Abstract
The aqueous extract of Jwarhar mahakashay Ayurvedic preparation (from the roots of Hemidesmus indicus R. Br., Rubia cordifolia L., Cissampelos pareira L.; fruits of Terminalia chebula Retz., Emblica officinalis Gaertn., Terminalia bellirica Roxb., Vitis vinifera L., Grewia asiatica L., Salvadora persica L. and granules of Saccharum officinarum L.) has been used as a traditional antipyretic. Experimental studies confirmed its antipyretic–analgesic effect with very low ulcerogenicity and toxicity. Flavonoids, glycosides and tannins were later found to be present in the extract. Detailed chemical investigations were undertaken after hydrolysis of extract using spectroscopic and chromatography methods to determine its active chemical constituent. UV-Visible spectroscopy showed absorbance maxima at 220 and 276 nm, while fourier transform infra-red investigations indicated an end carboxylic O–H structure at 2940 cm−1 suggesting the presence of glycoside-linked flavonoids. Thin layer chromatography and high performance liquid chromatography also confirmed the possibility of at least one major and two minor compounds in this abstract. Detailed examination using gas chromatography-mass spectrometry led to the identification of the principal component as 2-(1-oxopropyl)-benzoic acid, which is quite similar to the active compound found in the standard drug Aspirin (2-acetyl-oxybenzoic acid).
Keywords: Antipyretic, chromatography, spectroscopy, polyherbal preparation, Jwarahar mahakashay
INTRODUCTION
Traditional Indian systems of medicine such as Ayurveda are based on holistic treatment of diseases primarily relying on natural herbal drugs. Most Ayurvedic preparations are polyherbal in nature to take care of the multiple components of disease conditions. The group of antipyretic drugs has been defined in Jwarahar Mahakashay Sutra Sthana of Charak Samhita.[1] This Jwarhar mahakashay group of antipyretic drugs includes Sariva (Hemidesmus indicus R. Br.), Manjistha (Rubia cordifolia L.), Patha (Cissampelos pareira L.), Haritaki (Terminalia chebula Retz.), Amala (Phyllanthus emblica Gaertn.), Vibhitak (Terminalia bellirica Roxb.), Draksha (Vitis vinifera L), Parushak (Grewia asiatica L), Peelu (Salvadora persica L) and Sharkara (Saccharum officinarum L) plants. Many of these medicinal plants have been individually reported to exhibit diverse pharmacological actions such as antiinflammatory, analgesic, hepato-protective, antimicrobial and antiulcer properties as detailed in Table 1.[2-12] Analysis of the chemical constituents of these plants revealed the presence of tannins, triterpenoids, phenols, glycosides, sucrose, glucose, flavonoids and flavonidic glycosides as enlisted in Table 1.[13-17]
Table 1.
Pharmacological properties and chemical constituents of Jwarhar mahakashay drugs
| Name | Scientific name | Family | Parts used | Pharmacological properties Reported | Active chemical constituents |
|---|---|---|---|---|---|
| Sariva | Hemidesmus indicus R. Br. | Asclepiadaceae | Roots | Anti-infl ammatory, Anti-ulcer | Flavonoids, Triterpenoids, Tannin, Phytosterol,β-sitosterol |
| Sharkara | Saccharum offi cinarum L. | Poaceae | Granules | Anti-infl ammatory, analgesic | Flavonoids, Glucosides, Glycans, Glucose, Sucrose, Phenols |
| Patha | Cissampelos pareira L. | Menispermaceae | Roots | Tumour-inhibitor | Alkaloids, Berberin, Hayatine, Cissampareine |
| Manjistha | Rubia cordifolia L. | Rubiaceae | Fruits | Anti-infl ammatory, antibacterial | Glycosides, Anthraquinone, Rubiadin, Triterpenes |
| Draksha | Vitis vinifera L. | Vitaceae | Fruits | Anti-ulcer, hepatoprotective | Flavonoids, Glucose, Fructose, Glycosides, Polyphenols |
| Peelu | Salvadora persica L. | Salvadoraceae | Fruits | Anti-ulcer, anti-microbial | Tannin, Salvadorin, Glucose, Fructose |
| Parushak | Grewia asiatica L. | Tiliaceae | Fruits | Anti-malarial, anti-ulcer | Flavonoids, Tannin, Glucose, Glycosides |
| Haritaki | Terminalia chebula Retz. | Combretaceae | Fruits | Anti-microbial, purgative | Tannin, Triterpenes, Chebulinic acid, Glycosides |
| Vibhitak | Terminalia bellirica Roxb. | Combretaceae | Fruits | Hepato-protective, antihistaminic | Gallic acid, Glycosides, Chebulagic acid, Triterpenoids |
| Amala | Emblica offi cinalis Gaertn. | Euphorbiaceae | Fruits | Anti-ulcer, anti-infl ammatory, hepato-protective | Vitamin C, Elagic acid, Polyphenol, Glucose, Phyllembin |
We have already reported that the preliminary phytochemical analysis of the aqueous extract of a homogeneous mixture of the abovementioned plants prepared as laid down in Chikitsa Sthana of Charak Samhita (referred hereinafter as the research drug) had revealed the presence of tannins, reducing sugars, flavonoids, glycosides and salicylates.[18] During experimental study on rodents, this aqueous extract exhibited significant antipyretic–analgesic properties compared to the standard NSAIDS antipyretics such as Aspirin. It also exhibited significantly low ulcerogenicity and very low toxicity even at very high dosage compared to Aspirin.[18]
The present work aimed to perform a detailed chemical examination of the aqueous extract of Jwarhar mahakashay preparation and to identify pure chemical marker compounds in it using chromatographic and spectroscopy methods.
MATERIALS AND METHODS
General experimental procedures
UV-Visible spectroscopy scans and absorbance studies for assessing total polyphenol content were performed in Shimadzu UV-VIS-2550 model Spectrophotometer. Fourier transform infra-red spectroscopy (FTIR) studies were conducted in transmittance mode using FT/IR-670 Plus – JASCO model with DLATGS detector. The LC-6A Shimadzu system with UV-Visible spectroscopic detector and C18 column was used for high performance liquid chromatography (HPLC) analysis of the extract samples. Gas chromatography and mass spectroscopy (GC-MS) systematic evaluation was carried out in Shimadzu QP 5050 GC Mass and GC-17A Gas Chromatograph.
Plant materials
The roots of Sariva (Hemidesmus indicus R. Br.), Manjistha(Rubia cordifolia L.) and Patha (Cissampelos pareira L.), fruits of Haritaki (Terminalia chebula Retz.), Amla (Phyllanthus emblica Gaertn.), Vibhitak (Terminalia bellirica Roxb.), Draksha (Vitis vinifera L..), Parushak (Grewia asiatica L..) and Peelu (Salvadora persica L..), and Sharkara granules (Saccharum officinarum L.) were obtained from the Apothecary department of the Institute of Post-Graduate Ayurvedic Education and Research, Kolkata, during May 2006. These were authenticated and identified by the Department of Ethnobotany, Botanical Survey of India, Shibpur, Howrah, and a voucher specimen was deposited in the herbarium before their utilization. Plant parts were shade dried and coarsely powdered up to 40 mesh size. Equal portions by weight of all ingredients, as mentioned in Chikitsa Sthana of Charak Samhita for treatment of pyrexia, were homogenously mixed and subjected to Soxhlet extraction in refluxing distilled water.
The extraction was continued for 48 h using distilled water four times by weight of the crude drug mixture. The aqueous extract was filtered through calico cloth and was further concentrated to a semi-solid substance under reduced pressure in a rotary evaporator, which was then dried over water bath into a solid substance.
The extract yield from 300 g of plant powder mixture was 75 g. This Jwarhar mahakashay preparation was used for all systematic evaluation studies and preserved at the Division of Pharmaceutical Technology Laboratory, Department of Chemical Technology, Calcutta University, Kolkata, where the studies were carried out during 2006–2007.
Elemental analysis
Elemental analysis was performed to detect the presence of nitrogen, sulfur and halogens using routine chemical analysis techniques. A piece of metallic sodium was taken in a test tube, melted by slow heating and about 0.5 g of research drug was added and strongly heated for about 2 min. Twenty milliliter of distilled water was taken in a mortar and pastel, the red-hot test tube was broken and ground in mortar distilled water. The aqueous solution was filtered through Watman-40 filter paper and the filtrate was subjected to test for these elements.[19]
Analysis of phytochemical constituents
Systematic analysis using standard methods was done for ascertaining the presence of different phytochemical constituents such as alkaloids, amino acids, reducing sugars, tannins, saponins, anthraquinones, steroids, terpenoids, flavonoids and salicylates.[19]
Estimation of total polyphenol content
Total polyphenol content was estimated using the Folin–Ciocalteu method calibrated on Gallic acid.[20] Sample extracts of 500 µL were added to 500 µL of water, 5 mL of 0.2 N Folin–Ciocalteu reagent and 4 mL of 75 g /L∂1 saturated sodium carbonate solution and mixed in a cyclomixer. The absorbance was measured in the spectrophotometer at 765 nm after incubation for 2 h at room temperature. Quantification of total polyphenol content was done on the basis of a standard curve generated with 100, 200, 300 and 400 mg/L∂1 of Gallic acid.
Hydrolysis of research drug
The phytochemical tests on Jwarhar mahakashay preparation were strongly indicative of the presence of flavonoids, glycosides and sugars.[18] Since flavonoids normally exhibit antipyretic, analgesic and antiinflammatory properties, systematic and standard hydrolysis procedure was followed on the research drug for detailed investigation of its chemical constituents, especially flavonoids and glycosides. A 0.25 mg of research drug was dissolved in 0.3 mL – 2N HCl : MeOH (1 : 1 v/v), sealed in a screw-cap polypropylene tube and heated on a steam bath for 30 min. The mixture was extracted with equal volume of ethyl acetate; the upper organic layer was separately collected and was subsequently evaporated to dryness under reduced pressure.[21] The residue was dissolved in methanol and was simultaneously analysed in UV-Visible spectroscopy, thin layer chromatography (TLC), HPLC, FTIR and GC Mass Spectroscopy. The aqueous layer was analysed for sugar using Fehling's solution, which was found positive.
UV-visible spectroscopic scanning
The hydrolyzed sample of research drug was dissolved in (2% w/v) HPLC water, filtered and scanned in the spectrophotometer at medium speed in UV-Visible range for specific absorbance identification that might appear for its chemical constituents.[22] The characteristics of molecules to absorb radiations under specific wavelengths were scanned in the entire range of 190 to 800 nm. All prepared batches of research drug were routinely checked to match these UV absorbance band criterions for routine standardization purposes.
Fourier transform infra-red spectroscopy
FTIR scan of herbal medicaments provide for quality finger printing for identifiable absorbance bands due to presence of individual functional groups. Hydrolyzed sample of research drug was dried in rotary evaporator, mixed with KBr, palletized and subjected to FTIR studies in transmittance mode.[23]
Chromatography analysis
Different chromatography techniques such as TLC, HPLC, GC-Mass, etc. were used for evaluation and component standardization of hydrolyzed sample research drug.
Thin layer chromatography
The research drug was analysed using a glass plate coated with a thin layer of silica gel (194015 G, SISCO Research laboratories Pvt. Ltd., Mumbai, India) using different solvent mixtures. The different spots developed were visualized on coloration (like Iodine vapor exposure) and their Rf values were calculated.Rf values of components are indicative of specific character of molecule in the given environment of mobile and stationary phase. Best results were obtained with an ethyl acetate/butane/formic acid/water (30 : 20 : 10 : 40 v/v/v/v) solvent system.
High performance liquid chromatography
The HPLC flow rate was 0.5 ml/min using methanol/water (70 : 30) as the mobile phase solvent, under a pressure of 100 kgf/sq.cm, run time of 20 min and an injection volume of 20 µL. Analysis was performed at 220 nm and repeated at 276 nm, which are the two points where absorbance maxima were observed during the UV-Visible spectroscopy.
Gas chromatography and mass spectroscopy
For exact identification of principle components present in the research drug as chemical marker, systematic GC-MS evaluation was carried out using freshly prepared ethyl acetate extract of flavonidic hydrolyzed sample. Ethyl acetate extract was initially evaporated under reduced pressure at room temperature and the residual concentrate re-dissolved in HPLC methanol. Both injection temperature and interface temperature were maintained at 250° C, while the split ratio was set at 1 : 20. The flow rate of Helium in the column (ZB5 type, diameter 0.25 mm, length 30 m) was 0.6 mL/min and the carrier flow rate was 13 mL/min. The column temperature was initially set at 45°C and thereafter increased at the rate of 15°C/min till it reached 250°C. Mass spectroscopy was done in the range of 45 to 350 m/z and data were captured at time intervals of 0.5 s.[24]
RESULTS
Elemental analysis
Only sulfur was found present in research dry, while nitrogen and halogens were found to be absent.
Analysis of phytochemical constituents
Flavonoids, reducing sugar, tannins, glycosides and salicylates were found present, while alkaloids, steroids, terpenoids, amino acids, anthraquinones and saponins were found to be absent during analysis of its phytochemical constituents.
Estimation of total polyphenol content
The total polyphenol content in 1 mg of test drug was estimated to be 0.0535 mg of Gallic Acid Equivalents using the absorbance calibration curve generated with different concentrations of Gallic acid. Thus, the overall polyphenol content in Jwarhar mahakashay preparation was assessed as 5.35% (w/w) using the Folin–Ciocalteu method.
UV-visible spectroscopic scanning
The characteristics of molecules to absorb radiations under specific wavelengths were scanned in the entire range of 190 to 800 nm. The UV-Visible spectroscopic scan of the hydrolyzed sample is presented in Figure 1. Two absorbance maxima were observed at 276 and 220 nm.
Figure 1.
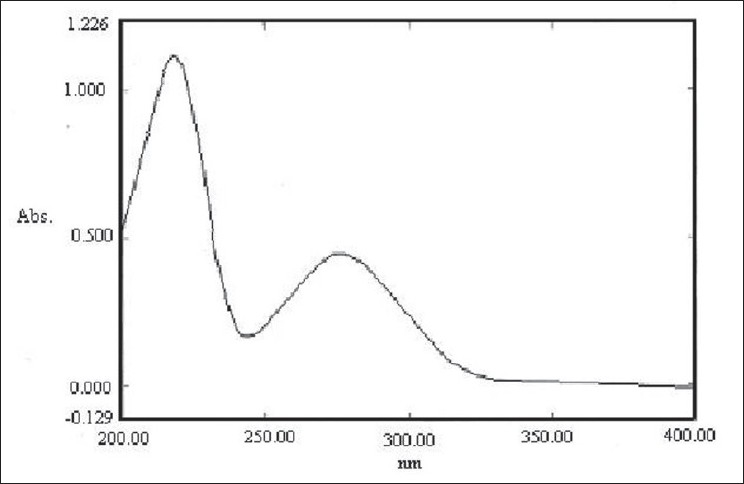
UV-visible spectroscopic scan of research drug
Fourier transform infra-red spectroscopy
Fourier Transform Infra-red Spectroscopy (FTIR) study of dry-powdered test drug in KBr pellet was investigated to identify the presence of functional groups. FTIR scan does not corroborate the presence of any nitrogenous functional group like -NH, -NHR, -NO, NO2 etc. However, characteristic broad alcoholic -OH in hydrogen-bonded structure was observed at 3360/cm. Such alcoholic-OH was confirmed for the presence of C–O stretching vibration at 1065/cm. Tautomeric C=O stretching was observed for aldehydic presence at 1647/cm. The FTIR results were presented in Figure 2. This end carboxylic O–H presence provided at 2940/cm is confirmatory to original chemical investigation for presence of glycoside-linked tannins or flavonoids.
Figure 2.
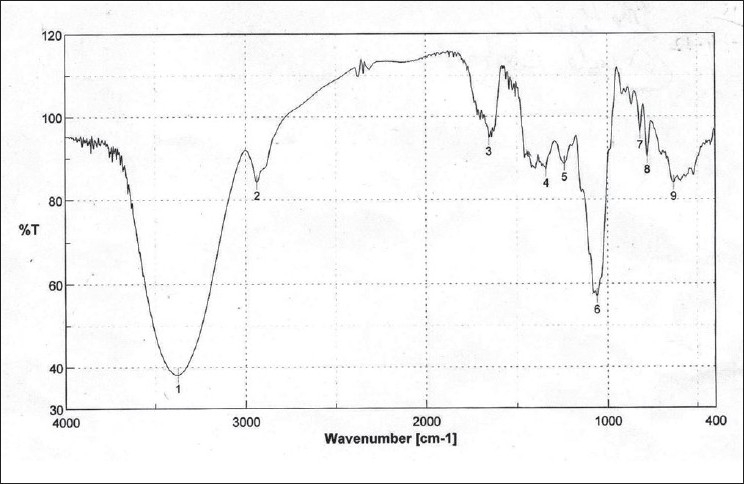
FTIR finger printing of research drug
Thin layer chromatography
After several runs in different solvent environments, flavonoids in hydrolyzate moved distinctly in flavonoid-specific solvent front ethyl acetate : butanone : formic acid : water (30 : 20 : 10 : 40) with Rf values of 0.344, 0.7444 and 0.967. Preliminary separation in TLC is suggestive of more than one flavonidic components.
High performance liquid chromatography
The HPLC analysis at 220 nm wavelength provided the majority component eluting at 8.7 min, while minor peaks appeared at 12.1, 16.2 and 24.3 min. Similarly, running HPLC finger printing at 276 nm provided a distinct pattern with the majority components appearing at 8.5 and 13.6 min. The results of HPLC analysis have been presented in Figures 3 and 4.
Figure 3.
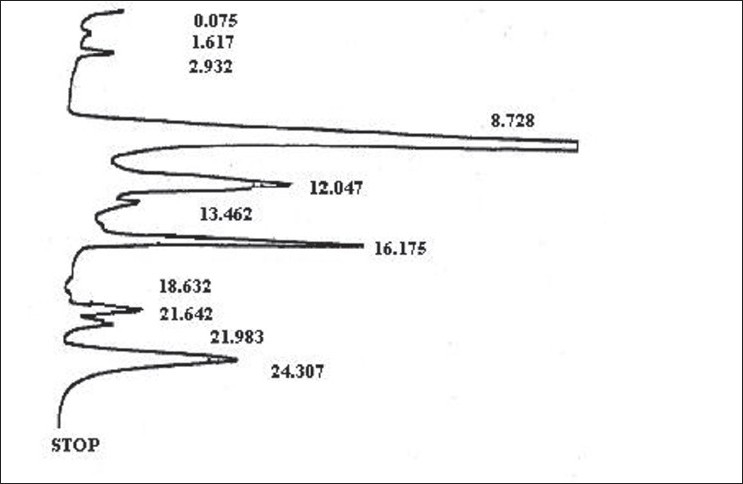
HPLC chromatogram of research drug at 220 nm
Figure 4.
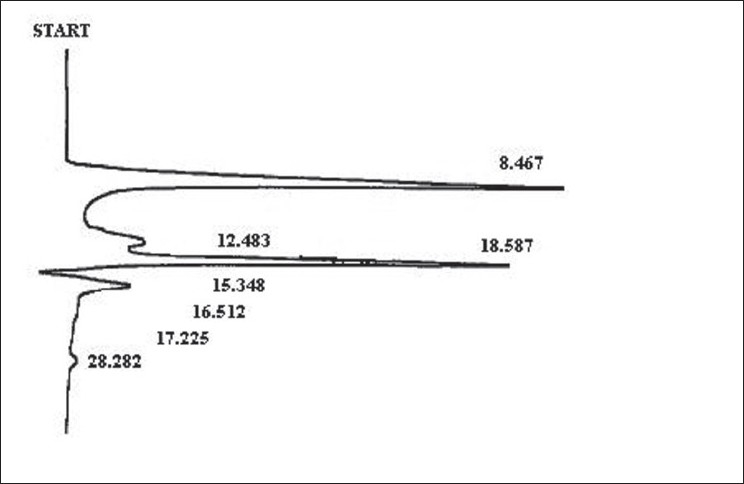
HPLC chromatogram of research drug at 276 nm
Gas chromatography and mass spectroscopy
The results of the GC–Mass Spectroscopy analysis are presented in Figures 5 and 6. Detailed analysis of concentrate provided one major and two minor components that were corroborated when chromatogram was zoomed. Detailed analysis of the two minor components was not performed since their relative intensity was found to be quite low (25.2 and 13.2%) as compared to the majority component (100%) as detailed in Figures 5 and 6. The majority component could be identified in comparison to Nist and Willey library as 2-(1-oxopropyl)-benzoic acid. This, considering all structural possibilities, could be one of the components that provided antipyretic–analgesic activity of the research drug since it is quite similar to the active ingredient 2-acetyl-oxybenzoic acid contained in Aspirin (acetylsalicylic acid), a common nonsteroidal antipyretic formulation.
Figure 5.
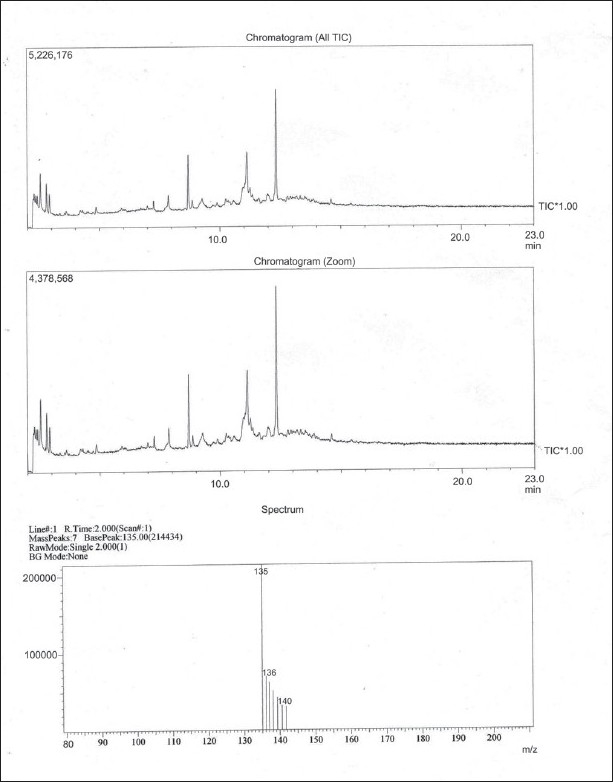
Results of GC–Mass spectroscopy of research drug
Figure 6.
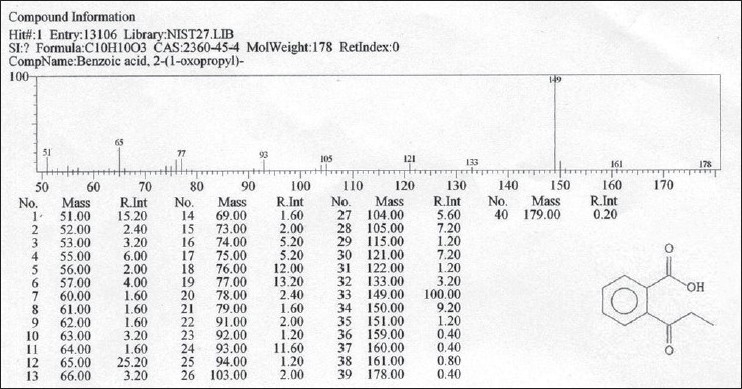
Identification of the majority component in research drug using GC–Mass analysis
DISCUSSION
Detailed chemical investigations were undertaken to analyse the basic chemical nature, to establish uniformity in quality standard of the research drug and to identify pure chemical marker compounds in it. Elemental analysis confirmed the presence of sulfur, while nitrogen and halogens were found to be absent. Preliminary phytochemical screening of the extract indicated the presence of tannins, reducing sugars, flavonoids, glycosides and salicylates.[18] Of these, flavonoids are well known for their ability to inhibit pain perception[25] and to exhibit antiinflammatory properties due to their inhibitory effects on enzymes involved in production of the chemical mediator of inflammation.[27] Flavonoids and its related compounds also exhibit inhibition of arachidonic acid peroxidation, which results in reduction of prostaglandin levels thus reducing the fever.[27] Since flavonoids exhibit several biological effects such as antiinflammatory, antimicrobial, antihepatotoxic and antiulcer activities,[2,28] it is likely that the antipyretic action of Jwarhar mahakashay preparation is primarily related to the presence of flavonoids. Therefore, standard hydrolysis procedure was followed for detailed investigation of its chemical constituents, especially flavonoids and glycosides, by using UV-Visible spectroscopy, TLC, HPLC, FTIR and GC–Mass Spectroscopy.
During UV-Visible spectroscopic scanning, absorbance maxima were observed at 276 and 220 nm. The FTIR scan indicated an end carboxylic O–H structure at 2940 cm−1 , which confirms the findings of the phytochemical investigation regarding the presence of glycoside-linked tannins or flavonoids in the research drug.
TLC and HPLC studies were performed for standardization and preliminary identification of the chemical parameter constituents in the research drug. Preliminary separation in Thin Layer Chromatography was suggestive of more than one flavonidic components. HPLC analysis at 220 and 276 nm wavelength exhibited three majority components eluting around 8, 12 and 13 min as represented by the local peaks in the resultant graphs.
Gas Chromatography–Mass Spectroscopy was finally done for exact identification of principle components present in Jwarhar mahakashay preparation as chemical marker by systematic evaluation of ethyl acetate extract of the sample. Detailed analysis of concentrate provided three major components that were corroborated when chromatogram was zoomed and the majority component could be identified in comparison to Nist and Willey library as 2-(1-oxopropyl)-benzoic acid.
The chemical analysis of the Jwarhar mahakashay preparation in order to ascertain the marker compounds indicated the presence of flavonoids, specifically the glycosidic flavonoids, which are well known for their antipyretic properties. Detailed analysis revealed the presence of at least one active compound, which is structurally quite similar to the active constituent of a well-known NSAIDs compound, the Aspirin.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shastri SN. Charak Samhita (Sutra Sthanam) Chaukhamba Bharati Academy, Varanasi 4. 1988:92–3. [Google Scholar]
- 2.Nijveldt RJ, van Nood E, von Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 3.Anoop A, Jagadeesan M. Biochemical studies on the anti-ulcerogenic potential of Hemidesmus indicus. R. Br. var. J Ethnopharmacol. 2003;84:149–56. doi: 10.1016/s0378-8741(02)00291-x. [DOI] [PubMed] [Google Scholar]
- 4.Perianayagam JP, Sharma SK, Joseph A, Christina AJ. Evaluation of anti-pyretic and analgesic activity of Emblica officinalis Gaertn. J Ethnopharmacol. 2004;95:83–5. doi: 10.1016/j.jep.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Ledon N, Casaco A, Rodriguez V, Cruz J, Gonzalez R, Tolon Z, et al. Anti-inflammatory and analgesic effect of a mixture of fatty acids isolated and purified from sugarcane wax oil. Planta Med. 2003;69:367–9. doi: 10.1055/s-2003-38880. [DOI] [PubMed] [Google Scholar]
- 6.Kasture SB, Kasture VS, Chopde CT. Anti-inflammatory activity of Rubia cordifolia roots. J Nat Rem. 2001;1:111–5. [Google Scholar]
- 7.Saito M, Hosoyama H, Ariga T, Kataoka S, Yamaji N. Antiulcer activity of grape seed extract and procyanindins. J Agric Food Chem. 1998;46:1460–4. [Google Scholar]
- 8.Monforte MT, Miceli N, Mondello MR, Sanogo R, Rossitto A, Galati EM. Antiulcer activity of Salvadora persica on experimental ASA-induced ulcer in rats: Ultrastructural modifications. Pharm Biol. 2001;39:289–92. [Google Scholar]
- 9.Anand KK, Singh B, Saxena AK, Chandan BK, Gupta VN. Hepatoprotective studies of a fraction from the fruits of Terminalia bellirica Roxb. on experimental liver injury in rodents. Phytother Res. 1994;8:287–92. [Google Scholar]
- 10.Valsaraj R, Pushpangadan P, Smitt UW, Adsersen A, Christensen SB, Sittie A, et al. New anti- HIV-1, antimalarial and antifungal compounds from Terminalia belerica. J Nat Prod. 1997;60:739–42. doi: 10.1021/np970010m. [DOI] [PubMed] [Google Scholar]
- 11.Asmawi MZ, Kankaanranta H, Moilaness E, Vapaatalo H. Anti-inflammatory activities of Emblica Officinalis Gaertn leaf extracts. J Pharmacol. 1993;45:581–4. doi: 10.1111/j.2042-7158.1993.tb05605.x. [DOI] [PubMed] [Google Scholar]
- 12.Gulati RK, Agarwal S, Agarwal SS. Hepatoprotectic studies on Phyllanthus emblica Linn and quercetin. Indian J Exp Biol. 1995;33:261–8. [PubMed] [Google Scholar]
- 13.Sharma PC, Yelne MB, Dennis TJ. Central Council for Research in Ayurveda and Siddha, Department of Indian system of medicine. New Delhi: Govt. of India; 2001. Database on medicinal plants used in Ayurveda; p. 1. 396; 3: 11, 158, 282; 5: 43, 172, 263. [Google Scholar]
- 14.Kirtikar KR, Basu BD. Indian Medicinal Plant. In: Basu LM, editor. Allahabad, India: 1984. p. 1. 95, 388, 607; 2: 1020, 1303, 1537; 3: 1596, 2221; 4: 2661. [Google Scholar]
- 15.Baltenweck-Guyot R, Trendel JM, Albrecht P, Chaeffer A. Glycosides and phenylpropanoid glycerol in Vitis vinifera cv. Gewürztraminer wine. J Agric Food Chem. 2000;48:6178–82. doi: 10.1021/jf0002600. [DOI] [PubMed] [Google Scholar]
- 16.Foo LY, Lu Y, Wong H. Biphenyl-linked biflavanoids from grape promace. Phytochemistry. 1998;47:1137–40. [Google Scholar]
- 17.Kundu AK, Mahato SB. Triterpenoids and their glycosides from Terminalia chebula. Phytochemistry. 1993;32:999–1002. [Google Scholar]
- 18.Gupta M, Shaw BP, Mukherjee A. Studies on antipyretic - analgesic and ulcerogenic activity of polyherbal preparation in rats and mice. Intl J Pharmacol. 2008;4:88–94. [Google Scholar]
- 19.Furniss BS, Hannaford AJ, Smith PW, Tatchell AR. Addison Wesley Longman Inc. 5th ed. Addison Wesley Longman Inc; 1989. Vogel's Textbook of Practical Organic Chemistry; p. 1205. [Google Scholar]
- 20.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58. [Google Scholar]
- 21.Hasler A, Sticher O, Meier B. Identification and determination of the flavonoids from Ginkgo biloba by high-performance liquid chromatography. J Chromatogr. 1992;605:41–8. [Google Scholar]
- 22.Trease GE, Evans WC. 14th ed. Harcourt Brace and Company (Asia) Pvt. Ltd; 1997. Pharmacognosy; p. 120. [Google Scholar]
- 23.Wells JI. Ellis Harwood Limited Publishers; 1988. Pharmaceutical Preformulations; p. 103. [Google Scholar]
- 24.Grob RL. John Wiley and Sons Inc; 1995. Modern Practice of Gas Chromatography; p. 103. [Google Scholar]
- 25.Sawadogo WR, Meda A, Lamien CE, Kiendrebeogo M, Guissou IP, Nacoulma OG. Phenolic content and antioxidant activity of six acanthaceae from Burkina Faso. J Biol Sci. 2006;6:249–52. [Google Scholar]
- 26.Oweyele VB, Oloriegbe YY, Balogun EA, Soladoye AO. Analgesic and anti-inflammatory properties of Nelsonia canescens leaf extract. J Ethnopharmacol. 2005;99:153–6. doi: 10.1016/j.jep.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Baumann J, Von Brucchau Sen F, Wurm G. Flavonoids and related compounds as inhibition of arachidonic acid peroxidation. Prostaglandins. 1980;20:627–39. doi: 10.1016/0090-6980(80)90103-3. [DOI] [PubMed] [Google Scholar]
- 28.Narayana KR, Reddy MS, Chaluvadi MR, Krishna DR. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol. 2001;33:2–16. [Google Scholar]


