Abstract
Fine particulate air pollutants, mainly their organic fraction, have been demonstrated to be associated with cardiovascular and respiratory health problems. Puerto Rico has been reported to have the highest prevalence of pulmonary diseases (e.g. asthma) in the US. The aim of this study was to assess, for the first time, the immunological response of human bronchial epithelial cells (BEAS-2B) to organic extracts isolated from air-borne particulate matter (PM2.5) in Puerto Rico. Organic extracts from PM2.5 collected throughout an 8-month period (2000-2001) were pooled (composite) in order to perform chemical analysis and biological activity testing. BEAS-2B cells were exposed to PM2.5 organic extract to assess cytotoxicity, levels of cytokines and relative gene expression of MHC-II, hPXR and CYP3A5. Our findings show that organic PM2.5 consist of toxic as well as bioactive components that can regulate the secretion of cytokines in BEAS-2B, which could modulate inflammatory response in the lung. Trace element analyses confirmed the presence of metals in organic extracts highlighting the relative high abundance of Cu and Zn in polar organic extracts. Polar organic extracts exhibited dose-dependant toxicity and were found to significantly induce the release of interleukin 6 (IL-6), IL-1β and IL-7 while significantly inhibiting the secretion of IL-8, G-CSF and MCP-1. Moreover, MHC-II transcriptional activity was up-regulated after 24h of exposure, whereas PXR and CYP3A5 were down-regulated. This research provides a new insight into the effects of PM2.5 organic fractions on specific effectors and their possible role in the development of respiratory inflammatory diseases in Puerto Rico.
Keywords: PM2.5, inflammation, PXR, CYP3A5, cytokines, toxicity
Introduction
Cardiovascular and respiratory illnesses related to air pollution have increased during the last few decades in the United States. Substantial evidence gathered through environmental and epidemiological studies show a strong association between fine particulate air pollution and health problems such as respiratory illnesses (e.g., respiratory track inflammation, asthma, acute bronchitis, and lung cancer) and cardiovascular disease mortality (Dockery et al., 1993; Delfino et al., 2005; Mehta et al., 2008). The smaller the air particle, the greater is its association to human adverse health effects. Fine particle constituents including organic fraction components, biological materials, and transition metals all of which are factors suggested to cause or exacerbate these adverse cardiopulmonary health conditions (Delfino et al., 2005; Mauderly and Chow, 2008).
Exposure to PM can induce airway inflammation giving rise to respiratory diseases as well as nasal damage (Molinelli et al., 2006; Thacker, 2006). An association between particulate matter with diameter ≤2.5um (PM2.5) and ≤10um (PM10) exposure, and the development of pulmonary inflammation through pro-inflammatory cytokines induction has been well documented (Pope, 2000a; Pope, 2000b). It is now known that cytokines can influence the airway inflammatory response and contribute to the development of lesions in respiratory diseases such as asthma and Chronic Obstructive Pulmonary Disease (COPD) (Fujii et al., 2001; Veranth et al., 2004). Various chemokines and cytokines have been described as playing a role in both initiating and controlling inflammation (Thacker, 2006). Interleukin 1 (IL-1), interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), among other cytokines, are known to be related to inflammation in lung injury (van Eeden et al., 2001). Organic particles, such as those originating from diesel (PM2.5 and PM10), can enhance or stimulate the secretion of a number of cytokines in both rat and human airway epithelial cells (Kennedy et al., 1998; Kawasaki et al., 2001; Oudin and Pugin, 2002; Takano et al., 2002; Baulig et al., 2003). In vitro studies using different PM2.5 constituents, especially diesel exhaust particles (DEPs), have confirmed that PM can induce the production of pro-inflammatory cytokines (Veranth et al., 2004; Becker et al., 2005). Studies conducted with soil-derived PM2.5 particles from several locations in the western United States showed an increased secretion of IL-6 and IL-8, but not TNF-α in bronchial epithelial cells (BEAS-2B), which suggest intrinsic differences between ambient dust and relate these differences to cell death and cytokines release leading to airway inflammation (Veranth et al., 2004).
The Major Histocompatibility Complex (MHC), also known as the Human Leukocyte Antigen (HLA), plays an essential role in immune function (Beck et al., 1999). This gene family is divided into three subgroups or classes, with two main types of MHC gene products: MHC class I (MHC-I) and MHC class II (MHC-II) molecules (Trowsdale and Campbell, 1992). Accumulating evidence suggests that epithelial cells can act as sentinels to inform other cells in the lung's mucosa of events occurring in the lumen and hence can actively participate in inflammatory/allergic processes. Epithelial cells, independent of their tissue of origin, can express increased levels of MCH-II during inflammation despite their constitutively low expression. MHC-II genes have been also identified as important determinants in pulmonary diseases caused by inorganic and organic compounds (Saltini et al., 1998). Therefore, we have evaluated the response of MHC-II transcriptional activity as a result of PM2.5 organic extract exposure.
The human pregnane X receptor (hPXR), which is a nuclear receptor, acts as a sensor for metabolism and disposition of a broad range of natural and synthetic endobiotics and xenobiotics in humans to prevent or reduce their toxic effects (Schote et al., 2007). This nuclear receptor is involved in the induction mechanisms of the cytochrome P450 3A (CYP3A), which in turn is one of the most important xenobiotic and drug metabolizing enzymes in humans (Quattrochi and Guzelian, 2001). Many exogenous compounds can induce PXR as well as a number of PXR-targeted genes. The expression of CYP3A5 has been reported in human lungs (Raunio et al., 2005), yet the expression pattern of CYP3A5 or PXR by organic components of PM2.5 has not been evaluated nor have any report been made on the effects of PM2.5 constituents.
Previous studies suggested that organic compounds in PM10 from Puerto Rico (from an urban/industrialized site) played a major role in the cytotoxicity observed with bronchial epithelial cells (Reyes et al., 2000; Molinelli et al., 2006), but did not explore the possible mechanism by which it occurred. High incidence of asthma in the Guaynabo area has been previously reported to be related with the proximity to some air pollution sources (Loyo-Berrios et al., 2007) and could also be related to high metal content as well as organic compounds found in fine particles (PM2.5) (Figueroa et al., 2006). The relative contribution of organic and inorganic constituents of these extracts to the immune response that can lead to inflammation and, consequently, pulmonary diseases, still need to be elucidated. Here we evaluated the possible contribution of polar and non-polar extracts of PM2.5 on cellular toxicity and the secretion of pro-inflammatory cytokines. Cytokine expression patterns in lung epithelial cells exposed to components of particulate air pollution have been previously reported (Ovrevik et al., 2009). However, to the best of our knowledge, this is the first study that evaluates the response of airway epithelial cells to organic extracts in PM2.5 from Puerto Rico, other than DEP. Here we evaluate the organic extracts obtained from PM2.5 at two locations previously studied in Puerto Rico (Figure 1), and their ability to induce the secretion of cytokines in BEAS-2B. We provide new evidence that the polar organic constituents of PM2.5 from an urban/industrialized site (Guaynabo) play a major role in cellular toxicity and mediate the increased release of the pro-inflammatory cytokines IL-6 and IL-1β, the transcriptional activation of MHC-II, and the suppression of genes involve in the metabolism of endobiotics and xenobiotics.
Figure 1. Graphical representation of the relative PM2.5 air sampling location on the island of Puerto Rico within the Caribbean region.
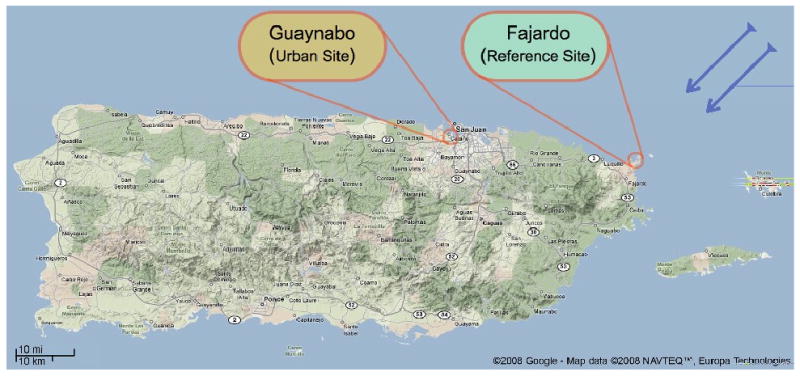
Sampling location for the urban/industrialized site (Guaynabo) is immediately south of the San Juan Harbor, while the sampling location for the reference site (Fajardo) is on the farthest northeast coastal tip of the island designated as a natural reserve (Cabezas de San Juan lighthouse). Blue arrows show the direction of trade winds. Figure edited from Google-Map data © 2008 NAVTEQ™, Europa Technology.
Materials and Methods
Cell Culture
The in vitro model used for the experiments was the SV40 large T antigen immortalized human bronchial epithelial cell line BEAS-2B, which is derived from normal human bronchial epithelium. BEAS-2B cells were purchased from ATCC (CRL-9609) and maintained with supplemented keratinocyte growth medium (KGM) bullet kit media (Lonza, Walkersville, MD). KGM is essentially keratinocyte basal medium (KBM) supplemented with human epidermal growth factor, insulin, hydrocortisone, calcium, bovine pituitary extract, and gentamicin sulfate amphotericin-B (GA-1000). The cells were grown at 37°C and 5% CO2, and fresh media was added every two to three days. Cells used for the experiments were between passages 46 and 49 from independent passages of cells that were grown from separate frozen stocks. For the experimental assays we used the KBM media with no supplements to avoid any effect of culture media on BEAS-2B cells response. All assays were performed at least in three separate wells with independent cell population (n=3). Error bars in graphs represent the SE of each mean.
Site Description
Airborne particulate matter (PM2.5) samples were collected at two monitoring stations (Guaynabo and Fajardo) designated by the Puerto Rico Environmental Quality Board (PREQB). The northern Guaynabo station is located in an urban/industrialized area at the Amelia ward of this municipality. This is a suburban location in the vicinity of a highly active commercial port zone. Some nearby facilities include two electrical power plants, a grain mill, an oil refinery (now closed, but active during the sampling period), a major commercial port characterized as a heavy truck trafficking zone and a major highway artery in the vicinity. The Fajardo station (consisting mainly of surrounding mangrove forest with light vehicle traffic) is located at the farthest northeast tip of the island designated as a natural reserve (Cabezas de San Juan lighthouse). The relative locations of these sites are illustrated in Figure 1.
Sampling Procedure of PM2.5
Teflon filters were used on a Fine Particulate Chemical Speciation Air Sampler (RAAS 2.5-400, Andersen Instruments Inc, Franklin, MA, USA) to capture PM2.5 particles in a period of a year (November 2000 to September 2001). The airflow rate set on the instrument was 17 L/min. Each filter represents the material collected in a 72h sampling period set to begin and end at midnight. The RAAS 2.5-400 monitors and records the ambient manifold and enclosure temperatures, barometric and pumps vacuum pressures, humidity, flow rates and volumes of each individual channel on the instrument. Teflon filters for PM2.5 were weighed and conditioned at a temperature of 20-23 ± 2°C and a humidity of 40 ± 5%.
Particulate Matter Organic extracts
All PM2.5 from filters were extracted by Soxhlet, based on EPA method 3540C (U.S. EPA, 1996). The organic PM2.5 were sequentially extracted using hexane as organic solvent for the non-polar fraction, followed by acetone in order to obtain the polar organic fractions. All filters from each month were combined, allowed to reflux for 24h with 175 ml of hexane and then 24h with 175ml acetone. The recovered solvent was dried by means of rotor evaporation to approximately 1-2ml and subsequently under a gentle stream of nitrogen. The vials with organic extracts were weighed and the content dissolved in dimethyl sulfoxide (DMSO) (Molinelli et al., 2006). Organic PM2.5 composites from organic extracts dissolved in DMSO, from each site, were then prepared from eight months throughout a period of a year (November 2000 to September 2001, specifically November and December of 2000, and March, April, May, July, August and September of 2001), to be used for further in vitro analyses.
Chemical Analysis
The samples were diluted up to 1mL with nitric acid trace metal grade and stored in plastic vials and refrigerated until analysis. Trace element analysis was carried out using an Inductively Coupled Plasma Mass Spectrometer (ICP-MS) 7500ce (Agilent Technologies), and EPA method 200.8. All samples were run in triplicates using Sc and Y as internals standards. The DMSO used as solvent was run as a method blank to correct from solvent contamination. To sustain a stable signal for analysis, the temperature of the spray chamber was set at 2°C.
Cytotoxicity
Cell viability after the different exposures was determined using the neutral red uptake bioassay (NRB; Sigma, St Louis MO) on BEAS-2B after a 24h exposure. Cells were seeded in a 96-wells plate at a density of approximately 25,000-cells/well. After 48h (85-90% of confluence) cells were exposed to KBM media containing different concentrations of organic extract composites from PM2.5 (1, 5, 10, 25, 50, and 100ug/ml), lipopolysacharides (10 μg/ml; LPS; Sigma, St Louis MO), Triton-X 100 (25ug/ml) was used as a positive control, and KBM alone or KBM containing 0.1% (v/v) DMSO (as an organic extract carrier) as controls. The use of KBM alone as a control was to demonstrate no artifacts resulting from the DMSO on BEAS-2B cells response. After removal of the treatment media, cell media containing 100ug/mL neutral red was added to each well with the exception of two blanks containing the appropriate media. Experiments were repeated at least twice to ensure reproducibility and statistical significance.
Treatment and quantification of human cytokines
BEAS-2B cells were plated for experiments in 24-wells plates at a cell density of 70,000 cells per well and maintained (media changed every 24h) for 48h until reaching 80%-90% of cell confluence. The effects of organic extracts (polar or non-polar) from PM2.5 were tested at concentrations of 1ug/ml, 50ug/ml, and 100ug/ml of organic extract composite from PM2.5, 10ug/ml LPS (positive control, Sigma), and KBM alone or containing 0.1% (v/v) DMSO as controls. The use of KBM alone as a control was to demonstrate no artifact resulting from the DMSO on BEAS-2B cells response. Cells were incubated in a total volume of 0.5ml of media for 24h before analyzing the supernatant for presence of cytokines. Cytokines/chemokines secretions were quantified in duplicates from each sample employing the Bio-Plex Human Cytokine 17-plex panel (Bio-Rad Lab. Inc.). The cytokines profile that was evaluated after exposure were interleukin-1 beta (IL-1β), IL-2, IL-4, IL-6, IL-8, IL-10, IL-5, IL-7, IL-12, IL-13, IL-17, granulocyte-monocyte colony stimulating factor (GM-CSF), interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), granulocyte colony stimulating factor (G-CSF), macrophage chemotactic protein-1 (MCP-1; MCAF) and MIP-1β. The Bio-Plex Manager software (Bio-Rad Lab. Inc.) and the Beadlyte software (Upstate) were employed for the data analysis. Each treatment set was made in three separate wells with independent cell population (n=3), repeated at least twice to ensure reproducibility and allow for statistical evaluation.
RNA extraction and relative real-time PCR
Total RNA was isolated from treated and untreated BEAS-2B human lung epithelial cells using TRIzol® reagent as described by the manufacturer (Invitrogen Life Technology, USA). Single-stranded cDNA from RNA samples were generated using SuperScript™ III First-Strand Synthesis SuperMix Kit (Invitrogen Life Technology, USA), after Turbo DNase treatment (Ambion). Relative gene expression was determined by real-time semi-quantitative PCR in an iQ-cycler (BioRad, Hercules, CA, USA) using 1ul of cDNA sample aliquot (100 ng of total mRNA) as a template with SybrGreen Super Mix (Molecular Probes, Leiden, The Netherlands). The quality of PCR product was monitored using post-PCR melt curve analysis at the end of the amplification cycles. The primers sets used for relative gene expression analysis were for HLA-DRα: sense 5′-gagtttgatgctccaagccctctccca-3′ and antisense 5′-cagaggccccctgcgttctgctgcaat-3′ as previously described (Beresford and Boss, 2001); for human PXR (NM_022002): sense 5′-aggagttgttcggcatcac-3′ and antisense 5′-ggcattgtcggctcttgg-3′; and for CYP3A5 as previously described (Nishimura et al., 2002). GAPDH gene expression was used as the internal control being a housekeeping gene and analyzed in each experiment for normalization using the primers as previously described (Schote et al., 2007). Relative fold inductions were calculated using the ΔΔCt formula (Schefe et al., 2006). All real-time RT-PCR assays for relative gene expression were repeated at least three times in duplicates from independent total RNA samples for the same treatment conditions.
Statistical analysis
Unpaired t-test or Student-Newman-Keuls Multiple Comparisons Test and analysis of variance tests were used to evaluate statistical differences between sample concentrations. Seasonal and sampling sites differences were also evaluated. All statistical analyses were performed using the GraphPad InStat version 3.0 computer software package (GraphPad Software Inc., San Diego, CA). Statistical significance was determined establishing a probability value of p < 0.05.
Results
Levels of PM2.5 collected from Fajardo and Guaynabo
Total monthly PM2.5 concentration obtained at the urban site (Guaynabo) was greater than that from the rural site (Fajardo). The yearly average concentration of PM2.5 (12-month period 2000-2001), collected at the urban site was 11.6 ug/m3, which is 30% greater than that at the rural site (8.5 ug/m3) (Jimenez-Velez et al., 2006). The average concentration of PM2.5 collected at the urban/industrialized site (eight months composite) was 10.982 ug/m3, 40.06% (SEM ±6.63%), higher than the rural site (7.890 ug/m3) (Figure 2). Moreover, the average monthly differences of PM2.5 for the year 2000 at the urban and rural sites were 4.598ug/m3 and 3.845 ug/m3, respectively. The average monthly difference of PM2.5 at the urban site was greater than the rural site, 5.309ug/m3 vs. 2.045ug/m3, respectively.
Figure 2. PM2.5 levels from Fajardo and Guaynabo.
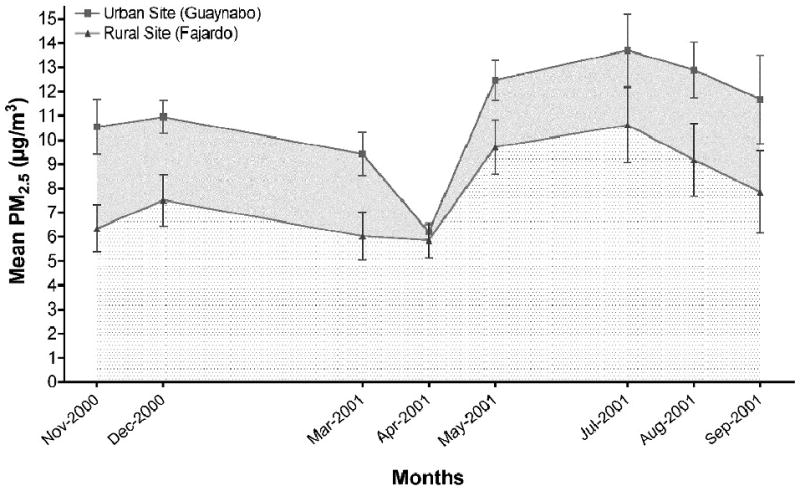
Average PM2.5 concentration collected at Guaynabo (solid squares) and Fajardo (solid triangles) for the eight-month period used for extract composites during 2000-2001. Error bars represent the SE of the mean.
Chemical Analysis
Collection of sufficient PM2.5 mass to conduct both the physicochemical characterization and biological assays has always been a limiting factor for this type of research. Although the small amount of material collected for this research, we were able to perform chemical analyses on PM2.5 organic extracts to confirm the presence of Vanadium (V), Nickel (Ni), Copper (Cu), Zinc (Zn), Arsenic (As), Cadmium (Cd), and Lead (Pb) from the extracts collected in both sites (Table 1). The polar organic extract (composite) from the urban site contained Cu and Zn in a concentration higher than the non-polar organic extracts from either the urban or the rural site. These trace metals are also found in polar organic extract from the rural site, but we were unable to quantify it due to the insufficient material (data not shown).
Table 1. Trace metals content within organic extracts from PM2.5 collected in Puerto Rico.
Trace metal concentrations (ng/ml) within polar and non-polar organic extracts from PM2.5 collected at two different sites (Guaynabo and Fajardo) in Puerto Rico. Results from the by Inductively Coupled Plasma Mass Spectrometer (ICP-MS) analysis were tabulated with its relative standard deviation (RSD).
| PM2.5 Samples | V (ng(ml) |
Ni (ng/ml) |
Cu (ng/ml) |
Zn (ng/ml) |
As (ng/ml) |
Cd (ng(ml) |
Pb (ng(ml) |
|---|---|---|---|---|---|---|---|
| Polar Organic - Guaynabo | 340.1 | 292.6 | 1401.0 | 985.4 | 528.1 | 216.8 | 354.7 |
| 8-months Composite | (1.46%) | (0.79%) | (3.40%) | (3.40%) | (0.45%) | (0.38%) | (0.24%) |
| Non-Polar Organic - Guaynabo | 347.9 | 386.9 | 370.2 | 418.8 | 521.3 | 192.7 | 358.3 |
| 8-months Composite | (2.05%) | (1.43%) | (2.47%) | (6.27%) | (0.51%) | (0.05%) | (0.35%) |
| Non-Polar Organic - Fajardo | 306.8 | 224 | 110.1 | 220.8 | 540.5 | 193.5 | 357.5 |
| 8-months Composite | (11.16%) | (6.93%) | (16.75%) | (53.58%) | (3.20%) | (0.37%) | (1.62%) |
| Polar Organic - Guaynabo | 320.8 | 208.4 | 674.3 | 438.8 | 528.4 | 194.1 | 365.5 |
| Jul-01 | (1.02%) | (0.54%) | (1.32%) | (1.77%) | (0.35%) | (0.17%) | (0.12%) |
| Non-Polar Organic - Guaynabo | 304.0 | 182.8 | 591.3 | 525.1 | 529.4 | 194.9 | 392.8 |
| Jul-01 | (0.20%) | (0.61%) | (0.31%) | (0.60%) | (0.25%) | (0.07%) | (0.21%) |
Toxicological analyses of organic extracts
Cytotoxicity assays using bronchial epithelial cells BEAS-2B exposed to urban polar organic extracts for 24h revealed higher toxicity than the rural site (figure 3a, 3b). The cytotoxicity begins around 25ug/ml and with the greatest effect at 100ug/ml. Conversely, the non-polar organic extracts from both sites showed no cytotoxicity at concentrations as high as 100ug/ml (figure 3c, 3d). These results demonstrate that the compounds responsible for cellular toxicity in the PM2.5 are associated with the polar fraction of the urban organic extract collected during this period. The polar extract from the rural site only exhibited toxicity at the high concentration (100ug/ml).
Figure 3. Cytotoxicity of polar and non-polar extracts composites collected from Guaynabo and Fajardo in BEAS-2B cell line.
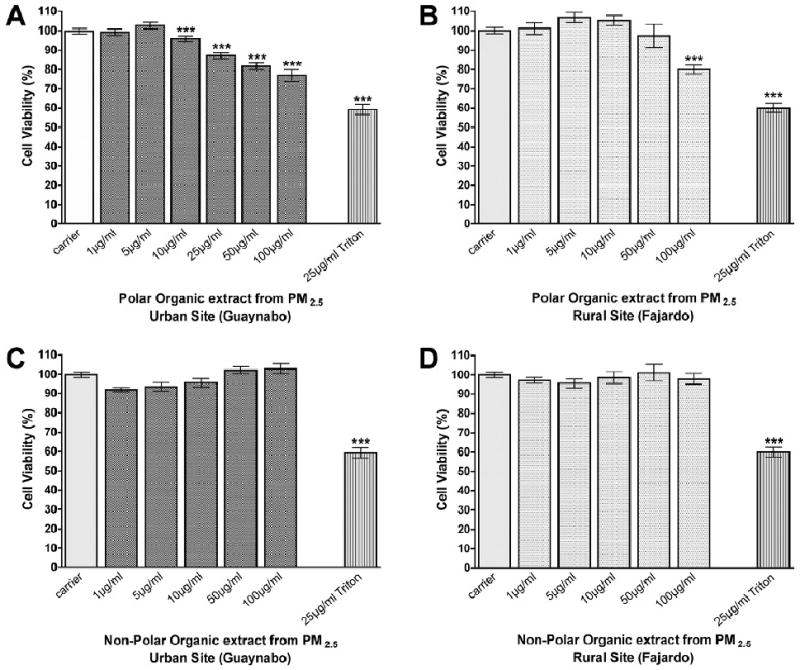
Viability of BEAS-2B cells exposed to different concentrations of polar extract composites from (A.) urban (Guaynabo) and (B.) rural (Fajardo), and non-polar extract composites from (C.) urban (Guaynabo) and (D.) rural (Fajardo). Cells viability was determined using the Neutral Red Bioassay. Asterisks (***) indicates statistical significance at p<0.001 and error bars illustrate the SE of the mean (n≥5).
Cytokines/chemokines released by BEAS-2B cells in response to organic extracts from PM2.5
Inflammatory responses in the lung have been observed after exposure to PM in both humans and animal studies (United States Environmental Protection Agency 2004). The cytokines secretion profile by immortalized human bronchial epithelial cells BEAS-2B exposed to organic extracts can provide information on the possible role of organic components in PM2.5 on respiratory illnesses due to inflammation. The effects on cytokines release by human bronchial epithelial cells due to exposure with organic extracts from PM2.5 collected at the urban and rural sites are summarized in Table 2. Six major cytokines were released or suppressed by these cells after organic extract exposure, these were, IL-6, IL-8, IL-1β, MCP-1 (MCAF), G-CSF and IL-7. The polar organic extract from PM2.5 induced the secretion of IL-6, IL-1β and IL-7 in BEAS-2B. Conversely, the same extract repressed the secretion of IL-8, MCP-1 (MCAF) and G-CSF. The pro-inflammatory cytokine TNF-α was present in all samples, but its secretion did not change after extract exposure (data not shown).
Table 2. Cytokine secretion by BEAS-2B cells after 24h exposure with polar organic extracts from PM2.5 collected in Puerto Rico.
Cytokine concentrations (pg/ml) secreted by BEAS-2B cells treated with polar organic extracts from PM2.5 collected at two different sites (Guaynabo and Fajardo) in Puerto Rico. Results are tabulated with the SE of the mean, considered statistically significant with p<0.05 and illustrated when there is a positive (+) or a negative (-) secretion pattern in response to the exposure (n = 3).
| Treatments | IL-6 (pg/ml) |
IL-8 (pg/ml) |
MCP-1 (MCAF) (pg/ml) |
IL-1β (pg/ml) |
G-CSF (pg/ml) |
IL-7 (pg/ml) |
|---|---|---|---|---|---|---|
| Control |
119.6 (±0.5) |
274.6 (±10.5) |
45.7 (±0.2) |
<LOW> |
146.1 (±2.7) |
4.9 (±0.8) |
| 50μg/ml - Guaynabo |
649.5 (±29.7)+++ |
235.1 (±6.8)--- |
14.2 (±1.1)--- |
4.9 (±0.7)+++ |
73.3 (±2.2)--- |
9.7 (±2.4) |
| 100μg/ml - Guaynabo |
603.1 (±9.0)+++ |
134 (±1.0)--- |
11.1 (±1.4)--- |
7.7 (±0.4)+++ |
39.1 (±2.7)--- |
11.8 (±1.5)++ |
| 50μg/ml - Fajardo |
522.7 (±19.9)+++ |
221.2 (±2.1)--- |
13.0 (±0.7)--- |
2.1 (±0.2)+ |
65.3 (±0.6)--- |
6.1 (±1.4) |
| 100μg/ml - Fajardo |
577.5 (±4.2)+++ |
136.7 (±4.7)--- |
11.7 (±1.0)--- |
7.6 (±0.3)+++ |
31.9 (±0.9)+++ |
11.2 (±0.4)++ |
| 10μg/ml - LPS |
942.8 (±10.9)+++ |
1274.1 (±10.9)+++ |
88.5 (±2.9)+++ |
2.3 (±0.1)+++ |
2066 (±83.5)+++ |
12.7 (±0.7)++ |
The interleukin 6 (IL-6) cytokine was vastly secreted after treatment when compared to the control (5 fold increase), specifically with polar organic extract from the urban and the rural site at 50ug/ml and 100ug/ml, respectively (Figure 4a). No cellular response to non-polar organic extract was observed at these concentrations from either site. Exposure to 100ug/ml of the non-polar organic extracts from the urban site caused an increment of only 1.57 fold (SEM ± 0.13) in IL-6; while no significant change (1.13 fold, SEM ±0.05) was observed with the rural extract (Figure 4a). These results were further confirmed at lower concentration of polar and non-polar organic extracts from the urban site during the month of July 2001 (Figure 4d).
Figure 4. Relative Secretion of IL-6, IL-8 and MCP-1 by BEAS-2B.
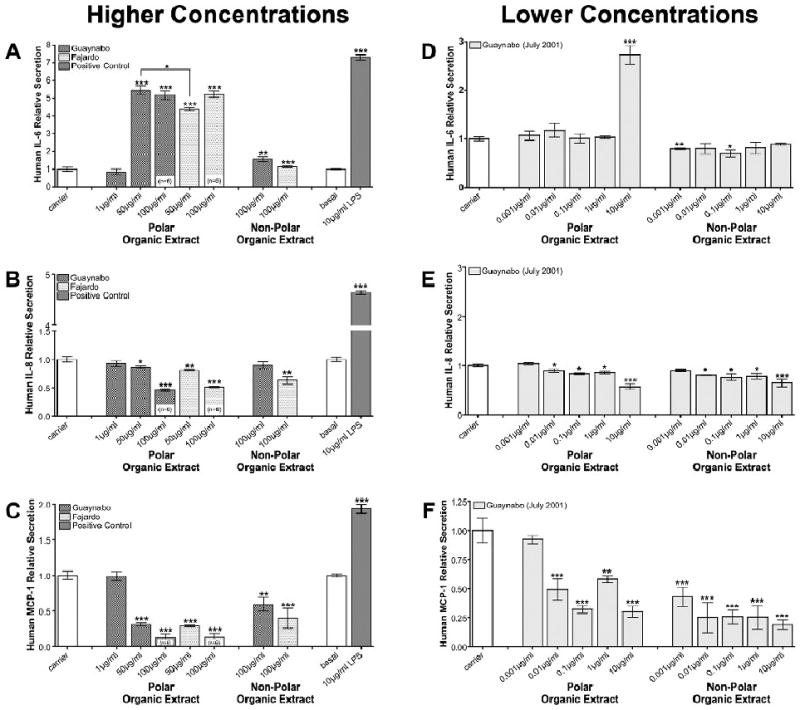
Measurement of relative secretion of (A) IL-6, (B) IL-8, and (C) MCP-1 after 24h exposure to polar and non-polar organic extracts composite from PM2.5 collected at the urban/industrialized (Guaynabo) and rural (Fajardo) sites in Puerto Rico. Measurement of relative secretion of (D) IL-6, (E) IL-8, and (F) MCP-1 after 24h exposure with polar and non-polar organic extracts from PM2.5 collected at the urban/industrialized site (Guaynabo, July 2001). Numbers in parenthesis indicate n values. Considered statistically significant at p<0.05 and error bars illustrate the SE of the mean (n≥3).
The general effects on IL-8 cell secretion by polar and non-polar extracts from both sites were suppressive in nature. The greatest effect was observed at the highest concentration of 100ug/ml (Figure 4b). This suppressive response was also confirmed at lower concentrations using extracts from the month of July 2001 (Figure 4e). In addition, significant inhibition of MCP-1 was obtained with both polar and non-polar organic extracts from either site (Figure 4c). Similar inhibition was detected at lower concentrations (0.01ug/ml) of urban organic extract from July 2001 (Figure 4f). G-CSF secretion was also reduced in a dose dependant manner upon exposure to polar organic extracts from either site (Table 2).
The absence of basal levels of IL-1β measured in our controls suggests that this pro-inflammatory cytokine is not secreted or is present at concentrations lower than 0.5pg/ml (the detection limit for the assay employed). However, exposure to polar organic extracts from either site induced the release of IL-1β in a dose dependant manner (Table 2). Furthermore, an increment in IL-7 was also observed after treatment with polar organic extract from either site (Table 2).
Relative expression of HLA-DRα, hPXR and CYP3A5 mRNA levels after organic extracts exposure
Polar organic extracts at 50ug/ml from either urban or rural site did not cause any significant change in HLA-DRα mRNA levels during the first 12h of exposure (Figure 5a). However, a 4-fold induction of HLA-DRα mRNA levels was evident after 24h of exposure with polar organic extracts (50ug/ml) from the urban site (Figure 6d). Conversely, the same concentration of polar organic extract (from either site) significantly repressed the human PXR mRNA levels after 12h and 24h (Figures 5b and 5e). Concomitant down-regulation of CYP3A5 transcriptional activity correlated with the human PXR repression after exposure (Figures 5c and 5f). However, basal transcriptional level of CYP3A5 was restored after 24h of exposure to rural polar organic extracts, whereas a down-regulation was still observed after 24h of exposure to the urban organic extracts (Figure 5f).
Figure 5. Relative up-regulation of HLA-DRα and down-regulation of hPXR and CYP3A5.
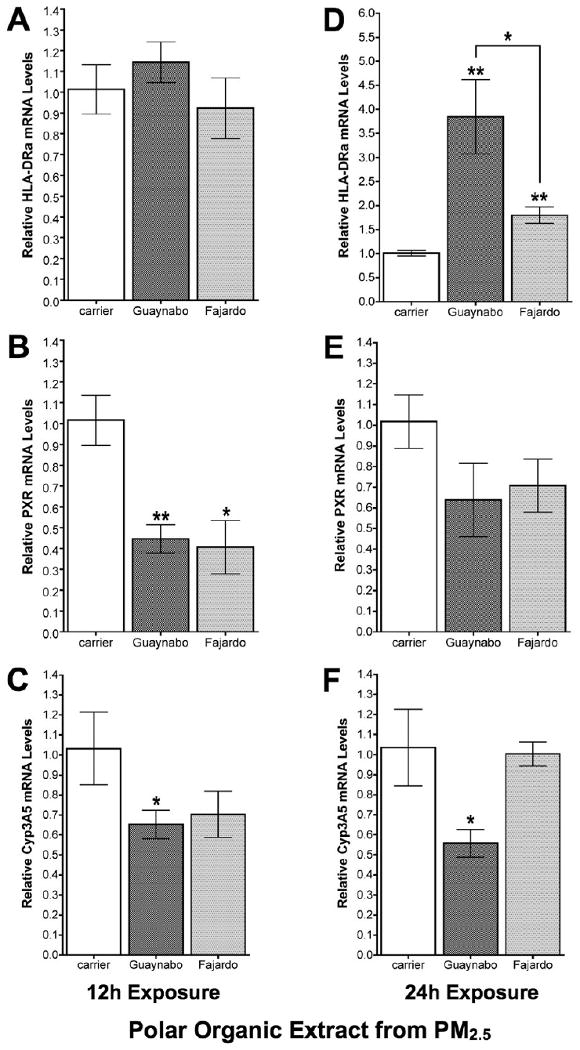
Relative mRNA levels of (A) HLA-DRα, (B) human PXR, and (C) CYP3A5 by means of two-step real time relative qPCR in BEAS-2B bronchial epithelial cells after 12h exposure with 50ug/ml of polar organic extracts from PM2.5 collected at the urban (Guaynabo) and rural (Fajardo) sites in Puerto Rico. Relative mRNA levels of (D) HLA-DRα, (E) human PXR, and (F) CYP3A5 after 24h exposure with 50ug/ml of polar organic extracts. Data normalized against GAPDH mRNA levels. Considered statistically significant at p<0.05 and error bars illustrate the SE of the mean (n≥3).
Discussion
The National Center for Environmental Health (United States Center for Disease Control), has reported that Puerto Rico has a higher overall prevalence of pulmonary diseases (e.g., asthma). A high incidence of asthma and other pulmonary illnesses have been previously reported in Puerto Rico, especially in the surroundings of the urban/industrialized area located in Guaynabo (Loyo-Berrios et al., 2007). Consistent with those reports, our group previously described that the polar organic extracts from PM10 in Puerto Rico collected in the same urban site are more cytotoxic than its counterpart rural site (Reyes et al., 2000; Molinelli et al., 2006). Fractionation of PM2.5 into polar and non-polar organic extracts can increase the resolution of its toxicological characterization and association with health effects. Polar organic extracts from PM2.5 obtained at the same urban site also showed significant cytotoxicity at 25ug/ml, with greater effect at 100ug/ml (Figure 3). These findings agree with other reports, which support the elevated associations between fine particulate air pollution and pulmonary/respiratory illnesses (Dockery et al., 1993; Reyes et al., 2000; Delfino et al., 2005; Figueroa et al., 2006).
This study investigates, for the first time, the ability of organic PM2.5 extracts from urban/industrialized and rural/reference sites in Puerto Rico to exert immunological response in exposed human respiratory tract cells. Polar organic extracts from PM2.5 collected in both sites contain components that induce the secretion of IL-6 and IL-1β in BEAS-2B cells. Although the bioactive organic components are endogenous to both sites, the response is amplified with the extracts from the urban site. IL-6 is a pro-inflammatory cytokine induced in response to environmental insults and plays an important role in acute inflammation in the lung (Thacker, 2006). Our results correlate well with other findings where the activation of IL-6 transcription has been observed in response to exposure to organic extracts from diesel exhaust particles, which are probably the major component of PM2.5 in urban areas (Baulig et al., 2003). BEAS-2B cells exposed with soil-derived PM2.5 from different sites within Utah, New Mexico and Texas, also showed a wide range of inductive potency to provoke the release of IL-6 and IL-8 (Veranth et al., 2006). Conversely, our findings show that the IL-8 secretion is suppressed by the polar organic extract from the urban site, which can be explained with a possible regulation of other genes or proteins that may interfere with IL-8 secretion. Other studies conducted with total PM2.5 from Mexico City showed that PM exposure induced the release of IL-6 and TNF-α in a monocytic cell line in a dose-dependent manner, which suggested that the observed toxicity could be mediated by transition metals (Osornio-Vargas et al., 2003). Our exposures did not affect the low levels of TNF-α basal secretion, but the secretion of interleukin 8 (IL-8), MCP-1 (MCAF) and G-CSF cytokines in BEAS-2B cells were suppressed by both polar and non-polar organic extracts composite from PM2.5 collected at both sites. The repressions of these cytokines may suggest that bronchial epithelial cells do not play a direct role in neutrophils and monocytes recruitment or in stimulating differentiation of granulocytes and neutrophils as an immune response to these organic extracts from Puerto Rico.
IL-1β has been described as a potent pro-inflammatory cytokine and mediator of a wide range of systemic human diseases (Dinarello, 2005; Koh et al., 2005; Allantaz et al., 2007). The polar organic extracts from PM2.5 collected at both stations in Puerto Rico triggered IL-1β secretion by bronchial epithelial (BEAS-2B) cells in vitro. The combined presence of IL-6 and IL-1β suggests that IL-6 secretion could be a secondary event initiated by IL-1β, which can induce (Allantaz et al., 2007; Palmqvist et al., 2008) and give stability to IL-6 mRNA transcript (Patil and Kirkwood, 2007). The secretion of IL-1β could also be considered as an early event in cardiovascular and respiratory illness due to its capacity to induce apoptosis, to inhibit myofibroblasts differentiation and repress cell proliferation in rat lung fibroblast (Zhang and Phan, 1999). VCAM-1 and ICAM-1 expression can be induced by IL-1 and IL-6 (Zambon et al., 2006). High serum levels of ICAM-1 have been reported in inflammatory airway diseases, especially in bronchial asthma (Kose et al., 2007).
The challenge still exists to obtain an adequate mass of material from PM2.5 to allow for both physicochemical characterization and biological assays. We recognize that a limitation of this study is the scarcity of a full specific organic and metal data on the extracts being tested. However, data on PM2.5 total mass and specific metals concentration in PM2.5 collected simultaneously from these same sites are described elsewhere (Figueroa et al., 2006). In previous studies we have characterized the trace elements in the polar and non-polar organic extracts from PM10 during winter 2000, these are listed as As, V, Cd, Ni, Pb, Iron (Fe) and Cu (Molinelli et al., 2006). The trace element analyses performed on PM2.5 extracts confirm the presence of these elements highlighting the relative high abundance of Cu and Zn in the polar organic extracts suggesting a possible contribution of these elements to the observed biological effect. A previous report showed that BEAS-2B cells exposed to Cu responded with an increase in IL-6 and IL-8 secretions; however, mucin proteins in the mucosal airway surfaces can reduce this observed IL-8 secretion (Kennedy et al., 1998). It has been reported that bronchial epithelial cell responses can vary depending on specific cell culture conditions including growth media. For example, BEAS-2B grown in KGM supplemented media can be more sensitive to induce IL-6 secretion in response to metals such as V, compared to LHC-9 media (Veranth et al., 2008). Therefore, we emphasize that the cell responses described in our work were obtained using KBM media (without any additional growth supplement).
A recent study demonstrated small increases in transcription of IL-6 and IL-8 genes in BEAS-2B cells exposed to airborne PM10 and PM2.5 from various sites across El Paso, Texas, during the months of August/September and October 2006, and January 2007 (Lauer et al., 2009). These increases in IL-6 and IL-8 transcriptions did not correlate with the polycyclic aromatic hydrocarbons (PAH) content in the PM used for exposure. However, the presence and effect of PAHs in PM extracts used were evidenced by the induction of detoxification genes such as CYP1A1 and several oxidative stress markers (Lauer et al., 2009). Similar to these results with a semi-polar organic extracts obtained from PM, our work indicates that PM2.5 polar extracts significantly increases IL-6 protein secretion in BEAS-2B cells, however only a slight increase was observed in response to non-polar PM2.5 extracts. Our research focused on the effects of the polar organic PM2.5 extracts on other immune and detoxification genes (HLA-DRα, PXR and CYP3A5 mRNA levels) and excluded analyses of PAH. PAH are volatile organics and a quantitative isolation was not considered in our extraction methods, limiting the possible extent of recovery, however, these are expected to appear in the non-polar organic extracts from PM2.5 particularly at the urban site. To test the effects of individual or groups of inorganic and organic constituents in PM2.5 from Puerto Rico was beyond the scope of our research. Our research however, does provide evidence that polar organic extracts from PM2.5, particularly those from the urban site can induce a series of pro-inflammatory cytokines.
MHC-II genes have been identified as important determinants of inflammation, especially in pulmonary diseases caused by the exposure to inorganic and organic compounds (Saltini et al., 1998). Hence, HLA-DRα mRNA levels can serve as an inflammation determinant. Our results indicate that the polar organic extracts from the urban site possess a greater inductive ability to provoke an immunological response in human lung epithelial cells. Simultaneous with this pro-inflammatory response, the transcription of HLA-DRα was also up-regulated. This is the first time that this effect has been described with particulate matter specifically with PM2.5 from Puerto Rico, and suggests that HLA-DRα expression in bronchial epithelial cell could be another determinant of pulmonary inflammation.
PXR is an orphan nuclear factor known to regulate the metabolisms of endogenous and exogenous organic compounds via the transcriptional induction of a myriad of genes including the CYP3A family among others. Activation of PXR can strongly induce the transcriptional activation of both CYP3A4 and CYP3A5 genes (Yeung et al., 2008), however, the predominant expression in the lung is CYP3A5 (Raunio et al., 2005). This fact supports the use of PXR and CYP3A5 transcriptional level as measurement of xenobiotic metabolism pathway activation in the lung. To the best of our knowledge this is the first time that the effects of PM2.5 extracts are investigated on PXR and CYP3A5 expression. The urban polar organic extracts of PM2.5 were found to down-regulate PXR transcription while up-regulating MHC-II. Interestingly, the transcriptional down-regulation of hPXR and CYP3A5 had been previously reported in smoking patients (Thum et al., 2006). Down-regulation of PXR and PXR-targeted genes in the intestine had been previously reported as a secondary effect of chronic inflammation (Zhou et al., 2006).
Activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) reciprocally inhibits PXR and its target genes by the disruption of the PXR/RXR heterodimers binding to its response element whereas inhibition of NF-kB enhances PXR activity (Gu et al., 2006; Zhou et al., 2006). These observations have not been reported to occur in lung, but can explain the induction of pro-inflammatory cytokines and concomitant repression of PXR and CYP3A5. The secretion of IL-1β and IL-6 suggests a possible activation of the NF-kB pathway in BEAS-2B cells after exposure to organic extract from PM2.5. The transcriptional up-regulation of HLA-DRα and the simultaneous down-regulation of human PXR can also be explained with the activation of the NF-kB pathway and subsequent up-regulation of IRF-1 as a downstream event in the IL-1β and IL-6 pathways (Beyaert et al., 1996; Madrid et al., 2001; Kroger et al., 2002; Dunne and O'Neill, 2003; Lee et al., 2006; Zhou et al., 2006). All of the mechanisms stated above are conceivable yet need to be demonstrated. Activation of NF-kB can also lead to the development of many pathological states involved in asthma as well as in chronic obstructive pulmonary disease (COPD) (Caramori et al., 2004).
This research states the grounds for future studies that will lead the way to explain the effects of specific components in organic extracts of PM2.5 from different areas of Puerto Rico, and also for a series of signal transduction experiments to elucidate the mechanisms by which specific components of airborne particulates may be involved. The identification of specific organic air pollutants on immune response could aid in the elucidation of the role of these compounds on the development of specific cardiovascular and respiratory diseases. High incidence of pulmonary illness in the Guaynabo's urban/industrialized areas correlates to the proximity of industrialized sources and air emissions (Loyo-Berrios et al., 2007), as well as with the secretion of pro-inflammatory cytokines that we found to be induced by organic compounds in PM2.5 from that same area. Secretion of IL-6 and IL-1β by bronchial epithelial cells in response to polar organic extracts from PM2.5 could play an important role in the pulmonary inflammatory response. Any possible role that PXR and CYP3A5 may play in the detoxification of organic xenobiotics in PM2.5 appears to be suppressed in the lung as a result of the triggered inflammation. The presence of these pro-inflammatory cytokines and the transcriptional induction of MHC-II suggest that organic extracts from PM2.5 can induce BEAS-2B to trigger an immune response that could be an early step orchestrating pulmonary inflammation and leading to cardiopulmonary illness.
Acknowledgments
The work presented here is part of the thesis dissertation work of Enrique Fuentes-Mattei, which participated in the conception, design and coordination of the study. Enrique Fuentes-Mattei was responsible for conducting the experiments, data analysis, interpretation of the results, and the writing of manuscript. E. Rivera was responsible for part of the data used in the results for in vitro citotoxicity studies. Adriana Gioda participated in the design of the study. Dr. Roman-Velazquez, Luis A. Alamo-Nole and Diana Sanchez-Rivera were responsible for conducting the chemical analysis experiments, its data analysis and interpretation. B.D. Jiménez-Vélez participated in the conception, design and coordination of the study. Dr. Jeremy Boss (Emory University School of Medicine) kindly provided the sequence for HLA-DRα primers. We thank our lab members for their comments, and Guermarie Velazquez-Torres and Sumaiyah Rehman for providing help in the proof reading and giving critical comments. We thank Dr. Elsa Cora and Dr. Silva for letting us use their tissue culture facilities. We especially thank Stephanie Cardona and Alan M. Preston for their critical revision of the writing. This work was supported in part by the grants: Minority Biomedical Research Support – Support of Continuous Research Excellence (MBRS-SCORE), National Institutes of Heath [grant number 5S06-GM008224]; Research Center in Minority Institutions (RCMI) Program at the University of Puerto Rico Medical Sciences Campus from the National Center for Research Resources, National Institutes of Heath [grant number G12RR03051]; Minority Biomedical Research Support – Research Initiative for Scientific Enhancement (MBRS-RISE), National Institutes of Heath [grant numbers 5R25GM061838-08, 2R25GM061838-09]; and the Center for Environmental and Toxicological Research at the University of Puerto Rico Medical Sciences Campus.
Abbreviations
- COPD
Chronic Obstructive Pulmonary Disease
- Cu
Copper
- CYP3A5
Cytochrome P450 3A5 oxidase
- DEPs
diesel exhaust particles
- DMSO
dimethyl sulfoxide
- G-CSF
granulocyte colony-stimulating factor
- IL
interleukin
- KBM
keratinocyte basal medium
- KGM
keratinocyte growth medium
- MCP-1
macrophage chemotactic protein-1
- MHC-II
major histocompatibility complex class II
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PM
particulate matter
- PM10
PM ≤10um
- PM2.5
PM ≤2.5um
- PREQB
Puerto Rico Environmental Quality Board
- PXR
pregnane X receptor
- hPXR
human PXR
- Zn
Zinc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allantaz F, Chaussabel D, Banchereau J, Pascual V. Microarray-based identification of novel biomarkers in IL-1-mediated diseases. Current opinion in immunology. 2007;19:623–632. doi: 10.1016/j.coi.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulig A, Sourdeval M, Meyer M, Marano F, Baeza-Squiban A. Biological effects of atmospheric particles on human bronchial epithelial cells. Comparison with diesel exhaust particles. Toxicol In Vitro. 2003;17:567–573. doi: 10.1016/s0887-2333(03)00115-2. [DOI] [PubMed] [Google Scholar]
- Beck S, Geraghty D, Inoko H, Rowen L. Complete sequence and gene map of a human major histocompatibility complex. The MHC Sequencing Consortium. Nature. 1999;401:921–923. doi: 10.1038/44853. [DOI] [PubMed] [Google Scholar]
- Becker S, Mundandhara S, Devlin RB, Madden M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: Further mechanistic studies. Toxicology and applied pharmacology. 2005;207:269–275. doi: 10.1016/j.taap.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Beresford GW, Boss JM. CIITA coordinates multiple histone acetylation modifications at the HLA-DRA promoter. Nat Immunol. 2001;2:652–657. doi: 10.1038/89810. [DOI] [PubMed] [Google Scholar]
- Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee JC, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- Caramori G, Adcock IM, Ito K. Anti-inflammatory inhibitors of IkappaB kinase in asthma and COPD. Curr Opin Investig Drugs. 2004;5:1141–1147. [PubMed] [Google Scholar]
- Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environmental health perspectives. 2005;113:934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Blocking IL-1 in systemic inflammation. The Journal of experimental medicine. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. The New England journal of medicine. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Dunne A, O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- Figueroa DA, Rodriguez-Sierra CJ, Jimenez-Velez BD. Concentrations of Ni and V, other heavy metals, arsenic, elemental and organic carbon in atmospheric fine particles (PM2.5) from Puerto Rico. Toxicology and industrial health. 2006;22:87–99. doi: 10.1191/0748233706th247oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Hayashi S, Hogg JC, Vincent R, Van Eeden SF. Particulate matter induces cytokine expression in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2001;25:265–271. doi: 10.1165/ajrcmb.25.3.4445. [DOI] [PubMed] [Google Scholar]
- Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. The Journal of biological chemistry. 2006;281:17882–17889. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- Jimenez-Velez BD, Gioda A, Fuentes-Mattei E. Organic and aqueous extracts from particulate matter (PM2.5) and their effect on the immunological response of human bronchial epithelial cells BEAS-2B. Metal Ions in Biology and Medicine: Proceedings of the 9th International Symposium on Metal Ions in Biology and Medicine Held; Lisboa, Portugal, Europe; May 21-24, 2006. 2006. pp. 267–272. [Google Scholar]
- Kawasaki S, Takizawa H, Takami K, Desaki M, Okazaki H, Kasama T, Kobayashi K, Yamamoto K, Nakahara K, Tanaka M, Sagai M, Ohtoshi T. Benzene-extracted components are important for the major activity of diesel exhaust particles: effect on interleukin-8 gene expression in human bronchial epithelial cells. American journal of respiratory cell and molecular biology. 2001;24:419–426. doi: 10.1165/ajrcmb.24.4.4085. [DOI] [PubMed] [Google Scholar]
- Kennedy T, Ghio AJ, Reed W, Samet J, Zagorski J, Quay J, Carter J, Dailey L, Hoidal JR, Devlin RB. Copper-dependent inflammation and nuclear factor-kappaB activation by particulate air pollution. Am J Respir Cell Mol Biol. 1998;19:366–378. doi: 10.1165/ajrcmb.19.3.3042. [DOI] [PubMed] [Google Scholar]
- Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. Journal of the American College of Cardiology. 2005;46:1978–1985. doi: 10.1016/j.jacc.2005.06.082. [DOI] [PubMed] [Google Scholar]
- Kose S, Karaman O, Islekel H, Uzuner N, Babayigit A, Olmez D, Altun Z, Turgut S, Tezcan D. Circulating adhesion molecules in sera of asthmatic children before and after steroid therapy. Allergy Asthma Proc. 2007;28:199–203. doi: 10.2500/aap.2007.28.2944. [DOI] [PubMed] [Google Scholar]
- Kroger A, Koster M, Schroeder K, Hauser H, Mueller PP. Activities of IRF-1. J Interferon Cytokine Res. 2002;22:5–14. doi: 10.1089/107999002753452610. [DOI] [PubMed] [Google Scholar]
- Lauer FT, Mitchell LA, Bedrick E, McDonald JD, Lee WY, Li WW, Olvera H, Amaya MA, Berwick M, Gonzales M, Currey R, Pingitore NE, Jr, Burchiel SW. Temporal-spatial analysis of U.S.-Mexico border environmental fine and coarse PM air sample extract activity in human bronchial epithelial cells. Toxicology and applied pharmacology. 2009;238:1–10. doi: 10.1016/j.taap.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Lee Y, Kim DS, Kwon HJ. Direct role of NF-kappaB activation in Toll-like receptor-triggered HLA-DRA expression. Eur J Immunol. 2006;36:1254–1266. doi: 10.1002/eji.200535577. [DOI] [PubMed] [Google Scholar]
- Loyo-Berrios NI, Irizarry R, Hennessey JG, Tao XG, Matanoski G. Air pollution sources and childhood asthma attacks in Catano, Puerto Rico. American journal of epidemiology. 2007;165:927–935. doi: 10.1093/aje/kwk088. [DOI] [PubMed] [Google Scholar]
- Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. The Journal of biological chemistry. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- Mauderly JL, Chow JC. Health effects of organic aerosols. Inhalation toxicology. 2008;20:257–288. doi: 10.1080/08958370701866008. [DOI] [PubMed] [Google Scholar]
- Mehta M, Chen LC, Gordon T, Rom W, Tang MS. Particulate matter inhibits DNA repair and enhances mutagenesis. Mutation research. 2008;657:116–121. doi: 10.1016/j.mrgentox.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinelli AR, Santacana GE, Madden MC, Jimenez BD. Toxicity and metal content of organic solvent extracts from airborne particulate matter in Puerto Rico. Environmental research. 2006;102:314–325. doi: 10.1016/j.envres.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Yoshitsugu H, Naito S, Hiraoka I. Evaluation of gene induction of drug-metabolizing enzymes and transporters in primary culture of human hepatocytes using high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi. 2002;122:339–361. doi: 10.1248/yakushi.122.339. [DOI] [PubMed] [Google Scholar]
- Osornio-Vargas AR, Bonner JC, Alfaro-Moreno E, Martinez L, Garcia-Cuellar C, Ponce-de-Leon Rosales S, Miranda J, Rosas I. Proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environmental health perspectives. 2003;111:1289–1293. doi: 10.1289/ehp.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin S, Pugin J. Role of MAP kinase activation in interleukin-8 production by human BEAS-2B bronchial epithelial cells submitted to cyclic stretch. Am J Respir Cell Mol Biol. 2002;27:107–114. doi: 10.1165/ajrcmb.27.1.4766. [DOI] [PubMed] [Google Scholar]
- Ovrevik J, Lag M, Holme JA, Schwarze PE, Refsnes M. Cytokine and chemokine expression patterns in lung epithelial cells exposed to components characteristic of particulate air pollution. Toxicology. 2009;259:46–53. doi: 10.1016/j.tox.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Palmqvist P, Lundberg P, Lundgren I, Hanstrom L, Lerner UH. IL-1beta and TNF-alpha regulate IL-6-type cytokines in gingival fibroblasts. J Dent Res. 2008;87:558–563. doi: 10.1177/154405910808700614. [DOI] [PubMed] [Google Scholar]
- Patil CS, Kirkwood KL. p38 MAPK signaling in oral-related diseases. J Dent Res. 2007;86:812–825. doi: 10.1177/154405910708600903. [DOI] [PubMed] [Google Scholar]
- Pope CA., 3rd Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who's at risk? Environmental health perspectives. 2000a;108 4:713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA., 3rd What do epidemiologic findings tell us about health effects of environmental aerosols? J Aerosol Med. 2000b;13:335–354. doi: 10.1089/jam.2000.13.335. [DOI] [PubMed] [Google Scholar]
- Quattrochi LC, Guzelian PS. Cyp3A regulation: from pharmacology to nuclear receptors. Drug Metab Dispos. 2001;29:615–622. [PubMed] [Google Scholar]
- Raunio H, Hakkola J, Pelkonen O. Regulation of CYP3A genes in the human respiratory tract. Chem Biol Interact. 2005;151:53–62. doi: 10.1016/j.cbi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Reyes DR, Rosario O, Rodriguez JF, Jimenez BD. Toxic evaluation of organic extracts from airborne particulate matter in Puerto Rico. Environmental health perspectives. 2000;108:635–640. doi: 10.1289/ehp.00108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltini C, Amicosante M, Franchi A, Lombardi G, Richeldi L. Immunogenetic basis of environmental lung disease: lessons from the berylliosis model. Eur Respir J. 1998;12:1463–1475. doi: 10.1183/09031936.98.12061463. [DOI] [PubMed] [Google Scholar]
- Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression's CT difference” formula. J Mol Med. 2006;84:901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- Schote AB, Turner JD, Schiltz J, Muller CP. Nuclear receptors in human immune cells: Expression and correlations. Mol Immunol. 2007;44:1436–1445. doi: 10.1016/j.molimm.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Takano H, Yanagisawa R, Ichinose T, Sadakane K, Yoshino S, Yoshikawa T, Morita M. Diesel exhaust particles enhance lung injury related to bacterial endotoxin through expression of proinflammatory cytokines, chemokines, and intercellular adhesion molecule-1. American journal of respiratory and critical care medicine. 2002;165:1329–1335. doi: 10.1164/rccm.2108122. [DOI] [PubMed] [Google Scholar]
- Thacker EL. Lung inflammatory responses. Veterinary research. 2006;37:469–486. doi: 10.1051/vetres:2006011. [DOI] [PubMed] [Google Scholar]
- Thum T, Erpenbeck VJ, Moeller J, Hohlfeld JM, Krug N, Borlak J. Expression of xenobiotic metabolizing enzymes in different lung compartments of smokers and nonsmokers. Environmental health perspectives. 2006;114:1655–1661. doi: 10.1289/ehp.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowsdale J, Campbell RD. Complexity in the major histocompatibility complex. Eur J Immunogenet. 1992;19:45–55. doi: 10.1111/j.1744-313x.1992.tb00047.x. [DOI] [PubMed] [Google Scholar]
- van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) American journal of respiratory and critical care medicine. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- Veranth JM, Cutler NS, Kaser EG, Reilly CA, Yost GS. Effects of cell type and culture media on Interleukin-6 secretion in response to environmental particles. Toxicol In Vitro. 2008;22:498–509. doi: 10.1016/j.tiv.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veranth JM, Moss TA, Chow JC, Labban R, Nichols WK, Walton JC, Watson JG, Yost GS. Correlation of in vitro cytokine responses with the chemical composition of soil-derived particulate matter. Environmental health perspectives. 2006;114:341–349. doi: 10.1289/ehp.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veranth JM, Reilly CA, Veranth MM, Moss TA, Langelier CR, Lanza DL, Yost GS. Inflammatory cytokines and cell death in BEAS-2B lung cells treated with soil dust, lipopolysaccharide, and surface-modified particles. Toxicol Sci. 2004;82:88–96. doi: 10.1093/toxsci/kfh248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EY, Sueyoshi T, Negishi M, Chang TK. Identification of Ginkgo biloba as a novel activator of pregnane X receptor. Drug Metab Dispos. 2008;36:2270–2276. doi: 10.1124/dmd.108.023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon A, Gervois P, Pauletto P, Fruchart JC, Staels B. Modulation of hepatic inflammatory risk markers of cardiovascular diseases by PPAR-alpha activators: clinical and experimental evidence. Arterioscler Thromb Vasc Biol. 2006;26:977–986. doi: 10.1161/01.ATV.0000204327.96431.9a. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1) Am J Respir Cell Mol Biol. 1999;21:658–665. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- Zhou C, Tabb MM, Nelson EL, Grun F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, Blumberg B. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116:2280–2289. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]


