Abstract
Objectives
We examined outcomes of patients with desmoid tumors receiving systemic therapy at a single institution to provide a basis for examination of newer agents.
Methods
We reviewed records of patients with desmoid tumors treated with chemotherapy at our institution. The activity of NSAIDs was not addressed. Patients without measurable disease, those receiving therapy we could not document, and those receiving prophylactic therapy were excluded.
Results
Sixty-eight patients received 157 lines of therapy. Nine patients died, 7 of progressive disease. The cohort was 62% female with median age 32.5, 32% with Gardner syndrome, median follow-up 63 months, and median of 2 lines of therapy. Intra-abdominal primary location was most common (44%). The greatest RECIST response rate was observed with anthracyclines and hormonal therapy and lowest with single agent dacarbazine/temozolomide or tyrosine kinase inhibitors, principally imatinib. In a multivariate analysis, only nodular gross morphology and presence of Gardner syndrome were the only tumor factors associated with greater time to progression.
Conclusions
Anti-estrogens and anthracycline-containing regimens are associated with a higher radiological response rate against desmoid tumors than other agents. Systemic therapy for desmoid tumors can be successful in patients with desmoids, and is a viable option in lieu of morbid or disabling surgery.
Keywords: Deep fibromatosis, desmoid tumor, systemic therapy, chemotherapy, hormonal therapy, retrospective analysis, anthracycline, imatinib
INTRODUCTION
Deep fibromatoses, also termed desmoid tumors, are rare, slow growing, histologically bland connective tissue neoplasms without metastatic potential1, 2. Incidence is estimated at 3-4 cases per million, with fewer than 1,000 cases diagnosed in the United States annually3. Desmoid tumors account for approximately 3% of all biopsy-analyzed soft-tissue tumors. Desmoids are typically diagnosed in patients between the ages of 15 and 60 years, more often in young adults, with a 2-3 fold female predominance. The incidence of desmoids is particularly high in patients with familial adenomatous polyposis (FAP); the reported incidence of desmoids in this patient population ranges widely (3.5-32%)3-7.
Although surgery remains the primary therapy for extra-abdominal and abdominal-wall desmoids, for static lesions observation alone is increasingly recommended, given the morbidity associated with resection and frequent recurrences8. Surgery is often not feasible for mesenteric desmoids because of the diffuse nature of the tumor throughout the mesentery in some patients, as well, a high risk of recurrence and frequent post-operative complications. Alternative options are therefore required for patients with tumors that are not amenable to surgery3, 9.
Though aggressive fibromatoses do not demonstrate metastatic potential, morbidity can lead to physical impairment, and mortality from the disease is occasionally observed from local infiltrative growth and tissue invasion, in particular with abdominal disease. Mortality is rare among patients with extra-abdominal desmoids. However, desmoids are associated with patient death in ~10% of patients with FAP3.
The natural history of this disease is increasingly well understood, but remains difficult to predict2, 8. Some patients progress despite all local-regional options for care. For patients with progressive disease no longer amenable to surgery or radiation, systemic therapy can play a significant role in improving clinical outcomes. To date, there are no comparative studies of systemic therapy to guide treatment of desmoid tumors. The purpose of this study was to analyze a highly selected group of patients with recurrent or unresectable desmoid tumors and their radiological responses to systemic treatment in order to guide treatment decisions when patients develop recurrences, and to provide a baseline for comparison for future clinical trials.
PATIENTS AND METHODS
Patient Selection
We identified 183 patients seen in medical oncology clinics at our hospital who received systemic therapy for a desmoid tumor between 1994 and 2007, using an institution IRB-approved Waiver of Authorization. Data were also available from an institutional database of patients with soft tissue tumors admitted for inpatient care, 304 of whom were admitted between 1994 and 2007. Case files and chemotherapy records were reviewed for all patients. A total of 115 patients were excluded because of incomplete records, administration of all chemotherapy outside our center, or the exclusive use of anti-inflammatory compounds as systemic therapy. This exclusion left 68 patients were analyzed for response to systemic treatment. Patient demographics, tumor characteristics, number of surgical resections, radiation therapy and systemic treatment response were analyzed. All pathology was reviewed at Memorial Sloan-Kettering Cancer Center and confirmed the diagnosis of deep fibromatosis (desmoid tumor). Measurements were extracted from departmental radiology reports; consulting radiologists at our institution confirmed measurements for images with incomplete data.
Demographics, definition of progression, and statistical methods
Data extracted as part of this retrospective analysis included age at diagnosis, gender, radiological desmoid morphology (nodular, diffuse), presence or absence of Gardner syndrome, primary site, primary size, other primary cancers diagnoses, use of radiation therapy, date of diagnosis, date of presentation, date of last follow up, survival date, survival status, lines of therapy, 1st and last dose dates of systemic therapies, duration of systemic therapy, reason for treatment discontinuation, date of last scan before initiation of a given systemic therapy, scan dates and outcomes on therapy, scan dates following discontinuation of therapy, date of progression, time to progression, overall survival, and best response to treatment by World Health Organisation (WHO) criteria10 and Response Evaluation Criteria in Solid Tumor (RECIST), version 1.011.
To calculate progression free survival, we defined progressive disease to be radiological worsening by the defined criteria (WHO or RECIST), or clinical progression in the setting of lesser radiological progression. For 142 of the 157 lines of therapy we had pre- and post-treatment radiographs. For the other 15, we had only radiology reports and clinic notes on which to base a determination of disease progression. Estimates of progression-free survival (PFS) were determined using Kaplan-Meier analysis (SPSS version 15.0), using a baseline scan date (performed within 0-5 weeks of the start of systemic therapy) as time 0 and restaging scan date as the progression date. Patients were censored for toxicity or surgical intervention. Overall survival data were compiled using the date of biopsy or date of initial surgical resection as time 0.
Prognostic significance of gender, primary site (abdomen vs. other), age (less than or greater than/equal to median), primary size, presence of Gardner syndrome, and nodular vs. diffuse desmoid type were examined by univariate analysis using a log-rank test. To examine the association between tumor features and PFS while adjusting for important prognostic factors, those variables found to be significant on univariate analysis at the 0.10 level were entered into a Cox proportional hazards model. Best radiological outcomes (partial response, stable disease, progression of disease) were compared between response criteria using a Fisher exact test. Best outcomes for different types of therapy (largest favorable percentage change in unidimensional or bi-dimensional tumor measurements) by response evaluation criteria (RECIST vs WHO) were compared using a Kruskal-Wallis or Wilcoxon signed rank test.
RESULTS
Patient population
Patient demographics are specified in Table 1. A total of 68 patients received 157 lines of radiologically well-documented systemic therapy at our institution between 1994 and 2008. The median age was 32.5 years (15-75) with a female predominance of 62% of the cases (n=42). Of the 68 patients, 11 had a primary tumor under 5 cm (16%), 19 (28%) between 5-10cm, 45, 34 (50%) over 10 cm, including 5 patients with multifocal desmoids (lesions affecting more than one primary site), as has been described previously12, and 4 (6%) with unknown primary size. The most common primary locations included intra-abdominal (n=30, 44%), followed by chest wall, n=11 (16%). Consistent with the high proportion of intra-abdominal primary tumors, 22 (32%) also carried a diagnosis of Gardner syndrome. Median follow up was 5.3 years (range 0.3-13.3). The primary size and anatomic site of patients treated with chemotherapy was different from patients from our institutional surgical database. Patients with desmoid tumors of the abdomen and retroperitoneum were over-represented in the chemotherapy cohort, and those with truncal desmoids under-represented in comparison to the patients admitted to hospital for care (Table 1). Not surprisingly, patients receiving chemotherapy had larger tumors overall in comparison to those who were admitted to hospital (generally for surgery).
Table 1.
Clinical characteristics of desmoid tumor patients treated with systemic therapy compared with those admitted to hospital (usually surgical patients)
| Number receiving systemic therapy (1994-2007) |
183 | ||||
| Number admitted for management (1994-2007) |
304 | ||||
| Excluded (incomplete records, therapy administered off site, anti-inflammatory agents only) |
115 | ||||
| Patients receiving all treatment on site |
68 | ||||
| Median age at presentation (range) |
32.5 (15-75) | 37 (15-79) | |||
| Number of women (%) | 42 (62%) | 203 (66%) | |||
| Median follow up, years (range) | 5.3 (0.3-13.3) | 3.3 (0.3-13.5) | |||
| Primary Size | < 5 cm (%) | 11 (16%) | 93 (31%) | ||
| 5-10 cm (%) | 19 (28%) | 114 (38%) | |||
| > 10 cm (%) | 34* (50%) | 84** (28%) | |||
| Unknown | 4 (6%) | 12 (4%) | |||
| Gardner syndrome | 22 (32%) | n.r. | |||
| Desmoid morphology | Diffuse | 17 (25%) | n.r. | ||
| Nodular | 51 (75%) | n.r. | |||
| Primary Site (%) | Head / Neck | 2 (3%) | 21 (5%) | ||
| Upper Extremity | 8 (12%) | 43 (9%) | |||
| Chest wall | 11 (16%) | 39 (9%) | |||
| Intra-abdominal | 36 (53%) | 66 (15%) | |||
| Trunk | 5 (7%) | 91 (20%) | |||
| Lower Extremity | 6 (9%) | 44 (10%) | |||
| Median number of surgeries (range) |
1 (0-5) | 1 (0-6) | |||
| No surgery (%) | 18 (26%) | n.r. | |||
| Median lines of therapy (range) | 2 (1-7) | n.r. |
includes 5 patients (7%) with multifocal disease
includes 13 patients (3%) with multifocal disease
n.r.: not recorded
Systemic therapy
While nonsteroidal anti-inflammatory agents (NSAIAs) were administered to some patients, we could not accurately document when the agents were administered, dosing, or compliance. Most commonly, patients were started on ibuprofen or sulindac following a resection as a prophylactic measure. We did not identify any patients with radiological responses to NSAIAs, and focused on other systemic therapy without recording their administration of NSAIAs.
Specific therapeutics and the order given were the physicians’ choices (Table 2). Patients who were treated had evidence of disease progression by physical examination or radiological examinations. The most common systemic therapies included tamoxifen, doxorubicin (including pegylated liposomal doxorubicin), and imatinib.
Table 2.
Type of chemotherapy administered and best RECIST and WHO responses
| Therapy administered | Lines of treatment given |
RECIST PR |
RECIST SD |
RECIST PD |
Median PFS† by RECIST (months), 95% CI |
WHO PR |
WHO SD |
WHO PD |
Median PFS† by WHO (months), 95% CI |
|---|---|---|---|---|---|---|---|---|---|
|
Anthracycline-
containing |
35 | 13 (37%) | 18 (51%) | 4 (11%) | * | 12 (34%) | 17 (43%) | 6 (23%) | * |
| Hormonal therapy | 26 | 6 (23%) | 17 (65%) | 3 (12%) | 12.0 (5.0-19.0) | 6 (23%) | 17 (65%) | 3 (12%) | 13.5 (6.8-20.2) |
|
Tyrosine Kinase
Inhibitor |
35 | 3 (9%) | 25 (71%) | 7 (20%) | 26.8 (0-61.4) | 2 (6%) | 22 (63%) | 11 (31%) | 21.4 (0-43.0) |
| DTIC or temozolomide | 16 | 2 (13%) | 12 (75%) | 2 (13%) | 14.3 (5.3-23.3) | 1 (6%) | 10 (63%) | 5 (31%) | 9.0 (0-19.2) |
|
Methotrexate (single
agent) |
12 | 4 (33%) | 6 (50%) | 2 (17%) | 9.4 (*) | 4 (33%) | 4 (33%) | 4 (33%) | * |
| Other cytotoxic agents | 8 | 0 | 7 (88%) | 1 (12%) | 3.7 (0-9.8) | 0 | 7 (88%) | 1 (12%) | 2.3 (0.5-4.1) |
|
Vinca-containing
combination |
10 | 2 (20%) | 6 (60%) | 2 (20%) | * | 2 (20%) | 5 (50%) | 3 (30%) | * |
| TOTAL | 142 | 30 (21%) | 91 (64%) | 21 (14%) | 14.1 (5.5-22.7) | 27 (19%) | 82 (58%) | 33 (23%) | 15.0 (5.6-24.4) |
Kaplan-Meier estimate
Median not reached, or not reached until last patient progressed, giving a meaningless value for the median or 95% CI.
CI: confidence interval
The 68 patients received 157 lines of therapy for which we could document clinical or radiological response. Of these, pre- and post-therapy images were available for review for 142 lines of therapy (i.e. radiological assessments 0-5 weeks before the start of therapy and scans after at least 6 weeks of therapy, except those with overt progression documented radiologically before 6 weeks). For the remaining 15 lines of therapy we used clinical and radiology records to determine time to progression. Radiological results were assessed by RECIST and WHO criteria. In many cases, after radiological (and symptomatic) improvement, systemic therapy was discontinued and patients followed radiologically and clinically. Time to progression was documented when there was progression by RECIST or WHO criteria, with patients being censored for drug toxicity or another local intervention.
The median lines of therapy given to a patient was 2 (range 1-7); median time to progression on all lines of therapy was 7.4 months (range 0.7-125). A total of 26 patients (38%) received one line of therapy, 22 (32%) received 2 lines, and 20 (29%) received 3 or more lines of therapy. The frequency of RECIST partial responses (PR) was different between arms of the study (p=0.048, Fisher exact test), suggesting choice of treatment was important to obtain a radiographic response.
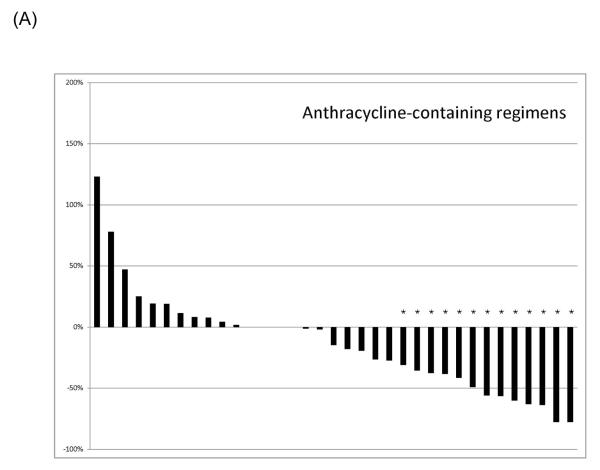
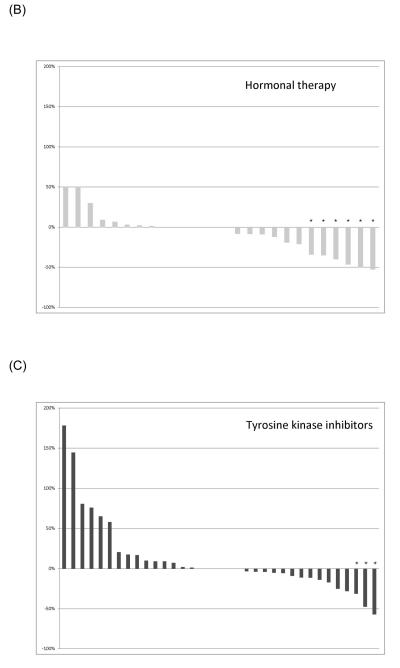
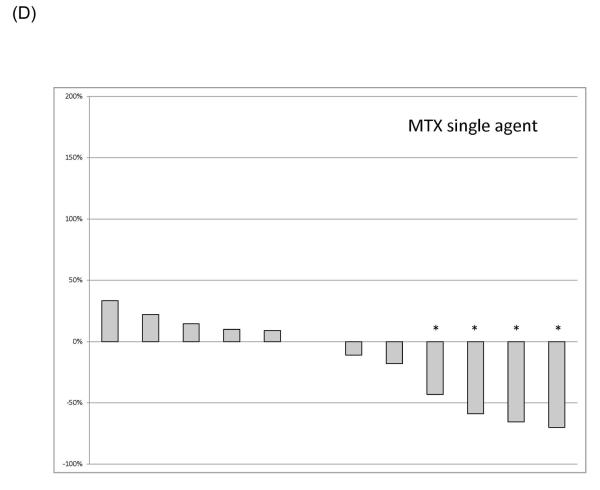
Responses were most common in patients receiving anthracycline-containing regimens. A waterfall plot of the best RECIST responses is indicated for different classes of systemic therapeutics in Figure 1. Twenty-eight patients of the 35 patients receiving anthracyclines received single agent doxorubicin in some form. Three of six patients receiving doxorubicin had a best result of RECIST PR, and 8/22 who received pegylated liposomal doxorubicin experienced a RECIST PR. Of the 35 patients receiving anthracyclines, one with a cumulative lifetime dose of 885 mg/m2 doxorubicin given over two years developed grade 3 cardiomyopathy, as did one patient (papillary muscle dysfunction) who received a total of 575 mg/m2 pegylated liposomal doxorubicin over 18 months; the latter patient also developed myelodysplastic syndrome, and is receiving darbepoetin support. Both had improvement in their cardiomyopathy with medical management.
Figure 1.
Best RECIST response of therapeutics used to treat desmoid tumors. (A) Anthracycline-containing regimens; (B) Tyrosine kinase inhibitors; (C) Vinca-containing combinations; (D) Hormonal therapy. An asterisk indicates a RECIST partial response.
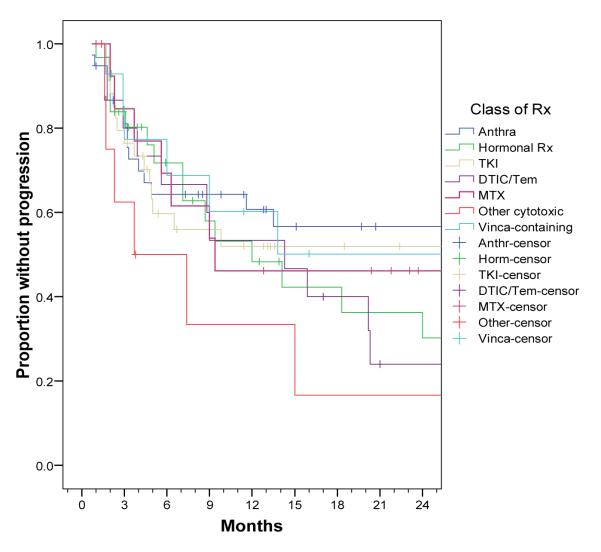
Progression-free survival (PFS)
PFS as observed for each line of systemic therapy is indicated in Figure 2, and median PFS by RECIST and by WHO criteria is indicated by type of therapy in Table 2. Given the selected nature of patients on the study, we did not examine type of systemic therapy or line of therapy and their relation to PFS. The variables independently predictive of superior PFS were nodular (vs. diffuse) type of desmoid, and presence of Gardner syndrome. On multivariate analysis, both variables remained statistically significant (Table 3).
Figure 2.
Progression free survival (RECIST) by type of systemic treatment
Table 3.
Univariate and multivariate analysis of variables associated with RECIST PFS.
| Univariate p-value |
Multivariate p-value (HR and 95% CI) |
|
|---|---|---|
| Gender | 0.93 | |
| Age | 0.60 | |
| Nodular (vs diffuse) | 0.008 | 0.002 (0.30 [0.14-0.63]) |
| Gardner syndrome (vs not) |
0.041 | 0.043 (0.57 [0.33-0.98]) |
| Primary site* | 0.39 | |
| Primary size† | 0.24 |
Italics: variables from univariate analysis selected for multivariate analysis
by anatomic location, intra-abdominal vs other
<5 cm, 5-10 cm, > 10 cm
HR: Hazard ratio
95% CI: Confidence interval for hazard ratio
RECIST and WHO responses
The best responses by WHO criteria and RECIST (Table 2) for each line of therapy were compared for patients in whom response data were available (n=142 lines of therapy). A best result of PR and PD was observed in 21% and 15% and by RECIST, respectively, and in 19% and 23% by WHO criteria. The median best radiological result for systemic therapy was 0% by WHO criteria and +3% by RECIST.
There was no apparent difference in best radiological outcome, comparing each best response result using RECIST and WHO criteria. (2-sided p-value=0.69, Wilcoxon signed rank test). In contrast to the response data by type of therapeutic agent, categorizing best radiological responses (PR, SD, or PD) by response criteria (WHO or RECIST) did not yield a significant difference (p=0.19, Fisher exact test)
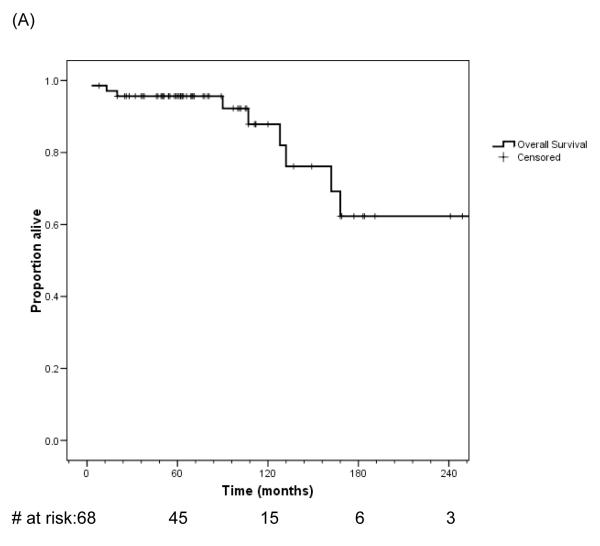
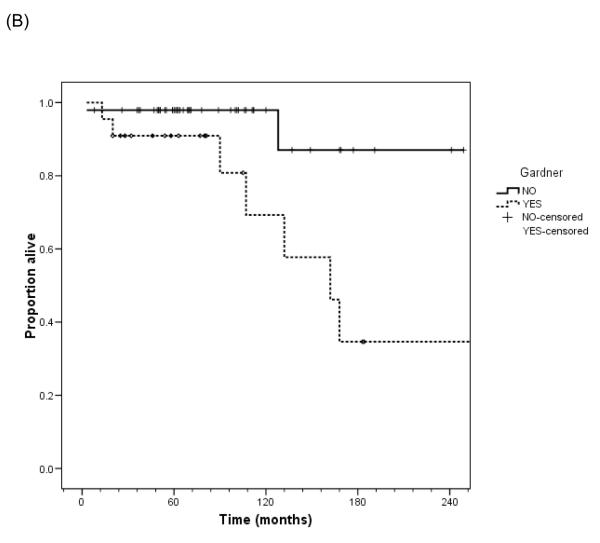
Overall survival
The high risk nature of this group of patients is indicated in Figures 3A and 3B. Nine patients died of local progression from their desmoid tumors. Two others died from primary peritoneal serous tumors associated with Gardner syndrome, but were censored in this analysis at time of death from their carcinomas. Eight of the nine patients died from a primary abdominal or abdominal wall desmoid; one died of local progression at the thoracic outlet. Seven of the patients had desmoids associated with Gardner syndrome, in comparison to the overall group of 22 of 68 patients with Gardner syndrome in association with their desmoid. Desmoid-related mortality was significantly associated with Gardner syndrome (p=0.01, log-rank test). Median survival of the patients with desmoid tumor in association with Gardner syndrome in this cohort was 13.5 years (95% confidence interval 9.3-17.7).
Figure 3.
Overall survival and after diagnosis of desmoid tumor in this patient cohort for all patients (A) and by presence or absence of Gardner syndrome (B).
DISCUSSION
Given the relative toxicity of the choices for systemic therapy, we generally treat patients with a course of hormonal therapy before considering cytotoxic agents or tyrosine kinase inhibitors. Beyond anti-estrogens13, an important report of the utility of the CyVADIC (cyclophosphamide, vincristine, doxorubicin and dacarbazine) has made a significant impact regarding systemic therapy for desmoid tumors14. The finding of patient responses to tyrosine kinase inhibitors15 raised the question of the relative activity of systemic agents in this patient population. Since there was not clear synergy between the drugs in this combination, we have generally opted to give single agents for patients with desmoid tumors in need of systemic therapy, occasionally using combinations for patients with more symptomatic tumors. Until there are randomized studies to examine one form of therapy against another, we hope that the data of this report will inform clinicians as to options for care when systemic therapy is needed.
Reservations regarding the present data, like those of any retrospective analysis, include the lack of randomized data, use of a non-standard metric for progression using both radiographic and clinical change, and collection of data over nearly 15 years, during which time options and quality of surgery, radiation therapy, radiology, and supportive medication have improved, potentially biasing any conclusions. Furthermore, the occasional observation of a patient with spontaneous improvement in their desmoid tumor, or responses to NSAIA, neither of which we take into account in this analysis, would only lower the observed response rates to systemic therapy noted here. These data show a higher radiological response rate of anthracycline based therapy and hormonal therapy, more than receptor tyrosine kinase (RTK)-directed agents or single agent dacarbazine or temozolomide against desmoids, consistent with other non randomized data from other centers9, 13-18.
We note that as single agents, both doxorubicin and liposomal pegylated doxorubicin are active against desmoid tumors. In an appropriate patient, typically failing hormonal therapy, liposomal pegylated doxorubicin presents a reasonable option with a lower risk of cardiac and other toxicity compared to the parent agent, and is now our typical 1st line of cytotoxic therapy for patients in need of such an agent. Given the favorable side effect profile and response data, hormonal therapy would appear to be the most appropriate comparator arm for a randomized study of systemic therapeutic agents.
The difficulty of managing desmoid tumors in association with Gardner syndrome is also underscored by these data, with a significant risk of death from desmoid tumor in this population in comparison to patients with desmoid tumors that arise sporadically in this population, despite the demonstration of an association with better PFS in desmoid tumor patients with Gardner syndrome vs. those who do not. It is also clear that mutation analysis of APC and CTNNB1 will be an important future analysis of these tumor samples19-23, which could potentially better inform choice of systemic therapy in the future.
The management of desmoid tumors remains multidisciplinary, necessitated by the diversity of patient presentations and treatment options2, 7. Caution must be exercised at every step of treatment given the potential morbidity of any therapy, be it surgery (functional loss, recurrence risk), radiation (loss of function as a late side effect, risk of secondary cancers such as sarcomas), or chemotherapy (cardiomyopathy, or treatment-associated myelodysplasia or leukemias, as observed in this study). We anticipate improvements in outcomes for patients as we learn about the molecular characteristics of molecularly heterogeneous condition. In the meantime, with these data we have demonstrated that systemic therapy is a viable option in lieu of potentially morbid and debilitating surgery for this frustrating diagnosis.
Acknowledgments
This work is supported in part by Program Project Grant P01 CA47179, and philanthropic contributions from Cycle for Survival and the Sarcoma Research Fund. We are grateful to members of the Department of Radiology at MSKCC who contributed their time for review of some of the scans included in this study, Paul Meyers for review of the manuscript, and Nicole Moraco, Alisa Pinkhasik, and Barbara MacLean for database support.
Footnotes
This work was previously presented in part at the 14th Annual Connective Tissue Oncology Society meeting, London, UK, November 13-15, 2008, and at the ASCO 45th Annual meeting, Orlando, FL, May 29-June 2, 2009.
REFERENCES
- 1.Alman BA, Pajerski ME, Diaz-Cano S, Corboy K, Wolfe HJ. Aggressive fibromatosis (desmoid tumor) is a monoclonal disorder. Diagn Mol Pathol. 1997;6(2):98–101. doi: 10.1097/00019606-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Lewis JJ, Boland PJ, Leung DH, Woodruff JM, Brennan MF. The enigma of desmoid tumors. Ann Surg. 1999;229(6):866–72. doi: 10.1097/00000658-199906000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosalkar HS, Torbert JT, Fox EJ, Delaney TF, Aboulafia AJ, Lackman RD. Musculoskeletal desmoid tumors. J Am Acad Orthop Surg. 2008;16(4):188–98. doi: 10.5435/00124635-200804000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Nieuwenhuis MH, De Vos Tot Nederveen Cappel W, Botma A, Nagengast FM, Kleibeuker JH, Mathus-Vliegen EM, et al. Desmoid tumors in a dutch cohort of patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6(2):215–9. doi: 10.1016/j.cgh.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Bertario L, Russo A, Sala P, Varesco L, Giarola M, Mondini P, et al. Multiple approach to the exploration of genotype-phenotype correlations in familial adenomatous polyposis. J Clin Oncol. 2003;21(9):1698–707. doi: 10.1200/JCO.2003.09.118. [DOI] [PubMed] [Google Scholar]
- 6.Clark SK, Neale KF, Landgrebe JC, Phillips RK. Desmoid tumours complicating familial adenomatous polyposis. Br J Surg. 1999;86(9):1185–9. doi: 10.1046/j.1365-2168.1999.01222.x. [DOI] [PubMed] [Google Scholar]
- 7.Lev D, Kotilingam D, Wei C, Ballo MT, Zagars GK, Pisters PW, et al. Optimizing treatment of desmoid tumors. J Clin Oncol. 2007;25(13):1785–91. doi: 10.1200/JCO.2006.10.5015. [DOI] [PubMed] [Google Scholar]
- 8.Bonvalot S, Eldweny H, Haddad V, Rimareix F, Missenard G, Oberlin O, et al. Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol. 2008;34(4):462–8. doi: 10.1016/j.ejso.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Gega M, Yanagi H, Yoshikawa R, Noda M, Ikeuchi H, Tsukamoto K, et al. Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol. 2006;24(1):102–5. doi: 10.1200/JCO.2005.02.1923. [DOI] [PubMed] [Google Scholar]
- 10.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Fong Y, Rosen PP, Brennan MF. Multifocal desmoids. Surgery. 1993;114(5):902–6. [PubMed] [Google Scholar]
- 13.Hansmann A, Adolph C, Vogel T, Unger A, Moeslein G. High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer. 2004;100(3):612–20. doi: 10.1002/cncr.11937. [DOI] [PubMed] [Google Scholar]
- 14.Patel SR, Evans HL, Benjamin RS. Combination chemotherapy in adult desmoid tumors. Cancer. 1993;72(11):3244–7. doi: 10.1002/1097-0142(19931201)72:11<3244::aid-cncr2820721118>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Heinrich MC, Joensuu H, Demetri GD, Corless CL, Apperley J, Fletcher JA, et al. Phase II, open-label study evaluating the activity of imatinib in treating life-threatening malignancies known to be associated with imatinib-sensitive tyrosine kinases. Clin Cancer Res. 2008;14(9):2717–25. doi: 10.1158/1078-0432.CCR-07-4575. [DOI] [PubMed] [Google Scholar]
- 16.Heinrich MC, McArthur GA, Demetri GD, Joensuu H, Bono P, Herrmann R, et al. Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumor) J Clin Oncol. 2006;24(7):1195–203. doi: 10.1200/JCO.2005.04.0717. [DOI] [PubMed] [Google Scholar]
- 17.Okuno SH, Edmonson JH. Combination chemotherapy for desmoid tumors. Cancer. 2003;97(4):1134–5. doi: 10.1002/cncr.11189. [DOI] [PubMed] [Google Scholar]
- 18.Baker LH, Wather K, Chugh R, Thomas D, Thall PF, Maki RG, et al. Activity of imatinib mesylate in desmoid tumors: Interim analysis of a Sarcoma Alliance for Research thru Collaboration (SARC) phase II trial. J Clin Oncol. 2004;22(14S) Abstract 9013. [Google Scholar]
- 19.Scott RJ, Froggatt NJ, Trembath RC, Evans DG, Hodgson SV, Maher ER. Familial infiltrative fibromatosis (desmoid tumours) (MIM135290) caused by a recurrent 3′ APC gene mutation. Hum Mol Genet. 1996;5(12):1921–4. doi: 10.1093/hmg/5.12.1921. [DOI] [PubMed] [Google Scholar]
- 20.Alman BA, Li C, Pajerski ME, Diaz-Cano S, Wolfe HJ. Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors) Am J Pathol. 1997;151(2):329–34. [PMC free article] [PubMed] [Google Scholar]
- 21.Lazar AJ, Tuvin D, Hajibashi S, Habeeb S, Bolshakov S, Mayordomo-Aranda E, et al. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173(5):1518–27. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caspari R, Olschwang S, Friedl W, Mandl M, Boisson C, Boker T, et al. Familial adenomatous polyposis: desmoid tumours and lack of ophthalmic lesions (CHRPE) associated with APC mutations beyond codon 1444. Hum Mol Genet. 1995;4(3):337–40. doi: 10.1093/hmg/4.3.337. [DOI] [PubMed] [Google Scholar]
- 23.Tejpar S, Nollet F, Li C, Wunder JS, Michils G, dal Cin P, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor) Oncogene. 1999;18(47):6615–20. doi: 10.1038/sj.onc.1203041. [DOI] [PubMed] [Google Scholar]