Abstract
In the pathogenesis of diabetic retinopathy, retinal mitochondria become dysfunctional resulting in accelerated apoptosis of its capillary cells. Matrix metalloproteinases2 (MMP2) is considered critical in the cell integrity and cell survival, and diabetes activates MMP2 in the retina and its capillary cells. The present study aims in elucidating the mechanism via which MMP2 contributes to the development of diabetic retinopathy. Using isolated bovine retinal endothelial cells, the effect of regulation of MMP2 (by its siRNA and pharmacological inhibitor) on superoxide accumulation and mitochondrial dysfunction was evaluated. Effect of inhibiting diabetes-induced retinal superoxide accumulation on MMP2 and its regulators was investigated in diabetic mice overexpressing mitochondrial superoxide dismutase. Inhibition of MMP2 ameliorated glucose-induced increase in mitochondrial superoxide and membrane permeability, prevented cytochrome c leakage from the mitochondria, and inhibited capillary cell apoptosis. Overexpression of MnSOD protected the retina from diabetes-induced increase in MMP2 and its membrane activator (MT1-MMP), and decrease in its tissue activator (TIMP-2). These results implicate that, in diabetes, MMP2 activates apoptosis of retinal capillary cells via mitochondrial dysfunction increasing their membrane permeability. Understanding the role of MMP2 in the pathogenesis of diabetic retinopathy should help lay ground for MMP2 targeted therapy to retard the development of retinopathy in diabetic patients.
Keywords: Diabetic retinopathy, MMP2, Mitochondria dysfunction, Oxidative stress
Diabetic retinopathy, a microvascular disease, causes progressive damage to the retina, and, if not controlled, could lead to blindness. Although many hyperglycemia-initiated metabolic and functional abnormalities are implicated in the pathogenesis of diabetic retinopathy, the exact mechanism of its development remains elusive. Studies have shown that retinal capillary cells and other non vascular cells undergo accelerated apoptosis before the appearance of histopathological lesions that are characteristic of diabetic retinopathy 1-4, but the exact mechanism responsible for their loss remains elusive.
In the development of diabetic retinopathy oxidative stress plays a major role, and sustained production of mitochondrial reactive oxygen species (ROS) are considered to be the active mediators in the regulation of apoptosis 5-8. Previous studies have postulated a link between mitochondria damage and the development of diabetic retinopathy 9-14. Regulation of mitochondrial ROS production has beneficial effects in preventing the activation of the major pathways that are implicated in the development of diabetic retinopathy, including protein kinase C and polyol pathway 5, 15, 16, but the exact mechanism involved in the pathogenesis of diabetic retinopathy is unclear.
Matrix metalloproteinase-2 (MMP2), one of the most ubiquitous members of the matrix metalloproteinase family, cleaves collagen type IV of the extracellular matrix maintaining equilibrium between matrix synthesis and degradation, thus providing a critical role in the cell integrity and cell survival 17. Clinical and experimental studies have shown increased expression of MMP2 in diabetic subjects 18-22. Recently we have demonstrated a significant role of MMP2 in the development of diabetic retinopathy; our results have shown that the activation of MMP2 is under the control of superoxide 23. However, the mechanism by which MMP2 activation is controlled in the retina in diabetes needs to be evaluated.
The present study is designed to test the hypothesis that MMP2 contributes to the pathology of diabetic retinopathy by inducing mitochondrial dysfunction and apoptosis of retinal capillary cells. Using both in vivo and in vitro models of diabetic retinopathy, we have investigated the possible mechanism by which MMP2 could contribute to the development of diabetic retinopathy. Using isolated retinal endothelial cells in culture, the effect of regulation of MMP2 is determined on ROS production, mitochondrial dysfunction and apoptosis. To confirm our in vitro results, we have investigated the effect of regulation of superoxide that the retina experience in diabetes on retinal MMP2 activity and its regulators in mice overexpressing mitochondrial superoxide dismutase (MnSOD).
Methods
Retinal endothelial cell isolation and culture
Endothelial cell were isolated from bovine retina and cultured on polystyrene dishes coated with 0.1%gelatin. Endothelial cells were grown in Dulbecco's Modified Eagle Medium (DMEM) containing 15% fetal bovine serum and 1% antibiotic/antimycotic at 37°C in 5% CO2 13, 24. The cells from 4th-7th passage were incubated in the DMEM containing 2% heat inactivated fetal calf serum, 10% Nu-serum, 50μg/ml heparin, 1μg/ml endothelial growth supplement and antibiotic/antimycotic supplemented with 5mM glucose or 20mM glucose for 4 days in the presence or absence of 10μM of MMP2 inhibitor I 23 (cis-octadecenoyl-N-hydroxylamide oleoyl-N-hydroxylamide; EMD Biosciences, Gibbstown, NJ). The medium and the inhibitors were replaced every other day. At the end of incubation medium was collected, and the cells were washed with phosphate buffer saline and collected. Osmotic controls included the cells incubated in identical experimental conditions with 20mM mannitol instead of 20mM glucose.
Mice
Group of age matched (8-10 weeks) mice overexpressing MnSOD (Tg) and their wild type (WT) littermates were made diabetic by streptozotocin injection (55mg/kg BW) for 5 consecutive days 10, 14. The mice, which presented blood glucose 250mg/dl or higher 3 days after the last injection were considered as diabetic. Mice were sacrificed approximately 6 month after induction of diabetes by overdose of pentobarbital (120mg/kg BW). One eye was used to isolate the retina (under dissecting microscope) for biochemical assay and the other eye for immunohistochemistry by incubating it in 10% paraformaldehyde for 30 minutes. The eye was washed with phosphate buffered saline (PBS), fixed it in OCT, and immediately frozen in liquid nitrogen for sectioning. These mice are being routinely used in our laboratory, and phenotype details are reported in our previous publications 14, 15. Treatment of the animals conformed to the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Research.
Transfection of cells with MMP2-siRNA
Endothelial cells (60-80% confluent) from 3rd - 4th passage were transfected using transfection reagent kit and MMP2 siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) as routinely done in our laboratory 25. The transfection complex, prepared by adding MMP2 siRNA and siRNA transfection reagent, was incubated for 30 minutes at room temperature, and the cells were incubated with this transfection complex for 8 hours at 37°C. Parallel incubations were carried out using non-targeting scrambled siRNA. After the transfection, the cells were rinsed with phosphate buffered saline and incubated in 5mM or 20mM glucose media for 4 days.
Isolation of mitochondria and cytosol
Mitochondria and cytosol fractions were prepared from retinal endothelial cells by differential centrifugation method as previously employed by us 10. Protein was determined by the Bicinchoninic Acid protein assay (Sigma-Aldrich, St Louis, MO).
Gelatin zymography
Gelatenolytic activity of MMP2 was estimated in culture media or in the retina by zymography technique 23. The samples (10-20μg protein) were electrophoresed under non-reducing conditions onto 10% SDS-PAGE gels polymerized with 1mg/ml gelatin. After washing the gel with 2.5% Triton X-100, it was incubated over night at 37°C in substrate buffer containing 50mM Tris–HCl, pH 8.0, 5mM CaCl2, and 0.02% NaN3. The gel was stained with Coomassie blue stained (Simply Blue Safe Stain; Invitrogen, Carlsbad, CA), and this was followed by destaining with distilled water. The band intensity of the active MMP2 (67kD) band was quantified by using Un-Scan-It Gel digitizing software.
Western blot analysis
Protein (25-40μg) was subjected to SDS-PAGE and transferred to nitrocellulose membrane. Immunodetection was performed using antibodies against cytochrome c and poly ADP ribose polymerase, PARP1/2 (Santa Cruz Biotechnology). Cox IV and β actin were used as the loading controls for mitochondria and cytosol fractions respectively.
Gene expression
Gene expression was determined using conventional semiquantitative PCR as routinely performed in our laboratory 23, 25. cDNA template (0.5μg) was added to 10pmol of forward and reverse primers (designed using Applied Biosystems software Primer Express 3.0 and synthesized by Integrated DNA Technologies, Coralville, IA), and 1 unit of GoTaq DNA polymerase (Promega, Madison, WI). The standard PCR conditions included 2 minutes at 50°C and 10 minutes at 95°C followed by 35 cycles of denaturation at 95°C for 15 seconds, and 1 minute of annealing at 60°C. The primers sequences for bovine cells were: Fwd 5′-CCTCCTGCTGGGGACGCTGC-3′ and Rev 5′-AGTCCTGGTGGCCTGACGGG-3′ for TIMP-2; and Fwd 5′-AACATCAAAGTCTGGGAAGG-3′ and Rev 5′-GAAGTTCTCGGTGTCCATCCA-3 for MT1-MMP. The primers used for mouse retina were: Fwd 5′-AGAGACTGGCTTAGGAGGGC -3′ and Rev 5′- ATGTCAGACAACCCGAGTCC-3′ for MMP2; Fwd 5′-AGTCAGGGTCACCCACAAAG 3′ and Rev 5′-GCATTGGGTATCCATCCATC- 3′ for MT1-MMP, and Fwd 5′-CTTCTCCGCCGGGTGCACTG- 3′ and Rev 5′-CAGCAGCGTGGCTAGCAGCA-3′ for TIMP-2. After the amplification, the DNA was run on a 1.2% agarose gel, and a 100bp DNA ladder was simultaneously run on each gel. The bands were visualized with the UVP Bio-Doc it Imaging System (UVP LLC, Upland, CA), and band intensities were quantified by Un-Scan-It software.
Mitochondrial ROS generation
MitoTracker Red CM-H2XROS (Molecular Probes-Invitrogen, CA), a cell permeable dye that sequesters in the mitochondria and emits fluorescence when oxidized was used to measure mitochondrial ROS. After the desired incubations, the cells were washed with fresh medium to remove non-adherent cells, and incubated with 400nM Mitotracker Red for 30 minutes. This was followed by extensive washing with DMEM, and mounting in Vectastain-DAPI mounting media (Vector Laboratories, Burlington, CA). The fluorescence was visualized using Olympus BX50 fluorescent microscope.
Mitochondrial permeability pore transition
Mitochondrial membrane potential collapses by a sudden increase in the permeability of the inner membrane to small ions and molecules resulting in swelling. This collapse of mitochondrial membrane potential was quantified spectrophotometrically using 5-20μg protein mitochondrial protein as previously reported by us 10. The transition induced by 400μM calcium chloride containing 75μM t-butylhydroperoxide was measured at 540 nm. The values were expressed as a percentage of swelling with respect to the maximum swelling achieved by exposure to external calcium.
Mitochondrial membrane potential
To measure mitochondrial membrane potential, cationic dye JC-1 (MitoPT, Immunohistochemistry Technologies, Bloomington, MN) was used. At the end of the incubation period, medium was removed and the cells were rinsed with DMEM and incubated with 1 × MitoPT staining for 15 minutes at 37°C. The cells were washed, and visualized under Olympus BX50 fluorescent microscope.
Apoptosis
Apoptosis was determined by Cell Death Detection ELISAPLUS (Roche Diagnostics, Indianapolis, IN) using antibodies directed against DNA and histones respectively as routinely used in our laboratory 13, 23.
Immunohistochemical analysis
Cryosection (10μm) were prepared from the mouse retina were blocked by incubating them with the blocking reagent containing (2.5 % bovine serum albumin and 5% serum from the host animal of the secondary antibody in PBS) for 30 minutes in a moist chamber. The slides were rinsed with PBS, and incubated for 1 hour with anti-MMP2 (goat polyclonal, Santa Cruz Biotechnology). After rinsing the slides with PBS, they were incubated with the secondary antibody (anti goat-FITC conjugated) for 1 hour. The slides were then rinsed with 50mM Tris-HCl buffer (pH 7.5), and counterstained with DAPI (Vector Laboratories). The sections were imaged with Olympus BX50 fluorescent microscope (10× magnification). Control slides included staining of the samples that were incubated under identical conditions except the primary antibody.
Statistical analysis
The results are presented as mean± SD and analyzed statistically using the nonparametric Kruskal-Wallis test followed by Mann-Whitney test for multiple group comparisons. Similar conclusions were achieved by using ANOVA with Fisher or Tukey.
Results
Retinal endothelial cells in culture
High glucose exposure activates MMP2 and damages mitochondria
Effect of high glucose on MMP2 and its regulators: Incubation of retinal endothelial cells in high glucose medium, as expected, increased MMP2 gelatinase activity by about 30-40% compared to the cells incubated in normal glucose medium (Figures 1a). In the same cell preparations, the gene expression of its membrane activator, MT1-MMP, was increased by 25% and that of TIMP 2 (intracellular inhibitor) was decreased by 70% (Figures 1b). ROS levels were significantly elevated as evidenced by enhanced staining of MitoTracker Red (Figure 2). Mitochondrial swelling was increased by over 4 fold (Figure 3a), and its membrane permeability transition, as determined by cationic fluorescent dye, was also increased significantly in the cells incubated in high glucose compared to the cells incubated in normal glucose (Figure 3b). The leakage of cytochrome c from mitochondria into cytosol was increased by 40%, and this was accompanied by a significant decrease in cytochrome c expression in the mitochondria (Figure 4). Apoptosis was increased by over 4 fold (Figure 5a), and the cleavage of PARP into its 85kD subunit by 40% (Figure 5b).
Figure 1.
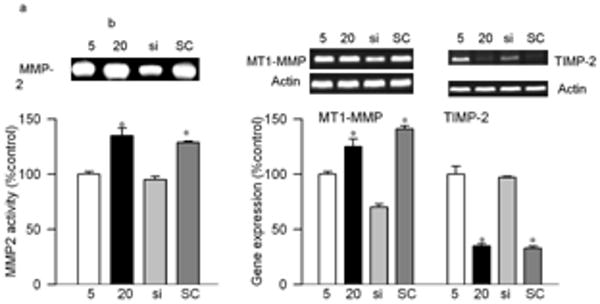
Effects of genetic manipulation of MMP2 on its glucose-induced activity and the regulators in retinal endothelial cells: Bovine retinal endothelial cells from passages 4th were incubated in 5 or 20mM glucose medium for 4 days in the presence or absence of MMP2-siRNA. At the end of incubation the medium was collected for gelatinolytic activity, and RNA was isolated from the cells by TRizol. (a) The gelatinase activity of MMP2 was determined in the medium by zymography technique (b) Gene expressions of MT1-MMP and TIMP-2 were quantified by semiquantitative PCR using the primers given in method section and were adjusted to the mRNA levels of β-actin in each sample. Each measurement was done in at least three different cell preparations. The values, represented as mean±SD, obtained from the cells incubated in 5mM glucose are considered as 100% (control). *P<0.05 compared to the values obtained from the cells incubated in 5mM glucose. 5mM and 20mM=cells incubated in 5mM glucose, or 2mM glucose respectively, si and SC= cells transfected with MMP2-siRNA or scrambled RNA respectively, followed by incubation in 20mM glucose for 4 days.
Figure 2.
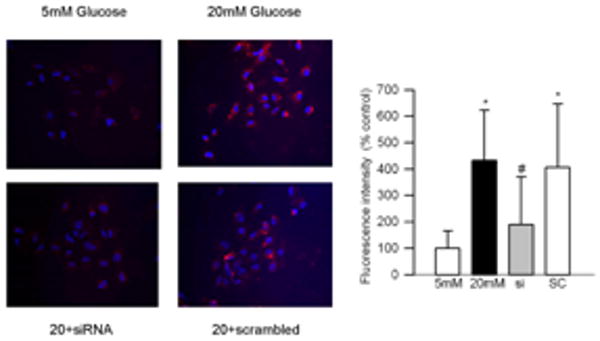
Effect of MMP2-siRNA on mitochondrial superoxide: Retinal endothelial cells, either transfected with MMP2-siRNA or un-transfected were incubated in 5mM or 20mM glucose for 4 days. After washing the cells with phosphate-buffered saline, they were stained with 400nM Mitotracker Red CM-H2XROS for 30 minutes. After removing the excessive stain by washing with phosphate-buffered saline, they mounted in vectashield mounting media and observed under fluorescence microscope at 20× magnification. The red fluorescence intensity was quantified using Image J software (NIH) Java (TM) Platform Version 6. The values obtained from the cells incubated in 5mM glucose are considered as 100% (control). * P<0.05 compared to the values obtained from the cells incubated in 5mM glucose and # P<0.05 compared to 20mM glucose: 5mM and 20mM=cells incubated in 5mM glucose, or 20mM glucose respectively, si and SC= cells transfected with MMP2-siRNA or scrambled RNA respectively, followed by incubation in 20mM glucose for 4 days.
Figure 3.
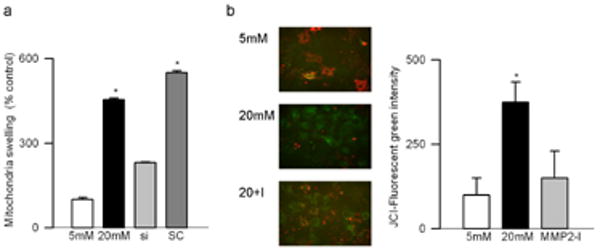
Effect of MMP2 inhibition on mitochondrial membranes: (a) Freshly isolated mitochondria prepared fromMMP2-siRNA transfected or un-transfected cells incubated in 5mM or 20mM glucose for 4 days were used to determine the collapse of their membrane potential by measuring the decrease in absorbance at 540 nm induced by calcium chloride. The extent of swelling was calculated as a percentage of swelling with respect to the maximum swelling achieved by exposure to external calcium. (b) Mitochondrial membrane potential was determined by cationic dye JC-1 in the cells incubated in 5mM glucose or 20mM glucose in the presence or absence of MMP2 inhibitor (MMP2-I). Green fluorescence represents the depolarized (monomer) mitochondria and red fluorescence=hyperpolarized (J aggregates) mitochondria. Green fluorescence intensity was determined by Image J software. Results are expressed as mean ± SD, *P<0.05 compared to the values obtained from the cells incubated in 5mM glucose. 5mM and 20mM=cells incubated in 5mM glucose, or 20mM glucose respectively, si and SC= cells transfected with MMP2-siRNA or scrambled RNA respectively, followed by incubation in 20mM glucose for 4 days, and MMP2-I=cells incubated in 20mM glucose medium containing MMP2 inhibitor.
Figure 4.
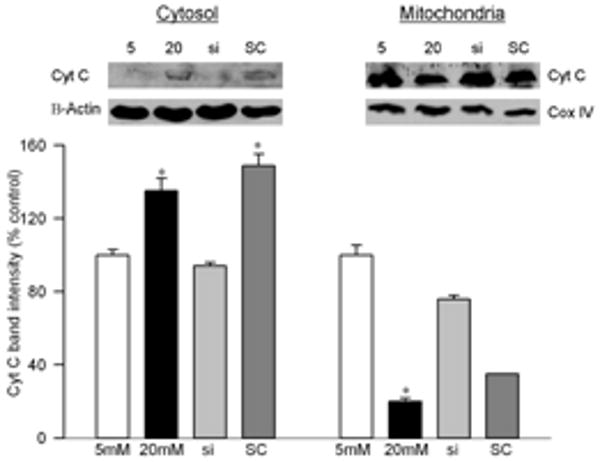
Effect of MMP2-siRNA on the leakage of cytochrome c from the mitochondria: The mitochondrial and cytosolic fractions were prepared from freshly harvested cells by differential centrifugation. Cytochrome c contents were determined by western blot in both mitochondrial and cytosolic fractions. The band intensities of cytochrome c were adjusted to the expression of the β-actin or Cox IV (cytosolic and mitochondrial fractions, respectively). The western blots are representative of three different experiments. The values obtained from cells incubated in 5mM glucose are considered as 100%. *P < 0.05 compared to the cells incubated in 5mM glucose.
Figure 5.
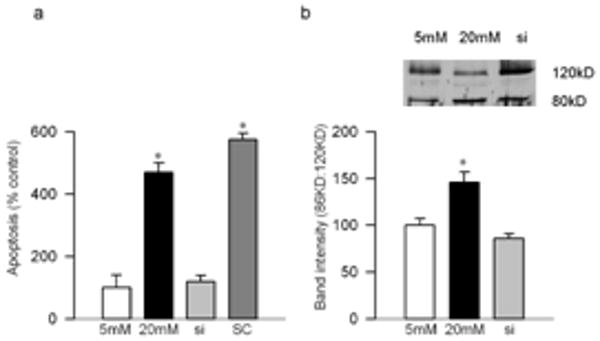
Effect of MMP2 inhibition on apoptosis of bovine retinal endothelial cells. (a) Apoptosis was measured by performing cell death ELISA by measuring cytoplasmic histone-associated-DNA- fragments using an assay kit from Roche Diagnostics. (b) PARP activation was measured by western blot technique, and the ratio of active PARP and pro-PARP was calculated. Each experiment was repeated using three separate cell preparation and the values obtained from the cells incubated in 5 mM glucose are considered as 100% (control). *P < 0.05 compared to the values obtained from the cells incubated in 5mM glucose. 5mM and 20mM=cells incubated in 5mM glucose, or 20mM glucose respectively, si and SC= cells transfected with MMP2-siRNA or scrambled RNA respectively, followed by incubation in 20mM glucose for 4 days.
Inhibition of MMP2 prevents glucose-induced mitochondrial dysfunction
Transfection of endothelial cells with MMP2-siRNA prevented glucose-induced increases in MMP2 activity and MT1-MMP gene expression, and decrease in TIMP-2; however, transfection of cells with the scrambled siRNA had no effect on any of these parameters (Figure 1). Glucose-induced increase in mitochondrial superoxide levels, and membrane swelling were ameliorated in the cells transfected with MMP2-siRNA (Figures 2 and 3). MMP2 inhibitor, which we have shown to inhibit glucose-induced increase in MMP2 activity and apoptosis of retinal capillary cells23, also abrogated increase in mitochondrial membrane potential (Figure 3b). Cytochrome c leakage from the mitochondria into the cytosol, which is increased in the endothelial cells in diabetes11, was significantly decreased by MMP2-siRNA (Figure 4), and in the same cells preparations, glucose-induced apoptosis was also ameliorated, and the cleavage of PARP was prevented (Figure 5).
Diabetic Mice
Overexpression of mitochondrial superoxide dismutase prevents diabetes-induced activation of MMP2 and its regulators
Diabetes in WT mice, as observed in rats 23, increased MMP2 gelatinase activity by over 80%, and its gene expression was elevated by 55-60% compared to the values obtained from non diabetic WT mice. In the same retina samples, MT1-MMP expression was increased by 60%, and TIMP-2 expression was decreased by about 25% (Figure 6). Immunostaining of MMP2, as detected by green FITC fluorescent staining, was also significantly increased in the retinal vessels and in several areas surrounding the retinal vessels in the sections obtained from WT-diabetic mice compared to age-matched normal mice (Figure 7). Overexpression of MnSOD prevented diabetes-induced activation of MMP2 and its regulators; MMP2 gelatinase activity, and gene expressions of MMP2, MT1-MMP and TIMP-2 were not statistically different in the retina from diabetic Tg and non diabetic Tg mice, and the values were similar to those obtained from WT-non diabetic mice (Figure 6). The intensity of FITC staining in the sections obtained from Tg-diabetic mice was significantly lower compared to WT-diabetic mice (Figure 7).
Figure 6.
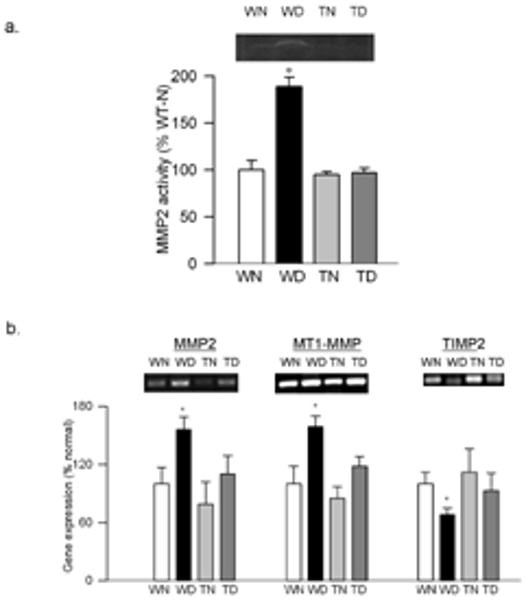
Effect of MnSOD overexpression on retinal MMP2 and its regulators: (a) The gelatinase activity of MMP2 was quantified by in-situ zymography using 10-15μg of retinal homogenate. (b) The gene expressions of MMP2, MT1-MMP and TIMP2 were quantified in the retina obtained from wild type and MnSOD over expressing mice maintained diabetic for 6 months and their age-matched controls. The levels of mRNA were adjusted to the mRNA levels of β-actin in each sample. Each measurement was done at least three times. The values obtained from WT nondiabetic mice are considered as 100%. Results are expressed as mean ± SD of at least six mice in each group. *P< 0.05 compared with WT non diabetic mice. WN and WD=wild type non diabetic and diabetic respectively, and TN and TD=MnSOD transgenic mice non diabetic and diabetic respectively.
Figure 7.
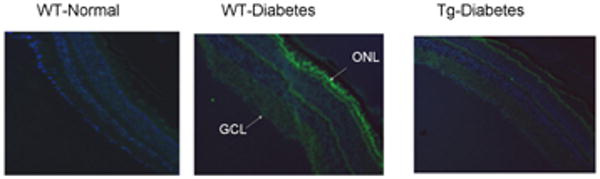
Immunostaining of MMP2: Cryosections from (a) WT-non diabetic (b) WT-diabetic, and (c) Tg-diabetic mice were subjected immunostaning using MMP2 antibodies (green), and DAPI (blue) was used to stain the nuclei. The sections were imaged at 10× magnification using Olympus BX50 fluorescent microscope, and the image is representative of 3 or more 5mice in each group. GCL= ganglion cell layer; ONL, outer nuclear layer.
Discussion
Diabetic retinopathy remains the major cause of blindness among young adults; despite extensive research in the field the precise mechanism(s) of its development remains unclear. In the development of diabetic retinopathy, accelerated apoptosis of retinal microvascular cells (pericytes and endothelial cells) and other non vascular cells is observed before other histopathology is detectable 1-4, or loss of vision is evident, and apoptosis of capillary cells predicts the pathology associated with diabetic retinopathy 3. Our recent study has suggested a pro-apoptotic role for MMP2 in the loss of retinal capillary cells in diabetes, and has shown that increase in MMP2 activity and its gene expression in diabetic rat retina and its microvessels is inhibited by the therapies that inhibit capillary cell apoptosis and the development of retinopathy 23. Here we elucidate the possible mechanism via which MMP2 increases apoptosis of retinal capillary cells. The exciting data demonstrate the importance of MMP2 in high glucose mediated mitochondrial dysfunction and apoptosis; inhibition of MMP2 ameliorates glucose-induced mitochondrial superoxide and dysfunction, and protects the mitochondria from leaking cytochrome c into the cytosol resulting in the inhibition of capillary cell apoptosis. From mouse model of diabetic retinopathy, we show that regulation of mitochondrial superoxide by overexpression of MnSOD, which prevents mitochondrial dysfunction and the development of diabetic retinopathy 10, also prevents diabetes-induced dysregulation of MMP2 and its regulators in the retina. Thus, the dysfunction of mitochondria could be one of the possible mechanism via which MMP2 contributes to the development of diabetic retinopathy.
Diabetes increases ROS levels in retina and its capillary cells, and increased oxidative stress is implicated in the development of diabetic retinopathy 9, 10, 14. ROS, by opening the mitochondrial permeability transition pores, alter the mitochondrial membrane potential, and this allows cytochrome c to leak from the mitochondria, subsequently activating apoptosis machinery 26, 27. Mitochondrial dysfunction is postulated to play a major role in the pathogenesis of diabetic retinopathy. We have shown that mitochondrial superoxide levels are elevated in the retina in diabetes, mitochondrial macromolecules are damaged, and cytochrome c is released out of the mitochondria 10, 11. Others have shown ROS to activate MMP2 in myocyte 28, and overexpression of MMP2 to increase the abnormalities in mitochondrial ultrastructure and lipid peroxidation in cardiac tissue 29. Recent study has demonstrated a pro-apoptotic role for MMP2 in the loss of retinal capillary cells in diabetes 23, our data now provide a possible mechanism by which MMP2 accelerates the apoptosis of retinal capillary cells: manipulation of MMP2 gene by its siRNA protects retinal endothelial cells from high glucose-induced increased superoxide accumulation and mitochondrial damage, and this prevents cytochrome c to leak into the cytosol preventing the apoptosis of the cells. These results strongly suggest that MMP2 upregulation in the retina in diabetes increases apoptosis of retinal capillary cells by collapsing mitochondrial membranes. In support, MT1-MMP is reported to sensitize endothelial cells to apoptosis via caspase-3 activation which can be abrogated by Bcl-2, thus suggesting the involvement of a mitochondrial pathway 30. Furthermore, we show that glucose-induced PARP activation in retinal endothelial cells is also under the control of MMP2. In support, the activation of MMP2 is reported to cleave the nuclear PARP 31, and because of significant cross talk between the nucleus and mitochondria, cleavage of PARP can result in apoptosis via mitochondrial pathway via releasing apoptosis-inducing factor from the mitochondria which ultimately facilitates the release of cytochrome c 26, 32.
In the mice overexpressing MnSOD diabetes fails to increase mitochondrial damage and protects the retinal vasculature from the pathology that is characteristic of diabetic retinopathy 10, 33. Our in vivo results show that protection of mitochondrial superoxide accumulation in the retina in diabetes by overexpression of MnSOD also prevents elevation in MMP2 activity and its regulators, and are consistent with the in vitro results demonstrating the role of retinal mitochondrial damage in the regulation of MMP2. In support, published reports from other laboratories have implicated the role of oxidative stress in the activation of MMP2 34, 35. Thus, it is conceivable to hypothesize that the protection of retinal mitochondrial damage in diabetes helps MMP2 from being activated, and this further keeps the apoptosis machinery in check. However, we recognize that there could be other members of the MMP family involved in the development of diabetic retinopathy, but our focus was to determine the mechanism by which MMP2, the most ubiquitous member, results in the apoptosis of retinal capillary cells in diabetes.
In summary, in this study we present a probable mechanism that allows MMP2 to act as pro-apoptotic in diabetic milieu, thus accelerating the loss of capillary cells resulting in the development of diabetic retinopathy. Understanding the role of MMP2 in the pathogenesis of diabetic retinopathy should help lay ground for MMP2 targeted therapy to retard the development of retinopathy, the sight threatening complication that diabetic patients are faced with.
Acknowledgments
We thank Mamta Kanwar and Yakov Shamailov for technical help, and Dr. Sally Madsen-Bouterse for help with the transfection experiments. This study was supported in part by grants from the National Institutes of Health, the Juvenile Diabetes Research Foundation, the Thomas Foundation, and Research to Prevent Blindness.
Grant Support: NIH-EY014370
List of abbreviations
- MMP2
Matrix metalloproteinase-2
- MnSOD
Manganese superoxide dismutase
- MT1-MMP
Membrane type-1 matrix metalloproteinase
- PARP
Poly (ADP-ribose) polymerase
- ROS
Reactive oxygen species
- siRNA
Small interfering RNA
- Tg
Transgenic
- TIMP2
Tissue inhibitor of metalloproteinases-2
- WT
Wild Type
Footnotes
Authors Disclosure Statements: No competing financial interests exist for both Ghulam Mohammad and Renu Kowluru
References
- 1.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kern TS, Tang J, Mizutani M, Kowluru R, Nagraj R, Lorenzi M. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: Comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000;41:3972–3978. [PubMed] [Google Scholar]
- 3.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008;172:1411–1418. doi: 10.2353/ajpath.2008.071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 6.Hammes HP. Pathophysiological mechanisms of diabetic angiopathy. J Diabetes Complications. 2003;17:16–19. doi: 10.1016/s1056-8727(02)00275-1. [DOI] [PubMed] [Google Scholar]
- 7.Kowluru RA, Kern TS, Engerman RL, Armstrong D. Abnormalities of retinal metabolism in diabetes or experimental galactosemia. III. Effects of antioxidants. Diabetes. 1996;45:1233–1237. doi: 10.2337/diab.45.9.1233. [DOI] [PubMed] [Google Scholar]
- 8.Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938–1942. doi: 10.2337/diabetes.50.8.1938. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 10.Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 11.Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Inves Ophthalmol Vis Sci. 2003;44:5327–5334. doi: 10.1167/iovs.03-0353. [DOI] [PubMed] [Google Scholar]
- 12.Kowluru RA. Diabetic retinopathy: Mitochondrial dysfunction and retinal capillary cell death. Antioxidants & Redox Signaling. 2005;7:1581–1587. doi: 10.1089/ars.2005.7.1581. [DOI] [PubMed] [Google Scholar]
- 13.Kowluru RA, Atasi L, Ho YS. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006;47:1594–1599. doi: 10.1167/iovs.05-1276. [DOI] [PubMed] [Google Scholar]
- 14.Kowluru RA, Kowluru V, Ho YS, Xiong Y. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Rad Biol Med. 2006;41:1191–1196. doi: 10.1016/j.freeradbiomed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Kanwar M, Kowluru RA. Role of glyceraldehyde 3-phosphate dehydrogenase in the development and progression of diabetic retinopathy. Diabetes. 2009;58:227–234. doi: 10.2337/db08-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikawa T, Kukidome D, Sonoda K, Fujisawa K, Matsuhisa T, Motoshima H, Matsumura T, Araki E. Impact of mitochondrial ROS production on diabetic vascular complications. Diabetes Res Clin Pract. 2007;77:S41–45. doi: 10.1016/j.diabres.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- 18.Das A, McGuire PG, Eriqat C, Ober RR, DeJuan E, Williams GA, et al. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Invest Ophthalmol Vis Sci. 1999;40:809–813. [PubMed] [Google Scholar]
- 19.Giebel SJ, Menicucci G, McGuire PG, Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest. 2005;85:567–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]
- 20.Jin M, Kashiwagi K, Iizuka Y, Tanaka Y, Imai M, Tsukahara S. Matrix metalloproteinases in human diabetic and nondiabetic vitreous. Retina. 2001;21:28–33. doi: 10.1097/00006982-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Shiau MY, Tsai ST, Tsai KJ, Haung ML, Hsu YT, Chang YH. Increased circulatory MMP-2 and MMP-9 levels and activities in patients with type 1 diabetes mellitus. Mt Sinai J Med. 2006;73:1024–1028. [PubMed] [Google Scholar]
- 22.Yang R, Liu H, Williams I, Chaqour B. Matrix metalloproteinase-2 expression and apoptogenic activity in retinal pericytes: implications in diabetic retinopathy. Ann N Y Acad Sci. 2007;1103:196–201. doi: 10.1196/annals.1394.000. [DOI] [PubMed] [Google Scholar]
- 23.Kowluru RA, Kanwar M. Oxidative stress and the development of diabetic retinopathy: Contributory role of matrix metalloproteinase-2. Free Rad Biol Med. 2009;46:1677–1685. doi: 10.1016/j.freeradbiomed.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowluru V, Kowluru RA. Increased oxidative stress in diabetes regulates activation of a small molecular weight G-protein, H-Ras, in the retina. Mol Vis. 2007;13:602–610. [PMC free article] [PubMed] [Google Scholar]
- 25.Kowluru RA, Kanwar M. Translocation of H-Ras and its implications in the development of diabetic retinopathy. Biochem Biophys Res Commun. 2009;387:461–466. doi: 10.1016/j.bbrc.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo CT, Hsu MJ, Chen BC, Chen CC, Teng CM, Pan SL, et al. Denbinobin induces apoptosis in human lung adenocarcinoma cells via Akt inactivation, Bad activation, and mitochondrial dysfunction. Toxicol Lett. 2008;177:48–58. doi: 10.1016/j.toxlet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Phaneuf S, Leeuwenburgh C. Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol. 2002;282:R423–R430. doi: 10.1152/ajpregu.00296.2001. [DOI] [PubMed] [Google Scholar]
- 29.Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: Rationale and therapeutic approaches. Ann rev Pharmac Toxico. 2007;47:211–242. doi: 10.1146/annurev.pharmtox.47.120505.105230. [DOI] [PubMed] [Google Scholar]
- 29.Zhoua HZ, Maa X, Graye MO, Zhua BQ, Nguyenb AP, Bakera AJ, et al. Transgenic MMP-2 expression induces latent cardiac mitochondrial dysfunction. Biochem Biophys Res Comm. 2007;358:189–195. doi: 10.1016/j.bbrc.2007.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langlois S, Tomasso G, Boivin D, Roghi C, Murphy G, Gingras D, et al. Membrane type 1-matrix metalloproteinase induces endothelial cell morphogenic differentiation by a caspase-dependent mechanism. Exp Cell Res. 2005;307:452–464. doi: 10.1016/j.yexcr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, et al. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18:690–692. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- 32.Dawson VL, Dawson TM. Deadly conversations: nuclear-mitochondrial cross-talk. J Bioenerg Biomembr. 2004;36:287–294. doi: 10.1023/B:JOBB.0000041755.22613.8d. [DOI] [PubMed] [Google Scholar]
- 33.Goto H, Nishikawa T, Sonoda K, Kondo T, Kukidome D, Fujisawa K, et al. Endothelial MnSOD overexpression prevents retinal VEGF expression in diabetic mice. Biochem Biophys Res Commun. 2008;15:814–820. doi: 10.1016/j.bbrc.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 34.Cox MJ, Sood HS, Hunt MJ, Chandler D, Henegar JR, Aru GM, Tyagi SC. Apoptosis in the left ventricle of chronic volume overload causes endocardial endothelial dysfunction in rats. Am J Physiol Heart Circ Physiol. 2002;282:H1197–H1205. doi: 10.1152/ajpheart.00483.2001. [DOI] [PubMed] [Google Scholar]
- 35.Lee SJ, Seo KW, Lee WS, Hong KW, Kim CD. 4-Hydroxynonenal enhances MMP-2 production in vascular smooth muscle cells via mitochondrial ROS-mediated activation of the Akt/NF-kappaB signaling pathways. Free Radic Biol Med. 2008;45:1487–92. doi: 10.1016/j.freeradbiomed.2008.08.022. [DOI] [PubMed] [Google Scholar]


