Abstract
The study addressed whether top-down control of visual cortex supports volitional behavioral control in a novel antisaccade task. The hypothesis was that anticipatory modulations of visual cortex activity would differentiate trials on which subjects knew an anti- versus a pro-saccade response was required. Trials consisted of flickering checkerboards in both peripheral visual fields, followed by brightening of one checkerboard (target) while both kept flickering. Neural activation related to checkerboards before target onset (bias signal) was assessed using electroencephalography. Pre-target visual cortex responses to checkerboards were strongly modulated by task demands (significantly lower on antisaccade trials), an effect that may reduce the predisposition to saccade generation instigated by visual capture. The results illustrate how top-down sensory regulation can complement motor preparation to facilitate adaptive voluntary behavioral control.
Keywords: attention, bias signal, EEG, saccade, antisaccade visual steady-state
Volitional control over gaze is critical for successfully navigating the environment. Paradigms like the antisaccade task require volitional cognitive control over otherwise prepotent responses (Munoz & Everling, 2004). Compared to a prosaccade task, where a glance is made to a peripheral target, antisaccade tasks require withholding a glance to a peripheral target and looking to that target's mirror image location. The ability to perform antisaccade tasks depends on prefrontal cortex (PFC) mediated top-down control (McDowell, Dyckman, Austin, & Clementz, 2008; Sweeney, Luna, Keedy, McDowell, & Clementz, 2007; Pierrot-Deseilligny et al., 2003), which makes antisaccade paradigms excellent probes of the neural substrates of flexible behavior (Miller & Cohen, 2001). Demonstrating relationships between behavioral performance and the neural dynamics of sensory processing and motor planning are important steps toward discerning how top-down control supports flexible behavioral regulation. The present study addressed this issue by measuring neural activity with high temporal resolution electroencephalography (EEG; Nunez & Srinivasan, 2006).
Abruptly-appearing visual stimuli capture attention (Yantis & Jonides, 1996), manifest as an increased activity in extrastriate neurons tuned for that particular spatial location (Colby & Goldberg, 1999). Neurophysiology studies indicate that suppression of reflexive saccades to novel visual targets during antisaccade tasks requires an anticipatory reduction of neural activity in saccade motor circuitry prior to stimulus appearance (Everling & DeSouza, 2005), an effect of top-down control mediated by PFC (Johnston & Everling, 2006). This process reduces the predisposition to move instigated by visual capture, and contributes to successful suppression of context-inappropriate saccades to peripheral targets on antisaccade trials (Munoz & Everling, 2004). While preparatory effects in the motor system are established in neurophysiology studies, possible analogous effects in the human visual system have been less systematically investigated (but see McDowell et al., 2005).
Reduced activity in visual cortex to antisaccade targets could complement anticipatory top-down inhibition in saccadic motor circuitry to support successful performance. The notion that top-down bias signals (what Desimone & Duncan, 1995, called the “attentional template”) begin their influence on perception in a preparatory manner has a history in the visual selective attention literature (see, e.g. Desimone & Duncan, 1995; Kastner & Ungerleider, 2000; Maunsell & Treue, 2006). There are various theories about the neural source(s) of such bias signals (i.e. which brain region or regions control early visual cortical responses by “informing” visual cortex of preferred stimulus features and/or locations), and their importance for optimizing behavioral performance by influencing the course of information flow early in visual processing (e.g., Miller & Cohen, 2001).
In the present study, the effects of top-down bias signals, defined as modifications of sustained sensory responses in neural mass activity in visual cortex, were assessed prior to peripheral target onset during anti- and pro-saccade trials using dense-array EEG. Use of the steady-state visual evoked potential (ssVEP) allowed for assessment of cortical facilitation/suppression of sensory processing in relation to possible peripheral target locations. The ssVEP is an electrocortical response to flickering stimuli, coming primarily from striate/extrastriate cortex (e.g., Clementz, Wang, & Keil, 2008; Di Russo, Taddei, Apnile, & Spinelli, 2006; Pastor, Artieda, Arbizu, Valencia, & Masdeu, 2003), where the frequency of brain activity equals the stimulus flicker rate. The ssVEP occurs at specific frequencies set by the experimenter via manipulating the frequency of the flickering visual stimuli, an advantage compared with visual evoked potentials (VEP) which measure changes over a wider frequency range (Makeig et al., 2002). This type of “frequency tagging” (Müller, Malinowski, Gruber, & Hillyard, 2003) allowed by ssVEPs facilitates identification of neural activity related to specific stimuli, and can be used to track neural activity in relation to multiple stimuli simultaneously (Clementz et al., 2008; Müller & Hubner, 2002; Müller et al., 2003; Regan & Regan, 2003; Wang, Clementz, & Keil, 2007). Using a ssVEP paradigm, the present experiment reveals neural modulation of visual cortex activity preceding correct antisaccades and illustrates the importance of PFC mediated top-down control of sensory cortices for the successful control of context-appropriate behaviors.
Materials and Methods
Participants
Fifteen healthy right-handed individuals (age range: 19 to 27 years; 8 females) recruited from the student population at the University of Georgia participated after providing written informed consent. Participants had normal or corrected-to-normal vision, had no evidence of neurological impairment, were free of psychiatric or substance use disorders (by self-report), and were given course credit for their participation. This project was approved by the Institutional Review Board at the University of Georgia.
Stimuli and experimental procedures
Stimuli were presented on a 21-inch flat-surface high resolution color monitor (60 Hz vertical refresh) situated 100 cm from the participant's nasion. Central fixation was a 2 deg white cross (see Figure 1). Peripheral stimuli were 4 deg square checkerboards that were centered at 9 deg left and right of central fixation. The checkerboards were composed of alternating gray and black boxes (each box was 1 deg square). The gray boxes were of equal luminance (5 cd/m2) and were presented against a dark background (<0.2 cd/m2).
Figure 1.
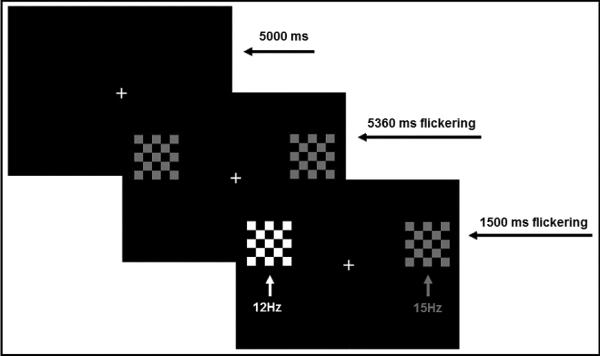
An example trial. All trials began with subjects fixating a central stimulus for 5000 ms (the fixation period). After this interval, checkerboards began simultaneous flickering left (12 Hz) and right (15 Hz) for 5360 ms while participants maintained fixation on the central stimulus (the preparatory period). At the end of this interval, one of the peripheral checkerboards increased in luminance for 1500 ms (the target; began the response period) which cued the participants to make the required response (towards the target on pro-trials and in the opposite direction on anti-trials).
Steady-state visual evoked potential
The ssVEP was used to assess sustained brain activity in relation to peripheral target locations during the period preceding target onset. At trial initiation (see Figure 1), participants fixated the central cross for 5000 ms (the fixation period; the long duration allows for settling of the ssVEP neural generators). Following the fixation period, peripheral checkerboards were presented that were square-wave luminance modulated (100% modulation depth) at two flickering frequencies (12 Hz in left visual field, 15 Hz in right visual field) for 5360 ms (the first common multiple of 12 and 15 Hz cycles after 5000 ms) so that possible lateralized changes in electrocortical facilitation and suppression could be tracked via the ssVEP. Subjects were instructed to remain fixated on the central cross during this preparatory period. At the end of the preparatory period, one of the peripheral checkerboards (randomly determined) increased in luminance to 20cd/m2 (defining target onset) signaling the subject to make a saccade response. The two checkerboards continued flickering at their respective frequencies for an additional 1500 ms (the saccadic response period), after which the peripheral checkerboards disappeared, leaving only the white cross on the screen to initiate the fixation period marking the start of a new trial.
Behavioral task requirements
Participants were presented with two blocks of stimuli. During one block they were required to generate a saccade as quickly and accurately as possible to the middle of the peripheral target checkerboard (prosaccade block). During the other block, participants were asked to generate a saccade as quickly and accurately as possible to the middle of the nontarget peripheral checkerboard without first making a saccade to the target checkerboard (antisaccade block). There were 50 trials to both the left and right checkerboards in each block; order of block presentation was counterbalanced.
Electrophysiological recording and data pre-processing
Data collection
EEG data were vertex-referenced and recorded using a 256-sensor Geodesic Sensor Net and NetAmps 200 amplifiers (EGI, Eugene, OR). Individual sensor impedances were kept below 50 kΩ(see Ferree, Luu, Russell, & Tucker, 2001). In addition, an electrolyte bridge test was conducted between all pairs of sensors prior to recording (Tenke & Kayser, 2001), and if there was evidence of bridging, sensors were adjusted until bridging was no longer evident (this was rarely required). Data were sampled at 500 Hz with an analog filter bandpass of 0.1–200 Hz. Sensors located at the outer canthi of each eye and below and above both eyes recorded horizontal and vertical eye movements and eye blinks. Following data collection, the 3D locations of sensors were acquired using a photogrammetry rig (EGI, Eugene, OR). The horizontal EEG eye sensors were used to measure saccadic response for each participant.
Data pre-processing
EEG sensors located at the neck and cheeks were excluded, leaving 207 sensors for data analyses. Raw data were visually inspected offline for bad sensor recordings (BESA 5.1, MEGIS Software, Gräfelfing, Germany). Bad sensors (no more than 5% of sensors for any subject) were interpolated using BESA's spherical spline interpolation method. Trials (6860 ms in length) with EEG signals greater than 100 μV and/or other artifacts were automatically eliminated from further processing (fewer than 25% of trials were eliminated for any subject for both pro- and anti-saccade conditions). The artifact-free data were then transformed to an average reference. The position data from the horizontal eye sensors for individual trials were visually inspected and scored for correct saccadic behavioral performance as in Dyckman and McDowell (2005). Saccadic latencies also were calculated (time from peripheral target onset to saccade initiation, see Dyckman & McDowell, 2005). Subjects made correct eye movements on 98% of prosaccade trials (reaction time M=375.0 ms, SD=19.1) and 92% of antisaccade trials (reaction time M=422.1 ms, SD=23.2). Given the low error rates, only correctly performed trials were analyzed.
Data analyses
Two approaches were used to quantify brain activity. First, standard VEP measures were used to evaluate the amplitude and spatial distribution of brain activity at the onset of visual stimulation (at the beginning of the preparatory period and before initiation of the steady-state response). Second, spectral measures were used to assess the phase stability and power of the ssVEP over time during the preparatory period.
VEP measures
The VEP data were used to assess for differences in brain activations, at the beginning of visual processing, between the pro-and anti-saccade conditions that could be related to similar effects observed in previous publications (Clementz, Brahmbhatt, McDowell, Brown, & Sweeney, 2007; McDowell et al., 2005). This was done as a validity check on the paradigm used here, which was different in style from previous EEG antisaccade studies. As the overwhelming frequency composition of early VEPs is below 10 Hz (Moratti, Clementz, Gao, Ortiz, & Keil, 2007), the EEG data were digitally low-pass filtered at 10 Hz (12 dB/octave rolloff), which also served to reduce possible confusion between the early VEPs and the initiation of the ssVEP (at 12 and 15 Hz). Next, VEPs were averaged time-locked to flicker onset separately for the pro- and anti-saccade trials. The VEP data were baseline corrected using the 200 ms pre-stimulus period, and VEP peaks occurring during the first 300 ms after stimulus onset (clearly prior to stabilization of the ssVEP) were compared between pro- and anti-saccade trials. Latencies of these peaks were determined by inspection of global field power plots (global field power is the root mean square of voltage over all EEG recording sensors at each data sampling point, which can yield an initial assessment of the presence and latency of above-baseline VEPs; Lehmann & Skrandies, 1984).
Comparisons of scalp potential amplitudes between pro- and anti-saccade trials were then conducted for each VEP peak (±4 ms; see Figure 3). For each comparison, a paired t-test was conducted separately for each EEG recording sensor. For significance thresholding, a clustering method (e.g., Forman et al., 1995) was used to take account of the non-independence of data from adjacent EEG sensors with significance levels determined based on the noise level of the data (estimated from the prestimulus period; see Krusemark, Campbell, & Clementz, 2008, for an example) and Monte Carlo simulations calculated using AlphaSim (Cox, 1996). To maintain the familywise alpha lower than .01, individual t-tests for a given sensor required at least six neighboring sensors with effects statistically significant at p<.035.
Figure 3.

Visual evoked potentials (VEPs) to onset of the flickering stimuli at the beginning of the preparatory period. The VEP waveforms on the left illustrate the evoked response effects for sensors close to the regions of significant voltage differences (time 0 indicates stimuli onset). The colored top-down view topographies (voltage maps, with scales of ±2 μV peak-to-peak) at the three VEP peaks are shown to the right of the waveforms for anti-and pro-saccades (black dots show the locations of Fz, Cz, and POz). Clusters of sensors with significant between-condition effects for the three peaks are indicated in gray shaded sensor distributions to the right of the topography plots (red dots show the locations of Fz, Cz, and POz, from top to bottom). The sLORETA solutions for the VEP voltages differences between anti- and pro-saccades are shown at the far right, with the label indicating which condition had stronger activity.
After VEP analyses calculated on voltage data at the sensors, we used standardized low-resolution brain electromagnetic tomography (sLORETA; Pascual-Marqui, 2002) to estimate brain regions involved in determining the between-condition differences on VEPs observed in the sensor space data (see Clementz et al., 2008; Krusemark et al., 2008). sLORETA is a modification of minimum norm least squares (Hämäläinen & Ilmoniemi, 1994) that uses the standardization of the minimum norm inverse solution to infer high probability regions of brain activation given the measured EEG data. sLORETA solutions yield pseudo-statistics that are not appropriate for determining strength of activity, but they provide accurate information about the regions of activity that can account for the voltage pattern recorded at the sensors (e.g., Soufflet & Boeijinga, 2005). The sLORETA calculations were performed using CURRY (Version 5.0, Neuroscan, Inc.). An averaged magnetic resonance (MR) image from the Montreal Neurological Institute (Collins, Neelin, Peters, & Evans, 1994) was used to construct a realistic head model (Fuchs, Kastner, Wagner, Hawes, & Ebersole, 2002) prior to source localization. The MR images were segmented into skin surface, inside of the skull, and cortex. A three compartment Boundary Element Method (BEM) model was then constructed; standard homogeneous conductivities were assumed for the skin, skull, and brain. For this BEM model, the average triangle edge lengths were 7.5 mm for the skin, 5.1 mm for the skull, and 3.1 mm for the brain compartment. Prior to source analysis calculations, the fiducial locations from the EEG data collection session were matched to the fiducial locations on the averaged segmented skin surface (using a least squares fitting procedure in CURRY). The sLORETA solutions were projected to the cortical surface.
Spectral measures
Previous work has demonstrated that, under typical circumstances, the ssVEP is determined primarily by increased inter-trial phase alignment (increased across-trial phase similarity of the EEG signals in relation to frequency of the visual flickering stimuli) without substantial changes in single trial power (Ding et al., 2006; Moratti et al., 2007). In the present study, however, subjects were required to impose sustained top-down control as a function of pro-versus anti-saccade task demands. The effect of this top-down control could be manifest on changes in either single trial power or inter-trial phase alignment, so both aspects of the ssVEP response were quantified for between-condition comparisons.
Single trial power of the steady state response across time was estimated by complex demodulation at the flickering rates (12 and 15 Hz) of the checkerboards for each trial (Regan & Regan, 1989). Complex demodulation is a time series analysis method for quantifying the power of oscillatory biosignals (similar to wavelet analysis). Complex demodulation yields power as a function of time for oscillations at frequencies of interest. It can be conceptualized as a band-pass filter which eliminates all other frequencies except for the selected one (Draganova & Popivanov, 1999; Haenschel, Baldeweg, Croft, Whittington, & Gruzelier, 2000; Hummel & Gerloff, 2004), allowing for quantification of time-dependent changes in power of a particular frequency component. Data were multiplied with 12-and 15-Hz sine and cosine functions. A Butterworth zero-phase low pass filter of 1 Hz was applied to the resulting time series before obtaining the vector length of the sine and cosine parts (separately for 12-and 15Hz signals) as a measure of time-varying power on single trials. The narrow low pass filter was chosen to obtain sufficient frequency resolution by separating the driving stimulus frequencies from each other and from background alpha activity. Finally, the single trial 12-and 15 Hz power estimates were averaged across trials for each subject.
To calculate inter-trial phase locking (ITPL) of the steady state response, as in our previous work (Moratti et al., 2007), each trial was submitted to the same complex demodulation analysis as described above. ITPL was determined by normalizing the phase vectors spanned by the sine and cosine parts of the complex demodulation components for each flicker frequency and time step by the corresponding length of the vectors. For each time point and flicker frequency, the normalized phase vectors were added across trials of each condition and subject. Thereafter, the sum of the phase vectors was divided by the corresponding number of trials resulting in the Rayleigh statistic R (Jammalamadaka & SenGupta, 2001). The R value is bound between 0 and 1. The higher the ITPL of an oscillatory response, the closer the R value will be to 1.
EEG data from 91 sensors over posterior cortex that captured the ssVEP (see Figure 2, and Clementz et al., 2008; Wang et al., 2007) were separately analyzed to obtain single trial power and ITPL values. Results were then averaged over these sensors to obtain single estimates of these dependent variables. The single trial power and ITPL values were standardized (mean 0, unit variance) across time for each subject. This was done by initially combining all the time points (from −500 to 5000 ms) for the pro- and anti-saccade conditions for each driving frequency individually (because 12 Hz power is normally larger than 15 Hz power, but we wanted these metrics to be on the same relative scale). Combining the pro- and anti-saccade conditions data into this standardization also allowed for a direct comparison of differences between conditions in standard units. Averaged values for the spectral measures were then calculated for 500 ms intervals, resulting in 11 time points for statistical analyses. Five hundred ms time bins were used because, given the filter type, low-pass filter frequency, and filter order (5th), the full width half maximum (FWHM) of the impulse response was estimated at 502 ms. No baseline adjustments were applied to these data because differences in baseline activity were possibly an important component of the analyses.
Figure 2.
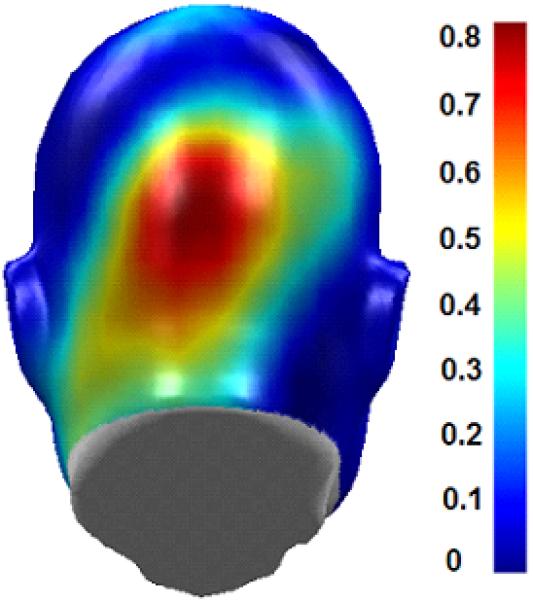
Head surface map (looking at the back of the head) of evoked visual response power to the steady-state stimuli (averaged over both frequencies and conditions). For display purposes, brain responses to the 12-and 15-Hz stimuli were placed on the same scale (maximum of 1) to adjust for between-frequency ssVEP power differences.
Results
VEPs to peripheral stimulus onset
At the initiation of the peripheral checkerboards flickering, there were three above baseline VEP peaks that were clearly identified from the global field power plots during both pro-and anti-saccade blocks (see Figure 3) prior to the onset of the ssVEPs. The average latency of these peaks was 135 ms (SE=0.9), 196 ms (SE=3.1), and 227 ms (SE=2.0); the latencies did not differ as a function of task condition.
Paired t-tests comparing brain activity for pro-and anti-saccade trials at the 135 ms peak revealed groups of sensors over superior parieto-frontal regions (see Figure 3) where participants had more extreme VEP amplitudes during anti- (average voltage in cluster=−1.52 μV, SD=.31) than pro-saccade trials (average voltage in cluster=−0.91 μV, SD=.30). The sLORETA solution on this between-condition voltage difference indicated increased activity in bilateral visual cortices and PFC during anti- compared to pro-saccade trials. Paired t-tests comparing brain activity for pro- and anti-saccade trials at the 196 ms peak revealed groups of sensors over bilateral inferior parieto-occipital regions (see Figure 3) where participants had more extreme VEP amplitudes during pro- (average voltage across both clusters=−1.15 μV, SD=.12) compared to anti-saccade trials (average voltage across both clusters=−0.94 μV, SD=.18). The sLORETA solution on this between-condition voltage difference indicated increased activity in bilateral middle occipital gyrus during pro- compared to anti-saccade trials. Paired t-tests comparing brain activity for pro- and anti-saccade trials at the 227 ms peak revealed groups of sensors over left and right superior and inferior parietal regions (see Figure 3) that had more extreme VEP amplitudes during anti- (average voltage across both clusters=1.10 μV, SD=0.1) than pro-saccade trials (average voltage across both clusters=0.52 μV, SD=0.1). The sLORETA solution on this between-condition voltage difference indicated increased activity in bilateral visual cortex and superior parietal lobe during anti- compared to pro-saccade trials.
ssVEP response to peripheral stimuli
We used saccade type (pro versus anti) by stimulus location (left versus right) by time interval (eleven 500 ms intervals beginning 500 ms before steady-state stimulus onset) repeated-measures ANOVAs to analyze (i) inter-trial phase locking (ITPL) and (ii) single trial spectral power in relation to the peripheral flickering checkerboards during the sustained preparatory period. For ITPL there was only a significant main effect of time interval, F(10,140)=13.6, p<.001, indicating that synchronization of neuronal firing increased from the beginning to the end of steady-state stimulation in both saccade conditions (see Figure 4). For single trial spectral power, there was a significant main effect of saccade type F(1,14)=9.9, p=.007, indicating that participants had differences in neural gain control of visual cortex signals between pro- and anti-saccade trials (see Figure 4). Four follow-up analyses were also conducted on single trial power: (1) activity in the 500 ms pre-stimulus period did not differ significantly between pro- and anti-saccade conditions, t(14)=1.83, p=.089, although the antisaccade condition tended to have lower power than the prosaccade condition even before stimuli onset; (2) during prosaccade trials, pre-stimulus activity did not differ significantly from averaged post-stimulus activity, t(14)=0.61, although power was higher after stimuli onset; (3) during antisaccade trials, pre-stimulus activity did not differ significantly from averaged post-stimulus activity, t(14)=1.69, p=.113, although power was lower after stimuli onset; and (4) averaged pro- and anti-saccade activities during the post-stimulus period were significantly different even after adjusting for differences in pre-stimulus activities, t(14)=2.92, p=.011.
Figure 4.

Inter-trial phase locking (ITPL; top plot) and single trial power (bottom plot) in relation to the flickering checkerboards during the preparatory period prior to the target onset. Time is on the x-axis and standardized ITPL and single trial power are on the y-axis for the top and bottom plots, respectively. The mean (SE) values are shown for 500 ms bins for both pro- (solid) and anti-saccade (dashed) trials. The checkerboards began flickering at 0 ms (indicated by the stimuli screen insert and “Flicker onset” label) and flickered through the entire post-stimuli onset time illustrated here.
Discussion
Executive control involves using learned rules to guide context appropriate behavioral responses and is supported by PFC (see Bunge, 2004, and Miller & Cohen, 2001, for a review). PFC ostensibly accomplishes this task by influencing activity of cortical and subcortical neurons involved in behavioral regulation via top-down control. Research on cognitive operations that require motor responses for their successful completion typically focuses on manifestations of top-down control in frontal cortical and subcortical motor output structures. This literature parallels a largely separate one documenting top-down influences on sensory perceptual systems in the control of visual spatial attention (Desimone & Duncan, 1995). The interplay between the top-down regulation of motor processes and external perceptual stimulus registration has been infrequently examined (but see Buchsman & Miller, 2007) despite its potential importance for efficiently influencing behavior guided by sensory cues (e.g., Yantis & Jonides, 1996).
The present study expands the literature on top-down regulatory control of motor preparation by measuring the neural correlates of visual sensory registration in preparation for antisaccade responses. Antisaccade tasks are well-suited for assessing the manifestations of top-down control on both sensory and motor structures in humans given an extensive understanding of the supporting neurophysiology through primate and human studies, because of their established lifespan developmental profile (e.g., Luna, Garver, Urban, Lazar, & Sweeney, 2004; Sweeney, Rosano, Berman, & Luna, 2001), their abnormality in psychiatric patients with executive control problems (Harris, Reilly, Keshavan, & Sweeney, 2006; Hutton et al. 2004; McDowell, Myles-Worsley, Coon, Byerley, & Clementz, 1999), and their deficits in patients with focal damage to PFC (Hamilton & Martin, 2005; Pierrot-Deseilligny et al., 2003; Ploner, Gaymard, Rivaud-Pechoux, & Pierrot-Deseilligny, 2005). Primate neurophysiology studies indicate that successful antisaccade performance requires a sustained anticipatory reduction of neural activity in specific regions of saccade motor circuitry prior to stimulus appearance (Everling & DeSouza, 2005), an effect related to top-down control imposed by PFC (Johnston & Everling, 2006). The results of the present experiment add complementary and unique information to this literature by demonstrating an analogous effect in the human visual system on correct antisaccade trials.
Stimulus Onset Effects: Manifestation of Cognitive Complexity
When subjects began processing the peripheral flickering stimuli that identified possible peripheral target locations, their initial visual responses (VEPs) were stronger during anti- than pro-saccade trials (at 135 and 227 ms) in visual and medial extrastriate, superior parietal, and mesial prefrontal brain regions, and stronger during pro- than anti-saccade trials (at 196 ms) in lateral extrastriate cortex (in the vicinity of middle occipital gyrus). These cortical activation differences early in sensory stimulus processing as a function of saccadic task demands are consistent with previous studies (e.g., Clementz et al., 2007, Dyckman et al., 2007; McDowell et al., 2005; Everling, Matthews, & Flohr, 2001). Indeed, it is typical to find stronger responses on antisaccade trials when brain regions outside of visual cortex are involved (e.g., Clementz et al., 2007; McDowell et al., 2005), and stronger responses during prosaccade trials when only extrastriate cortex is involved, presumably because of the need to immediately prepare a saccadic response to peripheral visual stimulus (e.g., Dyckman et al., 2007).
Increased neural activity in parieto-frontal circuitry during the initial phases of stimulus processing may be related to the extra cognitive and accompanying neurophysiological requirements associated with correct antisaccade performance. On correct antisaccade trials subjects must suppress a response to a peripheral stimulus, plan a response to an opposite visual field location, and then execute that response. The greater complexity of anti- versus pro-saccade tasks can yield greater initial involvement of dorsal visual stream circuitry (e.g., Curtis & D'Esposito, 2003; Dyckman et al., 2007; Ford, Goltz, Brown, & Everling, 2005; Ettinger et al., 2008;; Luna et al., 2001; Reynolds & Chelazzi, 2004). In addition, increased activity in PFC, a brain region supporting higher cognitive functions, such as attention, planning, spatial orientation, and behavioral restraint (Goldman-Rakic, 1995; Miller & Cohen, 2001), is also frequently reported on correct antisaccade trials (Dyckman et al., 2007; Ettinger et al., 2008; Ford et al., 2005).
Preparatory Period Effects: Manifestation of Top-Down Control on Visual Cortex
After the initial neural response to peripheral stimuli onset, single trial ssVEP power from visual cortex was also strongly modulated as a function of task demands. On prosaccade trials, the amount of neural activity in relation to the peripheral flickering stimuli was high throughout the preparatory interval and tended to increase as time of target onset neared. On antisaccade trials, however, amount of power was consistently lower than during prosaccade trials in relation to the peripheral flickering stimuli, and tended to decrease from the pre-stimulus period through the post-stimulus period. The ssVEP power difference between pro-and anti-saccade trials was especially striking given that (i) physical properties of sensory stimuli at the peripheral target locations were the same throughout the preparatory period, and (ii) this difference on single trial power was manifest in the context of highly similar ITPL over the course of the preparatory period. Results of the initial response to stimuli onset (VEP) and from the sustained effects captured by ssVEPs provide an illustration that these different approaches to assessing brain activity using EEG data may yield independent and complimentary information (e.g., Clementz et al., 2008; Müller & Hillyard, 2000). The phasic VEP largely indexed the increased cognitive control requirements associated with antisaccade tasks while the tonic ssVEP indexed the manifestation of contextually specific sensory bias signals necessary to support proper antisaccade performance.
In the present study, sustained top-down control in relation to the peripheral flickering checkerboards during correct antisaccade performance was manifest as electrocortical suppression of visual cortex activity (reduced single trial power), not as modification of inter-trial phase alignment. Alterations in synchronization of neural activity is theoretically a “gating” and/or filtering mechanism for modifying information flow through sensory systems (Hillyard & Anllo-Vento, 1998; Steinmetz, et al., 2000), and perhaps for coordinating timing between local and distant neural populations (Sauseng & Klimesch, 2008). During antisaccade tasks, accurately and precisely localizing the peripheral cue is critical to successful performance. Gating inflow of relevant sensory signals via altering neural synchronization, thus disrupting fidelity of sensory cue registration, would be counterproductive. Rather, modifying neural gain control seemed to be employed, manifest as changes in the power of neural activity in visual cortex in relation to peripheral stimuli. This strategy may help reduce the probability that neural activity in motor output circuitry will reach the threshold for movement generation in relation to the peripheral cue on an antisaccade trial (e.g., Munoz & Everling, 2004), but still allow for reasonable fidelity of stimulus registration via phase synchronization.
This reduction of visual cortex activity, as measured by reduced ssVEP power on antisaccade trials, is reminiscent of similar preparatory reductions seen in saccade motor circuitry (Everling & DeSouza, 2005), an effect attributed to top-down control imposed by PFC (Johnston & Everling, 2006). This attenuation during the preparatory period theoretically reduces the predisposition to movement generation instigated by visual capture, and contributes to successful suppression of contextually inappropriate saccades (i.e. error responses) to peripheral targets (Munoz & Everling, 2004). Anticipatory reductions in visual cortex activity based on expected behavioral response demands also parallel results in other literatures demonstrating visual spatial attentional modulation of context-dependent stimulus processing (Desimone & Duncam, 1995; Kastner & Ungerleider, 2000). This similar finding during an antisaccade task is consistent with a special functional significance associated with top-down regulation of sensory input to the motor systems as a complementary strategy for voluntarily controlling behavioral response generation in a context appropriate manner (see also Buschman & Miller, 2007). In addition, visual cortex (including striate and extrastriate visual regions) has access to the brainstem saccade generators through the superior colliculus (Collins, Lyon, & Kaas, 2005; Lock, Baizer, & Bender, 2003), so it might be expected that the response of neurons in striate and extrastriate regions could facilitate prediction of subsequent saccadic responses. Such effects have been demonstrated during sustained attention-related tasks (e.g. Chawla, Rees, & Friston, 1999; Driver & Frith, 2000; Pessoa, Kastner, & Ungerleider, 2003). It would be important in future studies, by virtue of either selecting for or using manipulations that increase behavior performance variance, to determine whether a quantified indicator of strength of top-down control (e.g., PFC activity) mediates the relationship between the observed anticipatory reductions in visual cortex activity and correct antisaccade responding.
Acknowledgments
This works was supported by grants from the United States Public Health Service (MH51129, MH001852).
References
- Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cogn Affect Behav Neurosci. 2004;4(4):564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 1999;2(7):671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Brahmbhatt SB, McDowell JE, Brown R, Sweeney JA. When does the brain inform the eyes whether and where to move? An EEG study in humans. Cereb Cortex. 2007;17(11):2634–2643. doi: 10.1093/cercor/bhl171. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Wang J, Keil A. Normal electrocortical facilitation but abnormal target identification during visual sustained attention in schizophrenia. J Neurosci. 2008;28(50):13411–13418. doi: 10.1523/JNEUROSCI.4095-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Collins CE, Lyon DC, Kaas JH. Distribution across cortical areas of neurons projecting to the superior colliculus in new world monkeys. Anat Rec A Discov Mol Cell Evol Biol. 2005;285(1):619–627. doi: 10.1002/ar.a.20207. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7(9):415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Taddei F, Apnile T, Spinelli D. Neural correlates of fast stimulus discrimination and response selection in top-level fencers. Neurosci Lett. 2006;408(2):113–118. doi: 10.1016/j.neulet.2006.08.085. [DOI] [PubMed] [Google Scholar]
- Ding J, Sperling G, Srinivasan R. Attentional modulation of SSVEP power depends on the network tagged by the flicker frequency. Cereb Cortex. 2006;16:1016–29. doi: 10.1093/cercor/bhj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganova R, Popivanov D. Assessment of EEG frequency dynamics using complex demodulation. Physiol Res. 1999;48(2):157–165. [PubMed] [Google Scholar]
- Driver J, Frith C. Shifting baselines in attention research. Nat Rev Neurosci. 2000;1(2):147–148. doi: 10.1038/35039083. [DOI] [PubMed] [Google Scholar]
- Dyckman KA, Camchong J, Clementz BA, McDowell JE. An effect of context on saccade-related behavior and brain activity. Neuroimage. 2007;36(3):774–784. doi: 10.1016/j.neuroimage.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Dyckman KA, McDowell JE. Behavioral plasticity of antisaccade performance following daily practice. Exp Brain Res. 2005;162(1):63–69. doi: 10.1007/s00221-004-2105-9. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Ffytche DH, Kumari V, Kathmann N, Reuter B, Zelaya F, Williams SC. Decomposing the neural correlates of antisaccade eye movements using event-related FMRI. Cereb Cortex. 2008;18(5):1148–1159. doi: 10.1093/cercor/bhm147. [DOI] [PubMed] [Google Scholar]
- Everling S, DeSouza JF. Rule-dependent activity for prosaccades and antisaccades in the primate prefrontal cortex. J Cogn Neurosci. 2005;17(9):1483–1496. doi: 10.1162/0898929054985455. [DOI] [PubMed] [Google Scholar]
- Everling S, Matthews A, Flohr H. Prestimulus cortical potentials predict the performance in a saccadic distractor paradigm. Clin Neurophysiol. 2001;112(6):1088–1095. doi: 10.1016/s1388-2457(01)00526-0. [DOI] [PubMed] [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clin Neurophysiol. 2001;112(3):536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MR, Everling S. Neural processes associated with antisaccade task performance investigated with event-related FMRI. J Neurophysiol. 2005;94(1):429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Kastner J, Wagner M, Hawes S, Ebersole JS. A standardized boundary element method volume conductor model. Clin Neurophysiol. 2002;113(5):702–712. doi: 10.1016/s1388-2457(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Baldeweg T, Croft RJ, Whittington M, Gruzelier J. Gamma and beta frequency oscillations in response to novel auditory stimuli: A comparison of human electroencephalogram (EEG) data with in vitro models. Proc Natl Acad Sci U S A. 2000;97(13):7645–7650. doi: 10.1073/pnas.120162397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamalainen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput. 1994;32(1):35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Hamilton AC, Martin RC. Dissociations among tasks involving inhibition: a single-case study. Cogn Affect Behav Neurosci. 2005;5(1):1–13. doi: 10.3758/cabn.5.1.1. [DOI] [PubMed] [Google Scholar]
- Harris MS, Reilly JL, Keshavan MS, Sweeney JA. Longitudinal studies of saccades in antipsychotic-naive first-episode schizophrenia. Psychol Med. 2006;36(4):485–494. doi: 10.1017/S0033291705006756. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci U S A. 1998;95(3):781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Saur R, Lasogga S, Plewnia C, Erb M, Wildgruber D, Grodd W, Gerloff C. To act or not to act. Neural correlates of executive control of learned motor behavior. Neuroimage. 2004;23(4):1391–1401. doi: 10.1016/j.neuroimage.2004.07.070. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Huddy V, Barnes TR, Robbins TW, Crawford TJ, Kennard C, Joyce EM. The relationship between antisaccades, smooth pursuit, and executive dysfunction in first-episode schizophrenia. Biol Psychiatry. 2004;56(8):553–559. doi: 10.1016/j.biopsych.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Jammalamadaka SR, SenGupta A. Topics in Circular Statistics. World Scientific Press; Singapore: 2001. World Scientific Publishing Co. Pte. Ltd. [Google Scholar]
- Johnston K, Everling S. Neural activity in monkey prefrontal cortex is modulated by task context and behavioral instruction during delayed-match-to-sample and conditional prosaccade-antisaccade tasks. J Cogn Neurosci. 2006;18(5):749–765. doi: 10.1162/jocn.2006.18.5.749. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Krusemark EA, Campbell WK, Clementz BA. Attributions, deception, and event related potentials: An investigation of the self-serving bias. Psychophysiology. 2008 doi: 10.1111/j.1469-8986.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W. Spatial analysis of evoked potentials in man--a review. Prog Neurobiol. 1984;23(3):227–250. doi: 10.1016/0301-0082(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295(5555):690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Treue S. Feature-based attention in visual cortex. Trends Neurosci. 2006;29(6):317–322. doi: 10.1016/j.tins.2006.04.001. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Dyckman KA, Austin BP, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 2008;68(3):255–270. doi: 10.1016/j.bandc.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JE, Kissler JM, Berg P, Dyckman KA, Gao Y, Rockstroh B, Clementz BA. Electroencephalography/magnetoencephalography study of cortical activities preceding prosaccades and antisaccades. Neuroreport. 2005;16(7):663–668. doi: 10.1097/00001756-200505120-00002. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Myles-Worsley M, Coon H, Byerley W, Clementz BA. Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology. 1999;36(1):138–141. doi: 10.1017/s0048577299980836. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moratti S, Clementz BA, Gao Y, Ortiz T, Keil A. Neural mechanisms of evoked oscillations: stability and interaction with transient events. Hum Brain Mapp. 2007;28(12):1318–1333. doi: 10.1002/hbm.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MM, Hillyard S. Concurrent recording of steady-state and transient event-related potentials as indices of visual-spatial selective attention. Clin Neurophysiol. 2000;111(9):1544–1552. doi: 10.1016/s1388-2457(00)00371-0. [DOI] [PubMed] [Google Scholar]
- Müller MM, Hubner R. Can the spotlight of attention be shaped like a doughnut? Evidence from steady-state visual evoked potentials. Psychol Sci. 2002;13(2):119–124. doi: 10.1111/1467-9280.00422. [DOI] [PubMed] [Google Scholar]
- Müller MM, Malinowski P, Gruber T, Hillyard SA. Sustained division of the attentional spotlight. Nature. 2003;424(6946):309–312. doi: 10.1038/nature01812. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5(3):218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. A theoretical basis for standing and traveling brain waves measured with human EEG with implications for an integrated consciousness. Clin Neurophysiol. 2006;117(11):2424–2435. doi: 10.1016/j.clinph.2006.06.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Pastor MA, Artieda J, Arbizu J, Valencia M, Masdeu JC. Human cerebral activation during steady-state visual-evoked responses. J Neurosci. 2003;23(37):11621–11627. doi: 10.1523/JNEUROSCI.23-37-11621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23(10):3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain. 2003;126(Pt 6):1460–1473. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- Ploner CJ, Gaymard BM, Rivaud-Pechoux S, Pierrot-Deseilligny C. The prefrontal substrate of reflexive saccade inhibition in humans. Biol Psychiatry. 2005;57(10):1159–1165. doi: 10.1016/j.biopsych.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Regan MP, Regan D. Techniques for investigating and exploiting nonlinearities in brain processes by recording responses evoked by sensory stimuli. In: Lu Z, Kaufman L, editors. Magnetic source imaging of the human brain. Lawrence Erlbaum Associates; Mahwah, NJ: 2003. pp. 135–157. [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci Biobehav Rev. 2008;32(5):1001–1013. doi: 10.1016/j.neubiorev.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Soufflet L, Boeijinga PH. Linear inverse solutions: simulations from a realistic head model in MEG. Brain Topogr. 2005;18(2):87–99. doi: 10.1007/s10548-005-0278-6. [DOI] [PubMed] [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404(6774):187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Keedy SK, McDowell JE, Clementz BA. fMRI studies of eye movement control: investigating the interaction of cognitive and sensorimotor brain systems. Neuroimage. 2007;36(Suppl 2):T54–60. doi: 10.1016/j.neuroimage.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Rosano C, Berman RA, Luna B. Inhibitory control of attention declines more than working memory during normal aging. Neurobiol Aging. 2001;22(1):39–47. doi: 10.1016/s0197-4580(00)00175-5. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. A convenient method for detecting electrolyte bridges in multichannel electroencephalogram and event-related potential recordings. Clin Neurophysiol. 2001;112(3):545–550. doi: 10.1016/s1388-2457(00)00553-8. [DOI] [PubMed] [Google Scholar]
- Wang J, Clementz BA, Keil A. The neural correlates of feature-based selective attention when viewing spatially and temporally overlapping images. Neuropsychologia. 2007;45(7):1393–1399. doi: 10.1016/j.neuropsychologia.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Attentional capture by abrupt onsets: new perceptual objects or visual masking? J Exp Psychol Hum Percept Perform. 1996;22(6):1505–1513. doi: 10.1037//0096-1523.22.6.1505. [DOI] [PubMed] [Google Scholar]


