Abstract
Objectives
To determine whether a Delirium Abatement Program (DAP) can shorten the duration of delirium among new admissions to post-acute care (PAC).
Design
Cluster randomized controlled trial.
Setting
Eight skilled nursing facilities specializing in PAC within a single metropolitan region.
Participants
Four hundred fifty-seven participants with delirium at PAC admission.
Intervention
The DAP consisted of four steps: 1) assessment for delirium within 5 days of PAC admission, 2) assessment and correction of common reversible causes of delirium, 3) prevention of complications of delirium, and 4) restoration of function.
Measurements
Eligible patients were screened by trained researchers. Those with Confusion Assessment Method defined delirium were eligible for participation via proxy consent. Two weeks and one month after enrollment, regardless of location, participants were re-assessed for delirium by researchers blind to intervention status.
Results
Nurses at DAP sites detected delirium in 41% of participants vs. 12% in usual care (UC) sites (p<.001) and completed DAP documentation in most delirium-detected participants. However, the DAP intervention had no impact on delirium persistence based on two measurements at 2 weeks (DAP 68% vs. UC 66%) and 1 month (DAP 60% vs. UC 51%), adjusted p values ≥ 0.20. Adjusting for baseline differences between DAP and UC participants and restricting analysis to delirium-detected DAP participants did not alter the results.
Conclusion
Detection of delirium improved at the DAP sites, however, the DAP had no impact on the persistence of delirium. This effectiveness trial demonstrated that a nurse-led DAP intervention was not effective in typical PAC facilities.
Keywords: Delirium, Outcomes, Post-acute care, Randomized trial
INTRODUCTION
Delirium (acute confusion) is a common, morbid, and costly geriatrics syndrome that affects one-third of hospitalized elders. (1) As evidence mounts that delirium may persist for weeks to months (2, 3), concern for delirium can no longer be restricted to acute hospitals. Of 15 million annual Medicare hospitalizations, 20% receive inpatient post-acute care (PAC). (4) We previously demonstrated a 16% delirium rate among new PAC admissions. (5) Thus, we estimate nearly 500,000 U.S. seniors annually are admitted to PAC facilities with delirium. We have demonstrated that resolution of delirium leads to functional recovery (6) and higher survival rates (7), yet over 50% of these patients are still delirious one month after PAC admission. (8) Failure to recover function in PAC often leads to long-term nursing home placement (9), with resulting negative impact on quality of life and cost.
Randomized trials employing multi-factorial risk reduction strategies in the hospital have successfully prevented incident delirium in general medical patients (10) and after hip fracture. (11) However, these trials had no impact on the persistence of delirium once it develops. It is likely that the typical hospital length of stay in the U.S. (median 4 days) (4) is too short to allow hospital-based treatment programs to impact the course of delirium. We hypothesized that most patients with persistent delirium are discharged to PAC facilities, where they stay several weeks or longer. Given its prevalence, persistence, morbidity, and costs, we developed and tested an intervention to reduce the duration of delirium in the PAC setting.
Our team brought together experts in evidence-based guidelines for management of delirium (12–14), and Resident Assessment Protocols for skilled nursing facilities. (15) Using our combined expertise, we developed the Delirium Abatement Program (DAP), a nurse-led program to improve detection and management of delirium among new admissions to PAC. (16) We tested the DAP using a cluster randomized controlled trial involving 8 Boston-area PAC facilities in which research staff taught facility caregivers how to implement key intervention steps, but did not directly intervene on patients. We report on the nursing implementation of the DAP, and its effect on the primary outcome of interest: persistence of delirium.
METHODS
Study Design, Facility Selection and Participant Accrual
We conducted a cluster randomized controlled trial in greater-Boston skilled nursing facilities. Facilities had to have at least 35 PAC admissions per month and leadership that supported study participation and randomization. They had to meet a minimum threshold for quality of care based on state survey results (17), and to have an appropriate matching facility. After matching on ownership status, size, and setting (urban vs. suburban), facilities were randomized to receive the DAP (16) or usual care.
Research personnel screened all new PAC admissions for trial eligibility. Eligible patients were ≥ 65 years old, admitted directly from an acute medical or surgical hospitalization, spoke English, were communicative prior to acute illness, had life expectancy > 6 months, did not have end-stage dementia or complete functional dependence prior to hospitalization, and lived within 25-miles of our research site.
Assenting eligible patients underwent a structured delirium assessment (18) conducted by trained researchers within 5 days of PAC admission (average time to interview = 2.5 days). In those who had delirium, family caregivers, acting as proxies, provided informed consent for trial participation using a protocol approved by the Institutional Review Board.
We used the Confusion Assessment Method (CAM), the most commonly used validated diagnostic instrument, to assess delirium. (19) The CAM algorithm requires an acute change in mental status with a fluctuating course, inattention, and either disorganized thinking or an abnormal level of consciousness. Prior to completing the CAM, trained research interviewers performed a structured cognitive assessment including the Mini-Mental State Examination (MMSE) (20), Digit Span (21), and the Delirium Symptom Interview (DSI). (22) The interviewers used data from these assessments to complete the CAM. This assessment protocol is highly reliable for the presence of delirium (inter-rater kappa=0.95). (18)
Proxy Baseline Assessments
Participant’s race, ethnicity and their living situation prior to hospitalization were ascertained from proxy baseline assessments. Proxies completed an interview version of the Charlson scale (23, 24) to quantify medical comorbidity. We used the Charlson item, “Has a doctor ever told you that your relative has Alzheimer’s Disease or related dementia?” along with ICD-9CM codes of dementia from the hospital discharge summary, to define “clinical dementia”. We asked the proxies to report on the participant’s pre-hospitalization self-care function using a modified version of the Activities of Daily Living Scale. (25, 26)
Administrative Advisory Panel
An Administrative Advisory Panel (AAP), consisting of facility administrative, nursing and medical leadership, was convened at each facility. The AAP met every 3 months at the DAP sites and every 6 months at the usual care sites. The AAP reviewed the processes of patient screening, consent, and follow-up, which were identical at all sites. At the DAP sites, the AAP also monitored adherence to the DAP. At these sites, an introductory letter was sent to all facility physicians and nurse practitioners informing them about the DAP, along with a semiannual newsletter to update personnel on DAP implementation and highlight key aspects of delirium detection and management.
Delirium Abatement Program (DAP)
Nursing implementation of the DAP began with the assessment of all PAC admissions for delirium. Our recommended assessment was based on six symptoms of delirium contained in Long term Care Resident Assessment Instrument, Version 2 (15, 16), and was performed completely blinded to the results of the research assessments described above. To address the fluctuating nature of delirium, if the initial screen was negative but the nurses detected a subsequent change in mental status, we recommended re-assessment of the patient.
For those whom the nurses detected delirium, the DAP continued with three additional steps: assessment and treatment of reversible causes of delirium, prevention and management of complications, and restoration of function. (16) The DAP was launched with a mandatory education program for all 3 nursing shifts. We gave a 50-minute comprehensive program to all licensed nurses, along with a post-test and nursing Continuing Education Units, and a 30-minute program to certified nursing assistants, with Certificates of Attendance.
In addition to the educational programs, we provided DAP facilities with: 1) admission assessment for delirium, 2) assessment for reversible causes of delirium, 3) delirium nursing plan of care, and, 4) a family caregiver pamphlet, “Guide to Understanding Delirium”. We also provided environmental modifications: 1) large face clocks, daily calendars, and orientation boards, 2) a portable radio and tape recorder with relaxation tapes, 3) incandescent lamps. Details of the use of these items are discussed elsewhere. (16)
Ongoing Education and Monitoring of DAP Implementation
Research project interventionists (senior nurses) visited each facility weekly to provide education for new staff and to measure adherence. We provided each DAP facility with a notebook containing all study documentation and “tip sheets” to assist with program implementation. With facility leadership, we identified a direct care provider on the PAC unit and gave them extra training and a second notebook. This Delirium Resource Nurse, based on the Geriatrics Resource Nurse model (27), worked closely with our project interventionists. We developed five measures of DAP implementation that were monitored on a quarterly basis: 1) nursing assessment for delirium within 5 days of PAC admission, 2) detection of delirium, 3) completion of the Assessment of Causes form, 4) completion of the delirium nursing care plan form, and 5) placement of at least 2 environmental modifications in the participant’s room. (16) The DAP facilities received small incentive payments (up to $700 every six months) based on their performance.
Comparison of delirium management at DAP and Usual Care facilities
A trained nurse performed a medical record review of all trial participants to assess key delirium management processes including: 1) Detection of delirium: At the DAP sites, this was based on the results of the nursing delirium assessments. At the usual care sites, we used two sources: documentation of delirium (or one of its synonyms) in the medical record or triggering of delirium in the admission Minimum Data Set (MDS) 2.0 assessment. (15) 2) Documentation of MD/NP contact for delirium or mental status changes in the medical record; 3) Evaluation and treatment of common reversible causes of delirium; 4) Prevention or management of common complications of delirium; 5) Restoration of function in delirious patients. Steps 3–5 had six specific sub-steps that we assessed in our medical record review. We performed identical reviews at the usual care sites to see if components of the DAP were performed as part of routine care.
Outcome Measures and Ascertainment
The primary outcome of this trial was the persistence of delirium at 2 weeks and 1 month after PAC admission. This was ascertained by research assessors using the CAM algorithm after structured interviews similar to those at PAC admission. (18) Importantly, these interviews were blinded to intervention status, and were performed regardless of the location of the participant (e.g., PAC, home, hospital, etc.). Since the rate of missing data at the DAP and usual care sites was nearly identical, only those participants with non-missing interviews were considered. Our study was powered to detect a 15% reduction in delirium persistence at 2 weeks at the DAP sites from a baseline rate of 75%. This power calculation incorporated a 16.7% loss to follow-up.
Data Safety and Monitoring
A Data Safety and Monitoring Board consisting of 3 senior clinical investigators convened every 6 months for the duration of the trial. No significant safety concerns were raised. Interim analysis of the primary study endpoints was performed at 50% and 75% enrollment. In neither case was a definitive conclusion reached; therefore, the study was allowed to recruit to its originally planned sample size.
Data Analysis
Conforming to recommended procedures for clinical trials (28), we compare the baseline characteristics of participants recruited at the DAP and usual care sites. Next, we describe DAP implementation and compare delirium management at DAP and usual care sites using medical record reviews. Finally, we compare delirium persistence at two weeks and one month between DAP and usual care sites. The outcomes comparisons are performed 3 ways: 1) unadjusted using chi-square tests, 2) adjusted for imbalances in baseline characteristics using logistic regression, and 3) adjusted for imbalances in baseline characteristics and accounting for clustering at the facility level. (29) Bivariable and multivariable statistical analyses using Generalized Estimating Equations (30) were performed using the SAS Statistical Package, Version 8.0. and STATA Statistical Package, Version 9.0. An alpha level of 0.05 (two-tailed) was used to determine statistical significance.
RESULTS
Overall Study Flow
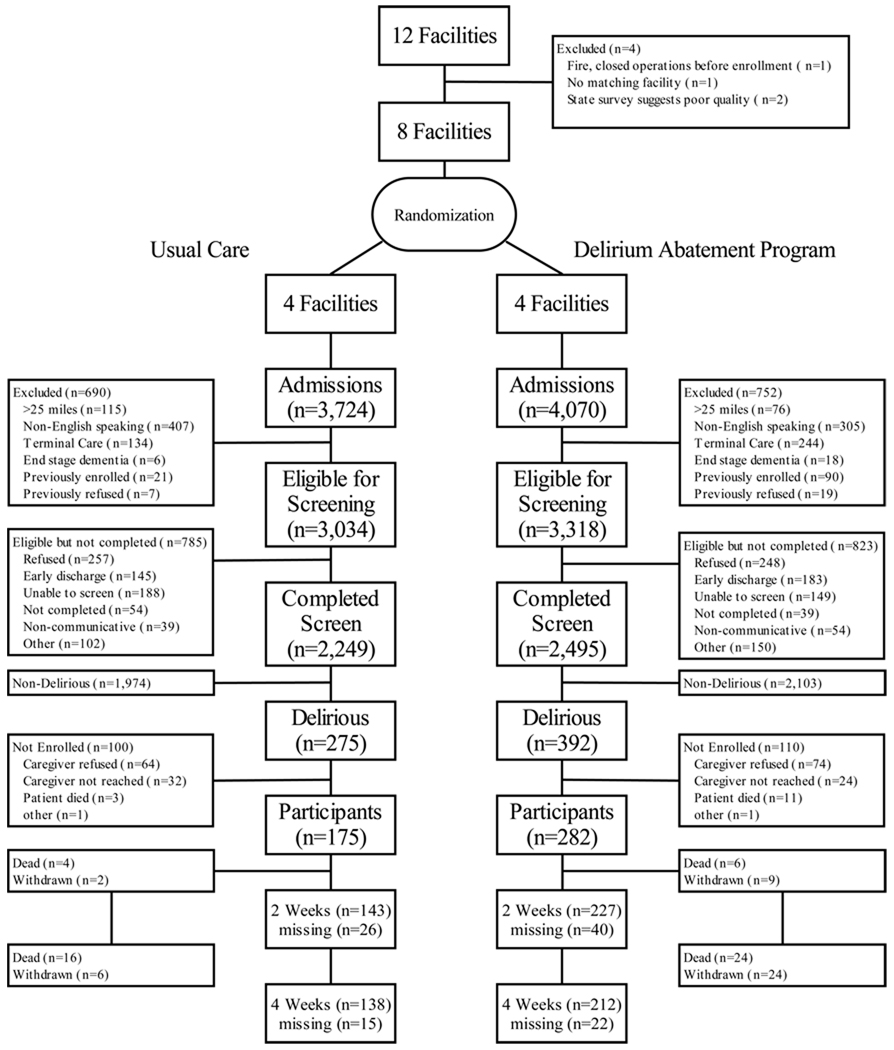
Figure 1 presents the detailed CONSORT figure. Twelve Boston-area facilities met eligibility requirements and were considered for trial participation. The study team visited all facilities and conducted interviews with facility administrative leadership. Of these12 facilities, 2 were excluded because subsequent state survey results documented poor quality. One suffered a major fire and had to close operations. A final facility was excluded because a suitable match was not found. The remaining eight facilities were matched into four pairs and one member of each pair was randomly allocated to the DAP or usual care.
Figure 1. CONSORT Figure for Randomized Trial.
This figure describes facility randomization and the screening, enrollment, randomization, and follow-up process for participants in the clinical trial.
Facilities were matched based on ownership status (profit vs. non-profit), size, and setting (urban vs. suburban).
Over the trial recruitment period (10/1/00-12/31/03), there were 7794 admissions at the 8 study sites. Of these, 3034 at the usual care sites and 3318 at the DAP sites were eligible for screening, and 2249 and 2495 respectively completed a delirium assessment. Reasons for screening ineligibility or inability to complete the screen are provided in Figure 1. Among those screened, 275 in the usual care sites and 392 in the DAP sites were delirious and trial eligible. Of these, 457 participants provided consent (via proxy) and were enrolled, 175 in the usual care sites and 282 in the DAP sites. Reasons for non-enrollment among the eligible delirious patients are provided in Figure 1. Details of subsequent flow of participants, including deaths, withdrawals, and missing follow-up interviews are also described in Figure 1.
Comparison of Baseline Participant Characteristics
Baseline characteristics of the DAP and usual care participants are described in Table 1. Mean age was nearly identical, though usual care participants were more likely to be female and nonwhite. DAP participants were slightly more likely to be living in the community. Total illness burden was nearly identical. DAP participants were more likely to have clinical dementia, and also had slightly more severe delirium at PAC admission based on the Memorial Delirium Assessment Scale score (Table 1). Of the baseline characteristics, only the differences in percentages of clinical dementia and white race were statistically significant (p<.01).
Table 1.
Baseline characteristics of population by intervention status
| Characteristic | DAP Group N=282 |
Usual Care Group N=175 |
|---|---|---|
| Female, N (%) | 174 (61) | 121 (69) |
| White race, N (%) | 272 (96) | 147 (84) |
| Community Residence, N (%) | 267 (95) | 159 (91) |
| Clinical dementia*, N (%) | 129 (46) | 56 (32) |
| Mean Age (years), (mean ± s.d.) | 83.8 ± 7.4 | 84.4 ± 7.2 |
| Mean Charlson comorbidity score†, (mean ± s.d.) |
2.6 ± 2.5 | 2.6 ± 2.2 |
| Mean pre-illness ADL Function (0–16, 16 fully independent)‡, (mean ± s.d.) |
13.2 ± 3.4 | 13.5 ± 3.1 |
| PAC Admission Delirium Severity (0–30, 30 most severe)^, (mean ± s.d.) |
12.8 ± 4.2 | 12.1 ± 4.1 |
Abbreviation: DAP: Delirium Abatement Program
Clinical dementia based on report of proxy informant from the intake Charlson comoribidty questionnaire ( 25) or an ICD code consistent with dementia in the hospital discharge summary
Based on the Charlson comorbidity interview administered to the participant’s next of kin ( 25)
ADL Function measured using the modified Katz Activities of Living Scale (27, 28), with 8 items, each scored 0, dependent, 1, requires assistance, 2 independent.
Delirium severity measured using the Memorial Delirium Assessment Scale (MDAS) (23)
Note: As per recommendations on the analysis of clinical trials, P values are not provided for this comparison table.
DAP Implementation
We performed 84 licensed-nursing and 58 certified-nursing assistant education programs at the DAP sites. Four hundred twenty-six out of 540 (79%) post-acute nursing and nursing assistant staff attended a DAP educational program. As described in the Methods, Ongoing Education and Monitoring section, this initial education was supplemented by weekly visits by a research nurse interventionist, and we trained an on-site Delirium Resource Nurse who worked closely with the project interventionists to monitor implementation of the program. We also conducted a total of 51 AAP meetings at the DAP sites. There was frequent turnover of key leadership so that we continually needed to orient new administrators and Directors of Nursing. Over the 39-month study duration, we interacted with 1–4 administrators and 1–4 Directors of Nursing at each of the DAP sites (median=3).
Adherence to the monitored steps of DAP implementation is described in Table 2. At the DAP facilities, delirium assessments were completed 75% of the time, and delirium was detected 41% of the time. We instructed PAC staff to perform subsequent DAP steps only in patients with delirium; the percentages of completion of these steps, the Assessment of Causes, Delirium Nursing Care plan, and placement of at least 2 environmental modifications in the room, were 80–93% of those with detected delirium.
Table 2.
Adherence rates with monitored steps of DAP implementation
| Implementation Step | DAP Participants N=282 N, (%) |
|---|---|
| DAP Delirium Assessment* Completed | 210 (75) |
| Delirium Triggered in Medical Record† | 116 (41) |
| Assessment of Causes of Delirium Completed‡ | 108 (38) |
| Nursing Plan of Care for Delirium Completed^ | 94 (33) |
| Environmental Modifications (2/3) Placed in Patient’s Room∥ | 100 (35) |
Abbreviation: DAP: Delirium Abatement Program
Completion of the structured delirium assessment provided as part of the DAP
Based on a positive identification of delirium in the structured delirium assessment
Completion of the structured assessment of causes of delirium provided as part of the DAP
Completion of the delirium nursing plan of care provided as part of the DAP
Placement of at least 2 out of 3 environmental modifications (orientation board, clock/calendar, and tape/radio player) provided as part of the DAP
Comparing delirium management in DAP vs. usual care sites (Table 3), we found the DAP improved detection of delirium (41% DAP versus 12% usual care, p <.001), though the majority of cases remained undetected at all facilities. However, for other key management steps, such as notification of MD/NP, documentation was lower at DAP than at usual care sites (13% vs. 21% respectively). Other than the completion of forms for which the DAP facilities received incentive payments, there was little evidence to suggest that more interventions were performed at DAP than at usual care sites (Table 3). For some practices, such as pressure ulcer and falls prevention, high levels of intervention existed at all sites due to pre-existing programs.
Table 3.
Comparison of DAP and Usual Care delirium management practices based on medical record review
| Key Steps of Delirium Management | DAP Group N=282 (%) |
Usual Care Group N=175 (%) |
|---|---|---|
| Step 1: Detection of Delirium | ||
| • Delirium Documentation in Medical Record* | 41 | 12 |
| • Notification of Physician/Nurse Practitioner of Delirium or Change in Mental Status | 13 | 21 |
| Step 2: Treat Reversible Causes of Delirium | ||
| • Medications | 32 | 42 |
| • Infection | 10 | 23 |
| • Fluid balance disorder—congestive heart failure or dehydration | 11 | 30 |
| • Inadequate Pain Control | 16 | 37 |
| • Urinary Retention | 25 | 16 |
| • Fecal Impaction | 3 | 20 |
| Step 3: Prevent or Manage Common Complications of Delirium | ||
| • Urinary Incontinence | 31 | 28 |
| • Pressure Ulcers | 99 | 94 |
| • Falls/Injury | 90 | 82 |
| • Sleep Problems | 4 | 6 |
| • Malnutrition | 75 | 76 |
| • Aspiration/Dysphagia | 47 | 57 |
| Step 4: Restore Function | ||
| • Provision of appropriate sensory aids | 8 | 9 |
| • Cognitive Re-orientation Program | 2 | 1 |
| • Continuity of Patient Care | 26 | 13 |
| • Family education about delirium | 1 | 1 |
| • Nursing-based rehabilitation/restoration plan | 9 | 12 |
| • Delirium Discharge Education | 1 | 0 |
Abbreviation: DAP: Delirium Abatement Program
Based on a positive identification of delirium in the structured delirium assessment for the DAP facilities, and documentation of delirium in the medical record or admission Minimum Data Set evaluation in the usual care facilities
Similar to Table 1, the goal is to compare DAP and usual care sites for clinically meaningful differences in implementation rates. Therefore, P values comparing the rates are not provided. Also, since there are no missing data and to improve table readability, only percentages (no N’s) are reported.
Comparison of Delirium Persistence at the DAP and Usual Care Sites
Table 4 shows that there was no difference in the persistence of delirium 2 weeks and 1 month after PAC admission in DAP and usual care sites. At one month, unadjusted rates suggest a trend toward greater delirium resolution in usual care, but this diminished after adjusting for baseline differences and clustering. Adjusting for or stratifying by variables with significant baseline differences, such as clinical dementia and race, did not alter the results. Delirium persistence in the 41% of DAP participants in whom delirium was detected also did not differ from the usual care participants. Finally, there were no differences in death rates at DAP and usual care facilities at 2 weeks (2.1% vs. 2.3%, respectively, p=.89) or 1 month (8.5% vs. 9.1%, p=.78).
Table 4.
Effect of the DAP on Primary Outcome: Delirium Status Two Weeks and One Month after PAC Admission
| Time Point | DAP Group N=282 Delirium Present (%) |
Usual Care Group N=175 Delirium Present (%) |
Unadjusted Risk Difference and 95% CI |
Adjusted Risk Difference and 95% CI* |
Adjusted Risk Difference and 95% CI† (with facility clustering) |
Unadjusted P value |
Adjusted P value* |
Adjusted P value† (with facility clustering) |
|---|---|---|---|---|---|---|---|---|
| PAC Admission |
282/282 (100) |
175/175 (100) |
- | - | - | - | - | - |
| Two Weeks | 154/227 (67.8) |
94/143 (65.7) |
2.1 (−7.8, 12.0) |
3.1 (−7.8, 14.1) |
3.2 (−18.4, 24.8) |
0.67 | 0.57 | 0.77 |
| One Month | 127/212 (59.9) |
70/138 (50.7) |
9.2 (−1.5, 19.8) |
7.8 (−4.2, 19.7) |
7.8 (−13.9, 29.4) |
0.09 | 0.20 | 0.48 |
Abbreviation: DAP: Delirium Abatement Program
Adjusted for age, gender, race, pre-hospitalization residence, clinical dementia, pre-illness functional status, and post-acute care (PAC) admission severity of delirium
Adjusted for the variables above, plus using robust variance estimators that account for clustering at the facility levels.
DISCUSSION
A systematic Delirium Abatement Program (DAP) (16) for new admissions to PACs did not shorten the duration of delirium. DAP facility nurses detected substantially more delirium than usual care nurses, and subsequent paperwork for which the facilities received incentive payments was completed in a high proportion of participants in whom delirium was detected. However, other delirium management practices for which the facilities did not receive incentives, such as nurses contacting physicians about delirium, were not performed at a higher rate at DAP sites than at usual care sites. We believe that it was this failure of the DAP facility staff to execute key steps in the intervention that explains our negative results.
Our trial should be interpreted in the context of other reported delirium interventions and the current U.S. skilled nursing facility environment. In 1999, Inouye and colleagues reported that the Hospital Elder Life Program successfully prevented incident delirium in hospitalized elders admitted to the general medical service (40% risk reduction). (10) This study employed Elder Life specialists funded by the study to carry out the intervention and achieved very high 87% adherence. Subsequently, we demonstrated that proactive geriatrics consultation reduced the incidence of delirium among elderly hip fracture patients (36% risk reduction). (11) Our study also had very high adherence (77%) with consultant’s recommendations. Neither of these interventions had an effect on the duration of delirium once it developed. Studies that have specifically attempted to reduce the duration of established delirium have been less successful. Cole et al. found that a specialized consultation service did not reduce the duration of delirium. (31) Notably, adherence to consultant’s recommendations was lower than in the 2 successful delirium prevention trials.
More recently, two interventions performed outside of the U.S. successfully shortened the duration of delirium. Lundstrom and colleagues, in Sweden, demonstrated that care on a specialized ward shortened the duration of delirium among elderly general medicine patients. (32) The intervention ward employed many of the same components as the DAP, but also involved a complete reorganization of nursing care. The mean length of stay in this study (in excess of 10 days) far exceeds that in U.S. hospitals. Kalisvaart and colleagues, in the Netherlands, found that 3 days of low-dose haloperidol, given prophylactically to high-risk hip surgery patients, shortened the duration of delirium. (33) Interestingly, both of these interventions began before delirium developed. Currently, there is no successful model shortening the duration of established delirium.
Seeking “real world” relevance, we did not select specialized, high quality facilities for our study. For-profit companies managed all eight facilities, and six of the eight had for-profit ownership. The average state inspection deficiency score for participating facilities was comparable to the overall state average. (17) Typical of skilled nursing facilities, our sites were plagued with staff turnover, understaffing, and use of agency personnel. (34, 35) Moreover, all but one site underwent turnover of key administrative leadership during our trial. Lack of stable facility leadership and nursing personnel made adoption of the DAP more challenging. Exit interviews conducted with nurses suggested that insufficient staffing and lack of continuity-of-care reduced adherence. (36) Analysis of our data suggests that incentive payments may have driven the modest behavioral change seen the trial.
Our trial has several important strengths. First, we screened nearly 5000 new PAC admissions with detailed mental status assessments, and our final sample of 457 represents the largest cohort of delirious patients ever enrolled in a research study. Second, our initial screening and outcome assessments were based on valid and highly reliable delirium assessments and outcomes were assessed blinded to intervention status. Third, though the cohort was enrolled in the PAC setting, participants were interviewed at follow-up regardless of setting. Finally, the DAP intervention was developed using published “best practices” for the detection and management of delirium (12–14) and our team included the developers of the federally-mandated long term care resident assessment instrument. (15)
Our results should be interpreted in light of several limitations. Given the nature of the DAP, we felt that cluster randomization was the only feasible allocation approach. While this led to a baseline imbalance in several variables, including pre-illness dementia, adjusting for or stratifying by these variables did not alter the study results. The clustering effect also widened the confidence intervals around the intervention effect (Table 4), but it is unlikely that increased sample size or improved statistical power would have altered our primary findings. While the DAP was intended to be implemented as a quality improvement intervention on a facility-wide basis, we were only able to ascertain adherence and outcomes in trial participants whose proxies provided informed consent to allow us to review medical records. We also only assessed outcomes at two weeks and one month and therefore may have missed fluctuations in delirium status between these time points.
Finally, and most importantly, we cannot determine whether the DAP would have shortened delirium had research-funded staff directly carried out all intervention steps. We carefully considered this approach when designing the trial, but rejected it because it would have been exceedingly logistically difficult and resource intensive to integrate research staff into clinical nursing practice at four DAP facilities. Our results demonstrate lack of effectiveness using skilled nursing facility-based staff working under current realities. However, our trial does not address the “proof of principle” question of whether the natural history of delirium in PAC can be altered by a systematic multi-component intervention.
In conclusion, our large, rigorously performed cluster randomized controlled trial demonstrated that the DAP did not shorten the duration of delirium in newly admitted PAC patients. Yet, the high prevalence, persistence and morbidity of delirium in PAC compel us to continue to design and test intervention strategies to improve the outcomes of these vulnerable patients. Our trial demonstrates that these strategies should be tested under carefully controlled conditions ensuring implementation of key intervention steps. The results of such a trial would tell us definitively whether the trajectory of patients admitted to PAC with delirium can be altered for the better.
ACKNOWLEDGMENTS
The authors express their appreciation to the faculty members of the Hebrew SeniorLife Institute for Aging Research who helped design the Delirium Abatement Program: John Morris, Ph.D., Katharine Murphy, R.N., Ph.D, and Lewis Lipsitz, M.D. We are also appreciative of the research nurses who assisted in teaching the Delirium Abatement Program at our intervention facilities: Caroli Flodstrom, R.N. M.S., Kathleen O’Neil R.N. M.S.N., Maryann Wallace, R.N, B.S.N, Ruth Carroll, R.N., B.S.N, and Celine Flynn, R.N., and for Judith Coulumbre, R.N., who completed all the medical record reviews. Finally, we express appreciation to the research assistants Monique Bussell, Kerry Clark, Kathryn Johnson, Maria Kereshi, Jennifer Kettell, Melissa McKenna, Mary Michaels and Sara Hooley for their efforts to screen, enroll and interview patients for this study. All of the above received financial compensation for their work on this study.
We are indebted to Russell Phillips, M.D., and Mary Beth Hamel, M.D. M.P.H. for their careful review and helpful suggestions in the preparation of this manuscript. Neither received financial compensation for their review of this manuscript.
This work was funded by a grant from the National Institute on Aging, RO1AG17649 and by a Paul Beeson Physician Faculty Scholarship in Aging Research (to Dr. Marcantonio.
Footnotes
Trial Registration: A Trial to Reduce Delirium in Aged Post Acute Patients is registered at www.clinicaltrials.gov (NCT00182936) and is no longer accepting participants.
Conflict of Interest
The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper. Dr. Marcantonio is a Paul Beeson Physician Faculty Scholar in Aging Research. The authors retained full autonomy in the preparation of this manuscript.
Authors Contribution
Marcantonio: Conception and design, acquisition of data, analysis and interpretation of data, drafting the manuscript, obtaining funding, supervision
Bergmann: Acquisition of data, analysis and interpretation of data, critical revision of manuscript, administrative, technical, or material support, supervision
Kiely: Analysis and interpretation of the data, critical revision of the manuscript, statistical analysis
Orav: Conception and design, analysis and interpretation of the data, critical revision of the manuscript, statistical analysis
Jones: Conception and design, analysis and interpretation of data, critical revision of the manuscript, statistical analysis, supervision
Dr. Marcantonio had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sponsor’s Role: The funding agencies had no role in the preparation of this manuscript. The authors retained full autonomy in the preparation of this manuscript.
References
- 1.Inouye SK. Current concepts: Delirium in older persons. New Eng J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 2.Levkoff SE, Evans DA, Liptzin B, et al. Delirium: the occurrence and persistence of symptoms among elderly hospitalized patients. Arch Int Med. 1992;152:334–340. doi: 10.1001/archinte.152.2.334. [DOI] [PubMed] [Google Scholar]
- 3.McCusker J, Cole M, Dendukuri N, et al. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med. 2003;18:696–704. doi: 10.1046/j.1525-1497.2003.20602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medicare Payment Advisory Commission (MEDPAC) A Data Book: Healthcare Spending and the Medicare Program: MEDPAC. 2005. [Google Scholar]
- 5.Kiely DK, Bergmann MA, Murphy KM, et al. Delirium among newly admitted post-acute facility patients: Prevalence, symptoms, and severity. J Gerontol A Biol Sci Med Sci. 2003;58A:441–445. doi: 10.1093/gerona/58.5.m441. [DOI] [PubMed] [Google Scholar]
- 6.Kiely DK, Jones RN, Bergmann MA, et al. The association between delirium resolution and functional recovery among newly admitted post-acute facility patients. J Gerontol A Biol Sci Med Sci. 2006;61A:204–208. doi: 10.1093/gerona/61.2.204. [DOI] [PubMed] [Google Scholar]
- 7.Kiely DK, Marcantonio ER, Inouye SK, et al. Persistent delirium predicts increased mortality. J Am Geriatr Soc. 2009;57:55–61. doi: 10.1111/j.1532-5415.2008.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiely DK, Bergmann MA, Jones RN, et al. Characteristics associated with delirium persistence among newly admitted post-acute facility patients. J Gerontol A Biol Sci Med Sci. 2004;59A:344–349. doi: 10.1093/gerona/59.4.m344. [DOI] [PubMed] [Google Scholar]
- 9.Marcantonio ER, Yurkofsky M. Principles of Geriatric Medicine and Gerontology. New York: McGraw-Hill Companies, Inc; 2003. Subacute Care. Chapter 16 in Hazzard, et. al. [Google Scholar]
- 10.Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Eng J Med. 1999;340:699–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 11.Marcantonio ER, Flacker JM, Wright JR, et al. Reducing delirium after hip fracture: A randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 12.Marcantonio ER. Management of Delirium. Oxford: Oxford University Press; 2002. Chapter 8 in Delirium in Old Age. [Google Scholar]
- 13.Marcantonio ER. Delirium module. Physician’ls Information and Education Resource. American College of Physicians-American Society of Internal Medicine. 2001 Revised, 2003. [Google Scholar]
- 14.Marcantonio ER. Delirium. 6th Edition. American Geriatrics Society; 2006. Chapter in Geriatrics Review Syllabus. [Google Scholar]
- 15.Morris JN, Murphy KM, Nonemaker SN. Long term care resident assessment instrument user’s manual, Version 2. Health Care Financing Administration; 1995. [Google Scholar]
- 16.Bergmann MA, Murphy KM, Kiely DK, et al. A model for management of delirious post-acute patients. J Am Geriatr Soc. 2005;53:1817–1825. doi: 10.1111/j.1532-5415.2005.53519.x. [DOI] [PubMed] [Google Scholar]
- 17.“Nursing Home Compare” at Medicare.gov. [last accessed January 25, 2007];
- 18.Simon SE, Bergmann MA, Jones RN, et al. Reliability of a structured assessment for non-clinicians to detect delirium among new admissions to post-acute care. J Am Med Dir Assoc. 2006;7:412–415. doi: 10.1016/j.jamda.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;21:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D. Psychological Corporation. New York: A Harcourt Assessment Company; 1989. Wechsler Adult Intelligence Scale-Revised Manual. [Google Scholar]
- 22.Albert MS, Levkoff SE, Reilly C, et al. The delirium symptom interview: An interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992;5:14–21. doi: 10.1177/002383099200500103. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged: the index of activities of daily living: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 26.“Functional Assessment” in Modules in clinical geriatrics. New York: American Geriatrics Society; 1997. [Google Scholar]
- 27.Fulmer TT. Grow your own experts in hospital elder care. Geriatr Nurs. 1991;12:64–66. doi: 10.1016/s0197-4572(09)90116-1. [DOI] [PubMed] [Google Scholar]
- 28.Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. Littleton, Mass: PSG Publishing Company, Inc; 1985. [Google Scholar]
- 29.Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clinical Trials. 2005;2:152–162. doi: 10.1191/1740774505cn076oa. [DOI] [PubMed] [Google Scholar]
- 30.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 31.Cole MG, McCusker J, Bellavance F, et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: A randomized trial. CMAJ. 2002;167:753–759. [PMC free article] [PubMed] [Google Scholar]
- 32.Lundstrom M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53 doi: 10.1111/j.1532-5415.2005.53210.x. 662-628. [DOI] [PubMed] [Google Scholar]
- 33.Kalisvaart KJ, de Jonghe JFM, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip surgery patients at risk for delirium: A randomized placebo-controlled trial. J Am Geriatr Soc. 2005;53:1658–1666. doi: 10.1111/j.1532-5415.2005.53503.x. [DOI] [PubMed] [Google Scholar]
- 34.Harrington C, Swan JH. Nursing home staffing, turnover, and case mix. Med Care Res Rev. 2003;60:366–392. doi: 10.1177/1077558703254692. [DOI] [PubMed] [Google Scholar]
- 35.Thompson TP, Brown HN. Turnover of licensed nurses in skilled nursing facilities. Nurs Econ. 2002;20:66–69. [PubMed] [Google Scholar]
- 36.Simon SE. When effectiveness falls short: Evaluation of a nursing-based trial to reduce delirium persistence among post-acute patients in skilled nursing facilities [Ph.D. dissertation] United States -- Massachusetts: Brandeis University, The Heller School for Social Policy and Management; 2005. [Accessed October 15, 2008]. Available from: Dissertations & Theses: A&I. Publication Number: AAT 3193098. [Google Scholar]