Abstract
Recent studies utilizing small molecule antagonists have revealed that the poly(ADP-ribose) polymerases (PARPs) Tankyrase 1 and 2 are critical regulators of canonical Wnt signaling in some cellular contexts. However, the absence of any activity during zebrafish embryogenesis suggested that the tankyrases may not be general/core components of the Wnt pathway. Here we show that Tnks1 and 2 are broadly expressed during mouse development and are essential during kidney and lung development. In the kidney, blockage of tankyrase activity phenocopies the effect of blocking production of all Wnt ligands. Tankyrase inhibition can be rescued by activation of β-catenin demonstrating its specificity for the Wnt pathway. In addition, treatment with tankyrase inhibitors appears to be completely reversible in some cell types. These studies suggest that the tankyrases are core components of the canonical Wnt pathway and their inhibitors should enjoy broad usage as antagonists of Wnt signaling.
Introduction
Wnts encode a family of secreted glycoproteins that play multiple roles in normal metazoan development. After binding to one of its receptors, the Wnt signal can be transduced down one of multiple different pathways that are roughly divided into canonical and non-canonical branches. The canonical branch utilizes beta-catenin as a transcriptional regulator while the non-canonical branches are beta-catenin independent. In the absence of ligand, cytoplasmic beta-catenin is destabilized by a number of proteins collectively known as the beta-catenin destruction complex. This complex includes the kinases glycogen synthase kinase (Gsk) 3beta and casein kinase (Csk), the scaffolding proteins Axin1 and 2 and the microtubule binding protein adenomatous polyposis coli (APC). Phosphorylation of the beta-catenin protein by the destruction complex targets the protein for degradation by the proteasome. In the presence of ligand, the destruction complex is inactivated, beta-catenin is dephosphorylated and the protein remains stable. The stabilized protein accumulates in the cytoplasm and passes into the nucleus where it can act as a transcriptional co-regulator (MacDonald et al., 2009).
Inappropriate activation of beta-catenin has been implicated in several human diseases. In the context of the kidney, it has been suggested to contribute to kidney cancers, cystic kidney diseases and fibrosis (Guillen-Ahlers, 2008; He et al., 2009; Koesters et al., 1999; Lal et al., 2008; Lin et al., 2003; Major et al., 2007; Qian et al., 2005; Romagnolo et al., 1999; Simons et al., 2005; Surendran et al., 2002). Due to the significant impact that beta-catenin related diseases have on human health, there has been great interest in identifying components of the pathway that are susceptible to attack by small molecules.
Utilizing high throughput compound screening strategies, an inhibitor of the Wnt/β-catenin pathway was identified and subsequently shown to be an antagonist of Poly (ADP-ribose) polymerases (PARPs) with high specificity for the tankyrase-1 and 2 proteins (Chen et al., 2009; Huang et al., 2009). Recently, it was revealed that Tankyrase-1 and 2 function to parsylate and subsequently de-stabilize Axin1 and 2 proteins (Huang et al., 2009). Treatment of cells with small molecule antagonists of the tankyrases has the effect of inappropriate degradation of beta-catenin. However, the general utility of these chemicals as Wnt pathway antagonists in mammalian tissues is not clear. Although tankyrase inhibition interferes with fin regeneration and maintenance of the gastrointestinal epithelium in adult zebrafish, it does not appear to effect Wnt-dependent development/patterning of the embryo suggesting that the cell types in which Tankyrase-1 and 2 are active is limited (Huang et al., 2009). However, it is also possible that the lack of an embryonic phenotype is simply the result of delayed manifestation of the compounds’ effect on Axin protein levels. Although mice lacking either Tnks genes are viable, co-deletion results in early embryonic lethality preventing analysis of gene function in later developmental events, including the formation of most organ systems (Chiang et al., 2008; Chiang et al., 2006). Thus, genetically based interrogation of Tnks function in adult tissues as a means to assess the utility of Tnks inhibitors as therapeutic agents is largely limited by the role of Tnks genes in development.
Canonical Wnt signaling plays several well-defined roles in development of the mouse urogenital system, including the mesenchymal to epithelial transition that leads to renal vesicle formation and branching of the ureteric buds (Merkel et al., 2007). This system is particularly well suited for testing the effects of the tankyrase inhibitors during embryonic development/tissue maintenance.
In this study we demonstrate that Tankyrase-1and 2 play essential roles in normal Wnt signaling during organ formation in the mouse. Our results suggest that the Tankyrase enzymes are likely core components of the normal Wnt signal transduction cascade in all cell types, and support the use of tankyrase inhibitors as therapeutic reagents. Our findings also reveal a utility of organotypic culture as a rapid and generally accessible method for characterizing small molecule activity in developmental processes.
Results
Correlation of Tnks expression and tissues with active Wnt signaling during development
Recent studies have suggested that the tankyrase proteins play an important role in regulation of the canonical Wnt pathway (Huang et al., 2009). To determine if these factors had a role in kidney development, we utilized in situ hybridization to evaluate the expression of Tnks1 and 2 mRNA. Kidney development initiates 11 days after fertilization when the ureteric bud branches from the Wolffian duct, invading a population of pre-specified mesenchymal cells known as the metanephric mesenchyme. Within the E11.5 kidneys, we observed high levels of expression of Tnks1 in the epithelium of the Wolffian ducts and ureteric bud (Supplementary Figure 1A). Tnks2 mRNA was significantly enriched in the ureteric epithelia and the surrounding metanephric mesenchyme (Supplementary Figure 1E). In newborn mouse kidneys, Tnks1 expression was maintained in the ureteric bud tips and was also expressed at high levels in the surrounding metanephric mesenchyme (Supplementary figure 1B). Tnks2 was maintained in the ureteric bud tips and the uninduced mesenchyme as well as in the comma- and S-shaped bodies (Supplementary Figure 1F).
In addition to its expression in the developing kidney, Tnks1 was expressed at high levels in both the epithelium and the mesenchyme of the E12.5 lung (Supplementary figure 1C), and the cells surrounding the E11.5 notochord, differentiated neurons of the neural tube, the dorsal root ganglia and the hindgut epithelium (Supplementary figure 1A and D). Tnks2 was expressed at high levels in the E12.5 lung and at lower levels in the mesenchyme (Supplementary figure 1G). At E11.5, Tnks2 mRNA was also expressed in the gut epithelium and surrounding mesenchyme and at high levels in the ventral half of the neural tube in a pattern that was non-overlapping with that of Tnks1 (Supplementary figure 1H). The expression of Tnks1 and 2 in the kidney, lung and neural tube correlate with described canonical Wnt activity (Carroll et al., 2005; Marose et al., 2008; Megason and McMahon, 2002; Rajagopal et al., 2008). Although more detailed characterization will need to be performed to determine relevance to Wnt activity in individual tissues/organ systems, the analysis performed on the kidney suggests that the tankyrases may be playing a general role in canonical Wnt signaling during formation of this organ. Although they show higher levels of expression in some cell types, Tnks1 and 2 mRNAs appear to be expressed at low levels throughout the embryo (data not shown).
Tnks antagonists disrupt development of cultured organs
Whether tankyrases affect Wnt signaling during mouse embryonic development has not been determined. The recent characterization of tankyrase inhibitors allowed us to address this issue in organ culture. We first tested whether the tankyrases were required for ureteric bud branching, a beta-catenin dependent process (Marose et al., 2008; Bridgewater et al., 2008). E11.5 kidneys were cultured in a range of concentrations of a tankyrase inhibitor, IWR1 (0.5-100uM). We compared the effects of IWR1 on branching morphogenesis to the effects of a porcupine inhibitor, IWP2 (porcupine is necessary for secretion of Wnt ligands) (Chen et al., 2009; Kadowaki et al., 1996). Treatment with either factor had a dosage dependent effect on branching morphogenesis (Figure 1A, B, Supplementary Figure 2A-E for IWR1 and 1E, F and Supplementary Figure 2F-I for IWP2. Graphical representation of dosage effects is in Figure 1K). IWR1 treatment significantly inhibited branching at all concentrations tested (p=0.002 for 0.5uM, p=0.0004 for 5uM, p=0.00095 for 50uM and p=0.00035 100uM respectively.). Increasing the dose beyond 100uM did not have a quantifiably increased effect. Therefore, we consider 100uM to be the lowest effective dose and performed all subsequent studies with this dose. Similarly, All tested concentrations of IWP2 also significantly inhibited branching morphogenesis, although there was no significant difference between 5 and 50 uM (p=0.37) concentrations, suggesting that in the context of UB branching, 5uM represented the lowest effective dosage and was utilized for all subsequent studies. There was no significant difference in the extent of branching morphogenesis between 5uM IWP2 and 100uM IWR1 treatments (p=0.12). The nearly 20-fold difference in the effective doses of these compounds correlates with the previously characterized differences in the concentration of these compounds that was required to reduce TOP-Flash activity to 50% of control levels (the IC50) (Chen et al., 2009).
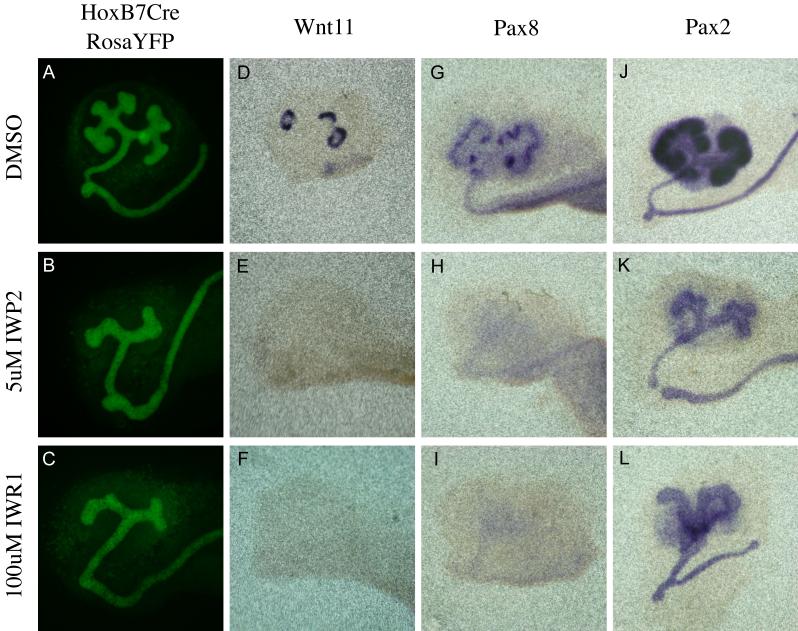
Figure 1. Tankyrase inhibition blocks branching morphogenesis and tubule induction similar to inhibition of the Wnt pathway.
Immunohistochemistry using anti-Ecadherin (Green) or anti-Laminin (Red) antibodies to evaluate branching morphogenesis in E11.5 kidneys that have been cultured for 48 hours in the presence of DMSO (A,C,E,G and I), 100uM IWR1 (B), 100uM IWR1-Exo (D), 5uM IWP2 (F), 50uM IWP7 (H) or 200uM XAV939 (J). Newly induced renal vesicles are indicated by arrows while the absence of any mesenchymally derived tubules are indicated by an arrow head. (K) A graphical depiction of the number of branching tips counted in kidneys cultured for 48 hours in IWP2 (blue line), IWR1 (red line) or XAV939 (green line).
In addition to branching of the ureteric bud, formation of the renal vesicles is also dependent on canonical Wnt signaling (Carroll et al., 2005; Park et al., 2007). To assess renal vesicle formation, treated kidneys were stained with antibodies to E-cadherin and Laminin (Figure 1 and Supplemental figure 2). Both compounds had a similar effect on renal vesicle formation to that seen in ureteric bud branching. Culture of E11.5 kidneys for 48 hours with either 100uM IWR1 or 5uM IWP2 completely blocked the formation of E-cadherin-positive, mesenchyme-derived structures (Figure 1A, B, E, F and data not shown). This does not appear to be due to a delay as RVs were still not present in treated kidneys after 5 days of culture (data not shown). The effect on renal vesicle formation also appeared to be dosage dependent (Supplemental figure 2A-E for IWR1 and F-I for IWP2).
To determine if the IWR1 effects were indeed the result of tankyrase inhibition, we performed a similar set of experiments with an unrelated tankyrase inhibitor, XAV939 (Huang et al., 2009). Similar to IWR1, XAV939 also had a dosage dependent effect on both branching morphogenesis and renal vesicle induction with 200uM being the lowest effective dose (Figure 1I-K and Supplemental Figure 2J-M). Treatment with 200uM of XAV939 was indistinguishable from 100uM IWR1 treatment (p=0.649 T-test, Supplemental Figure 3A).
Finally, to determine the general use of these compounds, we also tested their effects on another organ system where Tnks1 and 2 are expressed, beta-catenin signaling is required, and the organ can be cultured ex vivo: the developing lungs (Rajagopal et al., 2008). Similar to the results in cultured kidneys, tankyrase inhibition significantly repressed branching morphogenesis in cultured lung buds in a manner that was indistinguishable from IWP2 treatment (Supplementary Figure 4. Each image represents a dissected left lobe of and E11.5 lung rudiment cultured for 48 hours). Interestingly, cultured lung epithelia appeared to grow in size but failed to branch or remodel. Together, these data indicate that tankyrase activity is required for the development of both the kidneys and the lungs and that tankyrase inhibitors act effectively in the context of organ culture.
The IW compounds show little if any toxicity
Although renal vesicle induction and UB branching are both dependent on canonical Wnt signaling, it is possible that the phenotypes observed upon tankyrase or porcupine inhibition are due to non-specific and/or toxic side effects of the drugs. This possibility was addressed in several different ways. To address toxicity, we tested the effect of a diastereomer of IWR1, IWR1-exo. Although constitutionally identical to IWR1, IWR1-exo has an IC50 that is approximately 25 times less than IWR1 (Chen et al., 2009). If the effects of IWR1 are indeed specific, then we expect the effective dose of IWR1-exo to differ from that of IWR1 proportionate to the difference in their IC50s. If the effect is a toxic side effect, the effective dosage of the molecules should be identical. Addition of 100uM IWR1-exo to the culture media had an effect on branching morphogenesis although it was significantly reduced relative to the same concentration of IWR1 (p=0.001 T-test, Compare Figure 1B and D, Supplementary figure 3A). In addition, IWR1-exo treated kidneys induced renal vesicles in all cases (Figure 1D). The phenotype of 100uM IWR1-exo treated kidneys was very similar to that observed with 5uM IWR1 treatment. This difference closely corresponds to the 25 fold differences in their relative IC50s. More importantly, the significant differences between the effects elicited by treatment with equal concentrations of theses two diastereomers supports the claim that the effects of the compounds are not toxic side effects.
Similarly, addition of a 50uM dose of IWP7 (a compound that is structurally similar to IWP2 but that has little activity against the canonical Wnt/β-catenin pathway in vitro had no significant effect on ureteric bud branching or renal vesicle formation (Compare Figure 1F and H and Supplementary Figure 3B) suggesting the phenotype observed upon IWP2 treatment was also specific.
Finally, we observed no significant differences in the rates of apoptosis in kidneys treated with either IWR1 or IWP2 for 24 hours compared to DMSO treated controls (data not shown). These data further support the claim that the effect of these compounds is not due to toxicity.
Tankyrase inhibition specifically affects the expression Wnt/beta-catenin pathway targets
To test the specificity of the tankyrase inhibitors to blockade of the Wnt pathway, we examined treated kidneys at the molecular level. Beta-catenin is necessary for the expression of a number of ureteric bud specific factors (including Wnt11 and c-Ret) in the branching tips (Marose et al., 2008; Bridgewater et al., 2008). Culture of kidneys in either IWR1 or IWP2 resulted in a loss of both Wnt11 and c-Ret expression from the ureteric bud epithelium within 48 hours (Figure 2A-F). Wnt11 mRNA was undetectable within 24 hours of treatment (Figure 3D-F). It is important to note that the Ret positive cells seen in 2B and C do not reside in the kidney epithelium and appear to be extrarenal in origin. In stark contrast, expression of Pax2 and Wnt9b, factors that do not require beta-catenin signaling for their expression (Marose et al., 2008 and data not shown), was unaffected in the IW-treated Wolffian ducts/collecting ducts (Figure 2G-L).
Figure 2. Effect of Tankyrase inhibition on kidney development is similar to Wnt inhibition.
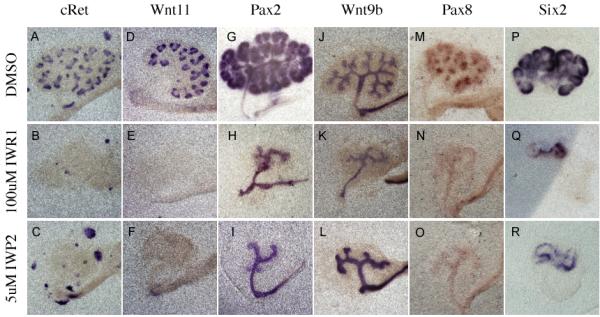
In Situ hybridization evaluating the expression of c-Ret (A-C), Wnt11 (D-F), Pax2 (G-I), Wnt9b (J-L), Pax8 (M-O), and Six2 (P-R) in e11.5 kidneys cultured for 48 hours in the presence of either DMSO (A, D, G, J, M, P and S), 100uM IWR1 (B, E, H, K, N, Q and T) or IWP2 (C, F, I,L, O, R and U).
Figure 3. Kidney development after 24 hour IW treatment.
Kidney development was visualized using YFP under the control of the HoxB7Cre transgene (A-C), antisense mRNA probes for Wnt11 (D-F), Pax8 (G-I) or Pax2 (J-L) in e11.5 kidneys cultured for 24 hours in DMSO (A, D, G and J), 5uM IWP2 (B, E, H and K) or 100uM IWR1 (C, F, J and L).
We next evaluated the effect of Tnks inhibition on the mesenchymal to epithelial transition that gives rise to the renal vesicles. Prior to their formation, the RV progenitors express Pax8, a direct transcriptional target of beta-catenin (Schmidt-Ott et al., 2007). E11.5 kidneys cultured for 48 hours in media supplemented with DMSO, formed multiple Pax8 positive cell clusters (Figure 2M). However, addition of either IWR1 or IWP2 to the media completely abolished mesenchymal Pax8 expression (Figure 2N and O). Mesenchymal Pax8 mRNA levels were undetectable within 24 hours of drug addition (Figure 3G-I) and were still undetectable after 96 hours of culture. Once again, Tnks inhibition had no effect on the initial expression of several genes that were previously shown to be independent of beta-catenin and/or Wnt signaling including Six2 and GDNF (Figure 2P, Q, and data not shown). However, the intensity of expression as well as the domains of expression for these genes did not expand over time (Figure 2Q and T), a phenotype similar to that observed in Wnt9b mutants (Carroll et al., 2005). This phenotype was identical when compared to treatment with the porcupine inhibitor IWP2 (Figure 2R and data not shown).
Interestingly tankyrase inhibition did affect the mesenchymal expression of Pax2. Previous studies showed that Pax2 mRNA and protein expression was unaffected in Wnt4 and Wnt9b mutants (Carroll et al., 2005; Stark et al., 1994). However, treatment with IWR1 resulted in greatly reduced levels of Pax2 after 24 and 48 hours of treatment (ureteric bud expression was unaffected, Figure 2G and H, 3J and L). To determine whether this was a non-specific effect or if Pax2 might be a previously undiscovered beta-catenin target, we also examined its expression in kidneys treated with the porcupine antagonist. Culture with IWP2 also blocked mesenchymal expression of Pax2 after 24 and 48 hours of treatment suggesting that Pax2 is a novel (direct or indirect) beta-catenin target in the metanephric mesenchyme (Compare Figure 2G to I and 3J to K).
All of the analysis performed to this point focuses on factors that are positively regulated by beta catenin in normal kidneys. As in independent indicator of the specificity of these factors, we wished to examine a factor that is normally negatively regulated by beta-catenin. Previous studies indicate that beta-catenin is necessary to maintain the ureteric bud in a progenitor state and that loss of beta-catenin results in precocious and ectopic expression of the alpha+ isoform of ZO-1 in the Wolffian duct and ureteric buds (Marose et al., 2008). We examined the expression of this factor in IW treated kidneys. As expected, no ZO-1alpha+ was observed in E11.5 kidneys cultured for 48 hours in DMSO (Figure 4A-D) suggesting these kidneys were still in an undifferentiated state. However, kidneys cultured in 100uM IWR1 or 5uM IWP2 for 48hrs resulted in the premature expression of ZO-1alpha+ in Wolffian duct derived epithelia (Figure 4G and K).
Figure 4. Tankyrase inhibition leads to premature differentiation.
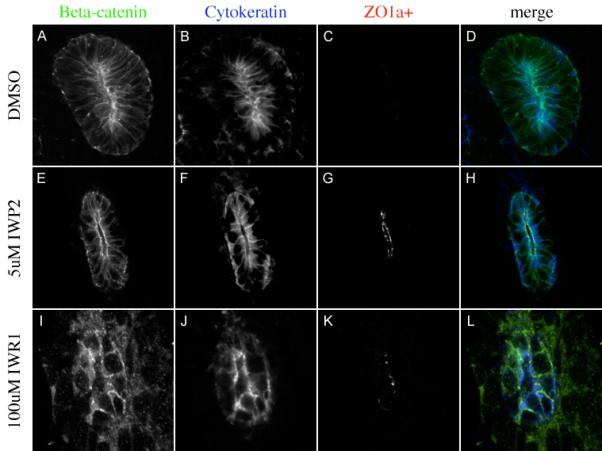
Immunohistochemistry evaluating the expression of the β-catenin (A, E, I green in D, H and L), the ureteric bud marker Cytokeratin (blue in B, F, J, blue in D, H and L) and the differentiation marker ZO1-α+ (C, G, K red in D, H and L) in kidneys cultured for 48 hours in DMSO (A-D), 5uM IWP2 (E-H) or 100uM IWR1 (I-L).
The effects of Tnks inhibition can be overcome by stabilization of beta-catenin
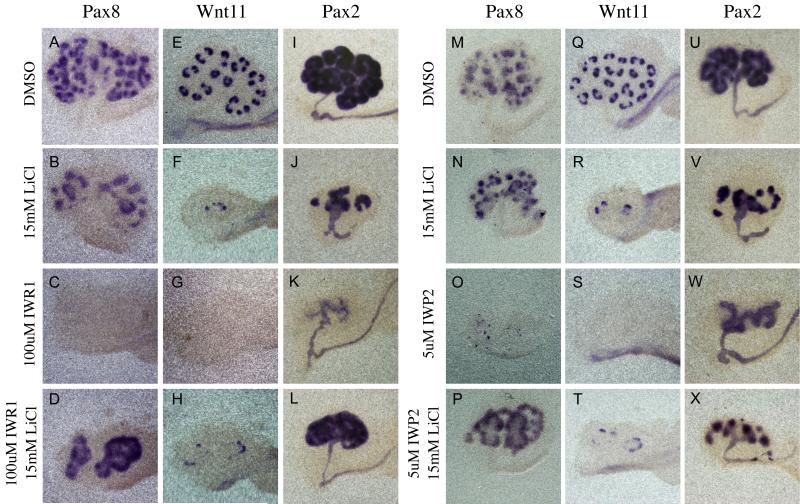
Although the results above indicate that the Wnt pathway is blocked upon tankyrase inhibition, we next sought to determine if the effects could be specifically attributed to loss of this pathway. To accomplish this, we examined the epistatic relationship between tankyrase and beta-catenin by supplementing IWR1 containing media with 15mM LiCl (a dosage that efficiently activates beta-catenin signaling in cultured kidneys). We assayed the effects of IWR1 and LiCl co-culture on both branching morphogenesis and renal vesicle induction. In agreement with previous studies showing that the expression of a stabilized form of beta-catenin in the ureteric bud blocked branching of the ureteric bud (Marose et al., 2008), treatment with LiCl also blocked branching. However, in contrast to removal of beta-catenin or treatment with IWP2 or IWR1, LiCl treated kidneys maintained the expression of Wnt11 and Pax8 (Figure 5B, F, N and R). Although addition of LiCl did not rescue branching morphogenesis, it was able to rescue the expression of Wnt11 and Pax8 in kidneys treated with IWR1 (Figure 4D and H). These results were indistinguishable from those generated by addition of LiCl to kidneys treated with IWP2 (Figure 5P and T). Finally, expression of a stabilized form of beta-catenin (Harada et al., 1999) in the mesenchyme (Rarβ2Cre;catnbexon3flox) rescued Pax8 expression in both IWR1 and IWP2 treated kidneys (data not shown).
Figure 5. Tankyrase inhibition specifically affects the canonical Wnt pathway.
In Situ hybridization comparing the expression of the canonical Wnt target genes, Pax8 (A-D, M-P), Wnt11 (E-H, Q-T) and Pax2 (I-L, U-X) in kidneys cultured for 48 hours in the presence of DMSO (A,E,I M, Q, U), 15mM LiCl (B,F,J N, R, V), 100uM IWR1 (C G, K), 5uM IWP2 (O, S, W), 100uM IWR1 and 15mM LiCl (D, H, L) or 5uM IWP2 and 15mM LiCl (P, T, X).
We next evaluated if the loss of Pax2 expression in IWR1 treated kidneys could be rescued by activation of β-catenin. Similar to the rescue of Pax8 and Wnt11, LiCl was also able to rescue the mesenchymal expression of Pax2 in both IWR1 and IWP2 treated kidneys (Figure 5 L and X) supporting the hypothesis that this gene is a novel target of β-catenin. Collectively, these data demonstrate that the effects of Tnks inhibition can be specifically attributed to destabilization of beta-catenin.
Transient disruption of Tnks activity induces irreversible changes in kidney development
To determine if the effects of IWR1 and IWP2 on kidney development were reversible, either by chemical withdrawal or stimulation of beta-catenin activity, we cultured kidneys for 24 hours in DMSO, IWR1 or IWP2, removed the compounds, then cultured for an additional 24 hours in media containing DMSO alone or DMSO with 15mM LiCl. Removal of IWR1 or IWP2 after 24 hours was not sufficient to rescue branching morphogenesis as visualized by either Wnt11 expression or visualization of the ureteric tree using HoxB7Cre;Rosa26YFP (Figure 6A-I and M-U, respectively). To test if re-stimulation of the Wnt pathway could rescue the defect, we exchanged the drug containing media after 24 hours with media containing 15mM LiCl for 24 hours. LiCl administration was sufficient to re-activate Wnt11 expression after 24 or 48 hours of both IWR1 and IWP2 treatment (Figure 6L, X and Supplemental figure 5 and 6) however due to the inhibitory effects of LiCl (see Figure 4), branching morphogenesis was not rescued (Figure 6K, W and Supplementary Figure 5 and 6). However, a 24-hour pulse with LiCl followed by culture in media alone was sufficient to rescue branching morphogenesis in kidneys treated with IWP2 for 24 or 48 hours (Supplementary figures 5, 6 and data not shown). After 72 hours of either IWR1 or IWP2 treatment, LiCL had no effects on branching morphogenesis as assessed by both morphology and Wnt11 expression (data not shown). Interestingly, culture with LiCl was never able to reverse the precocious expression of ZO-1α+ in the distal collecting ducts (data not shown) suggesting that once the differentiation program was initiated in the ureteric bud epithelium, simple reactivation of the Wnt pathway was not sufficient to reverse it.
Figure 6. The effect of Tankyrase inhibition on branching morphogenesis is reversible.
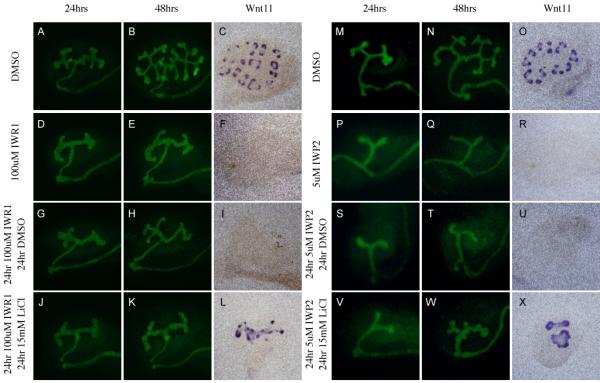
Evaluation of branching morphogenesis in kidneys cultured for 48 hours in DMSO (A-C, M-O), 100uM IWR1 (D-F), 5uM IWP2 (P-R), or sequentially for 24 hours each in 100uM IWR1 and DMSO (G-I), 100uM IWR1 and 15mM LiCl (J-L), 5uM IWP2 and DMSO (S-U), and 5uM IWP2 and 15mM LiCl (V-X). Branching morphogenesis was visualized using HoxB7Cre;RosaYFP mice (A,B,D,E,G,H,J,K,M,N,P,Q,S,T,V, and W). or antisense mRNA probes to Wnt11 (C,F,I,L,O,R,U and X).
In contrast to the situation for branching morphogenesis, removal of IWR1 after 24 hours was sufficient to completely rescue the tubule induction phenotype as assessed by Pax8 expression (Figure 7A-C). However, removal of IWP2 from the media at the same timepoint had no effect (Figure 7E-G). Removal of either drug after 48 or 72 hours did not result in rescue (data not shown). To test if re-stimulation of the Wnt pathway could reverse the phenotype, we once again exchanged the drug containing media after 24 hours with media containing 15mM LiCl for 24 hours. Replacement of either IWR1 or IWP2 with LiCl after 24 hours resulted in complete rescue of Pax8 expression (Figure 7D and H). Similar results were found after treatment with either molecule for 48 hours. However, after 72 hours of culture, LiCl was able to rescue tubulogenesis in IWR1 but not IWP2 treated kidneys (Figure 8). Similar to the findings with bud branching listed above, tubule development proceeds relatively normally in treated kidneys that received a 24-hour pulse of LiCl followed by culture in media alone (Supplementary Figure 5 and 6).
Figure 7. The effect of Tankyrase inhibition on tubulogenesis is reversible.
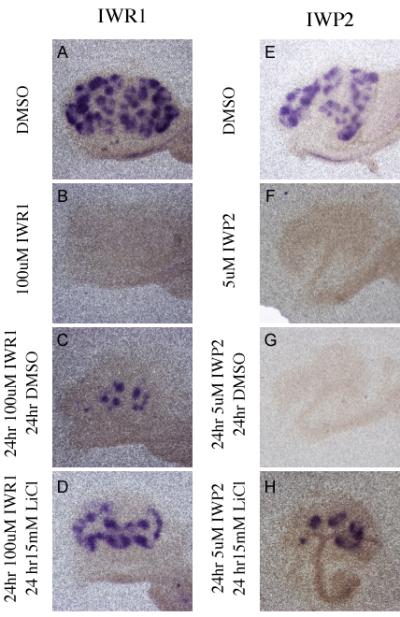
In Situ hybridization comparing the expression of Pax8 in kidneys cultured for 48 hours in DMSO (A, E), 100uM IWR1 (B), 5uM IWP2 (F), or sequentially for 24 hours each in 100uM IWR1 and DMSO (C), 5uM IWP2 and DMSO (G), 100uM IWR1 and 15mM LiCl (D), or 5uM IWP2 and 15mM LiCl (H).
Figure 8. Activation of beta-catenin rescues Tankyrase but not Porcupine inhibition after 72 hours.
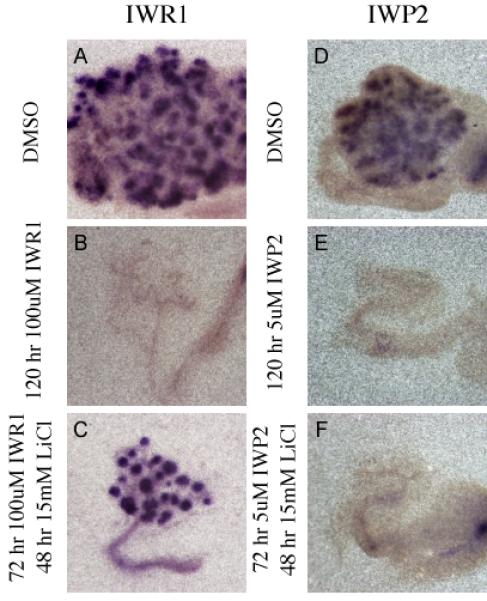
In Situ hybridization evaluating the expression of Pax8 mRNA (A-F) in kidney explants cultured for 120 hours in DMSO (A and D), 100uM IWR1 (B), 5uM IWP2 (E), or for 72 hours in 100uM IWR1 followed by 48 hours in 15mM LiCl (C) or for 72 hours in 5uM IWP2 followed by 48 hours in 15mM LiCl (F).
Discussion
Recent studies utilizing small molecule antagonists indicate that the Parps Tankyrase 1 and 2 PARsylate and subsequently deactivate Axin1 and 2, components of the beta-catenin destruction complex (Fearon, 2009; Huang et al., 2009). In the presence of the tankyrase inhibitors, the Axins are stabilized and beta-catenin is degraded. Tnks1 and 2 activities are essential for beta-catenin signaling in cell culture and during zebrafish gut and tail fin regeneration. However, a general requirement of Tnks enzymes for canonical/beta-catenin dependent Wnt signaling in mammalian development has not been determined. Here, we show the broad expression of Tnks1 and 2 mRNA in multiple areas of active Wnt signaling during the embryonic period of the mouse. Further, we show, using several individual small molecule antagonists and ex vivo organ culture, that tankyrase activity is required for normal embryonic development of the mouse kidney and lung. Finally, we demonstrate that the effects of the Tnks inhibitors on the kidney are the direct result of inhibition of the canonical/beta-catenin dependent Wnt pathway. These findings suggest that Tnks1 and 2 are core components of the Wnt transduction pathway during tissue development and maintenance.
The data presented here have uncovered a number of interesting findings related to Wnt signaling and kidney development. One such finding is that, in contrast to the situation in the regenerating zebrafish fin, tankyrase inhibition in the context of the developing mouse kidney is minimally reversible and the reversibility differs in distinct cell types. This limited reversibility of tankyrase inhibition in kidney seems to be attributable at least in part to the fact that reduced Wnt signaling in the context of the ureteric bud leads to premature differentiation of this tissue. These findings highlight differential roles for beta-catenin in diverse tissue types and perhaps are indicative of inherent differences between developing and regenerating tissues.
The distinct mode of action of the molecules utilized in this study should also allow researchers to tease apart canonical and non-canonical signaling events. Investigators can compare the blockade of all Wnt signaling (with IWP2), canonical signaling (with IWR1), or non-canonical signaling (with IWP2 supplemented with beta-catenin agonists). For instance, in this study, we found that at least in the context of tubule induction, the tankyrase inhibitors were always reversible by addition of LiCl while the porcupine inhibitors were minimally reversible. This finding may implicate a role for non-canonical Wnt signaling in maintaining the competence of the mesenchymal progenitor cells, a topic that is currently under investigation.
Relative to other commonly used Wnt inhibitors such as recombinant Dkk1, the small molecule inhibitors of tankyrase (as well as the inhibitors of porcupine) are extremely effective at inhibiting Wnt signaling in the context of organ culture (Iglesias et al., 2007; Marose et al., 2008). These molecules appear to completely block Wnt signaling at relatively low doses. In contrast, doses of Dkk1 up to 2ug/ml replaced every 12 hours had relatively mild effects on cultured kidneys (Data not shown). We predict this increased effectiveness will hold true for most, if not all, tissue types.
In addition to their potency, the IW molecules act rapidly (complete pathway repression is evident in less than 24 hours), appear to have no difficulty penetrating internal tissues and show little, if any, toxicity. This last point is supported by the fact that IW treated kidneys show defects very similar to Wnt and beta-catenin mutant kidneys and the phenotypes can be rescued (a the molecular level) by LiCl administration.
It is of note that the doses used in this study are in general 10-fold higher than those found to inhibit 50% of Wnt activity in cell culture. Interestingly, although XAV939 was found to be a much more potent inhibitor of tankyrase activity in vitro than IWR1, it was much less effective in the context of organ culture. The discrepancies in effectiveness of these compounds in vitro vs. in the context of organ culture may be the result of multiple factors. For instance, there may be differences in the half-lives of the compounds in the organ culture. Additionally the solubility of each factor will affect its effectiveness in the context of a complex, multi-cell layer tissue like the cultured kidney compared to cultured cells or a purely in vitro biochemical assay. The relatively high doses of these compounds required to completely block Wnt mediated events in organ culture may also indicate that very low levels of Wnt activity are sufficient to support normal development.
Finally, these compounds have additional advantages over current techniques of tissue specific gene ablation in that they do not depend on complicated, time consuming genetics or the efficiency of Cre mediated excision. For example, this study identified Pax2 as a potential beta-catenin target. This fact had not been revealed using traditional loss and gain of function studies. Further, with these compounds, the timing and degree of inhibition can be controlled and potentially reversed. Such studies can be undertaken in a relatively high throughput format utilizing small quantities of drug.
As TNKS1 and 2 mRNAs are present in multiple embryonic and adult tissues of humans, the Tnks inhibitors should be of wide use as biological and therapeutic reagents in multiple Wnt-related events and pathologies. In the kidney alone, beta-catenin misregulation has been implicated in Wilms’ tumors, nephronophthisis, kidney fibrosis and polycystic kidney disease (Benzing et al., 2007; Guillen-Ahlers et al., 2008; He et al.,, 2009; Koesters et al., 1999, Lal et al., 2008; Lin et al., 2003; Major et al., 2007; Qian et al., 2005; Simons et al., 2005; Surendran et al., 2002). Unfortunately, IWR1 appears to be rapidly metabolized when administered to adult mice, limiting its usefulness as an in vivo therapeutic reagent (Lu et al., 2009). However, the organ culture system will facilitate medicinal chemistry studies focused on identifying more stable derivatives that retain their effectiveness.
In sum, we have utilized small molecule antagonists of Tankyrase 1 and 2 to reveal a broad general role for these factors in Wnt signaling during embryonic development. Tankyrase activity appears essential for all known Wnt pathway activity in the context of the developing kidney. The ability to chemically target these proteins will significantly enhance efforts to abrogate pathological and non-pathological Wnt signaling. Such antagonists will be invaluable as biological and therapeutic reagents.
Experimental Procedures
IW culture
E11.5 urogenital systems were removed and bisected in sterile PBS and then the individual halves were cultured in 350ul of media at the air media interface on 24-well tissue culture treated, 6.5mm diameter, 8.0uM pore size Transwell filters (Corning, Cat. # 3422). The media (DMEM with 10% FBS and Pen/Strep) was supplemented with either DMSO or a dosage curve of IWR1, IWP2 or XAV939 (Maybridge, U.K.) and replaced with fresh media every 12, 24 or 48 hours. Replacement of the IWP2 containing media after 24 or 48 hours had moderate to no significant effect (respectively) on ureteric bud branching (data not shown) while replacement every 12 hours severely inhibited ureteric bud branching. All further studies with compounds were performed following a 12-hour replacement regimen. All treatments were repeated at least three times with a minimum of six individual kidneys from six distinct embryos each time. For lung bud culture, e11.5 lungs were dissected in sterile PBS and the left lobe of the lung bud isolated and cultured as described for 48 hours.
Mice
All mouse strains, including Rarβ2-Cre, HoxB7-Cre, Rosa26YFP, and Catnbexon3flox are as previously described (Harada et al., 1999; Kobayashi et al., 2005; Srinivas et al., 2001; Yu et al., 2009). Wild Type Swiss Webster outbred mice were obtained from Charles River.
Immunohistochemistry
Cultured kidneys were fixed overnight in 4% PFA at 4 degrees with gentle agitation. For wholemount staining, specimens were washed three times with PBS then dehydrated and rehydrated through a graded alcohol series, washed four times one hour each in 0.1% Triton-X/PBS at room temperature. Specimens were then blocked at room temperature for 4 hours in 5%FBS/0.1%Triton-X/PBS then incubated at 4 degrees overnight in primary antibody. The specimens were then washed 4 times one hour each in 0.1%Triton-X/PBS at room temperature and incubated at 4 degrees overnight in secondary antibody. The specimens were washed four times one hour each in PBS, equilibrated in Vectashield and photographed at 50X with a Zeiss fluorescent stereoscope and an Olympus DP71 camera. Tissues that were sectioned were washed 3 times with PBS and cryoprotected in 30% sucrose for 16 hours at 4 degrees C. Specimens were then embedded in OCT and cryosectioned at a thickness of 10uM. Specimens were washed 3 times in 0.1% TritonX/PBS for 5 minutes each wash and blocked for a minimum of 1 hour at room temperature in 5%FBS/0.1%TritonX/PBS. Sections were incubated in primary antibody for either 2 hours at room temperature or at 4 degrees overnight followed by 3 washes in 0.1%TritonX/PBS. Sections were then incubated in secondary antibody for 2 hours at room temperature, washed 3 times in PBS, mounted with Vectashield and examined by scanning laser confocal microscopy (Zeiss LSM-510). Tissue was incubated with the following antibodies: anti-pan cytokeratin (mouse, 1:500 Sigma), anti-beta-catenin (rabbit, 1:500 abcam), anti-ZO1a+ (rat, 1:500 Santa Cruz), anti-Laminin (Rabbit polyclonal, 1:500 Sigma) and anti-Ecadherin (Rat, 1:500 Zymed). Results shown are representative examples from one of three different stainings.
In situ hybridization
Both whole mount and section in situ hybridization was performed as previously described 19. Tissue was hybridized with the following antisense probes: Tnks1, Tnks2, cRet, Wnt11, Pax2, Wnt9b, Pax8, Six2, and GDNF. Restriction enzymes and polymerase have been previously described except for Tnks1 (Accession BC057370) and 2 (Accession CB249752) that were cut with EcoR1 and transcribed with T3 Polymerase.
Statistics
All statistical analysis was performed using the Students T-test.
Supplementary Material
Supplementary Figure 1: Tankyrase is expressed in ares of active Wnt signalling during embryonic development.
In Situ hybridization evaluating the expression of Tnks1 mRNA (A-D) or Tnks2 mRNA (E-H) in embryonic kidneys at E11.5 (A and E), newborn kidneys (B and F), E12.5 lungs (C and G) or E11.5 neural tube (D and H). The ureteric epithelium is labeled with an asterisk while arrows label the metanephric mesenchyme.
Supplementary Figure 2: IW molecules have a dosage dependent effect on branching morphogenesis.
Immunohistochemistry with antibodies against Ecadherin (Green) and Laminin (Red) evaluating branching morphogenesis in kidneys cultured in varying concentrations of IWR1 (A-E), IWP2 (F-I), or XAV939 (J-M). The presence of renal vesicles are indicated by arrows while the absence of renal vesicles are indicated by arrowheads.
Supplementary Figure 3: Evaluation of branching morphogenesis in cultured kidneys.
Graphical representation of the number of branching tips in kidneys cultured in the presence of Tankyrase inhibitors (IWR1 and XAV939) or the diasteriomer control IWR1-exo (A) or in the presence of IWP2 or a related compound IWP7 (B). n=15 kidneys from three independent experiments.
Supplementary Figure 4: Tankyrase inhbition blocks branching morphogenesis in cultured lungs similar to Wnt inhibition.
Immunohistochemistry with an antibody against Ecadherin (Green) to evaluate branching morphogenesis in left lung buds cultured in DMSO (A), IWP2 (B) or IWR1 (C). (D) Graphical representation of the quatification of lung branches after 48 hours of IW treatment. n=12 lung buds from 3 independent experiments.
Supplementary Figure 5: 24 hour pulse of LiCl reinitiates kidney development after IW treatment.
Evaluation of branching morphogenesis in kidneys cultured for 96 hours in DMSO (A-D, Q and U), 5uM IWP2 (E-H,R and V), or sequentially for 24 hours in 5uM IWP2 followed by 72 hours in 15mM LiCl (I-L, S and W) or for 24 hours in 5uM IWP2 followed by 24 hours in 15mM LiCl and 48 hours in DMSO (M-P, T and X). Branching morphogenesis was visualized using HoxB7Cre;RosaYFP mice (A-P). In Situ hybridization evaluating the expression of Pax8 mRNA (Q-T) or Wnt11 mRNA (U-X) was peformed on kidneys on treated explants upon completion of the 96 hour experiment. Results are representative of those found from 3 different experiments.
Supplementary Figure 6: Activation of beta-catenin is sufficient to rescue 48 hour IW treatment.
Evaluation of branching morphogenesis in kidneys cultured for 96 hours in DMSO (A-D, Q and U), 5uM IWP2 (E-H,R and V), or sequentially for 48 hours in 5uM IWP2 followed by 48 hours in 15mM LiCl (I-L, S and W) or for 48 hours in 5uM IWP2 followed by 24 hours in 15mM LiCl and 24 hours in DMSO (M-P, T and X). Branching morphogenesis was analyzed every 24 hours using HoxB7Cre;RosaYFP mice (A-P). In Situ hybridization evaluating the expression of Pax8 mRNA (Q-T) or Wnt11 mRNA (U-X) was peformed on kidneys on treated explants upon completion of the 96 hour experiment. Results are representative of those found from 3 different experiments.
Acknowledgements
We would like to thank members of the Carroll, Cleaver and Igarashi labs for their comments on this work. This work was supported by grants from the American Heart Association (0730236N), the N.I.H. (R01DK080004) and the Susan Irene Simons Kidney Cancer Foundation to T.C., grants from the NIGMS (R01GM079554) and Welch Foundation (Welch I-1596) to C.C. and the N.I.H (R01GM076398) and Welch Foundation to L.L.
References
- Benzing T, Simons M, Walz G. Wnt signaling in polycystic kidney disease. J Am Soc Nephrol. 2007;18:1389–98. doi: 10.1681/ASN.2006121355. [DOI] [PubMed] [Google Scholar]
- Bridgewater D, Cox B, Cain J, Lau A, Athaide V, Gill PS, Kuure S, Sainio K, Rosenblum ND. Canonical WNT/beta-catenin signaling is required for ureteric branching. Dev Biol. 2008;317:83–94. doi: 10.1016/j.ydbio.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–92. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–7. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YJ, Hsiao SJ, Yver D, Cushman SW, Tessarollo L, Smith S, Hodes RJ. Tankyrase 1 and tankyrase 2 are essential but redundant for mouse embryonic development. PLoS One. 2008;3:e2639. doi: 10.1371/journal.pone.0002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YJ, Nguyen ML, Gurunathan S, Kaminker P, Tessarollo L, Campisi J, Hodes RJ. Generation and characterization of telomere length maintenance in tankyrase 2-deficient mice. Mol Cell Biol. 2006;26:2037–43. doi: 10.1128/MCB.26.6.2037-2043.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon ER. PARsing the phrase “all in for Axin”- Wnt pathway targets in cancer. Cancer Cell. 2009;16:366–8. doi: 10.1016/j.ccr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Guillen-Ahlers H. Wnt signaling in renal cancer. Curr Drug Targets. 2008;9:591–600. doi: 10.2174/138945008784911813. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–76. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Iglesias DM, Hueber PA, Chu L, Campbell R, Patenaude AM, Dziarmaga AJ, Quinlan J, Mohamed O, Dufort D, Goodyer PR. Canonical WNT signaling during kidney development. Am J Physiol Renal Physiol. 2007;293:F494–500. doi: 10.1152/ajprenal.00416.2006. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–28. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 2005;132:2809–23. doi: 10.1242/dev.01858. [DOI] [PubMed] [Google Scholar]
- Koesters R, Ridder R, Kopp-Schneider A, Betts D, Adams V, Niggli F, Briner J, von Knebel Doeberitz M. Mutational activation of the beta-catenin proto-oncogene is a common event in the development of Wilms’ tumors. Cancer Res. 1999;59:3880–2. [PubMed] [Google Scholar]
- Lal M, Song X, Pluznick JL, Di Giovanni V, Merrick DM, Rosenblum ND, Chauvet V, Gottardi CJ, Pei Y, Caplan MJ. Polycystin-1 C-terminal tail associates with beta-catenin and inhibits canonical Wnt signaling. Hum Mol Genet. 2008;17:3105–17. doi: 10.1093/hmg/ddn208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A. 2003;100:5286–91. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Ma Z, Hsieh JC, Fan CW, Chen B, Longgood JC, Williams NS, Amatruda JF, Lum L, Chen C. Structure-activity relationship studies of small-molecule inhibitors of Wnt response. Bioorg Med Chem Lett. 2009;19:3825–7. doi: 10.1016/j.bmcl.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, Angers S, Moon RT. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–6. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- Marose TD, Merkel CE, McMahon AP, Carroll TJ. Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol. 2008;314:112–26. doi: 10.1016/j.ydbio.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–98. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Merkel CE, Karner CM, Carroll TJ. Molecular regulation of kidney development: is the answer blowing in the Wnt? Pediatr Nephrol. 2007;22:1825–38. doi: 10.1007/s00467-007-0504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134:2533–9. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- Qian CN, Knol J, Igarashi P, Lin F, Zylstra U, Teh BT, Williams BO. Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem. 2005;280:3938–45. doi: 10.1074/jbc.M410697200. [DOI] [PubMed] [Google Scholar]
- Rajagopal J, Carroll TJ, Guseh JS, Bores SA, Blank LJ, Anderson WJ, Yu J, Zhou Q, McMahon AP, Melton DA. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development. 2008;135:1625–34. doi: 10.1242/dev.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnolo B, Berrebi D, Saadi-Keddoucci S, Porteu A, Pichard AL, Peuchmaur M, Vandewalle A, Kahn A, Perret C. Intestinal dysplasia and adenoma in transgenic mice after overexpression of an activated beta-catenin. Cancer Res. 1999;59:3875–9. [PubMed] [Google Scholar]
- Schmidt-Ott KM, Masckauchan TN, Chen X, Hirsh BJ, Sarkar A, Yang J, Paragas N, Wallace VA, Dufort D, Pavlidis P, Jagla B, Kitajewski J, Barasch J. beta-catenin/TCF/Lef controls a differentiation-associated transcriptional program in renal epithelial progenitors. Development. 2007;134:3177–90. doi: 10.1242/dev.006544. [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–43. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Surendran K, McCaul SP, Simon TC. A role for Wnt-4 in renal fibrosis. Am J Physiol Renal Physiol. 2002;282:F431–41. doi: 10.1152/ajprenal.0009.2001. [DOI] [PubMed] [Google Scholar]
- Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon AP. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–71. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Tankyrase is expressed in ares of active Wnt signalling during embryonic development.
In Situ hybridization evaluating the expression of Tnks1 mRNA (A-D) or Tnks2 mRNA (E-H) in embryonic kidneys at E11.5 (A and E), newborn kidneys (B and F), E12.5 lungs (C and G) or E11.5 neural tube (D and H). The ureteric epithelium is labeled with an asterisk while arrows label the metanephric mesenchyme.
Supplementary Figure 2: IW molecules have a dosage dependent effect on branching morphogenesis.
Immunohistochemistry with antibodies against Ecadherin (Green) and Laminin (Red) evaluating branching morphogenesis in kidneys cultured in varying concentrations of IWR1 (A-E), IWP2 (F-I), or XAV939 (J-M). The presence of renal vesicles are indicated by arrows while the absence of renal vesicles are indicated by arrowheads.
Supplementary Figure 3: Evaluation of branching morphogenesis in cultured kidneys.
Graphical representation of the number of branching tips in kidneys cultured in the presence of Tankyrase inhibitors (IWR1 and XAV939) or the diasteriomer control IWR1-exo (A) or in the presence of IWP2 or a related compound IWP7 (B). n=15 kidneys from three independent experiments.
Supplementary Figure 4: Tankyrase inhbition blocks branching morphogenesis in cultured lungs similar to Wnt inhibition.
Immunohistochemistry with an antibody against Ecadherin (Green) to evaluate branching morphogenesis in left lung buds cultured in DMSO (A), IWP2 (B) or IWR1 (C). (D) Graphical representation of the quatification of lung branches after 48 hours of IW treatment. n=12 lung buds from 3 independent experiments.
Supplementary Figure 5: 24 hour pulse of LiCl reinitiates kidney development after IW treatment.
Evaluation of branching morphogenesis in kidneys cultured for 96 hours in DMSO (A-D, Q and U), 5uM IWP2 (E-H,R and V), or sequentially for 24 hours in 5uM IWP2 followed by 72 hours in 15mM LiCl (I-L, S and W) or for 24 hours in 5uM IWP2 followed by 24 hours in 15mM LiCl and 48 hours in DMSO (M-P, T and X). Branching morphogenesis was visualized using HoxB7Cre;RosaYFP mice (A-P). In Situ hybridization evaluating the expression of Pax8 mRNA (Q-T) or Wnt11 mRNA (U-X) was peformed on kidneys on treated explants upon completion of the 96 hour experiment. Results are representative of those found from 3 different experiments.
Supplementary Figure 6: Activation of beta-catenin is sufficient to rescue 48 hour IW treatment.
Evaluation of branching morphogenesis in kidneys cultured for 96 hours in DMSO (A-D, Q and U), 5uM IWP2 (E-H,R and V), or sequentially for 48 hours in 5uM IWP2 followed by 48 hours in 15mM LiCl (I-L, S and W) or for 48 hours in 5uM IWP2 followed by 24 hours in 15mM LiCl and 24 hours in DMSO (M-P, T and X). Branching morphogenesis was analyzed every 24 hours using HoxB7Cre;RosaYFP mice (A-P). In Situ hybridization evaluating the expression of Pax8 mRNA (Q-T) or Wnt11 mRNA (U-X) was peformed on kidneys on treated explants upon completion of the 96 hour experiment. Results are representative of those found from 3 different experiments.