Abstract
MfVEPs were recorded with a 22 deg radius, 60-sector pattern reversal dartboard stimulus (VERIS) at 6 contrast levels (10, 25, 35, 50, 75, 95%). Contrast response functions (CRFs) based on response amplitudes were adequately described by a simple hyperbolic function. The effect of reducing contrast on the amplitude was most apparent in the central 1 deg radius, which had a C50 (contrast at 50% of the maximum response) in excess of 50%, compared to values for C50 in more eccentric regions that were 30% or lower. Mean latency increased 6 (± 0.7 SE) ms from the highest to the lowest contrast tested, and did not vary sgnificantly with eccentricity.
Keywords: multifocal visual evoked potential, mfVEP, contrast, retinal eccentricity, contrast response function
Introduction
The multifocal visual evoked potential (mfVEP) is an objective measure of visual function that can be recorded non-invasively. The unique strength of the technique is that it allows simultaneous recording of local VEP responses from across the visual field, thereby providing spatially localized amplitude and latency information. Refinements in the analysis of mfVEP records have enhanced its clinical utility in the diagnosis of diseases affecting the optic nerve such as glaucoma and optic neuritis (Balachandran, Graham, Klistorner, & Goldberg, 2006; Fortune, Demirel, Zhang, Hood, Patterson, Jamil, Mansberger, Cioffi, & Johnson, 2007; Hood & Greenstein, 2003; Hood, Odel, & Zhang, 2000). As various peripheral ocular conditions such as refractive errors, lens opacities, corneal diseases or pre-ganglion cell abnormalities in the retina can cause a reduction in retinal image contrast, it is important to understand the relationship between the stimulus contrast and the mfVEP responses (Brown, 1993; Zadnik, Mannis, & Johnson, 1984). Previous studies have investigated the effects of contrast on pattern reversal VEP or mfVEP responses (Baseler & Sutter, 1997; Hasegawa & Abe, 2001; Hood, Ghadiali, Zhang, Graham, Wolfson, & Zhang, 2006; Katsumi, Tanino, & Hirose, 1985; Klistorner, Crewther, & Crewther, 1997; Maddess, James, & Bowman, 2005; Park, Zhang, Ferrera, Hirsch, & Hood, 2008; Rudvin, Valberg, & Kilavik, 2000; Souza, Gomes, Saito, da Silva Filho, & Silveira, 2007; Zadnik et al., 1984), with attention to the stimulus location in some of the studies (e.g. Baseler & Sutter, 1997, Hasegawa & Abe, 2001, Maddess et al, 2005). However, these studies did not provide a complete analysis of the change in the contrast response function (CRF) with eccentricity. In theory, the effect of contrast on local VEP responses should vary across the visual field as the distribution of retinal ganglion cells with different contrast-response characteristics changes with eccentricity (Curcio & Allen, 1990; Dacey, 1993; Kaplan & Shapley, 1986). To investigate whether the characteristics of the VEP CRF depend upon retinal eccentricity, we used the mfVEP technique to record 60 local VEP responses across a 22 degree radius of visual field in normal subjects for a range of stimulus contrasts. A report of this study has appeared previously in abstract form (Laron et al., Invest. Ophthalmol. Vis. Sci. 2008 49: E-Abstract 3311).
Methods
Subjects
Seven normal subjects participated in the study. Subjects ranged in age from 23 to 42 (mean ± SD: 28 ± 6), and had best corrected visual acuity of 20/25 or better. All subjects had a comprehensive eye examination and histories were taken prior to participating in the study, and were found to have no ocular or systemic conditions that could affect the visual system. Informed consent was obtained from all subjects. Procedures adhered to the tenets of Declaration of Helsinki, and the protocol was approved by the University of Houston Committee for the Protection of Human Subjects.
MfVEP procedures and data analysis
Stimulus (VERIS 51, mjVEP paradigm)
A dartboard pattern was presented on a 20” CRT monitor with a frame rate of 75 Hz. The pattern was comprised of 60 sectors scaled in size for cortical magnification (Figure 1A). Each sector had 16 checks (8 black and 8 white), and followed a pseudorandom sequence of reversal (m-sequence)(Sutter, 2001). Mean luminance of the pattern was fixed at 66 cd/m2, and the Michelson contrast was varied over 6 steps: 10, 25, 35, 50, 75, and 95%. Photopic luminance (cd/m2) of the stimulus was calibrated using a spot photometer (model LS-100, Minolta Camera Co., Ltd., Japan), and the Michelson contrast was calculated. The display was positioned so that the central 44.4 deg of the visual field were stimulated. Subjects viewed the display through their natural pupils with appropriate refractive corrections in place, and were instructed to maintain fixation at the stimulus center (marked as an “x”). The range of pupil sizes (4 – 5 mm in diameter) did not affect contrast sensitivity, because for photopic luminances, such as we used, and the low spatial frequency (here 2 cpd or lower), contrast sensitivity remains constant (De Valois, Morgan, & Snodderly, 1974). During recording, the eye position was monitored constantly by the examiner through the camera provided in the VERIS hardware.
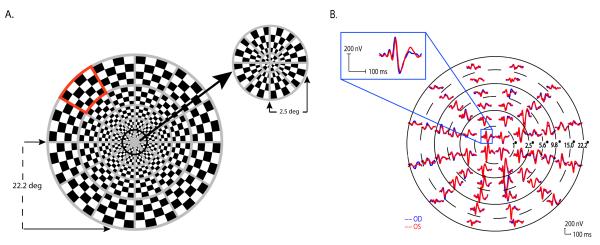
Figure 1.
(A) The mfVEP dartboard stimulus with one of the sectors marked in red. (B) MfVEP responses from the two eyes of a normal subject. The dashed and solid circles illustrate six concentric rings of increasing retinal eccentricity from 1° for the most central ring (ring 1), to 22.2° for the most peripheral ring (ring 6). The posttion of each of the 60 waveforms has been adjusted to enable better visualization. The inset shows the responses from one location on an expanded scale.
Electrode placement and recordings
Three channels were recorded simultaneously and three additional channels were derived mathematically using customized software generously provided by Dr. Donald Hood’s lab (Hood & Greenstein, 2003). The ground electrode was placed on the forehead, the reference electrode at the inion; the first channel electrode 4 cm above the inion; the second and third channel electrodes 1 cm above and 4 cm to the left and right of the inion. MfVEP was recorded in one eye from each subject with the other eye occluded. Stimuli were presented in order of increasing contrast to minimize adaptational effects. At each contrast level two 7-minute recordings from each subject were averaged for offline analysis. Subjects rested between recordings as needed to avoid fatigue.
Data analysis
The first slice of second-order kernels for responses were calculated by VERIS 5.1 (Electro-Diagnostic Imaging, San Mateo, CA) software and exported. All data analyses were performed with a customized software based on the ‘best channel’ responses as previously described (Hood & Greenstein, 2003). ‘Best channel’ responses were used to improve response amplitudes over those from single channel recordings, as has been demonstrated previously (Hood & Greenstein, 2003; Klistorner & Graham, 2000). Use of ‘best channels’ has been shown to be particularly important for locations along the lower horizontal meridian and in central sectors (Hood & Greenstein, 2003), and clearly would be helpful for responses to low contrast stimuli. It is likely that for a particular field location (i.e. sector), the ‘best channel’ remained the same as contrast was varied. In theory, the relative strength (and waveform) of the local signals across the field is determined mainly by the position and orientation of the underlying dipole (i.e., anatomical convolution of the cortex) relative to the electrodes associated with particular channels, and this would not be expected to change with contrast. Further, basic waveforms at the same location were essentially unaffected by stimulus contrast, except in amplitude, in previous studies using ‘best channel’ responses (Hood & Greenstein, 2003) or ‘single channel’ recordings (Hasegawa & Abe, 2001).
The mfVEP response amplitude is reported in the present study as signal to noise ratio (SNR). A sector’s SNR was calculated as the root-mean-square (RMS) amplitude of the signal window (45-150 ms) divided by the mean RMS amplitude of the noise window (325-430 ms in the record where stimulated activity was minimal) from all 60 sectors(Hood & Greenstein, 2003). Relative latency for the response in each sector was determined by calculating the cross-correlation of the subject’s waveform and a template built on the basis of 100 norms (Devers Eye Institute, Portland, OR)(Fortune, Zhang, Hood, Demirel, & Johnson, 2004; Hood, Ohri, Yang, Rodarte, Zhang, Fortune, & Johnson, 2004). The relative latency was the shift in milliseconds (ms) needed to achieve the best cross-correlation determined by the ‘xcorr’ function in MATLAB (The MathWorks Inc, Natick, MA).
Data analysis at different eccentricities
The mfVEP stimulus-response array was divided into 6 concentric rings of increasing eccentricity as shown in Figure 1B. Ring 1 included the central four sectors within an eccentricity of 1 degree radius. Ring 2 included 8 sectors which resided between 1 degree and 2.5 degrees. Rings 3, 4, 5, and 6 included sectors with increasing eccentricity, up to those between 15 and 22 degrees for ring 6. To evaluate the effects of eccentricity on contrast response characteristics, the SNRs or the latencies were pooled from all subjects for each ring and represented by the individual ring’s mean or median values. This allowed sufficient data points for analysis, especially in the case of ring 1 which included only four sectors.
Statistical analysis
The relationship between mfVEP SNR and contrast was evaluated by regression analyses (SigmaPlot 10, SYSTAT Software Inc. San Jose, CA) using a simple hyperbolic function
| (1) |
where R is the response amplitude measured by SNR, C is the stimulus contrast and C50 is the stimulus contrast that generates 50% of the maximum response (Rmax). This equation is sometimes called the Naka-Rushton equation, which has been widely used to fit stimulus response functions of retinal responses (Fulton & Rushton, 1978). The goodness-of-fit of the regression analysis was expressed as the coefficient of determination (R2), which indicates how much variability in the dependent variable can be accounted for by the regression function.
Previous studies have described the CRF for V1 single neurons (Albrecht, Geisler, Frazor, & Crane, 2002; Geisler & Albrecht, 1997) and mfVEP data (Hood et al., 2006; Park et al., 2008) using a more general form of the equation above
| (2) |
where the exponent n is an arbitrary constant. For comparison purpose, we also gave a brief report on fitting the CRF with equation (2).
Results
Response Amplitudes vs. Contrast
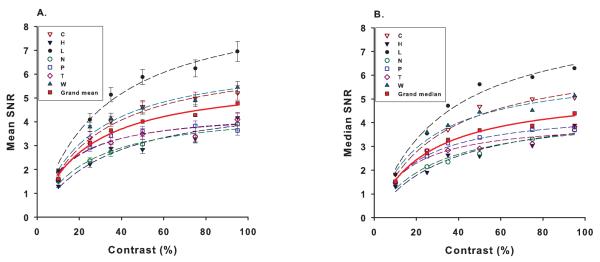
SNR, as defined in Methods section was used to measure the mfVEP response amplitudes. Figure 2 shows the mean and median SNR across the 60 sectors as a function of contrast for each of the 7 subjects. Both mean and median SNRs were plotted because of concern that the SNRs did not strictly follow a normal distribution, as we saw in our own data (not shown) and was reported previously (Hood & Greenstein, 2003). Mean SNR for our subjects ranged from 3.6 to 7 at 95% stimulus contrast, falling within the range reported previously for normal subjects (Fortune et al., 2004; Hood & Greenstein, 2003). The inter-subject variation of the SNRs was not obviously related to age or sex of our small sample of subjects who were mainly females (Table 1). The effect of contrast on SNR, for contrasts between 10% and 95%, was adequately described by a simple hyperbolic function (eqn. 1) with R2 ranging from 0.86-0.98 for means (Fig. 2A) and 0.89-0.98 for medians (Fig. 2B). C50 ranged from 14% to 33% for mean SNR, and 16% to 34% for median SNR (Table 1). Overall, the function from all subjects had a C50 of 23% (grand mean) and 24% (grand median).
Figure 2.
Mean (A) and median (B) mfVEP SNR for all 60 sectors as a function of contrast for each subject (n=7). The grand mean (A) and median (B) of responses from all subjects are shown as a solid red curve. The smooth curves represent fits with a simple hyperbolic function R = RmaxC/(C+C50). Error bars are ±SE.
Table 1.
Parameters from contrast response functions of each subject fitted with a sample hyperbolic function R = RmaxC/(C+C50), where R is the response amplitude measured by mean ( or median) SNR, C is the stimulus contrast, and C50 is the stimulus contrast that generates 50% of the maximum response (Rmax).
| Subject | Age/Sex | R2 | C50 | Rmax |
|---|---|---|---|---|
| C | 42 F | 0.98 (0.97) | 27 (30) | 6.8 (6.9) |
| H | 26 F | 0.93 (0.91) | 33 (34) | 5.2 (4.8) |
| L | 31 F | 0.98 (0.98) | 31 (33) | 9.1 (8.7) |
| N | 24 F | 0.97 (0.94) | 23 (27) | 4.6 (4.5) |
| P | 25 F | 0.94 (0.98) | 15 (19) | 4.5 (4.6) |
| T | 25 F | 0.86 (0.89) | 14 (16) | 4.5 (4.1) |
| W | 23 M | 0.96 (0.95) | 24 (23) | 6.8 (6.3) |
| Grand mean (median) |
28 | 0.99 (0.99) | 23 (24) | 5.9 (5.4) |
Previous studies have used R = RmaxCn/(Cn+C50n) (eqn. 2), allowing n to be a free parameter, for fitting the cortical CRFs measured with mfVEPs (Park et al., 2008) and in V1 neurons (Albrecht & Hamilton, 1982; Geisler & Albrecht, 1997). When fitted with eqn. 2, the grand average CRF from all our subjects showed results similar to those based on eqn. 1, with a C50 of 23% and n remaining very close to 1 (n = 1.06); and the grand median CRF had a C50 of 22% and n of 1.09. As the characteristics of CRFs were very similar whether the responses were measured using the mean or the median SNR; for simplicity, we will report the findings for SNR, from here on, referring only to the mean value.
Response Amplitudes vs. Contrast at Different Eccentricities
Figure 3 shows the effect of contrast on response amplitudes measured at different eccentricities. As described in the Methods, rings 1-6 had radii of 1, 2.5, 5.6, 9.8, 15, and 22 degrees of eccentricity respectively. The response amplitudes for each ring were calculated by taking the mean (Fig. 3) of the SNR in that ring from all subjects. The CRF for each ring was well described by the simple hyperbolic function (eqn. 1) with R2 ranging from 0.96 to 0.99(Table 2). C50 for ring 1 (central) was 56%, substantially higher than C50 for other rings where it ranged from 16% to 25% (Table 2). Rmax was greatest for ring 1 even though the mean SNR measured at 95% contrast for ring 1 was slightly lower but not significantly different from that for ring 2 (t-test, p = 0.36). Rmax declined monotonically with increasing eccentricity, and was least for ring 6. When eqn. 2 (n not fixed at 1) was used to fit the CRFs constructed from individual rings (R2: 0.98 to 0.99), the C50 (= 69%) and Rmax (= 10.4) of ring 1 (n = 0.93) were still higher than those from ring 2 -6 (C50: 17% - 28%, Rmax: 6.6 – 4.4, n = 1.67 – 0.68).
Figure 3.

Mean mfVEP SNR from all subjects for the six concentric rings at different eccentricities (see Figure 1). Error bars are ±SE. The smooth curves represent fits fitted with a simple hyperbolic function R = RmaxC/(C+C50). Error bars are ±SE.
Table 2.
Parameters from contrast response functions from different eccentricities (rings 1 – 6) fitted with a simple hyperbolic function R = RmaxC/(C+C50), where R is the response amplitude measured by mean (or median) SNR, C is the stimulus contrast, and C50 is the contrast that generates 50% of the maximum response (Rmax).
| Ring # | R2 | C50 | Rmax |
|---|---|---|---|
| 1 | 0.99 | 56 | 9.4 |
| 2 | 0.96 | 25 | 8.1 |
| 3 | 0.97 | 22 | 7.1 |
| 4 | 0.99 | 24 | 6.0 |
| 5 | 0.99 | 20 | 4.5 |
| 6 | 0.97 | 16 | 3.5 |
Latency vs. Contrast
In the present study, latency for each sector represents a relative latency compared to a template obtained from 100 normal subjects at 99% stimulus contrast (Hood et al., 2004). A positive value indicates a delayed (slower) response whereas a negative value denotes a faster response compared to the template. As initially done for analyzing SNR, mean and median latencies of all sectors from all subjects were calculated for each contrast level, and plotted as a function of contrast (Figure 4). For the highest contrast (95%), the mean latency was −0.62 ± 1.2, not statistically different from zero (t-test, p = −0.54). Overall, the mean or median latency (little difference) decreased by 6 ms to 7 ms when contrast was increased from 10% to 95%, similar to the change reported by Hood et al. (Hood et al., 2006). The large error bar at 10% contrast indicates large variability due to many sectors’ SNRs being too small for analysis as described by Hood and his colleagues (Hood & Greenstein, 2003; Hood et al., 2004). Figure 5 presents the change in latency as a function of contrast in the central 2.5 degrees (rings 1, 2) compared with the change in mean latency from rings 3 to 6. As illustrated, the effect of contrast on latency was similar in central and more eccentric rings.
Figure 4.

The effect of contrast on mfVEP latency (from all 60 sectors, mean (square) & median (circle) for al subjects). Error bars are ±SE.
Figure 5.

Squares show mean latency averaged over all subjects for rings 1 and 2 (0-2.5 deg eccentricity). Triangles show mean latency for rings 3-6 (2.5-22.2 eccentricity). Error bars are ±SE.
Discussion
The primary goal of this study was to examine whether the effect of contrast on mfVEP responses depends on retinal eccentricity. The CRFs from all subjects, despite variations in response amplitudes among them, showed saturating behavior similar to that previously described for mfVEPs (Baseler & Sutter, 1997; Hasegawa & Abe, 2001; Hood et al., 2006; Klistorner et al., 1997; Park et al., 2008).
A major finding of this study, as can be seen in Fig. 3, is that the CRF in ring 1 saturated at a higher contrast level than was found in more eccentric rings. Regardless of the equation used, the C50 for the central ring was much higher, always in excess of 50% contrast, than that for more peripheral rings where it never exceeded 30% and was as low as 15%. This finding is unlikely to be due to the differences in check size, which was scaled (in VERIS 5.1) to account for the cortical magnification factor across eccentricity. First, the check sizes in rings 1 and 2 were quite similar but the C50 values were distinctively different. Second, rings 2 to 6 had different check sizes but their C50 values were similar. Third, Hood et al (2006) varied check sizes by changing the number of checks in each sector from 2×2 (4 checks) to 8×8 (64 checks), and did not find a trend or significant difference in the C50 of CRFs.
The notable difference in the C50 in ring 1 compared to more eccentric rings is probably related to the unique anatomical/physiological properties of neurons in this area. In the fovea, there is minimal convergence in photoreceptor - bipolar - ganglion cell pathway (Curcio & Allen, 1990; Schein, 1988), and about 95% of the retinal ganglion cells are P cells (Dacey & Petersen, 1992; Dacey, 1993). Compared to M pathway neurons which are known to saturate at lower contrasts, P pathway neurons saturate at much higher contrasts (Derrington & Lennie, 1984; Kaplan & Shapley, 1986). The numerical advantage of P cells over M cells in the foveal region predicts a domination of P pathway behavior for the CRF in this region. Baseler and Sutter (1997) isolated two components (C1 and C2) in pattern reversal mfVEP waveforms whose contrast, chromatic and temporal characteristics are consistent with that of M and P pathways, respectively. For contrasts between 13% and 95%, the C2 component (P pathway) dominated the response at all eccentricities tested (up to 6.4 deg). The C2 dominance was most pronounced in the central 1 deg, with the ratio of C2 to C1 being 0.5-1 log unit higher than outside the central 1 deg. This implies that in this region P pathway neurons dominated the response even when the stimulation was not optimal for those neurons (Fig. 8 in Baseler and Sutter 1997). In the present study, the mfVEP amplitude was calculated using the RMS in the signal window which included both the C1 and C2 components described by Baseler and Sutter (1997). The distinctive behavior of the CRF in ring 1 was consistent with a larger dominance of C2 (P pathway) responses with in central 1 degree (Baseler & sutter, 1997), although both M and P pathway neurons would have contributed to responses at all eccentricities.
As the mfVEP is believed to be generated largely from the primary visual cortex (V1) (Hood & Greenstein, 2003), it is of particular interest to know whether the mfVEP responses reflect the spiking activities of V1 neurons. To investigate this, Hood et al. (2006) compared the mfVEP CRF with a linear V1 model that describes the average spiking activity, as a function of stimulus contrast, of 333 single cells recorded from macaque monkey V1 (Geisler & Albrecht, 1997). The V1 model and the mfVEP responses agreed for up to 40% contrast, but at higher stimulus contrasts the mfVEP responses deviated from the model, presenting faster saturation. One possible explanation for this discrepancy, as the authors proposed, is the different location of the visual neurons stimulated. The V1 model was based on neurons whose receptive fields were located within the central 5 deg whereas the mfVEP responses in Hood et al. (2006) were averaged across the 23 deg visual field tested.
In the present study, the mfVEP CRFs were compared for the different eccentricities established by the six rings (Fig. 3). To compare the shape of the mfVEP CRFs with the V1 model, the V1 model was multiplied by a constant (scaled); the mfVEP data from ring1, rings 1-2 and all rings were normalized to the amplitude at a mid contrast (35%) to bring the curves together then vertically shifted to match the V1 model. Figure 6 illustrates a comparison between the V1 model (gray solid curve), the mfVEP responses of the present study from ring 1 (central 1 deg, red squares), average of rings 1 and 2 (central 2.5 deg, dark blue circles), and the mfVEP responses averaged across the whole field (turquoise triangles). Curve fitting here used eqn. 2 because the V1 model was so described. The mfVEP responses from ring 1 followed the V1 model reasonably well up to about 75% contrast. As found by Hood et al., (2006) the average curve for the entire 22 deg field began to deviate from the model at about 40% contrast; as did the curve from rings 1 and 2 but to a slightly lesser extent.
Figure 6.

Contrast response functions fitted with R = RmaxCn/(Cn+C50n). V1 model adapted from Geisler and Albrecht; 1997 (solid gray line, C50 = 43%), mfVEP responses from ring 1 (red filled squares, C50 = 69%, R2 = 0.99), averaged mfVEP responses from rings 1 + 2 (dark blue open circles, C50 = 26%, R2 = 0.99) averaged mfVEP responses from all rings (turquoise open triangles, C50 = 21%, R2 = 0.99).
We do not have a good explanation for the deviation between the V1 model and the mfVEP average from rings 1-2. Considering a 0.5 deg fixation error during mfVEP recording (Zhang, Stevenson, Cheng, Laron, Kumar, Tong, & Chino, 2008), ring 2 is still within 5 deg of eccentricity. One possibility is that the spiking activity in the neuronal population used to construct the V1 model was dominated by neurons from the central 1 to 2 degrees. Another possibility is that one or more assumptions, as delineated by Hood et al. (2006) for comparing monkey V1 model to human VEP activity, are invalid because the stimulus conditions for the mfVEP differed from those for the single cell recordings used to produce the V1 model (i.e., flashed contrast reversal of checkerboards vs stimuli that were sinusoidally modulated in spatial and temporal frequency domains). In a recent paper, Park et al. (2008) reported an agreement between the CRFs of mfVEP and the blood-oxygen-level-dependent (BOLD) signal of functional MRI (fMRI), but not with that predicted by the V1 model of Geisler and Albrecht (1997). Park et al. (2008) proposed that the assumption behind a linear V1 model, i.e., parameters such as C50 and n remain constant over various stimulus conditions, is violated. Our results concur with their hypothesis and suggest that the assumption of the CRF being independent of eccentricity is also violated. Lastly, it is quite possible that the mfVEP reflects the underlying neurons’ integrated local field potentials more than their spiking activity, considering the good agreement between the contrast-sensitivity functions of mfVEP and BOLD fMRI. BOLD responses appear to be better correlated with local field potentials than spiking activity (Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001).
Conclusions
The mfVEP contrast response function in the central 1 deg radius of the fovea saturates at a substantially higher contrast level compared to those from more eccentric regions. The effect of contrast and eccentricity on latency is relatively small. For practical purposes, our results suggest that reducing contrast to about 50% will have a relatively small effect on mfVEP response amplitudes except for those within the central most retina. The general saturating nature of the mfVEP responses summed over areas where the central fovea makes a relatively small contribution to the response, as seen in our study, and by previous investigators (e.g. Hood et al., 2006; Park et al., 2008) implies that mid-contrast stimuli may be used to improve test sensitiveity in assessing diseases that affect central visual pathways up to V1.
Acknowledgments
This study was supported by NIH grants P30 EY07751, T35 007088, and a pilot grant from the National Multiple Sclerosis Society.
We wish to thank the subjects for their participation in this study, and Dr. Don Hood for his generosity with software.
References
- Albrecht DG, Geisler WS, Frazor RA, Crane AM. Visual cortex neurons of monkeys and cats: temporal dynamics of the contrast response function. Journal of Neurophysiology. 2002;88:888–913. doi: 10.1152/jn.2002.88.2.888. [DOI] [PubMed] [Google Scholar]
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. Journal of Neurophysiology. 1982;48:217–237. doi: 10.1152/jn.1982.48.1.217. [DOI] [PubMed] [Google Scholar]
- Balachandran C, Graham SL, Klistorner A, Goldberg I. Comparison of objective diagnostic tests in glaucoma: Heidelberg retinal tomography and multifocal visual evoked potentials. Journal of Glaucoma. 2006;12:110–116. doi: 10.1097/00061198-200604000-00006. [DOI] [PubMed] [Google Scholar]
- Baseler HA, Sutter EE. M and P components of the VEP and their visual field distribution. Vision Research. 1997;37:675–690. doi: 10.1016/s0042-6989(96)00209-x. [DOI] [PubMed] [Google Scholar]
- Brown NA. The morphology of cataract and visual performance. Eye (London, England) 1993;7:63–67. doi: 10.1038/eye.1993.14. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Allen KA. Topography of ganglion cells in human retina. The Journal of Comparative Neurology. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- Dacey D, Petersen M. Dendritic field size and morphology of midget and parasol ganglion cells of the human retina. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:9666–9670. doi: 10.1073/pnas.89.20.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. The mosaic of midget ganglion cells in the human retina. Journal of Neuroscience. 1993;13:5334–5355. doi: 10.1523/JNEUROSCI.13-12-05334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois RL, Morgan H, Snodderly DM. Psychophysical studies of monkey vision. 3. Spatial luminance contrast sensitivity tests of macaque and human observers. Vision Research. 1974;14:75–81. doi: 10.1016/0042-6989(74)90118-7. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. Journal of Physiology. 1984;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune B, Demirel S, Zhang X, Hood DC, Patterson E, Jamil A, Mansberger SL, Cioffi GA, Johnson CA. Comparing multifocal VEP and standard automated perimetry in high-risk ocular hypertension and eariy glaucoma. Investigative Ophthalmology & Visual Science. 2007;48:1173–1180. doi: 10.1167/iovs.06-0561. [DOI] [PubMed] [Google Scholar]
- Fortune B, Zhang X, Hood DC, Demirel S, Johnson CA. Normative ranges and specificity of the multifocal VEP. Documenta Ophthalmologica. 2004;109:87–100. doi: 10.1007/s10633-004-3300-5. [DOI] [PubMed] [Google Scholar]
- Fulton AB, Rushton WA. The human rod ERG: correlation with psychophysical responses in light and dark adaptation. Vision Research. 1978;18:793–800. doi: 10.1016/0042-6989(78)90119-0. [DOI] [PubMed] [Google Scholar]
- Geisler WS, Albrecht DG. Visual cortex neurons in monkeys and cats: detection, discrimination, and identification. Visual Neuroscience. 1997;14:897–919. doi: 10.1017/s0952523800011627. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Abe H. Mapping of glaucomatous visual field defects by multifocal VEPs. Investigative Ophthalmology & Visual Science. 2001;42:3341–3348. [PubMed] [Google Scholar]
- Hood D, Greenstein V. Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Progress in Retinal and Eye Research. 2003;22:201–251. doi: 10.1016/s1350-9462(02)00061-7. [DOI] [PubMed] [Google Scholar]
- Hood D, Odel J, Zhang X. Tracking the recovery of local optic nerve function after optic neuritis: a multifocal VEP study. Investigative Ophthalmology & Visual Science. 2000;41:4032–4038. [PubMed] [Google Scholar]
- Hood D, Ohri N, Yang E, Rodarte C, Zhang X, Fortune B, Johnson C. Determining abnormal latencies of multifocal visual evoked potentials: a monocular analysis. Documenta Ophthalmologica. 2004;109:189–199. doi: 10.1007/s10633-004-5512-0. [DOI] [PubMed] [Google Scholar]
- Hood DC, Ghadiali Q, Zhang JC, Graham NV, Wolfson SS, Zhang X. Contrast-response functions for multifocal visual evoked potentials: a test of a model relating V1 activity to multifocal visual evoked potentials activity. Journal of Vision. 2006;6:580–593. doi: 10.1167/6.5.4. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:2755–2757. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi O, Tanino T, Hirose T. Objective evaluation of binocular function with pattern reversal VER. I. Effect of contrast. Acta Ophthalmologica. 1985;63:706–711. doi: 10.1111/j.1755-3768.1985.tb01586.x. [DOI] [PubMed] [Google Scholar]
- Klistorner A, CrewWher DP, Crewther SG. Separate magnocellular and parvocellular contributions from temporal analysss of the multifocal VEP. Vision Research. 1997;37:2161–2169. doi: 10.1016/s0042-6989(97)00003-5. [DOI] [PubMed] [Google Scholar]
- Klistorner A, Graham SL. Objective perimetry in glaucoma. Ophthalmology. 2000;107:2283–2299. doi: 10.1016/s0161-6420(00)00367-5. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophyssological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Maddess T, James AC, Bowman EA. Contrast response of temporally sparse dichoptic multifocal visual evoked potentials. Visual Neuroscience. 2005;22:153–162. doi: 10.1017/S0952523805222046. [DOI] [PubMed] [Google Scholar]
- Park JC, Zhang X, Ferrera J, Hirsch J, Hood DC. Comparison of contrast-response functions from multifocal visual-evoked potentials (mfVEPs) and functional MRI responses. Journal of Vision. 2008;8:1–12. doi: 10.1167/8.10.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudvin I, Valberg A, Kilavik BE. Visual evoked potentials and magnocellular and parvocellular segregation. Visual Neuroscience. 2000;17:579–590. doi: 10.1017/s0952523800174085. [DOI] [PubMed] [Google Scholar]
- Schein SJ. Anatomy of macaque fovea and spatial densities of neurons in foveal representation. Journal of Comparative Neurology. 1988;169:479–505. doi: 10.1002/cne.902690403. [DOI] [PubMed] [Google Scholar]
- Souza GS, Gomes BD, Saito CA, da Silva Filho M, Silveira LC. Spatial luminance contrast sensitivity measured with transient VEP: comparison with psychophysics and evidence of multiple mechanisms. Investigative Ophthalmology & Visual Science. 2007;48:3396–3404. doi: 10.1167/iovs.07-0018. [DOI] [PubMed] [Google Scholar]
- Sutter EE. Imaging visual function with the multifocal m-sequence technique. Vision Research. 2001;41:1241–1255. doi: 10.1016/s0042-6989(01)00078-5. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Mannis MJ, Johnson CA. An analysis of contrast sensitivity in identical twins with keratoconus. Cornea. 1984;3:99–103. [PubMed] [Google Scholar]
- Zhang B, Stevenson SS, Cheng H, Laron M, Kumar G, Tong J, Chino YM. Effects of fixation instability on multifocal VEP (mfVEP) responses in amblyopes. Journal of Vision. 2008;8:1–14. doi: 10.1167/8.3.16. [DOI] [PubMed] [Google Scholar]