Abstract
Background
Adipose harbors a large depot of free cholesterol. However, a role for adipose in cholesterol lipidation of HDL in vivo is not established. We present the first evidence that adipocytes support transfer of cholesterol to HDL in vivo as well as in vitro and implicate ABCA1 and SR-BI, but not ABCG1, cholesterol transporters in this process.
Methods and Results
Cholesterol efflux from wild-type (WT), ABCA1−/−, SR-BI−/− and ABCG1−/− adipocytes to apoA-I and HDL3 were measured in vitro. 3T3L1-adipocytes, labeled with 3H-cholesterol, were injected intraperitoneally (IP) into WT, apoA-I transgenic and apoA-I−/− mice and tracer movement onto plasma HDL monitored. Identical studies were performed with labeled WT, ABCA1−/− or SR-BI−/− mouse-embryonic-fibroblast (MEF) adipocytes. The effect of TNFα on transporter expression and cholesterol efflux was monitored during adipocyte differentiation. Cholesterol efflux to apoA-I and HDL3 was impaired in ABCA1−/− and SR-BI−/− adipocytes respectively, with no effect observed in ABCG1−/− adipocytes. Injection IP of labeled 3T3L1-adipocytes resulted in increased HDL-associated 3H-cholesterol in apoA-I transgenic mice but reduced levels in apoA-I −/− animals. Injection IP of labeled ABCA1−/− or SR-BI−/− adipocytes reduced plasma counts relative to their respective controls. TNFα reduced both ABCA1 and SR-BI expression and impaired cholesterol efflux from partially-differentiated adipocytes.
Conclusions
These data suggest a novel metabolic function of adipocytes in promoting cholesterol transfer to HDL in vivo and implicate adipocyte SR-BI and ABCA1, but not ABCG1, in this process. Further, adipocyte modulation of HDL may be impaired in adipose inflammatory disease states such as type-2 diabetes.
Keywords: cholesterol, lipoproteins, adipocytes, atherosclerosis, inflammation
INTRODUCTION
Low plasma HDL cholesterol (HDL-C) is a key feature of obesity and insulin resistance1 and has a strong inverse relationship with atherosclerotic cardiovascular disease (CVD)2. Reduced cholesterol-lipidation of nascent HDL results in small, immature lipoprotein particles that are rapidly catabolized and excreted in the kidney. Thus, lipidation of HDL plays a role in supporting the atheroprotective functions of HDL in vivo3.
Lipidation of HDL is determined via a number of cholesterol transporters in several cholesterol-rich tissues. Although macrophage cholesterol efflux to HDL plays a major role in attenuating atherosclerosis, macrophages play a minor role in regulation of HDL-C levels4. In contrast, hepatic ATP binding cassette transporter 1 (ABCA1), through lipidation of apoA-I, is required for formation of nascent HDL particles5. Indeed, in cholesterol-rich tissues, both hepatic and extrahepatic, ABCA1 has discrete and essential roles in the maintenance of plasma HDL-C6, 7. ABCG1 mediates cholesterol efflux from macrophages to mature HDL particles8, 9 and may play a role in regulating plasma HDL-C levels10. In contrast to ABC transporters, SR-BI is a bidirectional transporter that plays a major role in hepatic uptake of HDL cholesterol ester11, 12. Although SR-BI is not believed to be important in macrophage cholesterol efflux to HDL13, a role in lipidating HDL via other peripheral SR-BI-expressing tissues14 has not been examined. In fact, the relative role of tissues, beyond liver, in HDL lipidation requires further definition.
Adipose tissue contains a very large pool of free cholesterol15, 16. In fact, adipocytes are known to support cholesterol efflux to HDL and apoA-I in vitro17, 18. Recent work shows ABCA1 and SR-BI are expressed in mature adipocytes and adipocyte cholesterol homeostasis may be regulated in a cell-specific manner19–21. Adipocyte cholesterol, therefore, represents a uniquely regulated and abundant depot for modulation of HDL-C levels. By extension, adipose inflammatory dysfunction in insulin-resistant states may impair adipocyte HDL lipidation and reduce circulating HDL-C levels in such settings.
In this study, we demonstrate that adipocytes are a regulated source of cholesterol transfer to HDL both in vitro and in vivo. In contrast to liver and macrophages, adipocyte cholesterol efflux is controlled by ABCA1 and SR-BI, but not ABCG1, and is suppressed, in a differentiation-dependent manner, by TNFα an inflammatory adipocytokine. In summary, we report a novel, adipocyte-dependent mechanism of cholesterol transfer to HDL that may uniquely contribute to reduced plasma HDL-C in adipose inflammatory settings.
MATERIALS AND METHODS
Cell culture
3T3L1 cells were differentiated to adipocytes as described previously22 (online supplement). Efflux studies were performed on days 0, 5 and 10 post-differentiation. Mouse embryonic fibroblasts (MEF) were isolated from 13.5–14.5 day embryos as described23 (online supplement). MEFs were grown to confluence prior to addition of differentiation media (as for 3T3L1 except addition of 1µM of PPARγ agonist, GW347845). Human SGBS adipocytes were cultured as previously described24 (online supplement). Bone-marrow macrophages (BMM) were isolated from mouse femurs and tibias and cultured in DMEM supplemented with 10% FBS and 30% L929 conditioned medium13.
Cholesterol efflux studies
Fully differentiated ABCA1−/−, ABCG1−/−, SR-BI−/− and littermate, wild-type control MEFs as well 3T3L1 and SGBS adipocytes were labeled with 3H-cholesterol (5µCi/mL) (Perkin-Elmer Analytical Sciences, Boston, MA) for 24h. Wild-type BMM were labeled with 5µCi/ml 3H-cholesterol, and loaded with 25µg/ml acLDL for 24h. After equilibration, 3H-cholesterol efflux from adipocytes and BMM to apoA-I (20µg/ml), HDL3 (50µg/ml), 5% human (for SGBS cells) or 5% mouse (for 3T3L1, MEF and BMM cells) serum and MEM control was assessed over 4h as described previously for macrophages25. In MEF-adipocyte studies cells were equilibrated overnight in MEM containing 0.2% BSA±LXR agonist (10µM). Cells were subsequently co-treated with LXR agonist ±BLT (10µM) or ±probucol (20µM) for 2h prior to efflux. The effect of TNFα (10ng/ml overnight) on cholesterol efflux from 3T3L1 cells was assessed at day 0 (pre), day 5 (~50% differentiated) or day 10 (mature) of adipocyte differentiation. Cell lipid was extracted with isopropanol and total cellular 3H-cholesterol was measured by liquid scintillation counting. Percent efflux to acceptors, minus MEM, is presented.
Adipocyte-transfer of cholesterol to HDL in vivo
We employed a modification of our published in vivo macrophage to HDL reverse cholesterol transport (RCT) model12, 26. Briefly, ] 3T3L1 adipocytes, or MEF-adipocytes derived from SR-BI−/−, ABCA1−/− or littermate wild-type mice, were labeled with 5µCi/mL 3H-cholesterol for 24h. Cells were washed, equilibrated in DMEM containing 0.2% BSA for 6h, lifted off the plate by incubation with EDTA (10mM) for 10min, centrifuged and resuspended in MEM. Adipocyte levels of 3H-cholesterol were measured by liquid scintillation counting. Within individual studies, equal numbers of 3H-cholesterol counts (~1.2×106 cpm in MEF and ~3×106 cpm in 3T3L1) and numbers of adipocytes (~2×106 MEFs and ~7×106 3T3L1) were injected into each recipient C57BL/6 Wild-Type mouse. Movement of 3H-cholesterol from intra-peritoneal injected adipocytes onto plasma HDL and through liver to bile/feces was monitored as previously described12, 26 (online supplement). Animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Effects of inflammation on expression of cholesterol transporters in adipose
Female C57BL/6 mice were injected intravenously with 10µg/kg LPS and after 6h mice were sacrificed by cervical dislocation. Adipose tissue was harvested.RNA and protein were isolated, and mRNA and protein expression of cholesterol transporters were assessed by Real-Time PCR and Western blotting respectively (online supplement). General laboratory methods
A description of laboratory methods including lipoprotein analysis, quantitative Real-Time PCR, immunoblot analysis and oil-red staining are presented in online supplement.
Statistical analysis
Data are reported as mean ± SEM. For experiments with multiple treatments, analysis of variance (ANOVA) was used to test for differences in groups means. For mouse experiments with multiple time-points, we performed two-way repeated measures analysis of variance (ANOVA) to test for differences in means between groups. When ANOVA was significant post-hoc Bonferroni corrected t-tests were applied. For comparison of data between two groups at a single time-point (liver, bile, feces, mRNA data), unpaired t-tests were performed. GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA) and Stata 9.0 software (Stata Corp, College Station, TX) were used for statistical analyses. Statistical significance is presented as *p<0.05, **p<0.01 and ***p<0.001 in all figures.
RESULTS
Cholesterol content and cholesterol transporter expression increase during adipocyte differentiation
During 3T3L1 differentiation from fibroblasts to adipocytes (Supplement Figure 1A), cellular cholesterol content increased ~2-fold (Supplement Table 1A), while mRNA expression (reduced delta Ct values relative to β-actin) of ABCA1, ABCG1 and SR-BI all increased (Supplement Table 1B). In fully differentiated adipocytes, SR-BI was abundant with similar expression to ABCA1, but with much lower-levels of ABCG1. Immunoblotting showed increased SR-BI and ABCA1 protein during differentiation, but ABCG1 protein was barely detectable in fully differentiated adipocytes (Supplement Figure 1B&C).
Mature mouse and human adipocytes efflux cholesterol to HDL acceptors
Mature murine (3T3L1) and human (SGBS) adipocytes supported cholesterol efflux to lipid acceptors apoA-I, HDL3 and serum (Supplement Figure 1D-E). Indeed, cholesterol efflux from mature adipocytes was comparable to that from bone-marrow macrophages (BMM) in parallel efflux studies (Supplement Figure 1F). Efflux to MEM from 3T3L1 (0.77±0.01%, n=3) and SGBS (0.42±0.044%, n=3) in the absence of acceptor was minimal.
Adipocyte cholesterol efflux is mediated via ABCA1 and SR-BI transporters
ABCA1−/−, ABCG1−/−, SR-BI−/− and littermate wild-type control MEFs were differentiated into adipocytes (Supplement Figure 2A) and cholesterol efflux studies performed. Initially, the role of individual transporters was probed pharmacologically in wild-type MEF adipocytes. Probucol, an inhibitor of ABCA1-mediated efflux27, reduced cholesterol efflux from MEF adipocytes to apoA-I and abolished LXR-enhanced cholesterol efflux to apoA-I (Figure 1A). Cholesterol efflux to HDL3 was reduced by BLT, an inhibitor of SR-BI28, suggesting adipocyte SR-BI mediates lipidation of mature HDL (Figure 1B).
Figure 1.
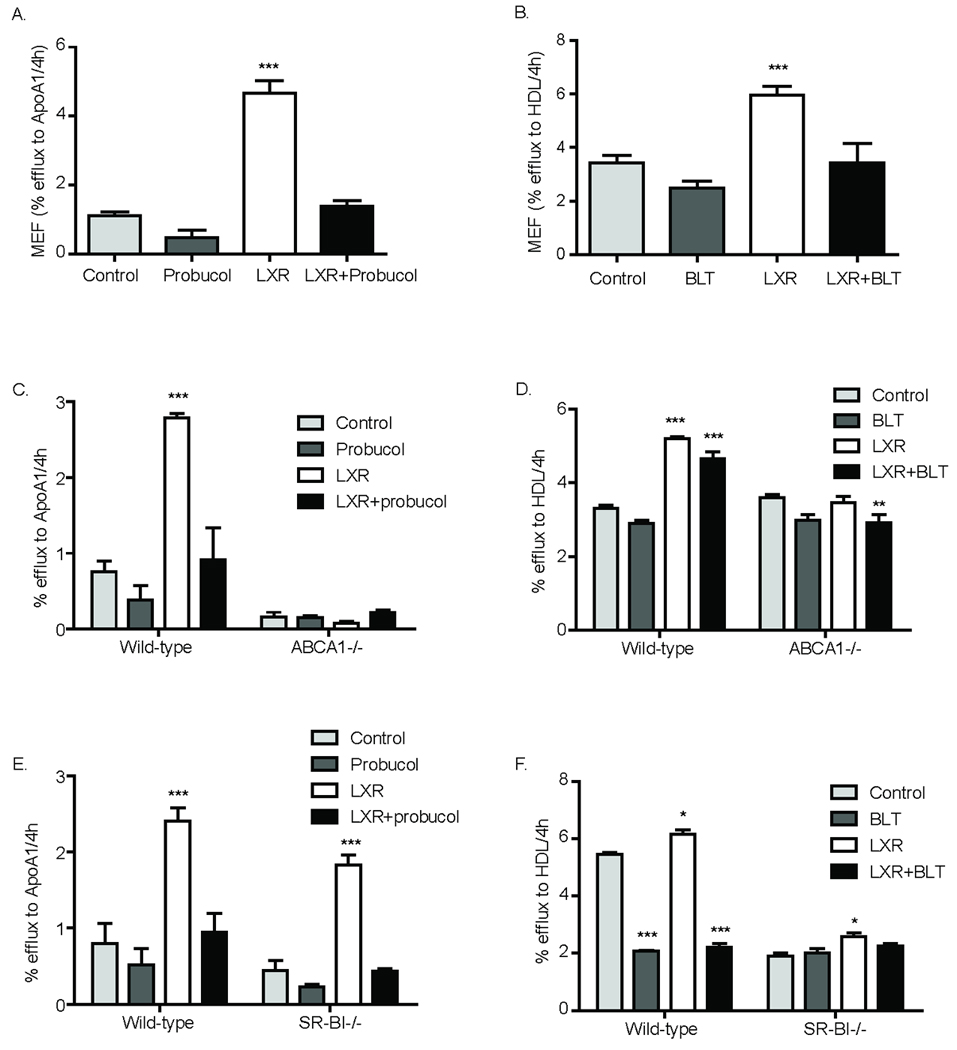
Mouse embryonic fibroblasts (MEF), derived from wild-type (WT), ABCA1−/− or SRB1−/− mice were differentiated and labeled overnight with 3H-cholesterol (5µCi/mL). Cells were equilibrated and treated overnight±LXR agonist (10µM) and co-treated±LXR agonist, ±BLT (10µM) or ±Probucol (20µM) for 2h. Cholesterol efflux from WT (A and B), WT and ABCA1−/− (C and D), and WT and SR-BI−/− (E and F) MEF adipocytes to apoA-I (20µg/ml) and HDL3 (50µg/ml) over 4h is presented; background efflux to MEM was subtracted. Efflux is presented as % total 3H-cholesterol loaded into cells (n=3–4, *p<0.05, **p<0.01, ***p<0.001 vs. control).
Similar to probucol effects (Figure 1C, left panel), ABCA1 deficiency reduced efflux to apoA-I with complete attenuation of LXR-induced efflux to apoA-I (Figure 1C, right panel). Probucol had no incremental effect on efflux to apoA-I in ABCA1−/− adipocytes. In contrast, absence of ABCA1 had no impact on cholesterol efflux to HDL3 (Figure 1D). These data suggest that (a) ABCA1 plays a central role in adipocyte cholesterol efflux to apoA-I, (consistent with macrophages13 and liver5, 29), (b) LXR-induced adipocyte cholesterol efflux is mediated via ABCA1, and (c) adipocyte ABCA1 plays a minor role in efflux to mature HDL.
SR-BI deficiency had little effect on cholesterol efflux to apoA-I and did not influence inhibition of efflux to apoA-I by probucol (Figure 1E). Lack of adipocyte SR-BI, however, resulted in marked reduction in cholesterol efflux to HDL3 (Figure 1F, right panel); this inhibition was almost identical to the BLT effect on efflux to HDL in wild-type adipocytes (Figure 1F, left panel). BLT had no incremental effect on efflux to HDL in SR-BI−/− adipocytes. These data suggest that (a) SR-BI plays an important role in adipocyte cholesterol efflux to mature HDL particles (in contrast to findings in macrophages13), and (b) SR-BI does not mediate basal or LXR-induced adipocyte cholesterol efflux to apoA-I.
ABCG1 does not regulate adipocyte cholesterol efflux in vitro
ABCG1 has been implicated in cholesterol efflux from macrophages8, 13 and liver10. Deficiency of ABCG1 in MEF adipocytes, however, had no effect on cholesterol efflux to any lipid acceptor (Figure 2A). As expected, real-time PCR analysis revealed a marked reduction in ABCG1 mRNA in knock-out cells (Figure 2B). However, ABCG1 protein expression was barely detectable in fully differentiated wild-type MEF adipocytes (Figure 2C). In fact, realtime analysis in MEFs, 3T3L1 adipocytes (Supplement Table 1), human-derived SGBS adipocytes as well as mouse (Supplement Table 2) and human adipose (Supplement Table 3) revealed very low expression of ABCG1 compared with ABCA1 and SR-BI and suggests that ABCG1 plays little role in adipocyte cholesterol homeostasis.
Figure 2.

MEF cells derived from ABCG1 or WT mice were differentiated and labeled overnight with 3H-cholesterol (5µCi/mL), equilibrated and then washed with PBS and efflux to apoA-I (20µg/ml), HDL3 (50µg/ml) or 5% serum was monitored over 4h. RNA and protein was extracted. (A) Efflux from WT and ABCG1−/− to ApoA-I, HDL3 and serum. (B) ABCG1 mRNA levels were markedly reduced in ABCG1−/− cells compared with WT control (n=3, ***p<0.001). (C) ABCG1 protein levels were barely detectable in WT and ABCG1−/− MEF adipocytes compared with positive control mouse liver lysate, positive control.
Adipocytes can transfer cholesterol to HDL in vivo
We modified our macrophage-to-feces RCT model12, 26 in order to examine adipocyte-to-HDL transport of cholesterol in vivo. We labeled fully differentiated adipocytes with 3H-cholesterol, injected labeled-adipocytes intraperitoneally (IP), and tracked movement of label onto plasma HDL and subsequently into liver and feces. First, we performed studies using 3T3L1 adipocytes injected into apoA-I transgenic (TG), apoA-I deficient or wild-type mice to establish proof-of-concept for this in vivo model. Movement of 3H-cholesterol from IP-adipocytes to plasma increased steadily in the first 24h (Figure 3A), tracked with the HDL fraction in each group (Figure 3C&D and Table 1A), and resulted in detectable tracer in liver (not shown) and feces (Figure 3B). Over-expression of apoA-I increased plasma and HDL counts consistent with enhanced adipocyte-to-HDL cholesterol movement whereas absence of apoA-I decreased plasma, HDL and fecal counts reflecting reduced movement of adipocyte-label to HDL. Notably, the time course and extent of 3H-cholesterol movement from adipocytes to HDL and through liver to feces was similar to our published findings for IP injection of macrophage-foam cells in apoA-I transgenic and null mice26, 30. These findings suggest that cholesterol movement from adipocytes into plasma in vivo is modulated by the circulating levels of HDL acceptor particles.
Figure 3.

3T3L1 adipocytes were labeled with 3H-cholesterol (5µCi/mL) overnight and equilibrated for 24h. Equal numbers of adipocytes (in 0.5mL) were intraperitoneally (IP)-injected into apoA-I transgenic (solid circle), apoA-I−/− (solid square) or wild-type (open circle) mice. Movement of 3H-cholesterol from IP-injected 3T3L1 adipocytes into (A) plasma and (B) feces was monitored over 48h (n=6, *p<0.05, **p<0.01, ***p<0.001 vs. wild-type animals). Pooled plasma was separated by FPLC and levels of (C) cholesterol mass and (D) 3H-cholesterol tracer was measured.
Table 1.
Plasma 3H-cholesterol counts associated with HDL fraction at 48h post adipocyte injection. Plasma was depleted of apoB-containing lipoproteins by PEG precipitation; counts in plasma pre-and post PEG precipitation were determined and the percentage counts in HDL fraction calculated. Data presented as mean ± SEM, n=3 for in vivo studies of (A) apoA-I modulation, (B) adipocyte ABCA1 deletion, and (C) adipocyte SR-BI deletion.
| Genotype | Percent 3H-cholesterol associated with HDL |
|
|---|---|---|
| (A) | Wild-Type | 70.8 ± 1.83 |
| ApoA-I Transgenic | 70.29 ± 5.21 | |
| ApoA-I −/− | 54.98 ± 7.17 | |
| (B) | WT MEF (ABCA1 study) | 76.21 ± 1.14 |
| ABCA1 −/− MEF | 81.63 ± 1.36 | |
| (C) | WT MEF (SR-BI study) | 82.35 ± 4.06 |
| SR-BI −/− MEF | 81.51 ± 5.83 | |
Adipocyte ABCA1 and SR-BI regulate adipocyte transfer of cholesterol to HDL in vivo
Movement of 3H-cholesterol, from IP-injected MEF-adipocytes derived from ABCA1−/− (Figure 4A&B) and SR-BI−/− (Figure 4C&D) mice, into plasma, onto HDL and into feces was significantly reduced compared to IP-injection of adipocytes derived from their wild-type littermates. As expected, the majority of plasma 3H-cholesterol counts was associated with the HDL fraction (Table 1B&C). These studies indicate that adipocytes, acting via functional ABCA1 and SR-BI, can transfer cholesterol to HDL in vivo.
Figure 4.

ABCA1−/− (A&B) or SR-BI−/− (C&D) MEF adipocytes and litter-mate wild-Type (WT) control MEF adipocytes were labeled with 3H-cholesterol (5µCi/mL) overnight and equilibrated. C57BL/6 WT mice were injected IP with WT (solid circle) or ABCA1−/− (open circle) MEF-adipocytes and levels of 3H-cholesterol tracer in (A) plasma and (B) feces was measured over 48h. C57BL/6 WT mice were injected with WT (solid circle) or SR-BI−/− (open circle) MEF-adipocytes and levels of 3H-cholesterol tracer in (C) plasma and (D) feces was measured. Levels of 3H-cholesterol are presented as % total cpm injected (n=12, *p<0.05, **p<0.01 and ***p<0.001 vs. animals injected with WT adipocytes).
Inflammation impairs cholesterol efflux from 3T3L1 adipocytes
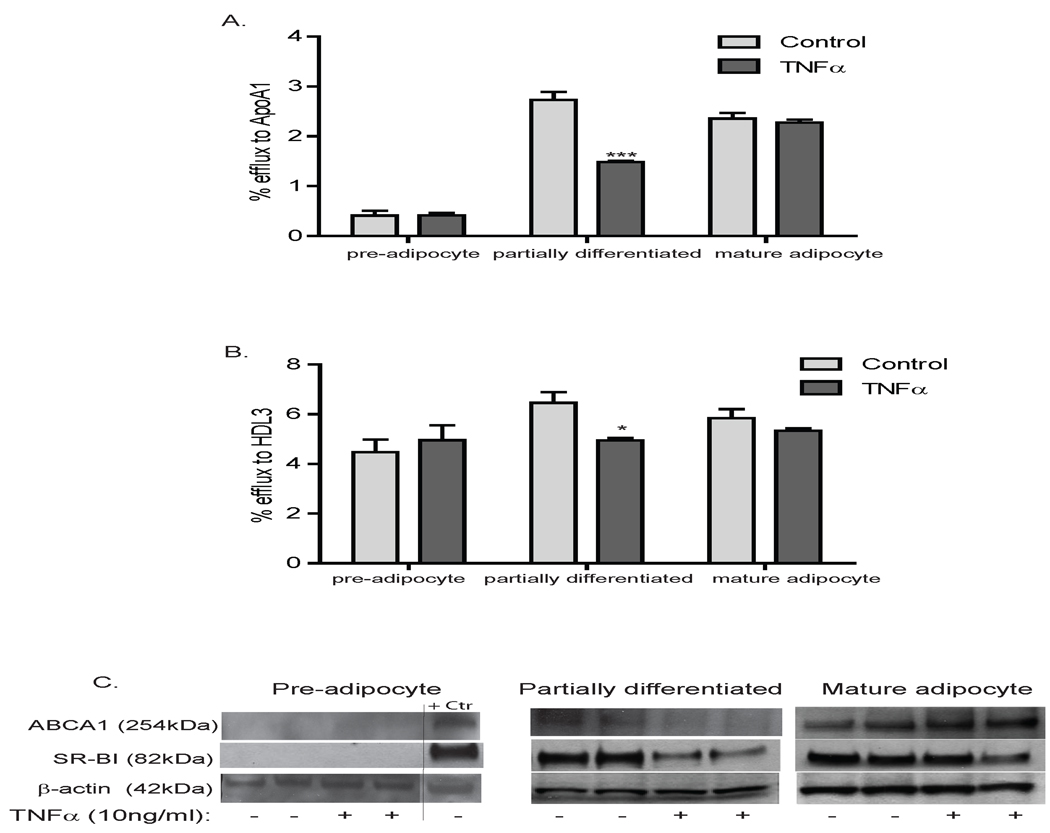
We examined whether TNFα, an inflammatory adipocytokine31, modulated 3T3L1 adipocyte cholesterol efflux in order to explore if adipocyte lipidation of HDL might be reduced during adipose inflammation, a cornerstone of central adiposity and insulin resistance32, 33. Because inflammatory modulation of adipocyte functions may be differentiation-dependent34, 35, we performed studies in pre (day-0), partially (day-5) and fully (day-10) differentiated adipocytes. Protein expression of ABCA1 and SR-BI cholesterol transporters increased during differentiation, whereas ABCG1 protein was barely detectable throughout differentiation (Supplement Figure 1B&C). Pre-adipocytes supported efflux to HDL3 but not to apoA-I (Figure 5A&B). Induction of PPARγ, adiponectin and lipoprotein lipase was suppressed by TNFα particularly in partially and fully differentiated adipocytes (Table 2). TNFα had little effect on expression of transporters and no effect on cholesterol efflux in pre-adipocytes (Figure 5A–C & Table 2). At day-5, however, TNFα inhibited efflux to apoA-I and HDL3 (Figure 5A&B) coincident with attenuation of ABCA1 and SR-BI protein expression (Figure 5C & Table 2). By day-10, there was no significant effect of TNFα on cholesterol efflux, consistent with lesser effect on ABCA1 and SR-BI in fully differentiated adipocytes (Figure 5A–C & Table 2).
Figure 5.
The effect of TNFα (10ng/ml) on cholesterol efflux from 3T3L1 cells during differentiation was assessed at day 0 (pre-adipocyte), day 5 (partially-differentiated) and day 10 (mature). Cells were labeled with 3H-cholesterol overnight prior to equilibration ±TNFα (10ng/ml). Efflux to (A) apoA-I (20µg/ml) and (B) HDL3 (50µg/ml) at day 0, 5 and 10 ±TNFα are presented. (C) Effects of TNFα vs. saline on ABCA1, ABCG1 and SR-BI protein levels at day 0, 5 and 10.
Table 2.
The effect of TNFα on ABCA1, ABCG1, SR-BI, adiponectin, lipoprotein lipase (LPL) and PPARγ mRNA expression in 3T3L1 cells during adipocyte differentiation. The ΔCt value between gene of interest and house-keeping β-actin gene is presented.
| ΔCt for gene of interest |
Pre-adipocyte | Partially (~50%) differentiated |
Mature adipocyte | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | TNFα | P | Control | TNFα | P | Control | TNFα | P | |
| ABCA1 | 4.7±0.1 | 4.9±0.1 | ns | 2.2±0.3 | 2.6±0.1 | ns | 1.5±0.2 | 3.7±0.7 | * |
| ABCG1 | 9.0±0.3 | 10.2±0.2 | * | 5.5±0.1 | 6.4±0.2 | * | 4.0±0.1 | 5.9±0.6 | * |
| SR-BI | 5.2±0.2 | 6.4±0.1 | ** | 0.9±0.1 | 2.1±0.1 | *** | 0.2±0.2 | 1.7±0.4 | * |
| Adiponectin | 13.1±1.1 | 14.8±1.4 | ns | −3.1±0.4 | −1.9±0.1 | * | −2.7±0.3 | −1.9±0.2 | ns |
| LPL | 0.5±0.4 | 1.9±0.03 | * | −4.8±0.2 | −3.3±0.1 | ** | −4.6±0.2 | −3.8±0.2 | * |
| PPARγ | 6.1±0.1 | 7.9±0.2 | ** | 1.1±0.1 | 2.1±0.1 | ** | 0.9±0.1 | 1.7±0.1 | ** |
The lower the ΔCt value, the greater the mRNA expression (*p<0.05, **p<0.01 and ***p<0.001 vs. control, n=3).
Finally, we assessed the effects of inflammation on mouse adipose expression of cholesterol transporters in vivo. Endotoxin administration (10µg/kg, IV) down-regulated adipose levels of SR-BI and ABCA1 mRNA and protein, with little effect on ABCG1 (Supplement Table 2 & Supplement Figure 2D).
DISCUSSION
Adipose tissue harbors a major pool of free cholesterol18 but its role in regulating circulating HDL-C is poorly understood. In this work, we present the first evidence that adipocytes transfer cholesterol to HDL in vivo as well as in vitro. We identified a differentiation-dependent role for ABCA1 and SR-BI, but not ABCG1, in adipocyte cholesterol efflux to apoA-I and mature HDL respectively and provide experimental evidence that both ABCA1 and SR-BI can regulate adipocyte cholesterol transfer to HDL in vivo. Finally, we show that adipocyte inflammation down-regulates transporters and impairs adipocyte cholesterol efflux to HDL. As adipose inflammation is a hallmark of central obesity and type-2 diabetes, loss of adipocyte-lipidation of HDL may directly contribute to lower HDL-C in these adipose inflammatory states.
Lipidation of HDL particles in vivo involves the coordinated effect of several tissues6, 7 likely involving cell-specific transporter functions. ABCA1 plays a major role in generation of nascent HDL particles5, 36 and maintenance of plasma HDL-C through integrated hepatic and peripheral tissue actions. Dramatic reductions in HDL-C levels are observed in the absence of ABCA16, 7 primarily because of loss of hepatic lipidation of liver-secreted apoA-I. However, peripheral ABCA1 also contributes to HDL-C6 through intestinal7 and brain37 ABCA1. Although macrophage cholesterol efflux to HDL plays a major role in attenuating atherosclerosis, macrophages contain a very small pool of cholesterol and do not regulate circulating HDL-C in vivo4. Because adipose tissue contains a large pool of free cholesterol15, 16, we hypothesized that adipocytes may play a unique role in cholesterol transfer to HDL both in vitro and in vivo. In fact, a role for adipose or involvement of non-ABCA1 transporters in peripheral lipidation of HDL has not been demonstrated.
Our findings support a model of adipocyte-specific regulation of cholesterol efflux to HDL acceptors. We identified a role for ABCA1 and SR-BI transporters in efflux to apoA-I and HDL respectively and demonstrated marked up-regulation of these proteins during adipocyte differentiation. Although ABCG1 promotes macrophage cholesterol efflux to mature HDL8, 9, 38 we found no evidence that ABCG1 protein is expressed in mature adipocytes or plays a role in adipocyte cholesterol efflux to HDL.
Using a modified version of our published macrophage-to-HDL reverse cholesterol transport model12, 26, we demonstrate that adipocytes are capable of transferring cholesterol to circulating HDL. Indeed, the timecourse and extent of cholesterol label movement onto HDL was similar to macrophages26, 39, 40. Further, cholesterol movement from IP-injected adipocytes to HDL was increased in apoA-I transgenic and reduced in apoA-I null mice. Adipocyte deficiency of ABCA1 or SR-BI reduced tracer movement onto HDL in vivo. Overall, our data provide indirect evidence for adipocyte regulation of HDL-C in vivo and suggest a role for SR-BI and ABCA1, but not ABCG1, in this process.
Recent studies suggest an underappreciated role for adipocyte cholesterol in adipose function and pathophysiologies19, 41, 42,43, 44. Zhao et al21 showed that primary adipocytes, isolated from rabbits fed a high-cholesterol diet or treated with statins, had altered cholesterol efflux to HDL that correlated with changes in SR-BI expression. They did not prove, however, that SR-BI was causal. Verghese and colleagues42 demonstrated that enhanced adipocyte cholesterol efflux to HDL occurs during lipolysis without change in SR-BI and ABCA1 expression. It is possible, however, that modulation of transporter function45–47 or membrane localization40 rather than change in protein level could mediate this efflux. Our studies provide novel data that go beyond prior correlative studies. We addressed directly the role of specific transporters and performed in vivo studies examining the potential for adipocytes and specific transporters to transfer cholesterol to HDL in vivo. Future work with adipose-specific, conditional modulation of ABCA1 and SR-BI in rodent models is required to confirm the importance of these transporters and adipose regulation of HDL cholesterol mass in vivo.
Our in vivo experimental model does have limitations including its non physiological nature, use of exogenous cells and reliance on cholesterol tracer rather than mass. The peritoneal space is a convenient experimental location in which cells are exposed to extracellular fluid that has many of the characteristics of extracellular fluid in other tissues. Importantly, this model has provided fundamental insights into the macrophage reverse cholesterol transport process12, 26. Work by Sehayek and colleagues48 demonstrates that the subcutaneous administration of macrophages provides a similar pattern of reverse cholesterol transport as the peritoneal cavity arguing against any unique properties for the peritoneum. Although adipocytes do not occur as single cells in the peritoneum, IP-injection of labeled adipocytes resulted in cholesterol-label movement to plasma HDL that was remarkably similar to that published for macrophages. Therefore, we doubt a systematic difference between adipocyes and macrophages in the intraperitoneal model.
We examined the impact of an inflammatory adipocytokine on adipocyte cholesterol efflux in order to explore if loss of adipocyte HDL-lipidation is one possible mechanism for reduced HDL-C in adipose-inflammatory settings33, 49. Because adipocyte susceptibility to inflammation34, 35 depends on adipocyte maturity, we examined TNFα effects during differentiation. TNFα impaired cholesterol efflux most in partially-differentiated adipocytes coincident with greatest suppression of ABCA1 and SR-BI. This is consistent with work by Chung et al. who reported that endotoxin impaired glucose transport maximally in partially-differentiated adipocytes34. We also found that endotoxemia down-regulated adipose SR-BI and ABCA1 in vivo. Thus, despite increased adipose mass and adipose cholesterol in obesity, attenuation of adipocyte-mediated HDL-lipidation may directly contribute to lower HDL-C in metabolic syndrome and type-2 diabetes (Figure 6).
Figure 6.
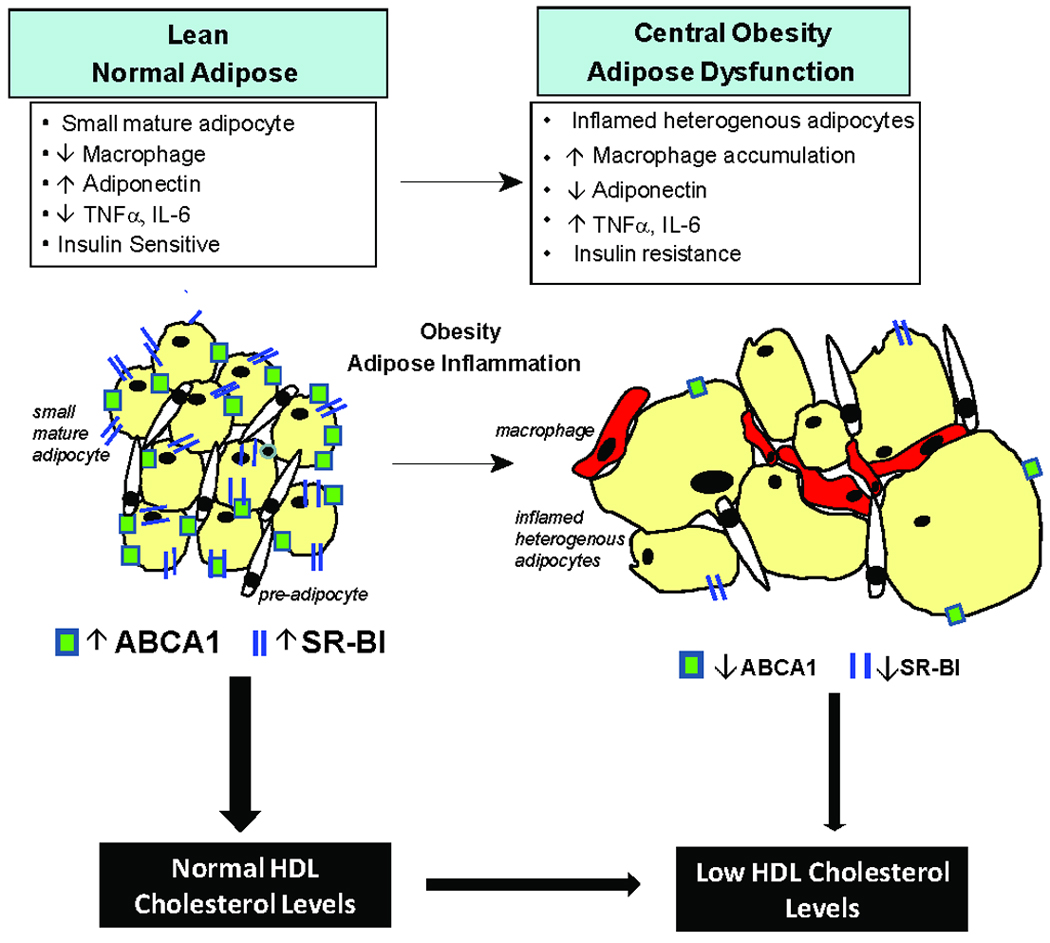
As adipose inflammation is a hallmark of central obesity and type-2 diabetes, loss of adipocyte lipidation of HDL may directly contribute to lower HDL-C levels in these inflammatory, insulin resistant states. Despite greater adipose mass and cholesterol content in adiposity, adipocyte inflammation is associated with reduced expression of the cholesterol efflux transporters, ABCA1 and SR-BI, and impaired cholesterol efflux to apoA-I and HDL particles.
In conclusion, adipocytes support transfer of cholesterol to HDL in vivo. This process is mediated by ABCA1 and SR-BI, but not ABCG1, and is attenuated in inflamed adipocytes. Our findings suggest adipocyte-specific cholesterol transporter functions and a role for mature adipose in maintenance of HDL-C levels. Conversely, adipose inflammation may attenuate adipocyte lipidation of HDL leading to lower HDL-C in metabolic syndrome and type-2 diabetes.
SUMMARY
Adipose tissue harbors a major pool of free cholesterol but its role in regulating circulating HDL-C is poorly understood. In this work, we present the first evidence that adipocytes transfer cholesterol to HDL in vivo as well as in vitro. We identified a differentiation-dependent role for the lipid-transporters ABCA1 and SR-BI, but not ABCG1, in adipocyte cholesterol efflux to apoA-I and mature HDL respectively. We also provide experimental evidence that both ABCA1 and SR-BI can regulate adipocyte cholesterol transfer to HDL in vivo. Finally, we show that adipocyte inflammation down-regulates transporters and impairs adipocyte cholesterol efflux to HDL. Our findings suggest a role for mature adipose in directly maintaining HDL-C levels. Conversely, adipose inflammation may attenuate adipocyte lipidation of HDL and may directly contribute to lower HDL-C in adipose inflammatory states such as central obesity and type-2 diabetes. Thus adipose tissue cholesterol homeostasis may be a direct therapeutic target for modulation of HDL levels in vivo.
Supplementary Material
ACKNOWLEGEMENTS
We would like to gratefully acknowledge Aisha Wilson, Maosen Sun, Edwige Edouard, and Leticia Pruscino for their technical expertise.
FUNDING SOURCES
This work was supported by the Alternative Drug Discovery Initiative award to the University of Pennsylvania from GlaxoSmithKline, a Clinical and Translational Science Award (UL1RR024134) from the National Center for Research Resources (NCRR) and a Diabetes and Endocrine Research Center (P20-DK 019525) award, both to the University of Pennsylvania, as well as RO1 HL-073278 (Dr Reilly) and P50 HL-083799-SCCOR (Dr Reilly).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Dr Reilly is the recipient of research grants from GlaxoSmithKline and Merck Research Laboratories. The other authors report no potential conflicts of interest.
REFERENCES
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Jama. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Gordon DJ, Rifkind BM. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 3.Brunham LR, Singaraja RR, Duong M, Timmins JM, Fievet C, Bissada N, Kang MH, Samra A, Fruchart JC, McManus B, Staels B, Parks JS, Hayden MR. Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:548–554. doi: 10.1161/ATVBAHA.108.182303. [DOI] [PubMed] [Google Scholar]
- 4.Haghpassand M, Bourassa PA, Francone OL, Aiello RJ. Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma HDL levels. J Clin Invest. 2001;108:1315–1320. doi: 10.1172/JCI12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singaraja RR, Van Eck M, Bissada N, Zimetti F, Collins HL, Hildebrand RB, Hayden A, Brunham LR, Kang MH, Fruchart JC, Van Berkel TJ, Parks JS, Staels B, Rothblat GH, Fievet C, Hayden MR. Both hepatic and extrahepatic ABCA1 have discrete and essential functions in the maintenance of plasma high-density lipoprotein cholesterol levels in vivo. Circulation. 2006;114:1301–1309. doi: 10.1161/CIRCULATIONAHA.106.621433. [DOI] [PubMed] [Google Scholar]
- 7.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klucken J, Buchler C, Orso E, Kaminski WE, Porsch-Ozcurumez M, Liebisch G, Kapinsky M, Diederich W, Drobnik W, Dean M, Allikmets R, Schmitz G. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc Natl Acad Sci U S A. 2000;97:817–822. doi: 10.1073/pnas.97.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Wiersma H, Nijstad N, de Boer JF, Out R, Hogewerf W, Van Berkel TJ, Kuipers F, Tietge UJ. Lack of Abcg1 results in decreased plasma HDL cholesterol levels and increased biliary cholesterol secretion in mice fed a high cholesterol diet. Atherosclerosis. 2009;206:141–147. doi: 10.1016/j.atherosclerosis.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Van Eck M, Twisk J, Hoekstra M, Van Rij BT, Van der Lans CA, Bos IS, Kruijt JK, Kuipers F, Van Berkel TJ. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J Biol Chem. 2003;278:23699–23705. doi: 10.1074/jbc.M211233200. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altmann SW, Davis HR, Jr, Yao X, Laverty M, Compton DS, Zhu LJ, Crona JH, Caplen MA, Hoos LM, Tetzloff G, Priestley T, Burnett DA, Strader CD, Graziano MP. The identification of intestinal scavenger receptor class B, type I (SR-BI) by expression cloning and its role in cholesterol absorption. Biochim Biophys Acta. 2002;1580:77–93. doi: 10.1016/s1388-1981(01)00190-1. [DOI] [PubMed] [Google Scholar]
- 15.Krause BR, Hartman AD. Adipose tissue and cholesterol metabolism. J Lipid Res. 1984;25:97–110. [PubMed] [Google Scholar]
- 16.Kovanen PT, Nikkila EA. Cholesterol exchange between fat cells, chylomicrons and plasma lipoproteins. Biochim Biophys Acta. 1976;441:357–369. doi: 10.1016/0005-2760(76)90233-2. [DOI] [PubMed] [Google Scholar]
- 17.Angel A, Yuen R, Nettleton JA. Exchange of free cholesterol between low density lipoproteins and human adipocytes. Can J Biochem. 1981;59:655–661. doi: 10.1139/o81-091. [DOI] [PubMed] [Google Scholar]
- 18.Prattes S, Horl G, Hammer A, Blaschitz A, Graier WF, Sattler W, Zechner R, Steyrer E. Intracellular distribution and mobilization of unesterified cholesterol in adipocytes: triglyceride droplets are surrounded by cholesterol-rich ER-like surface layer structures. J Cell Sci. 2000;113:2977–2989. doi: 10.1242/jcs.113.17.2977. [DOI] [PubMed] [Google Scholar]
- 19.Le Lay S, Robichon C, Le Liepvre X, Dagher G, Ferre P, Dugail I. Regulation of ABCA1 expression and cholesterol efflux during adipose differentiation of 3T3-L1 cells. J Lipid Res. 2003;44:1499–1507. doi: 10.1194/jlr.M200466-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Zhao SP, Dong SZ. Effect of tumor necrosis factor alpha on cholesterol efflux in adipocytes. Clin Chim Acta. 2008;389:67–71. doi: 10.1016/j.cca.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Zhao SP, Wu ZH, Hong SC, Ye HJ, Wu J. Effect of atorvastatin on SR-BI expression and HDL-induced cholesterol efflux in adipocytes of hypercholesterolemic rabbits. Clin Chim Acta. 2006;365:119–124. doi: 10.1016/j.cca.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Elmendorf JS, Chen D, Pessin JE. Guanosine 5'-O-(3-thiotriphosphate) (GTPgammaS) stimulation of GLUT4 translocation is tyrosine kinase-dependent. J Biol Chem. 1998;273:13289–13296. doi: 10.1074/jbc.273.21.13289. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Zhao L, Wang C, Lei B. Isolation and culture of mouse embryonic fibroblast. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34:344–346. [PubMed] [Google Scholar]
- 24.Wabitsch M, Brenner RE, Melzner I, Braun M, Moller P, Heinze E, Debatin KM, Hauner H. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int J Obes Relat Metab Disord. 2001;25:8–15. doi: 10.1038/sj.ijo.0801520. [DOI] [PubMed] [Google Scholar]
- 25.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–663. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 27.Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler Thromb Vasc Biol. 2004;24:2345–2350. doi: 10.1161/01.ATV.0000148706.15947.8a. [DOI] [PubMed] [Google Scholar]
- 28.Nieland TJ, Chroni A, Fitzgerald ML, Maliga Z, Zannis VI, Kirchhausen T, Krieger M. Cross-inhibition of SR-BI- and ABCA1-mediated cholesterol transport by the small molecules BLT-4 and glyburide. J Lipid Res. 2004;45:1256–1265. doi: 10.1194/jlr.M300358-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 30.Moore RE, Navab M, Millar JS, Zimetti F, Hama S, Rothblat GH, Rader DJ. Increased atherosclerosis in mice lacking apolipoprotein A-I attributable to both impaired reverse cholesterol transport and increased inflammation. Circ Res. 2005;97:763–771. doi: 10.1161/01.RES.0000185320.82962.F7. [DOI] [PubMed] [Google Scholar]
- 31.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 32.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 34.Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147:5340–5351. doi: 10.1210/en.2006-0536. [DOI] [PubMed] [Google Scholar]
- 35.Harkins JM, Moustaid-Moussa N, Chung YJ, Penner KM, Pestka JJ, North CM, Claycombe KJ. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134:2673–2677. doi: 10.1093/jn/134.10.2673. [DOI] [PubMed] [Google Scholar]
- 36.Vaisman BL, Lambert G, Amar M, Joyce C, Ito T, Shamburek RD, Cain WJ, Fruchart-Najib J, Neufeld ED, Remaley AT, Brewer HB, Jr, Santamarina-Fojo S. ABCA1 overexpression leads to hyperalphalipoproteinemia and increased biliary cholesterol excretion in transgenic mice. J Clin Invest. 2001;108:303–309. doi: 10.1172/JCI12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karasinska JM, Rinninger F, Lutjohann D, Ruddle P, Franciosi S, Kruit JK, Singaraja RR, Hirsch-Reinshagen V, Fan J, Brunham LR, Bissada N, Ramakrishnan R, Wellington CL, Parks JS, Hayden MR. Specific loss of brain ABCA1 increases brain cholesterol uptake and influences neuronal structure and function. J Neurosci. 2009;29:3579–3589. doi: 10.1523/JNEUROSCI.4741-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Wagner AC, Frank JS, Datta G, Garber D, Fogelman AM. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109:3215–3220. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- 40.Rotllan N, Ribas V, Calpe-Berdiel L, Martin-Campos JM, Blanco-Vaca F, Escola-Gil JC. Overexpression of human apolipoprotein A-II in transgenic mice does not impair macrophage-specific reverse cholesterol transport in vivo. Arterioscler Thromb Vasc Biol. 2005;25:e128–e132. doi: 10.1161/01.ATV.0000175760.28378.80. [DOI] [PubMed] [Google Scholar]
- 41.Izem L, Morton RE. Possible role for intracellular cholesteryl ester transfer protein in adipocyte lipid metabolism and storage. J Biol Chem. 2007;282:21856–21865. doi: 10.1074/jbc.M701075200. [DOI] [PubMed] [Google Scholar]
- 42.Verghese PB, Arrese EL, Soulages JL. Stimulation of lipolysis enhances the rate of cholesterol efflux to HDL in adipocytes. Mol Cell Biochem. 2007;302:241–248. doi: 10.1007/s11010-007-9447-0. [DOI] [PubMed] [Google Scholar]
- 43.Le Lay S, Krief S, Farnier C, Lefrere I, Le Liepvre X, Bazin R, Ferre P, Dugail I. Cholesterol, a cell size-dependent signal that regulates glucose metabolism and gene expression in adipocytes. J Biol Chem. 2001;276:16904–16910. doi: 10.1074/jbc.M010955200. [DOI] [PubMed] [Google Scholar]
- 44.Yvan-Charvet L, Bobard A, Bossard P, Massiera F, Rousset X, Ailhaud G, Teboul M, Ferre P, Dagher G, Quignard-Boulange A. In vivo evidence for a role of adipose tissue SR-BI in the nutritional and hormonal regulation of adiposity and cholesterol homeostasis. Arterioscler Thromb Vasc Biol. 2007;27:1340–1345. doi: 10.1161/ATVBAHA.106.136382. [DOI] [PubMed] [Google Scholar]
- 45.Haidar B, Denis M, Krimbou L, Marcil M, Genest J., Jr cAMP induces ABCA1 phosphorylation activity and promotes cholesterol efflux from fibroblasts. J Lipid Res. 2002;43:2087–2094. doi: 10.1194/jlr.m200235-jlr200. [DOI] [PubMed] [Google Scholar]
- 46.Tang C, Vaughan AM, Oram JF. Janus kinase 2 modulates the apolipoprotein interactions with ABCA1 required for removing cellular cholesterol. J Biol Chem. 2004;279:7622–7628. doi: 10.1074/jbc.M312571200. [DOI] [PubMed] [Google Scholar]
- 47.Yamauchi Y, Hayashi M, Abe-Dohmae S, Yokoyama S. Apolipoprotein A-I activates protein kinase C alpha signaling to phosphorylate and stabilize ATP binding cassette transporter A1 for the high density lipoprotein assembly. J Biol Chem. 2003;278:47890–47897. doi: 10.1074/jbc.M306258200. [DOI] [PubMed] [Google Scholar]
- 48.Sehayek E, Hazen SL. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler Thromb Vasc Biol. 2008;28:1296–1297. doi: 10.1161/ATVBAHA.108.165803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.