Abstract
The incomplete penetrance of mutations in BRCA1 and BRCA2 suggests that some combination of environmental and genetic factors modifies the risk of breast cancer in mutation carriers. The current study sought to identify possible interactions between established breast cancer risk factors and BRCA1 or BRCA2 mutations using a case-only study design. Breast cancer cases that had been tested for BRCA1 and BRCA2 mutations were identified from 11 collaborating centers. Comparisons of reproductive and lifestyle risk factors were made between women with breast cancer who were positive for BRCA1 mutations (n=283), BRCA2 mutations (n=204) or negative for both BRCA1 and BRCA2 mutations (n=894). Interaction risk ratios (IRRs) were calculated using multinominal logistic regression models. Compared with non-carriers, statistically significant IRRs were observed for later age at menarche among BRCA2 mutation carriers, for a greater number of pregnancies among both BRCA1 and BRCA2 mutation carriers, and for alcohol use among BRCA1 mutation carriers. Our data suggest that the risk for breast cancer among BRCA1 or BRCA2 carriers may be modified by reproductive characteristics and alcohol use. However, our results should be interpreted cautiously given the overall inconsistency in the epidemiologic literature on modifiers of BRCA1 and BRCA2.
Keywords: Breast cancer, BRCA1, BRCA2, mutations, modifiers
Introduction
Carriers of mutated forms of the BRCA1 and BRCA2 genes are at markedly higher risk for developing breast and ovarian cancer. Estimates of the lifetime risk for developing breast cancer for BRCA1 and BRCA2 mutation carriers range from approximately 50 to 80%. [1] Despite the high cancer risk associated with these mutations, their incomplete penetrance suggests that other factors, likely a combination of genetic variants and environmental exposures, influence the risk of a mutation carrier developing the disease.
Comparisons of cancers among BRCA1- and BRCA2-mutation carriers and non-mutation carriers show differences in clinical characteristics suggesting that they may arise from distinct etiologic pathways. [2,3] Breast cancers diagnosed in individuals with BRCA1 mutations tend to have a higher prevalence of the triple negative (negative for estrogen receptor (ER), progesterone receptor (PR) and HER2) subtype of breast cancer. [3,4] Although triple negative breast cancer is generally associated with poorer prognosis, [4] most studies show survival is not significantly worse for BRCA1 carriers than those without mutations, [5] providing further evidence that BRCA1 mutation-related cancers are distinct from both sporadic cancers and BRCA2-related cancers.
Differences in epidemiologic risk factors for BRCA1- and BRCA2-associated cancers as compared to risk factors for breast cancer in non-carriers may provide insight into factors that modify the penetrance of these genes. A number of investigations using a variety of study designs have been conducted in an attempt to identify modifiers of BRCA1 or BRCA2. The most common approach has been to compare established breast cancer risk factors between women with and without breast cancer in study populations comprised of BRCA1 and/or BRCA2 mutation carriers. [6–19] Other studies compared affected BRCA1 or BRCA2 mutation carriers to unaffected women without mutations, [20–22] or compared breast cancer cases with and without mutations. [20,21,23] The extent to which breast cancer risk factors among BRCA1 or BRCA2 carriers differ from risk factors for sporadic disease may be an indicator of factors that may modify the penetrance of mutated BRCA1 or BRCA2. To date, most reports have focused on reproductive and hormonal risk factors such as parity, breastfeeding and oral contraceptive use. No clear picture has yet emerged on whether the associations with these risk factors among BRCA1 or BRCA2 mutation carriers are similar or different to the established associations in sporadic breast cancer.
Although the majority of studies to date have examined breast cancer risk factors among affected and unaffected mutation carriers, this approach does not directly evaluate gene-environment interaction because the study population is comprised entirely of mutation carriers. A more direct approach to evaluating potential modifiers of BRCA1 and BRCA2 mutations is to compare the epidemiologic characteristics of women with BRCA1 or BRCA2 mutations who develop breast cancer to non-mutation carriers with breast cancer–a case-only design. In this report, we used a case-only analysis to evaluate differences in clinical characteristics and risk factors between breast cancers diagnosed in women with BRCA1 mutations, BRCA2 mutations and no known mutation. The goal of these analyses was to obtain a better understanding of differences in risk factors across genotypes, which ultimately could lead to prevention strategies that are tailored specifically to BRCA1 or BRCA2 mutation carriers.
Methods
Study Design
The Genetic and Environmental Modifiers of BRCA1/BRCA2 Study (GEMS) is a multi-center study coordinated at Duke University Medical Center that was designed to investigate genetic variants and epidemiologic risk factors that may affect the penetrance of BRCA1 and BRCA2 mutations. The study involved 11 centers in the United States and Australia that contributed data and biological samples for analysis. Cases included in the study were females aged 20 years or older who had been diagnosed with breast cancer and had been tested for BRCA1 or BRCA2 mutations. We included only women with known deleterious mutations as mutation-positive cases. Women who had variants of unknown significance but no known deleterious mutation were excluded from the analysis. The study was designed such that each case with a BRCA mutation would be frequency matched by center and age (±4 years) to two cases who tested negative, however in some instances there was only one negative for each positive case. To minimize survival bias, eligible cases had to have been tested for BRCA1 and/or BRCA2 mutations and have had epidemiologic data collected no later than three years after diagnosis. This analysis involved a total of 1381 cases of whom 283 are BRCA1 mutation positive (BRCA1+), 204 are BRCA2 mutation positive (BRCA2+) and 894 are negative for both BRCA1 and BRCA2 mutations (BRCA−). Four women who had both BRCA1 and BRCA2 mutations were excluded from the analyses. Mutation status was based on testing for BRCA1 and BRCA2 performed prior to enrollment in GEMS that was done in various laboratories using methods established at each center. Seventy-five percent of subjects had full-sequencing, 19% had partial sequencing (Jewish panel or family mutations), and 6% had various other tests such as protein truncation tests, single-strand conformation polymorphisms (SSCP), or denaturing high performance liquid chromatography (DHPLC). Among the women that had full sequencing, 65% of them had large deletion or duplication testing such as multiplex ligation-dependent probe analysis (MLPA). Participating centers and the number of cases contributed by each site are listed in Table 1.
Table 1.
Number of BRCA1 mutation positive, BRCA2 mutation positive and BRCA negative cases by participating site.
| Center | BRCA1+ | BRCA2+ | BRCA− | Total |
|---|---|---|---|---|
| Duke University Medical Center | 28 | 21 | 70 | 119 |
| Dana Farber Cancer Institute | 22 | 13 | 70 | 105 |
| Georgetown University | 25 | 14 | 79 | 118 |
| Johns Hopkins University | 13 | 8 | 42 | 63 |
| kConFab (Australia) | 43 | 49 | 133 | 225 |
| University of Southern California | 57 | 30 | 161 | 248 |
| University of Pennsylvania | 28 | 25 | 113 | 166 |
| Moffitt Cancer Center | 6 | 4 | 23 | 33 |
| University of North Carolina | 28 | 20 | 93 | 141 |
| University of Michigan | 4 | 3 | 11 | 18 |
| M.D. Anderson Cancer Center | 29 | 17 | 99 | 145 |
| TOTALS | 283 | 204 | 894 | 1381 |
All centers sent DNA or whole blood/cell lines from which DNA could be extracted to Duke University Medical Center. They also sent questionnaire data, pathology data from the breast cancer diagnosis, and mutation testing results. The study protocol was approved by the Institutional Review Boards at Duke University Medical Center and all participating centers.
Cases were identified both prospectively (newly diagnosed and tested) and retrospectively (previously diagnosed and tested), thus there was some variation in the questionnaire data that were obtained. Centers that prospectively enrolled cases for the GEMS study and were not administering a questionnaire to patients at their hereditary cancer clinic used a questionnaire developed for the GEMS study. Centers that prospectively enrolled cases but were already administering a questionnaire to their clinic patients added a supplement to their survey to obtain additional risk factor information. Centers that contributed retrospectively-identified cases for this analysis sent data from a previously administered risk factor questionnaire. Data from centers that did not use the GEMS questionnaire were mapped to the questions on the GEMS survey. Missing data in the analyses are due in most cases to questions not being asked on surveys administered to retrospectively identified cases.
Data analysis
The analysis took into account the different centers from which cases were recruited and the age matching within each center. All models treated BRCA1 or BRCA2 genotype as the response variable and included a random effect for center. We fit these unconditional logistic link multinomial response models using the GLIMMIX procedure in SAS (version 9.2, SAS Institute, Cary, North Carolina). The models used for characterizing relationships between demographic and clinical characteristics and BRCA1 or BRCA2 genotype were unadjusted (Table 2), while those used to evaluate epidemiologic factors as potential modifiers of BRCA1 or BRCA2 (Table 3) were age adjusted. The latter class of models were used to estimate the interaction risk ratios (IRRs) and 95% confidence intervals (CIs) for each parameter, where IRR =[P(D=1|BRCA+, E+)P(D=1|BRCA−, E−)]/[P(D=1|BRCA+, E−)P(D=1|BRCA−, E+)], with D=disease (breast cancer) and E=environmental exposure. The IRR is a ratio of risk ratios and can be expressed as the RR of disease given E among carriers to the RR of disease given E among non-carriers. [24,25] In these analyses, the IRRs are case-only comparisons and reflect heterogeneity in risk factors between case groups.
Table 2.
Demographic and clinical characteristics of breast cancer cases by BRCA1 or BRCA2 mutation status
| BRCA1+ (n=283) | BRCA2+ (n=204) | BRCA− (n=894) | p-value | ||
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | BRCA1+ vs BRCA− | BRCA2+ vs BRCA− | |
| Age at diagnosis (years) | |||||
| 22–29 | 18 (6.4) | 4 (2.0) | 37 (4.1) | 0.0004 | 0.09 |
| 30–39 | 111 (39.2) | 66 (32.4) | 248 (27.7) | ||
| 40–49 | 116 (41.0) | 89 (43.6) | 409 (45.8) | ||
| 50–59 | 27 (9.5) | 39 (19.1) | 142 (15.9) | ||
| 60+ | 11 (3.9) | 6 (2.9) | 58 (6.5) | ||
| Race | |||||
| White | 252 (89.1) | 184 (90.2) | 823 (92.1) | 0.4 | 0.5 |
| African-American | 11 (3.9) | 7 (3.4) | 28 (3.1) | ||
| Hispanic | 13 (4.6) | 6 (2.9) | 25 (2.8) | ||
| Asian or Pacific Islander | 3 (1.1) | 6 (2.9) | 12 (1.3) | ||
| Other | 4 (1.4) | 1 (0.5) | 6 (0.7) | ||
| Jewish | |||||
| Yes | 63 (23.3) | 44 (22.0) | 142 (16.2) | 0.01 | 0.02 |
| No | 207 (76.7) | 156 (78.0) | 737 (83.9) | ||
| Missing | 13 | 4 | 15 | ||
| Behavior | |||||
| Invasive | 266 (94.0) | 185 (90.7) | 791 (88.5) | 0.01 | 0.3 |
| In situ | 17 (6.0) | 19 (9.3) | 103 (11.5) | ||
| Stage | |||||
| 1 | 171 (70.1) | 120 (71.4) | 452 (64.3) | 0.2 | 0.4 |
| 2 | 60 (24.6) | 34 (20.2) | 188 (26.7) | ||
| 3 | 10 (4.1) | 11 (6.6) | 54 (7.7) | ||
| 4 | 3 (1.2) | 3 (1.8) | 9 (1.3) | ||
| Missing | 39 | 36 | 191 | ||
| Histologic grade | |||||
| Well differentiated | 44 (19.3) | 27 (17.9) | 145 (23.5) | <0.0001 | 0.2 |
| Moderately differentiated | 30 (13.2) | 51 (33.8) | 211 (34.1) | ||
| Poorly differentiated | 154 (67.5) | 73 (48.3) | 262 (42.4) | ||
| Missing | 55 | 53 | 276 | ||
| Estrogen receptor (ER) status | |||||
| Positive | 49 (20.6) | 125 (78.6) | 498 (72.8) | <0.0001 | 0.3 |
| Borderline | 8 (3.4) | 3 (1.9) | 18 (2.6) | ||
| Negative | 181 (76.1) | 31 (19.5) | 168 (24.6) | ||
| Missing | 45 | 45 | 210 | ||
| Progesterone receptor (PR) status | |||||
| Positive | 52 (22.0) | 97 (63.8) | 432 (64.6) | <0.0001 | 0.7 |
| Borderline | 12 (5.1) | 7 (4.6) | 22 (3.3) | ||
| Negative | 172 (72.9) | 48 (31.6) | 215 (32.1) | ||
| Missing | 47 | 52 | 225 | ||
| HER2 status | |||||
| Positive | 15 (10.3) | 16 (16.8) | 96 (22.6) | 0.01 | 0.3 |
| Borderline | 6 (4.1) | 5 (5.3) | 14 (3.3) | ||
| Negative | 125 (85.6) | 74 (77.9) | 315 (74.1) | ||
| Missing | 137 | 109 | 469 | ||
| Triple negative status (ER−/PR−/HER2−) | |||||
| Yes | 86 (59.3) | 17 (18.9) | 56 (13.6) | <0.0001 | 0.2 |
| No | 59 (40.7) | 144 (81.1) | 634 (86.4) | ||
| Missing | 138 | 43 | 204 | ||
| BRCAPRO probability of being a BRCA1 or BRCA2 mutation carrier | |||||
| <25% | 124 (44.6) | 120 (60.0) | 709 (81.1) | <0.0001 | <0.0001 |
| 25% to <50% | 45 (16.2) | 24 (12.0) | 69 (7.9) | ||
| 50% to <75% | 29 (10.4) | 24 (12.0) | 48 (5.5) | ||
| 75% to <90% | 24 (8.6) | 12 (6.0) | 27 (3.1) | ||
| >90% | 56 (20.1) | 20 (10.0) | 21 (2.4) | ||
| Missing | 5 | 4 | 20 | ||
Table 3.
Case-only interaction risk ratios (IRRs) and 95% confidence intervals (CI) comparing BRCA1+ or BRCA2+ breast cancer cases to BRCA- breast cancer cases.
| BRCA1+ | BRCA2+ | BRCA− | BRCA1+ vs BRCA− | BRCA2+ vs. BRCA− | |||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | IRR* | (95% CI) | IRR* | (95% CI) | |
| Age at menarche (years) | |||||||
| <11 | 57 (20.7) | 35 (17.6) | 182 (20.6) | 1.00 | Reference | 1.00 | Reference |
| 12–13 | 156 (56.7) | 98 (49.2) | 497 (56.2) | 0.98 | (0.69–1.39) | 1.02 | (0.67–1.56) |
| ≥14 | 62 (22.6) | 66 (33.2) | 205 (23.2) | 0.95 | (0.63–1.44) | 1.65 | (1.04–2.61) |
| Missing | 8 | 5 | 10 | ||||
| Full-term pregnancies | |||||||
| None | 81 (28.8) | 48 (23.5) | 281 (31.6) | 1.00 | Reference | 1.00 | Reference |
| 1 | 41 (14.6) | 35 (17.2) | 133 (15.0) | 1.09 | (0.71–1.68) | 1.55 | (0.95–2.52) |
| 2 | 96 (34.2) | 78 (38.2) | 299 (33.7) | 1.28 | (0.91–1.81) | 1.54 | (1.03–2.32) |
| ≥3 | 63 (22.4) | 43 (21.1) | 175 (19.7) | 1.54 | (1.04–2.28) | 1.46 | (0.90–2.35) |
| Missing | 2 | 0 | 6 | ||||
| Age at first full-term pregnancy | |||||||
| <20 years | 19 (9.6) | 15 (9.8) | 50 (8.3) | 1.00 | Reference | 1.00 | Reference |
| 20–24 years | 62 (31.5) | 42 (27.4) | 164 (27.3) | 1.03 | (0.56–1.90) | 0.86 | (0.44–1.67) |
| 25–29 years | 63 (32.0) | 41 (26.8) | 185 (30.7) | 0.83 | (0.45–1.53) | 0.73 | (0.37–1.43) |
| ≥30 years | 53 (26.9) | 55 (36.0) | 203 (33.7) | 0.63 | (0.34–1.17) | 0.90 | (0.46–1.74) |
| Missing | 3 | 3 | 5 | ||||
| Oral contraceptive use | |||||||
| Never | 47 (17.7) | 24 (12.2) | 120 (14.4) | 1.00 | Reference | 1.00 | Reference |
| Ever | 219 (82.3) | 173 (87.8) | 716 (85.7) | 0.70 | (0.48–1.02) | 1.19 | (0.74–1.91) |
| Missing | 17 | 7 | 58 | ||||
| Oral contraceptive duration | |||||||
| <12 months | 66 (26.4) | 40 (21.4) | 187 (23.9) | 1.00 | Reference | 1.00 | Reference |
| 12–35 months | 26 (10.4) | 29 (15.5) | 102 (13.0) | 0.69 | (0.41–1.15) | 1.30 | (0.76–2.22) |
| 36–59 months | 27 (10.8) | 10 (5.4) | 75 (9.6) | 0.95 | (0.56–1.61) | 0.60 | (0.28–1.27) |
| ≥60 months | 131 (52.4) | 108 (57.8) | 418 (53.5) | 0.81 | (0.57–1.15) | 1.13 | (0.74–1.71) |
| Missing duration (among users) | 16 | 10 | 54 | ||||
| Breastfeeding | |||||||
| Never | 32 (20.0) | 30 (22.3) | 97 (19.4) | 1.00 | Reference | 1.00 | Reference |
| Ever | 128 (80.0) | 101 (77.7) | 402 (80.6) | 0.88 | (0.56–1.39) | 0.76 | (0.47–1.23) |
| Missing | 40 | 25 | 108 | ||||
| BMI (kg/m2) 1 year before diagnosis | |||||||
| <25 | 98 (54.1) | 72 (53.0) | 309 (55.8) | 1.00 | Reference | 1.00 | Reference |
| ≥25 to <30 | 42 (23.2) | 38 (27.9) | 147 (26.5) | 0.99 | (0.65–1.51) | 1.09 | (0.70–1.70) |
| ≥30 | 41 (22.7) | 26 (19.1) | 98 (17.7) | 1.38 | (0.89–2.13) | 1.15 | (0.69–1.90) |
| Missing | 102 | 68 | 340 | ||||
| BMI (kg/m2) at age 18 | |||||||
| <25 | 138 (72.2) | 109 (75.7) | 413 (70.2) | 1.00 | Reference | 1.00 | Reference |
| ≥25 to <30 | 29 (15.2) | 25 (17.4) | 120 (20.4) | 0.76 | (0.48–1.20) | 0.81 | (0.50–1.31) |
| ≥30 | 24 (12.6) | 10 (6.9) | 55 (9.4) | 1.15 | (0.68–1.94) | 0.68 | (0.33–1.38) |
| Missing | 92 | 60 | 306 | ||||
| Alcohol use | |||||||
| No | 74 (28.0) | 49 (25.1) | 173 (20.9) | 1.00 | Reference | 1.00 | Reference |
| Yes | 190 (72.0) | 146 (74.9) | 656 (79.1) | 0.65 | (0.48–0.90) | 0.80 | (0.55–1.16) |
| Missing | 19 | 9 | 65 | ||||
| Smoking (Ever) | |||||||
| No | 175 (62.5) | 111 (55.0) | 514 (58.3) | 1.00 | Reference | 1.00 | Reference |
| Yes | 105 (37.5) | 91 (45.0) | 367 (41.7) | 0.87 | (0.66–1.15) | 1.14 | (0.84–1.56) |
| Missing | 3 | 2 | 13 | ||||
Adjusted for age and center.
Because these analyses are case-only comparisons, the IRRs are estimates of the interaction between the genetic factor (BRCA mutation) and the environmental factor provided that the two factors are independent in the population giving rise to the data (women with a family history of breast and/or ovarian cancer) and conditional on the factors adjusted for in the model (i.e., age). Since there is no traditional ‘control group’ of unaffected subjects, we are not able to assess main effects. For example, an IRR of 2.0 generated from this analysis for environmental factor E comparing BRCA1+ cases to BRCA− cases indicates that the relative risk of disease given E is twice as high among BRCA1+ individuals as it is among BRCA− individuals, but the strength of the association in BRCA− individuals (the main effect) is unknown. Similarly, an IRR of 1.0 for environmental factor E comparing BRCA1+ cases to BRCA− cases indicates that there is no interaction between BRCA1 mutation status and factor E, i.e., the RR of disease given E is the same for both BRCA1+ and BRCA− individuals. This could indicate that either the factor is unassociated with disease in both genotype groups or has a similar association (main effect) in both genotype groups.
Genetic testing for BRCA1 and BRCA2 mutations is specific, but its sensitivity is less than one with rates that vary by the method employed, gene and mutation type. As a result, we expect that test positives are truly positive for a deleterious mutation and that a fraction of the test negatives are also positive. This has the potential to result in biased estimates of effect. To assess the sensitivity of our results to misclassification of BRCA1 or BRCA2 genotype, we conducted a sensitivity analysis in which we repeated the above analyses after removing test negative subjects with pre-test probabilities of carrying a deleterious BRCA1 or BRCA2 mutation ranging from greater than 90% to greater than 25%. We used BRCAPRO as implemented in BayesMendel V2.0–2 to calculate each subject’s BRCAPRO probability assuming the penetrance functions found in Chen et al. [26] and Tai et al. [27] and implemented in the structure BRCApenet.metaDSL.2008. In calculating these probabilities, we conditioned on each subject’s first- and second-degree family history of breast and ovarian cancer as well as oophorectomy status, when known.
A factor, E, that is a modifier of penetrance may either result in a different risk of disease by genotype or shift the age at diagnosis distribution as a function of genotype, or both. Therefore, we also looked at whether the environmental factors modified the penetrance of BRCA1 and BRCA2 through a shift in the age at onset distributions by constructing multiple linear regression models with age at diagnosis as the response (dependent) variable and the independent variables gene mutation status (BRCA1+, BRCA2+, BRCA−), the environmental factor, and a cross-product gene by environmental factor interaction term. Statistically significant differences in the mean age at diagnosis by genetic and environmental factors provide evidence that the environmental factor acts as a genetic modifier. The analysis of mean age differences does not require the G by E independence assumption as stated for the case-only interaction analyses described above.
Results
Table 2 shows the demographic and clinical characteristics of the cases by BRCA mutation status. The BRCA1+ cases were slightly younger than the other case groups (mean age 41.2 years versus 44.0 for BRCA2+ cases and 44.1 for BRCA− cases). Only about 10% of the cases in each group were non-white, which is similar to the race/ethnic distribution of tested women reported by Myriad Genetics Laboratories. [28] The proportion of Jewish women was higher among the BRCA1+ and BRCA2+ cases than controls (p=0.01 and 0.02, respectively), reflecting the high prevalence of known founder mutations among women with Ashkenazi ancestry.
The BRCA1+ cases had clinical characteristics that were distinct from the BRCA2+ and the BRCA− cases. As previously reported, the stage distribution of cancers among BRCA1 mutation carriers was not statistically significantly different from the other two case groups, but they were more likely to have higher grade (poorly differentiated) and to be ER negative, PR negative, and HER2 negative. Among women with information on all three markers (ER, PR and HER2), 59.3% of the BRCA1+ cases were negative for all three, compared to a triple negative proportion of 18.9% for BRCA2+ and 13.6% for BRCA− cases. BRCA1+ cases had higher prior probabilities of being a mutation carrier than either BRCA2+ or BRCA− cases.
In Table 3, we present the case-only comparisons of the epidemiologic risk factors. Age at menarche showed a statistically significant difference in its association with BRCA2+ cancer as compared to BRCA− cases. A greater proportion of BRCA2+ cases had an age at menarche ≥14 years. The IRR of 1.65 (95% CI 1.04 – 2.61) comparing BRCA2+ to BRCA− cases indicates that the protective effect of late age at menarche typically observed for sporadic breast cancer was reduced among the BRCA2+ cases.
IRRs for number of full-term pregnancies were greater than one for both BRCA1+ and BRCA2+ cases, suggesting that the inverse relationship with pregnancy usually noted for breast cancer is attenuated or absent for BRCA1+ and BRCA2+ cases. There were no statistically significant differences between the case groups for breastfeeding, although the IRRs of 0.88 for BRCA1+ and 0.76 for BRCA2+ cases as compared to BRCA− cases suggest that breastfeeding has a protective effect regardless of BRCA mutation status. The IRRs for oral contraceptive use were not statistically significant and showed no clear trends with duration of use.
Among the other characteristics examined, the only statistically significant IRR observed was alcohol use among BRCA1+ cases (IRR=0.65, 95% CI 0.48–0.90), suggesting that the effect of alcohol on BRCA1+ cases as compared to BRCA− cases is weaker or absent. No clear interactions with BMI or smoking were observed.
We also performed multivariable analyses, with terms for age, age at menarche, number of pregnancies, oral contraceptive use, breastfeeding, alcohol use and smoking included in the model. Overall, results were not substantively different than the age-adjusted analyses.
In the sensitivity analysis, we repeated the modeling excluding test negative cases that had pre-test probabilities of being a mutation carrier based on BRCAPRO scores ranging from >90% (21 cases excluded) to >25% (165 cases excluded). No substantive differences in any of the IRRs were observed when excluding test negative cases that had a high pre-test probability of being a mutation carrier (data not shown). For example, the statistically significant IRR for late age at menarche that we observed for BRCA2 carriers (1.65, 95% CI 1.04–2.61) changed only slightly when we excluded cases that had high pre-test probabilities (IRRs ranged from 1.60 to 1.69). Therefore we conclude that misclassification of mutation status among test negative cases had minimal effect on our findings.
Results of the analyses that modeled the age at diagnosis by genetic and environmental factors are presented in Table 4. For each factor, we assessed whether each breast cancer risk factor modified the age at diagnosis according to the BRCA mutation status. For all but one of the factors examined, the p-values for gene-environment interaction were not statistically significant implying that the relationship between the risk factors and age at diagnosis did not vary according to BRCA mutation status. The only statistically significant interaction observed was for alcohol use. Among BRCA1+ and BRCA2+ cases, the age at diagnosis was younger for women who consumed alcohol whereas the age at diagnosis was slightly older for women who consumed alcohol among the BRCA− cases. Although the interaction p-value for mutation status by alcohol consumption was nominally statistically significant, we made no adjustment for multiple comparisons so these results should be interpreted cautiously.
Table 4.
Modeled age at breast cancer diagnosis by gene and risk factor status and p-values for gene effects (G), environmental factor effects (E) and gene by environment (GxE) interations.
| Risk Factor | Categories | BRCA1+ | BRCA2+ | BRCA− | P-values |
|---|---|---|---|---|---|
| # of full-term pregnancies | 0 | 38.2 | 41.9 | 41.3 | G: <0.0001 |
| 1 | 39.1 | 40.5 | 42.0 | E: <0.0001 | |
| 2 | 41.8 | 44.5 | 44.3 | GxE: 0.91 | |
| ≥3 | 42.8 | 44.7 | 46.1 | ||
| Age at last pregnancy | <20 years | 41.2 | 43.6 | 45.2 | G: 0.0004 |
| 20–24 years | 41.7 | 46.6 | 45.5 | E: 0.0087 | |
| 25–29 years | 41.8 | 40.8 | 43.3 | GxE: 0.37 | |
| ≥30 years | 40.6 | 42.7 | 43.4 | ||
| Age at menarche | <12 years | 41.4 | 43.9 | 43.5 | G: 0.0001 |
| 12 to 14 years | 40.2 | 42.3 | 43.2 | E: 0.34 | |
| >14 years | 40.0 | 43.4 | 42.9 | GxE: 0.86 | |
| Oral contraceptive duration of use | <12 months | 43.8 | 45.2 | 44.8 | G: <0.0001 |
| 12–35 months | 38.8 | 41.6 | 44.4 | E. <0.0001 | |
| 36–59 months | 38.3 | 44.6 | 43.5 | GxE: 0.20 | |
| >60 months | 39.6 | 41.9 | 42.2 | ||
| Breastfed | Never | 42.1 | 45.3 | 46.7 | G: 0.0016 |
| Ever | 41.3 | 42.4 | 43.8 | E: 0.014 GxE: 0.60 |
|
| BMI kg/m2 (1 year before diagnosis) | <25 | 40.3 | 43.1 | 42.9 | G: 0.0004 |
| 25 – <30 | 41.6 | 42.6 | 43.4 | E: 0.13 | |
| ≥30 | 41.3 | 45.7 | 45.9 | GxE: 0.14 | |
| BMI kg/m2 (at age 18) | Underweight | 40.9 | 44.2 | 45.5 | G: 0.0048 |
| Normal | 37.6 | 41.5 | 39.2 | E: 0.0076 | |
| Overweight | 40.9 | 42.9 | 43.2 | GxE: 0.65 | |
| Alcohol Use | Yes | 39.8 | 42.6 | 43.1 | G: 0.015 |
| No | 42.7 | 44.3 | 42.8 | E: 0.031 GxE: 0.047 |
|
| Smoking status | Never | 40.7 | 42.8 | 42.7 | G: <0.0001 |
| Ever | 40.3 | 43.2 | 44.0 | E: 0.54 GxE: 0.34 |
Discussion
In the classic situation of evaluating gene-environment interaction, the study population (case-control or cohort) involves eight groups of subjects: cases (1) and controls (2) that have neither the genetic nor environmental factor; cases (3) and controls (4) with the genetic factor but not the environmental factor; cases (5) and controls (6) with the environmental factor but not the genetic factors; and cases (7) and controls (8) with both the genetic factor and the environmental factor. Such a design allows the estimation of the effect of the gene, the effect of the environmental factor and the effect of both factors combined, allowing a determination of any interaction effects. In the published studies that have evaluated potential modifying factors of BRCA1 or BRCA2, none has included subjects with all eight of the possible combinations of disease status, gene and environmental exposure.
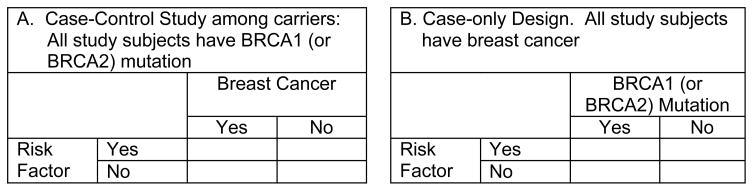
The design that has been used most frequently in the reports published to date, e.g. [6, 8,9,29] is a case-control study among carriers only, which is depicted in part A of Figure 1. Risk factors are compared between mutation carriers affected with breast cancer and unaffected carriers. The logORs generated in these studies describe the association with a given risk factor within a population of mutation carriers; hence they correspond to the sum of the population logOR of D given E and the population interaction logOR of D given E and G in the classical case-control or cohort study. If the associations are similar to established risk factors for sporadic breast cancer, the data may be interpreted as no evidence of gene by environment interaction. If the estimated associations are different, it is suggestive of interaction between the genetic and environmental factor.
Figure 1.
Study designs for evaluating modifiers of BRCA1/BRCA2
The case-only design used in this study, which is depicted in part B of Figure 1, compares risk factors between breast cancer cases who have a BRCA mutation and those who do not. The case-only odds of environmental exposure given genotype is mathematically equivalent to the IRR for disease given genotype and environmental exposures when those variables are independent in the population. [24,25] When this assumption is met, the case-only design has two distinct advantages over a full case-control study for estimating gene by environment interaction: it provides direct estimates of the interaction risk ratio and is more efficient, requiring fewer subjects.
Some investigations have combined aspects of both designs in case-control studies with multiple case groups, in which both case-control comparisons and case-only comparisons were made. [20,21] In these studies, case groups were defined by BRCA1 and/or BRCA2 genotype and the controls were all BRCA− (either based on testing or presumed to be negative if untested). [20,21] Lee [20] estimated odds of disease given a variety of environmental exposures for each case group and tested for heterogeneity in effects (which would suggest gene-environment interaction/modification). In addition, they carried out case-only analyses to formally test for modifiers. The report by Tryggvadottir et al. [21] which focused on the 999del5 Icelandic BRCA2 founder mutation, took a similar analytic tack in terms of utilizing case-only analysis to test for modifiers.
The different parameters estimated with the case-control design and the case-only design must be kept in mind when interpreting results from this study and comparing these results to other published studies. Only when (1) G and E are independent in the population conditional on the variables adjusted for in the modeling (age) and (2) the disease is rare for all combinations of the covariates can the case-only IRR be compared to an interaction OR generated by a classical case-control or cohort study. Because assumption 2 is clearly not met for BRCA1 or BRCA2 carriers, the IRRs we estimate are not directly comparable to interaction ORs but remain interpretable as RRs. [25]
Our data on pregnancy characteristics suggest that there is a weaker protective effect of pregnancy among mutation carriers than non-carriers. There was no evidence of significant risk modification with age at first pregnancy and BRCA mutation status. Several publications have evaluated the effect of pregnancy characteristics on breast cancer risk in mutation carriers. A recent paper describing the association between parity and breast cancer in BRCA1 and BRCA2 mutation carriers summarized the published literature. [29] When considering parity, results ranged from statistically significant inverse relationships among BRCA1 carriers [9] or pooled BRCA1 and BRCA2 carriers [30], to non-statistically significant inverse relationships among BRCA1 or BRCA2 carriers [8,9,20,29] to statistically significant positive relationships among BRCA1 [19] carriers. Similar inconsistencies in results were observed for age at first birth. Inverse associations with age at first birth were observed for BRCA2+ breast cancer in two studies [9] whereas no association was reported in other studies. [20,21,29] For BRCA1+ breast cancer, one study reported a statistically significant positive association with age at first birth [9] and two others reported no association. [9,29] There is no clear reason for the disparate findings between studies.
Other characteristics that we identified within our study as being potential modifiers are age at menarche in BRCA2 carriers and alcohol consumption in BRCA1 carriers. Our finding of a statistically significant IRR for BRCA2+ cancers (IRR=1.65, 95% CI 1.04 –2.61) suggests that later age at menarche is not as protective for BRCA2+ cancers as for BRCA− cancers. Several other published studies have reported on age at menarche in relation to breast cancer given BRCA mutation status. [10,16,19–21] In one case-control study of carriers only, age at menarche was not statistically significantly associated with breast cancer risk in either BRCA1+ or BRCA2+ carriers. [10] whereas two other studies reported inverse associations with age at menarche for BRCA1+ cancers. [16,19] The studies by Lee, et al. [20] and Tryggvadottir et al. [21] each conducted case-only analyses. Lee et al. [20] reported a significant IRR for age at menarche ≥14 years, when comparing BRCA1/2+ cases to BRCA− cases, but a non-significant p-value for trend. They observed no protective effect of later age at menarche among BRCA2 carriers, albeit with a small sample size, which is consistent with results from the present study. Tryggvadottir et al. reported that the interaction between age at menarche and BRCA2 mutation status was not statistically significant. [21]
To our knowledge, only one previous report, a case-control study among carriers, has described a relation between alcohol and BRCA1+ and BRCA2+ breast cancer, [12] and their results were not consistent with our findings. McGuire et al. [12] reported that ever use of alcohol was inversely associated with BRCA2+ cancer compared to unaffected carriers, but there was no trend between amount of alcohol and breast cancer risk. No significant associations were found with BRCA1+ cancer.
To date, the results of studies that have attempted to identify modifiers of BRCA1 or BRCA2 do not present a consistent picture of any factor that appears to interact with these genes. There are a number of possible reasons that various studies have reached different conclusions. Some of the inconsistency may be related to small sample sizes. [22, 23] There is also variability in the characteristics of the cases included in the studies. Some early reports that included both BRCA1 and BRCA2 mutation carriers did not perform separate analyses for BRCA1 and BRCA2. However, the evidence is quite compelling that BRCA1+ cancers have distinct clinical characteristics. Presuming that they arise from distinct biological pathways, differences in risk factors would be obscured when BRCA1+ and BRCA2+ cancers are evaluated as a single entity.
Other studies have been conducted within select populations, for example Tryggadottir et al. from Iceland [21] or Gronwald et al. from Poland [19] in which there is a preponderance of certain founder mutations that are distinct from those in other populations. Among studies that are multi-center collaborations with cases derived from a variety of populations, including the present study, there is a much greater spectrum of distinct mutations within the genes. If different factors act as modifiers of different mutations, it will be extremely challenging to detect such modifiers.
Our data provide evidence that the distributions of age at menarche, number of pregnancies, and alcohol consumption vary between breast cancer-affected carriers of a BRCA1 or BRCA2 mutation, suggesting these factors are potential modifiers of BRCA1 and/or BRCA2 mutations. However, the limitations of the study should be considered when interpreting the results of the analyses. Evaluations of potential modifiers of BRCA1 and BRCA2 rely on multi-center collaborations to achieve reasonable sample sizes, but the use of breast cancer cases from multiple sites also introduces variability that may not be completely controlled for in statistical analyses, including differences in testing methods, criteria for testing individuals and the type of epidemiologic data collected.
The women included in the study were tested for BRCA1 or BRCA2 mutations over a broad time span using a variety of methods, including full sequencing with or without the use of large delection/duplication testing, the Jewish panel only or family mutations only. It is possible that there were mutations among the test-negative cases that were not detected because of the testing methods used. The sensitivity analyses we performed in which we excluded test-negative cases with a high BRCAPRO probability of being mutation positive did not suggest our results were strongly influenced by false negative results, however the possibility of misclassification of mutation status remains a limitation.
It is also possible that the criteria for testing breast cancer cases for BRCA1 or BRCA2 mutations varied across the centers. All sites except the University of Southern California recruited women from high-risk clinics, but there may have been subtle differences in the characteristics of women tested across sites. Further, the recruitment from high-risk clinics means that the non-mutation carrriers are a select sub-group of sporadic breast cancers, and they could have a distinct set of risk factors.
The epidemiologic data collected across the sites also varied such that we were limited in exploring the effects of certain environmental factors. For example, differences across sites in how information on alcohol consumption or smoking was collected precluded us from doing more detailed analyses of the duration or timing of these exposures. Finally, although the number of subjects needed to detect statistically significant interactions is smaller with a case-only design, our analyses were limited by sample size considerations. With only 283 BRCA1+ and 204 BRCA2+ cases, it is possible that we had inadequate power to detect modifying factors that had modest effects.
Although we found evidence of potential modification of BRCA1 and BRCA2 by environmental factors, these results have to be interpreted in light of the overall lack of consistency in the literature regarding BRCA1 and BRCA2 modifiers. Much of this inconsistency is due, as we have shown, to use of different study designs and effect measures. The case-only design used in the present study allows us to estimate IRRs, which cannot be directly compared to interaction ORs estimated from a traditional case-control study of breast cancer given exposure and BRCA1 or BRCA2 mutations status. While there are compelling reasons to suspect that there are environmental modifiers of BRCA1 and BRCA2, the results from this study combined with the overall body of literature does not provide consistent evidence in result or form for any factor. Future work on the identification of modifiers of BRCA1 and BRCA2 will need to rely on collaborative efforts involving thousands of affected women.
Acknowledgments
This work was funded by the following grants and contracts from the National Cancer Institute, National Institutes of Health: P50-CA068438 Specialized Program of Research Excellence (SPORE) in breast cancer, Duke University Medical Center; Cancer Genetics Network (U01 CA78284 and HHSN261200744000C), Massachusetts General Hospital.
References
- 1.Fackenthal JD, Olopade OI. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer. 2007;7:937–48. doi: 10.1038/nrc2054. [DOI] [PubMed] [Google Scholar]
- 2.Breast Cancer Linkage Consortium. Pathology of familial breast cancer: differences between breast cancers in carriers of BRCA1 or BRCA2 mutations and sporadic cases. Breast Cancer Linkage Consortium. Lancet. 1997;349:1505–10. [PubMed] [Google Scholar]
- 3.Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26:4282–8. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–18. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 5.Bordeleau L, Panchal S, Goodwin P. Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat. 2010;119:13–24. doi: 10.1007/s10549-009-0566-z. [DOI] [PubMed] [Google Scholar]
- 6.Narod SA, Dube MP, Klijn J, et al. Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;94:1773–9. doi: 10.1093/jnci/94.23.1773. [DOI] [PubMed] [Google Scholar]
- 7.Jernstrom H, Lerman C, Ghadirian P, et al. Pregnancy and risk of early breast cancer in carriers of BRCA1 and BRCA2. Lancet. 1999;354:1846–50. doi: 10.1016/s0140-6736(99)04336-6. [DOI] [PubMed] [Google Scholar]
- 8.Cullinane CA, Lubinski J, Neuhausen SL, et al. Effect of pregnancy as a risk factor for breast cancer in BRCA1/BRCA2 mutation carriers. Int J Cancer. 2005;117:988–91. doi: 10.1002/ijc.21273. [DOI] [PubMed] [Google Scholar]
- 9.Andrieu N, Goldgar DE, Easton DF, et al. Pregnancies, breast-feeding, and breast cancer risk in the International BRCA1/2 Carrier Cohort Study (IBCCS) J Natl Cancer Inst. 2006;98:535–44. doi: 10.1093/jnci/djj132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang-Claude J, Andrieu N, Rookus M, et al. Age at menarche and menopause and breast cancer risk in the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol Biomarkers Prev. 2007;16:740–6. doi: 10.1158/1055-9965.EPI-06-0829. [DOI] [PubMed] [Google Scholar]
- 11.Kotsopoulos J, Lubinski J, Lynch HT, et al. Age at first birth and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2007;105:221–8. doi: 10.1007/s10549-006-9441-3. [DOI] [PubMed] [Google Scholar]
- 12.McGuire V, John EM, Felberg A, et al. No increased risk of breast cancer associated with alcohol consumption among carriers of BRCA1 and BRCA2 mutations ages <50 years. Cancer Epidemiol Biomarkers Prev. 2006;15:1565–7. doi: 10.1158/1055-9965.EPI-06-0323. [DOI] [PubMed] [Google Scholar]
- 13.Haile RW, Thomas DC, McGuire V, et al. BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;15:1863–70. doi: 10.1158/1055-9965.EPI-06-0258. [DOI] [PubMed] [Google Scholar]
- 14.Brohet RM, Goldgar DE, Easton DF, et al. Oral contraceptives and breast cancer risk in the international BRCA1/2 carrier cohort study: a report from EMBRACE, GENEPSO, GEO-HEBON, and the IBCCS Collaborating Group. J Clin Oncol. 2007;25:3831–6. doi: 10.1200/JCO.2007.11.1179. [DOI] [PubMed] [Google Scholar]
- 15.Jernstrom H, Lubinski J, Lynch HT, et al. Breast-feeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2004;96:1094–8. doi: 10.1093/jnci/djh211. [DOI] [PubMed] [Google Scholar]
- 16.Kotsopoulos J, Lubinski J, Lynch HT, et al. Age at menarche and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer Causes Control. 2005;16:667–74. doi: 10.1007/s10552-005-1724-1. [DOI] [PubMed] [Google Scholar]
- 17.Brunet JS, Ghadirian P, Rebbeck TR, et al. Effect of smoking on breast cancer in carriers of mutant BRCA1 or BRCA2 genes. J Natl Cancer Inst. 1998;90:761–6. doi: 10.1093/jnci/90.10.761. [DOI] [PubMed] [Google Scholar]
- 18.Kotsopoulos J, Olopado OI, Ghadirian P, et al. Changes in body weight and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2005;7:R833–43. doi: 10.1186/bcr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gronwald J, Byrski T, Huzarski T, et al. Influence of selected lifestyle factors on breast and ovarian cancer risk in BRCA1 mutation carriers from Poland. Breast Cancer Res Treat. 2006;95:105–9. doi: 10.1007/s10549-005-9051-5. [DOI] [PubMed] [Google Scholar]
- 20.Lee E, Ma H, McKean-Cowdin R, et al. Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;17:3170–8. doi: 10.1158/1055-9965.EPI-08-0396. [DOI] [PubMed] [Google Scholar]
- 21.Tryggvadottir L, Olafsdottir EJ, Gudlaugsdottir S, et al. BRCA2 mutation carriers, reproductive factors and breast cancer risk. Breast Cancer Res. 2003;5:R121–8. doi: 10.1186/bcr619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350–6. doi: 10.1158/1055-9965.EPI-04-0376. [DOI] [PubMed] [Google Scholar]
- 23.Ursin G, Henderson BE, Haile RW, et al. Does oral contraceptive use increase the risk of breast cancer in women with BRCA1/BRCA2 mutations more than in other women? Cancer Res. 1997;57:3678–81. [PubMed] [Google Scholar]
- 24.Piegorsch WW, Weinberg CR, Taylor JA. Non-hierarchical logistic models and case-only designs for assessing susceptibility in population-based case-control studies. Stat Med. 1994;13:153–62. doi: 10.1002/sim.4780130206. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt S, Schaid DJ. Potential misinterpretation of the case-only study to assess gene-environment interaction. Am J Epidemiol. 1999;150:878–85. doi: 10.1093/oxfordjournals.aje.a010093. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J ClinOncol. 2007;25:1329–33. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811–4. doi: 10.1093/jnci/djm203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480–90. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 29.Milne RL, Osorio A, Ramon y Cajal T, et al. Parity and the risk of breast and ovarian cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2009;119:221–32. doi: 10.1007/s10549-009-0394-1. [DOI] [PubMed] [Google Scholar]
- 30.Rebbeck TR, Wang Y, Kantoff PW, et al. Modification of BRCA1- and BRCA2-associated breast cancer risk by AIB1 genotype and reproductive history. Cancer Res. 2001;61:5420–4. [PubMed] [Google Scholar]