Abstract
β-Glucan particles (GPs) are purified Saccharomyces cerevisiae cell walls treated so that they are primarily β1,3-d-glucans and free of mannans and proteins. GPs are phagocytosed by dendritic cells (DCs) via the Dectin-1 receptor, and this interaction stimulates proinflammatory cytokine secretion by DCs. As the hollow, porous GP structure allows for high antigen loading, we hypothesized that antigen-loaded GPs could be exploited as a receptor-targeted vaccine delivery system. Ovalbumin (OVA) was electrostatically complexed inside the hollow GP shells (GP-OVA). Incubation of C57BL/6J mouse bone marrow-derived DCs with GP-OVA resulted in phagocytosis, upregulation of maturation markers, and rapid proteolysis of OVA. Compared with free OVA, GP-OVA was >100-fold more potent at stimulating the proliferation of OVA-reactive transgenic CD8+ OT-I and CD4+ OT-II T cells, as measured by in vitro [3H]thymidine incorporation using DCs as antigen-presenting cells. Next, immune responses in C57BL/6J mice following subcutaneous immunizations with GP-OVA were compared with those in C57BL/6J mice following subcutaneous immunizations with OVA absorbed onto the adjuvant alum (Alum/OVA). Vaccination with GP-OVA stimulated substantially higher antigen-specific CD4+ T-cell lymphoproliferative and enzyme-linked immunospot (ELISPOT) responses than that with Alum/OVA. Moreover, the T-cell responses induced by GP-OVA were Th1 biased (determined by gamma interferon [IFN-γ] ELISPOT assay) and Th17 biased (determined by interleukin-17a [IL-17a] ELISPOT assay). Finally, both the GP-OVA and Alum/OVA formulations induced strong secretions of IgG1 subclass anti-OVA antibodies, although only GP-OVA induced secretion of Th1-associated IgG2c antibodies. Thus, the GP-based vaccine platform combines adjuvanticity and antigen delivery to induce strong humoral and Th1- and Th17-biased CD4+ T-cell responses.
IMPORTANCE
Most licensed vaccines work by promoting protective antibody responses. However, for many infectious diseases, antibody-mediated protection appears to play a relatively minor role, and vaccination has met with limited success. While live-attenuated organisms generally elicit T-cell responses, their use in vaccines is limited by the potential for causing disease. Thus, there is an urgent need for new vaccine platforms that deliver antigens in such a manner as to promote strong T-cell-mediated responses. Here we designed a novel vaccine platform consisting of yeast-derived β-glucan particles (GPs) that combines antigen delivery and adjuvant activity. GPs loaded with the model antigen ovalbumin (OVA) stimulated robust humoral and T-cell responses in mice. In addition, the cellular response was Th1 and Th17 biased. This work has implications for the design of vaccines that stimulate biased T-cell responses as well as for understanding how immunity to fungal pathogens develops.
INTRODUCTION
β-Glucan particles (GPs) are purified Saccharomyces cerevisiae cell walls treated so that they are >85% β1,3-d-glucan polymers, ~2% chitin, and <1% lipids and protein, with the rest being mostly ash and moisture (1). β-Glucans are naturally found in fungi, algae, plants, and some bacteria. β-Glucans, especially β1,3-d-glucans, are key constituents of the cell walls of fungal and major fungal pathogen-associated molecular patterns (PAMPs) (2, 3). They are recognized by the pattern recognition receptor Dectin-1, a C-type lectin which has high levels of expression on phagocytes, including dendritic cells (DCs), macrophages, and neutrophils (4, 5). It has been previously demonstrated that GPs can be efficiently taken up by mouse bone marrow-derived DCs (BMDCs) in vitro (6). In addition, the hollow, porous GP structure allows for high antigen loading (7, 8). This suggests that GPs have the potential to be exploited as a targeted antigen delivery vehicle.
PAMPs have been extensively studied because of their importance in host defense against microbes. More recently, there has been a growing recognition of the potential role of PAMPs in vaccine development (9). PAMPs deliver a “danger” signal to DCs, resulting in DC activation and secretion of cytokines/chemokines, migration, maturation, antigen presentation, and costimulatory molecule expression. This, in turn, impacts B- and T-cell responses to antigens codelivered with PAMPs. Curdlan and yeast glucan particles have been shown to have this type of adjuvant activity (10, 11).
We hypothesized that antigen-loaded GPs could serve as a receptor-targeted vaccine delivery system that exploited the adjuvanticity of β1,3-d-glucan. The model antigen ovalbumin (OVA) was complexed in GPs and studied for its capacity to stimulate immunologic responses in mice in vitro and in vivo. We found that immunization with OVA complexed in GPs (GP-OVA) induced robust Th1- and Th17-biased T-cell responses and strong antibody responses.
RESULTS
Proteolysis of antigens loaded into GPs.
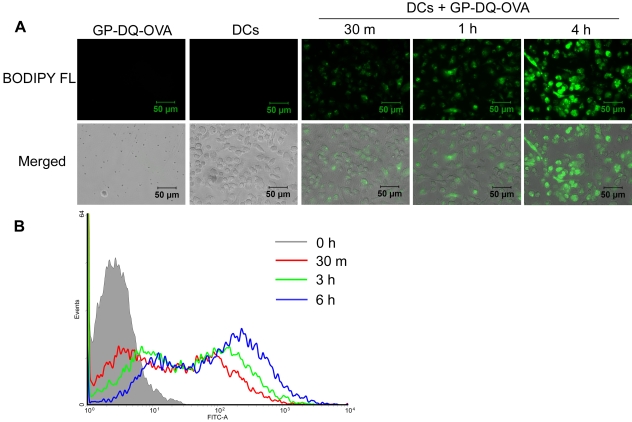
We previously demonstrated that GPs are efficiently taken up by BMDCs in vitro (6). In order to demonstrate that antigens encapsulated in GPs can be efficiently proteolysed by DCs, we loaded GPs with DQ-OVA. DQ-OVA consists of OVA that is heavily conjugated with BODIPY FL, resulting in self-quenching. Upon proteolytic degradation of DQ-OVA to single dye-labeled peptides, bright green fluorescence is observed. Incubation of GP-DQ-OVA with BMDCs resulted in the uptake of the particles and subsequent proteolytic degradation of DQ-OVA (Fig. 1). This was evidenced by progressively increased fluorescence over the time period studied by both epifluorescence microscopy and fluorescence-activated cell sorting (FACS) analysis.
FIG 1 .
Proteolysis of GP-DQ-OVA following uptake by BMDCs. GP-DQ-OVA particles (3:1 particle-to-BMDC ratio) were incubated with CD11c-purified day 8 BMDCs for the indicated times, washed, and examined by bright-field and epifluorescence microscopies (A) or collected and examined by FACS analysis (B). Photomicrographs are representative of two experiments. Histograms are representative of three experiments.
DC maturation.
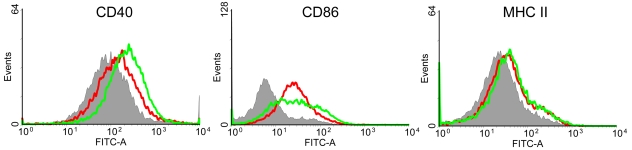
Optimal antigen presentation and subsequent CD4+ T-cell responses require DC maturation, a process that includes upregulation of costimulatory molecules and major histocompatibility complex class II (MHC-II) (12). Incubation of BMDCs with GP-OVA induced upregulation of two costimulatory molecules, CD40 and CD86, and MHC-II (Fig. 2). Lipopolysaccharide (LPS), a known DC maturation stimulus, also upregulated CD40, CD86, and MHC-II. The results suggest that GPs can have adjuvant activity when used to immunize animals.
FIG 2 .
DC maturation stimulated by GP-OVA. DCs were left unstimulated (gray area) or stimulated with GP-OVA (10:1 particle-to-BMDC ratio; red line) or LPS (1 µg/ml; green line). Surface expression of CD40, CD86, and MHC-II was analyzed by FACS. Data shown are representative histograms resulting from three independent experiments.
Antigen delivery activity of GP-OVA particles.
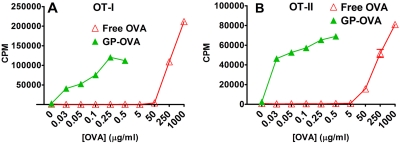
In order to demonstrate whether the antigens encapsulated in GPs can be efficiently presented by BMDCs, we performed in vitro T-cell proliferation assays, using OVA as the model antigen. CD8+ T cells obtained from “knock-in” transgenic OT-I mice (which recognize the OVA peptide in the context of MHC-I) and CD4+ T cells obtained from OT-II mice (which recognize the OVA peptide in the context of MHC-II) were stimulated with either free OVA or GP-OVA, using BMDCs as antigen-presenting cells (APCs). At concentrations from 0.03 to 0.5 µg/ml, free OVA failed to stimulate proliferation of either OT-I or OT-II T cells (Fig. 3A and B). In contrast, GP-OVA at those concentrations stimulated robust OT-I and OT-II T-cell proliferation (Fig. 3A and B). In order to achieve similar stimulation effects of GP-OVA, 100 times or higher concentrations of free OVA were required (Fig. 3A and B). These results demonstrate that antigens delivered in GPs were efficiently processed and presented by DCs.
FIG 3 .
Lymphocyte proliferation stimulated by free OVA compared to that stimulated by OVA complexed in GPs (GP-OVA). T cells (105 cells/well) purified from the lymph nodes and spleens obtained from OT-I (CD8+) (A) and OT-II (CD4+) (B) mice were incubated for 4 days with mitomycin C-treated BMDCs (104 cells/well) and free OVA or GP-OVA over the indicated OVA concentration range. For wells containing GP-OVA, 105 GP-OVA (containing 6, 10, 20, 50, or 100 µg OVA per 108 GPs) were added to yield the indicated concentration of OVA. [3H]thymidine was added 24 h before harvesting. Data are the means ± SE from a representative experiment (out of three); for each experiment, each sample was tested in triplicate.
Evaluation of GPs as an antigen delivery and adjuvant system.
The in vitro T-cell proliferation and DC maturation data suggest that GPs have the potential to serve as both an antigen delivery system and an adjuvant system. Therefore, in the next set of experiments, the immune responses in mice following immunization with GP-OVA were evaluated. Controls included OVA delivered in alum and unimmunized mice that received phosphate-buffered saline (PBS).
T-cell proliferation after immunization.
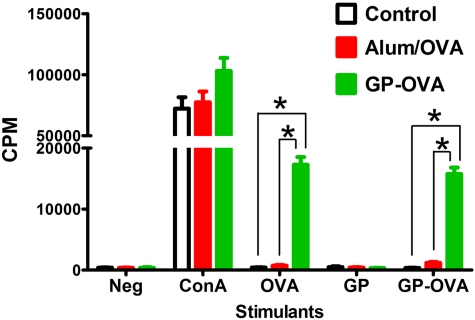
Two weeks after the last boost, CD4+ and CD8+ T cells were purified from lymph nodes and spleens and restimulated with OVA (in the form of either free OVA or GP-OVA) in an ex vivo T-cell proliferation assay. The mitogen concanavalin A (ConA) served as a positive control. CD4+ T cells obtained from mice immunized with GP-OVA proliferated in response to free OVA and GP-OVA at much higher levels than those that had received OVA in alum or PBS (Fig. 4). GPs alone (without OVA) did not stimulate T cells from any of the groups to proliferate, thus demonstrating that the CD4+ T-cell lymphoproliferation was specifically in response to OVA. Lymphoproliferation of CD8+ T cells from all immunization groups in response to stimulation with OVA was similar to that of unstimulated controls. This was not because the lymphocytes were incapable of proliferation, as the CD8+ T cells did proliferate when stimulated with ConA (data not shown).
FIG 4 .
OVA-specific ex vivo lymphoproliferative responses in vaccinated mice. CD4+ T cells were purified from lymph nodes and spleens of wild-type mice that received three injections of PBS (control), OVA admixed with alum (Alum/OVA), or OVA complexed in GPs (GP-OVA). Mitomycin C-treated BMDCs served as APCs. DCs and T cells were incubated for 4 day in the presence of no stimulants (Neg), 5 µg/ml of ConA, 50 µg/ml OVA, empty GPs (10:1 particle-to-BMDC ratio) or GP-OVA (10:1 particle-to-BMDC ratio; containing 0.5 µg/ml OVA [final concentration]). [3H]thymidine was added 24 h before harvesting. Results are the means ± SE from a representative experiment (out of three), with each sample tested in triplicate. *, P < 0.0001, comparing the indicated groups.
Skewing of Th responses.
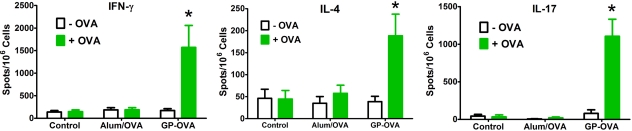
The ex vivo T-cell proliferation assay showed that GP-OVA induced strong T-cell responses in mice. To determine the Th bias of this response, gamma interferon (IFN-γ; Th1 type cytokine), interleukin-4 (IL-4; Th2 cytokine), and IL-17a (Th17 cytokine) enzyme-linked immunospot (ELISPOT) assays were performed using CD4+ T cells obtained from immunized mice. GP-OVA-immunized mice had considerably more IFN-γ and IL-17a ELISPOTs than IL-4 following ex vivo stimulation with OVA (Fig. 5). Consistent with the lymphoproliferation data, mice receiving OVA in alum had a near background number of spots. These data indicated that the CD4+ T-cell responses following immunization with GP-OVA were biased toward Th1 and Th17.
FIG 5 .
Skewing of Th responses following vaccination with GP-OVA. CD4+ T cells purified from vaccinated mice were incubated with mitomycin C-treated BMDCs as APCs, with 50 µg/ml OVA as the antigenic stimulus (+OVA) or without stimulus (−OVA). Results for IFN-γ and IL-4 are the means ± SE from three independent experiments, and results for IL-17a are the means ± SE from two independent experiments, with 4 to 6 mice/group. *, P < 0.05, comparing GP-OVA to controls or Alum/OVA with (+OVA) or without (−OVA) stimulus. Note that the y axis scale differs among groups.
Antibody responses.
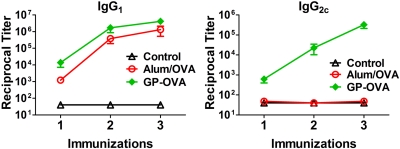
The above-described results showed that GP-OVA was a strong inducer of T-cell responses. Measurement of the antibody response to vaccination is also important because for many infections, antibodies mediate protection. In addition, determination of relative IgG1 and IgG2c subclass titers can give clues as to the polarization of Th responses, as IgG2c titers are associated with Th1 responses (13). Serum samples were analyzed for OVA-specific IgG1 and IgG2c by enzyme-linked immunosorbent assay (ELISA). Both classes of antibodies were demonstrated after just one immunization with GP-OVA (Fig. 6), and their levels were elevated after the second and third immunization. In contrast, OVA in alum elicited only IgG1 antibodies. These data are consistent with the Th1 skewing results observed by ELISPOT assays.
FIG 6 .
Antibody subclass titers following immunizations. Mice were bled 2 weeks after each immunization, and antibody subclasses were measured by ELISA. Data are the means ± SE, with five mice in each group. P < 0.05 by one-way ANOVA with Tukey’s multiple comparison test, comparing GP-OVA with any other group except for IgG1 after 2 or 3 immunizations.
DISCUSSION
DCs play central roles bridging innate and adaptive immunity and are the primary antigen-presenting cells initializing adaptive immunity. Targeted antigen delivery to DCs can result in enhanced antigen presentation by DCs. Both natural receptor ligands and receptor-specific antibodies have been used for targeted antigen delivery (14–18). GPs are naturally occurring ligands for Dectin-1, which is highly expressed on DCs. GPs are efficiently phagocytosed by BMDCs in a Dectin-1-dependent manner in vitro. In addition, engagement of GPs with Dectin-1 leads to proinflammatory cytokine production (6) and upregulation of costimulatory molecules on DCs, part of the process of DC maturation, which is essential for antigen presentation and induction of adaptive immunity.
Mice vaccinated with GP-OVA had robust humoral and Th1/Th17-skewed T-cell immune responses. The GP-based platform combines targeted antigen delivery and adjuvant activity and has several advantages compared to other targeting systems. First, the antigens are complexed into GPs noncovalently through electrostatic interaction. This eliminates the requirement for a chemical synthesis step whereby the antigen and targeting molecule are chemically conjugated. Second, because GPs themselves serve as adjuvants, the need for additional adjuvants is obviated.
Th1- and Th17-skewed cell-mediated immune responses appear to be important components of defenses against a wide range of fungal pathogens, including Cryptococcus neoformans, Aspergillus fumigatus, and Candida albicans (19–21). Recent studies suggest stimulation via Dectin-1 primes Th17, Th1, and cytotoxic T-cell responses (11, 22–25). Moreover, targeted delivery of antigen by conjugation to anti-Dectin-1 antibodies induced CD4+ and CD8+ T-cell responses at doses that antigen alone failed to produce a response (25). Taken together with the data presented herein, these results demonstrate the potential for β-glucan-based antigen delivery systems to stimulate robust T-cell-mediated immune responses.
We could not detect significant OVA-specific CD8+ T-cell proliferation in mice immunized with GP-OVA, OVA absorbed onto the adjuvant alum (Alum/OVA), or PBS. GPs are thought to be taken up by DCs via phagocytosis, following which fusion with endolysosomal compartments occurs (6). Thus, OVA delivered in GPs would be expected to be preferentially presented by the MHC-II pathway rather than cross-presented by the MHC-I pathway (26–29). However, this explanation does not account for our finding that GP-OVA can stimulate CD8+ T cells obtained from OT-I mice to proliferate ex vivo. It is possible that a low degree of cross-presentation may be sufficient to stimulate OT-I CD8+ cells to proliferate, given that these cells are already OVA responsive. Alternatively, the capacity for BMDCs to cross-present OVA delivered via GPs may be significantly greater than that of the DC populations that phagocytose GP-OVA in vivo. Strategies are being investigated to improve the cross-presentation of antigens delivered by GPs in order to enhance the CD8+ response.
Elicitation of memory responses is essential if vaccines are to confer long-lasting protection. Studies are under way to determine if delivery of antigen via the GP platform stimulates central and effector memory responses, and if so, the skewing of those responses. Recent studies with models of listeriosis suggest that antigen-specific Th1 cells were much more likely to enter the memory cell pool than Th17 cells (30). However, delivery of antigen via Dectin-1 may prove more effective at eliciting long-lived Th17 T cells, given the observation that circulating human blood contains memory Th17 cells specific for fungal antigens (31–33).
In addition to T-cell responses, vigorous antibody responses were observed following vaccination with GP-OVA. Detectable titers occurred following the administration of the priming dose, and the titers increased with each subsequent boost. In addition, whereas mice that received OVA in alum mounted only an IgG1 response, mice vaccinated with GP-OVA had both IgG1 and IgG2c responses. The latter subclass is associated with Th1 responses in the C57BL/6J mouse (13).
Data published by our group and others found undetectable IL-12p70 cytokine production following in vitro stimulation of BMDCs by β-glucans (6, 11, 34). IL-12p70 is a pivotal cytokine, guiding the differentiation of Th1 cells. Thus, the robust Th1 responses observed in vivo, following immunization with GP-OVA, and in other studies using OVA mixed with the β-glucan curdlan (11) would seem paradoxical. We speculate that several factors may contribute to this discrepancy. First, most of the in vitro studies used cultured BMDCs, which may not be fully representative of the heterogeneous DC populations that respond to β-glucans in vivo. Indeed, DC subsets vary greatly in their ability to process and present antigens (35–37). Second, cytokine secretion by macrophages responding to β-glucan stimulation could contribute to the Th skewing. Third, β-glucans are potent activators of the complement pathway (38), and responses to opsonized GPs likely differ from those to unopsonized GPs.
A low incidence of adverse events is critical for the advancement of vaccine candidates. β-Glucan preparations derived from fungi have a record of safety in both preclinical and human trials (39–41). In addition, GPs are derived from S. cerevisiae, which is regularly consumed by humans without ill effects. Although further testing is needed, these findings encourage the clinical development of GPs as a vaccine platform.
Finally, the structure of GPs permits loading of relatively large amounts of not only antigens but also other molecules with potential immunomodulatory activity, such as Toll-like receptor (TLR) ligands, small interfering RNA (siRNA), and cytokines (7, 8). This potentially allows manipulation of the magnitude and the qualitative nature of the vaccine response. For example, enhanced cytokine responses are seen when Dectin-1 and TLRs are cooperatively stimulated (6, 34). Thus, addition of a TLR ligand to GPs could lead to greater adjuvanticity. Similarly, siRNAs targeting negative regulators of proinflammatory pathways could potentially be added to the GP platform to inhibit the generation of regulatory T cells (42). Future studies will test these possibilities and determine the capacity of GPs loaded with microbial antigens to induce protective responses.
MATERIALS AND METHODS
Chemicals and cell culture media.
Chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless stated otherwise. RPMI 1640 media were obtained from Invitrogen Life Technologies (Carlsbad, CA). R10 medium consists of RPMI 1640 containing 10% fetal bovine serum (FBS; Tissue Culture Biologicals, Tulare, CA), 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM l-glutamine (Invitrogen), 0.5 µg/ml amphotericin B, and 55 µM 2-mercaptoethanol (2-ME; Invitrogen). Chicken OVA was purchased from Worthington Biochemical Corporation (Lakewood, NJ). Incubation of cells was performed at 37°C in humidified air containing with 5% CO2.
Mice.
Wild-type C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Transgenic OT-I and OT-II mice (Jackson Laboratory) were bred at the animal facility at the University of Massachusetts Medical School. Mice were specific pathogen free. All animal procedures were carried out under a protocol approved by the University of Massachusetts Medical School Institutional Use and Care of Animals Committee.
Mouse BMDCs.
BMDCs were made as shown in previous studies, with a slight alteration (43, 44). Briefly, bone marrow cells were isolated from the tibiae and femurs of 8- to 12-week-old mice and then cultured in R10 medium supplemented with 10% conditioned medium from the murine granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting J558L cell line. On days 3 and 6, fresh GM-CSF-supplemented R10 medium was added. On day 8, nonadherent cells were harvested and purified with CD11c+ magnetic beads (magnetic cell separation system; Miltenyi Biotec, Auburn, CA), per the protocol supplied by the manufacturer. Positively selected BMDCs were washed with RPMI 1640 three times, treated with 50 µg/ml mitomycin C for 30 min at 37°C, and finally washed three times with R10 medium before used as APCs in lymphoproliferation and ELISPOT assays.
GPs.
GPs were prepared from S. cerevisiae (Fleischmann’s baker’s yeast) using a series of alkaline and acidic extraction steps as previously described (1, 6, 8). Briefly, following centrifugation and washing in water, S. cerevisiae was subjected to two rounds of hot alkali extraction by heating for 1 h at 90°C in 1 M NaOH. The particles were suspended in water at pH 4.5, heated at 75°C for 1 h, and then successively washed with water (three times), isopropanol (four times), and acetone (two times). GPs were dried to yield a free-flowing light tan powder. To count the GPs or GP vaccine formulations, a 10-µg/ml suspension of particles in 0.9% saline was lightly sonicated, counted using a hemocytometer, and then kept in aliquots at −20°C until use. One microgram of GPs contains approximately 5 × 105 particles.
GP-OVA.
GPs containing complexed OVA (GP-OVA) were made as previously described, with a slight modification (8). Briefly, 10 mg of dry GPs were swollen with 50 µl of various concentrations of OVA dissolved in 0.9% saline for 2 h at 4°C to minimally hydrate the GPs, allowing OVA diffusion into the hollow GP cavity. The samples were then frozen at −80°C and lyophilized. To maximize OVA incorporation into the GP shells, the dry GP-OVA formulations were swollen, mixed with 50 µl of sterile water for 2 h at 4°C, and relyophilized. To trap OVA inside the GPs, the dry GP-OVA formulations were heated to 50°C and swollen with 50 µl of 25 mg/ml tRNA (derived from torula yeast, type VI) in 50 mM Tris-HCl, pH 8, 2 mM EDTA, and 0.15 M NaCl for 30 min, and then 500 µl of 10 mg/ml tRNA was added for 1 h at 50°C to complete the complexation reaction, trapping OVA inside the GPs. The suspension was centrifuged and washed three times in 0.9% saline, and particles were resuspended in 70% ethanol for 30 min, washed three additional times in sterile 0.9% saline, resuspended, counted, diluted to 5 × 108 particles/milliliter in 0.9% saline, and stored at −20°C. To calculate the amount of OVA incorporated into the GPs, the unbound OVA protein in the saline washes was measured by bicinchoninic acid (BCA) assay (Pierce, Rockford, IL) against an OVA standard. In addition, fluorescein-labeled OVA was prepared by reaction with fluorescein isothiocyanate (FITC) and used as a fluorescent tracer to estimate OVA incorporated in the GP-OVA particles and unbound in the saline washes. Typically, OVA incorporation was in excess of 90%. DQ-OVA (Invitrogen) was loaded into GPs in an manner identical to that of OVA, resulting in 100 µg DQ-OVA per 108 GP-DQ-OVA particles.
Proteolysis of GP-DQ-OVA by BMDCs.
CD11c-purified day 8 BMDCs were adhered to the bottom of a 35-mm glass-bottom culture dish (MatTek, Ashland, MA) (for microscopy) or to six-well plates (for FACS analysis) by incubation at 37°C for 1 h. GP-DQ-OVA particles were then added to cells at a ratio of 3 to 1 and incubated at 37°C for the indicated times. BMDCs in glass-bottom dishes were washed, fixed, and examined by bright-field and epifluorescence microscopies. BMDCs in six-well plates were collected after Versene (Invitrogen) treatment, washed, fixed, and examined by FACS analysis.
BMDC maturation.
CD11c-purified day 8 BMDCs (2 × 106 cells/well in six-well plates) were incubated with medium only, GP-OVA (10:1 particle-to-BMDC ratio), or LPS (1 µg/ml) for 24 h. Cells were collected after Versene treatment, washed, stained with FITC-conjugated anti-mouse CD40, CD86, or MHC-II antibodies (eBioscience, San Diego, CA), fixed, and examined by FACS analysis.
Purification of T-cell subsets.
Single-cell suspensions of harvested mouse spleens were obtained by grinding with a syringe plunger against a 70-µm cell strainer (BD, Bedford, MA). Red blood cells (RBCs) were then lysed using RBC lysis buffer (eBioscience). Single-cell suspensions of dissected brachial, inguinal, and mesenteric lymph nodes were accomplished by grinding with a syringe plunger against sterile fine stainless steel wire cloth mesh (Small Parts Inc., Miami Lakes, FL). CD4+ and CD8+ T cells were then purified from combined lymph node cells and splenocytes using CD4+ and CD8+ T-cell isolation kits (Miltenyi Biotec), according to the protocol supplied by the manufacturer.
T-cell proliferation assays.
Purified T cells (105 cells/well) were incubated with mitomycin C-treated BMDCs (104 cells/well) and the indicated stimuli for 4 days in round-bottom 96-well plates in 200 µl R10 medium. [3H]thymidine (1 µCi/well; PerkinElmer, Boston, MA) was added for the last 24 h of incubation. Each condition was studied in triplicate. Cells were collected on filter paper (Wallac, Turku, Finland) using a harvester (Tomtec, Hamden, CT), and [3H]thymidine incorporation was measured with a beta counter (Wallac 1450 Microbeta).
Mice and immunization.
For immunizations, groups of wild-type C57BL/6J mice (8 to 10 weeks old; 4 to 6 mice per group) received 100 µl of sterile PBS (control), 50 µg OVA in 50% Imject alum (Thermo Scientific, Rockford, IL) (Alum/OVA), or 50 µg OVA complexed in 5 × 107 GPs (GP-OVA) by subcutaneous injection over the abdomen. Mice were immunized three times at 3-week intervals (except for one experiment in which mice received four injections, with similar results) and euthanized 2 weeks after the last immunization, at which time spleens, lymph nodes, and blood were harvested for the T-cell and antibody assays described above and below. About 500 µl of blood samples was collected by heart puncture immediately after the mice were euthanized. Serum samples were prepared from the blood samples after clotting at 37°C for 1 h and stored at −20°C until antibody titers were evaluated by ELISA. Additionally, in one experiment, tail vein blood samples (100 to 200 µl) also were collected 2 weeks after the first and second immunizations.
ELISPOT assays.
Mouse IFN-γ, IL-4, and IL-17a ELISPOT assays were performed according to the manufacturer’s protocol (Mabtech, Mariemont, OH), with slight modifications. Briefly, 96-well MultiScreen HTS plates (Millipore, Billerica, MA) were used. Mouse IFN-γ, IL-4, and IL-17a capture antibodies at 5 µg/ml were used to coat the plates. Purified CD4+ T cells (105 cells or 2.5 × 104 cells per well), mitomycin C-treated BMDCs (104 cells/well), and stimulants were incubated at 37°C for 42 to 45 h. Plates were developed with BCIP (5-bromo-4-chloro-3-indolylphosphate)-NBT (nitroblue tetrazolium) solution. The spots were counted with an ImmunoSpot ELISPOT reader (Cellular Technology Ltd., Shaker Heights, OH).
OVA-specific antibody ELISA.
Serum samples obtained from immunized mice were serially diluted starting at 1:40 and then progressively diluted 2-fold. Round-bottom 96-well plates with high binding (Corning, Corning, NY) were coated with 50 µl of 10 µg/ml OVA in 0.1 M NaHCO3 overnight at 4°C, washed three times with PBS with 0.05% Tween 20, blocked with 100 µl 10% FBS in PBS for 1 h at room temperature, washed three times with PBS with 0.05% Tween 20, and incubated with 50 µl diluted mouse sera at room temperature for 2 h. Wells were then washed five times with PBS with 0.05% Tween 20, and incubated with biotin-conjugated rat anti-mouse IgG1 (1:500 dilution in 10% FBS in PBS) (BD Biosciences, San Jose, CA) or biotin-conjugated rat anti-mouse IgG2c (1:1,000 dilution in 10% FBS in PBS) (Jackson ImmunoResearch, West Grove, PA) at room temperature for 1 h. Then, the plates were washed five times with PBS-0.05% Tween 20, incubated with streptavidin-conjugated horseradish peroxidase (1:250 dilution in 10% FBS in PBS) (eBioscience) at room temperature for 1 h, washed five times with PBS-0.05% Tween 20, and then developed with tetramethylbenzidine solution (eBioscience). The optical density at 450 nm (OD450) was measured on a plate reader (Molecular Devices, Sunnyvale, CA). Each serum dilution was tested in duplicate, and the antibody titer was defined as the dilution factor just above the inflection point.
Statistics.
GraphPad Prism Software was used for preparation of figures and statistical analyses. Comparisons of groups were performed using a one-way analysis of variance (ANOVA) with the Tukey multiple correction test. Significance was defined as P values of <0.05.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants RO1 AI066087 (to S.M.L.) and RO1 AI025780 (to S.M.L.), the Worcester Foundation for Biomedical Research (to S.M.L.), the Juvenile Diabetes Research Foundation (to G.R.O.), and the Gates Foundation (to G.R.O.).
Footnotes
Citation Huang, H., G. R. Ostroff, C. K. Lee, C. A. Specht, and S. M. Levitz. 2010. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded β-glucan particles. mBio 1(3):e00164-10. doi:10.1128/mBio.00164-10.
REFERENCES
- 1. Hong F., Yan J., Baran J. T., Allendorf D. J., Hansen R. D., Ostroff G. R., Xing P. X., Cheung N. K., Ross G. D. 2004. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 173:797–806 [DOI] [PubMed] [Google Scholar]
- 2. Bowman S. M., Free S. J. 2006. The structure and synthesis of the fungal cell wall. Bioessays 28:799–808 [DOI] [PubMed] [Google Scholar]
- 3. Tsoni S. V., Brown G. D. 2008. Beta-glucans and dectin-1. Ann. N. Y. Acad. Sci. 1143:45–60 [DOI] [PubMed] [Google Scholar]
- 4. Brown G. D., Gordon S. 2001. Immune recognition. A new receptor for beta-glucans. Nature 413:36–37 [DOI] [PubMed] [Google Scholar]
- 5. Taylor P. R., Brown G. D., Reid D. M., Willment J. A., Martinez-Pomares L., Gordon S., Wong S. Y. 2002. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 169:3876–3882 [DOI] [PubMed] [Google Scholar]
- 6. Huang H., Ostroff G. R., Lee C. K., Wang J. P., Specht C. A., Levitz S. M. 2009. Distinct patterns of dendritic cell cytokine release stimulated by fungal beta-glucans and Toll-like receptor agonists. Infect. Immun. 77:1774–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aouadi M., Tesz G. J., Nicoloro S. M., Wang M., Chouinard M., Soto E., Ostroff G. R., Czech M. P. 2009. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 458:1180–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soto E. R., Ostroff G. R. 2008. Characterization of multilayered nanoparticles encapsulated in yeast cell wall particles for DNA delivery. Bioconjug. Chem. 19:840–848 [DOI] [PubMed] [Google Scholar]
- 9. Blander J. M., Medzhitov R. 2006. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440:808–812 [DOI] [PubMed] [Google Scholar]
- 10. Benach J. L., Habicht G. S., Holbrook T. W., Cook J. A. 1982. Glucan as an adjuvant for a murine Babesia microti immunization trial. Infect. Immun. 35:947–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. LeibundGut-Landmann S., Gross O., Robinson M. J., Osorio F., Slack E. C., Tsoni S. V., Schweighoffer E., Tybulewicz V., Brown G. D., Ruland J., Reis e Sousa C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630–638 [DOI] [PubMed] [Google Scholar]
- 12. Macagno A., Napolitani G., Lanzavecchia A., Sallusto F. 2007. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 28:227–233 [DOI] [PubMed] [Google Scholar]
- 13. Martin R. M., Brady J. L., Lew A. M. 1998. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6J and NOD mice. J. Immunol. Methods 212:187–192 [DOI] [PubMed] [Google Scholar]
- 14. Cruz L. J., Tacken P. J., Fokkink R., Joosten B., Stuart M. C., Albericio F., Torensma R., Figdor C. G. 2010. Targeted PLGA nano- but not microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. J. Control. Release 144:118–126 [DOI] [PubMed] [Google Scholar]
- 15. Lahoud M. H., Proietto A. I., Ahmet F., Kitsoulis S., Eidsmo L., Wu L., Sathe P., Pietersz S., Chang H. W., Walker I. D., Maraskovsky E., Braley H., Lew A. M., Wright M. D., Heath W. R., Shortman K., Caminschi I. 2009. The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses. J. Immunol. 182:7587–7594 [DOI] [PubMed] [Google Scholar]
- 16. Thacker E. E., Nakayama M., Smith B. F., Bird R. C., Muminova Z., Strong T. V., Timares L., Korokhov N., O’Neill A. M., de Gruijl T. D., Glasgow J. N., Tani K., Curiel D. T. 2009. A genetically engineered adenovirus vector targeted to CD40 mediates transduction of canine dendritic cells and promotes antigen-specific immune responses in vivo. Vaccine 27:7116–7124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vyas S. P., Goyal A. K., Khatri K. 2010. Mannosylated liposomes for targeted vaccines delivery. Methods Mol. Biol. 605:177–188 [DOI] [PubMed] [Google Scholar]
- 18. Wei H., Wang S., Zhang D., Hou S., Qian W., Li B., Guo H., Kou G., He J., Wang H., Guo Y. 2009. Targeted delivery of tumor antigens to activated dendritic cells via CD11c molecules induces potent antitumor immunity in mice. Clin. Cancer Res. 15:4612–4621 [DOI] [PubMed] [Google Scholar]
- 19. Cenci E., Mencacci A., Del Sero G., Bacci A., Montagnoli C., d’Ostiani C. F., Mosci P., Bachmann M., Bistoni F., Kopf M., Romani L. 1999. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J. Infect. Dis. 180:1957–1968 [DOI] [PubMed] [Google Scholar]
- 20. Huang W., Na L., Fidel P. L., Schwarzenberger P. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 190:624–631 [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y., Wang F., Tompkins K. C., McNamara A., Jain A. V., Moore B. B., Toews G. B., Huffnagle G. B., Olszewski M. A. 2009. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am. J. Pathol. 175:2489–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geijtenbeek T. B., Gringhuis S. I. . 2009. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9:465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leibundgut-Landmann S., Osorio F., Brown G. D., Reis e Sousa C. 2008. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood 112:4971–4980 [DOI] [PubMed] [Google Scholar]
- 24. Palm N. W., Medzhitov R. 2007. Antifungal defense turns 17. Nat. Immunol. 8:549–551 [DOI] [PubMed] [Google Scholar]
- 25. Carter R. W., Thompson C., Reid D. M., Wong S. Y., Tough D. F. 2006. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J. Immunol. 177:2276–2284 [DOI] [PubMed] [Google Scholar]
- 26. Heath W. R., Belz G. T., Behrens G. M., Smith C. M., Forehan S. P., Parish I. A., Davey G. M., Wilson N. S., Carbone F. R., Villadangos J. A. 2004. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 199:9–26 [DOI] [PubMed] [Google Scholar]
- 27. Rock K. L., Shen L. 2005. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol. Rev. 207:166–183 [DOI] [PubMed] [Google Scholar]
- 28. Savina A., Amigorena S. 2007. Phagocytosis and antigen presentation in dendritic cells. Immunol. Rev. 219:143–156 [DOI] [PubMed] [Google Scholar]
- 29. Shen L., Rock K. L. 2006. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr. Opin. Immunol. 18:85–91 [DOI] [PubMed] [Google Scholar]
- 30. Pepper M., Linehan J. L., Pagan A. J., Zell T., Dileepan T., Cleary P. P., Jenkins M. K. 2010. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol. 11:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Acosta-Rodriguez E. V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8:639–646 [DOI] [PubMed] [Google Scholar]
- 32. Levitz S. M. 2009. Th17 cells bounce off the fungal wall. Cell Host Microbe 5:311–313 [DOI] [PubMed] [Google Scholar]
- 33. van de Veerdonk F. L., Marijnissen R. J., Kullberg B. J., Koenen H. J., Cheng S. C., Joosten I., van den Berg W. B., Williams D. L., van der Meer J. W., Joosten L. A., Netea M. G. 2009. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 5:329–340 [DOI] [PubMed] [Google Scholar]
- 34. Dennehy K. M., Willment J. A., Williams D. L., Brown G. D. 2009. Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur. J. Immunol. 39:1379–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dudziak D., Kamphorst A. O., Heidkamp G. F., Buchholz V. R., Trumpfheller C., Yamazaki S., Cheong C., Liu K., Lee H. W., Park C. G., Steinman R. M., Nussenzweig M. C. 2007. Differential antigen processing by dendritic cell subsets in vivo. Science 315:107–111 [DOI] [PubMed] [Google Scholar]
- 36. Mount A. M., Smith C. M., Kupresanin F., Stoermer K., Heath W. R., Belz G. T. 2008. Multiple dendritic cell populations activate CD4+ T cells after viral stimulation. PLoS One 3:e1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steinman R. M., Banchereau J. 2007. Taking dendritic cells into medicine. Nature 449:419–426 [DOI] [PubMed] [Google Scholar]
- 38. Czop J. K., Austen K. F. 1985. Properties of glycans that activate the human alternative complement pathway and interact with the human monocyte beta-glucan receptor. J. Immunol. 135:3388–3393 [PubMed] [Google Scholar]
- 39. Novak M., Vetvicka V. 2008. Beta-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J. Immunotoxicol. 5:47–57 [DOI] [PubMed] [Google Scholar]
- 40. Weitberg A. B. 2008. A phase I/II trial of beta-(1,3)/(1,6) d-glucan in the treatment of patients with advanced malignancies receiving chemotherapy. J. Exp. Clin. Cancer Res. 27:40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Williams D. L., Sherwood E. R., Browder I. W., McNamee R. B., Jones E. L., Di Luzio N. R. 1988. Pre-clinical safety evaluation of soluble glucan. Int. J. Immunopharmacol. 10:405–414 [DOI] [PubMed] [Google Scholar]
- 42. Song X. T., Evel-Kabler K., Shen L., Rollins L., Huang X. F., Chen S. Y. 2008. A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat. Med. 14:258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lutz M. B., Kukutsch N., Ogilvie A. L., Rossner S., Koch F., Romani N., Schuler G. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77–92 [DOI] [PubMed] [Google Scholar]
- 44. Wozniak K. L., Levitz S. M. 2008. Cryptococcus neoformans enters the endolysosomal pathway of dendritic cells and is killed by lysosomal components. Infect. Immun. 76:4764–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]