Abstract
p120-catenin (p120) is required for cadherin stability and is thought to have a central role in modulating cell-cell adhesion. Several lines of evidence suggest that S/T phosphorylation may regulate p120 activity, but the upstream kinases involved have not been established, nor has a discreet measurable function been assigned to an individual site. To approach these issues, we have generated p120 phospho-specific monoclonal antibodies to several individual phosphorylation sites and are using them to pinpoint upstream kinases and signaling pathways that control p120 activity. Protein Kinase C (PKC) has been implicated as a signaling intermediate in several cadherin-associated cellular activities. Signaling events that activate PKC induce rapid phosphorylation at p120 Serine 879 (S879), suggesting that p120 activity is regulated, in part, by one or more PKC isoforms. Here, we find that physiologic activation of a G-protein coupled receptor (i.e., endothelin receptor), as well as several Receptor Tyrosine Kinases, induce rapid and robust p120 phosphorylation at S879, suggesting that these pathways crosstalk to cadherin complexes via p120. Using Va2 cells and PDGF stimulation, we show for the first time that PDGFR-mediated phosphorylation at this site is dependent on PKCα, a conventional PKC isoform implicated previously in disruption of adherens junctions.
INTRODUCTION
p120-catenin (hereafter referred to as p120) is the prototypic member of a p120 subfamily of Armadillo repeat (Arm) domain proteins, including ARVCF, delta-catenin, p0071, and the more distantly related plakophilins (reviewed in [1]). p120 was originally described as a substrate for Src- and Receptor-Tyrosine Kinases (RTKs) [2, 3] and was later identified as a catenin [4, 5], one of several cytoplasmic proteins that interact physically and functionally with cadherins [6]. The interaction is mediated by p120 Arm repeats 1–7 [7] and the juxtamembrane domain (JMD) of the cadherin cytoplasmic tail [8, 9]. The importance of this interaction is reflected in part by the high degree of conservation of the JMD among cadherins. Unlike p120, β-catenin and γ-catenin (or plakoglobin) bind tightly in a mutually exclusive fashion to the Carboxy-terminal (C-terminal) catenin binding domain (CBD) of cadherins. Through binding to α-catenin, they appear to physically and functionally link the cadherin complex to the actin cytoskeleton [10–15]. In contrast, p120 influences adhesive strength by regulating cadherin stability and retention at the cell surface [7, 16, 17].
Several lines of evidence suggest that p120 function is regulated by phosphorylation. Since p120 was first identified as a Src substrate, much of the data linked to p120 phosphorylation has focused on the tyrosine residues and the role of the amino-terminus (N-terminus) in the regulation of cadherin-associated phenotypes. Indeed, p120 is phosphorylated at numerous sites by various Src family kinases and RTKs [2–4, 18, 19]. Although the roles for individual phosphorylation sites have not been pinpointed, most of the phosphorylation occurs within a 300 amino acid segment of the N-terminus termed the “regulatory domain” [20, 21], a region implicated in several p120 activities, including adhesion [7, 22], coupling to Rho [23–25], and growth factor induced cell scattering [26]. Additionally, p120 is modified by serine/threonine (S/T) kinases [20, 21], and evidence suggests that p120 S/T phosphorylation may also regulate cell adhesion. In many epithelial and endothelial cell lines, pharmacologic activation of PKC by PDBu induces significant p120 S/T dephosphorylation [27, 28]. Several physiologically important ligands such as thrombin, LPA, histamine, and VEGF show the same effect, implying a role for p120 S/T phosphorylation in vascular permeability and leukocyte transcytosis [27–29]. Despite these observations, the signaling pathways mediating p120 phosphorylation are not well understood.
One obstacle is that PKC activation induces a combination of p120 S/T phosphorylation and dephosphorylation events affecting at least eight sites [21, 30, 31]. In an effort to address the complexities associated with p120 phosphorylation, we have mapped the individual p120 phosphorylation sites and have generated phospho-specific antibodies to these sites. Using these reagents, we can now delineate signaling pathways mediating p120 phosphorylation and perhaps better understand how these events are regulated.
A further complication is that PKC constitutes an entire family of S/T kinases involved in multiple cellular processes and signal transduction pathways regulating cell proliferation, migration, differentiation, apoptosis, and angiogenesis (reviewed in [32–35]). There are nine PKC genes, which encode for three classes of PKC isozymes: classical/conventional PKCs (cPKCS: PKCα, PKCβ, PKCγ), novel PKCs (nPKCs: PKCδ, PKCε, PKCη, PKCθ), and atypical PKCs (aPKCs: PKCζ, and PKCι/λ). Conventional PKCs contain a diacylglycerol (DAG)/phorbol ester-binding C1 domain and a calcium-binding C2 domain. Thus, they require both calcium and DAG or phorbol esters for activation. Novel PKCs lack the functional C2 domain required for calcium binding, and they are activated by phorbol esters or DAG but do not require calcium. Unlike the first two classes, atypical PKCs are not responsive to either calcium or DAG, and their exact mechanism of activation remains somewhat elusive. Another structurally similar S/T kinase, PKD1/PKCμ, was initially classified as a nPKC but was later grouped into a distinct kinase family. All PKC family members share a basic structural homology with an N-terminal regulatory domain and a C-terminal catalytic domain. Most PKCs are ubiquitously expressed, but some isoforms have distinct tissue distributions [35]. They also exhibit distinct subcellular localizations, activation methods, and substrate specificities. In general, inactive PKCs are cytoplasmic and translocate to various membrane compartments upon activation [36]. The classical method of PKC activation occurs through stimulation of RTKs or G-Protein Coupled Receptors (GPCRs). Receptor activation leads to tyrosine phosphorylation and activation of Phospholipase C (PLC), which leads to the production of DAG and intracellular calcium [37].
Given the previously described complexities of PKC in both phosphorylation and dephosphorylation of p120, phenotypes observed with the use of general PKC inhibitors/activators are difficult to attribute to a single phosphorylation event. Thus, it is important to use more directed studies to identify which PKC isoform(s) is responsible for initiating the signaling response. With the correlative evidence for involvement of p120 S/T phosphorylation in disease states of decreased adhesion triggered by inflammation, and with the known roles of PKC in cancer, it is likely that p120 is a critical component in numerous signaling pathways. As one approach to study upstream mediators of p120, we have chosen to examine the site with the most prominent PKC-induced modification, S879. This site is particularly interesting because of its distinction from the other S/T sites: S879 is the only S/T site phosphorylated in response to PKC activation, it is C-terminally located, and it is one amino acid upstream of an alternatively spliced exon of unknown function. With the robust PKC-induced phosphorylation and specific detection with mAb pS879, measuring phosphorylation at this site provides an excellent assay for determining upstream mediators of p120 activity. Given the potential coordinated p120 phosphorylation and dephosphorylation, it is likely that the upstream modifiers of S879 phosphorylation also affect other p120 phosphorylation events. Additionally, by better understanding the pathway(s) mediating p120 phosphorylation, we could use this information as a clue to the associated function.
Here, we have used a phospho-specific antibody to p120 S879 to identify signaling components that crosstalk to the cadherin complex via p120 S879. The data demonstrate that S879 phosphorylation is a rapid, tightly regulated event downstream of both RTK and GPCR activation. This phosphorylation is PKC-dependent, and we have identified PKCα as the critical mediator of S879 phosphorylation in Va2 cells. These data advance our understanding of the signaling events that regulate p120 and suggest a novel role for PKCα.
Materials and methods
Cell culture and cell lines
Cells were cultured in DMEM (Hyclone) containing 10% Fetalplex III (Hyclone) and 1% penicillin-streptomycin (Gibco/Invitrogen). A431 epidermoid carcinoma cells (human) and Va2 fibroblasts (human) were obtained from the American Type Culture Collection (ATCC). NHEM (human melanocytes) cells were obtained from Cascade Biologics, and SkMel28 (human melanoma) cells were a gift from Dr. Ann Richmond. Phoenix 293 cells (amphotropic) were obtained from Dr. Linda Sealy (Vanderbilt University) with permission from Dr. Garry Nolan (Stanford University). Phoenix 293 media contained heat-inactivated Fetalplex III.
Transfections, retroviral transductions, and constructs
For retroviral studies, plasmids were transfected into Phoenix 293 cells using the calcium phosphate transfection method as described previously [16]. Phoenix cells were then selected with puromycin to obtain a population of cells stably expressing the construct of interest and producing retrovirus. Following stable transfection of the construct of interest into the Phoenix 293 packaging cell line, virus was harvested and target cells were infected as described previously [16]. For shRNA knockdown studies, pRetro-Super (pRS) constructs were used. To produce stable cell lines, target cells were selected with puromycin.
The pSuper PKCδ shRNA (Addgene plasmid 10819) [38] was purchased from Addgene and was subcloned into pRS using EcoRI, HindIII restriction sites. The pSUPER.retro.puro PKCα (containing the double-stranded hairpin oligonucleotide AGGCUGAGGUUGCUGAUG) construct was provided by Dr. Mitch Denning (Loyola University Chicago) [39].
Lentiviral transductions
For lentiviral studies, PKCα (NM_002737) Mission human shRNA bacterial glycerol stocks were purchased from Sigma. Five PKCα-specific target sequences were provided. One day prior to transfection, ~2×106 HEK293T cells were plated into 100mm plates. 5–10 min. prior to transfection, 30µl of 25mM chloroquine was added to the plate. For each transfection, the following reagents were added in order to a 1.5ml tube: 20µg pLKO.1 shRNA vector, 15µg packaging plasmid (pCMV-dR7.74psPAX2), 6µg envelope plasmid (pMD2.G), 62µl 2M calcium chloride, sterile D/D water for total volume of 500µl, and 500µl 2XHBS, pH 7.0. Samples were mixed, and the cocktail was added dropwise to each plate. The transfection was allowed to proceed 7 hours at 37°C, and fresh medium was added to the plates.
Approximately 48 hours after transfection, lentivirus-containing media was harvested, and target cells were infected as described previously [16] except that 2mg/ml hexadimethrine was added to the virus instead of polybrene prior to infection. The day following infection, target cells were selected with puromycin. For all lentiviral transfections, pLKO-Tubro-GFP and pLKO-Non-target (Sigma) were used as controls.
Reagents and cell treatments
Bisindolylmaleimide I (Bis I) (B6292), Amphiregulin (AR) (A7080), Epidermal Growth Factor (EGF) (E9644), Endothelin-1 (ET-1) (E7764), and BAPTA-AM (A1076) were purchased from and Sigma-Aldrich. Platelet-Derived Growth Factor-BB (PDGF-BB) (GF018) was purchased from Chemicon/Millipore. Phorbol-12-myristate-13-acetate (PMA) (524400), Gö6983 (365251), and Gö6976 (365250) were purchased from EMD Biosciences. All reagents were prepared as a stock solution and stored in aliquots at −20°C until use.
Prior to treatment with PMA or growth factor, cells were serum starved in DMEM containing 0.1% FBS for 16 hours. PMA treatments were for 30 min, and PDGF and other growth factor treatments were for 10 min unless otherwise noted. Concentrations used, unless otherwise indicated, were 200nM PMA and 50ng/ml PDGF. For all inhibitor studies, cells were pre-treated with inhibitor for 5 min prior to the addition of agonist.
For calcium depletion studies, cells were serum starved 16 hours in 0.1% FBS and were then treated 30 min prior to and during PDGF stimulation with CFM, 2mM EGTA, or CFM containing 15µM BAPTA-AM.
Western blotting
Cells were washed once with PBS prior to lysis (5 min at 0°C) in RIPA buffer (50mM Tris, pH 7.4, 150mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 2mM EGTA, 5mM EDTA) containing the protease and phosphatase inhibitors (30mM NaF, 20mM sodium pyrophosphate, 1mM PMSF, 5ug/ml leupeptin, 2ug/ml aprotinin, and 1mM sodium orthovanadate). Lysates were cleared by microcentrifugation, and total protein levels between samples were measured by BCA assay (Pierce) and normalized. Whole cell lysate samples used for Western blotting were prepared by adding 10X Laemmli sample buffer (LSB) for a final concentration of 2X LSB and boiling for 5 minutes at 100°C.
Denatured proteins were separated by SDS-PAGE and transferred to PROTRAN nitrocellulose (Perkin Elmer) for Western blotting. Membranes were blocked in Odyssey blocking buffer (LI-COR) diluted 1:1 in Tris-Buffered Saline (TBS) for 1 hour and then incubated overnight at 4°C in primary antibody diluted in blocking solution. For Western blotting, primary antibodies were used at the following concentrations: Total p120 (F1αSH, 0.1 µg/ml) [4], pS879 (BD Transduction, 0.5 µg/ml) [31], anti-tubulin mAb DM1a (Sigma, 1:10000), anti-pERK (CST, 1:2000), anti-PKCδ (BD Transduction, 1:500), and anti-PKCα (BD Transduction, 1:1000). After TBS-Tween (TBST) washes, membranes were incubated for 30 minutes with labeled secondary antibodies diluted 1:10,000 in blocking solution. Secondary antibodies used were Alexa Fluor 680 Goat anti-Mouse IgG (Molecular Probes) and IRDye 800 Goat anti-Rabbit IgG (Rockland Labs). Membranes were imaged by scanning on an Odyssey Infrared Imaging System (LI-COR) using default settings. All antibodies were optimized for use on Odyssey to ensure accurate two-color imaging and lack of cross reactivity between the reagents used for simultaneous detection. Quantitation was performed using the Odyssey scanner and software. Total p120 and S879-phosphorylated p120 were quantitated separately, and levels of S879 phosphorylation were normalized for total p120.
RNA isolation and RT-PCR
Total RNA was isolated from fresh or frozen cell pellets using RNeasy kit with DNase treatment step according to the manufacturer’s recommendations (Qiagen). 2µg RNA equivalent, 1µl RNasin, and 2µl Oligo-dT were used for RNA denaturation at 70°C for 5min. First strand cDNA was synthesized in a 24µl volume containing 15.5µl denatured RNA, 5.0µl superscript II first strand buffer, 2.5µl 0.1M DTT, 10mM of each dNTP. The mixture was incubated at 42°C for 3 min, 1µl superscript II enzyme was added, then the mixture was incubated at 50°C for 1 hour, 72°C for 15 min, and 35°C for 3 min. 1µl RNase H was added and the mixture was incubated at 37°C for 30 min. cDNA was stored at −20°C.
The PCR mixture (25µl) mixture consisted of 0.5µM forward primer (100µM stock), 0.5µl reverse primer (100µM stock), 2µl cDNA, 9.5µl water, and 12.5µl GoTaq Green Master Mix (Promega). Sets of primers for isoform-specific PCR products were synthesized by IDT and have been previously described [40]. After initial denaturation at 94°C for 5 min, amplification was performed at 94°C for 1 min, 55°C for 2 min, and 72°C for 1 min for 35 cycles using a thermal cycler, followed by a final elongation step at 72°C for 10 min. The amplified PCR product was size-fractionated by electrophoresis on a 2% polyacrylamide gel stained with ethidium bromide and was imaged with the FluorChem imaging system (Alpha Innotech).
Results
PDGF induces p120 S879 phosphorylation in Va2 fibroblasts
p120 S879 phosphorylation is induced in response to phorbol ester treatment, but physiologically relevant signaling pathways have not been described. To establish a more physiologically relevant model system for studying the signal transduction pathways that mediate S879 phosphorylation, we screened numerous cell lines in response to a variety of receptor agonists. We found that in the Va2 human fibroblast cell line, as previously seen in multiple other cell lines, PMA induced robust S879 phosphorylation (Fig. 1A). We also found that stimulation with the PDGFR agonist, PDGF, induced striking S879 phosphorylation (Fig. 1A). To control for agonist activity, membranes were probed for phospho-ERK, a downstream effector of the PDGFR (Fig. 1A). To examine the kinetics of the PDGF-elicited signal, S879 phosphorylation was examined at indicated timepoints of PDGF stimulation (Fig. 1B). PDGF-induced S879 phosphorylation occurred within one minute of stimulation (Fig. 1B), consistent with the timeframe required for PKC activation by the classical model (described in the Introduction). The signal peaked at 3–5 minutes and returned to basal levels after 30 minutes. These data suggest that Va2 cells are a useful model for pinpointing the PKC isoform(s) responsible for p120 phosphorylation at S879.
Fig. 1. PDGF induces S879 phosphorylation in Va2 fibroblasts.
(A) Va2 human fibroblasts were treated with 200nM PMA for 30 minutes or with 50ng/ml PDGF for 10 minutes prior to lysis. Lysates were analyzed by SDS-PAGE and Western blotting using a total p120 antibody (F1αSH), pS879, and pERK. Equal loading was verified by probing for tubulin. (B) Timecourse of S879 phosphorylation in response to PDGF. Va2 cells were treated with 50ng/ml PDGF for the indicated times, and lysates were analyzed as indicated in (A). Onset and duration of S879 phosphorylation was analyzed by probing with pS879 antibody. To verify equal loading, both total p120 and tubulin antibodies were used.
PDGF-induced S879 phosphorylation is PKC-dependent
To establish whether S879 phosphorylation in Va2 cells is PKC-dependent, we down-regulated or inhibited PKC activity. A classic indicator of PKC dependence is the ability of prolonged phorbol ester exposure to down-regulate conventional and novel PKCs. In Fig. 2A, PKCs were down-regulated by treating Va2 cells with PMA for 24 hours (lanes 3, 4, 7 & 8). Cells were then re-stimulated with PMA (lanes 3 & 4) or PDGF (lanes 7 & 8), and the capacity of these signals to elicit S879 phosphorylation was examined by Western blotting. In control samples, both PMA and PDGF induced S879 phosphorylation (lanes 2 & 6). However, when PKC activity was down-regulated (24h PMA samples), S879 phosphorylation was markedly reduced (lanes 4 & 8). The level of S879 phosphorylation in the PMA stimulated lanes remained slightly above that of untreated control samples (lanes 1 & 5), probably because 24 hour PMA treatment does not completely abolish PKC activity. ERK signaling was also reduced (compare lanes 4 & 8 to lanes 2 & 6), consistent with observations by others [41]. These data suggest that prolonged phorbol ester exposure efficiently down-regulated PKC expression in this system, and that S879 phosphorylation was dependent on PKC.
Fig. 2. PDGF-induced S879 phosphorylation is PKC-dependent.
(A) Conventional and novel PKC isoform activity was down-regulated by 24 hour PMA (200nM) treatment in Va2 fibroblasts (lanes 3, 4, 7, & 8). Control and PKC-down-regulated cells were then left untreated (−) or were stimulated with fresh PMA (200nM) or PDGF (50ng/ml) as indicated (+). The level of S879 phosphorylation in response to stimulus was analyzed by SDS-PAGE and Western blot. To verify loading and to control for receptor activation, tubulin and pERK antibodies, respectively, were used. (B) Va2 cells were treated with the following PKC inhibitors 5 min prior to and during a 10 min incubation in the presence (+) or absence (−) of PDGF: 1µM BisI, 1µM Gö6983, or 1µM Gö6976. Cells were lysed and analyzed by SDS-PAGE and Western blot as indicated in (A).
Next, we examined the effect of specific PKC inhibitors on PDGF-induced S879 phosphorylation (Fig. 2B). Va2 cells were pre-treated with three PKC inhibitors in the absence (−) or presence (+) of PDGF. Bis I and Gö6983 are general PKC inhibitors, while Gö6976 is more specific for the conventional PKC isoforms, particularly at concentrations below 1µM. Treatment with all three inhibitors blocked PDGF-induced S879 phosphorylation (compare lanes 4, 6, & 8 to lane 2). The data from Figures 1 and 2 definitively show that PKC is a critical component in the pathway from PDGFR to p120 S879 phosphorylation.
A conventional, calcium-dependent PKC isoform mediates p120 S879 phosphorylation
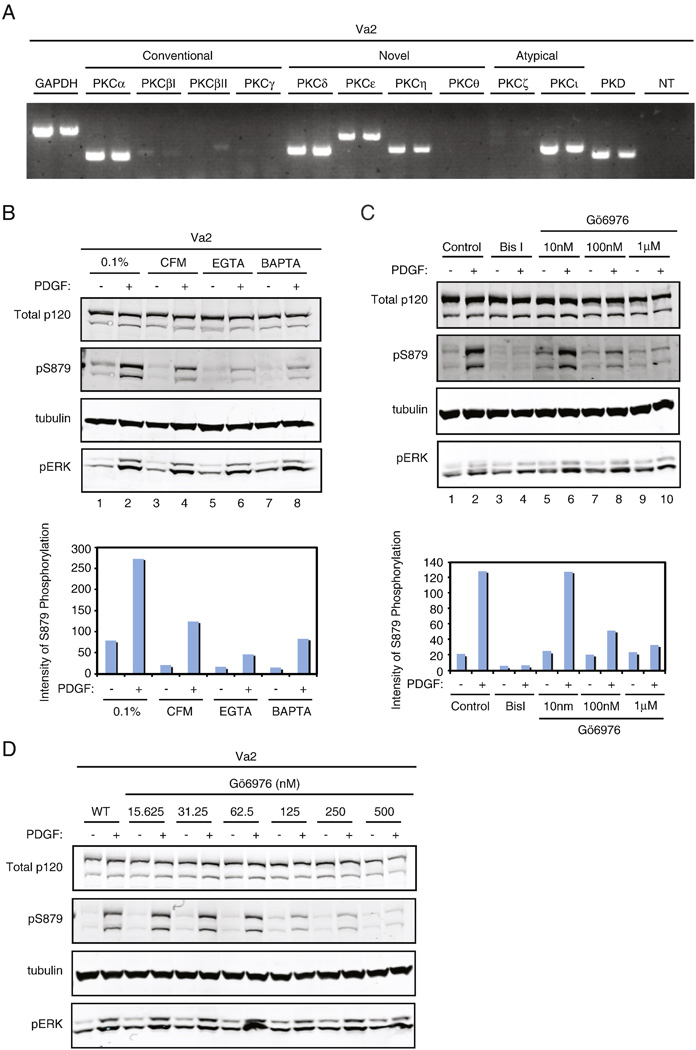
With the establishment of S879 phosphorylation as a PKC-dependent event, we next wanted to determine the PKC isoform expression profile in Va2 cells. RT-PCR was performed on mRNA isolated from Va2 cells using a panel of isoform-specific primers (Fig. 3A). Each PCR reaction was performed in duplicate. GAPDH was used as a positive control, and a PCR reaction containing no cDNA template (NT) was used as a negative control. Each PCR product was confirmed to be of expected size. Interestingly, Va2 fibroblasts express only six PKC isoforms at the mRNA level. The only conventional PKC isoform expressed is PKCα, while three novel isoforms (PKCδ, PKCε, and PKCη), one atypical isoform (PKCι), and PKD are also expressed. These data serve as an initial means of eliminating the PKC isoforms β, γ, and θ as candidate S879 kinases in Va2 cells.
Fig. 3. A conventional, calcium-dependent PKC isoform is responsible for p120 S879 phosphorylation.
(A) RNA from Va2 cells was isolated and reverse transcribed. The resultant cDNA was PCR-amplified using primers specific for each PKC isoform or for GAPDH as a positive control. Each PCR reaction was performed in duplicate. A reaction containing no cDNA template (NT) was used as a negative control. PKC isoform expression was visualized after electrophoresis in 2% agarose gels stained with ethidium bromide. (B) Va2 fibroblasts were incubated in serum starvation medium (0.1%), calcium free medium (CFM), EGTA (2mM), or CFM containing BAPTA-AM (15µM) for 30 min prior to and during a 10 min treatment in the absence (−) or presence (+) of 50ng/ml PDGF. S879 phosphorylation in response to PDGF was analyzed by SDS-PAGE and Western blot for each condition. Levels of S879 phosphorylation were quantitated by measuring band intensity using the Odyssey software package. pS879 band intensity was normalized to total p120, and the values are plotted below. Western blots are representative of three independent experiments. (C) Va2 cells were treated with 1µM of BisI or with the indicated concentrations of the conventional PKC isoform inhibitor, Gö6976, five minutes prior to and during incubation in the absence (−) or presence (+) of PDGF (50ng/ml). S879 phosphorylation was analyzed as indicated in (B), and S879 band intensity was quantitated and normalized to total p120. These data are represented graphically. (D) Va2 firoblasts were treated with two-fold serial dilutions of Gö6976 ranging from 500 nM to 15.625nM five min prior to and during a 10 min incubation in the absence (−) or presence (+) of PDGF. Lysate was analyzed by SDS-PAGE and Western blot. Levels of S879 phosphorylation were analyzed by using pS879 mAb. PDGF receptor activation was verified for each condition by analysis of pERK.
The activity of conventional PKC isoforms (α, β, γ) is calcium-dependent, while novel isoform (δ, ε, η, θ) activity is not. Therefore, calcium sensitivity was used to distinguish between conventional and novel PKC isoforms in phosphorylation of S879 (Fig. 3B). Va2 cells were treated with calcium free medium (CFM) (lanes 3 & 4), the calcium chelator EGTA (2mM) (lanes 5 & 6), or CFM in the presence of the intracellular chelator BAPTA-AM (15µM) (lanes 7 & 8). All treatments were performed in the absence (−) or presence (+) of PDGF. Incubation in CFM alone was sufficient to substantially reduce the levels of PDGF-induced S879 phosphorylation (lane 4), while phosphorylation was further reduced by EGTA or BAPTA treatments (lanes 6 & 8, respectively). Note that in all cases where S879 phosphorylation was blocked, ERK phosphorylation was still achieved (lanes 4, 6, & 8), indicating that PDGFR activation had occurred. Quantitatively, all methods of calcium depletion reduced S879 phosphorylation by at least two-fold compared to control, PDGF-stimulated conditions (Fig. 3B, graphically below). Both EGTA and BAPTA treatments (lanes 6 & 8, respectively) resulted in PDGF-induced S879 phosphorylation at or below the basal level of phosphorylation in untreated cells (lane 1). The fact that S879 phosphorylation was affected by multiple means of calcium depletion suggests that this phosphorylation is dependent on a conventional, calcium-dependent PKC isoform. However, depleting calcium affects multiple cellular processes in addition to the activation of PKC isoforms. Thus, it was unclear from these experiments whether the effects were specifically due to inactivity of PKCs.
To further implicate conventional PKC isoforms, Va2 cells were treated with the conventional PKC isoform inhibitor, Gö6976 (Fig. 3C & 3D). This inhibitor specifically blocks cPKC activity at concentrations below 1µM. At higher concentrations, PKD can be inhibited [42], but no selectivity towards nPKCs has been observed [43, 44]. Va2 cells were treated with three intermediate concentrations of Gö6976 (10nM, 100nM, and 1µM) as well as with Bis I (1µM) as a positive control for S879 inhibition, and S879 phosphorylation levels were determined by Western blotting (Fig. 3C). PDGF-induced phosphorylation of p120 at S879 was blocked at Gö6976 doses that specifically affect conventional PKC activity (lane 8). Quantitatively, there was over a two-fold decrease in PDGF-induced S879 phosphorylation following 100nM Gö6976 treatment, a level that was further reduced with the 1µM treatment (Fig. 3C, graphically below). Similar results were obtained in titration experiments with Gö6976 (Fig. 3D). Collectively, the data in Fig. 3 show that p120 S879 phosphorylation is dependent on a conventional PKC isoform. Because PKCα is the only cPKC expressed in Va2 cells (Fig. 3A), these data implicate PKCα in the phosphorylation of p120 S879.
Inhibition of S879 phosphorylation by selective ablation of PKCα
To pinpoint PKCα as the p120 S879 kinase in Va2 cells, we knocked down PKCα using a variety of shRNA constructs (Fig. 4). Five separate PKCα-specific lentiviral shRNA constructs were tested, and several exhibited significant knockdown of PKCα. These experiments were performed using constructs targeting different regions of PKCα, so our results are indeed specific for PKCα loss and are not due to off-target effects. The results from the two constructs with the most efficient targeting of PKCα (692 & 693) are shown (Fig. 4A). PDGF-induced S879 phosphorylation was blocked upon PKCα loss (lanes 4 & 6), and interestingly, S879 phosphorylation directly correlated with the level of PKCα knockdown obtained. To more effectively show this correlation, quantitative results are displayed graphically below. A low level of PKCα remained with lentiviral construct 693 (lanes 5 & 6), and consequently, low levels of PDGF-induced S879 phosphorylation were achieved. Alternatively, when PKCα was completely lost (lanes 3 & 4) PDGF-induced S879 phosphorylation only reached that of basal levels. Similar data were obtained with a retroviral PKCα shRNA construct in which a partial loss of PKCα was observed (Fig. 4B).
Fig. 4. PKCα phosphorylates p120 S879 in response to PDGF in Va2 cells.
(A) The Sigma Mission Lentiviral system was used to knock down PKCα expression. Five PKCα-specific shRNA constructs were individually assessed in Va2 fibroblasts as indicated in the Materials and methods section. The two constructs with the most efficient PKCα knockdown (692 & 693) are shown here. Wild type Va2 cells were used to control for normal PKCα levels and PDGF-induced S879 phosphorylation. Following treatment in the absence (−) or presence (+) of PDGF, cells were lysed and analyzed by SDS-PAGE and Western blot. Levels of PKCα knockdown were assessed by probing with a PKCα antibody. pS879 band intensity was quantified and normalized to total p120, and results are graphically represented below. (B) Va2 cells were stably transduced with a retroviral PKCα shRNA construct. Wild type (WT) and PKCα knockdown (pRS PKCα) Va2 cells were either control treated or stimulated with 50ng/ml PDGF for 10 min or 200nM PMA for 30 min as indicated. Cell lysates were analyzed by SDS-PAGE and Western blot using total p120, pS879, tubulin, pERK, and PKCα antibodies. S879 band intensities were quantified and normalized to total p120. Data are represented graphically below. (C) Va2 cells were retrovirally infected to stably express a shRNA against PKCδ. Cells were treated in the absence (−) or presence (+) of PDGF (50ng/ml), and lysates were analyzed by SDS-PAGE and Western blot. Knockdown efficiency was determined by probing with an antibody specific for PKCδ. The status of S879 phosphorylation was measured using mAb pS879. All results are representative of at least two independent experiments.
In contrast, shRNAs directed at other PKC isoforms had no effect on PDGF-induced S879 phosphorylation. Fig. 4C shows one such example for the novel PKC isoform, PKCδ. An shRNA construct directed against PKCδ was stably expressed in Va2 cells. The level of knockdown and the status of S879 phosphorylation were analyzed by Western blotting. Using an antibody to detect PKCδ, it is apparent that target protein expression was reduced by ~60–70%. However, the phosphorylation of S879 was not affected. In addition to the PKCδ knockdown experiment, we also expressed wild type, constitutive active, and kinase dead mutant versions of PKCη and PKD in Va2 cells with no observed changes in the S879 phosphorylation profile (data not shown). These data suggest that in Va2 cells, p120 S879 is not a promiscuous substrate for multiple PKC isoforms but is instead tightly regulated by PKCα.
Activation of other membrane receptor types induces p120 S879 phosphorylation
To determine the potential involvement of p120 S879 phosphorylation in other signaling pathways, we tested a variety of RTK and GPCR agonists in multiple cell lines. S879 phosphorylation was analyzed in A431 cells in response to two EGFR agonists, AR (Fig. 5A), and EGF (Fig. 5B). A431 cells were treated with PMA or with two-fold serial dilutions of AR (Fig. 5A). While AR-induced S879 phosphorylation was not nearly as robust as the PMA-induced signal, there was an evident dose response to the agonist. S879 phosphorylation continued to increase through the 800ng/ml dose while levels of ERK (a downstream effector of EGFR) phosphorylation remained constant regardless of the AR dose. The dose response can be effectively visualized by the quantitative data in the right panel. Similar results were obtained with EGF treatment of A431 cells (Fig. 5B). Thus, RTK activation through not only the PDGFR, but also the EGFR, can lead to p120 S879 phosphorylation.
Fig. 5. p120 S879 phosphorylation occurs in response to multiple RTK- and GPCR-stimulated pathways.
(A) A431 cells were treated with 200nM PMA or with Amphiregulin (AR) ranging from 25ng/ml to 800ng/ml prior to lysis and analysis by SDS-PAGE and Western blot using total p120, pS879, tubulin, and pERK antibodies. pS879 band intensity was quantified and normalized to total p120. Data are represented graphically in the right panel. (B) A431 cells were treated with 200nM PMA or with EGF ranging from 12.5ng/ml to 400ng/ml. Cells were lysed and analyzed as indicated in (A). (C) A431 cells were treated in the absence (−) or presence (+) of 200nM PMA for 30 min or in the absence (−) or presence (+) of 10nM Endothelin-1 for 10 minutes. SkMel28 (melanoma cells) and NHEM (primary melanocytes) were treated in the absence (−) or presence (+) of 10nM Endothelin-1 for 10 min. Cells were lysed and analyzed by SDS-PAGE and Western blot using total p120, pS879, and tubulin antibodies.
We also tested the ability of a GPCR agonist to initiate S879 phosphorylation. The GPCR agonist, endothelin-1 (ET-1), signals through the endothelin A and B receptors. The ability of ET-1 to induce S879 phosphorylation was analyzed for A431 cells, SkMel28 (human melanoma cells), and NHEM (primary human melanocytes) (Fig. 5C). PMA stimulation of A431 cells was used as a positive control (lane 2). Substantial phosphorylation of S879 was induced by ET-1 in primary NHEM cells (lane 8), but not in A431 (lane 4) or SkMel28 (lane 6). Primary melanocytes (NHEM) and SkMel28 cells express endothelin B receptors, but endothelin A receptors are downregulated in SkMel28 cells [45], which could account for reduced ET-1-induced S879 phosphorylation in these cells. We additionally screened a panel of colon and prostate cancer cell lines, all with reported endothelin receptor expression, for S879 phosphorylation in response to ET-1 treatment (data not shown). Like the SkMel28 cells, endothelin receptor activation via ET-1 did not result in S879 phosphorylation in the cell line panel. Remarkably, the level of endothelin-induced S879 phosphorylation in the NHEM line was equivalent to that induced in A431 cells by PMA (compare lane 2 to lane 8). This robust phosphorylation induced by a physiologically relevant agonist was somewhat surprising given the fact that PDGF- and EGF- induced S879 phosphorylation was well below that of the PMA-induced signal. Regardless, the fact that p120 is phosphorylated at S879 in response to both RTK and GPCR stimulation in multiple cell lines of various etiologies suggests a broadly relevant role in several signaling pathways.
Discussion
Several lines of evidence suggest that S/T phosphorylation regulates p120 activity, but the upstream kinases involved have not been established nor has a discreet measurable function been assigned to an individual site. To better understand how p120 is regulated, we have generated p120 phospho-specific monoclonal antibodies to several individual phosphorylation sites [30, 31, 46] and are using them to pinpoint upstream kinases and signaling pathways that control p120 activity.
In previous studies, phorbol esters (e.g., PMA, PDBu) were used to correlate overall changes in p120 S/T phosphorylation to changes in cellular activities associated with PKC activation [27, 28]. However, these data did not reflect the status of individual sites. For example, analysis of individual sites revealed that PMA simultaneously induces robust phosphorylation at S879 and dephosphorylation at other sites (e.g., S268, T310 and T916) [21, 30, 31]. Thus, the effects of PMA on p120 involve both kinases (presumably PKC family members) and phosphatases. Moreover, PMA activates multiple PKC isoforms as well as other DAG-responsive proteins, making it difficult to sort out downstream effects. Here, we have identified several ligand/receptor signaling models that induce rapid and robust p120 phosphorylation at S879 under physiologically relevant conditions. Using Va-2 cells and PDGF stimulation, we show for the first time that PDGFR-mediated phosphorylation at this site is dependent on PKCα, a conventional PKC isoform implicated previously in disruption of cadherin-mediated junctions [47–49].
As with many PKC isoforms, the biological responses associated with PKCα are not necessarily consistent across cell types. PKCα is anti-proliferative in several cell lines [50–53] but is associated with increased proliferation in tumor tissue of multiple origins and in glioma cells [35, 54]. The effects of PKCα activation are, therefore, not intrinsic to the kinase [55], but rather vary depending on cell-type specific substrates and perhaps oncogenic transformation. PKCα has been shown to regulate desmoplakin assembly at desmosomes [39] and can affect adherens junctions when overexpressed [47–49]. Thus, PKCα modulates both cell-cell adhesion and cell proliferation, activities that may be controlled in part through regulation of p120.
In addition to PMA, the RTK agonist, PDGF induces p120 S879 phosphorylation in Va2 cells. This phosphorylation is rapid (see Fig. 1B) and closely parallels the kinetics of tyrosine phosphorylation and activation of known PDGFR substrates such as PLCγ. Thus, a working model is that stimulation of PDGFR phosphorylates p120 S879 through PKCα via transient activation of PLCγ. The signaling cascade from PDGFR to p120 S879 is not limited to Va2 fibroblasts, as immunofluorescent analysis of PDGF-treated NIH3T3 fibroblasts also revealed S879 phosphorylation (data not shown). Although S879 phosphorylation in Va2 cells is dependent on PKCα, several attempts to directly phosphorylate p120 in vitro with purified PKCα were unsuccessful. Therefore, PKCα may act indirectly on p120 via one or more intervening kinases.
Interestingly, the most striking example of S879 phosphorylation observed in this study involved activation of a GPCR. Endothelin-1 is a vasoconstrictive peptide that binds to endothelin receptor type A and B GPCRs. In primary melanocytes (NHEM), the level of S879 phosphorylation induced by ET-1 was extraordinary. NHEM express PKCα, βI, βII, δ, ε, η, ι, and PKD as determined by RT-PCR analysis (data not shown). However, Gö6976 treatment did not inhibit ET-1-induced S879 phosphorylation in these cells (data not shown). These observations do not necessarily exclude the involvement of PKCα (or other conventional isoforms), but they strongly implicate the involvement of other PKC isoforms in ET-1 signaling to p120 in NHEM cells. Thus, PKC signaling to p120 in general and S879 in particular may be pathway- and/or cell type-specific. Surprisingly, ET-1 stimulation had little or no effect on S879 phosphorylation in several cancer cell lines tested. It is possible that the endothelin receptors are downregulated in these lines, as increased levels of ET-1 have been detected in various tumor samples [56]. Nonetheless, the NHEM data are clear and suggest that p120 might be an important target for the endothelin receptor and perhaps other GPCRs.
Both p120 S879 phosphorylation and ERK phosphorylation are dependent on PKC activity. Phosphorylation of ERK in these studies was mostly used to control for receptor activation, but in some cells, ERK activity is dependent on PKC [41]. Interestingly, in Va2 cells, ERK phosphorylation was blocked by BisI, but not Gö6983, even though both are general PKC inhibitors. Studies in NIH3T3 fibroblasts have shown that Raf-1, a kinase upstream of ERK, is directly phosphorylated by PKCα in response to TPA treatment [57]. In Va2 cells, however, PDGF-induced phosphorylation of ERK was unaffected by Gö6976 treatment, calcium depletion, or PKCα knockdown. Thus, it appears that PKCα is not an important upstream factor for ERK signaling in Va2 fibroblasts, and ERK phosphorylation did not correlate with S879 phosphorylation of p120.
Several members of the PKC family are known to have prominent roles in cancer, but the various isoforms are differentially expressed in tissues and display a wide variety of context-specific activation and translocation patterns [33, 35]. We found the available PKC inhibitors to be mostly inadequate as tools for discriminating one isoform from another. Likewise, PMA and other non-selective PKC activators have too many targets and side effects to use in studies aimed at pinpointing isoforms responsible for S879 phosphorylation. These and other issues highlight the importance of distinguishing between PKC isoforms on one hand, and identifying individual substrate effectors on the other. Our data show that PDGF-initiated signaling to p120 is dependent on PKCα. The level of phosphorylation of p120 on S879 was directly proportional to the amount of PKCα present, and phosphorylation was abolished in knockdown cell lines where PKCα was undetectable. Moreover, knockdown of other PKC isoforms had no effect PDGF-induced phosphorylation at S879. We have yet to identify a specific activity associated with S879 phosphorylation, but the identification of PKCα narrows the field considerably and may provide novel clues based on known roles of PKCα and its relationship to adherens junctions. Because PKCα was the only classical PKC isoform present in Va2 cells, it will be important in other cell lines to revisit other classical isoforms (i.e., PKCβ, PKCγ), but the current advance and the availability of powerful tools (e.g., shRNA constructs, antibodies) will accelerate the progress.
In conclusion, we have established physiologically relevant model systems for analysis of upstream signaling events that modify p120 function through phosphorylation. We found that at least two receptor types (RTKs and GPCRs) can induce rapid and significant phosphorylation of p120 at S879, and in Va2 cells we have pinpointed PKCα as the essential upstream PKC isoform in the pathway. The striking S879 phosphorylation induced by activation of the endothelin receptor implies an important and as yet unexplored role for GPCRs in regulating p120 activity.
Acknowledgments
We are grateful to Dr. Alex Toker for the PKCδ shRNA construct. This work was supported in part by the National Institutes of Health grants CA055724 and CA111947 (A.R.) and by the American Cancer Society grant RSG0424901CCG (M.D).
References
- 1.Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci. 2000;113(Pt 8):1319–1334. doi: 10.1242/jcs.113.8.1319. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989;9:629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds AB, Herbert L, Cleveland JL, Berg ST, Gaut JR. p120, a novel substrate of protein tyrosine kinase receptors and of p60v-src, is related to cadherin-binding factors beta-catenin, plakoglobin and armadillo. Oncogene. 1992;7:2439–2445. [PubMed] [Google Scholar]
- 4.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Miyazawa K, Kitamura N, Johnson KR, Wheelock MJ, Matsuyoshi N, Takeichi M, Fumiaki I. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. J Cell Biol. 1995;128:949–957. doi: 10.1083/jcb.128.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds AB, Daniel JM, Mo YY, Wu J, Zhang Z. The novel catenin p120cas binds classical cadherins and induces an unusual morphological phenotype in NIH3T3 fibroblasts. Exp Cell Res. 1996;225:328–337. doi: 10.1006/excr.1996.0183. [DOI] [PubMed] [Google Scholar]
- 7.Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. alpha-Catenin Is a Molecular Switch that Binds E-Cadherin-beta-Catenin and Regulates Actin-Filament Assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR, Wheelock MJ. Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J Cell Sci. 1997;110(Pt 8):1013–1022. doi: 10.1242/jcs.110.8.1013. [DOI] [PubMed] [Google Scholar]
- 12.Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R. The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc Natl Acad Sci U S A. 1991;88:9156–9160. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- 16.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Downing JR, Reynolds AB. PDGF, CSF-1, and EGF induce tyrosine phosphorylation of p120, a pp60src transformation-associated substrate. Oncogene. 1991;6:607–613. [PubMed] [Google Scholar]
- 19.Kanner SB, Reynolds AB, Parsons JT. Tyrosine phosphorylation of a 120-kilodalton pp60src substrate upon epidermal growth factor and platelet-derived growth factor receptor stimulation and in polyomavirus middle-T-antigen-transformed cells. Mol Cell Biol. 1991;11:713–720. doi: 10.1128/mcb.11.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariner DJ, Anastasiadis P, Keilhack H, Bohmer FD, Wang J, Reynolds AB. Identification of Src phosphorylation sites in the catenin p120ctn. J Biol Chem. 2001;276:28006–28013. doi: 10.1074/jbc.M102443200. [DOI] [PubMed] [Google Scholar]
- 21.Xia X, Mariner DJ, Reynolds AB. Adhesion-associated and PKC-modulated changes in serine/threonine phosphorylation of p120-catenin. Biochemistry. 2003;42:9195–9204. doi: 10.1021/bi034597h. [DOI] [PubMed] [Google Scholar]
- 22.Aono S, Nakagawa S, Reynolds AB, Takeichi M. p120(ctn) acts as an inhibitory regulator of cadherin function in colon carcinoma cells. J Cell Biol. 1999;145:551–562. doi: 10.1083/jcb.145.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 2002;129:3771–3782. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- 24.Castano J, Solanas G, Casagolda D, Raurell I, Villagrasa P, Bustelo XR, de Herreros AG, Dunach M. Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA. Mol Cell Biol. 2006 doi: 10.1128/MCB.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanagisawa M, Huveldt D, Kreinest P, Lohse CM, Cheville JC, Parker AS, Copland JA, Anastasiadis PZ. A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion and predicts metastatic disease. J Biol Chem. 2008 doi: 10.1074/jbc.M801192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cozzolino M, Stagni V, Spinardi L, Campioni N, Fiorentini C, Salvati E, Alema S, Salvatore AM. p120 Catenin is required for growth factor-dependent cell motility and scattering in epithelial cells. Mol Biol Cell. 2003;14:1964–1977. doi: 10.1091/mbc.E02-08-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratcliffe MJ, Rubin LL, Staddon JM. Dephosphorylation of the cadherin-associated p100/p120 proteins in response to activation of protein kinase C in epithelial cells. J Biol Chem. 1997;272:31894–31901. doi: 10.1074/jbc.272.50.31894. [DOI] [PubMed] [Google Scholar]
- 28.Ratcliffe MJ, Smales C, Staddon JM. Dephosphorylation of the catenins p120 and p100 in endothelial cells in response to inflammatory stimuli. Biochem J. 1999;338(Pt 2):471–478. [PMC free article] [PubMed] [Google Scholar]
- 29.Wong EY, Morgan L, Smales C, Lang P, Gubby SE, Staddon JM. Vascular endothelial growth factor stimulates dephosphorylation of the catenins p120 and p100 in endothelial cells. Biochem J. 2000;346(Pt 1):209–216. [PMC free article] [PubMed] [Google Scholar]
- 30.Xia X, Brooks J, Campos-Gonzalez R, Reynolds AB. Serine and threonine phospho-specific antibodies to p120-catenin. Hybrid Hybridomics. 2004;23:343–351. doi: 10.1089/hyb.2004.23.343. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan MH, Xia X, Wang X, Chronopoulou E, Gao GJ, Campos-Gonzalez R, Reynolds AB. Generation and characterization of a novel phospho-specific monoclonal antibody to p120-catenin serine 879. Hybridoma (Larchmt) 2007;26:407–415. doi: 10.1089/hyb.2007.0527. [DOI] [PubMed] [Google Scholar]
- 32.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 33.Mackay HJ, Twelves CJ. Protein kinase C: a target for anticancer drugs? Endocr Relat Cancer. 2003;10:389–396. doi: 10.1677/erc.0.0100389. [DOI] [PubMed] [Google Scholar]
- 34.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7:554–562. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
- 35.Martiny-Baron G, Fabbro D. Classical PKC isoforms in cancer. Pharmacol Res. 2007 doi: 10.1016/j.phrs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Kheifets V, Mochly-Rosen D. Insight into intra- and inter-molecular interactions of PKC: Design of specific modulators of kinase function. Pharmacol Res. 2007 doi: 10.1016/j.phrs.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvi F, Idkowiak-Baldys J, Baldys A, Raymond JR, Hannun YA. Regulation of membrane trafficking and endocytosis by protein kinase C: emerging role of the pericentrion, a novel protein kinase C-dependent subset of recycling endosomes. Cell Mol Life Sci. 2006 doi: 10.1007/s00018-006-6363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storz P, Doppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol. 2004;24:2614–2626. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bass-Zubek AE, Hobbs RP, Amargo EV, Garcia NJ, Hsieh SN, Chen X, Wahl JK, 3rd, Denning MF, Green KJ. Plakophilin 2: a critical scaffold for PKC alpha that regulates intercellular junction assembly. J Cell Biol. 2008;181:605–613. doi: 10.1083/jcb.200712133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oshevski S, Le Bousse-Kerdiles MC, Clay D, Levashova Z, Debili N, Vitral N, Jasmin C, Castagna M. Differential expression of protein kinase C isoform transcripts in human hematopoietic progenitors undergoing differentiation. Biochem Biophys Res Commun. 1999;263:603–609. doi: 10.1006/bbrc.1999.1425. [DOI] [PubMed] [Google Scholar]
- 41.Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Zheng S, Storz P, Min W. Protein kinase D specifically mediates apoptosis signal-regulating kinase 1-JNK signaling induced by H2O2 but not tumor necrosis factor. J Biol Chem. 2005;280:19036–19044. doi: 10.1074/jbc.M414674200. [DOI] [PubMed] [Google Scholar]
- 43.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 44.Koivunen J, Aaltonen V, Koskela S, Lehenkari P, Laato M, Peltonen J. Protein kinase C alpha/beta inhibitor Go6976 promotes formation of cell junctions and inhibits invasion of urinary bladder carcinoma cells. Cancer Res. 2004;64:5693–5701. doi: 10.1158/0008-5472.CAN-03-3511. [DOI] [PubMed] [Google Scholar]
- 45.Eberle J, Weitmann S, Thieck O, Pech H, Paul M, Orfanos CE. Downregulation of endothelin B receptor in human melanoma cell lines parallel to differentiation genes. J Invest Dermatol. 1999;112:925–932. doi: 10.1046/j.1523-1747.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- 46.Mariner DJ, Davis MA, Reynolds AB. EGFR signaling to p120-catenin through phosphorylation at Y228. J Cell Sci. 2004;117:1339–1350. doi: 10.1242/jcs.01001. [DOI] [PubMed] [Google Scholar]
- 47.Batlle E, Verdu J, Dominguez D, del Mont Llosas M, Diaz V, Loukili N, Paciucci R, Alameda F, de Herreros AG. Protein kinase C-alpha activity inversely modulates invasion and growth of intestinal cells. J Biol Chem. 1998;273:15091–15098. doi: 10.1074/jbc.273.24.15091. [DOI] [PubMed] [Google Scholar]
- 48.Masur K, Lang K, Niggemann B, Zanker KS, Entschladen F. High PKC alpha and low E-cadherin expression contribute to high migratory activity of colon carcinoma cells. Mol Biol Cell. 2001;12:1973–1982. doi: 10.1091/mbc.12.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandoval R, Malik AB, Minshall RD, Kouklis P, Ellis CA, Tiruppathi C. Ca(2+) signalling and PKCalpha activate increased endothelial permeability by disassembly of VE-cadherin junctions. J Physiol. 2001;533:433–445. doi: 10.1111/j.1469-7793.2001.0433a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frey MR, Saxon ML, Zhao X, Rollins A, Evans SS, Black JD. Protein kinase C isozyme-mediated cell cycle arrest involves induction of p21(waf1/cip1) and p27(kip1) and hypophosphorylation of the retinoblastoma protein in intestinal epithelial cells. J Biol Chem. 1997;272:9424–9435. doi: 10.1074/jbc.272.14.9424. [DOI] [PubMed] [Google Scholar]
- 51.Sun XG, Rotenberg SA. Overexpression of protein kinase Calpha in MCF-10A human breast cells engenders dramatic alterations in morphology, proliferation, and motility. Cell Growth Differ. 1999;10:343–352. [PubMed] [Google Scholar]
- 52.Frey MR, Clark JA, Leontieva O, Uronis JM, Black AR, Black JD. Protein kinase C signaling mediates a program of cell cycle withdrawal in the intestinal epithelium. J Cell Biol. 2000;151:763–778. doi: 10.1083/jcb.151.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oster H, Leitges M. Protein kinase C alpha but not PKCzeta suppresses intestinal tumor formation in ApcMin/+ mice. Cancer Res. 2006;66:6955–6963. doi: 10.1158/0008-5472.CAN-06-0268. [DOI] [PubMed] [Google Scholar]
- 54.Besson A, Yong VW. Involvement of p21(Waf1/Cip1) in protein kinase C alpha-induced cell cycle progression. Mol Cell Biol. 2000;20:4580–4590. doi: 10.1128/mcb.20.13.4580-4590.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakashima S. Protein kinase C alpha (PKC alpha): regulation and biological function. J Biochem. 2002;132:669–675. doi: 10.1093/oxfordjournals.jbchem.a003272. [DOI] [PubMed] [Google Scholar]
- 56.Grant K, Knowles J, Dawas K, Burnstock G, Taylor I, Loizidou M. Mechanisms of endothelin 1-stimulated proliferation in colorectal cancer cell lines. Br J Surg. 2007;94:106–112. doi: 10.1002/bjs.5536. [DOI] [PubMed] [Google Scholar]
- 57.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp UR. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]