1. Introduction
Comorbid substance use disorders among individuals with serious mental illnesses have gained much attention in recent decades, and research into the complex relationships between these disorders is accumulating. Substance use disorders are consistently reported at a higher rate among individuals with serious mental illnesses than in the general population (Siegfried, 1998; RachBeisel et al., 1999; Drake et al., 2002; Buckley & Brown, 2006). Among individuals with schizophrenia, comorbid substance misuse is associated with poorer outcomes, including more severe psychotic symptoms, poorer adherence to medications, greater potential for violence, a higher likelihood of housing instability, and more frequent hospital admissions (Dixon, 1999; Hunt et al., 2002; Westermeyer, 2006; Winklbaer et al., 2006; Compton et al., 2007). As research into the complex relationships between serious mental illnesses and substance use disorders progresses, more information is needed on the temporal sequencing of symptoms and substance use, cumulative exposure to specific substances, and problematic behaviors related to use. Inquiry into these subjects would be facilitated by new methods for collecting comprehensive data on substance use.
Current approaches to measuring substance use are varied and each methodology has advantages and limitations. Objective approaches to assessing substance use include measurement of substance-related biomarkers in urine, blood, or hair. Screening for such substances in urine and blood is generally considered a valid and reliable approach, but is time-limited, only yielding data on substances used in the days (or for some substances, like cannabis, in the weeks) prior to data collection. Hair analysis (Kintz et al., 2006; Pragst & Balikova, 2006) may provide data over a longer period of time (e.g., weeks to months) compared to other biological sources, but this method is costly and only feasible if subjects are able to provide an adequate quantity of hair. Furthermore, the validity of biological assays has been questioned, due to the susceptibility of results being influenced by multiple factors outside of the individual’s substance-use, such as their metabolism (O’Farrell & Maisto, 1987; Harrison, 1997; Buchanan et al. 2002).
In terms of interview-based measurement, collateral reports are valuable, but friends and family members are often incompletely aware of loved ones’ substance use patterns. Self-report measures are widely used, non-invasive, and may be the best method for obtaining long-term, retrospective data on substance use (O’Farrell & Maisto, 1987). The accuracy of self-report data has been questioned by many, due to social desirability or underreporting biases and difficulties with recall (O’Farrell & Maisto, 1987; Brown et al., 1992; Harrison, 1997). However, many self-report instruments have demonstrated reliability and validity, giving valuable insight into a broad scope of drug-use behaviors, including onset, progression, frequency, amounts, and consequential behaviors (O’Farrell & Maisto, 1987; Sobell & Sobell, 1992; Harrison, 1997; Weiss et al. 1998).
A variety of self-report measures are available, but none provide year-by-year data on substance intake since initiation of use. Many brief screening instruments elicit information about current and recent substance use, including the Michigan Alcoholism Screening Test (MAST; Selzer, 1971), the CAGE Questionnaire (Mayfield et al., 1974), the Drug Abuse Screening Test (DAST; Skinner, 1982), and the Dartmouth Assessment of Lifestyle Instrument (Rosenberg et al., 1998). The Addiction Severity Index (McLellan et al., 1980) is often used to measure substance misuse in greater detail, though it may not be appropriate for use with individuals with serious mental illnesses (Carey et al., 1997; Zanis et al., 1997). The time-line follow-back approach is a well-respected technique for measuring recent substance use, but is not intended for use beyond the preceding year (Sobell & Sobell, 1992). The Composite International Diagnostic Interview Substance Abuse Module is a valid and reliable instrument for collecting data on the onset of substance use, the presence of substance use disorders, and both frequency and quantity of consumption, but only during the first year and heaviest year of use (Wittchen 1994; Compton et al. 1996; Atkan et al. 1997). Similarly, the Alcohol Use Disorder and Associated Disabilities Interview Schedule elicits extensive information about substance use disorders and frequency and quantity of use in selected years. However, with a primary focus on alcohol, this instrument does not collect adequate information on other substances to derive a dose amount, which relies both on frequency and quantity of use (Grant et al. 1995). The Retrospective Alcohol and Other Substance Use Measure does collect year-by-year data on the frequency and quantity of alcohol use and the frequency of drug consumption, but it is limited to the five years prior to the interview and does not provide data for each individual illicit substance used (Windle, 2005).
While each of the above-mentioned instruments is a valuable tool for measuring substance use and abuse, most focus primarily on behaviors or consequences of substance use, and none are designed to collect extensive, standardized information on the quantity of substances consumed throughout the entire course of a person’s substance use history. Many research questions pertaining to comorbid substance use disorders among individuals with serious mental illnesses require a thorough assessment of substance use, including cumulative lifetime use, beyond that which can be measured using the aforementioned assays, screening and severity instruments, and interviewing approaches. For example, our ongoing research seeks to address the ways that pre-illness cannabis use may influence the early course, including the age at onset, of psychotic disorders (Compton & Ramsay, 2009; Compton et al., 2009a). In light of substantial limitations of available instruments, and a nearly complete absence of methodologies that allow a derivation of continuous measures that estimate a “dose” of exposure, this report describes development and initial validation of two novel, multidimensional measures of substance use, the Lifetime Substance Use Recall (LSUR) and Longitudinal Substance Use Recall for 12 Weeks (LSUR-12) Instruments. In addition to providing extensive and granular data on all substances used over the course of the lifetime, these new interviewer-administered instruments provide structured visual tools to aid recall and enhance the validity of this retrospective data. The objectives of the present report are to describe the new instruments and provide data on validity of scores derived from them.
2. Methods
2.1. Development of the two measures
2.1.1. The Lifetime Substance Use Recall (LSUR) Instrument
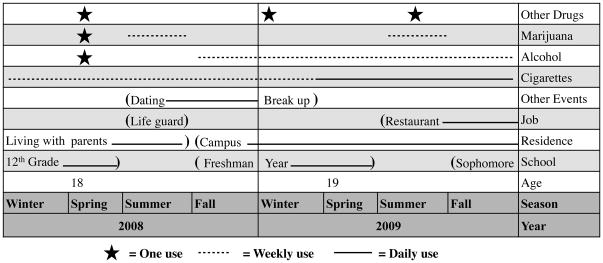
The LSUR is comprised of the LSUR Timeline, LSUR Interview Guide, and LSUR Scoring Sheet. A novel component of this instrument is the use of the Timeline, which is completed collaboratively with the patient, to inform and guide data collection. This Timeline, an example of which is depicted in Figure 1, enables the participant and interviewer to track participants’ age, school status, employment, residence, and other important life events over each calendar year. This serves as the basis for the questions asked by the researcher using the LSUR Interview Guide (and the numerical values recorded on the LSUR Scoring Sheet) as data collection tied to specific contexts may trigger more precise memories or estimates of substance use in the preceding years. On the Timeline, (see Figure 1) vertical lines delineate each calendar year, and space is provided to indicate the beginning and end of activities and trends in tobacco, alcohol, and drug use. A standard format is suggested, with a star indicating first or very occasional use, dotted horizontal lines indicating weekly use, and solid horizontal lines indicating daily use. The format of the Timeline was developed and refined based on pilot testing within the population of interest (hospitalized patients with first-episode psychosis).
Figure 1.
Snapshot of an example timeline used to facilitate recollection and accurate measurement of past substance use
This figure represents the timeline of a participant who is 19 years old, in college, works in a restaurant, and lives on campus. He/she had a prior job as a life guard, and was in a dating relationship from the summer of 2008 to the early spring of 2009. At the end of 2009, he/she used alcohol on a weekly basis and tobacco on a daily basis. The participant used cannabis on a weekly basis during the summers of 2008 and 2009, but not during the school year. He/she used another drug on three occasions in the past two years.
The LSUR Interview Guide was developed to facilitate the collection of calendar-year-specific estimates of nicotine, alcohol, and drug use, beginning with the year of first use and repeating for each year until the present. During Step 1, information is collected on first use, first weekly use, and first daily use of tobacco, alcohol, cannabis, and any other drugs that were used, including abused prescription medications. In Step 2, the average number of days of use per month is collected (and recorded on the LSUR Scoring Sheet) for each year from the year of first use to present. For tobacco, participants indicate whether they primarily smoked cigarettes, cigars, or another form of tobacco. Then, the average number of units per day of use is collected for each year, recorded in terms of cigarettes or cigars, standard drinks, and joint-equivalents. If participants report using blunts or other forms of cannabis, they are asked to convert those into joint-equivalents. Additionally, the portion of time smoking cannabis within a group of people and by oneself, the size of an average group, and the average units of use in each context is gathered. Use of other drugs is recorded in standard units (e.g., one rock of crack cocaine, one ecstasy pill). Key constructs and derived scores of the LSUR are described in Table 1. While lifetime doses are presented in the present study, other aggregate could be derived from the data (e.g., doses during specific years or time periods, frequency of use, average amounts used, and other summary scores based on the variables of interest).
Table 1.
Key Constructs and Derived Scores of the LSUR and LSUR-12 and their Operationalizations
| The Lifetime Substance Use Recall (LSUR) Instrument | |
| Ages at onset of nicotine use, alcohol use, cannabis use, and other druga use | Subjects are asked both their age and the year when they: (1) first used each substance, (2) began using each substance on a weekly basis, and (3) began using each substance on a daily basis |
| Lifetime nicotine dose | For each year since first use, subjects report: (1) the average number of days of smoking per month, and (2) the average number of cigarettes/cigarsb per day of smoking. These are multiplied for a yearly dose, and yearly scores are summed for a lifetime dose.c |
| Lifetime alcohol dose | For each year since first use, subjects report: (1) the average number of days of drinking per month, and (2) the average number of standard drinksd per day of drinking. These are multiplied for a yearly dose, and yearly scores are summed for a lifetime dose.c |
| Lifetime cannabis dosee | For each year since first use, subjects report: (1) the average number of days of smoking per month, and (2) the average number of joint-equivalentsf per day of smoking. These are multiplied for a yearly dose, and yearly scores are summed for a lifetime dose.c |
| Alcohol-related problem behaviors | Subjects are asked the number of: (1) fights or heated arguments while drinking, (2) fights or heated arguments about alcohol, (3) times having trouble with the law while drinking or because of alcohol, (4) black-outs, and (5) attempts at cutting back. These are summed. |
| Cannabis-related problem behaviorse | Subjects are asked the number of: (1) fights or heated arguments while smoking, (2) fights or heated arguments about cannabis, (3) times having trouble with the law related to cannabis, and (4) attempts at cutting back. These are summed. |
| The Longitudinal Substance Use Recall for 12 Weeks (LSUR-12) Instrument | |
| Past 12-week nicotine dose | For each of the past 12 weeks, subjects report: (1) the number of days of smoking, and (2) the average number of cigarettes/cigarsb per day of smoking. These are multiplied for a weekly dose, and weekly scores are summed for a past 12-week dose. |
| Past 12-week alcohol dose | For each of the past 12 weeks, subjects report: (1) the number of days of drinking, and (2) the average number of standard drinksd per day of drinking. These are multiplied for a weekly dose, and weekly scores are summed for a past 12-week dose. |
| Past 12-week cannabis dosee | For each of the past 12 weeks, subjects report: (1) the number of days of smoking, and (2) the average number of joint-equivalentsf per day of smoking. These are multiplied for a weekly dose, and weekly scores are summed for a past 12-week dose. |
Other drugs are examined individually, not as an “other drug” category.
For nicotine, the unit of measurement is determined by asking participants how they typically consume this substance (e.g., cigarettes, cigars, other).
In calculating the initial year of use and the current year of use, the number of months of use are taken into account.
Alcohol is measured in standard drink units (12 oz beer = 5 oz wine = 1.5 oz liquor).
Lifetime dose of the other drug, other drug-related problem behaviors, and past 12-week other drug dose are calculated in the same manner.
Cannabis consumption is measured in joint-equivalents. Joints are considered approximately the size of a cigarette.
2.1.2. The Longitudinal Substance Use Recall for 12 Weeks (LSUR-12) Instrument
The LSUR-12 is comprised of the LSUR-12 Calendar, LSUR-12 Interview Guide, and LSUR-12 Scoring Sheet. Thus, rather than the Timeline used in the LSUR, the LSUR-12 employs a Calendar to guide a more granular assessment of the amount of substances used during the 12 weeks preceding the interview. The Calendar can be used to capture routine schedules (e.g., work or school) significant events (e.g., holidays, travel) and dates when the participant recalls a significant increase or decrease in substance use (e.g., binges or periods of abstinence).
The LSUR-12 Interview Guide and LSUR-12 Scoring Sheet resemble those of the first instrument, but capture data on the use of nicotine, alcohol, cannabis, and other drugs for the 12 weeks preceding the interview date. For each week, participants are asked to recall or estimate the number of days of use for each substance used. They are then asked to estimate the average number of standard units (e.g., cigarettes, drinks, joints) used per day of use during that week. Similar to the LSUR, the LSUR-12 elicits information on cannabis use in groups and individually. Table 1 shows key constructs and derived scores of the LSUR-12. The LSUR-12 can yield other summary scores of interest, such as use during specific weeks, frequency of use, or average amount consumed on days of use.
2.2. Validation plan
A detailed a priori plan for assessing validity guided the selection of concurrently administered measures such that validity of scores from both instruments was determined by demonstrating associations that would be expected based on previous literature. That is, tests of the validity of LSUR and LSUR-12 scores pertaining to nicotine use were planned in relation to two accepted measures of nicotine dependence, one demographic feature (years of educational attainment), and one personality trait (neuroticism). Validity of scores pertaining to alcohol use relied on two previously studied instruments assessing alcohol misuse, the formal diagnosis of alcohol use disorders, and two personality traits (neuroticism and conscientiousness). Finally, validity of scores of cannabis use were tested by determining associations with two widely used instruments assessing drug use and dependence, the formal diagnosis of cannabis use disorders, a demographic factor (number of past incarcerations), the same two personality traits, and to the extent possible, results from a urine toxicology screen. Regarding the selection of personality traits, multiple reports indicate that nicotine, alcohol, and illicit drug use are associated with higher levels of neuroticism and lower conscientiousness (Trull & Sher, 1994; Sher et al., 2000; Chassin et al., 2004; Trull et al. 2004; Anderson et al., 2007). Greater substance use severity (e.g., younger age at initiating habitual consumption and greater levels of lifetime and recent use) was expected to be positively associated with neuroticism and negatively related to conscientiousness.
A set of key scores from the LSUR and LSUR-12 was chosen for validity testing, including age at onset of weekly or daily use, lifetime dose, and past 12-week dose. These measures were considered separately for nicotine, alcohol, and cannabis. Additionally, scores were calculated for alcohol- and cannabis-related problem behaviors. The chosen measures are shown in Tables 3, 4, and 5, with comparisons between measures expected a priori to have small-to-medium effect sizes differentiated from those expected to have medium-to-large ones.
Table 3.
Associations between Key Nicotine-Related Variables of the LSUR and LSUR-12 and Hypothesized Validating Measures
| Nicotine-Related Variables, Sample Sizes Used for Hypothesis Tests, and Descriptive Statistics d | Expected Medium-to-Large Effect Size | Expected Small-to-Medium Effect Size | ||
|---|---|---|---|---|
| HONC a | FTND b | Years of Education | NEO-FFI c Neuroticism | |
| Age at onset of daily nicotine use (17.7±3.6) | N/A | N/A | ρ=.162 p=.346 n=59 |
ρ=−.262 p=.141 n=33 |
| Lifetime nicotine dose (15,610±27,210) | ρ=.852 p<.001 n=54 |
ρ=.796 p<.001 n=53 |
ρ=−.351 p=.007 n=59 |
ρ=.185 p=.197 n=50 |
| Past 12-week nicotine dose (493±1,557) | ρ=.827 p<.001 n=54 |
ρ=.802 p<.001 n=53 |
N/A | ρ=.177 p=.241 n=51 |
Hooked on Nicotine Checklist
Fagerström Test for Nicotine Dependence
Neuroticism, Extraversion, Openness Five-Factor Inventory
Mean and standard deviation
Table 4.
Associations between Key Alcohol-Related Variables of the LSUR and LSUR-12 and Hypothesized Validating Measures
| Alcohol-Related Variables, Sample Sizes Used for Hypothesis Tests, and Descriptive Statistics e | Expected Medium-to-Large Effect Size | Expected Small-to-Medium Effect Size | |||
|---|---|---|---|---|---|
| RETROSUB a | Brief MAST b | SCIDc Alcohol Use Disorder | NEO-FFI Neuroticism d | NEO-FFI Conscientiousness d | |
| Age at onset of weekly alcohol use (17.5±4.0) | N/A | N/A |
t=1.20 p=.245 (d=.486) n=27 |
ρ=−.42 8 p=.029 n=26 |
ρ=.304 p=.136 n=26 |
| Lifetime alcohol dose (4,021±8,652) | ρ=.589 p<.001 n=43 |
ρ=.497 p<.001 n=49 |
U=59.0 p<.001 (θ=.105) n=54 |
ρ=.182 p=.211 n=49 |
ρ=−.269 p=.061 n=49 |
| Alcohol-related problem behaviors (7.3±18.8) | N/A | ρ=.559 p<.001 n=48 |
U=73.5 p<.001 (θ=.135) n=53 |
ρ=.376 p=.008 n=49 |
ρ=−.267 p=.063 n=49 |
| Past 12-week alcohol dose (62±209) | ρ=.661 p<.001 n=46 |
N/A | N/A | ρ=.188 p=.187 n=51 |
ρ=−.151 p=.291 n=51 |
Retrospective Use of Alcohol and Other Substances.
Michigan Alcoholism Screening Test, Brief Version
Structured Clinical Interview for DSM-IV Axis I Disorders
Neuroticism, Extraversion, Openness Five-Factor Inventory
Mean and standard deviation
Table 5.
Associations between Key Cannabis-Related Variables of the LSUR and LSUR-12 and Hypothesized Validating Measures
| Cannabis-Related Variables, Sample Sizes Used for Hypothesis Tests, and Descriptive Statisticse | Expected Medium-to-Large Effect Size | Expected Small-to-Medium Effect Size | ||||
|---|---|---|---|---|---|---|
| RETROSUB a | Brief DAST b | SCIDc Cannabis Use Disorder | Number of Past Incarcerations | NEO-FFI d Neuroticism | NEO-FFI d Conscientiousness | |
| Age at onset of daily cannabis use (16.8±2.8) | N/A | N/A |
t=1.83 p=.080 (d=.731) n=27 |
ρ=−.022 p=.911 n=29 |
ρ=−.462 p=.018 n=26 |
ρ=.219 p=.283 n=26 |
| Lifetime cannabis dose (5,141±13,225) | ρ=.486 p=.001 n=43 |
ρ=.551 p<.001 n=50 |
U=153.0 p<.001 (θ=.210) n=54 |
ρ=.597 p<.001 n=58 |
ρ=.199 p=.167 n=50 |
ρ=−.176 p=.223 n=50 |
| Cannabis-related problem behaviors (4.5±6.1) | N/A | ρ=.681 p<.001 n=54 |
U=209.0 p=.017 (θ=.310) n=52 |
ρ=.428 p=.001 n=56 |
ρ=.157 p=.285 n=48 |
ρ=−.241 p=.099 n=48 |
| Past 12-week cannabis dose (105±227) | ρ=.634 p<.001 n=45 |
N/A | N/A | N/A | ρ=.109 p=.445 n=51 |
ρ=−.015 p=.917 n=51 |
Retrospective Use of Alcohol and Other Substances
The Drug Abuse Screening Test, Brief Version
Structured Clinical Interview for DSM-IV Axis I Disorders
Neuroticism, Extraversion, Openness Five-Factor Inventory
Mean and standard deviation
2.3. Setting and sample
After development, pilot testing, and refinement of the two instruments, a validation study was conducted with 60 research participants from an ongoing study on the early course of psychotic disorders (Compton et al., 2008; Monte et al., 2008; Compton et al., 2009a; 2009b; 2009c; 2009d; 2009e; Stewart et al., 2009). Participants were recruited from two public-sector hospital settings that serve an urban, predominantly African American population. All participants were English-speaking, 18–40 years of age, and currently or very recently hospitalized for first-episode psychosis. Those with known mental retardation, a Mini-Mental State Examination (Folstein et al., 1975, Cockrell & Folstein, 1988) score of <24, prior outpatient treatment for psychosis lasting >3 months, or prior hospitalization for psychosis >3 months prior to index hospitalization were excluded. Patients receiving a confirmed clinical diagnosis of a substance-induced psychotic disorder or psychotic disorder due to a general medical condition also were excluded.
2.4. Procedures and materials
Data came from detailed clinical research assessments (typically lasting 6–7 hours divided over 2–3 days) completed as part of an ongoing study of pre-illness cannabis use and the early course of psychotic disorders. As part of this evaluation, the LSUR and LSUR-12 required an average of 27.9±20.4 and 12.3±8.5 minutes, respectively, to complete; the entire battery of instruments pertaining to this validation study required an average of 74.5±43.7 minutes. Interviews were conducted once patients were adequately acclimated to the inpatient unit and psychotic symptoms were stabilized enough to allow for informed consent and participation, typically beginning between hospital days 3 and 7 (mean: 5.3±3.8). The study was approved by all relevant institutional review boards, and subjects gave written informed consent prior to participating.
Nicotine dependence was measured using the Hooked on Nicotine Checklist (HONC; DiFranza et al., 2002; Wellman et al., 2004) and the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991). The HONC consists of 10 yes/no items developed to detect loss of autonomy over nicotine use (Wellman et al., 2004). It has been demonstrated to have good internal consistency (α=.82) (Wheeler, 2004; Wellman, 2006a). The FTND is a 6-item questionnaire that rates dependence on nicotine (Etter, 1999). This instrument has been shown to have satisfactory internal consistency (α>.70) and test-retest stability (Pomerleau, 1994; Wellman, 2006a). Both measures are widely used, have good reliability and validity (Wellman et al., 2006a; Wellman et al., 2006b), and take less than five minutes to administer.
Alcohol abuse was measured with the brief version of the Michigan Alcoholism Screening Test (Brief MAST; Pokorny et al., 1972), an instrument consisting of 10 yes/no items on alcohol-related problem behaviors. This instrument has demonstrated good validity, categorizing 90% of self-identified alcoholics as such in one sample (Gibbs, 1983). Drug abuse was measured with the brief version of the Drug Abuse Screening Test (Brief DAST; Skinner, 1982), which consists of 20 yes/no items. Both instruments have good internal consistency (α=.89 and .88, respectively), and take less than five minutes to administer (Pokorny et al., 1972; Skinner, 1982).
The Retrospective Use of Alcohol and Other Substances (RETROSUB; Windle, 2005) instrument was used to assess the frequency and amount of alcohol and drug use during the five years prior to assessment. The RETROSUB measures substance use on a year-by-year basis, using questions about life events during each year (e.g., “Where did you live?”) to enhance participants’ recollection of substance use. (Of note, the LSUR Timeline and the LSUR-12 Calendar similarly link participants’ reporting of substance use to life events, but use a visual format that is completed collaboratively with the patient rather than relying solely on verbally presented questions about such life events.) Test-retest reliability and intraclass correlation coefficients for RETROSUB scores are high (.85 and .81, respectively; Windle, 2005).
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First et al., 1995) was used to determine the presence of psychotic disorders and substance use disorders. The SCID has high concordance with clinical diagnoses and is the gold-standard format for accurately establishing diagnoses in research settings. Rating of the SCID was based on a chart review and collateral interviews with one or two family members when available, in addition to the detailed semi-structured diagnostic interview.
Personality correlates of substance use were measured using the Neuroticism, Extraversion, Openness Five-Factor Inventory (NEO-FFI), a widely used, 60-item, Likert scaled, self-report instrument with well-established subscales for five personality constructs: agreeableness, neuroticism, conscientiousness, openness, and extraversion. Developed as a shortened form of the NEO Personality Inventory-Revised, the NEO-FFI has good internal consistency within each domain (α=.68–.86), convergent and divergent validity, and correlations between the short and long versions of each domain ranging .88–.94 (Costa & McCrae, 1992).
Although other laboratory assays were not conducted, results of the routine, clinically conducted urine toxicology screen were abstracted from the medical record when available. Finally, to examine correlations between participants’ and family members’/informants’ reports of alcohol and cannabis-related problem behaviors from the LSUR, a brief accompanying instrument was developed for use with family members/informants who participated in an in-depth research administration as part of the ongoing study (primarily to gather collateral information about the patients’ emerging psychotic disorder). Informants reported problem behaviors—using the same items as those in the LSUR—associated with each substance over the participants’ lifetime.
2.5. Data analyses
Distributional properties and descriptive statistics of all variables were carefully examined. Of note, many of the substance use variables presented in Tables 3–5 were right-skewed and therefore violated normality assumptions. For this reason, where appropriate, non-parametric tests of significance were employed. All reported p values were based on two-tailed tests of significance. A priori hypothesis tests addressing validity relied on Spearman correlations, independent samples Student’s t-tests, and Mann-Whitney U-tests. Data imputation methods were not used for sparse missing data points; each hypothesis test was conducted with the available sample size.
Effect sizes, rather than statistical significance, were of primary interest. We considered small-to-medium correlations or effect sizes as r, ρ, or d = |.20–.50| and medium-to-large effect sizes as r, ρ, or d > |.50|. For Mann-Whitney U-tests, a proxy effect size was computed using θ=U/mn, where m and n represent the sample sizes of the two groups (Newcombe, 2006). Although the a priori target sample size of 50 participants would provide reasonable power (approximately 57%) to identify a small effect size (.30) as statistically significant (using two-tailed tests and p≤0.05 as the criterion for establishing significance), this sample size would yield clearly adequate power to identify medium (.45) and large (.60) effects (approximately 92% and >99% power, respectively). Furthermore, the final sample size exceeded the target of 50.
3. Results
3.1. Demographic and diagnostic characteristics of the study sample
Demographic characteristics of the sample (n=60) are presented in Table 2. The mean age was 23.5±4.6 years and the majority of participants were male (42, 70.0%) and African American (55, 91.7%). Twenty-seven (45.0%) had not graduated high school, which is consistent with the high rate of school drop-out described in a previous first-episode sample from this setting (Goulding et al., 2009). At the time of admission, 42 participants (70.0%) were unemployed. SCID-based diagnoses for psychotic disorders are shown in Table 2. Consistent with the previous first-episode sample from this setting (Stewart et al., 2009), alcohol and cannabis use disorders were prevalent in the sample, also given in Table 2.
Table 2.
Demographic and Diagnostic Characteristics of the Study Sample (n=60)
| Age, years | 23.5±4.6 |
|---|---|
| Gender, male | 42 (70.0%) |
| Race/ethnicity | |
| African American | 55 (91.7%) |
| Caucasian | 2 (3.3%) |
| Other (Nigerian, Bi-racial, Indian) | 3 (5.0%) |
| Highest educational attainment | |
| Some high school, but did not graduate | 21 (35.0%) |
| General equivalency diploma | 6 (10.0%) |
| High school graduate | 11 (18.3%) |
| Some college, but did not graduate | 19 (31.7%) |
| College graduate | 3 (5.0%) |
| Employment status the month prior to hospitalization | |
| Unemployed | 42 (70.0%) |
| Employed part-time or full-time | 18 (30.0%) |
| SCID psychotic disorder diagnoses | |
| Schizophrenia, paranoid type | 21 (35.0%) |
| Schizophrenia, undifferentiated type | 7 (11.7%) |
| Schizophrenia, disorganized type | 2 (3.3%) |
| Schizoaffective disorder, depressive type | 9 (15.0%) |
| Schizoaffective disorder, bipolar type | 2 (3.3%) |
| Schizophreniform disorder | 8 (13.3%) |
| Psychotic disorder not otherwise specified | 8 (13.3%) |
| Brief psychotic disorder | 2 (3.3%) |
| Delusional disorder | 1 (1.7%) |
| SCID alcohol use disorder diagnoses (n=56) | |
| None | 42 (75.0%) |
| Current abuse | 2 (3.6%) |
| Abuse in the past 5 years | 2 (3.6%) |
| Current dependence | 6 (10.7%) |
| Dependence in the past 5 years | 4 (7.1%) |
| SCID cannabis use disorder diagnoses (n=55) | |
| None | 26 (47.3%) |
| Current abuse | 7 (12.7%) |
| Abuse in the past 5 years | 2 (3.6%) |
| Current dependence | 12 (21.8%) |
| Dependence in the past 5 years | 8 (14.6%) |
3.2. Descriptive statistics for key nicotine-related variables of the LSUR and LSUR-12, and associations with hypothesized validating measures
Among 35 participants who reported daily nicotine use, the mean age at initiating daily use was 17.7±3.6 years (Table 3). The mean lifetime nicotine dose among smokers was over 15,000 cigarettes, equating to approximately 780 packs, and the past 12-week nicotine dose amounts to an average of approximately two packs per week. Lifetime and past 12-week doses measured by the LSUR and LSUR-12 were highly correlated with the HONC and FTND scores (ranging from ρ=.796 to ρ=.852). The number of years of education completed was negatively correlated with the lifetime nicotine dose, as predicted (ρ=−.351). Small effect-size correlations (ρ=|.177–.262|) between the key LSUR nicotine-related variables and neuroticism were observed in the expected directions.
3.3. Descriptive statistics for key alcohol-related variables of the LSUR and LSUR-12, and associations with hypothesized validating measures
Among 26 participants who reported weekly alcohol use, the mean age at initiating weekly use was 17.5±4.0 years. Weekly, rather than daily, alcohol use was examined because of the relatively low prevalence of daily alcohol use. As shown in Table 4, the mean lifetime alcohol dose was over 4,000 standard drinks. The past 12-week alcohol dose was 62±209, or about five drinks per week. The hypothesized validating score for the LSUR lifetime alcohol dose from the RETROSUB was the past 5-year alcohol use amount (days per month drinking multiplied by the number of drinks per day when drinking), and the hypothesized validating score for the LSUR-12 past 12-week alcohol dose was the past 1-year alcohol use amount from the RETROSUB. As predicted, there were medium-to-large correlations with lifetime (ρ =.589) and past 12-week (ρ =.661) alcohol doses. The LSUR can be used to obtain total alcohol consumption for the five years preceding assessment, which had an even stronger correlation with the RETROSUB (ρ=.768, p<.001, n=43). Some of the remaining variability in the two measures may be due to truncation of the consumption reported in the RETROSUB (e.g., any use less than 12 times per year is set to zero), whereas infrequent use is captured by the LSUR.
Expected correlations with the brief MAST score were observed for lifetime alcohol dose and alcohol-related problem behaviors (ρ=.497 and ρ=.559). Participants who were determined through the SCID to have an alcohol use disorder currently or during the past five years reported a significantly younger age at onset of weekly use, a significantly greater lifetime alcohol dose, and more problem behaviors than those without a SCID-defined alcohol use disorder. Of the hypothesized correlations with neuroticism, age at onset of weekly alcohol use and the number of problem behaviors were in the small-to-medium range in the expected direction (ρ=−.428 and ρ=.376), and several meaningful correlations (though not statistically significant) with conscientiousness were observed, as shown in Table 4.
3.4. Descriptive statistics for key cannabis-related variables of the LSUR and LSUR-12, and associations with hypothesized validating measures
Among participants reporting daily cannabis use, the mean age at initiating daily use was 16.8±2.8 years (Table 5). The mean lifetime cannabis dose was over 5,000 joint-equivalents, and the past 12-week cannabis dose was 105±227, or nearly nine joint-equivalents per week. By necessity, the hypothesized validating score from the RETROSUB for the LSUR lifetime cannabis dose was the total days of use of illicit substances during the past five years, and the hypothesized validating score for the LSUR-12 cannabis dose was the RETROSUB total days of use of illicit substances during the past year. The expected medium-to-large associations with lifetime cannabis dose (ρ=.486) and past 12-week cannabis dose (ρ=.634) were observed. The LSUR can also estimate the number of days of cannabis use in the five years preceding the assessment, and for this, a stronger correlation was noted with the number of days of drugs use measured by the RETROSUB (ρ=.726, p<.001, n=41). Some of the remaining variability between these LSUR and RETROSUB variables may be accounted for by other drug use, as 36.7% of the sample reported using at least one other drug, and the RETROSUB does not collect data separately on cannabis and other drugs.
As hypothesized, lifetime cannabis dose and cannabis-related problem behaviors demonstrated medium-to-large correlations with the Brief DAST score (ρ=.551 and ρ=.681), shown in Table 5. Participants with a SCID-based diagnosis of cannabis abuse or dependence currently or during the past five years had a significantly younger age at onset of daily use, and had a significantly higher lifetime cannabis dose and more problem behaviors than those without a cannabis use disorder. The number of past incarcerations was correlated with the lifetime cannabis dose and number of cannabis-related problem behaviors reported (ρ=.597 and ρ=.428). Of the hypothesized correlations with neuroticism, age at onset of daily use was significantly correlated in the expected direction (ρ=−.462). As predicted, several small-to-medium correlations were observed pertaining to conscientiousness, again in the expected directions. Although urine toxicology screen results were available for only 17 participants, those testing positive for cannabis had a significantly higher past 12-week cannabis dose (U=7.0, p=.009) than those testing negative.
3.5. Correlations between patients’ and family members’/informants’ reports of problem behaviors
Forty participants (66.7%) had an informant who participated in a collateral interview about the patient’s emerging psychotic disorder and who had seen the patient at least weekly for ≥3 years when the patient was 12–18 years old (only these informants were deemed eligible a priori to provide information on substance use-related problem behaviors). Informants’ and patients’ reported number of problem behaviors were moderately correlated for both alcohol (ρ=.474, p=.015, n=26) and cannabis (ρ =.399, p=.053, n=24).
4. Discussion
The LSUR and LSUR-12 were developed as multidimensional, retrospective measures of substance use that rely on the LSUR Timeline and the LSUR-12 Calendar, respectively, to facilitate detailed data collection. These instruments are potentially useful tools in addressing research questions that require a comprehensive assessment of substance use onset, progression, frequency, amounts, and consequential behaviors. Although a number of measures of substance use—many of which are applicable to populations with comorbid substance use disorders and serious mental illnesses—are available, the structure and use of these instruments vary widely. Many instruments, such as the MAST and DAST, focus on problem behaviors stemming from substance use. Instruments such as the SCID are employed to make clinical diagnoses of abuse and dependence, which are tied to behavior more than onset, progression, and quantities consumed. While such instruments are clinically useful, they do not collect data on doses or cumulative exposure over time. Other measures, like urine toxicology screens, examine current or recent substance use, but are time-limited, capturing only a specified window prior to the assessment. To study the impact of substance use on developmental pathways, more information is needed on the earliest instances of consumption and on dosages during specific developmental stages.
The formulation of the LSUR was informed by all of the above-mentioned measures of substance use and related problem behaviors. This new instrument extends the collection of data beyond the scope of these other instruments by collecting data separately on each substance used and by extending the time period of inquiry to first-ever use for each substance. The structure and administration of the LSUR also acknowledges literature suggesting that interviewing (versus paper and pencil approaches) and establishing a collaborative rapport with participants improves the validity of data (RachBeisel et al., 1999).
Among instruments that collect data on the amount of substances consumed in the past, there is an inevitable trade-off between the time required to administer the instrument and the amount of data collected, such that instruments that take less time may only collect limited approximated data on ordinal scales while longer instruments might allow an assessor to collect detailed information yielding continuous variables. The LSUR and LSUR-12 required an average of about 30 and 15 minutes, respectively, to complete, though the length of administration varies prominently by the number of substances used and the duration and extent of such use. This length of time may not be feasible in many research settings, but may be merited for some subjects of inquiry, such as those seeking new knowledge about longer-term patterns of substance use and their relationships with biological or psychosocial development.
Several methodological limitations must be acknowledged in interpreting the present results. First, the sample size was relatively small, though it appears to have been sufficient for demonstrating validity of scores derived from the two new measures. The a priori sample size of 50 was exceeded overall, although some hypothesis tests had to, by necessity, rely on smaller sample sizes. Second, because the overarching study is based at urban, public-sector, inpatient psychiatric units that serve a low-income, socially disadvantaged, predominantly African American population, results may not be generalizable to dissimilar populations. However, there is no obvious reason why the new measures would produce valid scores in this setting but not in others. Third, only select psychometric properties were examined, given that the focus was on validity. Empirical data on inter-rater and test-retest reliability are necessary to further document the utility of this approach. However, the LSUR and LSUR-12 were designed to collect data in a highly consistent and structured way (e.g., the use of Interview Guides, Scoring Sheets, the LSUR Timeline, and the LSUR-12 Calendar ). Finally, tests of validity in this analysis were primarily based on correlations with self-report instruments, with only a small sub-sample available for objective verification of recent substance use via urine toxicology analysis. This would be an important component to include for all participants in future studies of validity, particularly for the LSUR-12, through which data on very recent use is collected.
This study demonstrates the validity of scores derived from the two measures in the context of young adults with first-episode psychosis. Validity should be established in other populations before making use of the new methodology in samples unlike the one involved in this analysis. Such careful development and demonstration of the psychometric properties of instruments may advance the fields of addiction and comorbidity research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KG, Tapert SF, Moadab I, Crowley TJ, Brown SA. Personality risk profile for conduct disorder and substance use disorders in youth. Addictive Behaviors. 2007;32:2377–2382. doi: 10.1016/j.addbeh.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. British Journal of Medicine. 2002;35:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktan GB, Calkins RF, Ribisl KM, Kroliczak A, Kasim RA. Test-retest reliability of psychoactive substance abuse an dependence diagnoses in telephone interviews using a modified diagnostic interview schedule-substance abuse module. American Journal of Drug and Alcohol Abuse. 1997;23:229–248. doi: 10.3109/00952999709040944. [DOI] [PubMed] [Google Scholar]
- Brown J, Kranzler HR, DelBoca FK. Self-reports by alcohol and drug abuse inpatients: Factors affecting reliability and validity. British Journal of Addictions. 1992;87:1013–1024. doi: 10.1111/j.1360-0443.1992.tb03118.x. [DOI] [PubMed] [Google Scholar]
- Buchan BJ, Dennis ML, Tims FM, Diamond GS. Cannabis Use: Consistency and Validity of Self-Report, On-Site Urine Testing and Laboratory Testing. Addiction. 2002;(Supp 1):98–108. doi: 10.1046/j.1360-0443.97.s01.1.x. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Brown ES. Prevalence and consequences of dual diagnosis. Journal of Clinical Psychiatry. 2006;67:e01. doi: 10.4088/jcp.0706e01. [DOI] [PubMed] [Google Scholar]
- Carey KB, Cocco KM, Correia CJ. Reliability and validity of the Addiction Severity Index among outpatients with severe mental illness. Psychological Assessment. 1997;9:422–428. [Google Scholar]
- Chassin L, Flora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: The effects of familial alcoholism and personality. Journal of Abnormal Psychology. 2004;113:483–498. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE) Psychopharmacological Bulletin. 1988;24:689–692. [PubMed] [Google Scholar]
- Compton MT, Chien VH, Leiner AS, Goulding SM, Weiss PS. Mode of onset of psychosis and family involvement in help-seeking as determinants of duration of untreated psychosis. Social Psychiatry and Psychiatric Epidemiology. 2008;43:975–982. doi: 10.1007/s00127-008-0397-y. [DOI] [PubMed] [Google Scholar]
- Compton MT, Gordon TL, Goulding SM, Esterberg ML, Carter T, Leiner AS, Weiss PS, Druss B, Walker EF, Kaslow NJ. Patient-level predictors and clinical correlates of duration of untreated psychosis in an urban, predominantly African American, first-episode sample. Journal of Clinical Psychiatry. 2009d doi: 10.4088/JCP.09m05704yel. in press. [DOI] [PubMed] [Google Scholar]
- Compton MT, Goulding SM, Gordon TL, Weiss PS, Kaslow NJ. Family-level predictors and correlates of the duration of untreated psychosis in African American first-episode patients. Schizophrenia Research. 2009b;115:338–345. doi: 10.1016/j.schres.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton MT, Goulding SM, Walker EF. Cannabis use, first-episode psychosis, and schizotypy: A summary and synthesis of recent literature. Current Psychiatry Reviews. 2007;3:161–171. [Google Scholar]
- Compton MT, Goulding SM, Walker EF. Characteristics of the retrospectively assessed prodromal period in hospitalized patients with first-episode nonaffective psychosis: Findings from a socially disadvantaged, low-income, predominantly African American population. Journal of Clinical Psychiatry. 2009e doi: 10.4088/JCP.08m04678yel. in press. [DOI] [PubMed] [Google Scholar]
- Compton MT, Kelley ME, Ramsay CE, Pringle M, Goulding SM, Esterberg ML, Stewart T, Walker EF. Association of pre-onset cannabis, alcohol, and tobacco use with the age at onset of prodrome and age at onset of psychosis in first-episode patients. American Journal of Psychiatry. 2009a;166:1251–1257. doi: 10.1176/appi.ajp.2009.09030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton MT, Ramsay CE. Impact of pre-onset cannabis use on the age at onset of prodromal and psychotic symptoms. Primary Psychiatry. 2009;16:35–43. [Google Scholar]
- Compton MT, Ramsay CE, Shim RS, Goulding SM, Gordon TL, Weiss PS, Druss B. Health services determinants of the duration of untreated psychosis among African-American first-episode patients. Psychiatric Services. 2009c;60:1489–1494. doi: 10.1176/ps.2009.60.11.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Cottler LB, Dorsey KB, Spitznagel EL, Magera DE. Comparing assessments of DSM-IV substance dependence disorders using CIDI-SAM and SCAN. Drug and Alcohol Dependence. 1996;41:179–187. doi: 10.1016/0376-8716(96)01249-5. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. The NEO Personality Inventory Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, Coleman M, Wood C. Measuring the loss of autonomy over nicotine use in adolescents: The DANDY (Development and Assessment of Nicotine Dependence in Youth) Study. Archives of Pediatrics and Adolescent Medicine. 2002;156:397–403. doi: 10.1001/archpedi.156.4.397. [DOI] [PubMed] [Google Scholar]
- Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophrenia Research. 1999;35:S93–S100. doi: 10.1016/s0920-9964(98)00161-3. [DOI] [PubMed] [Google Scholar]
- Drake RE, Wallach MA, Alverson HS, Mueser KT. Psychosocial aspects of substance abuse by clients with severe mental illness. Journal of Nervous and Mental Disease. 2002;190:100–106. doi: 10.1097/00005053-200202000-00006. [DOI] [PubMed] [Google Scholar]
- Etter J, Duc TV, Perneger TV. Validity of the Fagerström Test for Nicotine Dependence and the Heaviness of Smoking Index among relatively light smokers. Addiction. 1999;94:269–281. doi: 10.1046/j.1360-0443.1999.94226910.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders. Clinician Version (SCID-CV) Washington, D.C: American Psychiatric Press, Inc; 1996. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gibbs LE. Validity and reliability of the Michigan Alcoholism Screening Test: a review. Drug and Alcohol Dependence. 1983;12:279–285. doi: 10.1016/0376-8716(83)90071-6. [DOI] [PubMed] [Google Scholar]
- Goulding SM, Victoria VH, Compton MT. Prevalence and correlates of school drop-out prior to initial treatment of nonaffective psychosis: Further evidence suggesting a need for supported education. Schizophrenia Research. 2009 doi: 10.1016/j.schres.2009.09.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Harford TC, Dawson DA, Chou PS, Pickering RP. The alcohol use disorder and associated disabilities interview schedule (AUDADIS): reliability of alcohol and drug modules in a general population sample. Drug and Alcohol Dependence. 1995;39:37–44. doi: 10.1016/0376-8716(95)01134-k. [DOI] [PubMed] [Google Scholar]
- Harrison L. The validity of self reported drug use in survey research: An overview and critique of research methods. In: Harrison L, Hughes A, editors. The validity of self reported drug use: Improving the accuracy of survey estimates. Rockville, MD: National Institute on Drug Abuse; 1997. pp. 17–36. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hunt GE, Bergen J, Gashir M. Medication compliance and comorbid substance abuse in schizophrenia: impact on community survival 4 years after a relapse. Schizophrenia Research. 2002;54:253–264. doi: 10.1016/s0920-9964(01)00261-4. [DOI] [PubMed] [Google Scholar]
- Kintz P, Villain M, Cirimele V. Hair analysis for drug detection. Therapeutic Drug Monitoring. 2006;28:442–446. doi: 10.1097/01.ftd.0000211811.27558.b5. [DOI] [PubMed] [Google Scholar]
- Mayfield D, McLeod G, Hall P. The CAGE Questionnaire: Validation of a new alcoholism screening instrument. American Journal of Psychiatry. 1974;131:1121–1123. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: The Addiction Severity Index. Journal of Nervous and Mental Disease. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Monte RC, Goulding SM, Compton MT. Premorbid functioning of patients with first-episode nonaffective psychosis: A comparison of deterioration in academic and social performance, and clinical correlates of Premorbid Adjustment Scale scores. Schizophrenia Research. 2008;104:206–213. doi: 10.1016/j.schres.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe RG. Confidence intervals for an effect size measure based on the Mann-Whitney statistic. Part 1: General issues and tail-area-based methods. Statistics in Medicine. 2006;25:543–557. doi: 10.1002/sim.2323. [DOI] [PubMed] [Google Scholar]
- O’Farrell TH, Maisto SA. The utility of self report and biological measures of drinking in alcoholism treatment outcome studies. Advances in Behaviour Research and Therapy. 1987;9:91–125. [Google Scholar]
- Pokorny AD, Miller BA, Kaplan HB. The Brief MAST: A shortened version of the Michigan Alcoholism Screening Test. American Journal of Psychiatry. 1972;149:342–345. doi: 10.1176/ajp.129.3.342. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerström Tolerance Questionnaire and the Fagerström Test for Nicotine Dependence. Addictive Behaviors. 1994;19:33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Pragst F, Balikova MA. State of the art in hair analysis for detection of drug and alcohol abuse. Clinica Chimica Acta. 2006;370:17–49. doi: 10.1016/j.cca.2006.02.019. [DOI] [PubMed] [Google Scholar]
- RachBeisel J, Scott J, Dixon L. Co-occurring severe mental illness and substance use disorders: A review of recent Research. Psychiatric Services. 1999;50:1427–1434. doi: 10.1176/ps.50.11.1427. [DOI] [PubMed] [Google Scholar]
- Rosenberg SD, Drake RE, Wolford GL, Mueser KT, Oxman TE, Vidaver RM, Carrieri KL, Luckoor R. Dartmouth Assessment of Lifestyle Instrument (DALI): A substance use disorder screen for people with severe mental illness. American Journal of Psychiatry. 1998;155:232–238. doi: 10.1176/ajp.155.2.232. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. American Journal of Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: Systematic review. Journal of Psychopharmacology. 2005;19:187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: A prospective study. Journal of Consulting and Clinical Psychology. 2000;68:818–829. [PubMed] [Google Scholar]
- Siegfried N. A review of comorbidity: Major mental illness and problematic substance use. Australian and New Zealand Journal of Psychiatry. 1998;32:707–717. doi: 10.3109/00048679809113127. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The Drug Abuse Screening Test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Smit F, Boiler L, Cuijpers P. Cannabis use and the risk of later schizophrenia: A review. Addiction. 2004;99:425–430. doi: 10.1111/j.1360-0443.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Humana Press Inc; Totowa, New Jersey: 1992. pp. 41–72. [Google Scholar]
- Stewart T, Goulding SM, Pringle M, Esterberg ML, Compton MT. A descriptive study of nicotine, alcohol, and cannabis use in urban, socially-disadvantaged, predominantly African American patients with first-episode nonaffective psychosis. Clinical Schizophrenia and Related Psychoses. 2010;3:217–225. [Google Scholar]
- Trull TJ, Sher KJ. Relationship between the five-factor model of personality and Axis I disorders in a nonclinical sample. Journal of Abnormal Psychology. 1994;103:350–360. doi: 10.1037//0021-843x.103.2.350. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Waudby CJ, Sher KJ. Alcohol, tobacco, and drug use disorders and personality disorder symptoms. Experimental and Clinical Psychopharmacology. 2004;12:65–75. doi: 10.1037/1064-1297.12.1.65. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Najavits LM, Greenfield SF, Soto JA, Shaw SR, Wyner D. Validity of substance use self-reports in dually diagnosed outpatients. American Journal of Psychiatry. 1998;155:127–128. doi: 10.1176/ajp.155.1.127. [DOI] [PubMed] [Google Scholar]
- Wellman KC, Fletcher KE, Wellman R, DiFranza J. Screening adolescents for nicotine dependence: The Hooked on Nicotine Checklist. Journal of Adolescent Health. 2004;35:225–230. doi: 10.1016/j.jadohealth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Wellman RJ, DiFranza JR, Pbert L, Fletcher KE, Flint A, Young MH, Druker S. Comparison of the psychometric properties of the Hooked on Nicotine Checklist and the modified Fagerström Tolerance Questionnaire. Addictive Behavior. 2006a;31:486–495. doi: 10.1016/j.addbeh.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Wellman RJ, Savageman JA, Godiwala S, Savageau N, Friedman K, Hazelton J, DiFranza JR. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependance in adult smokers. Nicotine and Tobacco Research. 2006b;8:575–580. doi: 10.1080/14622200600789965. [DOI] [PubMed] [Google Scholar]
- Westermeyer J. Comorbid schizophrenia and substance abuse: A review of epidemiology and course. American Journal on Addictions. 2006;15:345–355. doi: 10.1080/10550490600860114. [DOI] [PubMed] [Google Scholar]
- Wheeler KC, Fletcher KE, Wellman RJ, DiFranza JR. Screening adolescents for nicotine dependence: The Hooked on Nicotine Checklist. Journal of Adolescent Health. 2004;35:225–230. doi: 10.1016/j.jadohealth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Windle M. Retrospective use of alcohol and other substances by college students: psychometric properties of a new measure. Addictive Behaviors. 2005;30:337–342. doi: 10.1016/j.addbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Winklbaur B, Ebner N, Sachs G, Thau K, Fischer G. Substance abuse in patients with schizophrenia. Dialogues in Clinical Neuroscience. 2006;8:37–43. doi: 10.31887/DCNS.2006.8.1/bwinklbaur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H. Reliability and validity studies of the WHO-Composite International Diagnostic Interview (CIDI): A critical review. Journal of Psychiatric Research. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Zanis DA, McLellan AT, Corse S. Is the Addiction Severity Index a reliable and valid assessment instrument among clients with severe and persistent mental illness and substance abuse disorders? Community Mental Health Journal. 1997;33:213–227. doi: 10.1023/a:1025085310814. [DOI] [PubMed] [Google Scholar]