Abstract
The notion that family support may buffer individuals under adversity from poor outcomes has been theorized to have important implications for mental and physical health, but little is known about the biological mechanisms that explain these links. We hypothesized that adults who grew up in low socioeconomic status (SES) households but who experienced high levels of maternal warmth would be protected from the pro-inflammatory states typically associated with low SES. 53 healthy adults (ages 25–40) low in SES early in life were assessed on markers of immune activation and systemic inflammation. Genome-wide transcriptional profiling also was conducted. Low early life SES individuals who had mothers who expressed high warmth toward them exhibited less Toll-like receptor-stimulated production of interleukin 6, and reduced bioinformatic indications of pro-inflammatory transcription factor activity (NF-κB) and immune activating transcription factor activity (AP-1) compared to those who were low SES early in life but experienced low maternal warmth. To the extent that such effects are causal, they suggest the possibility that the detrimental immunologic effects of low early life SES environments may be partly diminished through supportive family climates.
Keywords: socioeconomic status, maternal warmth, immune
INTRODUCTION
Individuals lower in socioeconomic status (SES) consistently suffer poorer mental and physical health. They are at heightened risk for numerous mental health problems, including depression, anxiety, and disruptive disorders 1, 2, as well as for a variety of chronic medical illnesses, including cardiovascular disease, arthritis, and some cancers 3, 4. These relationships are robust across the lifespan 5, 6, as well as across both countries that do and do not have universal health care systems 7. These patterns make low SES one of the most robust risk factors for disease and disability.
Researchers have long speculated, however, that there are individuals who are able to thrive, despite confronting serious adverse circumstances such as being low in SES. This is the notion of resilience, that is, individual characteristics or situational processes that can buffer those facing adversity from poor outcomes 8, 9. In children, the types of factors that have most consistently been found to be protective against outcomes such as mood and antisocial disorders include supportive relationships with others such as close family relationships and authoritative parenting 10–13.
What has remained less clear, however, are the biological processes by which supportive families may protect against disease and disability related to adversity. One of the leading mechanistic hypotheses about how SES exerts its effects on health is via inflammation 14. Research shows, for example, that in healthy adults, low SES is associated with systemic inflammatory profiles, such as elevated levels of C-reactive protein (CRP) and interleukin 6 (IL-6) in circulation 15, 16. In children, low SES is associated with increased cytokine production in response to in vitro mitogen challenge in patients with inflammation-related diseases such as asthma 17, 18. More recently, functional genomics studies have indicated that several different types of social adversity, including low SES, social isolation, and chronic stress, are all associated with heightened signaling of pro-inflammatory transcription control pathways in both children and adults 19–23.
In turn, inflammation has been implicated as a key pathway in numerous illnesses ranging from depression to cardiovascular disease 24–27. For example, inflammation is known to contribute to the progression of coronary heart disease by facilitating the growth and rupturing of atherosclerotic plaque, and activating coagulation processes involved in thrombogenesis 26, 28. Mounting evidence indicates that inflammatory cytokines can also induce depressive symptoms 29, 30. Administration of inflammatory cytokines evokes a constellation of sickness behaviors that resembles depression, including anhedonia, social withdrawal, anorexia, and hyposomnia 31. In adults, those who are administered inflammatory cytokines as part of medical treatments are at greater risk for experiencing depressive symptoms, and this in turn can be prevented prophylactically by administering antidepressants 32.
Although low SES is associated with a pro-inflammatory profile, researchers have argued that it, and other physiological concomitants of adversity, can be offset by individual characteristics or supportive processes 33–35. No studies that we are aware of, however, have directly tested this hypothesis. Further, it is not known whether any buffering effects of positive early life factors could have lasting effects on biology into adulthood (the focus of previous research has been on current psychosocial factors that mitigate the biological profile of adversity in children and adults 36–38). In the animal literature, high levels of maternal licking/grooming and arched back nursing early in life buffer rats from elevated physiological responses to stressors later in life as adults 39–41. These findings suggest that early life maternal care may serve as one type of support factor that protects against detrimental biological responses in adult life.
In the present study, we hypothesized that among adults who faced adverse circumstances early in life (low SES), those who experienced high levels of maternal warmth in childhood would show reduced indications of pro-inflammatory signaling compared to those who experienced low maternal warmth. More specifically we hypothesized that as adults, low early life SES individuals who experienced high maternal warmth would show: (1) reduced activity of a key pro-inflammatory transcription factor, NF-κB, in bioinformatic analyses of genome-wide transcriptional profiles of peripheral blood mononuclear cells (PBMC); (2) reduced PBMC responsivity to in vitro stimulation, as indicated by lower expression of the pro-inflammatory cytokine IL-6; and (3) reduced systemic inflammation, as indicated by lower levels of circulating CRP. We tested secondary hypotheses that other transcription control pathways involved in immune activation, such as AP-1, would also show indications of reduced activity in low early life SES individuals who experienced high maternal warmth in childhood.
METHODS
Sample
Participants were 53 adults recruited from Vancouver, BC through postings in local media and pubic transit. To be eligible, they had to be 25–40 years of age and in good health, defined as being free of infection the past four weeks and without a history of psychiatric or chronic physical illnesses (by self-report). Participants also had to have been raised in a low-SES household in early life (details below). 26 were in the high maternal warmth group, and 27 were in the low maternal warmth group (details below). Participants represent the low SES subset of a larger study, described in more detail in 22. The project was approved by the University of British Columbia’s Research Ethics Board and all subjects gave written consent before participating.
Socioeconomic Status (SES)
SES was defined according to occupational status using the United Kingdom’s National Statistics Socio-economic Classification 42. This study focused on individuals who fell into the low SES category, based on their parents having routine, manual, or lower supervisory occupations. For classification of early-life SES, we contacted each subject’s mother or father by telephone, and inquired about their occupation (and their spouse’s) during their child’s first five years of life (including the in utero period). Given that social status is often ascribed to households rather than individuals, we used the highest occupation in the family to categorize SES, a common approach in the SES literature 43. Average early-life SES for this sample was 6.0 (SD=1.3) on a 1–7 scale, with lower numbers indicating lower prestige occupations.
Maternal Warmth
Maternal warmth experienced in childhood was measuring using the Parent Bonding Inventory (PBI) 44. This inventory consists of a subscale assessing maternal warmth/care. This scale consists of 12 items querying the quality of their relationship with their mother during childhood (e.g., “My mother spoke to me in a warm and friendly voice.”). Participants reported on a 4-point scale how true each statement was of their own experiences. The PBI has demonstrated adequate test-retest reliability (.60–.79), and has demonstrated adequate validity when scores were compared between twins, as well as with interview-based ratings 45. In addition, it has been used on other studies investigating family relationships and physiological measures 46, 47. In our studies, internal consistency of this scale was .94, and 6 month test-retest reliability was .84. Participants in the top half of the score distribution were coded as high maternal warmth (n=26), and those in the bottom half were coded as low maternal warmth (n=27).
Transcriptional Profiles
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood through density-gradient centrifugation, lysed, and then homogenized in QiaShredder Spin Columns (Qiagen; Mississauga, ON). Lysates were frozen at −80° C until RNA was extracted using AllPrep DNA/RNA kits (Qiagen). RNA purity and integrity were verified using an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA). 50 ng of RNA was later assayed on an Illumina Beadstation 500 using HumanRef-8 v3.0 Expression Beadchips (Illumina; San Diego, CA) for a randomly selected subgroup of 30 low early life SES individuals (15 high and 15 low maternal warmth). The assays were performed at the Genome Quebec Innovation Centre. The raw data were log-2 transformed and differentially expressed genes were identified as those showing ≥20% difference in mean expression levels between low and high maternal warmth groups (corresponding to a false discovery rate of < 5%; 48). The data are deposited in Gene Expression Omnibus (Accession number: GSE15180).
To identify the upstream signal transduction pathways that underlie differential gene expression, we used a 2-sample variant of the Transcription Element Listening System (TELiS; http://www.telis.ucla.edu; 49. TELiS analyzes differential gene expression data in terms of the prevalence of transcription factor-binding motifs (TFBMs) within the promoters across all differentially expressed genes. This approach can accurately identify the activation of specific hormone or cytokine signaling pathways based on the resulting pattern of gene induction, which occurs selectively in genes bearing TFBMs responsive to transcription factors activated through that pathway. Analyses utilized aggregate indices that had been pooled across 9 different technical specifications involving variations of promoter length and TFBM match stringency 49.
Confirmation of Microarray Findings
To validate microarray findings, three transcripts that showed upregulation in the microarray analyses, and are known to be key genes involved in inflammation (listed under Gene Ontology annotation ‘inflammatory response’ or ‘regulation of inflammatory response’) were independently assayed by qRT-PCR using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). Genes analyzed included: CCL2, OLR1, and PTGS2. Assays for each sample were carried out in duplicate using an Applied Biosystems Prism 7000 Sequence Detection System according to the manufacturer’s recommended 1-step thermal cycling protocol. Threshold cycle numbers for each analyte were normalized to β-actin prior to analysis.
Markers of Inflammation
Systemic inflammation was assessed through serum levels of C-reactive protein (CRP). A high-sensitivity, chemiluminescent assay was performed on an IMMULITE 2000 (Diagnostic Products Corporation, Los Angeles, CA). This assay has an inter-assay coefficient of variation of 2.2% and a lower detection threshold of .19 mg/L. Because values were not normally distributed, CRP was log-transformed. To model the dynamics of inflammatory signaling pathways under immune challenge, we quantified PBMC production of IL-6 following stimulation with TLR ligands 50. Both IL-6 production and CRP assays were conducted on the larger sample of 53. Whole blood was drawn into Vacutainer Cell Preparation Tubes (Becton-Dickinson; Oakville, ON) and PBMCs were isolated through centrifugation, washed, and resuspended in R10 medium (Sigma; Saint Louis, MO), and 5×105 PBMCs were incubated with TLR ligands following established protocols 50, and as detailed in 22 (See Table S3 in the online supplement; purchased from Cedarlane; Burlington, ON). Cells were incubated for 24 hours at 37° C in a 5% CO2 atmosphere, after which supernatants were harvested and frozen at −80° C until analysis. The samples were later assayed in duplicate for the inflammatory cytokine IL-6 using commercially available ELISA Development kits from R&D Systems (#DY206E; Minneapolis, MN). These kits have detection thresholds of 5 pg/ml and intra- and inter-assay coefficients of variation < 5%.
Potential Confounds
We also collected information on demographic and behavioral factors that could serve as potential confounds. Demographic variables included age, gender, ethnicity and current SES. Behavioral factors included smoking status, alcohol use, exercise 51, and body mass index.
RESULTS
Sample Characteristics
Low early life SES participants who experienced low vs. high maternal warmth did not differ in demographic factors of age, gender, ethnicity, or current SES (all p’s>.1), or behavioral factors including smoking, drinking, exercise, and body mass index (all p’s>.1). See Table 1. Among the subsample on whom gene expression microarrays were conducted, those who experienced low vs. high maternal warmth also did not differ on any factors from Table 1, including age (p=.38), gender (p=.47), ethnicity (p=.46), current SES (p=1.0), smoking (p=.41), drinking (p=.70), exercise (p=.42), or body mass index (p=.56).
Table 1.
Descriptive Information
| Low Warmth | High Warmth | p | |||||
|---|---|---|---|---|---|---|---|
| % | M | SD | % | M | SD | ||
| Age | 34.44 | 4.58 | 32.42 | 5.39 | .15 | ||
| Gender (% female) | 63 | 65 | .85 | ||||
| Ethnicity (% Caucasian) | 63 | 58 | .70 | ||||
| Current SES (1–9 scale) | 4.30 | 2.14 | 5.04 | 1.95 | .20 | ||
| Smokers (%) | 18 | 19 | .95 | ||||
| Alcohol (drinks/week) | 2.80 | 6.23 | 3.06 | 4.94 | .87 | ||
| Exercise (hours/week) | 3.10 | 4.28 | 5.10 | 8.24 | .27 | ||
| Body mass index | 25.55 | 4.39 | 23.73 | 3.88 | .12 | ||
Note: p value indicates significance of differences between low warmth and high warmth groups.
In addition, the subsample on whom gene expression microarrays were conducted was similar to the larger sample (no differences between those who were in the microarray substudy and those who were not on age: p=.93, gender: p=.48, ethnicity: p=.29, current SES: p=.82).
Transcriptional Dynamics
A total of 491 transcripts showed a 1.20-fold or larger difference in expression between low early life SES participants experiencing high versus low maternal warmth (corresponding to a false-discovery rate of 5%; 48). Tables S1 and S2 in the online supplement lists these genes. Of the genes that were differentially expressed, 330 were upregulated and 161 were downregulated in participants who grew up with high maternal warmth.
Primary Hypothesis
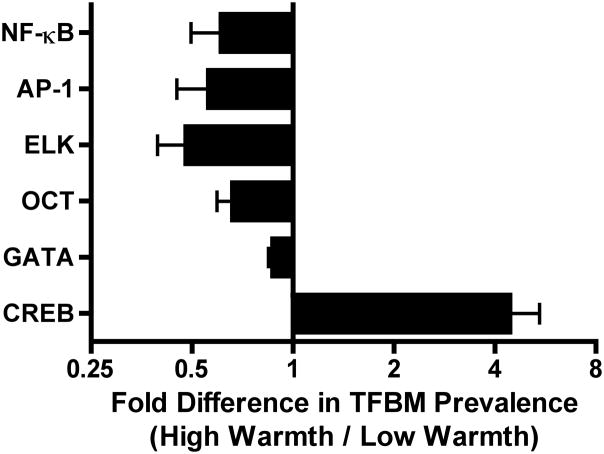
Results of promoter-based bioinformatics analysis indicated that there was significant downregulation of genes bearing response elements for the transcription factor NF-κB (average 0.61-fold difference in V$NFKAPPAB65_01 prevalence, SE = .11, p = .02) in low early life SES participants who experienced high maternal warmth compared to those who experienced low maternal warmth. See Figure 1 and Table 2.
Figure 1.
Fold differences in the prevalence of transcription factor binding motifs (TFBM) for NF-κB, AP-1, OCT, ELK, GATA, and CREB in promoters of genes differentially expressed among individuals from low early life SES backgrounds who experienced either low or high levels of childhood maternal warmth. For example, for NF-κB, high warmth individuals exhibited a .61 fold difference, or a 39% decrease, in TFBM prevalence compared to high warmth individuals. High warmth individuals also exhibited a 44% decrease in AP-1, a 52% decrease in ELK, a 34% decrease in OCT, and a 13% decrease in GATA TFBM prevalence, along with a 4.4 fold increase in CREB TFBM prevalence, compared to low warmth individuals.
Table 2.
Unadjusted and adjusted fold differences in prevalence of transcription factor binding motifs (high maternal warmth relative to low maternal warmth groups)
| Unadjusted Fold difference | p | Adjusted Fold difference | p | |
|---|---|---|---|---|
| NF-κB | .61 | .02 | .62 | .001 |
| AP-1 | .56 | .01 | .68 | .02 |
| CREB | 4.43 | .0001 | 3.28 | .001 |
| GATA3 | .87 | .001 | .86 | .01 |
| OCT | .66 | .005 | .72 | .002 |
| ELK1 | .48 | .006 | .60 | .02 |
Note: Adjusted analyses control for age, gender, ethnicity, current socioeconomic status, exercise, alcohol intake, body mass index, and leukocyte subset distributions.
Secondary Analyses
Our secondary hypothesis was that in addition to NF-κB, differences in the activity of immune activating transcription factors such as AP-1 would also be evident between the two groups. Results revealed that low early life SES individuals who experienced high maternal warmth displayed downregulated expression of genes bearing TFBMs for AP-1 compared to those who experienced low maternal warmth (average 0.56-fold difference in V$AP1_Q4 prevalence, SE=.11, p = .01). See Figure 1 and Table 2.
Exploratory Analyses
In addition to the primary hypotheses above, we performed exploratory bioinformatics analyses to evaluate whether maternal warmth among low early life SES individuals was associated with altered activity of any other transcription-control pathways. Participants high in maternal warmth showed a relative upregulation of genes responsive to the CREB pathway (average 4.43-fold difference in V$CREBP1_Q2, SE=1.01, p=.0001). They also showed a relative downregulation of genes bearing TFBMs for the GATA3 transcription factor (average 0.87-fold difference in V$GATA3_01, SE=.02, p=.001), OCT family factors (average 0.66-fold difference in V$OCT1_06, SE=.06, p=.005), and the ELK1 transcription factor (average 0.48-fold difference in V$ELK1_01, SE=.08, p=.006). See Figure 1 and Table 2.
In contrast, there were no differences between maternal warmth groups on genes bearing TFBMs for the glucocorticoid receptor, GR (average .89-fold difference in V$GR_Q6, SE=.11, p=.36).
To identify common functional characteristics of differentially expressed genes, we conducted additional exploratory Gene Ontology analyses using GOstat (http://gostat.wehi.edu.au). Gene Ontology categories over-represented among genes upregulated in low warmth individuals included innate immune response (GO:0045087), cytokine production (GO:0001816), IκB kinase/NF-κB cascade (GO:0007249), and chemotaxis (GO:0006935). Functional characteristics of genes upregulated in high warmth individuals included cell development (GO:0048468), transcription activator activity (GO:0016563), and intracellular signaling cascade (GO:0007242). These patterns mirror the results of the transcription control pathway analyses in suggesting that high maternal warmth buffers the activation of genes involved in inflammation and immunologic activation.
Confirmatory RT-PCR
To verify results of the microarray expression analysis, we used real-time RT-PCR (qRT-PCR) to assess relative quantities of mRNA for three genes identified as up- or down-regulated in low SES individuals from high warmth families. The results were concordant in all three cases; among individuals with high maternal warmth, there was a relative upregulation of CCL2 and OLR1, and a relative downregulation of PTGS2 (all p’s < .0001). See Figure S1 in the online supplement.
Confounds
To ensure that variation in the distribution of leukocyte subsets within the PBMC pool did not contribute to the observed disparities, we performed complete blood counts on participants’ whole blood. Gene expression profiles were statistically adjusted to control for individual differences in leukocyte subset distributions. Downregulation of NF-κB in high maternal warmth participants remained statistically significant in analyses controlling for leukocyte subset distributions (p=.001), as did downregulation of AP-1, GATA, OCT, and ELK, and upregulation of CREB (all p’s<.05).
We next considered the possibility that demographic or behavioral factors might account for the above differences. Gene expression profiles were statistically adjusted to control for age, gender, ethnicity, current socioeconomic status, exercise, alcohol intake, and body mass index. Downregulation of NF-κB in high maternal warmth participants remained statistically significant (p=.002), as did downregulation of AP-1, GATA, OCT, and ELK, and upregulation of CREB (all p’s<.05). See Table 2.
Immune Activation
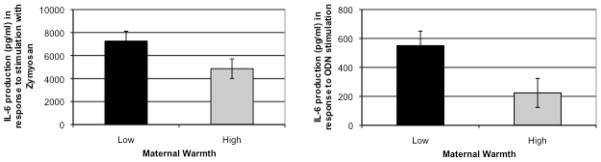
To evaluate whether the leukocyte transcriptional disparities identified above gave rise to differential systemic immune activation, we assessed serum levels of the inflammatory biomarker CRP and PBMC production of the inflammatory cytokine IL-6 following ex vivo stimulation with ligands to Toll-like receptors on the larger sample. Low early life SES individuals who experienced high maternal warmth showed directionally lower levels of CRP (log transformed M=−.31, SD=.44) compared to low early life SES individuals who experienced low maternal warmth (M=−.05, SD=.66), but these differences were not statistically significant (t=1.62, p=.11). In addition, their PBMCs responded differently to TLR stimulation. High maternal warmth individuals produced significantly less IL-6 in response to TLR2/TLR6 stimulation with the ligand Zymosan than did those with low maternal warmth (t=2.04, p<.05). PBMCs from high maternal warmth individuals also produced significantly less IL-6 in response to TLR-9 stimulation with ODN (t=2.26, p<.05). No differences were found for other ligands. See Figure 2.
Figure 2.
Differences in IL-6 production in response to TLR2/TLR6 stimulation by Zymosan and in response to TLR9 stimulation by ODN for individuals from low early life SES backgrounds who experienced either low or high levels of childhood maternal warmth.
DISCUSSION
We found that in a sample of adults who grew up in low SES homes, maternal warmth during childhood was associated with reduced pro-inflammatory signaling. As adults, those who experienced high maternal warmth showed reduced immune cell responsivity to challenge (as indicated by less PBMC stimulated production of IL-6), and at the functional genomic level, reduced bioinformatic indications of activity by pro-inflammatory (NF-κB) and immune-activating (AP-1) transcription factors compared to their low warmth counterparts. These findings are significant because they suggest the possibility that the excess inflammatory activity linked to low SES may be mitigated, in part, by the presence of a warm and supportive family climate early in life.
These results are consistent with the notion that adverse social environments engender heightened signaling of pro-inflammatory transcription control pathways in childhood and adulthood 19–23 as well as heightened systemic inflammation in adulthood 52, 53, and that maternal warmth experienced during childhood may serve to reduce these states. Our results add to recent theoretical discussions about how positive psychological and support factors may be able to biologically protect those who experience adversity from poor health outcomes 35, 54, 55, and complement research that has shown protective effects of biological traits such as genetic polymorphisms under certain adverse conditions 56, 57. Furthermore, given the causal role that inflammatory cytokines are hypothesized to play in major depression 29, 30, the reduced inflammatory signaling seen among high maternal warmth individuals suggests the possibility that supportive family environments early in life could potentially be protective against later depression in part because of their effects on inflammatory pathways.
Previous human research on the biological profiles associated with positive psychosocial factors experienced under adversity has largely focused on neuroendocrine or cardiovascular risk outcomes. For example, a program to teach parenting skills to foster parents produced greater declines in cortisol among maltreated children in comparison to children in foster care whose parents did not receive the program 58. Similarly, maltreated children who have high ego resilience, as well as psychologically healthy adult survivors of child abuse, show different profiles of cortisol and functioning of the hypothalamic pituitary adrenal axis compared to those without resilience characteristics 59, 60. Positive relationships with others also have been linked to less detrimental neuroendocrine and cardiovascular outcomes. For example, effects of current cumulative adversity on allostatic load (a composite of cardiovascular and neuroendocrine measures) did not occur in children who experienced high maternal warmth 36. Adults who experience positive relationships currently did not show detrimental effects of low maternal warmth on adult allostatic load 37. Additionally, high quality family or social relationships mitigated the relationship between low SES or parental loss and cardiovascular risk (e.g., blood pressure, cholesterol profiles) in adults 61–62. Finally, the relationship between current SES and glycosylated hemoglobin in adults has been found to be stronger among those currently low in psychological well-being 38. The present study is unique both in focusing on early life factors and their links with adult biology, as well as in demonstrating a biological signature associated with maternal warmth that spans from systemic inflammatory markers down to indicators of functional genomic activity.
In addition to our hypotheses relating to NF-κB and AP-1, exploratory analyses also revealed downregulation of the OCT, ELK, and GATA family of transcription factors, as well as upregulation of CREB. Interestingly, these same pathways have emerged in several other studies of the transcription control pathways linked to social adversity 19, 22, 23, 63. CREB is activated by a variety of different physiologic receptor systems, some of which would not be consistent with the other patterns seen in this study. However, one CREB modulator that may be relevant to the present findings is the neurotrophin Brain-Derived Neurotrophic Factor (BDNF, which in addition to being found in the brain, can also be produced by immune cells 64). Low levels of BDNF have been implicated in depression, and conversely, antidepressant treatments (both pharmacologic and psychotherapeutic) have been found to increase BDNF 65, 66. Moreover, increases in BDNF are linked to activation of CREB, and increases in CREB phosphorylation are also seen with antidepressant treatment 67, 68. This suggests the possibility that the upregulation of CREB seen in the present study could have implications for protection against depression. In addition, activated CREB can inhibit NF-κB-mediated transcription by competing for transcriptional co-factors such as p300/CBP 69. Activated CREB may reflect increased upstream activity of PKA, which can also inhibit NF-κB 70. Given the inhibitory effects of PKA/CREB on NF-κB, the CREB activation observed in this study may be one plausible mechanism for reciprocal reductions in NF-κB-mediated gene expression.
In the present study, we did not find evidence that impaired glucocorticoid signaling played a role in the differential pro-inflammatory activity of low-versus high-maternal warmth subjects (there were no significant differences in the expression of genes with response elements for the glucocorticoid receptor, GR). This is intruiging because previous studies have suggested that the effects of adverse social environments on pro-inflammatory signaling pathways may operate in part via increased glucocorticoid resistance 19, 20, 22. Patterns for CREB were also different, and taken together, these findings suggest that while adverse social environments and protective support factors both appear to have effects at the genomic level, each has its own specific mediational fingerprint. That is, low SES appears to increase pro-inflammatory transcription control pathways via increased glucocorticoid resistance, whereas maternal warmth appears to reduce pro-inflammatory transcription signaling via other pathways that do not involve the GR.
The present analyses focus on cis-regulatory activity, and thus cannot definitively rule out the possibility that altered GR signaling might contribute to the observed NF-κB inhibition through non-transcriptional mechanisms (e.g., protein-protein interactions) 71. However, GR transcriptional regulation and protein-protein interactions tend to correlate because they are both induced by glucocorticoid ligation of the GR 71. In the absence of any detectable change in GR-mediated transcription, the most parsimonious interpretation of the data is that GR signaling alterations are not driving the observed inhibition of NF-κB.
Despite differences in immune cell responsivity to challenge and bioinformatic indications of pro-inflammatory signaling, we found no statistically significant differences by maternal warmth in CRP levels, although group means were in the hypothesized direction. Because this was a healthy and relatively young sample, this could mean that differences at the level of systemic inflammation take time to emerge. Alternatively, it could be that larger samples are required to detect differences at this level.
Controlling for a variety of demographic and behavioral factors did not substantially affect study results. This indicates that the differences between low and high maternal warmth participants cannot be attributed to differences in gender, age, ethnicity, or various health behaviors. In addition, because the low and high maternal warmth groups did not differ on current SES, the results observed in this study do not appear to be due to effects of current socioeconomic environments on inflamatory signaling pathways.
Strengths of the present study include the empirical testing of theoretical models of biology profiles associated with maternal warmth using a well-defined molecular mechanism for disease risk. A second strength lies in the in-depth assessment of pro-inflammatory profiles ranging from indicators of systemic inflammation to genome-wide microarray analyses of gene expression. A third strength lies in the confirmation of early life SES status from parents, rather than from the study participants themselves. Limitations include the small sample size (although it is similar to other microarray studies of social adversity 19, 21, 23), the retrospective nature of assessments of parental warmth and early life SES, and the inability to infer causality based on the study design. Future studies should follow clinical outcomes over time to characterize the implications of these immune and gene expression profiles for psychiatric illnesses, and as well to determine the degree to which maternal warmth early in childhood protects low SES individuals, much as higher SES status does. In addition, the potential benefits of paternal, in addition to maternal, warmth would be important to establish. Finally, it would be important to identify the underlying biological mechanisms by which early life factors contribute to persistent effects on inflammatory signaling by exploring the role of processes such as epigenetics 72.
In sum, the present study found that high maternal warmth among those low in SES early in life was associated with reduced PBMC response to TLR stimulation, and at the genomic level, reduced bioinformatic indications of pro-inflammatory transcription factor activity. Given that pro-inflammatory states have been strongly implicated in both mental health disorders such as depression29, as well as in physical illnesses such as cardiovascular disease 26, this suggests that individuals who grow up low in SES but who have warm maternal figures may be less likely to develop a number of mental and physical illnesses later in life. If true, these findings could have important public policy and public health implications. Working to alleviate poverty, as lofty and important a goal as this is, has remained an intractable problem in our society. Complementing this effort, encouraging and teaching parenting behaviors that facilitate warm emotional climates, even in the face of adversity, might prove to be a supporting, effective target of intervention (as suggested by cross-fostering and environmental manipulation studies in previous animal research 40, 73). To the extent that promoting such environments early in children’s lives can be shown to have causal protective effects, we may one day be able to partially ameliorate the inflammatory and subsequent health effects of low early life SES environments through increasing the warmth and supportiveness of family relationships in at-risk children.
Supplementary Material
Acknowledgments
Supported by NIH grants HD058502, CA116778, and AG107265; BC Ministry of Child and Family Development via the Human Early Learning Partnership; and the Allergy, Genes, & Environment Research Network.
Footnotes
Conflict of Interest
All authors declare no conflicts of interest.
References
- 1.Dohrenwend BP, Levav I, Shrout PE, Schwartz S, Naveh G, Link BG, et al. Socioeconomic status and psychiatric disorders: The causation-selection issue. Science. 1992;255:946–952. doi: 10.1126/science.1546291. [DOI] [PubMed] [Google Scholar]
- 2.Johnson JG, Cohen P, Dohrenwend BP, Link BG, Brook JS. A longitudinal investigation of social causation and social selection processes involved in the association status and psychiatric disorders. J Abnorm Psychol. 1999;108:490–499. doi: 10.1037//0021-843x.108.3.490. [DOI] [PubMed] [Google Scholar]
- 3.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, et al. Socioeconomic status and health: The challenge of the gradient. American Psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Marmot MG, Shipley MJ, Rose G. Inequalities in death-specific explanations of a general pattern? Lancet. 1984;i:1003–1006. doi: 10.1016/s0140-6736(84)92337-7. [DOI] [PubMed] [Google Scholar]
- 5.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: How and why do these relationships change with age? Psychological Bulletin. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 6.House JS, Kessler RC, Herzog AR. Age, socioeconomic status, and health. Milbank Quarterly. 1990;68:383–411. [PubMed] [Google Scholar]
- 7.Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health: No easy solution. Journal of the American Medical Association. 1993;269:3140–3145. [PubMed] [Google Scholar]
- 8.Masten AS, Garmezy N, Tellegen A, Pellegrini DS, Larkin K, Larsen A. Competence and stress in school children: The moderating effects of individual and family qualities. Journal of Child Psychiatry. 1988;29:745–764. doi: 10.1111/j.1469-7610.1988.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 9.Masten AS. Ordinary magic: Resilience processes in development. American Psychologist. 2001;56:227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- 10.Masten AS, Obradovic J. Competence and resilience in development. Ann N Y Acad Sci. 2006;1094:13–27. doi: 10.1196/annals.1376.003. [DOI] [PubMed] [Google Scholar]
- 11.Luthar SS, Sawyer JA, Brown PJ. Conceptual issues in studies of resilience: Past, present, and future research. Ann N Y Acad Sci. 2006;1094:105–115. doi: 10.1196/annals.1376.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masten AS, Shaffer A. How families matter in child development: Reflections from research on risk and resilience. In: Clarke-Stewart A, Dunn J, editors. Families count: Effects on child and adolescent development. New York, NY: Cambridge University Press; 2006. pp. 5–25. [Google Scholar]
- 13.Repetti RL, Taylor SE, Seeman T. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- 14.Chen E, Miller GE. Social context as an individual difference in psychoneuroimmunology_. In: Irwin MR, editor. Psychoneuroimmunology. 4. Boston, MA: Elsevier; 2007. pp. 497–508. [Google Scholar]
- 15.Panagiotakos DB, Pitsavos C, Manios Y, Polychronopoulos E, Chrysohoou CA, Stefanadis C. Socio-economic status in relation to risk factors associated with cardiovascular disease, in healthy individuals from the ATTICA study. European Journal of Cardiovascular Prevention & Rehabilitation. 2005;12:68–74. [PubMed] [Google Scholar]
- 16.Hemingway H, Shipley M, Mullen MJ, Kumari M, Brunner E, Taylor M, et al. Social and psychosocial influences on inflammatory markers and vascular function in civil servants (The Whitehall II study) Am J Cardiol. 2003;92:984–987. doi: 10.1016/s0002-9149(03)00985-8. [DOI] [PubMed] [Google Scholar]
- 17.Chen E, Fisher EB, Jr, Bacharier LB, Strunk RC. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosom Med. 2003;65:984–992. doi: 10.1097/01.psy.0000097340.54195.3c. [DOI] [PubMed] [Google Scholar]
- 18.Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: The role of psychological stress. Journal of Allergy and Clinical Immunology. 2006;117:1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 19.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen E, Miller GE, Walker HA, Arevalo JM, Sung CY, Cole SW. Genome-wide transcriptional profiling linked to social class in asthma. Thorax. 2009;64:38–43. doi: 10.1136/thx.2007.095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutgendorf SK, DeGeest K, Sung CY, Arevalo JM, Penedo F, Lucci, et al. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun. 2009;23:176–183. doi: 10.1016/j.bbi.2008.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol. 2002;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- 25.Lesperance F, Frasure-Smith N, Theroux P, Irwin M. The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. Am J Psychiatry. 2004;161:271–277. doi: 10.1176/appi.ajp.161.2.271. [DOI] [PubMed] [Google Scholar]
- 26.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 27.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 28.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 29.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 33.Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci. 2006;1071:379–396. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- 34.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 35.Curtis WJ, Cicchetti D. Moving research on resilience into the 21st century: theoretical and methodological considerations in examining the biological contributors to resilience. Dev Psychopathol. 2003;15:773–810. doi: 10.1017/s0954579403000373. [DOI] [PubMed] [Google Scholar]
- 36.Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43:341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- 37.Singer B, Ryff CD. Hierarchies of life histories and associated health risks. Ann N Y Acad Sci. 1999;896:96–115. doi: 10.1111/j.1749-6632.1999.tb08108.x. [DOI] [PubMed] [Google Scholar]
- 38.Tsenkova VK, Love GD, Singer BH, Ryff CD. Socioeconomic status and psychological well-being predict cross-time change in glycosylated hemoglobin in older women without diabetes. Psychosom Med. 2007;69:777–784. doi: 10.1097/PSY.0b013e318157466f. [DOI] [PubMed] [Google Scholar]
- 39.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 40.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 41.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Statistics OoN. The National Statistics Socio-Economic Classification. Hampshire, England: Palgrave MacMillan; 2005. [Google Scholar]
- 43.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: Concepts, methodologies, and guidelines. Annual Review of Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 44.Parker G. Parental characteristics in relation to depressive disorders. Br J Psychiatry. 1979;134:138–147. doi: 10.1192/bjp.134.2.138. [DOI] [PubMed] [Google Scholar]
- 45.Parker G. The Parental Bonding Instrument. A decade of research. Soc Psychiatry Psychiatr Epidemiol. 1990;25:281–282. doi: 10.1007/BF00782881. [DOI] [PubMed] [Google Scholar]
- 46.Luecken LJ. Parental caring and loss during childhood and adult cortisol responses to stress. Psychology and Health. 2000;15:841–851. [Google Scholar]
- 47.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cole SW, Galic Z, Zack JA. Controlling false negative errors in microarray differential expression analysis: A PRIM approach. Bioinformatics. 2003;19:1808–1816. doi: 10.1093/bioinformatics/btg242. [DOI] [PubMed] [Google Scholar]
- 49.Cole SW, Yan W, Galic Z, Arevalo J, Zack JA. Expression-based monitoring of transcription factor activity: The TELiS database. Bioinformatics. 2005;21:803–810. doi: 10.1093/bioinformatics/bti038. [DOI] [PubMed] [Google Scholar]
- 50.Hirschfeld AF, Bettinger JA, Victor RE, Davidson DJ, Currie AJ, Ansermino JM, et al. Prevalence of Toll-like receptor signalling defects in apparently healthy children who developed invasive pneumococcal infection. Clinical Immunology. 2007;122:271–278. doi: 10.1016/j.clim.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Paffenbarger RS, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Medicine and Science in Sports and Exercise. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the Coronary Artery Risk Development in Young Adults Study. Biological Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luthar SS, Brown PJ. Maximizing resilience through diverse levels of inquiry: Prevailing paradigms, possibilities, and priorities for the future. Development and Psychopathology. 2007;19:931–955. doi: 10.1017/S0954579407000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 57.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 58.Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: impact on children’s behavior, neuroendocrine activity, and foster parent functioning. J Am Acad Child Adolesc Psychiatry. 2000;39:1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 59.Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: a multilevel perspective. Dev Psychopathol. 2007;19:787–809. doi: 10.1017/S0954579407000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 61.Luecken LJ, Rodriguez AP, Appelhans BM. Cardiovascular stress responses in young adulthood associated with family-of-origin relationship experiences. Psychosom Med. 2005;67:514–521. doi: 10.1097/01.psy.0000160466.10397.18. [DOI] [PubMed] [Google Scholar]
- 62.Vitaliano PP, Scanlan JM, Zhang JP, Savage MV, Brummett B, Barefoot J, et al. Are the salutogenic effects of social supports modified by income? A test of an “added value hypothesis”. Health Psychology. 2001;20:155–165. [PubMed] [Google Scholar]
- 63.Cole SW, Arevalo JMG, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proceedings of the National Academy of Sciences. doi: 10.1073/pnas.0911515107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 66.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 67.Koch JM, Hinze-Selch D, Stingele K, Huchzermeier C, Goder R, Seeck-Hirschner M, et al. Changes in CREB phosphorylation and BDNF plasma levels during psychotherapy of depression. Psychother Psychosom. 2009;78:187–192. doi: 10.1159/000209350. [DOI] [PubMed] [Google Scholar]
- 68.Koch JM, Kell S, Hinze-Selch D, Aldenhoff JB. Changes in CREB-phosphorylation during recovery from major depression. J Psychiatr Res. 2002;36:369–375. doi: 10.1016/s0022-3956(02)00056-0. [DOI] [PubMed] [Google Scholar]
- 69.Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 1997;159:5450–5456. [PubMed] [Google Scholar]
- 70.Takahashi N, Tetsuka T, Uranishi H, Okamoto T. Inhibition of the NF-kappaB transcriptional activity by protein kinase A. Eur J Biochem. 2002;269:4559–4565. doi: 10.1046/j.1432-1033.2002.03157.x. [DOI] [PubMed] [Google Scholar]
- 71.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 72.Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 73.Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behavioral Neuroscience. 2007;121:1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.