Abstract
Natural regulatory T cells (nTregs) are defined by their inherent ability to establish and maintain peripheral self-tolerance. In recent years, the development of nTregs has come under close examination with the advent of FOXP3-GFP reporter mice that pinpointed the initiation of FOXP3 expression within the thymus. The mechanism and pathway of nTreg development has only recently been studied in detail and to a large degree still remains unclear. In this review, we will discuss our current understanding of nTreg lineage choice and development from a cellular and intracellular standpoint.
Keywords: Regulatory T cell, FOXP3, TCR, co-stimulation, cytokines, thymus, peripheral tolerance
Introduction
For several decades, the idea of a cell population that has an inherent ability to suppress a variety of immunological relevant cells has been hotly debated. However, in the last 20 years, it has now been firmly established that Tregs are a vital player in establishing peripheral self-tolerance. Sakaguchi and colleagues elegantly demonstrated that the depletion of a CD5+ splenocyte subpopulation could elicit multiorgan autoimmunity1. CD5+ T cells were further distinguished by the presence of CD4+, CD45 RBlow and finally CD25+, the high-affinity subunit of the IL-2 receptor.2,3,4 It was also determined that neonatal thymectomy three days after birth blocked the emergence of peripheral CD4+CD25+ cells, confirming the thymic origin of Tregs.3
In 2003, Forkhead Box P3 protein (FOXP3) was identified as a unique marker for Tregs which was predominantly expressed within CD25+CD4+ T cells.5,6 FOXP3 is a transcription factor required for the development and function of CD4+CD25+ Tregs.7 It was later established that Tregs are not only required in protection from multiorgan autoimmunity, but also play an important role in specific autoimmune conditions including inflammatory bowel disease (IBD) and type 1 diabetes.8,9
FOXP3 has been the focus of considerable interest since its discovery in humans in 1982 10 and in mice in 1949,11,12 before it was linked to Tregs. It was determined that a mutation found within FOXP3 in humans led to immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. As the name describes, IPEX syndrome is an X-linked recessive disorder caused by mutations in the Forkhead Box P3 (FOXP3) gene located at Xq11.23-Xq13.3 of the X chromosome.10,13 Immunopathology associated with the IPEX syndrome becomes apparent around the first month after birth and includes eczema or psoriatiform lesions, watery diarrhea, type 1 diabetes mellitus, excessive cytokine production and chronic inflammation leading to death.14 To date, there are over 20 known FOXP3 mutations resulting in the manifestation of IPEX syndrome. The majority of the mutations are missense mutations found in the DNA-binding domain of FOXP3. Various mutations within FOXP3 can lead to multiple dysregulations including a loss in transcriptional repression, mRNA stability of FOXP3, DNA binding, and FOXP3 dimerization.15
Mice that carry the lethal scurfy mutation also have defective FOXP3 and are characterized as runted with thickening and scaling of the ears and scaling of eyelids, feet, and tails.11,16 High quantities of lymphocyte infiltrates are present in the skin and liver, and these mice are anemic with splenomegaly, hepatomegaly and lymphadenophathy.12,17 All male mice with the scurfy mutation die between three and four weeks of age. Interestingly, heterozygous females remain healthy and are only carriers of the lethal FOXP3 mutation and lymphoproliferative disorder. It was established that the symptoms of the scurfy mouse were T cell mediated and the scurfy-like phenotype could be replicated in T cell-deficient animals by transfer of T cells from scurfy mice.16 Together these studies first established FOXP3+ T cells as the primary cell type that prevents severe autoimmunity. However, it was only recently established that FOXP3 is the master regulator of Treg function and development. While significant advances have been made, much remains to be defined regarding Treg develop in both the thymus and the periphery. The remainder of this review will focus on recent advances in understanding lineage choice of natural Tregs (nTregs) within the thymus.
T cell development
In order to discuss how Tregs develop in the thymus, it is critical to understand the basis of T cell development as a whole. In 1961 Jacques Miller first characterized the thymus as the place for lymphocyte development.18,19 Following this landmark discovery, Miller went on to describe the role of the thymus and its direct relationship with immunological tolerance.20 The more comprehensive terms of “central tolerance” and “peripheral tolerance” took into account the deletion of autoreactive T cells in the thymus before they reach the periphery (central tolerance) and the control or suppression of autoreactive T cells that escape deletion and circulate in the periphery (peripheral tolerance).21,22 Both central and peripheral tolerance are crucial in preventing the onset autoimmunity. Tregs are generally regarded as the key mediator of peripheral tolerance, where they are able to suppress self-reactive T cells and efficiently prevent autoimmunity.23 As nTregs originate in the thymus, it is thought they initially undergo the same selective pressure and developmental checkpoints as conventional (αβ) T cells (Tconv).
Two vital checkpoints that occur very close together, if not simultaneously, during thymocyte development are positive selection and negative selection. At this point in development, randomly rearranged TCRs act in concert with CD4 and CD8 co-receptors to interact preferentially with either MHC class I or MHC class II.24,25 The ligation of TCR with cognate peptide-MHC allows for a cacophony of signaling events and the development of immature thymocytes into either CD8+ or CD4+ single positive (SP) T cells, respectively. This engagement of TCR/co-receptor with MHC is vital in generating a T cell population that can distinguish self from non-self. Understanding the detailed mechanism of positive selection has garnered considerable attention over the last two decades. From these studies it was discovered that over 97% of all thymocytes that become DP cells will die by their failure to recognize, at any level, the presence of MHC and therefore will not receive survival signals through the TCR26. These cells are said to die by neglect. The remaining thymocytes are thought to survive initially by the recognition of low-affinity peptides as described in the “kinetic signaling” model.27 In this model it is proposed that signal intensity allows for positive selection and then signal duration allows for CD4/CD8 lineage commitment. Recently, a variation of the kinetic signaling model has been proposed in which serial low affinity triggering by peptide-MHC complexes can promiscuously ligate enough TCRs to generate a persistent signal.28 In opposition to this model, it has been shown that MHC class I-restricted thymocytes lacking CD8 can be driven to choose the CD8 lineage by interacting with high-avidity ligands.29 Furthermore, CD4+CD8lo/− thymocytes are not always observed within TCR transgenic mice as seen in some mice that express MHC class I specific TCRs.30 Both of these rare observations may only present themselves in default pathways to allow survival of thymocytes when selective pressures are applied. Regardless of the detailed events in positive selection, less than 1% of all thymocytes will recognize self-peptide at high enough affinity/avidity to receive survival signals but also low enough to avoid the activation threshold for apoptosis.31,32
The recognition of self-peptide with high affinity/avidity by the TCR will direct the thymocyte towards controlled apoptosis and deletion.21 This process is also known as negative selection. An alternative to negative selection and deletion is the ability of these cells to undergo TCR editing and subsequently lose their autoreactive TCR and become anergic.33 Recently, a fourth pathway has been described detailing the development of T cells selected against high affinity peptides that normally induce negative selection, sometimes referred to as agonist selection.34 These cell types include NKT cells, CD8αα intestinal intraepithelial lymphocytes (IEL), and possibly CD4+CD25+ regulatory T cells and γδ T cells. It is still unclear how the intracellular signaling and genetic signatures differ between those cells selected on high affinity peptides versus low affinity peptides during positive selection. A more detailed review of the signaling environment believed to mediate nTreg development will be discussed in a later section.
TCR signaling in thymocyte development
The αβ TCR is composed of two polypeptides that contain variable regions specifically designed to ligate peptide-bound MHC. In the thymus, the αβ TCR is expressed on thymocytes entering the DP stage of development. The TCR itself is unable to transmit any intracellular signals from external stimulation due to short cytoplasmic tails, however, it does associate with the CD3 complex (εγ,δε and ζζ chains) that mediate signal transduction.35 Each of the four (ε,γ,δ,ζ) chains in the TCR-CD3 complex contain immunotyrosine activation motifs (ITAMs) that can be phosphorylated by protein tyrosine kinases to recruit downstream signaling molecules. It was not known if the 10 CD3 associated ITAMs were functionally redundant, and because of the high number of ITAMs found in the ζ subunit (6 ITAMS), it was presumed that the CD3ζ chains held the most control over the TCR signaling threshold.36 To address this question, a scalable model of TCR signaling was proposed and tested, confirming that the CD3ζ chain is indeed critical for the signaling events needed in the establishment of central tolerance.37 In this model, lowering the number of functional ITAMs within the TCR:CD3 complex translates into lower TCR signal strength and loss in negative selection of autoreactive cells. The reduction in the number of ITAMs led to a breakdown in central tolerance and widespread autoimmunity. However, at this time it is not clear what impact, if any, individual ITAMs have on the signaling requirements for Treg development. When CD3εζ−/− bone marrow was transduced with either 10 functional ITAMs or 4 functional ITAMs and used to reconstitute Rag1-deficient mice, there did not appear to be any difference in the proportion of FOXP3+ Tregs or their ability to suppress in vitro.37 Further characterization of the role of individual ITAMs is needed to address if any particular ITAM or combination of ITAMs are necessary in the development of FOXP3+ Tregs.
From these studies, it appears that the relatively high number of ITAMs found within the CD3 complex is needed to ensure a critical threshold of signaling possibly through the recruitment and activation of certain downstream signaling molecules such as the tandem Src homology 2 (SH2) domain-containing ζ-chain-associated protein of 70 kDa (ZAP-70).38 It has been shown that recruitment and activation of the ZAP-70 substrate, linker of activated T cells (LAT), is essential in the development of FOXP3+ T cells in the thymus and the periphery.39 In this study a key tyrosine residue was mutated (Y136F), inhibiting the binding of phospholipase Cγ1 (PLCγ1) to activated LAT, causing a complete block in Treg development. However, it appears there is also a partial block in thymocyte development in Y136F mice and this may lead, indirectly, to the loss of FOXP3 expression and impairment of Treg development.
The CD3-associated ITAMs may also have distinct signaling responsibilities following TCR ligation. The TCR must translate extracellular stimuli into an intracellular response of a particular fate and by using finely tuned signals emanating from the TCR/CD3 complex, T cells can decide whether to proliferate, die or simply survive. The 21 kDa form of the CD3ζ-chain is in a constitutively phosphorylated state, possibly acting as a hair trigger for TCR signaling events including differentiation and survival.40,41 The other CD3-associated ITAMs, ε,γ and δ, are thought to be more difficult to phosphorylate42 and may have specific roles in mediating activation of downstream effector molecules. For example, it has been shown that the CD3δ chain is necessary for ERK signaling and lipid raft recruitment while the CD3γ chain is necessary for ERK signaling leading to positive selection of thymocytes. Collectively, these data indicate the need for a threshold of signaling within developing thymocytes and potentially mediating signals necessary for development of Tregs. Clearly, the CD3-associated ITAMs have differential roles in TCR signaling and T cell development, and in combination, the ten ITAMs allow for a determined strength of signal or threshold to initiate pre-TCR signaling, central tolerance, homeostatic expansion, and maintenance of T cells.
Co-stimulatory molecules and Treg development
As previously mentioned, nTreg development is thought to take place solely in the thymus, however, it is currently unknown what signaling pathway(s) allow(s) for positive selection and survival of Tregs and their escape from deletion. It is widely known that FOXP3 expression does not commence until day 3 in neonates and it has been postulated that the lack of an organized thymic architecture may lead to the inability of developing thymocytes to receive the proper co-stimulatory signals for FOXP3 expression.43 Many co-stimulatory signals have been implicated in the development and lineage commitment of nTregs including: CD28 ligation by CD80/CD86, IL2R, thymic stromal-derived lymphopoietin receptor (TSLPR), CD154, glucocorticoid-induced tumour necrosis factor receptor (GITR), and STAT5 signaling.44–48 For instance, nTregs are somewhat resistant to apoptotic signals, and this is in part due to the expression of GITR.49 GITR may not be the only molecule responsible for the resistance of apoptosis in Tregs as anti- and pro-apoptotic molecules Bim and Bcl2 also appear to have a hand in Treg development.50 For example, it was reported that Bim−/− mice have an increase in total Tregs while there is an increase in the frequency of FOXP3+ Tregs in Bcl2 transgenic mice.
Another co-stimulatory pathway necessary for nTreg development is mediated by the interaction of CD28 with CD80/CD86. In mice that lack either CD80/CD86 or CD28, there is a marked reduction of FOXP3+ Tregs.51 It was later shown that CD28 has cell intrinsic and extrinsic roles in Treg development.52 Extrinsically, without CD28 expression there is a reduction in global IL-2 production leading to insufficient IL-2R signaling and reduction in FOXP3 expression. Intrinsically, CD28 costimulation is required for Treg precursors as shown in an experiment where Cd28−/− HSC’s have reduced numbers of FOXP3+ Tregs in the presence of exogenous IL-2.52
It is well known that nTregs constitutively express the high-affinity IL-2R chain CD25, however they are unable to secrete IL-2 themselves.53 IL-2 appears to have a significant role in development and maintenance of Tregs as is evident from IL-2Rβ-deficient mice that have a loss of functional Tregs.46 In addition, when there is a lack of IL-2 or the IL-2R, there is a 50% reduction in thymic Tregs. The incomplete block in Treg development indicates IL-2 signaling through STAT5 is only a part of several signals needed for optimal Treg development.47,54 Based upon these observations, a two-step model of nTreg development has been postulated.55,56 In this model, recognition of high affinity self-peptide by developing thymocytes upregulates CD25 allowing for the rescue of these cells by IL-2- and STAT5-mediated signaling within the medulla and their progression into the Treg lineage.
In addition to IL-2R signaling, ligation of TGFβR also appears to be involved in development and maintenance of nTregs. Initially, the role of TGFβ was unclear as mice deficient in TGFβ or TGFβ receptor II had normal numbers of Tregs in adult thymi.57 However, after close examination of mice with T cell restricted deletion of TGFβ receptor I, it was found that numbers of CD4+CD25+FOXP3+ thymocytes were greatly reduced in young mice, between days 3 and 5 of age.58 It appears the reduction in CD4+CD25+FOXP3+ thymocytes is only temporary, and the numbers of thymic FOXP3+ Tregs rapidly recover due to increased production of IL-2. Also important to note is that CD4+CD25+FOXP3+ thymocytes were completely lost when mice lacking both TGFβRI and IL-2 were generated. From this work it is apparent that TGFβ is an important upstream mediator of FOXP3 expression although other signals, including TCR signaling, are necessary.
Spatial and temporal development of nTregs
Visualizing and pinpointing the initial expression of FOXP3 within the thymus was greatly facilitated by the generation of FOXP3-GFP reporter mice, which have broadened our understanding of nTreg development.7 Using these mice it was clearly established that FOXP3 expression is predominantly seen at the CD4 SP stage of development. However, a small percentage of GFP+ cells were also seen in the DP and CD8 SP populations, and, interestingly, at the immature DN stage of thymocyte development. In support of this notion, studies have identified a FOXP3+CD4−CD8−(DN) population within the human thymus that was αβTCR and pTCRα negative.59 These FOXP3 expressing cells were also negative for γδTCR, monocyte, B cell or NK cell markers. Interestingly the FOXP3+ DN cells were only found in the cortex region of the thymus.
Determining where within the thymus FOXP3 is first expressed has been hotly debated, as both the thymic medulla and cortex may be responsible for nTreg development.60–63 For example, one recent study implicated expression of the autoimmune regulator gene (Aire) within medullary thymic epithelial cells (mTECs) as a critical cell type in nTreg development.64 In this study, medullary regions of MHC class II negative mTECs had significantly less FOXP3+ Tregs compared to MHC class II positive regions. Furthermore, using GFP as a marker for FOXP3 expression, it was determined that the overwhelming majority of GFP+ cells are found within the medulla of the thymus.7 In addition, it has been shown that thymic stromal-derived lymphopoietin (TSLP) produced in the medullary region of the human thymus is critical for Treg development44 giving credence to the notion that nTreg development is medullary. It is important to note that thymic dendritic cells (tDCs) also make TSLP and these cells may infrequently be found in the thymic cortex.65 Another study examined the role of MHC class II expressed in the cortex in nTreg development and demonstrated that mice expressing MHC-II only in the cortex were able to give rise to nTregs, suggesting that nTregs receive TCR signals early in development and require a combination of other co-stimulatory signals to mature into functionally suppressive nTregs.61,63,66 To address the ability of the cortex to support Treg development, a study was performed where thymic migration from the cortex to the medulla was blocked by administering pertussis toxin and therefore inhibiting G protein-coupled receptor signaling. In these treated mice there was an accumulation of CD4SP FOXP3+ thymocytes within the cortex indicating the thymic cortex is sufficient to initiate FOXP3 expression.63 It was also determined that about 25% of total FOXP3+ Tregs found within the thymus are CD4+CD8+ double positive thymocytes. Finally, in the case of CCR7 deficient mice, which have impaired thymocyte trafficking from the cortex to the medulla, there is an accumulation of SP cells, as well as, an increase in FOXP3+ Tregs in the cortex.63,67 Clearly nTregs are capable of developing within the cortical region of the thymus, but require other co-stimulatory signals for lineage commitment and survival.
Within the cortex, DP thymocytes interact primarily with low affinity self-antigens expressed on cTEC’s while other cells including fibroblasts and tDCs may be presenting higher affinity peptides.68 One could theorize that DP thymocytes actively engage high affinity self-peptide/MHC, possibly by the association with an infrequent tDC population or other APCs in the cortex, which initiates expression of FOXP3.
It is thought that a major contributor of negative selection and T cell tolerance is mediated by expression of the autoimmune regulator gene (Aire).69 Aire is expressed in thymic medullary epithelial cells (mTEC) and this allows for expression of many, but not all peripheral-tissue antigens that would otherwise be absent during negative selection. It is possible that interactions with AIRE-expressing mTECs could also initiate FOXP3 expression within CD4 SP immature thymocytes.64 However, AIRE expressing mTECs undergo rapid turnover, allowing for cross-presentation of antigens by traveling tDCs.70 The infrequent tDCs may then present AIRE antigens in the cortex allowing for nTreg development at the DP stage. Taken together, these data indicate that initiation of FOXP3 expression within DP and SP thymocyte populations may be due to a combination, and possibly an accumulation, of signals mediated by the TCR, cytokine receptors and co-stimulatory molecules. From these observations a variation of the two-step model has been proposed71 where nTreg development begins with a population of pre-Tregs generated by unknown mechanisms. After successful rearrangement of the TCR, pre-Tregs will recognize a diverse repertoire of self and foreign antigens but only progress to mature nTregs by the accumulation of TCR-dependent and independent signals including a pathway that allows for survival under negative selection conditions.45 In this model, TCR dependent and independent signals have variable levels of importance within any give developing nTreg.
MicroRNA’s and phosphatases
One area that needs closer inspection is the role of microRNAs (miRNAs) and phosphatases, as mediators of gene regulation and ultimately TCR signal strength during Treg development72,73 and how they might affect FOXP3 expression. Recent studies have shown that the miRNA, miR-181a, represses multiple tyrosine phosphatases within mature T cells leading to dysregulated TCR signaling.72 These studies also found differential levels of miR-181a expression within the various stages of thymocyte development, coinciding with TCR signaling checkpoints and sensitivity to cell fates. One phosphatase found to be sensitive to miR181a, DUSP6, has also been described to have a role in regulating TCR-mediated ERK activation, and subsequently, the signaling threshold required for thymocyte positive selection.74 Other miRNAs have been implicated in Treg fitness and homeostasis.73,75 For instance, it has been suggested that miR-155 is necessary for the regulation of Treg homeostasis by targeting SOCS1, which allows Tregs to become sensitive to IL-2.73 Dicer, a protein involved in miRNA processing, was shown to be critically important in FOXP3+ Treg development as well as their peripheral maintenance.75,76 It is likely that other regulators of activation will be uncovered and it will be of great interest to determine if any contribute to the regulation of FOXP3 expression.
Regulatory T cell repertoire
In the last few years there has been a heated debate about whether TCRs expressed by Tregs have a higher propensity to recognize self-antigen or have a restricted repertoire in comparison to Tconv cells.62,77,78 In support of higher self-reactivity of nTreg TCRs, it was shown that there is an enhancement in nTreg development when agonist self-peptide is presented in the thymus.60 However it was subsequently determined, following analysis of individual TCRs expressed by Tregs and Tconv cells, that neither preferentially recognizes self-antigen as their cognate antigen.79 Moreover, isolated Tregs have been shown to be reactive to not only self-peptide, but also antigens derived from bacteria, viruses and parasites, neo-antigens and allo-antigens (Reviewed in ref. 79). However, it is possible that these analyses did not take into account the differences in TCR repertoire expressed by natural versus induced Tregs. In a recent study that was performed in mice with a fixed TCRβ and freely rearranged TCRα, there appeared to be no difference in Treg TCR repertoire compared to Tconv TCR repertoire.78 Moreover, the reported αβ TCR diversity of Tregs in mouse and human appears to be comparable in terms of TCR variable region usage (Vβ or Vα) between Tconv and Tregs.80,81 In addition, when TCR transgenic mice were engineered from an isolated and cloned Treg TCR, these mice did not generate any Tregs or mature T cells in the periphery.82 Taken together, the current data suggest a diverse TCR repertoire that may rival that of conventional αβT cells. However, the analysis of a greater pool of isolated naive Treg and naïve Tconv TCRs will ultimately reveal if there is any skewing of the Treg repertoire towards self or non-self. Currently, no reliable method has been described to isolate and sequence large number of TCRs from individual naïve Tregs and Tconv cells (ie. from the current approaches which sequence several hundred TCR to approaches that can sequence several million). Most methods employ the use of single cell sorting or by isolating Tregs and Tconv cells from antigen-primed environments. To fully appreciate the complete Treg repertoire, innovative approaches need to be developed to distinguish nTreg from iTregs in order to determine TCR diversity between these two Treg populations.
nTregs versus iTregs
Although nTregs are perhaps more abundant within a quiescent immune system, iTregs may play an important role in maintaining proper immune function and regulation.83 While nTregs need costimulation via CD28, iTregs do not need any costimulation.84 This is not surprising considering the inflammatory environment and milieu of cytokines that are present at the presumed site of iTreg development. Currently there are two types of iTregs; type 1 regulatory T cells (Tr1) and T helper-3 T cells (Th3). Tr1 cells are induced by IL-10 and suppress via the same cytokine along with the production of TGFβ85, but they lack FOXP3 expression.86 In certain cases, Tr1 cells can secrete additional cytokines such as IL-5 and IFNγ. In contrast, Th3 cells are induced by TGFβ and express FOXP3 as well as TGFβ after conversion into the Treg phenotype.87 Th3 cells are predominately found within the intestinal tissue and mice lacking Th3 cells at this tissue site will develop spontaneous autoimmunity (Reviewed in 88). Both types of iTregs can control the development of autoimmunity and promote transplantation tolerance.89,90 The distinction between nTregs and iTregs may be in their cognate antigen. Whereas nTregs are selected on endogenous self-peptide and may be responsible for controlling autoreactive T cells before tolerance is broken, iTregs are more likely to be found at sites of inflammation where it makes sense to convert activated T cells into suppressor cells.84,91 Interestingly, many iTregs are found at mucosal sites and gut-associated lymphoid organs, where there is a tendency for a steady state of activation and exposure to foreign antigen.
Summary
Over the last 10 years there have been many advances in understanding the role of FOXP3 in Treg function and development.92 It has been shown that both nTregs and iTregs are vital in controlling and mediating suppression in autoimmune and inflammatory disease models, such as IBD, T1D and EAE.89 It is becoming increasingly clear that nTregs are a distinct lineage pathway determined at the DP stage of thymocyte development due in part to co-stimulatory signals initiating FOXP3 expression. Prevailing evidence suggests that nTregs development may shadow the process used by αβ T cells63,71,79, where early TCR signals in concert with co-stimulatory signals at the DP stage of development dictate a divergence from Tconv development and induce FOXP3 expression and Treg development. The advent of reporter mice highlighted FOXP3 expression at early time points within the thymus, and revealed the location nTreg development. FOXP3 expression in both the cortex and medulla suggests that selection of Tregs may begin in the cortex. Engagement of rare tDC or other APCs found in the cortex may provide the early TCR signal for Treg lineage development. Understanding the relationship between APCs and Treg progenitors at this initial engagement is key to understanding the earliest signals involved in Treg differentiation.
There are many questions regarding Treg lineage development that remain unanswered. (1) What co-stimulatory and or cytokine signals are responsible for Treg lineage commitment beyond what is already known? (2) What are the upstream regulators of FOXP3 and the dominant signaling pathways that determine Treg lineage fate? (3) Is there a unique APC that mediates Treg development? (4) Is the nTreg TCR repertoire more restricted compared to Tconv cells and how do nTreg and iTreg repertoires compare? (5) Just as there are induced Tregs in the periphery, is it possible that CD4 SP FOXP3− cells in the medulla can become induced FOXP3+ Tregs? More, specifically, are there iTregs in the CD4 SP stage of thymocyte development? The recent explosion of genome and transcriptome sequencing technologies, and other advances, will undoubtedly lead to a more complete understanding of nTreg and iTreg development and homeostasis and help address these questions.
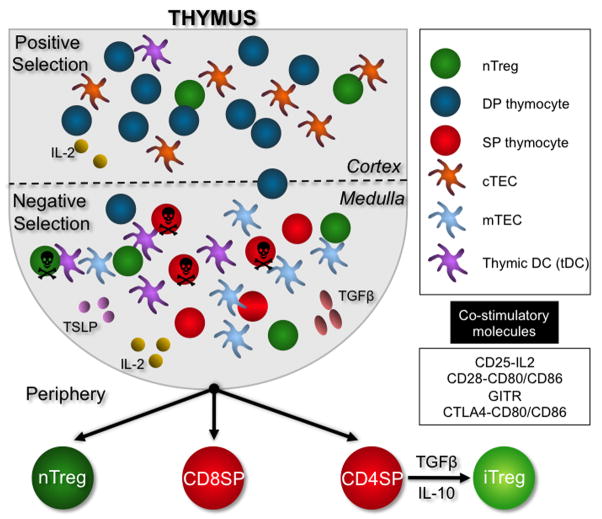
Figure 1.
As double positive (DP) thymocytes navigate the cortex of the thymus, they will encounter cortical thymic epithelial cells (cTECs) and rare thymic dendritic cells (tDCs). Only a few DPs will encounter cognate peptide/MHC and with co-stimulation by CD28 or other unknown mechanisms, upregulate FOXP3 and progress to the medulla. Most DPs, however, will undergo positive selection and progress to the medulla as FOXP3- cells. Within the medulla, lingering DPs and the more abundant single positive (SP) CD4 and CD8 thymocytes will encounter AIRE+ medullary thymic epithelial cells (mTECs) and tDCs. mTECs and tDCs present higher affinity peptides that allow for deletion of potentially autoreactive thymocytes. They also provide the co-stimulation necessary for the initiation of FOXP3 expression. tDCs and perhaps other antigen presenting cells (APCs) provide a source for the IL-2, TSLP and TGFβ molecules necessary but not required for nTreg development.
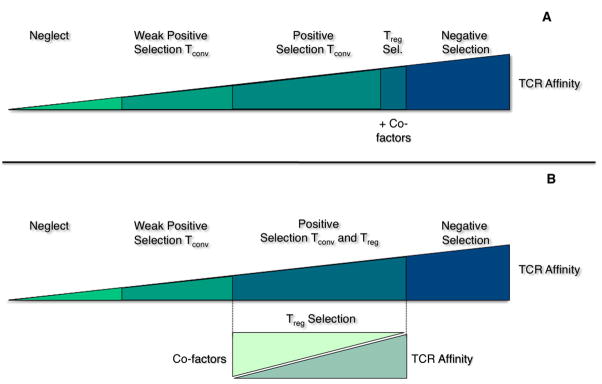
Figure 2.
Schematic diagram of nTreg development as it relates to T cell receptor (TCR) affinity and co-factors. The current model (A) of nTreg development indicates selection is mediated by high TCR affinity interactions with cognate peptide/MHC (pMHC) plus additional co-factors such as CD28-CD80/CD86 and IL-2 found in the medulla. A more recent model proposes nTreg development can occur in both the medulla and cortex based upon a balance between TCR/pMHC and multiple co-factors. In this model (B), nTregs that have high affinity for pMHC will need less co-factors such as the cytokines, TGFβ and IL-2 or co-stimulatory molecules, GITR and CD28.
Acknowledgments
MB is supported by a Hartwell Fellowship. DAAV is supported by the National Institutes of Health (NIH) (AI39480, AI52199, AI072239), a Cancer Center Support CORE grant (CA21765) and the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161 (1):72. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith H, Sakamoto Y, Kasai K, Tung KS. Effector and regulatory cells in autoimmune oophoritis elicited by neonatal thymectomy. J Immunol. 1991;147 (9):2928. [PubMed] [Google Scholar]
- 3.Sakaguchi S, et al. T cell-mediated maintenance of natural self-tolerance: its breakdown as a possible cause of various autoimmune diseases. J Autoimmun. 1996;9 (2):211. doi: 10.1006/jaut.1996.0026. [DOI] [PubMed] [Google Scholar]
- 4.Powrie F, Mason D. Subsets of rat CD4+ T cells defined by their differential expression of variants of the CD45 antigen: developmental relationships and in vitro and in vivo functions. Curr Top Microbiol Immunol. 1990;159:79. doi: 10.1007/978-3-642-75244-5_5. [DOI] [PubMed] [Google Scholar]
- 5.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]; Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299 (5609):1057. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 6.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4 (4):337. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 7.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202 (7):901. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coombes JL, et al. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 9.Shevach EM, et al. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 10.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27 (1):18. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 11.Lyon MF, et al. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proc Natl Acad Sci U S A. 1990;87 (7):2433. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godfrey VL, Wilkinson JE, Rinchik EM, Russell LB. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci U S A. 1991;88 (13):5528. doi: 10.1073/pnas.88.13.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27 (1):20. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 14.Torgerson TR. Regulatory T cells in human autoimmune diseases. Springer Semin Immunopathol. 2006;28(1):63. doi: 10.1007/s00281-006-0041-4. [DOI] [PubMed] [Google Scholar]; van der Vliet HJ, Nieuwenhuis EE. IPEX as a result of mutations in FOXP3. Clin Dev Immunol. 2007;2007:89017. doi: 10.1155/2007/89017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7 (4):305. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey VL, Rouse BT, Wilkinson JE. Transplantation of T cell-mediated, lymphoreticular disease from the scurfy (sf) mouse. Am J Pathol. 1994;145 (2):281. [PMC free article] [PubMed] [Google Scholar]
- 17.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27 (1):68. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 18.Miller JF. Analysis of the thymus influence in leukaemogenesis. Nature. 1961;191:248. doi: 10.1038/191248a0. [DOI] [PubMed] [Google Scholar]
- 19.Miller JF. The discovery of thymus function and of thymus-derived lymphocytes. Immunol Rev. 2002;185:7. doi: 10.1034/j.1600-065x.2002.18502.x. [DOI] [PubMed] [Google Scholar]
- 20.Miller JF. Role of the Thymus in Immunity. Br Med J. 1963;2 (5355):459. doi: 10.1136/bmj.2.5355.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer E. Negative selection--clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3 (5):383. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 22.Stockinger B. T lymphocyte tolerance: from thymic deletion to peripheral control mechanisms. Adv Immunol. 1999;71:229. doi: 10.1016/s0065-2776(08)60404-6. [DOI] [PubMed] [Google Scholar]
- 23.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16 (2):81. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Kaye J, et al. Selective Development of Cd4+ T-Cells in Transgenic Mice Expressing a Class-Ii Mhc-Restricted Antigen Receptor. Nature. 1989;341 (6244):746. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 25.Scott B, Bluthmann H, Teh HS, Vonboehmer H. The Generation of Mature T-Cells Requires Interaction of the Alpha, Beta T-Cell Receptor with Major Histocompatibility Antigens. Nature. 1989;338 (6216):591. doi: 10.1038/338591a0. [DOI] [PubMed] [Google Scholar]
- 26.Huesmann M, Scott B, Kisielow P, von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991;66 (3):533. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 27.Bosselut R, Feigenbaum L, Sharrow SO, Singer A. Strength of signaling by CD4 and CD8 coreceptor tails determines the number but not the lineage direction of positively selected thymocytes. Immunity. 2001;14 (4):483. doi: 10.1016/s1074-7613(01)00128-5. [DOI] [PubMed] [Google Scholar]
- 28.Valitutti S, et al. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375(6527):148. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]; Borovsky Z, Mishan-Eisenberg G, Yaniv E, Rachmilewitz J. Serial triggering of T cell receptors results in incremental accumulation of signaling intermediates. J Biol Chem. 2002;277 (24):21529. doi: 10.1074/jbc.M201613200. [DOI] [PubMed] [Google Scholar]
- 29.Goldrath AW, Hogquist KA, Bevan MJ. CD8 lineage commitment in the absence of CD8. Immunity. 1997;6 (5):633. doi: 10.1016/s1074-7613(00)80351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogquist KA. Signal strength in thymic selection and lineage commitment. Curr Opin Immunol. 2001;13 (2):225. doi: 10.1016/s0952-7915(00)00208-9. [DOI] [PubMed] [Google Scholar]
- 31.Allen PM. Peptides in positive and negative selection: a delicate balance. Cell. 1994;76 (4):593. doi: 10.1016/0092-8674(94)90497-9. [DOI] [PubMed] [Google Scholar]
- 32.Santori FR, et al. Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity. 2002;17 (2):131. doi: 10.1016/s1074-7613(02)00361-8. [DOI] [PubMed] [Google Scholar]
- 33.McGargill MA, Derbinski JM, Hogquist KA. Receptor editing in developing T cells. Nat Immunol. 2000;1 (4):336. doi: 10.1038/79790. [DOI] [PubMed] [Google Scholar]
- 34.Baldwin TA, Hogquist KA, Jameson SC. The fourth way? Harnessing aggressive tendencies in the thymus. J Immunol. 2004;173 (11):6515. doi: 10.4049/jimmunol.173.11.6515. [DOI] [PubMed] [Google Scholar]
- 35.Janeway CA., Jr The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- 36.Pitcher LA, van Oers NS. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 2003;24 (10):554. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Holst J, et al. Scalable signaling mediated by T cell antigen receptor-CD3 ITAMs ensures effective negative selection and prevents autoimmunity. Nat Immunol. 2008;9 (6):658. doi: 10.1038/ni.1611. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, et al. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92 (1):83. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 39.Koonpaew S, Shen S, Flowers L, Zhang W. LAT-mediated signaling in CD4+CD25+ regulatory T cell development. J Exp Med. 2006;203 (1):119. doi: 10.1084/jem.20050903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420 (6914):429. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 41.van Oers NS, Killeen N, Weiss A. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR zeta in murine thymocytes and lymph node T cells. Immunity. 1994;1 (8):675. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 42.Straus DB, Weiss A. The CD3 chains of the T cell antigen receptor associate with the ZAP-70 tyrosine kinase and are tyrosine phosphorylated after receptor stimulation. J Exp Med. 1993;178 (5):1523. doi: 10.1084/jem.178.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JM, Rudensky A. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol Rev. 2006;212:86. doi: 10.1111/j.0105-2896.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Q, et al. Delayed functional maturation of natural regulatory T cells in the medulla of postnatal thymus: role of TSLP. BMC Immunol. 2006;7:6. doi: 10.1186/1471-2172-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spence PJ, Green EA. Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc Natl Acad Sci U S A. 2008;105 (3):973. doi: 10.1073/pnas.0709071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malek TR, et al. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17 (2):167. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 47.Burchill MA, et al. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178 (1):280. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 48.Maggi E, et al. Thymic regulatory T cells. Autoimmun Rev. 2005;4 (8):579. doi: 10.1016/j.autrev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 49.McHugh RS, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16 (2):311. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 50.Pandiyan P, Lenardo MJ. The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biol Direct. 2008;3:6. doi: 10.1186/1745-6150-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12(4):431. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]; Tang Q, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171 (7):3348. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 52.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6 (2):152. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 53.Fehervari Z, Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol. 2004;16(2):203. doi: 10.1016/j.coi.2004.01.004. [DOI] [PubMed] [Google Scholar]; Su L, et al. Murine CD4+CD25+ regulatory T cells fail to undergo chromatin remodeling across the proximal promoter region of the IL-2 gene. J Immunol. 2004;173 (8):4994. doi: 10.4049/jimmunol.173.8.4994. [DOI] [PubMed] [Google Scholar]
- 54.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6 (11):1152. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 55.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28 (1):100. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burchill MA, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28 (1):112. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25(3):441. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]; Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25 (3):455. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9 (6):632. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 59.Tuovinen H, et al. Cutting edge: human CD4-CD8- thymocytes express FOXP3 in the absence of a TCR. J Immunol. 2008;180 (6):3651. doi: 10.4049/jimmunol.180.6.3651. [DOI] [PubMed] [Google Scholar]
- 60.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2 (4):301. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 61.Bensinger SJ, et al. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J Exp Med. 2001;194 (4):427. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med. 2004;200 (10):1221. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liston A, et al. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci U S A. 2008;105 (33):11903. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aschenbrenner K, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8 (4):351. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe N, et al. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436(7054):1181. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]; Ladi E, et al. Thymocyte-dendritic cell interactions near sources of CCR7 ligands in the thymic cortex. J Immunol. 2008;181 (10):7014. doi: 10.4049/jimmunol.181.10.7014. [DOI] [PubMed] [Google Scholar]
- 66.Ribot J, et al. Shaping of the autoreactive regulatory T cell repertoire by thymic cortical positive selection. J Immunol. 2007;179 (10):6741. doi: 10.4049/jimmunol.179.10.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kurobe H, et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24 (2):165. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 68.Ignatowicz L, Kappler J, Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84(4):521. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]; Hugo P, Kappler JW, Marrack PC. Positive selection of TcR alpha beta thymocytes: is cortical thymic epithelium an obligatory participant in the presentation of major histocompatibility complex protein? Immunol Rev. 1993;135:133. doi: 10.1111/j.1600-065x.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]; Pawlowski T, Elliott JD, Loh DY, Staerz UD. Positive selection of T lymphocytes on fibroblasts. Nature. 1993;364(6438):642. doi: 10.1038/364642a0. [DOI] [PubMed] [Google Scholar]; Yasutomo K, et al. The duration of antigen receptor signalling determines CD4+ versus CD8+ T-cell lineage fate. Nature. 2000;404 (6777):506. doi: 10.1038/35006664. [DOI] [PubMed] [Google Scholar]
- 69.Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23 (2):227. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 70.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204 (11):2521. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pacholczyk R, Kern J. The T-cell receptor repertoire of regulatory T cells. Immunology. 2008;125 (4):450. doi: 10.1111/j.1365-2567.2008.02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li QJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129 (1):147. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Lu LF, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30 (1):80. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bettini ML, Kersh GJ. MAP kinase phosphatase activity sets the threshold for thymocyte positive selection. Proc Natl Acad Sci U S A. 2007;104 (41):16257. doi: 10.1073/pnas.0705321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cobb BS, et al. A role for Dicer in immune regulation. J Exp Med. 2006;203 (11):2519. doi: 10.1084/jem.20061692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou X, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205 (9):1983. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsieh CS, et al. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7(4):401. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]; Fazilleau N, Bachelez H, Gougeon ML, Viguier M. Cutting edge: size and diversity of CD4+CD25high Foxp3+ regulatory T cell repertoire in humans: evidence for similarities and partial overlapping with CD4+CD25- T cells. J Immunol. 2007;179(6):3412. doi: 10.4049/jimmunol.179.6.3412. [DOI] [PubMed] [Google Scholar]; Wong J, et al. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007;178 (11):7032. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 78.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25 (2):249. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 79.Pacholczyk R, et al. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27 (3):493. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujishima M, Hirokawa M, Fujishima N, Sawada K. TCRalphabeta repertoire diversity of human naturally occurring CD4+CD25+ regulatory T cells. Immunol Lett. 2005;99 (2):193. doi: 10.1016/j.imlet.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 81.Pacholczyk R, Kraj P, Ignatowicz L. Peptide specificity of thymic selection of CD4+CD25+ T cells. J Immunol. 2002;168 (2):613. doi: 10.4049/jimmunol.168.2.613. [DOI] [PubMed] [Google Scholar]
- 82.DiPaolo RJ, Shevach EM. CD4+ T-cell development in a mouse expressing a transgenic TCR derived from a Treg. Eur J Immunol. 2009;39 (1):234. doi: 10.1002/eji.200838772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17 (6):638. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6 (12):1219. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 85.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389 (6652):737. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 86.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18 (2):120. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 87.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 88.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lan RY, Ansari AA, Lian ZX, Gershwin ME. Regulatory T cells: development, function and role in autoimmunity. Autoimmun Rev. 2005;4 (6):351. doi: 10.1016/j.autrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 90.Maloy KJ, et al. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197(1):111. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zheng SG, et al. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172 (3):1531. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 91.Jaeckel E, Kretschmer K, Apostolou I, von Boehmer H. Instruction of Treg commitment in peripheral T cells is suited to reverse autoimmunity. Semin Immunol. 2006;18 (2):89. doi: 10.1016/j.smim.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 92.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8 (5):457. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]