Abstract
In order for the opportunistic Gram-negative pathogen Pseudomonas aeruginosa to cause an airway infection, the pathogen interacts with epithelial cells and the overlying mucous layer. We examined the contribution of the biofilm polysaccharide Psl to epithelial cell adherence and the impact of Psl on proinflammatory signaling by flagellin. Psl has been implicated in the initial attachment of P. aeruginosa to biotic and abiotic surfaces, but its direct role in pathogenesis has not been evaluated (L. Ma, K. D. Jackson, R. M. Landry, M. R. Parsek, and D. J. Wozniak, J. Bacteriol. 188:8213–8221, 2006). Using an NF-κB luciferase reporter system in the human epithelial cell line A549, we show that both Psl and flagellin are necessary for full activation of NF-κB and production of the interleukin 8 (IL-8) chemokine. We demonstrate that Psl does not directly stimulate NF-κB activity, but indirectly as a result of increasing contact between bacterial cells and epithelial cells, it facilitates flagellin-mediated proinflammatory signaling. We confirm differential adherence of Psl and/or flagellin mutants by scanning electron microscopy and identify Psl-dependent membrane structures that may participate in adherence. Although we hypothesized that Psl would protect P. aeruginosa from recognition by the epithelial cell line A549, we instead observed a positive role for Psl in flagellin-mediated NF-κB activation, likely as a result of increasing contact between bacterial cells and epithelial cells.
IMPORTANCE
Pseudomonas aeruginosa is the predominant airway pathogen causing morbidity and mortality in individuals affected by the genetic disease cystic fibrosis. P. aeruginosa can also cause severe pneumonia, burn wound infections, and sepsis, making its overall impact on human health significant. The attachment of P. aeruginosa to host tissues, often leading to recalcitrant biofilm infections, and inflammation induced by flagellin are both important mechanisms of virulence. We explored the role of the biofilm polysaccharide Psl in the pathogenesis of P. aeruginosa and found that Psl is required for surface adherence to A549 epithelial cells, and as an adhesin, it facilitates flagellin-mediated NF-κB activation. This work was done to better understand the initial events of infection and revealed that a biofilm polysaccharide contributes to inflammation in a novel manner.
INTRODUCTION
In lung infections, the Gram-negative opportunist Pseudomonas aeruginosa initially encounters airway epithelial cells and their associated covering of mucus, which is characteristically thick and dehydrated in cystic fibrosis (CF) patients (1). Psl is a mannose-rich polysaccharide adhesin involved in biotic and abiotic surface attachment and biofilm formation, and it is hypothesized to be important in adherence to epithelial cells early in infection, likely by facilitating interactions between bacteria (2–7). Although its role in P. aeruginosa pathogenesis has not been explored, Psl may have direct stimulatory or inhibitory effects on the host immune response, or it may function primarily to facilitate interactions between P. aeruginosa and host cells. An intact flagellum is also required for attachment to a variety of surfaces, including respiratory mucins, and for surface motility associated with biofilm formation (8–12). In addition to the role of flagella in attachment, the flagellar structural subunit FliC elicits proinflammatory signaling through Toll-like receptor 5 (TLR5), resulting in activation of the NF-κB transcription factor among other factors (13–15). We hypothesized that if Psl protects against immune recognition, then a psl mutant would generate a greater NF-κB response in A549 epithelial cells than the parental strain would. However, we observed that Psl is required for full activation of NF-κB and that this phenotype is likely a result of Psl-mediated adherence facilitating TLR5 signaling by FliC.
Psl does not directly stimulate NF-κB activation in A549/NF-κB-luc cells.
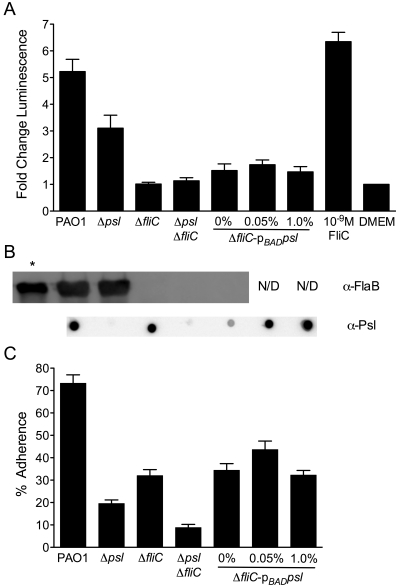
Initial experiments using A549 cells stably transfected with an NF-κB-responsive luciferase reporter (A549/NF-κB-luc cells), cultured in 24-well plates as described previously (16), showed a decrease in NF-κB activation in A549 cells infected with P. aeruginosa lacking Psl compared to the parental strain expressing Psl (M. S. Byrd and B. Pang, unpublished data). To determine whether the effect of Psl on NF-κB activation is direct, A549/NF-κB-luc cells (~4 × 105 cells/well) were infected in triplicate with mid-log-phase P. aeruginosa at a multiplicity of infection (MOI) of 10 (strains are described in Text S1 in the supplemental material). The plates were centrifuged (800 × g, 5 min) and then incubated for 1 h at 37°C with 5% CO2 before fresh medium with gentamicin (100 µg/ml) was added overnight. Luminescence was measured using the Luciferase assay system per the manufacturer’s instructions (Promega), and the change in luciferase activity (fold change) over medium alone, normalized to the MOI, was then determined. As expected, P. aeruginosa PAO1 induced a robust increase in luminescence over the luminescence of the medium alone, similar to the response to exogenous Salmonella FliC (final concentration of 10-9 M) (Fig. 1A). An isogenic fliC mutant did not induce luminescence over that of medium alone, in keeping with NF-κB-dependent signaling of TLR5 in response to flagellin (17). Removing only Psl resulted in a significant decrease in luminescence compared to P. aeruginosa PAOl (Fig. 1A) (P < 0.001, Tukey’s test following one-way analysis of variance [ANOVA]), even though this strain retains flagellin expression at a level equivalent to that of strain PAO1 (Fig. 1B). Eliminating Psl expression in the ΔfliC background did not alter luminescence, suggesting that the activation of NF-κB is due to the presence of flagellin and not Psl (Fig. 1A). To confirm the lack of direct Psl signaling, psl genes were induced in the ΔfliC mutant under the control of the arabinose-inducible araC-pBAD cassette (6). At none of the l-arabinose (l-Ara) concentrations tested did luminescence increase over that of the ΔfliC mutant or the Δpsl ΔfliC mutant (Fig. 1A), despite an increase in Psl (Fig. 1B). One explanation is that Psl increases adherence but is not sensed by the epithelial cells, with NF-κB activation occurring via FliC-dependent TLR5 signaling. Another intriguing possibility is that Psl modulates the level of FliC monomers available for signaling, although preliminary data do not support this. Internalization of bacteria by A549 cells probably does not contribute to the difference in NF-κB activity, because no strain internalized more than 0.5% of the inoculum (see Fig. S1 in the supplemental material). Production of IL-8 as a functional readout of NF-κB activity revealed a trend similar to that of luminescence, with the Δpsl mutant displaying a defect in IL-8 comparable to that of the ΔfliC mutant, confirming that the decrease in NF-κB activity due to the loss of Psl represents a biologically relevant difference in the innate immune response (see Fig. S2 in the supplemental material).
FIG 1 .
NF-κB activation in A549/NF-κB-luc cells by P. aeruginosa. (A) Fold change in luminescence over DMEM, normalized to an MOI of 10 for P. aeruginosa PAO1, Δpsl and ΔfliC single and double mutants, and the arabinose-inducible ΔfliC araC-pBADpsl mutant grown in 0, 0.05, or 1.0% l-Ara. Purified Salmonella FliC (final concentration of 10-9 M) was used as a positive control. Values are means plus standard errors of the means (error bars) for three experiments. (B) Western blots of whole-cell lysates against P. aeruginosa flagellin type B (using an antibody against FlaB [α-FlaB]) and Psl immunoblot of 1.5-µl EDTA extracts (α-Psl), corresponding with strains from panel A. Conditions for flagellin Western blotting and Psl immunoblotting are in Text S1 in the supplemental material. In the leftmost lane with an asterisk, purified P. aeruginosa type B flagellin (1 µg) was used as a positive control. N/D, not done. (C) Percent adherence of strains from panels A and B compared to inocula by plate counts following washing and lysis with 1% saponin. Values are means plus standard errors of the means (error bars) for three experiments.
Psl and flagellin both facilitate adherence to A549/NF-κB-luc cells.
To address the possible contribution of Psl-mediated adherence to NF-κB activation by flagellin, A549/NF-κB-luc cells were infected with P. aeruginosa as in luciferase experiments, immediately washed five times, and then vigorously lysed with 1% saponin to release bacterial cells. Percent adherence was determined by plate counts of lysates compared to inocula. Adherence of P. aeruginosa PAO1 was nearly 75%, while the Δpsl mutant displayed approximately 20% adherence (Fig. 1C), consistent with the previously identified roles of psl in surface adherence and biofilm initiation on glass, polyvinyl chloride (PVC), mucin, and epithelial cells (3, 4, 6, 7, 18). The fliC mutant also showed decreased adherence (32%), as flagella are required for reversible surface attachment (9, 11). Not surprisingly, the Δpsl ΔfliC mutant adhered even less (<10%), evidence supporting a model where both flagella and Psl are important in reversible and more-permanent attachment, respectively (19). To determine whether increasing Psl expression can rescue the fliC mutant adherence phenotype, production of Psl was induced with l-Ara in the ΔfliC mutant as described for luciferase assays (Fig. 1C). At all l-Ara concentrations tested, adherence increased compared to the adherence of the Δpsl ΔfliC mutant, but not significantly above that of the ΔfliC mutant (Fig. 1C). This suggests that the adherence defect due to loss of flagella cannot be overcome by simply increasing Psl production, despite levels of Psl expression at 0.05% and 1.0% l-Ara similar to or higher than that of strain PAO1 (Fig. 1B). The increase in adherence at 0% l-Ara over that of the Δpsl ΔfliC double mutant is likely due to leaky expression of the araC-pBAD promoter, since the chemically defined Jensen’s medium used for all experiments lacks l-Ara (20). The weakly detectable level of Psl, as seen in the anti-Psl immunoblot (Fig. 1B), may represent the minimum expression required for adherence in this system.
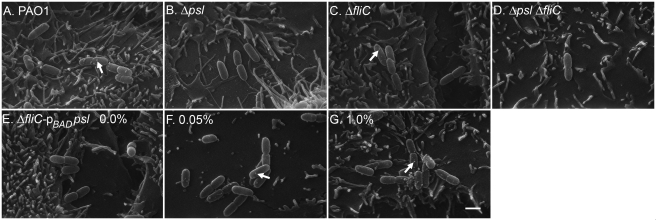
To visualize the contribution of Psl and flagellin to P. aeruginosa adherence to A549/NF-κB-luc cells, the adherence experiment was repeated with cells seeded onto glass coverslips. Two replicate wells were used to measure percent adherence, while following washing, the third coverslip was processed for scanning electron microscopy (SEM) analysis as described previously (2). Adherent bacteria were counted in 20 predetermined fields for each sample, and percent adherence was calculated. Aggregates of P. aeruginosa PAO1 were distributed evenly across A549/NF-κB-luc cells, while Δpsl and ΔfliC mutants were present in smaller, sparsely distributed groups (Fig. 2A to C). The psl mutant did not display extensive cell-cell contact, which is in agreement with observations after lectin staining, suggesting that Psl mediates cell-cell adherence along the long axis (5). The Δpsl ΔfliC double mutant could be found only as sporadic single cells, correlating with the lowest percent adherence measured by plate counts and SEM (Fig. 2D; see Fig. S3 in the supplemental material). In the absence of induction, ΔfliC pBADpsl mutant cells were present in isolated groups similar to what was seen for Δpsl and ΔfliC mutant cells (Fig. 2E). When Psl production was induced by the addition of l-Ara at either 0.05% or 1.0%, ΔfliC pBADpsl cells were observed adhering with a distribution similar to that of strain PAO1, though in larger clusters at 0.05% and 1.0% l-Ara (Fig. 2F and G). Despite the fact that similar percentages of adherence were found by plate counts and SEM, SEM revealed a difference in the cluster size and adherence of the ΔfliC pBADpsl mutant dependent on l-Ara concentration, with substantially greater adherence at 1.0% l-Ara than for the ΔfliC mutant (see Fig. S3 in the supplemental material). This is likely due to aggregates of induced cells appearing as individual CFUs by plate counts but discernible as individual cells by SEM. Regardless of technique, overexpression of Psl did not fully restore adherence to PAO1 levels, confirming the requirement for both Psl and flagellin in epithelial cell adherence.
FIG 2 .
Representative SEM images of adherent P. aeruginosa on A549/NF-κB-luc cells infected with P. aeruginosa PAO1 (A), Δpsl mutant (B), ΔfliC mutant (C), Δpsl ΔfliC mutant (D), and ΔfliC pBADpsl mutant (E to G) at three different concentrations of l-Ara, 0% (E), 0.05% (F), and 1.0% (G). The white arrows indicate Psl-dependent membrane structures. Bar, 1 µm.
In all panels of Fig. 2, cells expressing Psl at the level P. aeruginosa PAO1 did or higher (Fig. 2A, C, F, and G) show what appear to be membrane protrusions approximately 100 nm in diameter (indicated by arrows), while cells expressing little to no Psl (Fig. 2B, D, and E) lack these structures. It is not known whether these protrusions contain Psl or whether these protrusions are membrane vesicles produced as a result of a Psl or adherence-mediated signal (21). Further work will determine what role, if any, these Psl-associated structures have in adherence. We conclude from these experiments that full adherence and NF-κB activation in A549/NF-κB-luc cells requires Psl. During an infection, the presence of Psl would both aid initial attachment and increase FliC-mediated inflammation, whether by simply increasing interactions between bacterial cells and epithelial cells or by altering FliC disassociation from P. aeruginosa. This would make Psl a potential target for reducing further damage in the already inflamed airways in CF patients and in other P. aeruginosa infections where Psl-mediated adherence is critical.
SUPPLEMENTAL MATERIAL
ACKNOWLEDGMENTS
We thank Gayle Foster and Jim Turner for technical assistance and Ken Grant for SEM assistance. Steven Mizel generously provided Salmonella and P. aeruginosa type B purified flagellins.
This work was supported by Public Health Service grants AI061396 and HL058334 (D.J.W.) and NRSA fellowship AI07870002 (M.S.B.).
Footnotes
Citation Byrd, M. S., B. Pang, M. Mishra, W. E. Swords, and D. J. Wozniak. 2010. The Pseudomonas aeruginosa exopolysaccharide Psl facilitates surface adherence and NF-κB activation in A549 cells. mBio 1(3):e00140-10. doi:10.1128/mBio.00140-10.
REFERENCES
- 1. Govan J. R., Deretic V. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Byrd M. S., Sadovskaya I., Vinogradov E., Lu H., Sprinkle A. B., Richardson S. H., Ralston B., Parsek M. R., Anderson E. M., Lam J. S., Wozniak D. J. 2009. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol. Microbiol. 73:622–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedman L., Kolter R. 2004. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol. 186:4457–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jackson K. D., Starkey M., Kremer S., Parsek M. R., Wozniak D. J. 2004. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 186:4466–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma L., Conover M., Lu H., Parsek M. R., Bayles K., Wozniak D. J. 2009. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 5(3):e1000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma L., Jackson K. D., Landry R. M., Parsek M. R., Wozniak D. J. 2006. Analysis of Pseudomonas aeruginosa conditional Psl variants reveals roles for the Psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J. Bacteriol. 188:8213–8221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsukawa M., Greenberg E. P. 2004. Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J. Bacteriol. 186:4449–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lillehoj E. P., Kim B. T., Kim K. C. 2002. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. Am. J. Physiol. Lung Cell. Mol. Physiol. 282:L751–L756 [DOI] [PubMed] [Google Scholar]
- 9. O’Toole G. A., Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295–304 [DOI] [PubMed] [Google Scholar]
- 10. Ramphal R., Arora S. K. 2001. Recognition of mucin components by Pseudomonas aeruginosa. Glycoconj. J. 18:709–713 [DOI] [PubMed] [Google Scholar]
- 11. Sauer K., Camper A. K., Ehrlich G. D., Costerton J. W., Davies D. G. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scharfman A., Arora S. K., Delmotte P., Van Brussel E., Mazurier J., Ramphal R., Roussel P. 2001. Recognition of Lewis x derivatives present on mucins by flagellar components of Pseudomonas aeruginosa. Infect. Immun. 69:5243–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayashi F., Smith K. D., Ozinsky A., Hawn T. R., Yi E. C., Goodlett D. R., Eng J. K., Akira S., Underhill D. M., Aderem A. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099–1103 [DOI] [PubMed] [Google Scholar]
- 14. Honko A. N., Mizel S. B. 2005. Effects of flagellin on innate and adaptive immunity. Immunol. Res. 33:83–101 [DOI] [PubMed] [Google Scholar]
- 15. Zhang Z., Louboutin J. P., Weiner D. J., Goldberg J. B., Wilson J. M. 2005. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect. Immun. 73:7151–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoon S. S., Mekalanos J. J. 2008. Decreased potency of the Vibrio cholerae sheathed flagellum to trigger host innate immunity. Infect. Immun. 76:1282–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weimer E. T., Lu H., Kock N. D., Wozniak D. J., Mizel S. B. 2009. A fusion protein vaccine containing OprF epitope 8, OprI, and type A and B flagellins promotes enhanced clearance of nonmucoid Pseudomonas aeruginosa. Infect. Immun. 77:2356–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryder C., Byrd M., Wozniak D. J. 2007. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr. Opin. Microbiol. 10:644–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Merritt J. H., Brothers K. M., Kuchma S. L., O’Toole G. A. 2007. SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function. J. Bacteriol. 189:8154–8164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jensen S. E., Fecycz I. T., Campbell J. N. 1980. Nutritional factors controlling exocellular protease production by Pseudomonas aeruginosa. J. Bacteriol. 144:844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McBroom A. J., Kuehn M. J. 2007. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63:545–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.