data not shown). Based on these observations, we speculate that the binding epitopes in DC-SIGN for mouse MAbs MMD2 and MMD3 are identical, but this epitope is different from those of rat MAbs BMD10, 11, and 30.
3.5. BMD10, BMD30, and MMD3 detect DC-SIGN in acetone-fixed cells
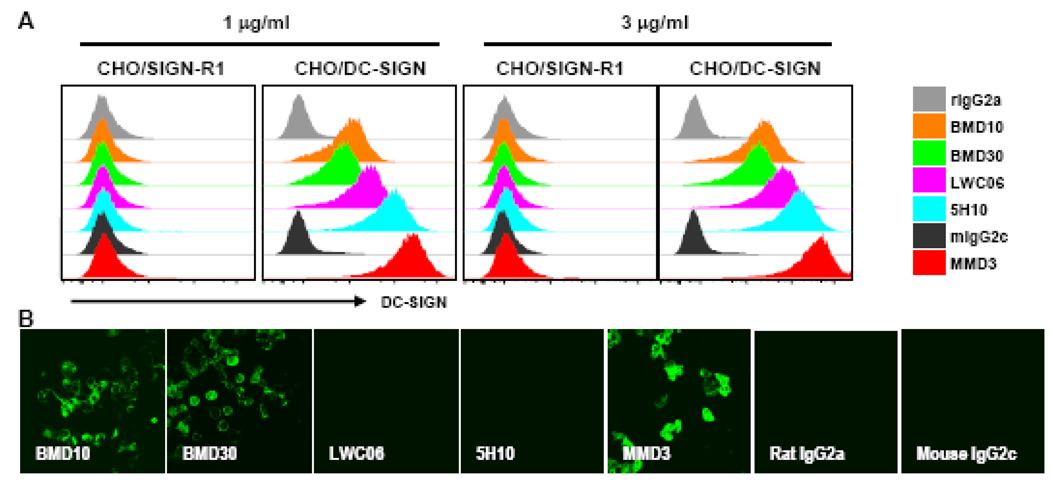
We compared the surface binding activities of the new BMD and MMD MAbs with 5H10 and LWC06. As shown in Figure 4A, all MAbs bound specifically to CHO/DC-SIGN but not CHO/SIGN-R1 cells. We also fixed cells with acetone, which is widely used to fix cryosections of animal tissues. To this end, CHO/DC-SIGN and CHO/Neo cells were grown on cover slides for 24 hrs, fixed with cold acetone for 10 min, and stained with each MAb. In contrast to formaldehyde fixation, BMD10, BMD30, and MMD3 were able to stain CHO/DC-SIGN cells with acetone fixation (Fig. 4B) but not control CHO/Neo cells (data not shown). However, neither 5H10 nor LWC06 stained CHO/DC-SIGN cells different than the isotype control (Fig 4B).
Figure 4. Comparison of different anti-DC-SIGN MAbs for immunolabeling.
(A)Anti-DC-SIGN MAbs that have been previously reported plus the new MAbs reported here were used to stain CHO/DC-SIGN or CHO/SIGN-R1 cells at 1 µg/ml and 3 µg/ml; labeling was assessed by FACS.(B) As in (A), but CHO/DC-SIGN cells grown on cover slides and fixed with acetone were stained. The binding was visualized with Streptavidin-APC in (A) and Streptavidin-Alexa 488 in (B).
3.6. DC-SIGN molecules are sensitive to collagenase treatment
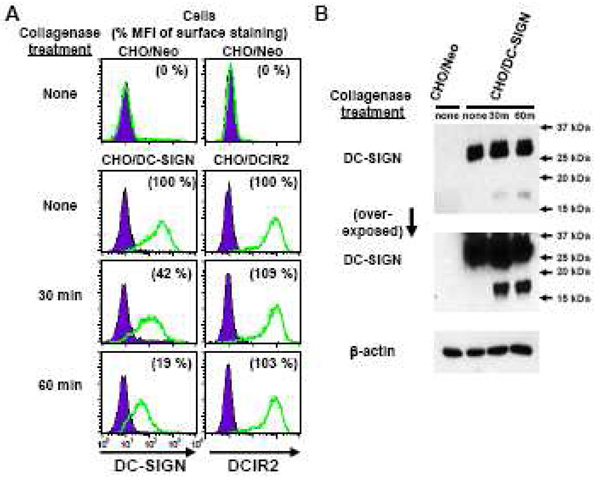
During our previous study to identify and isolate SIGN-R1 positive cells from mouse spleen (Kang et al., 2003), we became aware that significant amounts of SIGN-R1 molecules were degraded following a standard procedure (Inaba et al., 1998) to make tissue cell suspensions using collagenase treatment, when monitored by Western blot with anti-SIGN-R1 PAb-R1C13 (data not shown). When we tested if mouse DC-SIGN was also sensitive to collagenase treatment, using the newly generated MAbs and PAb-DSCYT14, we found by FACS analysis, that 30 min treatment with collagenase removed more than 50% of DC-SIGN molecules from the surface of CHO/DC-SIGN cells (Fig. 5A). When the same CHO/DC-SIGN cells, treated with collagenase and used in FACS analysis, were lysed and blotted with PAb-DSCYT14, the degraded cytosolic fragment (~17 kDa) of DC-SIGN appeared but not in CHO/DC-SIGN cells without collagenase treatment (Fig. 5B), and similar results were obtained with/without trypsin treatment of CHO/DC-SIGN cells (data not shown). This indicates that collagenase destroys DC-SIGN molecules on cell surfaces. Meanwhile, the surface expression of DCIR2, another C-type lectin molecule found on DCs, was not changed by collagenase treatment of CHO/DCIR2 cells (Fig. 5A).
Figure 5. Mouse DC-SIGN molecules are sensitive to collagenase.
(A) CHO cells expressing mouse DC-SIGN, DCIR2, or control Neomycin (Neo) were incubated without (none) or with collagenase (400 units/ml) for 30 or 60 min, and the reaction was stopped by adding EDTA (10 mM final concentration). The halves of treated CHO cells were, then, stained with antibodies as indicated for FACS analysis. Relative levels of surface expression for each molecule were calculated as the percent (%) value of each MFI compared to the MFI of the CHO/DC-SIGN, or CHO/DCIR2 cells stained without collagenase treatment.
(B) The remaining halves of CHO/Neo and CHO/DC-SIGN cells treated with/without collagenase from (A) were harvested and lysed. Equivalent amounts of cell lysates were boiled in sample buffer and separated in a 15 % SDS-PAGE gel prior to Western blot analyses with PAb-DSCYT14 and anti-β-actin antibody.
4. Discussion
DCs are comprised of different subsets, which can express distinct C-type lectins. The differential expression level of various C-type lectin receptors, as detected by MAbs, has effectively marked these subsets. For example, most of the initial markers used to identify DCs are C-type lectins expressed by DC subsets such as DEC205, DCIR2, and Langerin (Vremec and Shortman, 1997; Cheong et al., 2007; Dudziak et al., 2007). DC-SIGN is a C-type lectin with a type II transmembrane structure, i.e. a carbohydrate recognition domain (CRD) at the COOH-terminus. Although the role of human DC-SIGN has been investigated in many contexts, its mouse counterpart has not been studied because of a lack of good antibodies.
Recently, two MAbs against mouse DC-SIGN became available (Caminschi et al., 2006) (eBioscience). With MAb 5H10, mouse DC-SIGN was detected in a minor population of DCs from lymphoid tissue cell suspensions by FACS analysis (O'Keeffe et al., 2002; Caminschi et al., 2006). However, we find that these MAbs do not react with DC-SIGN in acetone fixed cells, which probably explains why this lectin has not been localized previously in tissue sections. The new MAbs reported here overcome this obstacle since they react specifically with DC-SIGN in acetone fixed cells.
Competition studies reveal that the new mouse and rat mAbs define at least 3 immunogenic regions in the extracellular domain of mouse DC-SIGN, although the epitopes seen by the new mouse MMD2 and MMD3 MAbs lead to 10 fold higher binding.
These new MAbs offer means to study DC-SIGN expressing cells in vivo. In addition, these MAbs will allow for the rapid and selective targeting to DC-SIGN expressing cells in vivo. For example, we will clone the heavy and light chains of BMD and MMD MAbs so that they can be engineered to encode defined protein antigens, to test if ligation of DC-SIGN in vivo leads to more efficient antigen presentation, as described previously for engineered MAbs to DEC205/CD205 (Hawiger et al., 2001; Boscardin et al., 2006; Trumpfheller et al., 2006), DCIR2 (Dudziak et al., 2007; Soares et al., 2007), and Langerin/CD207 (Idoyaga et al., 2008).
Acknowledgments
We thank Judy Adams for preparing the figures; Yoonkyung Do, Hyein Koh, Patrick Seo, Sung Ho Park, and Jung Heon Jo for technical help; Juan Carcamo, Syeda Rizvi, Francisco Berguido, Jay Overholser, and Frances Weis-Garcia at the Monoclonal Antibody Core Facility of the Rockefeller University and Memorial Sloan-Kettering Cancer Center for help with hybridoma production; and Alison North at the Rockefeller University Bio-Imaging Resource Center for help with confocal microscopy. We wish to acknowledge the NIH-sponsored Mutant Mouse Regional Resource Center (MMRRC) National System as the source of genetically-altered DC-SIGN/CD209a knockout mice for use in this study. The mice were produced and deposited to the MMRRC by the Consortium for Functional Glycomics supported by the National Institute of General Medical Sciences (GM62116). We were supported by NIH Grants to RMS (AI 13013, AI 40045, AI 057158) and CGP (AI 057158), by funds from the New York Community Trust to CC (The Francis Florio funds for blood diseases), and by Fundação para a Ciência e Tecnologia, Ph.D. scholarship to IM (SFRH/BD/41073/2007).
Abbreviations
- BME
β-mercaptoethanol
- CHO cells
Chinese hamster ovary cells
- CRD
carbohydrate recognition domain
- DCs
dendritic cells
- ECD
extracellular domain
- FACS
fluorescence-activated cell sorter
- FBS
fetal bovine serum
- MAb
monoclonal antibody
- MFI
mean fluorescence intensity
- OD
optical density
- OPD
o-phenylenediamine dihydrochloride
- ORF
open reading frame
- PAb
polyclonal antibody
- PECs
peritoneal exudate cells
- PEG
polyethylene glycol
- PVDF
olyvinylidine difluoride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez CP, Lasala F, Carrillo J, Muniz O, Corbi AL, Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 2002;76:6841–6844. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Boscardin SB, Hafalla JC, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U, Morrot A, Zavala F, Steinman RM, Nussenzweig RS, Nussenzweig MC. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J. Exp. Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminschi I, Corbett AJ, Zahra C, Lahoud M, Lucas KM, Sofi M, Vremec D, Gramberg T, Pohlmann S, Curtis J, Handman E, van Dommelen SL, Fleming P, Degli-Esposti MA, Shortman K, Wright MD. Functional comparison of mouse CIRE/mouse DC-SIGN and human DC-SIGN. Int. Immunol. 2006;18:741–753. doi: 10.1093/intimm/dxl011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminschi I, Lucas KM, O'Keeffe MA, Hochrein H, Laabi Y, Brodnicki TC, Lew AM, Shortman K, Wright MD. Molecular cloning of a C-type lectin superfamily protein differentially expressed by CD8α− splenic dendritic cells. Mol. Immunol. 2001;38:365–373. doi: 10.1016/s0161-5890(01)00067-0. [DOI] [PubMed] [Google Scholar]
- Cheong C, Idoyaga J, Do Y, Pack M, Park SH, Lee H, Kang YS, Choi JH, Kim JY, Bonito A, Inaba K, Yamazaki S, Steinman RM, Park CG. Production of monoclonal antibodies that recognize the extracellular domain of mouse Langerin/CD207. J. Immunol. Methods. 2007;324:48–62. doi: 10.1016/j.jim.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenares M, Puig-Kroger A, Pello OM, Corbi AL, Rivas L. Dendritic-cell specific ICAM-3 grabbing nonintegrin (DC-SIGN, CD209), a C-type surface lectin in human dendritic cells, is a receptor for Leishmania amastigotes. J. Biol. Chem. 2002;16:16. doi: 10.1074/jbc.M205270200. [DOI] [PubMed] [Google Scholar]
- Curtis BM, Scharnowske S, Watson AJ. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA. 1992;89:8356–8360. doi: 10.1073/pnas.89.17.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz V, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- Galustian C, Park CG, Chai W, Kiso M, Bruening SA, Kang YS, Steinman RM, Feizi T. High and low affinity carbohydrate ligands revealed for murine SIGN-R1 by carbohydrate array and cell binding approaches, and differing specificities for SIGN-R3 and langerin. Int. Immunol. 2004;16:853–866. doi: 10.1093/intimm/dxh089. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, van Vliet SJ, Engering A, 't Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu. Rev. Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TBH, Krooshoop DJEB, Bleijs D, van Vliet SJ, van Duijnhoven GCF, Grabovsky V, Alon R, Figdor CG, van Kooyk Y. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 2000a;1:353–357. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TBH, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GCF, Middel J, Cornelissen ILMHA, Nottet HSLM, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell specific HIV-1 binding protein that enhances trans-infection of T cells. Cell. 2000b;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TBH, Torensma R, van Vliet SJ, van Duijnhoven GCF, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000c;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, Park CG, Steinman RM. Langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J. Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G. Current Protocols in Immunology. John Wiley & Sons, Inc.; 1998. Isolation of dendritic cells; pp. 3.7.1–3.7.15. [DOI] [PubMed] [Google Scholar]
- Kang Y-S, Yamazaki S, Iyoda T, Pack M, Bruening S, Kim JY, Takahara K, Inaba K, Steinman RM, Park CG. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int. Immunol. 2003;15:177–186. doi: 10.1093/intimm/dxg019. [DOI] [PubMed] [Google Scholar]
- Kang YS, Do Y, Lee HK, Park SH, Cheong C, Lynch RM, Loeffler JM, Steinman RM, Park CG. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell. 2006;125:47–58. doi: 10.1016/j.cell.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Kang YS, Kim JY, Bruening SA, Pack M, Charalambous A, Pritsker A, Moran TM, Loeffler JM, Steinman RM, Park CG. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc. Natl. Acad. Sci. USA. 2004;101:215–20. doi: 10.1073/pnas.0307124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier JL, Arenzana-Seisdedos F, Despres P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003;4:1–6. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, Anders EM, Wu L, Lahoud MH, Henri S, Scott B, Hertzog P, Tatarczuch L, Shortman K. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8+ dendritic cells only after microbial stimulus. J. Exp. Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CG, Chwae YJ, Kim JI, Lee JH, Hur GM, Jeon BH, Koh JS, Han JH, Lee SJ, Park JW, Kaslow DC, Strickman D, Roh CS. Serologic responses of Korean soldiers serving in malaria-endemic areas during a recent outbreak of Plasmodium vivax. Am. J. Trop. Med. Hyg. 2000;62:720–725. doi: 10.4269/ajtmh.2000.62.720. [DOI] [PubMed] [Google Scholar]
- Park CG, Lee SY, Kandala G, Choi Y. A novel gene product that couples TCR signaling to Fas (CD95) expression in activation-induced cell death. Immunity. 1996;4:583–91. doi: 10.1016/s1074-7613(00)80484-7. [DOI] [PubMed] [Google Scholar]
- Park CG, Takahara K, Umemoto E, Yashima Y, Matsubara K, Matsuda Y, Clausen BE, Inaba K, Steinman RM. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int. Immunol. 2001;13:1283–1290. doi: 10.1093/intimm/13.10.1283. [DOI] [PubMed] [Google Scholar]
- Park SH, Cheong C, Idoyaga J, Kim JY, Choi JH, Do Y, Lee H, Jo JH, Oh YS, Im W, Steinman RM, Park CG. Generation and application of new rat monoclonal antibodies against synthetic FLAG and OLLAS tags for improved immunodetection. J. Immunol. Methods. 2008;331:27–38. doi: 10.1016/j.jim.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailleux L, Schwartz O, Herrmann J-L, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman CJ, Lagrange PH, Gicquel B, Neyrolles O. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, Steinman RM, Schlesinger S, Marovich MA. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpfheller C, Finke JS, Lopez CB, Moran TM, Moltedo B, Soares H, Huang Y, Schlesinger SJ, Park CG, Nussenzweig MC, Granelli-Piperno A, Steinman RM. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J. Exp. Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Die I, van Vliet SJ, Nyame AK, Cummings RD, Bank CM, Appelmelk B, Geijtenbeek TB, van Kooyk Y. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13:471–478. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- Vremec D, Shortman K. Dendritic cells subtypes in mouse lymphoid organs. Cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J. Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- Zhang P, Skurnik M, Zhang SS, Schwartz O, Kalyanasundaram R, Bulgheresi S, He JJ, Klena JD, Hinnebusch BJ, Chen T. Human dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin (CD209) is a receptor for Yersinia pestis that promotes phagocytosis by dendritic cells. Infect. Immun. 2008a;76:2070–2079. doi: 10.1128/IAI.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SS, Park CG, Zhang P, Bartra SS, Plano GV, Klena JD, Skurnik M, Hinnebusch BJ, Chen T. Plasminogen activator Pla of Yersinia pestis utilizes murine DEC-205 (CD205) as a receptor to promote dissemination. J Biol Chem. 2008b;283:31511–31521. doi: 10.1074/jbc.M804646200. [DOI] [PMC free article] [PubMed] [Google Scholar]