Abstract
Objective
After univentricular Fontan conversion, systemic venous pressure serves as the sole driving force for transpulmonary blood flow. Consequently, systemic venous return is markedly altered and ventricular filling is subnormal. The mechanisms and time course of systemic adaptation to Fontan conversion are incompletely understood. We hypothesized that acute elevation in systemic venous pressure induces an adaptive response similar to conversion to a univentricular Fontan circulation.
Methods
Adjustable vessel occluders were placed around the superior and inferior vena cavae in juvenile sheep. After one-week recovery, occluders were tightened to acutely increase and maintain systemic venous pressure at 15 mmHg (n=6), simulating one-stage Fontan conversion. Control animals (n=4) received identical surgery, but venous pressure was not manipulated.
Results
Cardiac index decreased significantly (3.9±1.0 to 2.7±0.7 ml/min/m2, P<0.001), then normalized to Control at 2 weeks. Circulating blood volume increased (100±9.4 vs. 85.5±8.4 ml/kg, P=0.034) as a persistent response. Cardiac reserve improved, and was not different from Control, by week 3. Resting heart rate decreased in both groups. Oxygen extraction (A-VO2 difference) and neurohormonal mediators increased transiently, then normalized by week 2.
Conclusions
Adaptation to global elevation in systemic venous pressure to Fontan levels is complete within 2 weeks. Increased blood volume and reduced heart rate are persistent responses. Increased oxygen extraction and neurohormonal upregulation are temporary responses which normalize with recovery of cardiac output. With improved physiologic understanding of systemic adaptation to Fontan conversion, approaches to single ventricle palliation can be more objectively assessed and optimized.
Introduction
Fontan repair of functional single ventricle is performed using a staged surgical approach. This strategy, which has evolved on a clinical basis, remains problematic [1]. Although stage II volume unloading of the single ventricle provides early partial ventricular volume reduction and improvement in hypoxemia, counter to assumptions, it is not associated with improved late ventricular function or exercise performance [2]. Thus, the majority benefit of interim staging exists early, likely from takedown of the systemic-to-pulmonary arterial shunt. Furthermore, evidence increasingly suggests that early Fontan conversion is associated with improved long-term ventricular function, incidence of normal sinus rhythm, and freedom from AV valve insufficiency (all presumably secondary to decreased interval of ventricular volume overload) [1,2]. This is consistent with current trends towards earlier repair. As Fontan conversion at an early age may improve long-term outcome by limiting the period of ventricular volume overload and hypoxemia associated with an interim-staged approach, best long-term status may derive from avoidance of ventricular volume load or use of a shunt altogether: Hence, early primary repair [3].
In select patients, Fontan conversion can be performed in one stage with excellent results [4]. This has fallen out of favor due to improved safety margin and early outcomes achieved with interim palliation (earlier take-down of the systemic arterial shunt, with partial improvement in hypoxemia, partial reduction in ventricular volume load, and partial return to series circulation). Perhaps more importantly, however, staged conversion imposes much less physiologic stress on the systemic venous, capillary, and interstitial circulation with partial (SVC), rather than global (SVC + IVC), increase in systemic venous pressure. Because decisions to perform Fontan staging have historically revolved around downstream pulmonary vascular resistance (ironically exacerbated by shunt physiology), the upstream systemic aspects of Fontan conversion have been largely overlooked.
Coupled with these questions, evolving circulatory support concepts are making compression of staging and earlier conversion to Fontan an increasingly realistic consideration [5]. Temporary mechanical cavopulmonary assist can support the time-limited process of systemic and interstitial adaptation to global increase in systemic venous pressure. While it may improve the margin of safety for direct Fontan conversion, the duration required to bridge to unsupported Fontan is not known. The goal of this study is to define the physiologic events and timeframe to Fontan steady state - with respect to the systemic circulation.
Material and Methods
Animal model
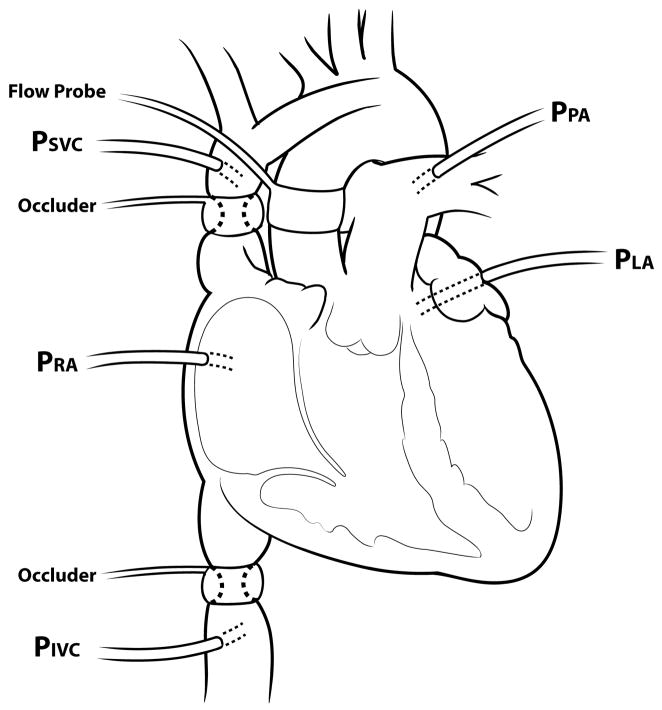
Juvenile sheep (3 mo., n=10) underwent right thoracotomy. The right and left azygous veins were ligated. Transthoracic pressure monitoring lines were placed in the pulmonary artery, aorta, superior vena cava (SVC), inferior vena cava (IVC), right atrium, and left atrium. An ultrasonic flowprobe (Transonic, Ithaca, NY) was placed around the ascending aorta. Percutaneous adjustable vessel occluders (Fine Science Tools, Foster City, CA) were placed around the juxtacardiac IVC and SVC (Figure 1). During one week recovery, normal hemodynamics were maintained. Transthoracic pressures (zeroed at olecranon; head neutral) and cardiac output were monitored daily. Animals were allowed unrestricted access to food and water.
Figure 1.
Experimental model (PSVC: pressure superior vena cava; PIVC: pressure inferior vena cava; PRA: pressure right atrium; PLA: pressure left atrium; PPA: pressure pulmonary artery).
Systemic Venous Pressure Elevation
On experimental day 0 (day of intervention), baseline hemodynamics were measured. In 6 animals, vena caval occluders were incrementally tightened over 15–20 minutes to achieve SVC and IVC pressure 14–15 mmHg. This was selected as the upper limit of “normal” systemic venous pressure in patients with Fontan, a range which allows documentation of hemodynamic response with few clinical symptoms. Aortic, SVC, and IVC samples were drawn before and after the pressure increase for oxygen saturation (OSM3 hemoximeter, Radiometer, Westlake, OH). Venous pressure was monitored twice daily for 5 days to ensure consistency; beyond Day 5, occluder adjustment was seldom required. Control animals (n=4) were identically monitored, with no manipulation of venous pressure.
Assessment of Cardiac Reserve
Dobutamine infusion (10 mcg/kg/min, 20 min) was used to quantify cardiac reserve at 5 time points: Study 1) Experimental day -1 (one day prior to venous restriction) for baseline; Study 2) Experimental day 3; Study 3) Day 7; Study 4) Day 14; and Study 5) Day 21. Hemodynamic variables were recorded at 5 minute intervals, including 5 minutes after termination. Aortic, SVC, and IVC samples were drawn before and after dobutamine infusion for oxygen saturation.
Cerebral Oximetry
Cerebral oximetry was monitored using near infrared spectroscopy (Somanetics Inc, Troy, MI) in 4 experimental animals at the time of venous pressure increase and during dobutamine challenges.
Circulating Blood Volume
Blood volume was determined at the time of surgery, the day following venous pressure intervention, and prior to all dobutamine studies using dilution technique (Evan’s Blue dye) [6]. Absorbance of plasma drawn from the SVC was measured using a standard spectrophotometric curve. Total blood volume (ml/kg) was calculated from plasma volume and hematocrit (Blood volume = Plasma volume/(1−Hematocrit)). Animals were weighed the same day of blood volume determination.
Neurohormonal Markers
Serum and plasma were collected for antidiuretic hormone (ADH), aldosterone, angiotensin II, epinephrine, norepinephrine, and brain natriuretic peptide (BNP) determination prior to administering Evan’s Blue dye or dobutamine on Studies 1–5, as well as the day following venous pressure increase. Samples were frozen at − 80C and batch analyzed (Anilytics Inc., Gaithersburg, MD).
Statistical analysis
Results are reported as mean ± standard deviation. Data was analyzed by pairwise comparison within and between control and experimental groups, including time factor for all time points, by two-way ANOVA with correction for repeated measures using Student-Neumal-Kuels post-hoc test. Cerebral oxygen saturation data was analyzed by regression. Significance was defined as P ≤0.05. Statistical analysis was performed with Sigmastat software (Systat, Richmond, CA).
The experimental protocol was approved by the Animal Care and Use Committee of the Indiana University School of Medicine. Animals received humane care in accordance with the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication 86–23, revised 1996).
Results
Hemodynamic response to acute elevation in systemic venous pressure
The 3-fold elevation in systemic venous pressure was surprisingly well-tolerated; only one animal became unsteady and needed to lie down. In all instances, venous pressure of at least 12 mmHg was achieved at the initial intervention; goal pressure was achieved in most. Pressure adjustments (4–5 mmHg) were common in the afternoon of experimental day 0, but were subsequently few and minor (< 2–3 mmHg). SVC and IVC pressures were maintained at 15 mmHg over the duration of the study (Figure 2). Cardiac index decreased significantly in the Experimental group, but normalized by Day 14 to Control (Figure 3). Systemic arterial blood pressure was not affected by increased systemic venous pressure, although heart rate did decrease significantly in Control animals over the last two weeks of the study (Table EI). A trend toward lower mean pulmonary arterial pressure in Experimental animals reached significance on experimental days 2, 4, 5, 7, 8, 10, and 16. Pulmonary vascular resistance increased in the Experimental group in concert with decreased right ventricular preload and cardiac index.
Figure 2.
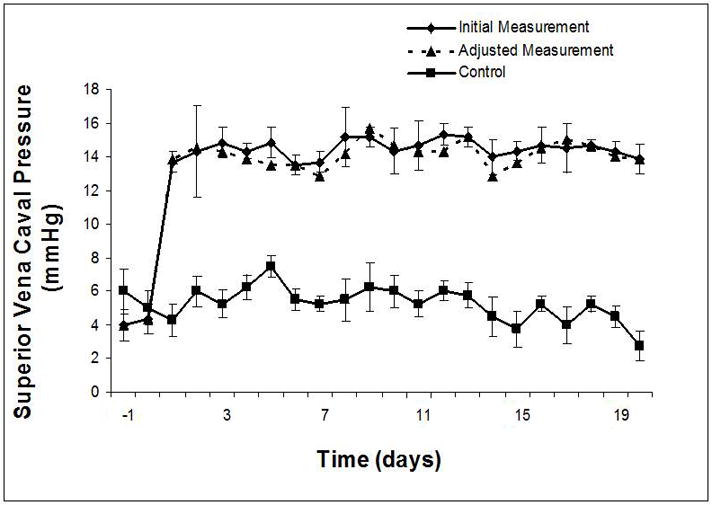
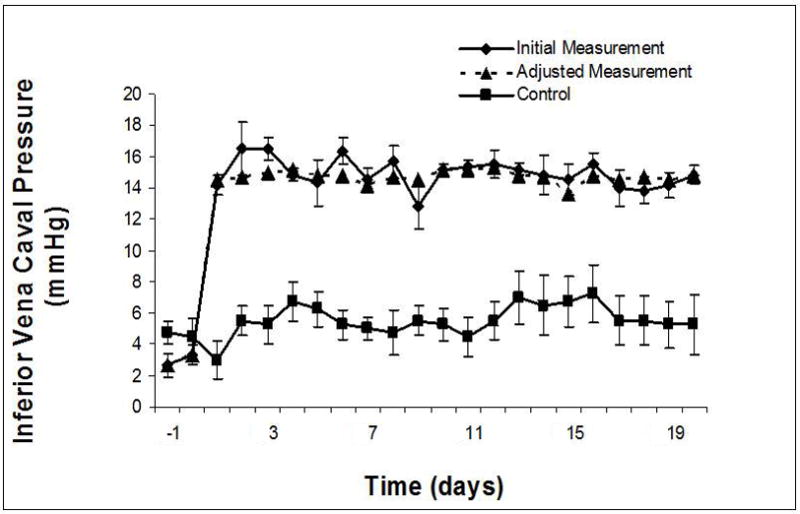
Systemic venous pressure: increased and maintained at goal range (14–15 mmHg) in SVC (A) and IVC (B) distributions days 1–20. Adjusted measurement indicates adjustment required in vena caval restriction to maintain measured systemic venous pressure within goal parameters after the initial value at that time interval had been determined.
Figure 3.
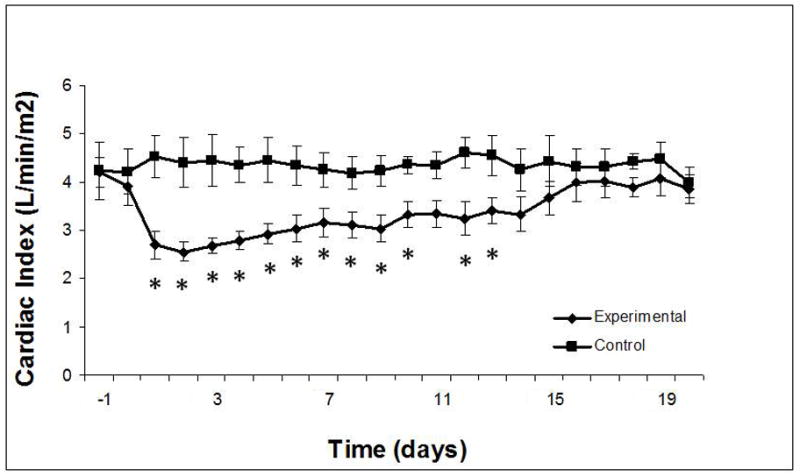
Cardiac index: significant reduction after intervention, with normalization by day 14. * significant difference between experimental and control groups (P ≤0.05).
Cardiac reserve
A significant heart rate response occurred in both groups to dobutamine infusion, with no between-group difference (Figure E1A, B). In the Experimental group, cardiac index was significantly decreased in comparison to Control at baseline for studies 2 and 3, and after dobutamine for studies 2, 3, and 4 (Figure E1C, D). Controls had a significant increase in cardiac index from baseline for all 5 studies, but Experimental animals did not significantly augment cardiac index in response to dobutamine during studies 2 and 3.
Blood Volume
Blood volume increased in the Experimental group and was significantly higher than baseline on Days 3, 7, 14, and 20. Blood volume was significantly higher than Control on days 14 and 20 (Figure 4).
Figure 4.
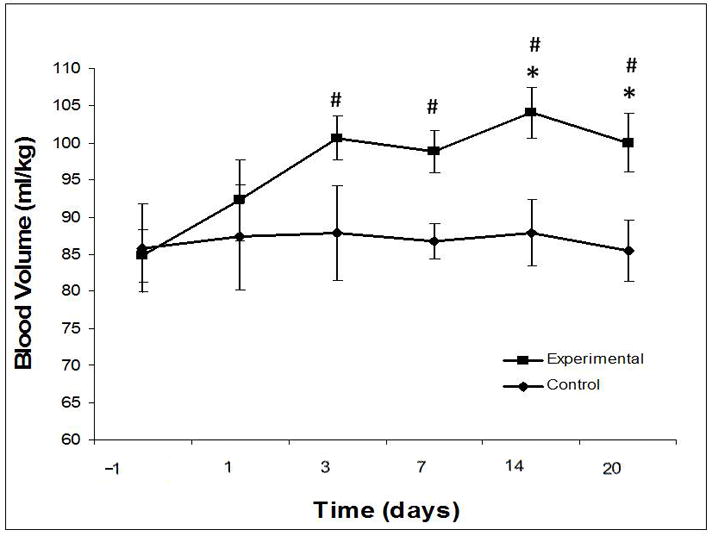
Blood volume. * significant difference (P ≤0.05) from control. # significant difference (P ≤0.05) from baseline.
Neurohormonal markers
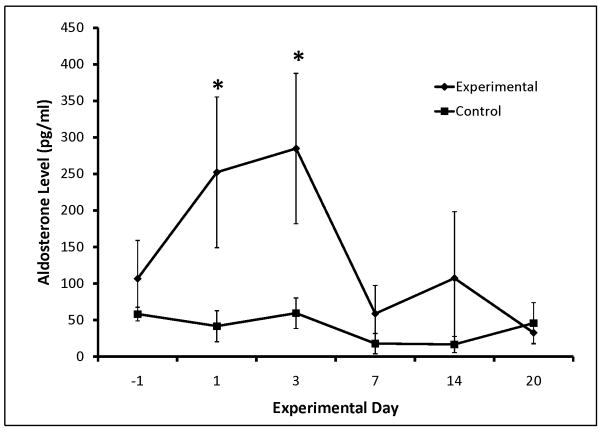
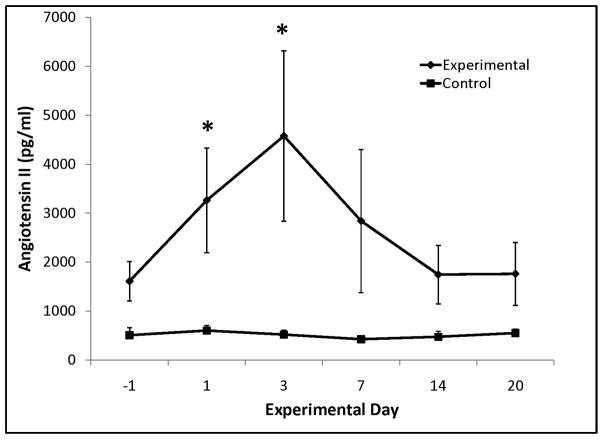
Aldosterone and angiotensin II were significantly elevated in the Experimental group early following the increase in systemic venous pressure, but normalized to Control after week 1 (Figure 5). ADH also increased early, but was not significant (Table EII). No statistical difference was seen in BNP, epinephrine, and norepinephrine levels in Experimental vs. Control groups (Table EII).
Figure 5.
Serum aldosterone (A) and angiotensin II (B). * significant difference (P ≤0.05) from control.
Blood gases and cerebral oximetry
Aortic oxygen saturation (Aortasat), SVCsat, and IVCsat data are presented in Table EI. SVC oxygen saturation (SVCsat) was significantly decreased at baseline for studies 2 and 3, and increased significantly in response to dobutamine during studies 2, 3, and 4 (Figure E2B). IVC oxygen saturation (IVCsat) was significantly decreased at baseline for all studies, and did not increase in response to dobutamine (Figure E2D). There was no change in SVCsat or IVCsat in Controls (Figure E2A,C). Cerebral saturation increased in response to dobutamine in all cases (Figure E2E). Oxygen extraction (Aortasat – SVCsat or IVCsat) was calculated. Significant group differences in SVC oxygen extraction were seen on Days 1, 2, 3, 7 (Figure E3A). Group differences in IVC oxygen extraction were seen on Days 2 and 3 (Figure E3B). Cerebral oximetry correlated with SVC and IVC oxygen saturation both before and after the increase in venous pressure and dobutamine studies. A total of 48 measurements were taken each for cerebral oximetry, SVCsat, and IVCsat using 4 experimental animals. Cerebral oximetry values correlated significantly with SVCsat (R=0.789, P<0.001) and IVCsat (R=0.525, P<0.001) (Figure E4).
Necropsy
Left and right azygous vein ligation was confirmed in all cases. There was no evidence of venous thrombus. Small right pleural effusion (<100 cc) was present in most animals. One animal exhibited significant ascites (6L).
Comment
With increasing numbers of Fontan survivors reaching adulthood, the long-term sequelae of single ventricle Fontan palliation is now recognized an emerging public health concern [7]. Regression from compensated to uncompensated systemic venous hypertension is integral to the pathogenesis of both early and late Fontan failure [8,9]. Although long proposed, medical therapies aimed at controlling fluid retention, decreasing pulmonary resistance, and optimizing ventricular function via increased contractility and/or afterload reduction, remain limited and suboptimal.
Systemic compensatory mechanisms undoubtedly play an important role in Fontan homeostasis, including hemodynamic, interstitial, oncotic, neuroendocrine, and renin-angiotensin axis regulation. These mechanisms are poorly understood. Venous hypertension, construed as an immutable consequence of Fontan physiology, has been clinically approached as a problem to minimize or avoid. An impediment to objective resolution of this problem is that chronic models of Fontan do not exist; mechanisms underlying Fontan homeostasis and failure are not well-defined. Complete [10] and partial right heart bypass [11] in the context of mechanical support of cavopulmonary flow have been reported, but none have achieved chronic survival, limiting the ability to test therapies or draw conclusions beyond the perioperative period.
Hemodynamic response to restricted systemic venous return
In this study, systemic venous pressure was acutely and globally (both SVC and IVC distributions) elevated in awake, conscious animals as a systemic physiologic mimic of one-stage Fontan conversion. This differs from clinical staged Fontan conversion where pressure elevation in the SVC and IVC distributions are punctual events separated in time, and occurs in a setting of general anesthesia and cardiopulmonary bypass-associated myocardial dysfunction and capillary leak. Without superimposed capillary leak and third spacing, the findings in this study suggest that one-stage Fontan conversion may be readily achievable once perioperative fluid shifting is complete and lung parenchymal function is optimized. Normalization of cardiac reserve may reflect recovered ability to fill the right ventricle due to increased preload, although this is not borne out by measured left atrial pressure values. This demonstrates adaptation beyond resting cardiac index, for which increased blood volume presumably plays a key role.
Heart rate response to restricted systemic venous return
Heart rate disturbances and autonomic dysfunction are well-documented late after Fontan [12]. Sinus node dysfunction and bradycardia have been attributed to surgical injury near the atrial pacemaker complex. In this study, there was no difference in heart rate between Control and Experimental animals at any time point. There was an overall trend towards decreasing heart rate for both groups that reached significance after week 2 for Control animals. The etiology of the heart rate decrease is unclear, but it may be attributed to acclimatization to the testing procedures or post-surgical recovery. Both groups retained equivalent ability to increase heart rate in response to dobutamine, with no difference in basal epinephrine and norepinephrine levels, indicating no endogenous catecholamine response to systemic venous restriction. Counter to expectations that an increase in heart rate will occur in response to reduced cardiac output after Fontan, a decrease in heart rate may represent a natural compensatory mechanism aimed to prolong diastolic filling time. It may also be secondary to increased cerebral venous pressure. This has clinical implications for low resting heart rate in Fontan patients; recent studies have, in fact, independently associated low resting heart rate with higher functional status [13,14].
Cerebral oximetric response to restricted systemic venous return
Despite the inability to increase cardiac index in response to dobutamine, cerebral oximetry values and SVCsat increased during dobutamine infusion. IVCsat remained unchanged or decreased. This indicates that blood flow is preferentially shifted to the cerebral circulation in response to dobutamine. SVCsat and IVCsat both correlated well with cerebral oximetry, but oximetry values more closely approximated the change in SVCsat in response to dobutamine when initial saturation was subnormal. In agreement with clinical findings, cerebral oximetry strongly predicts SVCsat [15].
Neuroendocrine response to restricted systemic venous return
The neurohormonal response to intervention includes the following: 1) upregulation of the renin-angiotensin-aldosterone system (RAAS) by increase in angiotensin II and aldosterone, with subsequent normalization beyond one week (Figure 5); 2) increased blood volume in the period that RAAS is upregulated, and which persists after angiotensin II and aldosterone have normalized. Therefore, adaptive mechanisms beyond RAAS upregulation must remain in play to maintain increased blood volume. There is likely a shift in RAAS over time since its role in chronic Fontan physiology appears to differ from that in the immediate postoperative period.
In animal models, acute and chronic restriction of the IVC alone has been used to induce low cardiac output and an antinatriuretic response [16–18]. In these studies, diminished cardiac output, ascites formation, increased total peripheral resistance, decreased sodium and potassium excretion, and decreased glomerular filtration rate, and antinatriuresis are secondary to decrease in cardiac output and sodium retention mediated by RAAS. RAAS response has been examined clinically early and late following Fontan. It is upregulated early following Fontan and bidirectional Glenn cavopulmonary anastomosis [19]. It has been implicated in the development of effusions, but trials using angiotensin converting enzyme inhibitors have mixed results. Chronically after Fontan, adrenergic tone and RAAS is upregulated [20], although in one study blood volume was not significantly different from biventricular circulations [21]. A randomized controlled trial of angiotensin converting enzyme inhibitor administration late after Fontan demonstrated no improvement in exercise performance [22].
Is staged palliation the best long-term strategy?
Interim staged palliation may represent a trade-off between improved early outcomes, but worsened long-term status. Basal health of the pulmonary vascular bed, in part a reflection of previous staging procedures in which a shunt was utilized [23], is the critical inflection point upon which ultimate Fontan upstream systemic venous pressure and downstream ventricular filling depend. As a result, a palliative strategy designed to preserve ventricular function and basal pulmonary vascular resistance is of prime importance. Because a strategy of shunt palliation and intermediate staging may induce irreversible alterations in the myocardium prior to volume unloading, it can be argued that this may worsen candidacy for Fontan conversion and long-term functional status [1,2]. Consideration of approaches which may reduce, or eliminate, use of a shunt and/or interval staging is timely, clinically relevant, and merits investigation.
The case for direct Fontan repair
The best strategy may be primary Fontan repair with no staging at all: Precisely how this can be accomplished is a provocative and complex matter. Minimizing or avoiding Fontan staging (combined Stage-2 and -3, or Stage 1, 2, and 3 Fontan conversion) by performing primary Fontan repair, with temporary mechanical cavopulmonary assist has compelling theoretical advantages [10,3]. The interval of ventricular volume overload and hypoxemia is reduced or eliminated, which may reduce the incidence of ventricular hypertrophy and diastolic dysfunction. Additionally, minimization of hypoxemia and early return to normoxia would reduce the stimulus for pulmonary hypertension and pathologic pulmonary vascular remodeling. Finally, the interstage interval and a 2nd and or 3rd open-heart procedure, all significant risk factors for mortality, would be obviated by direct conversion to Fontan.
Duration of support as bridge to Fontan
Adaptation to Fontan venous pressure is complete within 2 weeks; this is a reasonable duration within which percutaneous mechanical circulatory support can be applied. During support, 2-ventricle physiologic conditions are replicated, with no unsafe upper limit to oxygenation, a more stable series circulation, and no ventricular volume overload. An obvious concern is risk of elevated pulmonary resistance in newborns. However, in normal human neonates, pulmonary resistance falls rapidly to within 10% of adult values within the first 2–3 days of life [24,25]. Experimental data that we have generated in newborn lambs with mechanically assisted cavopulmonary blood flow also support this, with normalization of pulmonary resistance in response to mechanical cavopulmonary assist within 8 hours [3]. Physiologic details remain to be determined, but, under ideal physiologic conditions, neonates are clearly capable of maintaining low pulmonary resistance. Adjuncts, such as selective pulmonary vasodilators, may facilitate transition from supported to unsupported neonatal Fontan repair of single ventricle.
Limitations
This is not a true model of a Fontan circulation; it is rather a model of systemic venous hypertension in a biventricular circulation. While the model produces chronic elevation in systemic venous pressure, it differs in important ways from a Fontan procedure. The right ventricle is maintained in the circulation, and the downstream resistor to venous return is fixed anatomic obstruction rather than physiologic pulmonary resistance. Blood is still pumped to the lungs by the right ventricle, rather than being driven passively by systemic venous pressure. The interactions of respiration, gravity, IVC and hepatic venous and transpulmonary blood flow, all of which are important in the Fontan circulation, are not accounted for. Additionally, the effects of pulmonary resistance on systemic impedance when added in series to the systemic circulation are not examined or accounted for.
Absent an existing chronic model of Fontan, however, it provides a reasonable estimate of the systemic transitional events which occur in one-stage Fontan conversion and may serve for study of systemic Fontan pathophysiology. In this analysis, selective restriction of SVC and IVC return to model intermediate staging was not performed; studies of this nature must account for venous collateral shunting to the opposing unrestricted venous territory. Because the study was performed in juvenile animals, firm conclusions regarding neonatal adaptation to elevated systemic venous pressure cannot be drawn. The findings in this study elucidate the mechanisms of global adaptation to Fontan-level venous pressure, and define the time course for this transition to stably occur. This information may be useful to guide optimization strategies for Fontan palliation.
Supplementary Material
Acknowledgments
Supported by NIH Grant #HL080089 (MDR), Research Support Funds Grant, IUPUI (MDR), Riley Children’s Foundation grant #08-05 (MDR), and with facility support by NIH Grant #C06 RR10601.
Footnotes
Presented in part at the Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting, May 2008, Honolulu, HI (CDM).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Backer CL. The Fontan procedure. Our odyssey continues. J Am Coll Cardiol. 2008;52:114–6. doi: 10.1016/j.jacc.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 2.Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, et al. Contemporary outcomes after the Fontan procedure. A pediatric heart network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodefeld MD, Boyd JH, Myers CD, Presson RG, Wagner WW, Brown JW. Cavopulmonary assist in the neonate: an alternative strategy for single-ventricle palliation. J Thorac Cardiovasc Surg. 2004;127:705–11. doi: 10.1016/j.jtcvs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Hsu DT, Quaegebeur JM, Ing FF, Silber EJ, Lamour JM, Gersony WM. Outcome after the single-stage, nonfenestrated Fontan procedure. Circulation. 1997;96(suppl II):II-335–40. [PubMed] [Google Scholar]
- 5.Rodefeld MD, Coats B, Fisher T, Giridharan GA, Chen J, Brown JW, Frankel SH. Cavopulmonary assist for the univentricular Fontan circulation: von Karman viscous impeller pump. Abstract AATS Boston. 2009 doi: 10.1016/j.jtcvs.2010.04.037. manuscript in review, JTCVS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wamberg S, Sandgaard NC, Bie P. Simultaneous determination of total body water and plasma volume in conscious dogs by the indicator dilution principle. J Nutr. 2002;132:1711S–3S. doi: 10.1093/jn/132.6.1711S. [DOI] [PubMed] [Google Scholar]
- 7.Williams R, Pearson GD, Barst RJ, Child JS, del Nido P, Gersony WM, et al. Report of the National Heart, Lung, and Blood Institute Working Group on Research in Adult Congenital Heart Disease. J Am Coll Cardiol. 2006;47:701–7. doi: 10.1016/j.jacc.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 8.Rychik J, Spray TL. Strategies to treat protein-losing enteropathy. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2002;5:3–11. doi: 10.1053/pcsu.2002.31498. [DOI] [PubMed] [Google Scholar]
- 9.Marino BS. Outcomes after the Fontan procedure. Curr Opin Pediatr. 2002;14:620–6. doi: 10.1097/00008480-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Rodefeld MD, Boyd JH, Myers CD, LaLone BJ, Bezruczko AJ, Potter AW, Brown JW. Cavopulmonary assist: circulatory support for the univentricular Fontan circulation. Ann Thorac Surg. 2003;76:1911–6. doi: 10.1016/s0003-4975(03)01014-2. [DOI] [PubMed] [Google Scholar]
- 11.Tsuda S, Sasaki T, Maeda K, Riemer RK, Reichenbach SH, Reinhartz O. Recovery during mid-term mechanical support of Fontan circulation in sheep. ASAIO J. 2009;55:406–11. doi: 10.1097/MAT.0b013e3181a0a570. [DOI] [PubMed] [Google Scholar]
- 12.Davos CH, Francis DP, Leenarts MF, Yap SC, Li W, Davlouros PA, et al. Global impairment of cardiac autonomic nervous activity late after the Fontan operation. Circulation. 2003;108(Suppl 1):II180–5. doi: 10.1161/01.cir.0000087946.47069.cb. [DOI] [PubMed] [Google Scholar]
- 13.Blaufox AD, Sleeper LA, Bradley DJ, Breitbart RE, Hordorf A, Kanter RJ, et al. Functional status, heart rate, and rhythm abnormalities in 521 Fontan patients 6 to 18 years of age. J Thorac Cardiovasc Surg. 2008;136:100–7. doi: 10.1016/j.jtcvs.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camposilvan S, Milanesi O, Stellin G, Pettenazzo A, Zancan L, D’Antiga L. Liver and cardiac function in the long term after Fontan operation. Ann Thorac Surg. 2008;86:177–82. doi: 10.1016/j.athoracsur.2008.03.077. [DOI] [PubMed] [Google Scholar]
- 15.Kirshbom PM, Forbess JM, Kogon BE, Simsic JM, Kim DW, Raviele AA, et al. Cerebral near infrared spectroscopy is a reliable marker of systemic perfusion in awake single ventricle children. Pediatr Cardiol. 2007;28:42–5. doi: 10.1007/s00246-006-1389-x. [DOI] [PubMed] [Google Scholar]
- 16.Schrier RW, Humphreys MH. Factors involved in antinatriuretic effects of acute constriction of the thoracic and abdominal inferior vena cava. Circ Res. 1971;29:479–89. doi: 10.1161/01.res.29.5.479. [DOI] [PubMed] [Google Scholar]
- 17.Schrier RW, Humphreys MH, Ufferman RC. Role of cardiac output and the autonomic nervous system in the antinatriuretic response to acute constriction of the thoracic superior vena cava. Circ Res. 1971;29:490–8. doi: 10.1161/01.res.29.5.490. [DOI] [PubMed] [Google Scholar]
- 18.Lifschitz MD, Schrier RW. Alterations in cardiac output with chronic constriction of thoracic inferior vena cava. Am J Physiol. 1973;225:1364–70. doi: 10.1152/ajplegacy.1973.225.6.1364. [DOI] [PubMed] [Google Scholar]
- 19.Francois K, Bove T, DeGroote K, Panzer J, Vandekerchove K, Suys B, et al. Pleural effusions, water balance mediators and the influence of lisinopril after completion Fontan procedures. Eur J Cardiothorac Surg. 2009;36:57–62. doi: 10.1016/j.ejcts.2009.02.059. [DOI] [PubMed] [Google Scholar]
- 20.Inai K, Nakanishi T, Nakazawa M. Clinical correlation and prognostic predictive value of neurohumoral factors in patients late after the Fontan operation. Am Heart J. 2005;150:588–94. doi: 10.1016/j.ahj.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Kelley JR, Mack GW, Fahey JT. Diminished venous vascular capacitance in patients with univentricular hearts after the Fontan operation. Am J Cardiol. 1995;76:158–63. doi: 10.1016/s0002-9149(99)80049-6. [DOI] [PubMed] [Google Scholar]
- 22.Kouatli AA, Garcia JA, Zellers TM, Weinstein EM, Mahony L. Enalapril does not enhance exercise capacity in patients after Fontan procedure. Circulation. 1997;96:1507–12. doi: 10.1161/01.cir.96.5.1507. [DOI] [PubMed] [Google Scholar]
- 23.Khambadkone S, Li J, de Leval MR, Cullen S, Deanfield JE, Redington AN. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation. 2003;107:3204–8. doi: 10.1161/01.CIR.0000074210.49434.40. [DOI] [PubMed] [Google Scholar]
- 24.Morin FC, Egan E. Pulmonary hemodynamics in fetal lambs during development at normal and increased oxygen tension. J Appl Physiol. 1992;73:213–8. doi: 10.1152/jappl.1992.73.1.213. [DOI] [PubMed] [Google Scholar]
- 25.Soifer S, Schreiber M, Heymann M. Leukotriene antagonists attenuate thromboxane-inducible pulmonary hypertension. Pediatr Res. 1989;26:83–7. doi: 10.1203/00006450-198908000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.