Abstract
Multidrug resistance (MDR) is a major impediment to the success of cancer chemotherapy. P-glycoprotein is an important and the best-known membrane transporter involved in MDR. Several strategies have been used to address MDR, especially P-glycoprotein-mediated drug resistance in tumors. However, clinical success has been limited, largely due to issues regarding lack of efficacy and/or safety. Nanoparticles have shown the ability to target tumors based on their unique physical and biological properties. To date, nanoparticles have been investigated primarily to address P-glycoprotein and the observed improved anticancer efficacy suggests that nanomedicinal strategies provide a new opportunity to overcome MDR. This article focuses on nanotechnology-based formulations and current nanomedicine approaches to address MDR in tumors and discusses the proposed mechanisms of action.
Keywords: Brij, drug delivery, efflux transporters, lipid formulation, nanoparticle, P-glycoprotein, Pluronic, vitamin E TPGS
Cancer is a leading cause of death worldwide, accounting for 7.9 million deaths in 2007 [201]. Over 85% of human cancers are solid tumors. Surgery and radiation are the initial treatments for most cancers. Chemotherapy with cytotoxic agents is used as an adjuvant therapy or a single-agent therapy especially for the management of recurrent disease. There are a number of chemotherapeutic agents with established first-line activity against cancer, with the anthracyclines [1] and taxanes [2] generally considered the most active. The relative benefits and toxicities of individual anticancer agents or combinations must be considered, as well as the treatment history and clinical status of the patient. Toxicity is one of the most critical issues in chemotherapy since most anticancer agents lack selective efficacy in cancer tissue. Another problem with conventional chemotherapy pertains to the challenge of delivery. The effectiveness of cancer chemotherapy in solid tumors depends on adequate delivery of therapeutic agents to tumor cells. The biological properties of the solid tumor, which limit the penetration of drugs into neoplastic cells distant from tumor vessels, include abnormal and heterogeneous tumor vasculature, interstitium, interstitial fluid pressure (IFP) and cell density. However, even if anticancer drugs are located in the tumor interstitium, they can have limited efficacy because cancer cells are able to develop mechanisms of resistance. Drug resistance has emerged as a major obstacle limiting the therapeutic efficacy of chemotherapeutic agents. These mechanisms allow tumors to evade chemotherapy. For example, multidrug resistance (MDR), a term to describe the broad-spectrum resistance to chemotherapy in human cancer, is one of the most important problems in chemotherapy. MDR is the phenomenon in which exposure of tumor cells to a single cytotoxic agent accounts for cross-resistance to other structurally unrelated classes of cytotoxic agents. ATP-binding cassette (ABC) transporters are transmembrane proteins that utilize the energy of ATP hydrolysis to shuttle various substrates across the cell membrane. Normally, ABC transporters function as pumps that extrude toxins and drugs out of the cell. To date, there are approximately 49 known transporters in the ABC family that are classified into seven different families (ABC A through ABC G) [3,4]. However, not all have been shown to have a role in MDR. Table 1 provides a summary of the most pertinent MDR-related ABC transporters and their most studied drug substrates [5]. P-glycoprotein (P-gp) is one of the most well-described ABC transporters and is overexpressed in the plasma membrane of MDR tumor cells. P-gp is capable of effluxing various anticancer drugs, such as doxorubicin and paclitaxel, out of the cells. P-gp inhibitors (e.g., verapamil) have been developed to overcome P-gp-mediated MDR. However, some P-gp inhibitors do not have good selectivity and also block normal cell function of P-gp, for example, in the intestines or at the blood–brain barrier (BBB), and therefore increase toxicity. A refinement of this concept is the incorporation of both the therapeutic drug and the P-gp inhibiting agent into the same drug carriers (e.g., nanoparticles) for simultaneous delivery into the cell.
Table 1.
Multidrug resistance-related ATP-binding cassette transporters and their substrates.
| Subfamily | Genes | Established anticancer drugs | Published target for nanomedicine? | Ref. |
|---|---|---|---|---|
| ABC1 | ABC A2/ABC2/STGD | Estramustine, mitoxantrone | No | |
| ABCA3/ABC3 | Daunorubicin, Ara-C, mitoxantrone and etoposide | No | ||
| MDR/TAP | ABC B1/MDR1/P-GP/PGY1 | Anthracyclines, vinca alkaloids, taxanes, etoposide, teniposide, imatinib, irinotecan, SN-38, bisantrene, colchicines, methotrexate, mitoxantrone, saquinivir, actinomycin D and others | Yes | [113–117,122–124, 127–132, 137–139,141, 143–145,152–154] |
| ABC B4/MDR3/PFIC3/PGY3 | Daunorubicin, doxorubicin, vincristine, etoposide and mitoxantrone | No | ||
| ABC B5 | Doxorubicin, camptothecin, 10-OH camptothecin and 5-FU | No | ||
| ABC B11/BSEP/PFIC2/SPGP | Paclitaxel | No | ||
| CFTR/MRP | ABC C1/MRP1 | Anthracyclines, vinca alkaloids, methotrexate, antifolate antineoplastic agents, etoposide, imatinib, irinotecan, SN-38, arsenite, colchicine, mitoxantrone, saquinivir and others | Yes | [53] |
| ABC C2/MRP2/CMOAT | Vinca alkaloids, cisplatin, etoposide, doxorubicin, epirubicin, metotrexatetaxanes, irinotecan, SN-38, topotecan, arsenite, mitoxantrone and saquinivir | No | ||
| ABC C3/MRP3/CMOAT2 | Etoposide, tenoposide and metotrexate | No | ||
| ABC C4/MRP4/MOATB | 6-mercaptopurine, 6-thioguanine, irinotecan, SN-38, topotecan, AZT, metotrexate and PMEA | No | ||
| ABC C5/MRP5/MOATC | 6-mercaptopurine, 6-thioguanine, irinotecan, 5-FU, cisplatin, AZT, metotrexate and PMEA | No | ||
| ABC C6/MRP6/MOATE/PXE | Etoposide, doxorubicin, daunorubicin, teniposide and cisplatin | No | ||
| ABC C10/MRP7 | Taxanes and vinca-alkaloids | No | ||
| ABC C11/MRP8 | 6-mercaptopurine, 5-FU and PMEA | No | ||
| WHITE | ABC G2/BCRP/MXR/ABCP | Mitoxantrone, camptothecin, anthracycline, etoposide, teniposide imatinib, flavopiridol, bisantrene, methotrexate, AZT and others | No | |
5-FU: 5-fluorouracil; ABC: ATP-binding cassette; AZT: 3′-azido-2′,3′-deoxythymidine; CTFR: Cystic fibrosis transmembrane regulator; MDR: Multidrug resistance; MRP: Multidrug resistance protein; PMEA: 9-(2-phosphonylmethoxyethyl)adenine; TAP: Transporter associated with antigen presentation.
Adapted from [5].
Nanoparticles have been developed to enhance the intracellular concentration of drugs in cancer cells while avoiding toxicity in normal cells using both passive and active targeting. The nanosize particles can pass through leaky and hyperpermeable tumor vasculature and accumulate in the tumor vicinity utilizing the enhanced permeability and retention (EPR) effect. Moreover, vascular permeability-enhancing factors, such as bradykimin, nitric oxide and VEGF, facilitate extravasation of macromolecular drugs within tumor tissues, as well as surrounding normal tissues. Furthermore, the tumor interstitium is also characterized by the absence of an anatomically well-defined functioning lymphatic network. Therefore, the clearance of nanoparticles via lymphatics is generally seriously compromised in neoplastic tissues, so that an additional retention of nanoparticles in the tumor interstitium has been observed. This particular concept known as the EPR effect results in intratumoral drug accumulation, which is even higher than that observed in plasma and other tissues. In addition to passive targeting by the EPR effect, active targeting may also be pursued by targeting nanoparticles with a tumor cell-specific ligand. More importantly, it has been suggested that nanoparticles may be able to circumvent P-gp-mediated resistance. The mechanisms of carriers to overcome P-gp have been proposed based on various drug delivery systems, including N-(2-hydroxypropyl)methacrylamide (HPMA) drug conjugates, Pluronic® micelles, hybrid lipid nanoparticles, lipid-based nanocapsules and nanoparticles, liposomes and cyanoacrylate-type nanoparticles. Reported mechanisms included enhancement of cellular uptake of drug via endocytosis and ion-pair formation, ATP depletion, influence of function and expression of P-gp, and change of P-gp downstream signaling pathways.
Barriers in chemotherapy for the treatment of solid tumors
The development of therapeutic drugs against cancer has been focused on finding innovative approaches to selectively deliver anticancer drugs to solid tumors, while minimizing injury to healthy tissues. However, the efforts are confronted by physiological barriers at the tumor level and drug resistance at the cellular level.
Physiological characteristics of solid tumors related to drug delivery include: abnormal blood vessel architecture and function; interstitial fluid pressure, also known as interstitial hypertension; and lack of lymphatics [6,7]. Unlike normal microvessels, tumor vessels resulting from angiogenesis are dilated, tortuous and heterogeneous in their spatial distribution [8–10]. The flow of blood through tumor vessels varies according to tumor types and microenvironment, so that the penetration of drugs in solid tumors is difficult. The disorganized structure of solid tumors causes poor drug delivery to the central region in large tumors. Consequently, the microenvironment in solid tumors may also contribute to drug resistance by limiting drug penetration, thereby exposing the tumor to lower than efficacious concentration [11]. Nonetheless, large interendothelial junctions, increased numbers of fenestrations and abnormal basement membranes are often found in tumor vessels. The ‘leakiness’ of tumor vessels generates gap openings that are significantly larger than those observed in normal tissues [11–14]. This vascular permeability in solid tumors leads one to develop tumor-targeting carriers that are small enough to enter through tumor vascular openings, without passing through those in normal tissues. The abnormal structure and function of blood and lymphatic vessels in solid tumors cause IFP elevation whereas IFP in normal tissues is approximately 0 mm/Hg. An increase in tumor IFP reduces the transcapillary pressure gradient from the vascular compartment to interstitial space of tumors [15]. Furthermore, uniform elevation of IFP results in a negligible flow inside tumors [16]. Thus, the uniformly elevated IFP impedes the delivery of therapeutic drugs across both the blood vessel wall and interstitium in solid tumors. Studies have demonstrated that there are no functional lymphatic vessels inside solid tumors [17–19]. Functional lymphatic vessels are present in the tumor margin and the peritumoral tissue to mediate tumor growth and metastases. The lack of lymphatics in tumors reduces the effective outflow of interstitial fluid, partially contributing to the increase of tumor IFP. However, the positive aspect of the absence of lymphatics for drug delivery is that drugs can not be drained out of tumors via the lymphatic system once they locate inside tumors. Moreover, since a blood supply is crucial for growth, tumor cells have the ability to recruit new blood vessels in various ways through a process termed angiogenesis. The fact that the survival of a tumor critically depends on its blood supply provides a common opportunity for the destruction of solid tumors if drug concentrations in the bloodstream remain high for an appropriate period.
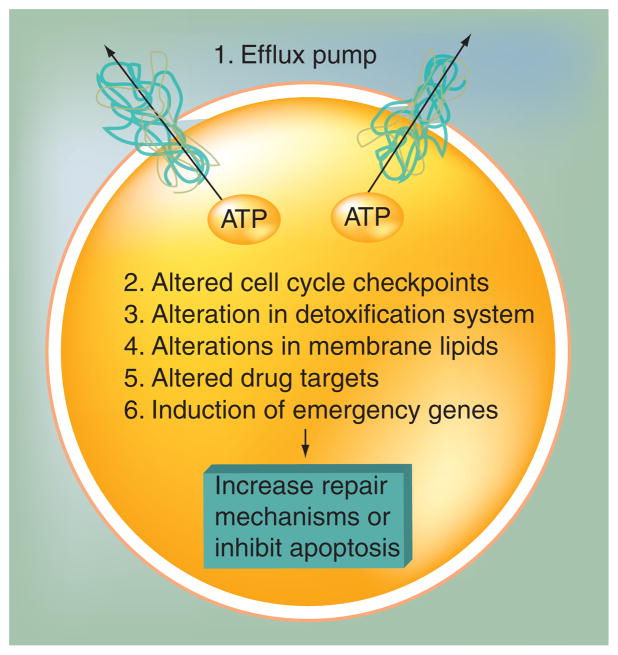
Therapeutic drugs will face another major barrier – drug resistance – after they penetrate in tumors. MDR remains the main cause of the failure of the use of conventional chemotherapy to treat common solid tumors [20]. Frequently, resistance is intrinsic to the cancer, but as therapy becomes more and more effective, acquired resistance has also become common. A variety of mechanisms contribute to acquired drug resistance to a broad range of anticancer drugs, including: expression of one or more energy-dependent transporters that bind to and efflux anticancer drugs from cells, leading to insensitivity to drug-induced apoptosis [21]; altered targets; and induction of drug-detoxifying mechanisms [22]. A summary of the reported mechanisms of drug resistance is presented in Figure 1. The initial MDR was reported in the late 1960s and early 1970s. Drug-resistant mammalian cell lines were then established for studying this phenomenon. A common feature of these drug-resistant cell lines was the overexpression of P-gp when compared with the drug-sensitive parent cell lines [23,24]. So far, overexpression of drug transporters is the most common reason for MDR. P-gp is an important and best-known membrane transporter involved in MDR. In humans, closely related genes, MDR1 and MDR2 or MDR3, encode highly homologous P-gp. However, only the MDR1 gene has been linked to the MDR. The MDR1 gene is highly expressed in many clinically resistant tumors. In some cases its expression at diagnosis has been proven to be an adverse prognostic factor. In other cases, MDR1 appeared after relapse from remission, suggesting that P-gp was a survival mechanism in a subclone of cancer cells that eventually reoccured [25–30]. Studies in leukemias, myelomas and some childhood cancers demonstrated that P-gp expression correlated with poor response to chemotherapy. Moreover, the early clinical trials testing P-gp modulation in acute leukemia in the presence of P-gp modulators resulted in clinical relapse and reduction of P-gp expression [31,32]. These clinical evidences demonstrated that P-gp plays a significant role in clinical drug resistance.
Figure 1. Summary of the mechanisms in which cultured cancer cells have been shown to become resistant to cytotoxic anticancer drugs.
The efflux pumps at the plasma membrane include P-glycoprotein, multidrug resistance protein family members and breast cancer resistance protein. Adapted from [42].
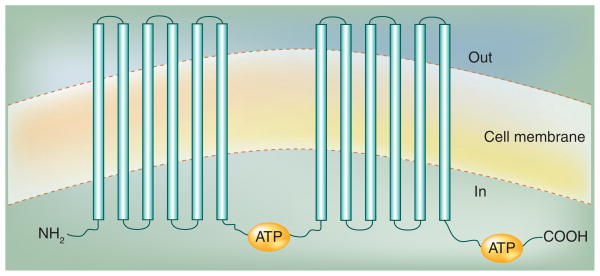
P-glycoprotein is an ATP-dependent transmembrane transporter that can transport a broad range of structurally unrelated compounds out of the cells. The human P-gp is a 170-kDa protein containing 1280 amino acids. The topology of P-gp was proposed to contain 12 transmembrane domains with six extracellular loops and two hydrophilic regions (ATP-binding domains), containing nucleotide-binding domains that are characteristics of the ABC family of transporters (Figure 2) [33–36]. P-gp is expressed at significant levels in human normal tissues, such as the biliary canaliculi of the liver, the proximal tubules of the kidneys, the small intestine, the colon and the adrenal cortex [37,38]. The activity of P-gp in normal tissues suggested an important role by transepithelial transport to prevent cytotoxic compounds in the environment and diet from entering the body, although the physiological role of P-gp remains a subject of speculation. Studies in P-gp-knockout mice demonstrated that P-gp had no exclusive and essential physiological function on fertility. The mice grew and developed into adulthood normally. However, these mice were very sensitive to MDR-related anticancer drugs. Moreover, more of these drugs accumulated in the CNS of the mice, indicating a major role of P-gp at the BBB [39–41].
Figure 2. Topology model of P-glycoprotein.
Each hydrophobic domain is followed by a hydrophilic domain (ATP-binding domain) containing a nucleotide-binding site that is located at the cytoplasmic face of the membrane and couples ATP hydrolysis.
P-gp can remove many different drugs from cells to decrease intracellular accumulation of anticancer drugs. Drugs may be pumped out by P-gp as they enter the plasma membrane, or even as they are inside the cells. These drugs include many of the commonly used natural product anticancer drugs, such as doxorubicin, vinblastine, etoposide and paclitaxel, as well as many commonly used pharmaceuticals, ranging from antiarrhythmics and antihistamines to cholesterol-lowering statins and HIV protease inhibitors [5,42]. Although the detailed mechanism of drug efflux by P-gp is unknown, the predicted transport cycle of P-gp involves the change of structure of ATP-binding domains after binding of drug substrates, stimulation of ATP hydrolysis, and rearrangement of P-gp shape caused by ATP binding and hydrolysis. The final step involving the conformational change of P-gp results in the release of the drug into the extracellular space. ATP hydrolysis is necessary for resetting of P-gp [43].
Seven strategies to overcome MDR
The strategies to overcome MDR are summarized in the following sections.
Modification of chemotherapy regimens
Noncross-resistant chemotherapeutic regimens utilize the largest number of active agents at the highest possible doses, assuming that mutations conferring drug resistance will not convey resistance to all of the agents in the regimen and, also, high-dose chemotherapy regimens could be given to cancer patients. This approach assumes that despite resistance to standard doses of anticancer drugs, a dose–response relationship still exists for these tumors and that high doses of chemotherapy might overcome this resistance.
Inactivation of MDR-associated genes by targeting specific mRNA for degradation
Antisense oligonucleotides and catalytic RNAs have successfully reduced P-gp, MRP and BCRP expression and sensitized drug-resistant cells [44–47]. The most recent development targeted to mRNA degradation is based on the RNA interference post-transcriptional gene silencing mechanism. Both siRNA (which has a transient effect) and shRNA (which has longer term effects) silence gene expression of P-gp, MRP and BCRP in MDR cancer cells [48–53]. To limit the exposure of normal cells to the inhibitor (siRNA) and the anticancer drug and maximize synergy, both P-gp-targeted siRNA and paclitaxel were coencapsulated into poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles. Biotin was also attached to the nanoparticles to target biotin receptors on cancer cells. In vitro and in vivo studies demonstrated that the biotin-functionalized nanoparticles encapsulating paclitaxel and siRNA partially overcame tumor drug resistance [54].
Use of monoclonal antibodies for P-gp
A monoclonal antibody to P-gp has been shown to inhibit tumor growth in an athymic nude mouse model [55,56]. However, there are some issues with the antibody approach; for example, MRK-16, a specific monoclonal antibody for P-gp, may target MDR1-expressing cells in normal tissues. Thus, the potential of this approach to lead to unacceptable toxicities could be overlooked in certain mouse models.
Development of new anticancer drugs that are not substrates of P-gp
The modified drug analogs can affect the binding of analogs to P-gp, and consequently P-gp cannot recognize the analogs. Two taxane analogs, DJ-927 (Phase I) [57,58] and ortataxel (Phase II) [59,60], designed to overcome drug resistance, have been evaluated in clinical trials. Other novel taxane analogs, such as BMS-184476 (Phase I) [61,62] and RPR 109881A (Phase II) [63,64], were also claimed to have a broad spectrum of activity both in sensitive and resistant tumor cell lines.
Use of inhibitors of ABC transporters to reverse MDR
This is an important approach receiving a great deal of attention. The inhibitors of ABC transporters can be classified into two groups. Some inhibitors can transport themselves and then act as competitive antagonists. The others are not transported but affect transporter function. The mechanism of reversal of transporters is discussed later.
Use of nanotechnology-based formulations & nanomedicine approaches to overcome MDR
Recently, several different formulations encapsulating anticancer drugs that are P-gp substrates have been developed. Paclitaxel vitamin E emulsion (TOCOSOL), containing the P-gp inhibitor D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) as an excipient, was evaluated in a Phase II clinical trial for drug resistance. The results showed promising efficacy when TOCOSOL was administrated weekly in patients with refractory cancers [65]. Partial reversal of drug resistance was also observed when liposomal doxorubicin was given to cell cultures [66]. This topic is discussed in greater detail in the sections below.
Inhibition of MDR using peptides
Synthetic P-gp-derived peptides corresponding to fragments from the extracellular loops of the murine P-gp were coupled to polyethylene glycol (PEG) and inserted into liposomes. These peptides have been shown to reverse MDR. After immunization with these peptide-loaded liposomes and treatment with doxorubicin, an increase of 83% survival time was observed in mice inoculated with P388R cells. No auto-immune responses were detected in immunized mice. However, complete eradication of tumors did not occur. These results indicate a potential new approach to break immune tolerance towards MDR1 protein and consequently modulate sensitivity of resistant tumors to chemotherapy [67–69].
The clinical success has been limited in the attempts to overcome MDR. A large number of a new generation of drug analogs have been developed as non-P-gp substrates. However, most taxane analogs have not demonstrated greater therapeutic indexes in vivo, or have shown toxicity to normal tissues, although they were designed and shown not to be P-gp substrates [62]. Another means to overcome MDR is to develop ABC transporter inhibitors. Studies on compounds, including the calcium channel blocker verapamil, to reverse vincristine resistance in murine leukemia cells opened the door for the development of inhibitors of P-gp to overcome P-gp-mediated drug resistance [70,71]. The therapeutic results from previous clinical trials using first- and second-generation inhibitors of ABC transporters have generally been negative or only modestly positive and have not fulfilled the promise of the preclinical data. There are several reasons for the failure of many of these inhibitors to show beneficial effects. One of the reasons for failure is that the inhibitors are weak and nonspecific. The requirement for the use of high inhibitor concentration (e.g., >10 μM verapamil) was not achievable in clinical trials. Additional toxicities of anticancer drugs due to the inhibition of transporters in normal tissues remain a concern for the more potent inhibitors. Another reason for failure is the observed programmed pharmacokinetic drug interaction resulting from coadministration of inhibitors and anticancer drugs. For example, a modest pharmacokinetic interaction between verapamil and doxorubicin in humans was observed [72]. In addition, some second-generation inhibitors of P-gp are substrates of cytochrome P-450. Competition of these P-gp inhibitors for cytochrome P-450-mediated oxidative reactions may lead to undesirable pharmacokinetic interactions [73]. Increased toxicity of the anticancer drugs may occur due to both pharmacokinetic effects and inhibition of a protective function of P-gp in normal tissues. To date, clinical trials with the third generation inhibitors are ongoing, including trials with compounds LY335979-targeted P-gp, GF120918-targeted P-gp and BCRP, R101933-targeted P-gp and XR9576-targeted P-gp and MRP1. However, toxicity issues are still associated with this generation. For example, XR9576 demonstrated high potency in both in vitro and in vivo studies and showed no effect on the pharmacokinetics of several anticancer drugs in a Phase I study. However, a Phase III study in non-small-cell lung cancer has been stopped due to increased toxicity [74]. Therefore, the contribution of these inhibitors to reverse clinical drug resistance needs to be defined in clinical trials.
Nano-based drug delivery systems for cancer
In the pharmaceutical field, the development of the first viable nanocarrier dates back approximately 40 years, when the first example of a liposome was described [75]. However, the most important scientific advancements on development of nanoscale vehicles with distinct physical and biochemical properties for drug delivery applications have only taken place within the last two decades. The most common examples of these nanoparticles include polymeric nanoparticles, dendrimers, nanoshells, liposomes, lipid-based nanoparticles, magnetic nanoparticles and virus nanoparticles. One of the most appealing properties of nanoparticles is their size, resulting in distinct features that are not available from individual molecules alone or equivalent materials at a larger scale. Nanoparticles have emerged as one approach to overcome both the lack of specificity of conventional drugs and delivery barriers in solid tumors [76–82].
Nanoparticles have the potential to improve the therapeutic index of currently available drugs by increasing drug efficacy, lowering drug toxicity and achieving steady state therapeutic levels of drugs over an extended period of time. Nanoparticles can also improve drug solubility and drug stability, allowing the development of potentially effective new chemical entities that have been stalled during the preclinical or clinical development owing to suboptimal pharmacokinetic or biochemical properties. Finally, the flexible surface chemistry of nanoparticles also allows for possible conjugation of targeting ligands for active targeted delivery.
A schematic representation for different mechanisms by which nanoparticles enhance drug delivery to solid tumors is shown in Figure 3 [76]. The effectiveness of cancer therapy in solid tumors depends on adequate delivery of the therapeutic agent to tumor cells. Many anticancer drugs have a marginal selectivity for malignant cells because they target the reproductive apparatus in cells having high proliferation rates. However, anticancer drugs having this mechanism of action result in high toxicities against rapidly dividing normal cells, such as hair follicles, germ cells and hematopoietic cells. Other anticancer drugs are distributed nonspecifically in the body where they affect cancer and normal cells. Therefore, the side effects associated with chemotherapy limit the dose achievable to the tumor and also result in suboptimal treatment due to excessive toxicities. Thus, the delivery of drugs to solid tumors remains difficult owing to the physiological barriers, as described above.
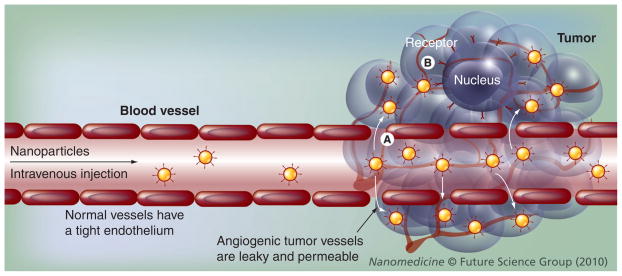
Figure 3. Enhanced drug delivery to solid tumors using nanoparticles.
(A) Passive targeted delivery. After intravenous injection, nanoparticles accumulate in tumors through leaky and permeable tumor vasculature and impaired lymphatic system (e.g., enhanced permeability and retention effect). (B) Active targeted delivery. Ligand-coated nanoparticles bind to a cancer cell receptor resulting in cell-specific recognition and improved drug delivery to solid tumors.
The role of tumor vasculature as a potential target for solid tumors has been elucidated since the late 1970s. In terms of the EPR effect, the small physical dimensions of nanoparticles enable them to partially penetrate through biological and physiological barriers of tumors that are unique to tumors and normally impermeable for larger particles. In this manner, passive targeted delivery to tumors based on the EPR effect may be achieved due to the physicochemical properties of a carrier and the pathophysiological condition of a target. For such a passive targeting mechanism to take place, physiochemical parameters of nanoparticles, including particle size and surface properties, are crucial. The desirable nanoparticles should have a certain particle size along with long blood circulation. The pore cut-off size of extravasation through transvascular gaps in most experimental tumors is in the range of 380–780 nm depending on the tumor types, whereas the tight endothelial junctions of normal vessels typically are between 1 and 10 nm [11,83]. It was proposed that transvascular transport of the particles in the tumors resulted from interendothelial or transendothelial open junctions rather than by endothelial phagocytosis or vesicles. To achieve this extravasation in solid tumors, an average particle size of less than 300 nm was suggested to be a requirement [11]. It should be noted there has been some recent debate as to whether the EPR effect is an artifact of mouse xenograft models, and whether this same type of effect exists in human cancers or even in genetically engineered mouse models [84,85]. If, in fact, human tumors lack the inherent leakiness required for the accumulation of nanoparticles by the EPR effect, then this will have profound effects on future nanomedicinal strategies for targeting solid tumors. As a consequence, one may have to develop strategies to enhance the convection of nanoparticles from the vasculature across the endothelial membrane to gain access to the solid tumors. Alternatively, one may have to employ anticancer strategies targeting the endothelium adjacent to solid tumors that may lead to an anticancer effect (e.g., using nanoparticles to deliver potent antiangiogenic factors perhaps in combination with drug[s]).
Another important prerequisite for passive targeted delivery is that the plasma concentration of the drug must remain high and long; meaning that drug-coated nanoparticles must be sustained in the bloodstream long enough to reach or recognize solid tumors. Nanoparticles, unlike microspheres (>1 μm), are small enough to be safely dosed via intravenous administration. In the bloodstream, a major defense system of the body, the reticuloendothelial system (RES), which is also known as the mononuclear phagocytes system, rapidly removes nanoparticles from the blood and becomes a major obstacle to long circulation of nanoparticles. The first step of clearance of nanoparticles by RES is opsonization, in which nanoparticles are recognized as foreign particles and are coated with opsonin proteins to make them more recognizable to phagocytic cells. Phagocytic cells mainly include Kupffer cells of the liver and fixed macrophages of the spleen. A large number of researchers have been motivated to develop ‘stealth’ nanoparticles to avoid the uptake of nanoparticles by the RES. It has been repeatedly demonstrated that the opsonization of hydrophobic particles occur more quickly than that of hydrophilic nanoparticles, owing to the enhanced adsorbability of blood serum proteins on hydrophobic surfaces [86,87]. Modification of the nanoparticle surface with chains of hydrophilic and flexible polymers can shield nanoparticles from the opsonins and, therefore, prevent elimination by the cells of the RES. The most commonly used polymers are PEG, poly(ethylene oxide) and poly(propylene oxide), or their combinations. Such polymers can be put on the nanoparticle surface by covalently grafting, entrapping and adsorption. However, there are no absolute rules or methods available to completely and effectively block the opsonization of nanoparticles although research in this area has been ongoing for over 30 years. Modification of nanoparticle surface is complicated and case dependent. The thickness of the layer is crucial but hard to control and will vary within different types of nanoparticles and coating strategies. However, it is noteworthy that the effective stealth properties provided by these barrier layers depend largely on the PEG molecular weight, surface chain density and conformation [88]. In addition to clearance of nanoparticles by phagocytosis, nanoparticles are also subjected to splenic filtration, a non-phagocytic uptake process. The width of interendothelial cell slits of the spleen is approximately 200–500 nm [89]. A study of the biodistribution of polystyrene nanospheres (60–250 nm), coated or uncoated with hydrophilic polymers, was carried out in rats. In this study, coating of nanospheres with hydrophilic polymers dramatically reduced uptake of particles by liver, regardless of particle sizes. However, coated nanospheres were sequestered by the spleen, apparently depending on the particle size. The size and deformability of nanoparticles play critical roles in their clearance by this mechanism in human spleens. For rigid nanoparticles, the size to achieve long circulation should not exceed 200 nm. Otherwise, nanoparticles should be deformable enough to pass splenic filtration [90,91].
The innovations in combinatorial chemistry and high throughput screening, focused on achieving potency and high activity of small molecule anticancer drugs, are leading to a shift of the chemical and physical properties of new chemical entities towards more lipophilic and poorly water-soluble molecules. As a result, approximately 40% of potential drug candidates never enter further development, such as a formulation development stage, due to their poor water solubility [92]. The advances of lipid-based nanoparticle systems for improved drug delivery offer a great potential for the administration of anticancer drugs. The main advantages of these systems includes low toxicity of carriers themselves, solubilization of poorly water-soluble drugs, high drug loading, and protection of drugs against chemical and biological degradation related to the administration route, prolonged circulation, targeting delivery and controlled release. In addition, more than one anticancer drug may be coencapsulated into colloidal carriers for combined cancer chemotherapy [93]. Lipid-based nanoparticles are generally comprised of biocompatible and biodegradable lipids and, therefore, may be less toxic than many types of polymeric nanoparticles prepared from synthetic polymers. Once the particle size is reduced to sub-micron levels, the surface area of the nanoparticles increases substantially, which the saturation solubility also increases with the reduction of particle size, leading to an increase in the dissolution rate. The encapsulation of drugs into the carriers provides protection of the drugs from the influence of physiological conditions. Furthermore, the carrier may have prolonged circulation; thus, the required therapeutic levels of the drugs in the blood for the extended time interval, and a better targeting effect, may be more readily achieved. In addition, by choosing the excipients and compositions of the nanoparticles, controlled release can be explored to further reduce acute toxicity. As described previously, nanoparticles may be passively targeted to solid tumors by the EPR effect or even actively targeted by placing the ligand on the surface of nanoparticles. Lipid-based nanoparticles have become an increasingly important field of research for delivering anticancer drugs in terms of these potential benefits. The primary types of lipid-based nanoparticles investigated have been liposomes, micelles, nanoemulsions, nanocapsules and solid lipid nanoparticles. These, as well as some other important systems, will be discussed in the following sections. All are potential drug carriers administered by different routes, including oral [94,95], topical [96,97] and parenteral [98,99].
Drug delivery systems to overcome P-gp-mediated drug resistance & their possible mechanisms
As mentioned previously, MDR is a major impediment to the success of cancer chemotherapy. P-gp was the first multidrug transporter discovered, and remains the best characterized and most clinically relevant to date. As such the majority of clinical trials have focused on P-gp, as highlighted in Table 1. P-gp is the product of the MDR1 gene and effluxes drugs without chemical modification. Approximately 50% of the anticancer drugs used clinically today are substrates of P-gp [100]. The emergence of drug resistance has made many of the chemotherapy drugs ineffective. Different strategies attempting to overcome P-gp-mediated drug resistance have been developed as previously described. Therefore, this section focuses on overcoming P-gp-mediated drug resistance using drug delivery systems. Unlike the potentially more serious effects of the active P-gp inhibitors, drug delivery systems may be inhibitors of P-gp with low pharmacological activity and reduced side effects. The formulations discussed here to overcome MDR are summarized in Table 2 and will be highlighted in the following sections.
Table 2.
Formulations to overcome multidrug resistance and their proposed mechanisms.
| Formulations | Proposed mechanisms | Status | Ref. |
|---|---|---|---|
| Liposomes | Endocytosis | In vitro | [117] |
| Interaction of liposomes with P-gp | In vitro | [114] | |
| Coencapsulation of a P-gp inhibitor (verapamil) | In vitro | [123] | |
| Polymethacrylate NPs | Endocytosis | In vitro | [133] |
| Polyisohexylcyanoacrylate NPs | No endocytosis | In vitro | [134–136] |
| Saturation of P-gp by high concentration of the drug | |||
| Formation of an ion pair between NP degradation product and the drug | In vivo | ||
| Polymer–lipid hybrid NPs | Phagocytosis | In vitro | [132] |
| AOT–alginate NPs | Not established | In vitro | [129] |
| Solutol HS-15-based lipid nanocapsules | Interaction of the released intracellular free solutol HS-15 with MDR efflux pump | In vitro | [138] |
| Redistribution of intracellular cholesterol | In vivo | ||
| Lipid-based nanoparticles containing Brij 78 | Endocytosis | In vitro | [140,141] |
| Inhibition of P-gp | In vivo | ||
| ATP depletion | |||
| HPMA copolymer–doxorubicin conjugates | Endocytosis | In vitro | [143–149] |
| Inhibition of drug detoxification systems | In vivo | ||
| Inhibition of cellular defensive mechanisms | |||
| Pluronic® block copolymer micelles | Change in membrane microviscosity | In vitro | [152–161] |
| Inhibition of drug efflux transporters | In vivo | ||
| ATP depletion | Phase II | ||
| Influence of cell apoptosis signaling | |||
| Inhibition of the GSH–GST detoxification system | |||
| Inhibition of mitochondria respiratory chain and decrease of oxygen consumption | |||
AOT: Sodium bis(2-ethylhexyl) sulfosuccinate; GSH: Glutathione; GST: Glutathione-S-transferase; HPMA: N-(2-hydroxypropyl)methacrylamide; MDR: Multidrug resistance; NP: Nanoparticle; P-gp: P-glycoprotein.
P-gp inhibition by surfactant-based formulations
In 1972, a published study demonstrated that Tween 80 enhanced the cytotoxicity of actinomycin D and daunomycin in Chinese hamster resistant cells [101]. Since this report, a number of lipid and polymeric excipients present in pharmaceutical formulations have been reported to modulate the activity of P-gp. P-gp, an efflux pump located in the apical membranes of intestinal absorptive cells, can reduce the absorption of drugs and consequently decrease the oral bioavailability. Thus, the lipid formulation strategy for enhancing absorption of drugs that are P-gp substrates became an attractive topic and has been extensively reviewed for oral delivery [100,102–104]. Caco-2 and MDCK cells expressing P-gp are the most widely used cell models to study the oral absorption. Most of the surfactants inhibiting P-gp are nonionic. They can be divided into two classes according to the chemical structure [102]. The first class of surfactants exhibit a hydrophilic head group and a hydrophobic tail responsible for membrane anchoring, including triglycerides, Cremophor EL, Solutol HS-15, vitamin E TPGS, Tween 80 and Brij 35. The second class of surfactants, which lack a typical membrane anchor, includes PEG and polyethylene oxide (EO)–polypropylene oxide (PO) block copolymers (Pluronics or poloxamers). All surfactant inhibitors contain a unit that comprises hydrogen bond acceptor groups such as ester groups and polyoxyethylene sequences, which could form hydrogen bonds with the transmembrane sequences of P-gp, which are rich in hydrogen bond donor groups. Provided that the binding affinity of surfactants is higher than that of the drug, the surfactants may be used to inhibit P-gp and, therefore, enhance drug absorption. The inhibitory effects of surfactants on P-gp efflux are related to the structure of surfactants, such as the length of the hydrophilic chain and hydrophilic–lipophilic balance (HLB). TPGS 1000 has been reported to influence drug efflux well below its reported critical micelle concentration (CMC) of 0.02 wt% [105]. A structure–activity relationship study was carried out to understand the influence of PEG chain length on apical efflux transporters in Caco-2 cell monolayers [106]. TPGS analogs containing different PEG chain lengths (molecular weight from 200 to 6000 Da) were synthesized. The results suggested that PEG chain length was essential to influence rhodamine 123 efflux in vitro. TPGS 1000 turned out to be one of the most potent analogs to inhibit P-gp efflux. The effect of HLB values of excipients on P-gp modulation was also evaluated for their effects on the uptake of epirubicin in Caco-2 cells [107]. Surfactants with enhanced efficacy in this study, including Tween 20, Tween 80, Brij 30 and Myrj 52, consisted of a polyethylene and intermediate hydrocarbon chain. The characteristics of the surfactants allow them to partition between lipid bilayers and P-gp domains. The optimal HLB value to enhance epirubicin uptake was in the range of 10 to 17. A related study on Pluronic block copolymers with varying length of EO and PO segments was performed in bovine brain microvessel endothelial cells [108]. The most efficacious block copolymers exhibited intermediate length of 30–60 PO blocks and a relatively hydrophobic structure (HLB <20; e.g., Pluronic P85 with 40 PO blocks and an HLB of 16). The common mechanisms of surfactants to inhibit P-gp may include binding competition of drugs with surfactants resulting from an interaction between surfactants and P-gp, and membrane fluidization leading to an indirect protein destabilization [108–111]. However, the latter mechanism may not be the case for some surfactants. For example, TPGS tends to make lipid bilayers more, rather than less, rigid [110].
Liposomes
Liposomes have been, and continue to be, the most intensively researched colloidal drug delivery systems even for more than four decades after their discovery. Liposomes are normally composed of phospholipids that spontaneously form multilamellar, concentric bilayer vesicles, with layers of aqueous media separating the lipid layers. The particle size of small unilamellar vesicles, which are comprised of a single, lipid outer layer with an aqueous core, is in the range 20–80 nm. The surface of the liposomes may be charged or uncharged based on the selection of different phospholipids. There are many methods to prepare liposomes, including precipitation [112]. Liposomes may be used to load both hydrophobic and hydrophilic drugs. Hydrophilic drugs reside in the aqueous core, whereas hydrophobic drugs tend to remain in the lipid layers. Hydrophobic drugs are added during the formation of liposomes. Hydrophilic drugs may also be loaded during formation, but for charged drugs the pH-gradient method may be used wherein a pH gradient between the internal and external aqueous domains drives the drug into the interior of the liposomes by partitioning through the membrane. Liposomes have poor loading capacity for hydrophobic drugs that cannot be dissolved in sufficient amounts in the phospholipid bilayer or sequestered in the liposome core. Furthermore, after intravenous administration, such drugs often rapidly partition from the bilayers into cells, or bind to serum proteins, preventing accumulation at the target site. Several liposomal drugs are now marketed including Ambisome® (amphotericin B), Doxil® (doxorubicin hydrochloride) and Visudyne® (verteporfin) to name a few.
Liposomal delivery systems have been shown to inhibit P-gp efflux [66,113–117]. The proposed mechanisms included bypassing P-gp through an endocytosis pathway [117] and direct interaction with P-gp. The interaction of liposomes with P-gp was proved by complete inhibition of photoaffinity labeling of P-gp by azidopine [114]. However, other studies demonstrated that liposomes had limited effectiveness in addressing P-gp-mediated resistance in laboratory in vitro models of cellular resistance and in clinical studies [118–121]. Liposome formulations containing both an anticancer drug and a P-gp inhibitor have been studied recently. The results showed that liposomal coencapsulated drugs had better responses in both in vitro and in vivo resistant models compared with a single drug [122–124]. Moreover, liposomal targeting delivery systems have been investigated to overcome P-gp-mediated drug resistance [123]. For example, doxorubicin and verapamil were coencapsulated into liposomes with 95 and 70% loading efficiency, respectively. To achieve active targeting, human transferrin (Tf) was conjugated to the liposomes to target Tf receptors, which are overexpressed in leukemia cells. In resistant leukemia K562 cells (Tf receptor+), Tf-conjugated coloaded liposomes showed 5.2- and 2.8-times greater cytotoxicity than nontargeted coloaded liposomes and Tf-conjugated doxorubicin liposomes, respectively. It was concluded that TfR-targeted liposomes coloaded with doxorubicin and verapamil were effective in selective targeting and reversal of drug resistance in cells [123].
Polymer, lipid nancapsules & nanoparticles
Nanocapsules have a liquid core (generally an oil) surrounded by a polymeric membrane structured by polymers or a combination of hydrophilic/lipophilic surfactants. Vegetable oils and triglycerides with medium- and long-chain fatty acids are the common components for the lipid cores. The drugs are confined to the lipid core, which serves as a reservoir to allow a high drug loading for hydrophobic drugs and a slow release profile. Thus, nanocapsules are pharmaceutically attractive for water-insoluble drugs.
Solid lipid nanoparticles made from biodegradable or biocompatible solid lipids were developed in the beginning of the 1990s as an alternative colloidal carrier system for controlled drug delivery. Solid lipid nanoparticles are matrix systems in which the drug is physically and uniformly dispersed. The release of a drug incorporated in the lipid matrix occurs due to degradation of the particles by lipases present at the site of injection, leading to a prolonged release of drugs from the solid lipid nanoparticles [125]. A comprehensive review on solid lipid nanoparticles can be found in the literature [126].
A number of studies have investigated encapsulation of anticancer drugs into polymer nanoparticles and lipid nanoparticles (or nanocapsules) to overcome P-gp-mediated drug resistance [127–132]. Among them, polyalkylcyanoacrylate nanoparticles were the earliest ones investigated in resistant cell lines [130]. The results showed that nonbiodegradable polymethacrylate nanoparticles can be internalized by an endocytosis process and reverse P-gp-mediated drug resistance in vitro [133]. For in vivo studies, biodegradable doxorubicin-loaded polyisohexylcyanoacrylate (PIHCA) nanoparticles were developed. These PIHCA nanoparticles showed more cytotoxicity than free doxorubicin in doxorubicin-resistant C6 cells. Later on, more rapidly biodegradable PIBCA nanoparticles were formulated to load doxorubicin. Doxorubicin uptake from PIBCA nanoparticles was different than that from PIHCA nanoparticles, as doxorubicin-loaded PIBCA nanoparticles caused higher cellular doxorubicin uptake than free doxorubicin. In addition, it was demonstrated that PIBCA nanoparticles did not enter cells via an endocytosis pathway and efflux of doxorubicin in nanoparticles had a similar profile with free doxorubicin. Mechanistic studies found that nanoparticles could deliver a high concentration of doxorubicin close to or adhered onto the cell membrane, resulting in saturation of P-gp; the formation of an ion pair between cyanoacrylic acid (a nanoparticle degradation product) and doxorubicin could also mask the positive charge of doxorubicin and facilitate diffusion of doxorubicin across cell membranes [134,135]. However, in vivo studies using MDR tumors demonstrated that these nanoparticles were not efficacious in vivo, perhaps due to poor delivery to the tumors [136]. A new polymer–lipid hybrid nanoparticle (PLN) system was used to increase the cytotoxicity of doxorubicin in resistant cells [132]. Doxorubicin uptake and retention from doxorubicin-loaded nanoparticles were significantly enhanced compared with free doxorubicin. Blank PLNs did not improve doxorubicin uptake and retention in resistant MDA-MB-435/LCC6MDR1 cells. These results indicated that the PLNs did not influence P-gp activity by themselves. The results also revealed that phagocytosis was an important pathway for PLN to enter the cells. Based on this pathway, doxorubicin-loaded PLNs could bypass P-gp, leading to enhanced doxorubicin uptake in resistant cells [137]. Recently, sodium bis(2-ethylhexyl) sulfosuccinate (AOT)–alginate nanoparticles were evaluated for their potential to overcome P-gp-mediated drug resistance. Doxorubicin-loaded AOT–alginate nanoparticles enhanced the cytotoxicity of doxorubicin in resistant NCI/ADR-RES cells. It was observed that: the uptake of rhodamine was significantly increased using rhodamine-entrapped nanoparticles in resistant cell; blank nanoparticles improved rhodamine accumulation in a dose-dependent manner in resistant cells; and the enhancement in rhodamine accumulation was not due to membrane permeabilization. However, the mechanism of AOT–alginate nanoparticles to overcome P-gp-mediated drug resistance has not been established [129]. As mentioned previously, the surfactant solutol HS-15, a mixture of free PEG 660 and PEG 660 hydroxystearate, could inhibit P-gp. Solutol HS-15-based lipid nanocapsules (LNC) containing paclitaxel or etoposide were studied for their potential to overcome MDR [138,139]. Paclitaxel-loaded LNCs were shown to significantly reduce cancer cell survival in comparison with Taxol in 9L cells and F98 cells. Solutol HS-15 on its own did not improve the effects of paclitaxel. Similarly, paclitaxel-loaded LNCs significantly reduced tumor mass in vivo, whereas Taxol did not have a significant effect in an MDR-expressing F98 subcutaneous glioma model. The mixture of solutol HS-15 and paclitaxel did not improve tumor responses in this in vivo model. This indicated the importance of the nanocarrier itself for the anticancer effect on MDR. The study also showed that LNC internalization was not mediated by clathrin-dependent endocytosis. It was assumed that LNC endocytosis involves one or both of the two known cholesterol-enriched membrane microdomains. The consecutive intracellular cholesterol movements would constitute the core of the LNC inhibitory effects on MDR. Thus, according to these mechanism studies, the investigators proposed that the inhibition of the MDR efflux pump by LNCs could result from the interaction of the released intracellular free Solutol HS-15 with MDR efflux pump and redistribution of intracellular cholesterol [138]. Recently, doxorubicin and paclitaxel-loaded lipid-based nanoparticles containing Brij 78 as a surfactant were used to overcome P-gp-mediated drug resistance. These drug-loaded nanoparticles showed six- to nine-fold lower IC50 values in P-gp overexpressing human cancer cells than those of free drugs [140]. Paclitaxel nanoparticles showed marked anticancer efficacy in a nude mouse HCT-15 xenograft model via intratumoral injection [141] and in a nude mouse NCI/ADR-RES xenograft model after intravenous injection [140] as compared to all control groups. A series of in vitro cell assays were used to understand the underlining mechanisms. Enhanced uptake and prolonged retention of doxorubicin were observed with nanoparticle-based formulations in P-gp-overexpressing cells. Calcein acetoxymethylester assays and ATP assays confirmed that Brij 78 and blank nanoparticles inhibited P-gp and transiently depleted ATP. The change in the mitochondrial potential and mitochondrial swelling were observed to be dominant in MDR cells, suggesting Brij 78 and nanoparticles influence the mitochondrial respiratory chain. It was concluded that nanoparticles may be used to target both drug and biological mechanisms to overcome MDR via P-gp inhibition and ATP depletion [140]. It is noteworthy that the drugs delivered into MDR cells by PLGA nanoparticles are susceptible to efflux by P-gp [142]. PLGA nanoparticles were taken up by cells via endocytosis, resulting in an increase of cellular concentration of the drug encapsulated into the nanoparticles. Following entry, nanoparticles were retained in the cytoplasm for a sustained period of time and slowly released the drug in the cellular cytoplasm. However, paclitaxel-loaded PLGA nanoparticles did not show significant cytotoxicity in resistant NCI/ADR-RES cells. It was proved that P-gp activity did not affect the uptake and retention of nanoparticles themselves. Thus, the inefficiency of paclitaxel-loaded PLGA nanoparticles could result from the active efflux of the drug in cytoplasm by P-gp. P-gp could not only extract the drug when the drug diffused into the cell through the lipid bilayer, but also pump out the drug present in the cytoplasm. Consequently, the efficiency of overcoming P-gp based on endocytosis of nanoparticles may be limited.
Polymer–drug conjugates
Poly(N-[2-hydroxypropyl]methacrylamide) (polyHPMA) and HPMA copolymers have been proposed by several groups as potential drug delivery systems. HPMA is a water-soluble, non-immunogenic synthetic polymer. HPMA copolymer–doxorubicin conjugates have shown the potential to overcome drug resistance [143–145]. A series of studies on HPMA–doxorubicin conjugates addressed multiple mechanisms of MDR in addition to P-gp-mediated drug resistance. After the HPMA–doxorubicin conjugate was internalized by an endocytosis pathway, the spacer between the polymer and the drug was cleaved by an enzymatic hydrolysis reaction in the lysosomal compartment of the cells, resulting in the release of the drug from the conjugate. Chronic exposure of sensitive A2780 cells to HPMA–doxorubicin conjugates did not induce MDR as measured by quantification of MDR1 gene expression; inhibition of MPR gene expression and a decrease of resistance against Taxol was evident [146]. By contrast, repeated exposure to free doxorubicin led to an increased resistance to doxorubicin and Taxol, and upregulation of the MDR1 gene. Further in vitro mechanistic studies on overcoming MDR revealed that HPMA–doxorubicin conjugates inhibited: drug detoxification systems by suppressing the expression of genes encoding glutathione and UDP; and cellular defensive mechanism by activating apoptosis signaling pathways and downregulating the expression of the bcl-2 protein family and mechanisms of DNA repair, replication and synthesis leading to more DNA damage [147,148]. These results indicated that underlying mechanisms triggered by macromolecular carriers can modulate the biological response of the cell at a molecular level, resulting in an overall increased cytotoxicity. The ability of HPMA–doxorubicin conjugates to overcome MDR in vivo was demonstrated in solid tumor mouse models of sensitive human ovarian carcinoma A2780 and resistant A2780/AD tumors [149]. HPMA–doxorubicin conjugates significantly decreased tumor size by 28-fold in sensitive tumors and 18-fold in resistant tumors, whereas free doxorubicin only reduced tumor size by 2.8-fold in sensitive tumors and had no effect in the resistant tumor model as compared with the control. The underlying mechanisms were also investigated for the in vivo study. An enhanced accumulation of HPMA–doxorubicin conjugates in the tumor was observed and attributed to the EPR effect. The permeability of blood vessels decreased concomitantly with the downregulation of vascular growth and permeability (VEGF) gene in polymer-treated tumors. The other proposed in vitro mechanisms, such as downregulation of the expression of genes responsible for the activity of efflux pumps, detoxification and apoptosis, were also demonstrated in the in vivo studies.
Pluronic micelles
Micelles are the most basic colloidal drug delivery systems and are formed spontaneously in nature. In the body, colloidal micellar species comprising endogenous surfactants and lipid digestion products (i.e., bile salt mixtures) facilitate the absorption of highly insoluble fatty acids and fat soluble vitamins. Micelles have a particle size normally within a 5–100 nm range, are thermodynamically stable and form spontaneously by association of amphiphilic molecules, such as surfactants, under defined concentrations and temperatures. The concentration of a monomeric amphiphile at which micelles appear is termed the critical micelle concentration (CMC). The formation of micelles is driven by the decrease of free energy in the system owing to the removal of hydrophobic fragments from the aqueous environment and the re-establishment of the hydrogen bond network in water. Hydrophobic fragments of the amphiphilic molecules form the core of a micelle, while hydrophilic moieties form the shell of the micelle. When used as drug carriers in aqueous media, micelles solubilize molecules of poorly soluble nonpolar drugs within the micelle core, while polar drugs could be adsorbed on the micelle surface, and substances with intermediate polarity distribute along surfactant molecules in intermediate positions. One limitation of micellar systems is the relatively low hydrophobic volume of the interior of micelles, leading to limited drug loading. Another limitation of conventional micellar systems is the danger of drug precipitation upon the dilution of the solubilized drug with physiological fluids after parenteral administration. The dilution of the formulation by physiological fluids may cause the disassociation of the micelles as the concentration of the surfactants used to solubilize the drugs may be lower than their CMC.
More recently, polymer micelles prepared from amphiphilic copolymers for solubilization of poorly soluble drugs as an alternative to lipid-based surfactant systems have attracted much attention. Polymer micelles offer a more versatile structure, biodegradability and lower CMC, which may lead to better in vivo stability and more conjugation chemistries for linking ligands to the surface of the colloidal system. Polymer micelles are self-assembled from block copolymers comprising a hydrophobic block (e.g., polylactic acid) with a hydrophilic block. As a result of a common progression of development of ‘stealth’ systems for intravenous administration, PEGylation approaches were used to form stealth micelles to enhance circulation time. Furthermore, the PEG corona can act as a diffusion barrier for hydrophobic drugs to reduce the burst release characteristic of drug-loaded micelles [150]. Thus, the hydrophilic block on the copolymer typically contains PEG segments with a molecular weight from 1 to 15 kDa. Similar to micelles formed with conventional surfactants, polymeric micelles comprise the core of the hydrophobic blocks stabilized by the corona of hydrophilic chains in water. However, compared with the conventional micelles, polymeric micelles are more stable upon the relatively high dilution conditions experienced in vivo. For example, some amphiphilic copolymers have CMC values as low as 10−6 M [151].
Pluronics are inert block copolymers comprising of poly(EO) (hydrophilic) and poly(PO) (hydrophobic). Pluronics are different from HPMA copolymers due to their amphiphilic nature. Their surfactant properties allow them to self-assemble into micelles with a hydrophobic PO inner core and a hydrophilic EO outer shell. However, similar to HPMA copolymers, Pluronic block copolymer micelles have also been shown to overcome drug resistance. Extensive studies with Pluronic block copolymer micelles have been reviewed [152–154]. SP1049C-containing doxorubicin in the mixed micelles of Pluronic L61 and F127 is in clinical trials to treat metastatic adenocarcinoma of the upper GI tract. In addition, SP1049C showed more efficient accumulation in tumors than free doxorubicin, while distribution of SP1049C in normal tissues was similar to that of doxorubicin [155]. Efficacy of SP1049C was confirmed in in vivo experiments in both sensitive and resistant tumor models, including P388 and P388/ADR murine leukemia, Sp2/0 and Sp2/0-Dnr murine myeloma, 3LL-M27 Lewis lung carcinoma, MCF-7 and MCF-7/ADR human breast carcinomas, and KBv human oral epidermoid carcinoma [153,155]. However, the toxicity of SP1049C was similar to that of free doxorubicin. This suggested that SP1049C did not improve the toxicity profile of free doxorubicin (e.g., cardiotoxicity), which was improved by liposomal doxorubicin. However, there were no additional side effects reported for SP1049C. Disintegration of micelles in biological fluids upon dilution to a concentration below its CMC is a common concern regarding using micelles for drug delivery. The biodistribution and pharmacokinetics of Pluronic P85 micelles suggested that the elimination of P85 was controlled by the renal elimination of P85 unimers and not by the rate of micelle disintegration [156]. However, micelle disintegration had been reported with other Pluronic micelles. To further address the potential of micelle disintegration, Pluronic L121 and Pluronic P-105 micelles were chemically modified to form a network or crosslink. Therefore, the CMC of the micelles was greatly reduced and the stability was enhanced, while their ability to inhibit P-gp function still remained [157,158].
The complex mechanisms associated with the effects of Pluronic block copolymers on MDR cells have been thoroughly studied. These mechanisms include altering membrane microviscosity [159,160]. It was suggested that unimers (single block copolymer molecules) are responsible for biological modification as the effect of Pluronic copolymer occurred at concentrations below their CMC. The hydrophobic PO chains of Pluronic unimers insert into the hydrophilic regions of the membrane, resulting in alterations of the membrane structure, and decrease in its microviscosity (membrane fluidization); inhibiting drug efflux transporters, such as P-gp and MRPs, through inhibition of P-gp ATPase activity; and depleting intracellular ATP levels [159–161]. As these pumps are energy dependent, attenuation of these pumps was related to energy deprivation and abolishment of pump-associated ATPase activity. Thus, it can be surmized that Pluronic-mediated direct and indirect energy depletion leads to cessation of the operation of efflux pumps, and consequently sensitizes resistant cell lines to chemotherapeutic agents. A linear correlation between the extent of ATP depletion and chemosensitization elicited was established, further influencing cell apoptosis signaling. Doxorubicin-loaded Pluronic block copolymer P85 significantly enhanced the pro-apoptotic activity of the drug and prevented the activation of the antiapoptotic cellular defense [162]; decreasing glutathione (GSH) intracellular levels and glutathione-S-transferase (GST) activity, indicating the inhibition of the GSH–GST detoxification system [163]; and inhibited mitochondria respiratory chain and decreased oxygen consumption in MDR cells, accompanied by a decrease in mitochondria membrane potential, production of reactive oxygen species and release of cytochrome C. Eventually, this results in Pluronic-enhanced drug-induced apoptosis [164].
Conclusion & future perspective
The increasing importance of overcoming MDR in tumors has become emphasized in the last few decades. Great improvements in the application of nanotechnology as drug delivery systems have offered the possibility of more potential treatments for MDR. Commonly used pharmaceutical excipients have been explored for the ability to inhibit P-gp. This has, in turn, led to the development of several nanoparticle-based drug delivery systems that have incorporated these excipients to overcome MDR. Despite the fact that the mechanisms to overcome MDR using these nanoparticles are complicated and not fully understood, improved anticancer efficacy on MDR in tumors has been confirmed in vitro and in vivo. Moreover, some drug-loaded nanoparticles to treat MDR in tumors are now in human clinical trials. It is therefore anticipated that current development of nanoparticles may provide new strategies for the treatment of MDR.
However, many challenges remain for nanomedicine to overcome MDR. Drug-loaded nanoparticles still have the chance to distribute within normal tissues. Most of the current nanoparticles aimed at MDR utilize the EPR effect to pursue passive targeted delivery to solid tumors. Therefore, more efficacious targeting strategies for nanoparticles are still needed. Active targeted delivery could be achieved by attaching tumor specific antibodies or other ligands on the nanoparticle surface, or using external techniques (e.g., ultrasound, to enhance drug uptake at the tumor site). There also exists the need to continue to understand the biological mechanisms by which these nanomedicines overcome MDR. Although more research articles are published about the mechanisms, relatively few in vivo studies have been performed to date. More detailed studies on mechanisms would help direct the application of current delivery systems or lead to the discovery of alternative novel delivery systems. Furthermore, MDR itself is a complex phenomenon and contains different mechanisms. P-gp remains one of the best studied mechanisms, so most of the current formulation approaches have focused on P-gp-mediated drug resistance. Additional understanding of the mechanisms of how nanoparticles address the biological aspects of MDR may lead to novel nanoparticles that could effectively address MDR as well as other potential resistance mechanisms.
Executive summary.
Barriers in chemotherapy for the treatment of solid tumors
Physiological characteristics of solid tumors present a barrier in chemotherapy as they result in poor drug delivery and therapeutic outcomes.
Many cancer drugs will face multidrug resistance.
Strategies to overcome P-glycoprotein-mediated drug resistance
-
Strategies include:
Modification of chemotherapy regimens
Inactivation of MDR-associated genes by targeting specific mRNA for degradation
Development of new anticancer drugs that are not substrates of P-glycoprotein (P-gp)
The use of inhibitors of P-gp to reverse P-gp-mediated drug resistance
The use nanotechnology-based formulations and nanomedicine approaches to overcome P-gp-mediated drug resistance
Inhibition of P-gp-mediated drug resistance using monoclonal antibodies or peptides
Nano-based drug delivery systems for cancer
Nanoparticles may target solid tumors based on the enhanced permeability and retention effect to enhance tumor uptake and accumulation.
Lipid-based nanoparticles offer a great potential to formulate poorly water soluble anticancer drugs.
Drug delivery systems to overcome P-gp-mediated drug resistance & their possible mechanisms
Pharmaceutical excipients have shown the ability to inhibit P-gp and enhance drug uptake.
Several drug delivery systems have been investigated to overcome MDR, including surfactant-based formulations, liposomes, polymer and lipid nanocapsules and nanoparticles, polymer–drug conjugates and micelles.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported, in part, by grant R01 CA115197 from the NIH to Russell J Mumper. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Cortes-Funes H, Coronado C. Role of anthracyclines in the era of targeted therapy. Cardiovasc Toxicol. 2007;7(2):56–60. doi: 10.1007/s12012-007-0015-3. [DOI] [PubMed] [Google Scholar]
- 2.Piccart M. The role of taxanes in the adjuvant treatment of early stage breast cancer. Breast Cancer Res Treat. 2003;79(Suppl 1):S25–S34. doi: 10.1023/a:1024393926965. [DOI] [PubMed] [Google Scholar]
- 3.Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8 (5):411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- 4.Leonard GD, Polgar O, Bates SE. ABC transporters and inhibitors: new targets, new agents. Curr Opin Invest Drugs. 2002;3 (11):1652–1659. [PubMed] [Google Scholar]
- 5.Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 2010;596:47–75. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- 6.Campbell RB. Tumor physiology and delivery of nanopharmaceuticals. Anticancer Agents Med Chem. 2006;6(6):503–512. doi: 10.2174/187152006778699077. [DOI] [PubMed] [Google Scholar]
- 7.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74(2–3):72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endrich B, Reinhold HS, Gross JF, Intaglietta M. Tissue perfusion inhomogeneity during early tumor growth in rats. J Natl Cancer Inst. 1979;62(2):387–395. [PubMed] [Google Scholar]
- 9.Hamberg LM, Kristjansen PE, Hunter GJ, Wolf GL, Jain RK. Spatial heterogeneity in tumor perfusion measured with functional computed tomography at 0.05 microliter resolution. Cancer Res. 1994;54(23):6032–6036. [PubMed] [Google Scholar]
- 10.Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48(10):2641–2658. [PubMed] [Google Scholar]
- 11.Hobbs SK, Monsky WL, Yuan F, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95(8):4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin P, Casarett G. Microcirculation of tumors. I. Anatomy, function, and necrosis. Clin Radiol. 1966;17(3):220–229. doi: 10.1016/s0009-9260(66)80027-2. [DOI] [PubMed] [Google Scholar]
- 13.Shubik P. Vascularization of tumors: a review. J Cancer Res Clin Oncol. 1982;103(3):211–226. doi: 10.1007/BF00409698. [DOI] [PubMed] [Google Scholar]
- 14.Yuan F, Dellian M, Fukumura D, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55(17):3752–3756. [PubMed] [Google Scholar]
- 15.Aukland K, Reed RK. Interstitial–lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73(1):1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990;50(15):4478–4484. [PubMed] [Google Scholar]
- 17.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60(16):4324–4327. [PubMed] [Google Scholar]
- 18.Padera TP, Stoll BR, Tooredman JB, Capen D, Di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427(6976):695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 19.Padera TP, Kadambi A, Di Tomaso E, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296(5574):1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 20.Saijo N. Chemotherapy: the more the better? Overview. Cancer Chemother Pharmacol. 1997;40(Suppl):S100–S106. doi: 10.1007/s002800051070. [DOI] [PubMed] [Google Scholar]
- 21.Reed JC. Bcl-2: Prevention of apoptosis as a mechanism of drug resistance. Hematol Oncol Clin North Am. 1995;9(2):451–473. [PubMed] [Google Scholar]
- 22.Morrow CS, Cowan KH. Glutathione s-transferases and drug resistance. Cancer Cells. 1990;2(1):15–22. [PubMed] [Google Scholar]
- 23.Ling V, Thompson LH. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- 24.See YP, Carlsen SA, Till JE, Ling V. Increased drug permeability in Chinese hamster ovary cells in the presence of cyanide. Biochim Biophys Acta. 1974;373(2):242–252. doi: 10.1016/0005-2736(74)90148-5. [DOI] [PubMed] [Google Scholar]
- 25.Arao S, Suwa H, Mandai M, et al. Expression of multidrug resistance gene and localization of P-glycoprotein in human primary ovarian cancer. Cancer Res. 1994;54(5):1355–1359. [PubMed] [Google Scholar]
- 26.Arceci RJ. Clinical significance of P-glycoprotein in multidrug resistance malignancies. Blood. 1993;81(9):2215–2222. [PubMed] [Google Scholar]
- 27.Bell DR, Gerlach JH, Kartner N, Buick RN, Ling V. Detection of P-glycoprotein in ovarian cancer: a molecular marker associated with multidrug resistance. J Clin Oncol. 1985;3(3):311–315. doi: 10.1200/JCO.1985.3.3.311. [DOI] [PubMed] [Google Scholar]
- 28.Dalton WS, Grogan TM, Rybski JA, et al. Immunohistochemical detection and quantitation of P-glycoprotein in multiple drug-resistant human myeloma cells: Association with level of drug resistance and drug accumulation. Blood. 1989;73(3):747–752. [PubMed] [Google Scholar]
- 29.Pirker R, Wallner J, Geissler K, et al. MDR1 gene expression and treatment outcome in acute myeloid leukemia. J Natl Cancer Inst. 1991;83(10):708–712. doi: 10.1093/jnci/83.10.708. [DOI] [PubMed] [Google Scholar]
- 30.Sato H, Preisler H, Day R, et al. MDR1 transcript levels as an indication of resistant disease in acute myelogenous leukaemia. Br J Haematol. 1990;75(3):340–345. doi: 10.1111/j.1365-2141.1990.tb04346.x. [DOI] [PubMed] [Google Scholar]
- 31.List AF, Spier C, Greer J, et al. Phase I/II trial of cyclosporine as a chemotherapy-resistance modifier in acute leukemia. J Clin Oncol. 1993;11(9):1652–1660. doi: 10.1200/JCO.1993.11.9.1652. [DOI] [PubMed] [Google Scholar]
- 32.Marie JP, Bastie JN, Coloma F, et al. Cyclosporin A as a modifier agent in the salvage treatment of acute leukemia (AL) Leukemia. 1993;7(6):821–824. [PubMed] [Google Scholar]
- 33.Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 34.Kast C, Canfield V, Levenson R, Gros P. Transmembrane organization of mouse P-glycoprotein determined by epitope insertion and immunofluorescence. J Biol Chem. 1996;271(16):9240–9248. doi: 10.1074/jbc.271.16.9240. [DOI] [PubMed] [Google Scholar]
- 35.Loo TW, Clarke DM. Membrane topology of a cysteine-less mutant of human P-glycoprotein. J Biol Chem. 1995;270(2):843–848. doi: 10.1074/jbc.270.2.843. [DOI] [PubMed] [Google Scholar]
- 36.Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F. The functions and structure of ABC transporters: implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR) Curr Drug Targets. 2006;7(7):893–909. doi: 10.2174/138945006777709520. [DOI] [PubMed] [Google Scholar]
- 37.Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci USA. 1987;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schinkel AH, Mol CA, Wagenaar E, Van Deemter L, Smit JJ, Borst P. Multidrug resistance and the role of P-glycoprotein knockout mice. Eur J Cancer. 1995;31A(7–8):1295–1298. doi: 10.1016/0959-8049(95)00130-b. [DOI] [PubMed] [Google Scholar]
- 40.Schinkel AH, Smit JJ, Van Tellingen O, et al. Disruption of the mouse MDR1A P-glycoprotein gene leads to a deficiency in the blood–brain barrier and to increased sensitivity to drugs. Cell. 1994;77(4):491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 41.Schinkel AH, Wagenaar E, Van Deemter L, Mol CA, Borst P. Absence of the MDR1A P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96(4):1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42▪▪.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. Detailed review on the mechanisms of drug resistance in tumors. [DOI] [PubMed] [Google Scholar]
- 43.Loo TW, Clarke DM. Recent progress in understanding the mechanism of P-glycoprotein-mediated drug efflux. J Membr Biol. 2005;206(3):173–185. doi: 10.1007/s00232-005-0792-1. [DOI] [PubMed] [Google Scholar]
- 44.Nadali F, Pourfathollah AA, Alimoghaddam CK, et al. Multidrug resistance inhibition by antisense oligonucleotide against MDR1/mRNA in P-glycoprotein expressing leukemic cells. Hematology. 2007;12 (5):393–401. doi: 10.1080/10245330701283991. [DOI] [PubMed] [Google Scholar]
- 45.Ren Y, Wang Y, Zhang Y, Wei D. Overcoming multidrug resistance in human carcinoma cells by an antisense-oligodeoxynucleotide–doxorubicin conjugate in vitro and in vivo. Mol Pharm. 2008;5(4):579–587. doi: 10.1021/mp800001j. [DOI] [PubMed] [Google Scholar]
- 46.Stewart AJ, Canitrot Y, Baracchini E, et al. Reduction of expression of the multidrug resistance protein (MRP) in human tumor cells by antisense phosphorothioate oligonucleotides. Biochem Pharmacol. 1996;51(4):461–469. doi: 10.1016/0006-2952(95)02220-1. [DOI] [PubMed] [Google Scholar]
- 47.Kawabata S, Oka M, Shiozawa K, et al. Breast cancer resistance protein directly confers SN-38 resistance of lung cancer cells. Biochem Biophys Res Commun. 2001;280(5):1216–1223. doi: 10.1006/bbrc.2001.4267. [DOI] [PubMed] [Google Scholar]
- 48.Krühn A, Wang A, Fruehauf JH, Lage H. Delivery of short hairpin RNA by transkingdom RNA interference modulates the classical ABCB1-mediated multidrug-resistant phenotype of cancer cells. Cell Cycle. 2009;8(20):3349–3354. doi: 10.4161/cc.8.20.9845. [DOI] [PubMed] [Google Scholar]
- 49.Xia Z, Zhu Z, Zhang L, et al. Specific reversal of MDR1/P-gp-dependent multidrug resistance by RNA interference in colon cancer cells. Oncol Rep. 2008;20(6):1433–1439. [PubMed] [Google Scholar]
- 50.Stege A, Krühn A, Lage H. Overcoming multidrug resistance by RNA interference. Methods Mol Biol. 2010;596:447–465. doi: 10.1007/978-1-60761-416-6_20. [DOI] [PubMed] [Google Scholar]
- 51.Duan Z, Brakora KA, Seiden MY. Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells. Mol Cancer Ther. 2004;3(7):833–838. [PubMed] [Google Scholar]
- 52.Lv H, He Z, Liu X, Yuan J, Yu Y, Chen Z. Reversal of BCRP-mediated multidrug resistance by stable expression of small interfering RNAs. J Cell Biochem. 2007;102(1):75–81. doi: 10.1002/jcb.21276. [DOI] [PubMed] [Google Scholar]
- 53.Saad M, Garbuzenko OB, Minko T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine. 2008;3(6):761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials. 2010;31:358–365. doi: 10.1016/j.biomaterials.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe T, Naito M, Kokubu N, Tsuruo T. Regression of established tumors expressing P-glycoprotein by combination of adriamycin, cyclosporine derivatives, and MRK16 antibodies. J Natl Cancer Inst. 1997;89(7):512–518. doi: 10.1093/jnci/89.7.512. [DOI] [PubMed] [Google Scholar]
- 56.Goda K, Fenyvesi F, Bacsó Z, et al. Complete inhibition of P-glycoprotein by simultaneous treatment with a distinct class of modulators and the UIC2 monoclonal antibody. J Pharmacol Exp Ther. 2006;320(1):81–88. doi: 10.1124/jpet.106.110155. [DOI] [PubMed] [Google Scholar]
- 57.Ono C, Takao A, Atsumi R. Absorption, distribution, and excretion of DJ-927, a novel orally effective taxane, in mice, dogs, and monkeys. Biol Pharm Bull. 2004;27(3):345–351. doi: 10.1248/bpb.27.345. [DOI] [PubMed] [Google Scholar]
- 58.Shionoya M, Jimbo T, Kitagawa M, Soga T, Tohgo A. DJ-927, a novel oral taxane, overcomes P-glycoprotein-mediated multidrug resistance in vitro and in vivo. Cancer Sci. 2003;94(5):459–466. doi: 10.1111/j.1349-7006.2003.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cassinelli G, Lanzi C, Supino R, et al. Cellular bases of the antitumor activity of the novel taxane IDN 5109 (BAY59–8862) on hormone-refractory prostate cancer. Clin Cancer Res. 2002;8(8):2647–2654. [PubMed] [Google Scholar]
- 60.Polizzi D, Pratesi G, Monestiroli S, et al. Oral efficacy and bioavailability of a novel taxane. Clin Cancer Res. 2000;6(5):2070–2074. [PubMed] [Google Scholar]
- 61.Altstadt TJ, Fairchild CR, Golik J, et al. Synthesis and antitumor activity of novel c-7 paclitaxel ethers: discovery of BMS-184476. J Med Chem. 2001;44(26):4577–4583. doi: 10.1021/jm0102607. [DOI] [PubMed] [Google Scholar]
- 62.Rose WC, Fairchild C, Lee FY. Preclinical antitumor activity of two novel taxanes. Cancer Chemother Pharmacol. 2001;47(2):97–105. doi: 10.1007/s002800000241. [DOI] [PubMed] [Google Scholar]
- 63.Gelmon KA, Latreille J, Tolcher A, et al. Phase I dose-finding study of a new taxane, RPR 109881a, administered as a one-hour intravenous infusion days 1 and 8 to patients with advanced solid tumors. J Clin Oncol. 2000;18(24):4098–4108. doi: 10.1200/JCO.2000.18.24.4098. [DOI] [PubMed] [Google Scholar]
- 64.Kurata T, Shimada Y, Tamura T, et al. Phase I and pharmacokinetic study of a new taxoid, RPR 109881a, given as a 1-hour intravenous infusion in patients with advanced solid tumors. J Clin Oncol. 2000;18(17):3164–3171. doi: 10.1200/JCO.2000.18.17.3164. [DOI] [PubMed] [Google Scholar]
- 65.Lissianskaya A, Gershanovich M, Ognerubov N, Golubeva O, Pratt J. Paclitaxel injectable emulsion: Phase 2a study of weekly administration in patients with platinum-resistant ovarian cancer. Proc Am Soc Clin Oncol. 2004;22:5047. [Google Scholar]
- 66.Sadava D, Coleman A, Kane SE. Liposomal daunorubicin overcomes drug resistance in human breast, ovarian and lung carcinoma cells. J Liposome Res. 2002;12(4):301–309. doi: 10.1081/lpr-120016196. [DOI] [PubMed] [Google Scholar]
- 67.Gatouillat G, Odot J, Balasse E, et al. Immunization with liposome-anchored pegylated peptides modulates doxorubicin sensitivity in P-glycoprotein-expressing p388 cells. Cancer Lett. 2007;257(2):165–171. doi: 10.1016/j.canlet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Hamada H, Tsuruo T. Functional role for the 170- to 180-kDa glycoprotein specific to drug-resistant tumor cells as revealed by monoclonal antibodies. Proc Natl Acad Sci USA. 1986;83(20):7785–7789. doi: 10.1073/pnas.83.20.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mechetner EB, Roninson IB. Efficient inhibition of P-glycoprotein-mediated multidrug resistance with a monoclonal antibody. Proc Natl Acad Sci USA. 1992;89(13):5824–5828. doi: 10.1073/pnas.89.13.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in p388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41(5):1967–1972. [PubMed] [Google Scholar]
- 71.Tsuruo T, Iida H, Yamashiro M, Tsukagoshi S, Sakurai Y. Enhancement of vincristine- and adriamycin-induced cytotoxicity by verapamil in p388 leukemia and its sublines resistant to vincristine and adriamycin. Biochem Pharmacol. 1982;31(19):3138–3140. doi: 10.1016/0006-2952(82)90097-1. [DOI] [PubMed] [Google Scholar]
- 72.Kerr DJ, Graham J, Cummings J, et al. The effect of verapamil on the pharmacokinetics of adriamycin. Cancer Chemother Pharmacol. 1986;18(3):239–242. doi: 10.1007/BF00273394. [DOI] [PubMed] [Google Scholar]
- 73.Bates S, Kang M, Meadows B, et al. A Phase I study of infusional vinblastine in combination with the P-glycoprotein antagonist PSC 833 (valspodar) Cancer. 2001;92(6):1577–1590. doi: 10.1002/1097-0142(20010915)92:6<1577::aid-cncr1484>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 74.Nobili S, landini I, Giglioni B, Mini E. Pharmacological strategies for overcoming multidrug resistance. Curr Drug Targets. 2006;7(7):861–879. doi: 10.2174/138945006777709593. [DOI] [PubMed] [Google Scholar]
- 75.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 76▪.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. Detailed review on nanoparticles for cancer therapy. [DOI] [PubMed] [Google Scholar]
- 77.Alexis F, Rhee JW, Richie JP, Radovic-Moreno AF, Langer R, Farokhzad OC. New frontiers in nanotechnology for cancer treatment. Urol Oncol. 2008;26(1):74–85. doi: 10.1016/j.urolonc.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 78.Jang SH, Wientjes MG, Lu D, Au JL. Drug delivery and transport to solid tumors. Pharm Res. 2003;20(9):1337–1350. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 79.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 2008;26(1):57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 80.Thierry B. Drug nanocarriers and functional nanoparticles: applications in cancer therapy. Curr Drug Deliv. 2009;6(4):391–403. doi: 10.2174/156720109789000474. [DOI] [PubMed] [Google Scholar]
- 81.Gindy ME, Prud’homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv. 2009;6(8):865–878. doi: 10.1517/17425240902932908. [DOI] [PubMed] [Google Scholar]
- 82.Pridgen EM, Langer R, Farokhzad OC. Biodegradable, polymeric nanoparticle delivery systems for cancer therapy. Nanomedicine. 2007;2(5):669–680. doi: 10.2217/17435889.2.5.669. [DOI] [PubMed] [Google Scholar]
- 83.Karakotchian M, Fraser IS. An ultrastructural study of microvascular inter-endothelial tight junctions in normal endometrium. Micron. 2007;38(6):632–636. doi: 10.1016/j.micron.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 84.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2010 doi: 10.1016/j.addr.2010.03.011. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 85.Gopinathan A, Tuveson DA. The use of GEM models for experimental cancer therapeutics. Dis Model Mech. 2008;1(2–3):83–86. doi: 10.1242/dmm.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carrstensen H, Muller RH, Muller BW. Particle size, surface hydrophobicity and interaction with serum of parenteral fat emulsions and model drug carriers as parameters related to RES uptake. Clin Nutr. 1992;11(5):289–297. doi: 10.1016/0261-5614(92)90006-c. [DOI] [PubMed] [Google Scholar]
- 87.Norman ME, Williams P, Illum L. Human serum albumin as a probe for surface conditioning (opsonization) of block copolymer-coated microspheres. Biomaterials. 1992;13(12):841–849. doi: 10.1016/0142-9612(92)90177-p. [DOI] [PubMed] [Google Scholar]
- 88.Owens DE, Peppas NA. Opsonization, biodistribution and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 89.Chen LT, Weiss L. The role of the sinus wall in the passage of erythrocytes through the spleen. Blood. 1973;41(4):529–537. [PubMed] [Google Scholar]
- 90.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 91.Moghimi SM, Porter CJ, Muir IS, Illum L, Davis SS. Non-phagocytic uptake of intravenously injected microspheres in rat spleen: influence of particle size and hydrophilic coating. Biochem Biophys Res Commun. 1991;177(2):861–866. doi: 10.1016/0006-291x(91)91869-e. [DOI] [PubMed] [Google Scholar]
- 92.Stegemann S, Leveiller F, Franchi D, De Jong H, Linden H. When poor solubility becomes an issue: from early stage to proof of concept. Eur J Pharm Sci. 2007;31(5):249–261. doi: 10.1016/j.ejps.2007.05.110. [DOI] [PubMed] [Google Scholar]
- 93.Liu P, Wang B, Weili Qiao JL. Multi-anticancer drugs encapsulated in the micelle: a novel chemotherapy to cancer. Med Hypotheses. 2008;71(3):379–381. doi: 10.1016/j.mehy.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 94.Benny O, Fainaru O, Adini A, et al. An orally delivered small-molecule formulation with antiangiogenic and anticancer activity. Nat Biotechnol. 2008;26(7):799–807. doi: 10.1038/nbt1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sarmento B, Martins S, Ferreira D, Souto EB. Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomed. 2007;2(4):743–749. [PMC free article] [PubMed] [Google Scholar]
- 96.Cosco D, Celia C, Cilurzo F, Trapasso E, Paolino D. Colloidal carriers for the enhanced delivery through the skin. Expert Opin Drug Deliv. 2008;5(7):737–755. doi: 10.1517/17425247.5.7.737. [DOI] [PubMed] [Google Scholar]
- 97.Mainardes RM, Urban MC, Cinto PO, et al. Colloidal carriers for ophthalmic drug delivery. Curr Drug Targets. 2005;6(3):363–371. doi: 10.2174/1389450053765914. [DOI] [PubMed] [Google Scholar]
- 98.Constantinides PP, Chaubal MV, Shorr R. Advances in lipid nanodispersions for parenteral drug delivery and targeting. Adv Drug Deliv Rev. 2008;60(6):757–767. doi: 10.1016/j.addr.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 99.Tamilvanan S. Oil-in-water lipid emulsions: implications for parenteral and ocular delivering systems. Prog Lipid Res. 2004;43(6):489–533. doi: 10.1016/j.plipres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 100▪.Constantinides PP, Wasan KM. Lipid formulation strategies for enhancing intestinal transport and absorption of P-glycoprotein (P-gp) substrate drugs: in vitro/in vivo case studies. J Pharm Sci. 2007;96(2):235–248. doi: 10.1002/jps.20780. Detailed review on using lipid formulation to overcome P-glycoprotein in order to enhance oral drug delivery. [DOI] [PubMed] [Google Scholar]
- 101.Riehm H, Biedler JL. Potentiation of drug effect by Tween 80 in Chinese hamster cells resistant to actinomycin D and daunomycin. Cancer Res. 1972;32(6):1195–1200. [PubMed] [Google Scholar]
- 102.Seelig A, Gerebtzoff G. Enhancement of drug absorption by noncharged detergents through membrane and P-glycoprotein binding. Expert Opin Drug Metab Toxicol. 2006;2(5):733–752. doi: 10.1517/17425255.2.5.733. [DOI] [PubMed] [Google Scholar]
- 103.Bogman K, Zysset Y, Degen L, et al. P-glycoprotein and surfactants: effect on intestinal talinolol absorption. Clin Pharmacol Ther. 2005;77(1):24–32. doi: 10.1016/j.clpt.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Foger F, Hoyer H, Kafedjiiski K, Thaurer M, Bernkop-Schnurch A. In vivo comparison of various polymeric and low molecular mass inhibitors of intestinal P-glycoprotein. Biomaterials. 2006;27(34):5855–5860. doi: 10.1016/j.biomaterials.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 105.Dintaman JM, Silverman JA. Inhibition of P-glycoprotein by D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) Pharm Res. 1999;16(10):1550–1556. doi: 10.1023/a:1015000503629. [DOI] [PubMed] [Google Scholar]
- 106.Collnot EM, Baldes C, Wempe MF, et al. Influence of vitamin E TPGS poly(ethylene glycol) chain length on apical efflux transporters in Caco-2 cell monolayers. J Control Release. 2006;111(1–2):35–40. doi: 10.1016/j.jconrel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 107.Lo YL. Relationships between the hydrophilic-lipophilic balance values of pharmaceutical excipients and their multidrug resistance modulating effect in Caco-2 cells and rat intestines. J Control Release. 2003;90(1):37–48. doi: 10.1016/s0168-3659(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 108.Batrakova EV, Li S, Alakhov VY, Miller DW, Kabanov AV. Optimal structure requirements for Pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J Pharmacol Exp Ther. 2003;304(2):845–854. doi: 10.1124/jpet.102.043307. [DOI] [PubMed] [Google Scholar]
- 109.Loe DW, Sharom FJ. Interaction of multidrug-resistant chinese hamster ovary cells with amphiphiles. Br J Cancer. 1993;68(2):342–351. doi: 10.1038/bjc.1993.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rege BD, Kao JP, Polli JE. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci. 2002;16(4–5):237–246. doi: 10.1016/s0928-0987(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 111.Yamazaki T, Sato Y, Hanai M, et al. Non-ionic detergent Tween 80 modulates VP-16 resistance in classical multidrug resistant K562 cells via enhancement of VP-16 influx. Cancer Lett. 2000;149(1–2):153–161. doi: 10.1016/s0304-3835(99)00355-9. [DOI] [PubMed] [Google Scholar]
- 112.Dass CR. Drug delivery in cancer using liposomes. Methods Mol Biol. 2008;437:177–182. doi: 10.1007/978-1-59745-210-6_9. [DOI] [PubMed] [Google Scholar]
- 113.Mayer LD, Shabbits JA. The role for liposomal drug delivery in molecular and pharmacological strategies to overcome multidrug resistance. Cancer Metastasis Rev. 2001;20(1–2):87–93. doi: 10.1023/a:1013108524062. [DOI] [PubMed] [Google Scholar]
- 114.Rahman A, Husain SR, Siddiqui J, et al. Liposome-mediated modulation of multidrug resistance in human HL-60 leukemia cells. J Natl Cancer Inst. 1992;84(24):1909–1915. doi: 10.1093/jnci/84.24.1909. [DOI] [PubMed] [Google Scholar]
- 115.Thierry AR, Dritschilo A, Rahman A. Effect of liposomes on P-glycoprotein function in multidrug resistant cells. Biochem Biophys Res Commun. 1992;187(2):1098–1105. doi: 10.1016/0006-291x(92)91310-m. [DOI] [PubMed] [Google Scholar]
- 116.Warren L, Jardillier JC, Malarska A, Akeli MG. Increased accumulation of drugs in multidrug-resistant cells induced by liposomes. Cancer Res. 1992;52(11):3241–3245. [PubMed] [Google Scholar]
- 117.Ford JM, Hait WN. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990;42(3):155–199. [PubMed] [Google Scholar]
- 118.Booser DJ, Esteva FJ, Rivera E, et al. Phase II study of liposomal annamycin in the treatment of doxorubicin-resistant breast cancer. Cancer Chemother Pharmacol. 2002;50(1):6–8. doi: 10.1007/s00280-002-0464-0. [DOI] [PubMed] [Google Scholar]
- 119.Hu YP, Henry-Toulme N, Robert J. Failure of liposomal encapsulation of doxorubicin to circumvent multidrug resistance in an in vitro model of rat glioblastoma cells. Eur J Cancer. 1995;31A(3):389–394. doi: 10.1016/0959-8049(94)00493-o. [DOI] [PubMed] [Google Scholar]
- 120.Rivera E, Valero V, Esteva FJ, et al. Lack of activity of stealth liposomal doxorubicin in the treatment of patients with anthracycline-resistant breast cancer. Cancer Chemother Pharmacol. 2002;49(4):299–302. doi: 10.1007/s00280-001-0405-3. [DOI] [PubMed] [Google Scholar]
- 121.Wang Y, Eksborg S, Lewensohn R, Lindberg A, Liliemark E. In vitro cellular accumulation and cytotoxicity of liposomal and conventional formulations of daunorubicin and doxorubicin in resistant K562 cells. Anticancer Drugs. 1999;10(10):921–928. doi: 10.1097/00001813-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 122.Li X, Lu WL, Liang GW, et al. Effect of stealthy liposomal topotecan plus amlodipine on the multidrug-resistant leukaemia cells in vitro and xenograft in mice. Eur J Clin Invest. 2006;36(6):409–418. doi: 10.1111/j.1365-2362.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 123.Wu J, Lu Y, Lee A, et al. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J Pharm Pharm Sci. 2007;10(3):350–357. [PubMed] [Google Scholar]
- 124.Wang J, Goh B, Lu W, et al. In vitro cytotoxicity of stealth liposomes co-encapsulating doxorubicin and verapamil on doxorubicin-resistant tumor cells. Biol Pharm Bull. 2005;28(5):822–828. doi: 10.1248/bpb.28.822. [DOI] [PubMed] [Google Scholar]
- 125.Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 126.Manjunath K, Reddy JS, Venkateswarlu V. Solid lipid nanoparticles as drug delivery systems. Methods Find Exp Clin Pharm. 2005;27(2):127–144. doi: 10.1358/mf.2005.27.2.876286. [DOI] [PubMed] [Google Scholar]
- 127.Sahoo SK, Labhasetwar V. Enhanced antiproliferative activity of transferrin-conjugated paclitaxel-loaded nanoparticles is mediated via sustained intracellular drug retention. Mol Pharm. 2005;2(5):373–383. doi: 10.1021/mp050032z. [DOI] [PubMed] [Google Scholar]
- 128.Barraud L, Merle P, Soma E, et al. Increase of doxorubicin sensitivity by doxorubicin-loading into nanoparticles for hepatocellular carcinoma cells in vitro and in vivo. J Hepatol. 2005;42(5):736–743. doi: 10.1016/j.jhep.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 129.Chavanpatil MD, Khdair A, Gerard B, et al. Surfactant-polymer nanoparticles overcome P-glycoprotein-mediated drug efflux. Mol Pharm. 2007;4(5):730–738. doi: 10.1021/mp070024d. [DOI] [PubMed] [Google Scholar]
- 130.Bennis S, Chapey C, Couvreur P, Robert J. Enhanced cytotoxicity of doxorubicin encapsulated in polyisohexylcyanoacrylate nanospheres against multidrug-resistant tumour cells in culture. Eur J Cancer. 1994;30A(1):89–93. doi: 10.1016/s0959-8049(05)80025-5. [DOI] [PubMed] [Google Scholar]
- 131.Colin De Verdiere A, Dubernet C, Nemati F, Poupon MF, Puisieux F, Couvreur P. Uptake of doxorubicin from loaded nanoparticles in multidrug-resistant leukemic murine cells. Cancer Chemother Pharmacol. 1994;33(6):504–508. doi: 10.1007/BF00686509. [DOI] [PubMed] [Google Scholar]
- 132.Wong HL, Rauth AM, Bendayan R, et al. A new polymer–lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm Res. 2006;23(7):1574–1585. doi: 10.1007/s11095-006-0282-x. [DOI] [PubMed] [Google Scholar]
- 133.Astier A, Doat B, Ferrer MJ, et al. Enhancement of adriamycin antitumor activity by its binding with an intracellular sustained-release form, polymethacrylate nanospheres, in U-937 cells. Cancer Res. 1988;48(7):1835–1841. [PubMed] [Google Scholar]
- 134.Colin De Verdiere A, Dubernet C, Nemati F, et al. Reversion of multidrug resistance with polyalkylcyanoacrylate nanoparticles: towards a mechanism of action. Br J Cancer. 1997;76(2):198–205. doi: 10.1038/bjc.1997.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pepin X, Attali L, Domrault C, et al. On the use of ion-pair chromatography to elucidate doxorubicin release mechanism from polyalkylcyanoacrylate nanoparticles at the cellular level. J Chromatogr B Biomed Sci Appl. 1997;702(1–2):181–191. doi: 10.1016/s0378-4347(97)00362-9. [DOI] [PubMed] [Google Scholar]
- 136.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54(5):631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 137.Wong HL, Bendayan R, Rauth AM, Xue HY, Babakhanian K, Wu XY. A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. J Pharmacol Exp Ther. 2006;317(3):1372–1381. doi: 10.1124/jpet.106.101154. [DOI] [PubMed] [Google Scholar]
- 138.Garcion E, Lamprecht A, Heurtault B, et al. A new generation of anticancer, drug-loaded, colloidal vectors reverses multidrug resistance in glioma and reduces tumor progression in rats. Mol Cancer Ther. 2006;5(7):1710–1722. doi: 10.1158/1535-7163.MCT-06-0289. [DOI] [PubMed] [Google Scholar]
- 139.Lamprecht A, Benoit JP. Etoposide nanocarriers suppress glioma cell growth by intracellular drug delivery and simultaneous P-glycoprotein inhibition. J Control Release. 2006;112(2):208–213. doi: 10.1016/j.jconrel.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 140.Dong X, Mattingly CA, Tseng MT, et al. Doxorubicin and paclitaxel-loaded lipid-based nanoparticles overcome multidrug resistance by inhibiting P-glycoprotein and depleting ATP. Cancer Res. 2009;69(9):3918–3926. doi: 10.1158/0008-5472.CAN-08-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koziara JM, Whisman TR, Tseng MT, Mumper RJ. In vivo efficacy of novel paclitaxel nanoparticles in paclitaxel-resistant human colorectal tumors. J Control Release. 2006;112(3):312–319. doi: 10.1016/j.jconrel.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 142.Chavanpatil MD, Patil Y, Panyam J. Susceptibility of nanoparticle-encapsulated paclitaxel to P-glycoprotein-mediated drug efflux. Int J Pharm. 2006;320(1–2):150–156. doi: 10.1016/j.ijpharm.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 143.Omelyanenko V, Kopeckova P, Gentry C, Kopecek J. Targetable HPMA copolymer-adriamycin conjugates. Recognition, internalization and subcellular fate. J Control Release. 1998;53(1–3):25–37. doi: 10.1016/s0168-3659(97)00235-6. [DOI] [PubMed] [Google Scholar]
- 144.Nori A, Kopecek J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv Drug Deliv Rev. 2005;57(4):609–636. doi: 10.1016/j.addr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 145▪.Kopecek J, Kopeckova P, Minko T, Lu Z. HPMA copolymer-anticancer drug conjugates: design, activity and mechanism of action. Eur J Pharm Biopharm. 2000;50(1):61–81. doi: 10.1016/s0939-6411(00)00075-8. Detailed review on N-(2-hydroxypropyl) methacrylamide–drug conjugates to overcome drug resistance. [DOI] [PubMed] [Google Scholar]
- 146.Minko T, Kopeckova P, Kopecek J. Chronic exposure to HPMA copolymer-bound adriamycin does not induce multidrug resistance in a human ovarian carcinoma cell line. J Control Release. 1999;59(2):133–148. doi: 10.1016/s0168-3659(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 147.Minko T, Kopeckova P, Kopecek J. Comparison of the anticancer effect of free and HPMA copolymer-bound adriamycin in human ovarian carcinoma cells. Pharm Res. 1999;16(7):986–996. doi: 10.1023/a:1018959029186. [DOI] [PubMed] [Google Scholar]
- 148.Minko T, Kopeckova P, Kopecek J. Preliminary evaluation of caspases-dependent apoptosis signaling pathways of free and HPMA copolymer-bound doxorubicin in human ovarian carcinoma cells. J Control Release. 2001;71(3):227–237. doi: 10.1016/s0168-3659(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 149▪▪.Minko T, Kopeckova P, Kopecek J. Efficacy of the chemotherapeutic action of HPMA copolymer-bound doxorubicin in a solid tumor model of ovarian carcinoma. Int J Cancer. 2000;86(1):108–117. doi: 10.1002/(sici)1097-0215(20000401)86:1<108::aid-ijc17>3.0.co;2-8. Reports the in vivo mechanistic studies on a resistant tumor model treated with N-(2-hydroxypropyl)methacrylamide–drug conjugates. [DOI] [PubMed] [Google Scholar]
- 150.Piskin, Kaitian X, Denkbas EB, Kucukyavuz Z. Novel PDLLA/PEG copolymer micelles as drug carriers. J Biomater Sci. 1995;7(4):359–373. doi: 10.1163/156856295x00373. [DOI] [PubMed] [Google Scholar]
- 151.La SB, Okano T, Kataoka K. Preparation and characterization of the micelle-forming polymeric drug indomethacin-incorporated poly(ethylene oxide)-poly(β-benzyl L-aspartate) block copolymer micelles. J Pharm Sci. 1996;85(1):85–90. doi: 10.1021/js950204r. [DOI] [PubMed] [Google Scholar]
- 152.Alakhov VY, Moskaleva E, Batrakova EV, Kabanov AV. Hypersensitization of multidrug resistant human ovarian carcinoma cells by Pluronic P85 block copolymer. Bioconjug Chem. 1996;7(2):209–216. doi: 10.1021/bc950093n. [DOI] [PubMed] [Google Scholar]
- 153.Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130(2):98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154▪.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers for overcoming drug resistance in cancer. Adv Drug Deliv Rev. 2002;54(5):759–779. doi: 10.1016/s0169-409x(02)00047-9. Detailed review on how Pluronic micelles overcome resistance, including the mechanisms. [DOI] [PubMed] [Google Scholar]
- 155.Alakhov VY, Klinski E, Li S, et al. Block copolymer-based formulation of doxorubicin. From cell screen to clinical trials. Colloids Surf B Biointerfaces. 1999;16:113–134. [Google Scholar]
- 156.Batrakova EV, Li S, Li Y, Alakhov VY, Elmquist WF, Kabanov AV. Distribution kinetics of a micelle-forming block copolymer Pluronic P85. J Control Release. 2004;100(3):389–397. doi: 10.1016/j.jconrel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 157.Pruitt JD, Husseini G, Rapoport N, Pitt WG. Stabilization of Pluronic P-105 micelles with an interpenetrating network of N,N-diethylacrylamide. Macromolecules. 2000;33:9306–9309. [Google Scholar]
- 158.Yang TF, Chen CN, Chen MC, Lai CH, Liang HF, Sung HW. Shell-crosslinked Pluronic l121 micelles as a drug delivery vehicle. Biomaterials. 2007;28(4):725–734. doi: 10.1016/j.biomaterials.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 159.Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of Pluronic effect on P-glycoprotein efflux system in blood–brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299(2):483–493. [PubMed] [Google Scholar]
- 160.Batrakova EV, Li S, Li Y, Alakhov VY, Kabanov AV. Effect of Pluronic P85 on ATPase activity of drug efflux transporters. Pharm Res. 2004;21(12):2226–2233. doi: 10.1007/s11095-004-7675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Batrakova EV, Li S, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: selective energy depletion. Br J Cancer. 2001;85(12):1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Minko T, Batrakova EV, Li S, et al. Pluronic block copolymers alter apoptotic signal transduction of doxorubicin in drug-resistant cancer cells. J Control Release. 2005;105(3):269–278. doi: 10.1016/j.jconrel.2005.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Batrakova EV, Li S, Alakhov VY, Elmquist WF, Miller DW, Kabanov AV. Sensitization of cells overexpressing multidrug-resistant proteins by Pluronic P85. Pharm Res. 2003;20(10):1581–1590. doi: 10.1023/a:1026179132599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Alakhova DY, Rapoport NY, Batrakova EV, et al. Differential metabolic responses to Pluronic in MDR and non-MDR cells: a novel pathway for chemosensitization of drug resistant cancers. J Control Release. 2009;142(1):89–100. doi: 10.1016/j.jconrel.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 201.WHO: cancer. 2008 www.who.int/cancer/en/