Abstract
Objective
Radioimmunotherapy of cancer with radiolabeled antibodies has shown promise. We evaluated an anti-CD25 monoclonal Antibody, 7G7/B6, armed with 90Y as a potential radioimmunotherapeutic agent for CD25-expressing lymphomas.
Materials and Methods
The lymphoma model was established by subcutaneous injection of 1 × 107 SUDHL-1 cells into nude mice. The biodistribution of 111In-7G7/B6 and therapeutic studies with 90Y-7G7/B6 were performed in the tumor-bearing mice.
Results
Therapy using 90Y-7G7/B6 prolonged survival of the SUDHL-1 lymphoma-bearing mice significantly, as compared with either untreated mice or the mice treated with 90Y-11F11, a radiolabeled isotype-matched control antibody (p < 0.001). All of the mice in the control and the 90Y-11F11 treatment groups died by days 18 and 24, respectively. In contrast, 30% of the mice in the low-dose group (75 μCi of 90Y-7G7/B6/mouse) and 75% in the high-dose group (150 μCi of 90Y-7G7/B6/mouse) became tumor free and remained healthy for greater than 6 months.
Conclusions
Our findings suggested that 90Y-7G7/B6 is a potentially useful radioimmunotherapeutic agent for the treatment of patients with CD25-expressing lymphomas.
Key words: radioimmunotherapy, monoclonal antibody, CD25, lymphoma, β-emitter
Introduction
Harnessing the immune system to treat cancer is a major goal of immunotherapy. Passive immunotherapy using monoclonal antibodies (mAbs) have come of age, with 21 therapeutic mAbs approved by the U.S. Food and Drug Administration (FDA), including eight directed toward the treatment of cancer.1 A limitation in the use of certain mAbs is that they are often poor cytocidal agents. Therefore, mAbs have been armed with cytokines, chemotherapeutic agents, toxins, and radionuclides to augment their efficacy as tumor cytotoxic agents.2–4 A pivotal issue to be addressed in all systemic radioimmunotherapy trials is the selection of a mAb that targets an antigen expressed by a tumor and thereby defines the type of malignancy chosen as the target for radioimmunotherapy. In the present study, we have chosen an epitope of human interleukin (IL)-2Rα identified by the mAb, 7G7/B6, as our target for radioimmunotherapy. The scientific basis for this choice of the α-subunit of the IL-2R is that virtually no normal resting cells, with the exception of CD4+ CD25+ T regs, express this receptor subunit, whereas this receptor is expressed by a high proportion of the abnormal cells in certain forms of lymphoid neoplasms, including adult T-cell leukemia/lymphoma (ATL), anaplastic large-cell lymphoma (ALCL), and Hodgkin's lymphoma.5–7 We reported on the production of the murine anti-Tac mAb (MAT) that identifies the human IL-2Rα subunit and blocks the interaction of IL-2 with its receptor.8 The unmodified MAT was utilized in the treatment of patients with ATL, a malignancy of mature CD4+ CD25+ suppressor T-lymphocytes, and 7 of the 19 patients responded to therapy.7 To augment the efficacy, the antibody was armed with 90Y. In a subsequent study, 9 of 16 of the evaluable patients with ATL responded with a partial or complete response to 90Y-MAT.9 In a recent study, patients with relapsed or refractory Hodgkin's lymphoma were treated with repeated infusions of 15 mCi of 90Y-humanized anti-Tac (daclizumab). In 30 Hodgkin's lymphoma patients treated with 90Y-daclizumab, there were 6 with progressive disease, 5 with stable disease, 7 with partial responses, and 12 with complete responses (Janik JE and Waldmann TA, unpublished observations). Although these results were very encouraging, neither the daclizumab nor 90Y-daclizumab as monotherapy was curative for ATL.9 A paradigm is emerging that for cancer therapy, the addition of two therapeutic agents that function via different mechanisms may be greater than additive in their cytotoxic action leading to malignant cell death.10–16 In our previous therapeutic trials, we obtained improved therapeutic efficacy by combining daclizumab at receptor-saturating doses with radioimmunotherapy in an ATL model.14,15,17 Our future goal is to use two mAbs directed toward different epitopes of the CD25 antigen: one to block IL-2 binding to yield antibody-dependent cellular cytotoxicity (ADCC) and cytokine deprivation-mediated cell death and the other armed with a strong β-emitting radionuclide, 90Y, to provide tumor cytoreduction by irradiation mediated by therapeutic radionuclides delivered at high specific activity by the mAb to leukemic cell surfaces. The aim of the present study was to determine if 90Y-7G7/B6, a mAb directed toward an epitope on CD25 other than that defined by daclizumab, was effective in the treatment of a lymphoma model to provide the scientific basis for the subsequent use of 90Y-7G7/B6 in a combination regimen with saturating doses of daclizumab.
Materials and Methods
Monoclonal antibodies
7G7/B6 is a mouse IgG2a mAb directed toward an epitope of the IL-2Rα subunit other than the IL-2 binding site that is identified by daclizumab.8 The 7G7/B6 was purified from supernatants of a hybridoma (American Type Culture Collection, Manassas, VA), using ImmunoPure Protein A columns (Pierce, Rockford, IL). 11F11 is also a mouse IgG2a, which recognizes the Shiga-like toxin II of enterohemorrhagic Escherichia,18 and it was used in this study as an isotype-matched control antibody. The hybridoma-producing 11F11 mAb was obtained from Alison D. O'Brien, Ph.D. (Department of Microbiology, Uniformed Services University of Health Science, Bethesda, MD). UPC10, a murine IgG2a, which does not recognize resting or activated peripheral blood mononuclear cells or cell lines including T-cell, B-cell, and monocyte populations, was used as a nonspecific agent to block the nonspecific binding of radiolabeled 7G7/B6 in liver and spleen in nude mice. The plasmocytoma-producing UPC10 was obtained from Michael Potter (National Cancer Institute, Bethesda, MD). Daclizumab was obtained from Hoffmann-La Roche (Nutley, NJ).
Radiolabeling of monoclonal antibodies
The 7G7/B6 and 11F11 were conjugated with 2-(p-isothiocyanato-benzyl)-cyclohexyl-diethylenetriaminepenta-acetic acid (CHX-A”). The conjugation of the antibodies with CHX-A” was performed as previously described.19,20 7G7/B6-CHX-A” and 11F11-CHX-A” were labeled with 111In (Amersham Corporation, Arlington Heights, IL) at specific activities of 3–5 μCi/μg (0.111–0.185 MBq/μg) for biodistribution experiments and with 90Y (NEN, Boston, MA) at specific activities of 10–30 μCi/μg (0.37–1.11 MBq/μg) for therapeutic studies, as described previously.19,20 The 7G7/B6 was labeled with 125I for the internalization study at a specific activity of 12 μCi/μg (0.444 MBq/μg) by using the chloramine-T method.
Tumor cell lines and mouse model
Kit225IG3 is a leukemic T-cell line, which expresses CD25 on the cell surfaces. We used this cell line for the bindability assay. SUDHL-1 (a kind gift from S. Morris, St. Jude Children's Research Hospital, Memphis, TN) is a human anaplastic large-cell lymphoma (ALCL) cell line, which also expresses CD25 on the cell surfaces. The tumor model was established by the subcutaneous (S.C.) injection of 1 × 107 SUDHL-1 cells into the right flank of female nude mice.15 Both cell lines were maintained in RPMI-1640 containing 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 μg/mL of streptomycin in an atmosphere containing 5% CO2. All animal experiments were approved by the National Cancer Institute Animal Care and Use Committee (NCI ACUC) and were performed in accordance with the NCI ACUC guidelines. NCI-Frederick is accredited by AAALAC international and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accord with the procedures outlined in the “Guide for Care and Use of Laboratory Animals” (National Research Council; 1996; National Academy Press; Washington, DC.)
Immunoreactivity assay
The immunoreactivity of 90Y-7G7/B6 was evaluated as described previously,21 using the CD25-positive kit225IG3 cells, and compared with 111In-7G7/B6. Briefly, radiolabeled 7G7/B6 (5 ng) was incubated with an increasing number of kit225IG3 cells (2 × 104–1 × 107) with or without unlabeled 7G7/B6 (25 μg/tube) inhibition at 4°C for 1 hour. After centrifugation, the supernatant was aspirated and the radioactivity bound to the cells was quantitated in a γ-counter (Wallac, Turku, Finland).
Internalization and modulation study
The internalization of 125I-labeled 7G7/B6 by SUDHL-1 and kit225IG3 cells was evaluated by using an in vitro method described previously.22 The modulation of CD25 from SUDHL-1 and kit225IG3 cells was analyzed by flow cytometry. The cells were cultured with medium alone or with medium containing unmodified 7G7/B6 at a concentration of 20 μg/mL for 24 hours. Then, the cells were washed and aliquots of 0.5–1 × 106 cells were incubated with or without daclizumab (1 μg/50 μL) on ice for 45 minutes. The cells were washed and then stained with a Flourescein isothiocyanate (FITC)-labeled antihuman IgG antibody (BD Biosciences, San Jose, CA). After washing, the cells were analyzed by using a FACScan flow cytometry (Becton Dickinson, San Jose, CA).
Biodistribution study
SUDHL-1 lymphoma-bearing mice were injected intravenously (i.v.) with 10 μg of 111In-7G7/B6 or 111In-11F11. At intervals after injection, groups of 4 or 5 mice were killed, and the tumors and organs were taken, weighed, and counted in a γ-counter. The percentage of the injected dose per gram of tissue (%ID/g) was calculated for each organ and normalized to a 20-g mouse. All mice were coinjected with 400 μg of UPC10 to block the nonspecific binding of mouse IgG2a in the liver and spleen of nude mice.
Definition of maximum tolerated dose
Prior to the initiation of radioimmunotherapy, the maximum tolerated dose (MTD) of 90Y-7G7/B6 was determined in healthy nude mice without tumor. Four groups of 5 healthy nude mice received doses of 100, 150, 200, and 300 μCi (3.7, 5.6, 7.4, and 11.1 MBq) of 90Y-7G7/B6, respectively. All of the mice received a coinjection of 400 μg of UPC10. The body weights and the complete blood counts were measured before and after treatment (initially at weekly and, subsequently, at monthly intervals).
Therapeutic study
The therapeutic study was performed in SUDHL-1-bearing nude mice. Groups of 10 mice were injected i.v. with 75 or 150 μCi (2.78 or 5.55 MBq) of 90Y-7G7/B6 or 75 μCi (2.78 MBq) of 90Y-11F11 or 200 μL of phosphate-buffered saline (PBS). The tumor progression was monitored by measuring tumor size in two orthogonal dimensions twice per week for 3 weeks after treatment and then once per week. The tumor volume was calculated by using the formula ½ (long dimension) (short dimension)2. Body weight and survival of the SUDHL-1-bearing mice were monitored throughout the experiment. The therapeutic study was repeated with the PBS control and 150 μCi (5.55 MBq) of 90Y-7G7/B6 in the same tumor model, and the results from these two sets of studies were pooled together. The mice in the 90Y-7G7/B6 and 90Y-11F11 groups received a coinjection of 400 μg of UPC10.
Statistical analysis
Tumor volumes at different time points for the different treatment groups were analyzed by using the t-test for unpaired data. Statistical significance of differences in survival of the mice in different treatment groups was determined by the log-rank test, using the GraphPad Prism program (GraphPad Software, San Diego, CA).
Results
Immunoreactivity of 90Y-7G7/B6
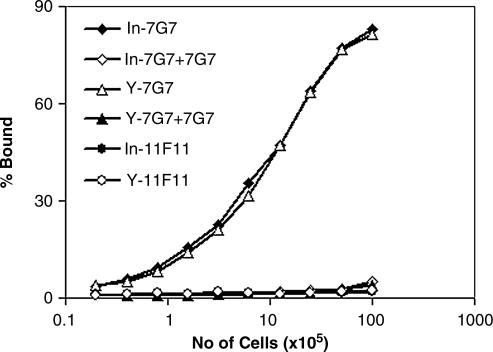
For a radiolabeled antibody to be effective, the labeling procedure should not compromise antibody specificity. We tested the bindability of 90Y-7G7/B6 and compared it with 111In-7G7/B6 in vitro. The proportion of 90Y-7G7/B6 that bound to kit225IG3 cells was very similar to that observed with 111In-7G7/B6 (Fig. 1). The maximal bindings for 90Y-7G7/B6 and 111In-7G7/B6 were greater than 80% of the added radiolabeled antibodies. The bindings were specifically inhibited by unmodified 7G7/B6 (Fig. 1). Both 111In-11F11 and 90Y-11F11 did not bind to kit225IG3 cells.
FIG. 1.
Immunoreactivity of 90Y-7G7/B6 and 111In-7G7/B6. The cell-binding assay of the radiolabels was performed as described in Materials and Methods. Both 90Y-7G7/B6 and 111In-7G7/B6 bound to CD25-positive kit225IG3 cells similarly and the bindings were inhibited by the addition of a 1000-fold greater concentration of unmodified 7G7/B6. 90Y-11F11 and 111In-11F11 did not bind to kit225IG3 cells.
Internalization of 125I-labeled 7G7/B6 and modulation of CD25
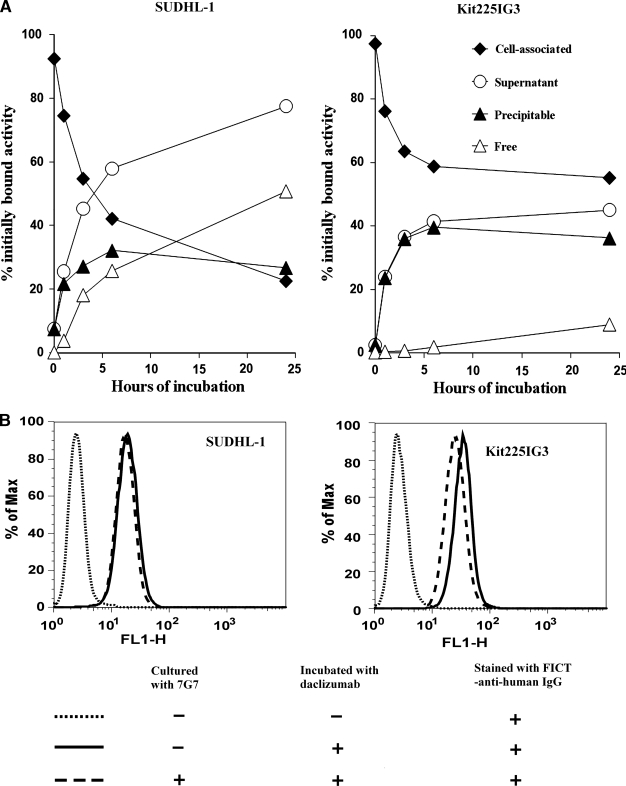
It is important for the combination regimen of 90Y-labeled 7G7/B6 with unmodified daclizumab that the administered 90Y-labeled 7G7/B6 does not cause the modulation of the CD25 from the cell surface and does not affect the binding of daclizumab to the tumor cells. The internalization of 125I-labeled 7G7/B6 by SUDHL-1 and kit225IG3 cells was investigated. After surface labeling, the cell-associated radioactivity of both cell lines was more than 90% initially (Fig. 2A). As the cell-associated radioactivity decreased with time, the radioactivity in supernatant increased accordingly. The internalization rates of 125I-labeled 7G7/B6 by the two cell lines were different (Fig. 2A). At 24 hours after incubation, more than 50% of initially bound activity was free iodine in the supernatant with SUDHL-1 cells, which reflects the release of iodine from the cells after internalization and processing of the radiolabeled 7G7/B6 (Fig. 2A). In contrast, the amount of free iodine in the supernatant with kit225IG3 cells was less than 10% at 24 hours of incubation (Fig. 2A). However, incubation of SUDHL-1 and kit225IG3 cells with medium containing unmodified 7G7/B6, at a concentration of 20 μg/mL for 24 hours, did not cause a significant modulation of CD25 from the cell surface and did not affect the binding of daclizumab to the cells (Fig. 2B).
FIG. 2.
Internalization of 125I-labeled 7G7/B6 and modulation of CD25. (A) Internalization of 125I-7G7/B6 by SUDHL-1 and kit225IG3 cells. Cells were incubated with 125I-7G7/B6 for 1 hour at 4°C for surface labeling. After washing, the cells were incubated at 37°C for 0, 1, 3, 6, and 24 hours. The relative percentages of radioactivity associated with cells and in the supernatant, which was further differentiated by methanol precipitation as free and precipitable, are depicted as a function of time. The data represent the mean of triplicates. (B) Flow cytometric analysis showed that the binding of daclizumab to SUDHL-1 and kit225IG3 cells was not affected meaningfully by incubation of the cells with unmodified 7G7/B6 at a concentration of 20 μg/mL for 24 hours.
Biodistribution
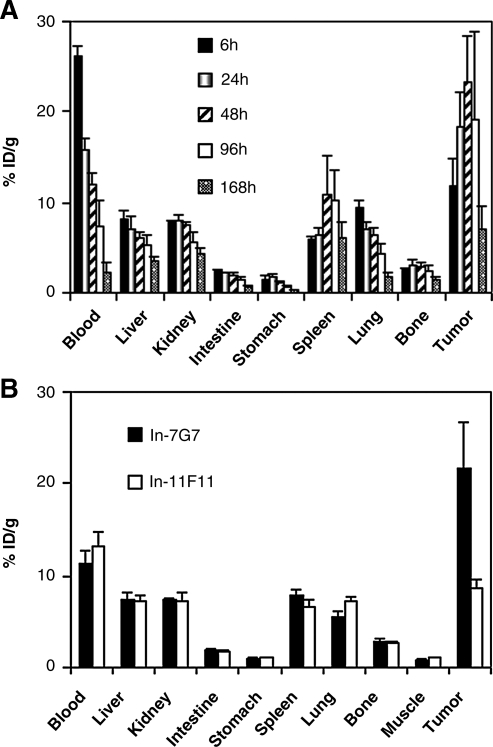
Biodistribution data of 111In-7G7/B6 and 111In-11F11 in SUDHL-1-bearing mice are shown in Figure 3. After the injection of 111In-7G7/B6, the blood concentration of radioactivity reduced with time, while the tumor uptake of radioactivity increased with the peak uptake at 48 h (Fig. 3A). Compared with 111In-7G7/B6, 111In-11F11 showed a similar tissue distribution, except for the much lower tumor uptake of radioactivity (Fig. 3B).
FIG. 3.
Biodistribution of 111In-7G7/B6 and 111In-11F11 in SUDHL-1-bearing nude mice. (A) Biodistribution of 111In-7G7/B6 at different time points after injection. (B) Comparison of the biodistributions of 111In-7G7/B6 and 111In-11F11 at 48 hours after injection. After the injection of 111In-7G7/B6, the blood concentration of radioactivity reduced with time, while the tumor uptake of radioactivity increased with the peak uptake at 48 hours. Compared with 111In-7G7/B6, 111In-11F11 showed a similar tissue distribution, except for the much lower tumor uptake of radioactivity. The data are expressed as the percent injected dose per gram of tissue. Bars represent the mean ± standard deviation.
Definition of MTD
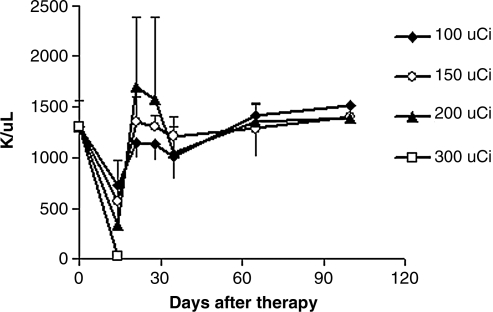
In the experiment defining the MTD of 90Y-7G7/B6 in normal nude mice, the animal body weights did not show significant changes with doses of 200 μCi or less of 90Y-7G7/B6 (data not shown). The platelet counts were reduced in a dose-related manner (Fig. 4). The nadir occurred at 2 weeks and recovered 3 weeks after radiation treatment. The white blood cell (WBC) counts showed a similar pattern as the platelet counts (data not shown). Three (3) of 5 mice in the 300-μCi group died approximately 2 weeks after the treatment. The mice that received 200 μCi or less of 90Y-7G7/B6 remained healthy for greater than the 6-month period of observation.
FIG. 4.
Platelet counts were measured in normal nude mice that received different doses of 90Y-7G7/B6. The platelet counts were reduced in a dose-related manner. The nadir occurred at 2 weeks with recovery at 3 weeks after the administration of 90Y-7G7/B6. Three (3) of 5 mice in the 300-μCi group died approximately 2 weeks after treatment. The mice receiving 200 μCi or less of 90Y-7G7/B6 remained healthy for greater than 6 months.
Therapeutic study
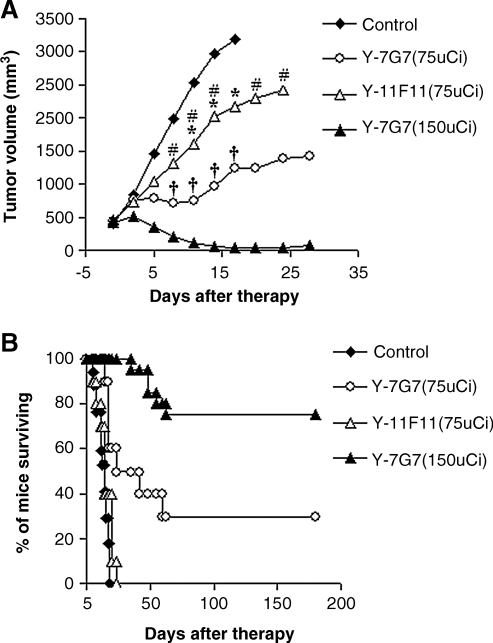
Radioimmunotherapy with 90Y-7G7/B6 was performed in SUDHL-1 lymphoma-bearing mice. SUDHL-1 tumors in the control group grew rapidly, from 0.4 cm3 at the initiation of the experiment, to ≥2 cm3 within 3 weeks (Fig. 5A), and these mice were sacrificed according to our animal protocol. Treatment with 75 μCi of 90Y-7G7/B6 inhibited the tumor growth significantly, when compared with the control group (Fig. 5A; p < 0.001). By increasing the radiation dose from 75 to 150 μCi, the tumor growth was halted and the tumor volume decreased (Fig. 5A). Further, survival of the mice in the treatment groups was significantly prolonged, when compared with the control groups (Fig. 5B; p < 0.01). Three (3) of the 10 mice in the 75 μCi of 90Y-7G7/B6 group and 75% of the mice (15 of 20) in the 150 μCi of 90Y-7G7/B6 group became tumor free and remained healthy for greater than 6 months (Fig. 5B). To confirm the specificity of the therapeutic effect of 90Y-7G7/B6, we used the irrelevant mouse IgG2a monoclonal antibody, 11F11, armed with 90Y in the same model. Although the treatment with 75 μCi of 90Y-11F11 slowed down the tumor growth, when compared with the control group (Fig. 5A; p < 0.01), survival of the mice in this group was not prolonged significantly, when compared with the control group (p > 0.1). There were significant differences in tumor size (p < 0.05) and survival (p < 0.001) of the mice between the 90Y-7G7/B6 (75 μCi) and 90Y-11F11 (75 μCi) groups.
FIG. 5.
Therapeutic study of 90Y-7G7/B6 in the SUDHL-1 model. (A) Tumor volume. (B) Kaplan-Meier survival plot of the SUDHL-1-bearing nude mice. Treatment with 90Y-7G7/B6 inhibited the SUDHL-1 lymphoma growth significantly, as seen by tumor size and prolonged survival of the SUDHL-1-bearing mice, when compared with the control and 90Y-11F11 groups. *p < 0.01, †p < 0.001, compared with the control group; #p < 0.05, compared with the 90Y-7G7/B6 (75-μCi) group.
Discussion
In the present study, 90Y-7G7/B6 showed efficacy as a single radioimmunotherapeutic agent in the treatment of SUDHL-1 lymphoma-bearing mice. The 90Y-7G7/B6 treatment prolonged the survival of the SUDHL-1 lymphoma-bearing mice significantly, as compared with either the untreated mice or mice treated with 90Y-11F11, a radiolabeled isotype-matched control antibody. A pivotal issue in defining an optimal radioimmunotherapeutic agent is to consider the nature of the radionuclide used in relation to the nature of the disease being treated. An α-emitting radionuclide, because of its high-linear energy transfer and short-path length, is superior to a β-emitting radionuclide for the treatment of small tumors, including micrometastases, individual tumor cells, and leukemia.14,15,17,23–27 However, such α-emitters are not effective against large tumor masses.27 Previously, we demonstrated that 211At-7G7/B6 was effective in the MET-1 murine leukemia model of human ATL but was ineffective in treating the SUDHL-1 lymphoma model.17,27 In contrast, β-emitters may be preferable in the treatment of large tumor masses.2,15,28 Therapeutic benefits of β-emitters result from “crossfire.” Therefore, one of the advantages of β-emitters is their ability to bypass tumor-antigen heterogeneity and poor penetration of mAbs into tumor masses. In clinical situations such as Hodgkin's lymphoma, β-emitting radionuclides linked to mAbs eliminated nontargeted tumor cells through the crossfire effect emanating from neighboring antigen-bearing cells that have been targeted by the radiolabeled mAb (Janik JE and Waldmann TA, unpublished observations).
An additional pivotal issue to be addressed in all systemic radioimmunotherapy trials is the selection of the mAb that targets the tumor and thereby defines the target for radioimmunotherapy. In the present study, we have chosen an epitope of human IL-2Rα identified by 7G7/B6 as our target for radioimmunotherapy. The 7G7/B6 mAb recognizes an epitope on IL-2Rα other than that identified by daclizumab.8 Therefore, the two non-cross-competing mAbs could be combined for treatment of CD25-expressing leukemias and lymphomas. In our previous therapeutic trials, we obtained therapeutic efficacy by employing daclizumab in IL-2Rα receptor-saturating doses.16,29,30 Further, we showed efficacy in clinical trials that used 90Y-daclizumab in the treatment of lymphoma (Janik JE and Waldmann TA, unpublished observations). However, with a single-radiolabeled mAb it is difficult to obtain the complementary actions of receptor-saturating doses of daclizumab to yield ADCC and IL-2 deprivation-mediated apoptotic leukemic cell death in conjunction with the tumor cytoreduction provided by irradiation mediated by therapeutic radionuclides (e.g., 90Y) delivered at high specific activity by the mAb to the leukemic cell surfaces. To address this limitation inherent in systemic radioimmunotherapy, our long-term goal is to use daclizumab at receptor-saturating doses in conjunction with small quantities of 7G7/B6 armed at high specific activity with 90Y for the treatment of CD25-expressing leukemia and lymphomas.
Conclusions
The encouraging results of the present study that involved a single dose of 90Y-7G7/B6 in the CD25-expressing SUDHL-1 lymphoma model suggest that 90Y-7G7/B6 is a potential therapeutic agent for the treatment of CD25-expressing lymphomas. Further, the present study, taken in conjunction with previous studies on the effectiveness of receptor-saturating doses of daclizumab, provides the scientific basis for the subsequent combination of 90Y-7G7/B6 with receptor-saturating doses of daclizumab in human clinical trials to provide the desired two independent cytotoxic actions for the treatment of CD25-expressing leukemias and lymphomas.
Acknowledgments
The authors thank Nhat Le for the labeling of 90Y-7G7/B6. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project has been funded, in whole or in part, with federal funds from the National Cancer Institute, NIH (under contract N01-CO-12400). The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Disclosure Statement
I confirm that no competing Financial conflicts exist—Thomas A. Waldmann, M.D.
References
- 1.Waldmann TA. Effective cancer therapy through immunomodulation. Annu Rev Med. 2006;57:65. doi: 10.1146/annurev.med.56.082103.104549. [DOI] [PubMed] [Google Scholar]
- 2.Milenic DE. Brady ED. Brechbiel MW. Antibody-targeted radiation cancer therapy. Nat Rev Drug Discov. 2004;3:488. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann T. ABCs of radioisotopes used for radioimmunotherapy: Alpha- and beta-emitters. Leuk Lymph. 2003;44(Suppl 3):S107. doi: 10.1080/10428190310001623685. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann TA. Immunotherapy: Past, present and future. Nat Med. 2003;9:269. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 5.Janik JE. Morris JC. Pittaluga S, et al. Elevated serum-soluble interleukin-2 receptor levels in patients with anaplastic large cell lymphoma. Blood. 2004;104:3355. doi: 10.1182/blood-2003-11-3922. [DOI] [PubMed] [Google Scholar]
- 6.Morris JC. Waldmann TA. Advances in interleukin-2-receptor-targeted treatment. Ann Rheum Dis. 2000;59(Suppl 1):i109. doi: 10.1136/ard.59.suppl_1.i109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldmann TA. White JD. Goldman CK, et al. The interleukin-2 receptor: A target for monoclonal antibody treatment of human T-cell lymphotrophic virus I-induced adult T-cell leukemia. Blood. 1993;82:1701. [PubMed] [Google Scholar]
- 8.Rubin LA. Kurman CC. Biddison WE, et al. A monoclonal antibody, 7G7/B6, binds to an epitope on the human interleukin-2 (IL-2) receptor that is distinct from that recognized by IL-2 or anti-Tac. Hybridoma. 1985;4:91. doi: 10.1089/hyb.1985.4.91. [DOI] [PubMed] [Google Scholar]
- 9.Waldmann TA. White JD. Carrasquillo JA, et al. Radioimmunotherapy of interleukin-2R alpha-expressing adult T-cell leukemia with yttrium-90-labeled anti-Tac. Blood. 1995;86:4063. [PubMed] [Google Scholar]
- 10.Coiffier B. Lepage E. Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. NEJM. 2002;346:235. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 11.Slamon DJ. Leyland-Jones B. Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. NEJM. 2001;344:783. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 12.Tan C. Waldmann TA. Proteasome inhibitor PS-341, a potential therapeutic agent for adult T- cell leukemia. Cancer Res. 2002;62:1083. [PubMed] [Google Scholar]
- 13.Wu K. Wang C. D'Amico M, et al. Flavopiridol and trastuzumab synergistically inhibit proliferation of breast cancer cells: Association with selective cooperative inhibition of cyclin D1-dependent kinase and Akt signaling pathways. Mol Cancer Ther. 2002;1:695. [PubMed] [Google Scholar]
- 14.Zhang M. Yao Z. Garmestani K, et al. Pretargeting radioimmunotherapy of a murine model of adult T-cell leukemia with the alpha-emitting radionuclide, bismuth 213. Blood. 2002;100:208. doi: 10.1182/blood-2002-01-0107. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M. Zhang Z. Garmestani K, et al. Pretarget radiotherapy with an anti-CD25 antibody-streptavidin fusion protein was effective in therapy of leukemia/lymphoma xenografts. Proc Natl Acad Sci U S A. 2003;100:1891. doi: 10.1073/pnas.0437788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M. Zhang Z. Goldman CK, et al. Combination therapy for adult T-cell leukemia-xenografted mice: Flavopiridol and anti-CD25 monoclonal antibody. Blood. 2005;105:1231. doi: 10.1182/blood-2004-05-1709. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z. Zhang M. Garmestani K, et al. Effective treatment of a murine model of adult T-cell leukemia using 211At-7G7/B6 and its combination with unmodified anti-Tac (daclizumab) directed toward CD25. Blood. 2006;108:1007. doi: 10.1182/blood-2005-11-4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perera LP. Marques LR. O'Brien AD. Isolation and characterization of monoclonal antibodies to Shiga-like toxin II of enterohemorrhagic Escherichia coli and use of the monoclonal antibodies in a colony enzyme-linked immunosorbent assay. J Clin Microbiol. 1988;26:2127. doi: 10.1128/jcm.26.10.2127-2131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi H. Wu C. Yoo TM, et al. Evaluation of the in vivo biodistribution of yttrium-labeled isomers of CHX-DTPA-conjugated monoclonal antibodies. J Nucl Med. 1998;39:829. [PubMed] [Google Scholar]
- 20.Milenic DE. Garmestani K. Chappell LL, et al. In vivo comparison of macrocyclic and acyclic ligands for radiolabeling of monoclonal antibodies with 177Lu for radioimmunotherapeutic applications. Nucl Med Biol. 2002;29:431. doi: 10.1016/s0969-8051(02)00294-9. [DOI] [PubMed] [Google Scholar]
- 21.Yao Z. Sakahara H. Zhang M, et al. Radioimmunoimaging of colon cancer xenografts with anti-Tn monoclonal antibody. Nucl Med Biol. 1995;22:199. doi: 10.1016/0969-8051(94)00092-x. [DOI] [PubMed] [Google Scholar]
- 22.Yao Z. Garmestani K. Wong KJ, et al. Comparative cellular catabolism and retention of astatine-, bismuth-, and lead-radiolabeled internalizing monoclonal antibody. J Nucl Med. 2001;42:1538. [PubMed] [Google Scholar]
- 23.Jurcic JG. Larson SM. Sgouros G, et al. Targeted alpha-particle immunotherapy for myeloid leukemia. Blood. 2002;100:1233. [PubMed] [Google Scholar]
- 24.Macklis RM. Kinsey BM. Kassis AI, et al. Radioimmunotherapy with alpha-particle-emitting immunoconjugates. Science. 1988;240:1024. doi: 10.1126/science.2897133. [DOI] [PubMed] [Google Scholar]
- 25.McDevitt MR. Ma D. Lai LT, et al. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294:1537. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 26.McDevitt MR. Sgouros G. Finn RD, et al. Radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med. 1998;25:1341. doi: 10.1007/s002590050306. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M. Yao Z. Zhang Z, et al. The anti-CD25 monoclonal antibody 7G7/B6, armed with the {alpha}-emitter 211At, provides effective radioimmunotherapy for a murine model of leukemia. Cancer Res. 2006;66:8227. doi: 10.1158/0008-5472.CAN-06-1189. [DOI] [PubMed] [Google Scholar]
- 28.Yao Z. Zhang M. Axworthy DB, et al. Radioimmunotherapy of A431 xenografted mice with pretargeted B3 antibody-streptavidin and 90Y-labeled 1,4,7,10-tetraazacyclododecane-N,N′,N″,N″′-tetraacetic acid (DOTA)-biotin. Cancer Res. 2002;62:5755. [PubMed] [Google Scholar]
- 29.Phillips KE. Herring B. Wilson LA, et al. IL-2R alpha-directed monoclonal antibodies provide effective therapy in a murine model of adult T-cell leukemia by a mechanism other than blockade of IL-2/IL-2R alpha interaction. Cancer Res. 2000;60:6977. [PubMed] [Google Scholar]
- 30.Zhang M. Zhang Z. Garmestani K, et al. Activating Fc receptors are required for antitumor efficacy of the antibodies directed toward CD25 in a murine model of adult T-cell leukemia. Cancer Res. 2004;64:5825. doi: 10.1158/0008-5472.CAN-04-1088. [DOI] [PubMed] [Google Scholar]