Abstract
Metal homeostasis and resistance in bacteria is maintained by a panel of metal sensing transcriptional regulators that collectively control transition metal availability and mediate resistance to heavy metal xenobiotics, including AsIII, CdII, PbII and HgII. The ArsR family constitutes a superfamily of metal sensors that appear to conform to the same winged helical, homodimeric fold, that collectively “sense” a wide array of beneficial metal ions and heavy metal pollutants. The genomes of many actinomycetes, including the soil dwelling bacterium Streptomyces coelicolor and the human pathogen Mycobacterium tuberculosis, encode over ten ArsR family regulators, most of unknown function. Here, we present the characterization of a homolog of M. tuberculosis CmtR (CmtRMtb) from S. coelicolor, denoted CmtRSc. We show that CmtRSc, in contrast to CmtRMtb binds two monomer mol equivalents of PbII or CdII to form two pairs of trigonal S3 coordination complexes per dimer. Metal site 1 conforms exactly to the α4C site previously characterized in CmtRMtb while metal site 2 is coordinated by a C-terminal vicinal thiolate pair, Cys110 and Cys111. Biological assays reveal that only CdII and, to a lesser extent, PbII mediate transcriptional derepression in the heterologous host M. smegmatis in a way that requires metal site 1. In contrast, mutagenesis of metal site 2 ligands Cys110 or Cys111 significantly reduces CdII responsiveness, with no detectable effect on PbII sensing. The implications of these findings on the ability to predict metal specificity and function from metal-site “signatures” in the primary structure of ArsR family proteins are discussed.
The concentrations of first-row d-block transition metal ions and other heavier, toxic di- and trivalent metal ions and metalloids that play no biological role can vary dramatically in the immediate environment of a bacterial community. Although some are essential, and enable proteins to adopt their native three-dimensional structures or function as cofactors for metalloenzyme catalysis, an excess beyond that required by normal cellular metabolism can also be strongly deleterious to cell viability (1). As a result, metal-specific regulatory systems have evolved that detect metal-sufficiency or toxicity and control the intracellular availability of the different metal ions as well as purge any toxic elements or compounds from the cytosol. In free-living microorganisms, such detoxification/resistance systems are particularly important because it permits these organisms to survive by adaptation to potentially harsh environmental conditions, e.g., soil and water heavily contaminated by heavy metal salts (2) and/or the phagosome of mammalian host cells for certain microbial pathogens (3). Large, highly polarizable and thiophilic metal ions such as HgII, CdII and PbII are highly toxic, in part because they are capable of forming high affinity, kinetically long-lived complexes with protein cysteine thiolates found in native zinc binding sites, thereby rendering them nonfunctional. Such proteins include canonical zinc finger proteins and 5-aminolevulinic acid dehydratase (ALAD) in mammals (4, 5).
CmtR is a CdII sensing SmtB/ArsR (or ArsR) family metalloregulatory repressor from Mycobacterium tuberculosis (6–9). In addition to CdII, M. tuberculosis CmtR (denoted CmtRMtb here) senses PbII in the heterologous host M. smegmatis and has been shown to form Cys-thiolate rich metal coordination complexes with CdII, PbII and even ZnII in vitro (6, 7). CmtR possesses a unique pair of α4C metal binding sites (metal ligands derived from the α4 helix and the C-terminal tail; see Fig. 1B) (6, 8, 10, 11) structurally distinct from the CdII/PbII sites of another SmtB/ArsR CdII/PbII sensor, Staphylococcus aureus plasmid pI258-encoded CadC, which contains functional α3N and nonfunctional α5 metal sites (1, 12, 13). The solution structure of the CmtR-CdII complex reveals a homodimer with metal bound to Cys102' from the C-terminal tail region of one subunit and Cys57, Cys61 in the helix α4 from the other subunit; PbII is predicted to bind to the same pair of sites (7, 8) (Fig. 1). Cys102 plays an accessory role in stabilizing the coordination complex while Cys57 and Cys61 anchor it, contributing most of the metal binding affinity (7). However, Cys102 does function as a key allosteric metal ligand in mediating the disassembly of oligomeric CmtR-cmt O/P1 oligomeric complexes in vitro (7, 8), and for derepression in vivo (6), although the structural and dynamic changes induced in the dimer upon Cys-102 CdII binding remain to be characterized.
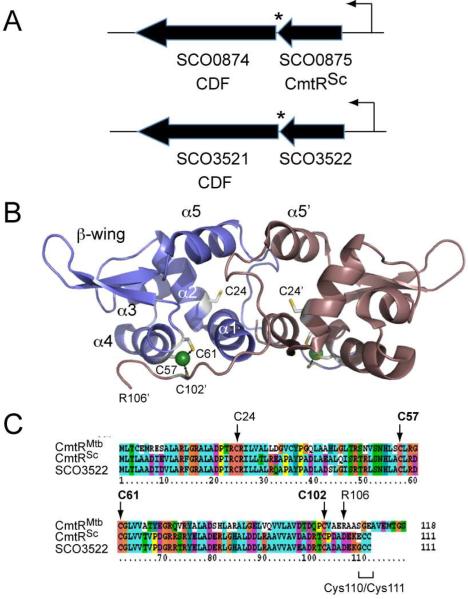
Figure 1. Genomic region of CmtR homologs in S. coelicolor, solution structure and multiple sequence alignment of M. tuberculosis CmtR.
(A) Genomic region around S. coelicolor CmtRSc (SCO0875) and SCO3522; each gene is separated by a single TGA termination codon (*) from homologous downstream genes SCO0874 and SCO3521 that encode putative CDF-family heavy metal transporters. The surrounding genomic regions are completely unrelated in the two loci. (B) Ribbon diagram of the solution structure of the M. tuberculosis CmtR-CdII complex (2jsc) with individual protomers shaded slate and violet and the two symmetry-related CdII ions colored green (8). The secondary structural units are labeled, with the side-chains of C24 and the CdII-coordinating residues, C57, C61 and C102' (prime designation, opposite subunit) highlighted in stick. The most C-terminal residue in the structural model of each protomer is R106. (C) Multiple sequence alignment of CmtRMtb, CmtRSc and the product of SCO3522. CdII-coordinating residues in CmtRMtb are highlighted in bold, with other residues as in panel B.
Recent in vivo experiments using M. tuberculosis suggest that CmtRMtb binds cooperatively to four binding sites in an extended 90-bp region upstream of the cmtR-Rv1993c-cmtA operon, to inhibit the interaction of RNA polymerase with the promoter region (9). These studies also suggest that CdII is the sole inducer in M. tuberculosis, a situation that contrasts with findings in the heterologous host, M. smegmatis (6, 9). The cmtA gene encodes a deduced metal transporting P1B-ATPase efflux pump, which is proposed to efflux toxic metal ions from the bacterial cytosol (6, 9). Therefore, increasing cellular CdII concentration triggers derepression of the cmtR-Rv1993c-cmtA operon, resulting in increased concentrations of CmtA in the plasma membrane which is thought to export CdII from the cytosol against a metal concentration gradient (9).
Streptomyces coelicolor A3(2) is representative of a ubiquitous group of soil-dwelling, filamentous Gram-positive bacteria. This highly adaptable organism undergoes a broad range of metabolic processes and biotransformations, and is noted for its natural antibiotic production (14). Like M. tuberculosis, S. coelicolor belongs to the taxonomy order of Actinomycetales (14, 15). Although each has very different lifestyles, both encode elaborate metal detoxification and efflux systems. As a human pathogen, M. tuberculosis must adapt to various microenvironmental niches, including the phagosome of infected macrophages where it encounters changes in metal availability (3, 16–19). S. coelicolor must also sense and respond to fluctuating metal levels within soils (20–22). Notably, the sequenced strains of M. tuberculosis and S. coelicolor possess multiple deduced ArsR family regulators, ten (10) and fourteen, respectively, as well as representatives of many of the other metalloregulatory protein classes (1, 23). The diversity of the metal-responsive regulators in these two organisms is therefore likely to reflect their different ecological niches and the different survival strategies employed to avoid metal stress. A search for protein homologs of M. tuberculosis CmtR returned two open reading frames in S. coelicolor A3(2) corresponding to locus tags SCO0875 and SCO3522. The gene products of SCO0875 and SCO3522 possess only ten amino acid differences between them, and each shares ≈50% identity with M. tuberculosis CmtR. Both SCO0875 and SCO3522 are immediately upstream of deduced cation diffusion facilitator (CDF) family integral membrane metal transporters (Fig. 1A), with predicted roles in the transport of the more thiophilic metal ions (24). SCO0875 was chosen for detailed study herein and designated CmtRSc.
CmtRSc shares four conserved cysteine residues with CmtRMtb which include the three CdII ligands (Cys57, Cys61 and Cys102) required for metal binding and allosteric regulation (6) as well as C24 in the α2 helix (Fig. 1A). As a result, we expected to observe at least partially similar metal binding profiles for CmtRMtb and CmtRSc. However, CmtRSc contains two additional cysteines arranged as C-terminal vicinal pair (Cys110 and Cys111) that could also be involved in metal coordination (Fig. 1C). It was therefore of interest to functionally and structurally characterize CmtRSc and compare its properties to that of CmtRMtb. We show here that the CmtRSc homodimer harbors a second pair of high affinity Cys-thiolate rich CdII/PbII coordination sites relative to CmtRMtb that involves metal coordination by Cys110 and Cys111. We further show that wild-type CmtRSc, is a bona fide CmtR that is highly selective for CdII/PbII in the heterologous host M. smegmatis, the same host used to characterize CmtRMtb, with no other metal ions, including HgII and ZnII, capable of inducing transcriptional derepression. Strikingly, the second pair of CdII/PbII sites unique to CmtRSc is required for full CdII-responsiveness in M. smegmatis, but does not alter PbII sensing under the same conditions. The structural and functional implications of these findings for the prediction of metal-sensing sites in ArsR family metal sensors are discussed.
MATERIALS AND METHODS
Construction of wild-type and C110G/C111S CmtRSc overexpression plasmids
To create pET3a-CmtRSc, the CmtRSc coding region was amplified by PCR from Streptomyces violaceoruber genomic DNA (M145, used in to sequence the S. coelicolor genome (14) using primers V (5'-AAAAACATATGGTGCTGACTCTCGCTGCCGATATC-3') and VI (5'-AAAAAGCTAAGCTCAGCAGCACTCCTTCTCGTC-3') and cloned into pET3a (Novagen) between the NdeI and BPU1102I restriction sites using standard methods. The plasmid encoding C110G/C111S CmtRSc was generated by site-directed mutagenesis using the QuikChange kit (Stratagene) and primers VII (5'-GGACGAGAAGGAGGGCTCCTGAGCAATAACTAGC-3') and primer VIII (5'-GCTAGTTATTGCTCAGGAGCCCTCCTTCTCGTCC-3') using pET3a-CmtRSc as the template. The integrity of all expression plasmids was verified by DNA sequencing.
Purification of wild type and mutant CmtRSc
CmtRSc and C110G/C111S CmtRSc were purified to homogeneity using a procedure previously described for CmtRMtb (7). The theoretical molecular weight for CmtRSc is 12.2 kDa and the extinction coefficient was calculated to be 3730 M−1•cm−1.
Free thiol determination
A standard DTNB1 colorimetric assay was used to determine the number of free thiols in CmtRSc (25). 25 μL 2.5 mM DTNB solution was added separately into 400 μL 10–15 μM protein. After a 30 min incubation in the anaerobic chamber, the concentration of thiolate anion was quantified at 412 nm (ε=13,600 M−1•cm−1) after subtraction of the absorbance of the final dialysis buffer with same concentration of DTNB. The number of free thiols in wild-type CmtRSc was 5.8 ± 0.2 (6 expected) and 3.0 ± 0.2 (4 expected) for C110G/C111S CmtRSc.
Atomic absorption spectroscopy
The concentrations of all metal titrants were determined using a Perkin-Elmer Analyst 700 atomic absorption spectrophotometer operating in flame mode using a different hollow cathode lamps specific for each metal (25). ZnII was detected at 213.9 nm (slit=0.7 nm), CdII was detected at 228.8 nm (slit = 0.7 nm) and PbII was detected at 283.3 nm (slit = 0.7 nm).
Analytical Sedimentation Equilibrium Ultracentrifugation
All experiments were carried out on a Beckman Optima XL-A analytical ultracentrifuge with the rotor speed set to 20,000 rpm at 25.0 °C. Ultracentrifuge cells were assembled in the anaerobic glove box and contained 5.0 μM CmtRSc wild-type or 8.0 μM C110G/C111S CmtRSc in buffer (10 mM Hepes, 0.4 M NaCl, and 0.1 mM EDTA at pH 7.0). Scans were monitored by absorbance at 232 nm or 233 nm for the wild-type and C110G/C111S CmtRSc, respectively, with the final seven scans extracted and subjected to simultaneous fitting using Ultrascan II 8.0. A partial specific volume (ν) of 0.7354 ml/g (predicted by Sednterp 1.07 software, www.bbri.org/RASMB/rasmb.html) and a buffer density (ρ) of 1.0 g/ml were used in the analysis. The data were globally and simultaneously fitted to either a one component ideal species model or a monomer-dimer equilibrium model using eqs. 1 and 2, respectively:
| (Eq. 1) |
| (Eq. 2) |
where X = cell radius, Xr = reference radius, A = amplitude of monomer*, M = molecular weight of monomer*, E = extinction coefficient, R = gas constant, T = temperature, B = baseline offset*, ω = angular velocity, L = optical pathlength, K1,2 = monomer–dimer equilibrium constant*, and * indicates this parameter can be floated during parameter optimization (26). Fits to the monomer-dimer model did not significantly improve the goodness-of-fit, consistent with a low concentration of monomeric species under these solution conditions; thus, K1,2≥106 M−1.
CdII, PbII optical absorption spectroscopy
All metal binding experiments were carried out anaerobically at ambient temperature (~25°C) using a Hewlett-Packard model 8452A spectrophotometer (12, 27). For CdII titrations, apo proteins were diluted using final dialysis buffer S to ≈ 50 μM in 800 μL and loaded into an anaerobic cuvette fitted with a Hamilton gas-tight adjustable volume syringe containing 0.5 mM CdII titrant in the glove box. Complete optical spectra of apo protein were collected from 200–900 nm 1–2 min after each addition of a known aliquot (5–15 μL) of CdII. Corrected spectra were obtained by subtraction of the apo protein spectra from each spectrum obtained after addition of metal ion, and further corrected for dilution. PbII titrations were done in exactly the same way except that 10 mM bis-Tris, 0.4 M NaCl, pH 7.0 was used as the buffer. Bis-Tris is a weakly chelating buffer that can prevent excess PbII from forming PbII(OH)2 precipitate (4). CdII competition experiment performed with EDTA was carried out as described above except 200–300 μM chelator was present. The competition binding curves were fit to the model using Dynafit:
where P is the monomer concentration of CmtRSc and KCd-EDTA (pH 7.0) = 8.31 × 1013 M−1 under these conditions using a pH-dependent log KCd=16.5 and pKas of 9.52 and 6.13 for EDTA (28).
ZnII binding experiments
The zinc chelator dye magfura-2 (KZn = 5.0 × 107 M−1 at pH 7.0 and 25°C) was used as a colorimetric competitor for ZnII binding by wild-type and C110G/C111S CmtRSc as previously described (29). 30 μM CmtRSc and ≈15 μM magfura-2 was used for all experiments. The data were fit to a competitive binding model assuming two nonequivalent, noninteracting metal binding sites per CmtRSc protomer for CmtRSc wild-type by DynaFit:
where KZn-magfura2 = 5.0 × 107 M−1 under these conditions. For C110G/C111S CmtRSc, data were fitted as above to a competitive binding model assuming one metal binding site per protomer or two per dimer (without KPZn2).
Construction of promoter-lacZ fusions, site-directed mutagenesis and β-galactosidase assays
CmtRSc upstream sequences and the coding region (539 bp) were amplified from S. coelicolor genomic DNA using primers 5'- GAAAGTACTGCGGGTAGTGGCGATGTGATCC-3' and 5'-GAAGGTACCGCGGTCATTCAGCAGC-3', and ligated to pGEM-T prior to subcloning into the ScaI/KpnI site of pJEM15 (30) to create a transcriptional fusion with lacZ. QuickChange XL (Stratagene) site-directed mutagenesis was subsequently employed to generate derivatives with the following codon substitutions in CmtRSc: Cys24, Cys57, Cys61, Cys102, Cys110, and Cys111, each to Ser; and Arg16 to a UGA stop codon. All generated plasmid constructs were checked by sequence analysis. Mycobacterium smegmatis mc2155 was used as an actinomycete host for reporter gene assays. The lacZ fusion constructs were introduced into M. smegmatis and transformants selected on Luria-Bertani (LB) agar supplemented with 25 μg mL−1 kanamycin as described previously (30). β-galactosidase assays were performed as described (31) in triplicate on at least three separate occasions. Cells were grown at 37 °C with shaking in LB broth or Sauton medium containing 0.05% (v/v) Tween 80 and kanamycin (25 μg ml−1) supplemented with the indicated concentration of metal salt (described in individual experiments) for ~20 h immediately prior to assays. The metal salts used were ZnSO4, CoSO4, NiCl2, CdCl2, Pb(C2H3O2)2, CuSO4, AsNaO2 and HgCl2.
RESULTS
Wild-type and C110G/C111S CmtRSc are stable homodimers at low protein concentrations
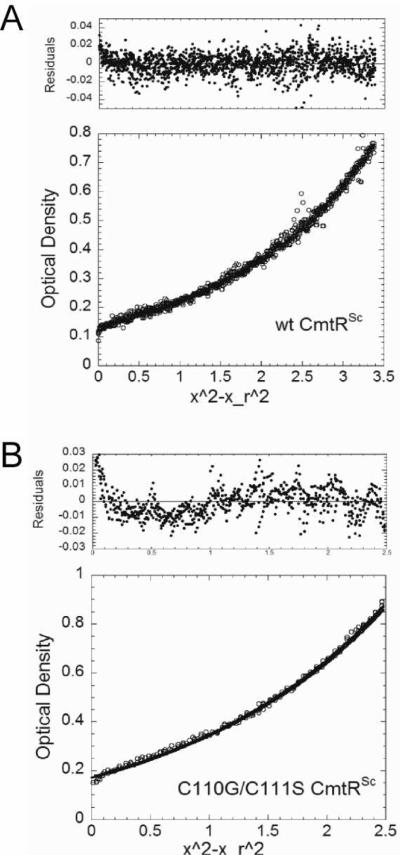
Both CmtRSc and its C110G/C111S mutant were subjected to analytical equilibrium sedimentation ultracentrifugation at 20,000 rpm at 5 μM and 8 μM monomer concentration, respectively, and absorbance recorded at 232 nm, with representative results shown in Fig. 2. Each set of experimental data that had reached equilibrium was first subjected to a global simultaneous fit to a single ideal species model indicated by a solid line. The apparent molecular weights obtained from these data (Fig. 2) are consistent with both apo-CmtRSc and apo-C110G/C111S CmtRSc being predominantly homodimeric under these conditions.
Figure 2. Analytical sedimentation equilibrium ultracentrifugation of CmtRSc and C110G/C111S CmtRSc.
(A) 5.03 μM monomer wild-type CmtRSc. (B) 8.0 μM monomer C110G/C111S CmtRSc. Filled symbols in upper panels represent an overlay of data collected during the last seven scans and indicate that equilibrium had been reached. The solid line represents the global simultaneous fit for a single ideal species model using Ultrascan. For wild-type CmtRSc, the fitted Mw is 23,820 Da (theoretical dimer Mw = 24,378 Da), variance = 1.0689 e−4. For C110G/C111S CmtRSc, the fitted Mw is 25,690 Da (theoretical dimer Mw = 24,252 Da), variance = 5.8026 e−5. Conditions: 10 mM Hepes, 0.4 M NaCl, and 0.1 mM EDTA at pH 7.0, 25.0 °C, and 20,000 rpm rotor speed.
CmtRSc possesses two CdII binding sites per monomer while C110G/C111S CmtRSc possesses one
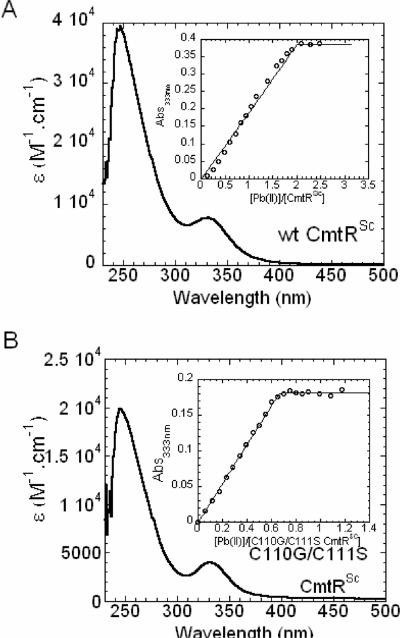
Apoprotein-subtracted CdII absorbance spectra of CmtRSc are shown in Fig. 3. The saturated CdII spectra is characterized by an intense ligand-to-metal charge transfer (LMCT1) at 240 nm, similar to that observed for CmtRMtb (7). Binding isotherms (insets) were obtained by plotting the corrected absorbance at 240 nm as a function of total [CdII] over protein monomer ratio. The maximum molar absorptivity at 240 nm for CmtRSc is ≈ 31,000 M (monomer)−1 cm−1 which is two-fold larger than wild-type mycobacterial CmtRMtb (ε ≈ 16,000 M−1 cm−1) (Fig. 3A) (7). In addition, CmtRSc exhibits a 2:1 (CdII:CmtRSc monomer) binding stoichiometry (Fig. 3A), in contrast to CmtRMtb which is known to bind one metal per protomer (7). Nonlinear least-squares fits of the binding isotherms to a simple 2:1 independent-site metal binding model returns only a lower limit of the binding affinity given the stoichiometric nature of these binding curves (KCd > ≈5 × 107 M−1) (Fig. 3A).
Figure 3. CdII titrations of wild-type and C110G/C111S CmtRSc.
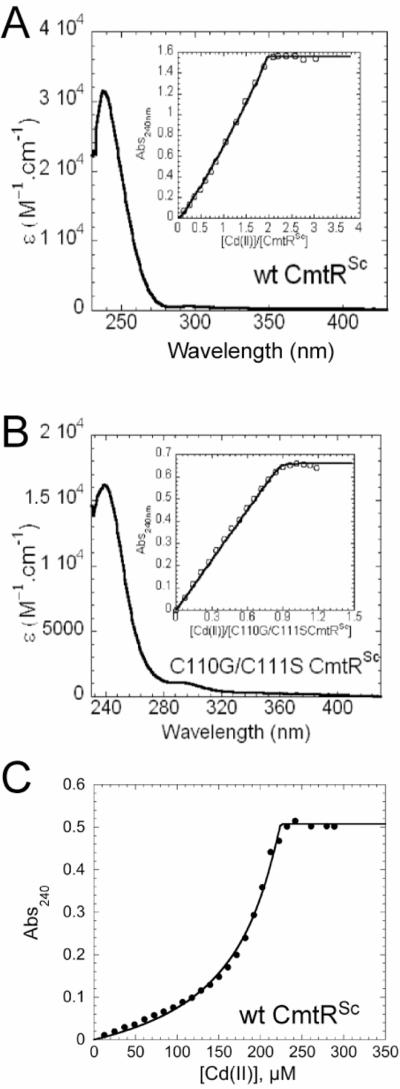
(A) Apoprotein subtracted difference spectrum of wild-type CmtRSc (50.7 μM monomer). (B) Apoprotein subtracted difference spectrum of C110G/C111S CmtRSc (44.8 μM monomer). CmtRSc variants were titrated anaerobically with increasing concentrations of CdII. Inset, CdII binding isotherm plotted as change in A240 vs. [CmtRSc variant monomer]. (C) CdII-EDTA competition binding isotherm in which 20.9 μM wild-type CmtRSc was titrated with CdII in the presence of 227 μM EDTA. Conditions: 10 mM Hepes, 0.4 M NaCl, pH 7.0, 25°C.
The presence of an additional CdII site in the CmtRSc homodimer coupled with twice the Cys S−→CdII molar absorptivity prompted us to investigate if the two vicinal cysteine residues (Cys110 and Cys111) at the C-terminus donate thiolate ligands to the second CdII ion. We measured the CdII absorption spectrum of C110G/C111S CmtRSc, where the two C-terminal cysteines were converted to their analogous residues in CmtRMtb (Fig. 1C). As expected, C110G/C111S CmtRSc behaves much like CmtRMtb, given a ≈0.9:1 CdII:C110G/C111S monomer stoichiometry and an identical monomer molar absorptivity of ≈ 16,000 M−1 cm−1 (Fig. 3B) (7), which is precisely half that of wild-type CmtRSc. As expected, the binding of CdII is stoichiometric under these conditions and thus returns only a lower limit of the metal binding affinity (Fig. 3B). Given a molar absorptivity of ≈5500 M−1 cm−1 per Cd-S coordination bond (32–34), it is reasonable to conclude that C110G/C111S CmtRSc possesses one CdII site (termed metal site 1) that is identical to the previously characterized canonical α4C site CmtRMtb, while wild-type CmtRSc possesses two spectroscopically similar CdII binding sites (metal sites 1 and 2).
The CdII affinities of each metal site per monomer in wild-type CmtRSc were estimated by carrying out a CdII titration experiment in the presence of a known concentration of the metal chelator EDTA (Fig. 3C), with KCd-EDTA = 8.3 × 1013 M−1 under these solution conditions (pH 7.0, 25.0 °C). A nonlinear least squares fit of a simple competition model assuming two non-equivalent, non-interacting sites per protomer gives KCd1 = 3.0 (±0.1) × 1013 M−1, KCd2 = 3.6 (±0.5) × 1012 M−1. These data taken together confirm that CmtRSc has two pairs of structurally similar high affinity metal sites per homodimer, which differ in macroscopic affinity by ≈10-fold. Previous findings with CmtRMtb reveal that Cys57, Cys61 and Cys102 are ligands to CdII and form trigonal pyramidal coordination geometry (S3) or distorted tetrahedral coordination geometries (S3O), with Cys102 not as strongly bound as the other thiolate ligands (6–8) (see Fig. 1B). Considering these three cysteines are conserved in CmtRMtb and CmtRSc, they likely create a CdII site indistinguishable to that in CmtRMtb and is denoted metal site 1. In contrast, CdII site 2 clearly requires coordination by Cys110 and Cys111.
CmtRSc possesses two PbII binding sites and the same C110G/C111S mutation abolishes one
Anaerobic PbII titration experiments were performed using ≈50 μM apo-wild-type CmtRSc (Fig. 4A) and C110G/C111S CmtRSc (Fig. 4B) and the apoprotein-subtracted difference spectra are shown. The saturated PbII spectra of both proteins are characterized by an intense absorption in the far-ultraviolet and a long-wavelength absorption band with maximum at 333 nm, identical to that of CmtRMtb (7) and report on ligand-to-metal charge transfer (S− 3p→Pb 6p) and intraatomic (Pb 6s2→Pb 6sp) electronic transitions (35, 36). Binding isotherms (insets) were obtained by plotting the corrected absorbance at 333 nm as a function of total [PbII]/protein monomer ratio. As expected, for wild-type CmtRSc PbII binding is saturable at ≈ 2:1 ratio over [CmtRSc] monomer (Fig. 4A); in contrast, this stoichiometry drops to ≈ 0.7 PbII/monomer for C110G/C111S CmtRSc (Fig. 4B). These PbII stoichiometries are largely consistent with the relative monomer molar absorptivities at 333 nm (ε333) of ≈7900 M−1 cm−1 and ≈3950 M−1 cm−1 for wild-type CmtRSc and C110G/C111S CmtRSc, respectively (Fig. 4). Interestingly, the PbII-thiolate molar absorptivity of C110G/C111S CmtRSc appears to be about one-half that of CmtRMtb (7) for reasons that are not clear, although the fractional stoichiometries in each case may complicate this. Furthermore, unlike for CdII-binding, the extent to which molar absorption wavelength and intensity reports on coordination number and geometry for PbII-thiolate complexes has not yet been firmly established (12, 36–39). In any case, the simplest conclusion is that substitution of Cys110 and Cys111 with non-liganding residues abolishes PbII binding to metal site 2, as is the case for CdII.
Figure 4. PbII titrations of CmtRSc and C110G/C111S CmtRSc.
(A) Apoprotein subtracted difference spectrum of wild-type CmtRSc (50.8 μM). (B) Apoprotein subtracted difference spectrum of C110G/C111S CmtRSc (53.7 μM). CmtRSc variants were titrated anaerobically with increasing concentrations of PbII. Inset, PbII binding isotherm plotted as change in A333 vs. [CmtRSc variant monomer] Conditions: 10 mM Bis-Tris, 0.4 M NaCl, pH 7.0, 25.0 °C.
CmtRSc binds two ZnII per monomer as determined by chelator competition experiments with magfura-2
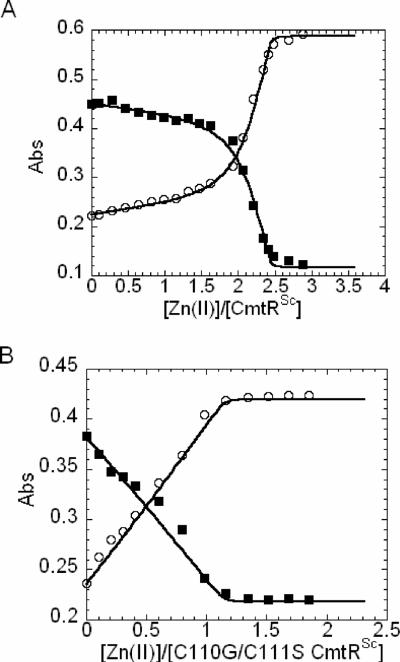
The zinc indicator dye magfura-2 (KZn•mag-fura-2 = 5.0 × 107 M−) was used as a competitor of ZnII binding to CmtRSc and the C110G/C111S mutant to determine the ZnII binding stoichiometry and affinity constant KZn (11). Figure 5 shows representative titrations of ZnII into mixtures of wild-type CmtRSc (Fig. 5A) and C110G/C111S CmtRSc (Fig. 5B) and magfura-2. The solid curve represents a fit to a model that describes the binding of two ZnII ions to the wild-type CmtRSc monomer, considering each monomer-metal binding as an independent event (Fig. 5A). For wild-type CmtRSc, the estimated parameters are similar for both zinc ions, with KPZn = 5.3 (±1.8) ×108 M− and KPZn2 = 6.7 (±1.4) ×108 M−. These binding affinities differ from that determined previously for the CmtRMtb dimer, which showed strong negative cooperativity within the dimer with one site in ≈1010 M− range and the other in ~105 M−1 range under the same solution conditions (7). For the C110G/C111S CmtRSc mutant, competition titration curves were fit to a model that describes the binding of one ZnII ion to the C110G/C111S CmtRSc monomer (Fig. 5B), with an estimated affinity of KPZn = 5.5 (±1.2) × 107 M−1.
Figure 5. ZnII titrations of CmtRSc and C110G/C111S CmtR Sc using magfura-2 as an indicator.
(A) 30 μM CmtRSc and 14.7 μM magfura-2 and (B) 30 μM C110G/C111S CmtRSc and 15 μM magfura-2 were present. The empty circles represent A325 and the filled squares represent A366. The solid line represents a global non-linear least square fit to a model that incorporates the stepwise binding of two ZnII (defined by KPZn and KPZn2) to a CmtRSc monomer using Dynafit. The titration for C110G/C111S CmtRSc was fitted using one ZnII to protein monomer binding model. The following parameters were obtained for wild-type CmtRSc: KPZn = 5.3 (±1.8) × 108 M−1 (a lower limit under these conditions), KPZn2 = 6.7 (±1.4) × 108 M−1. For C110G/C111S CmtRSc, KPZn = 5.5 (±1.2) × 107 M−1. Conditions: 10 mM Hepes, 0.4 M NaCl at pH 7.0 and 25.0 °C.
Cadmium and lead alleviate CmtRSc mediated repression
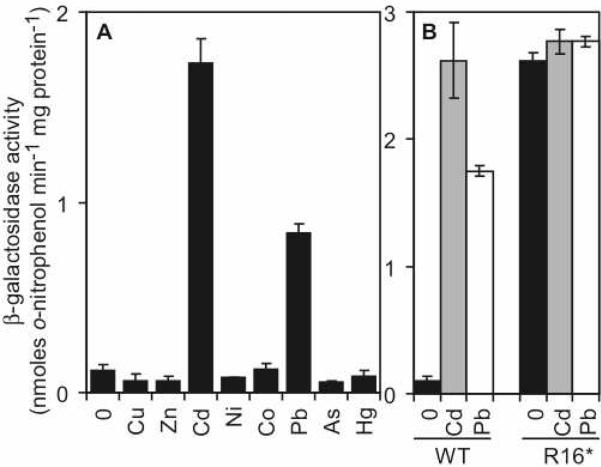
M. smegmatis mc2155 was previously used to characterize CmtRMtb and hence was exploited as a heterologous host for the characterization of CmtRSc, thereby allowing direct comparison of these two regulators within the same cytosol. M. smegmatis mc2155 and S. coelicolor are closely related actinomycetes, with similarly high GC-contents, and recognition of Streptomyces operator-promoter elements by the mycobacterial transcription machinery (40, 41) has been exploited previously to characterize S. coelicolor genes in vivo (41). To determine which, if any, metals are sensed by CmtRSc in vivo, a 539-bp DNA fragment including the cmtRSc operator-promoter and coding region was fused to a promoterless lacZ in plasmid pJEM15 and introduced into M. smegmatis mc2155. β-galactosidase activity was measured following growth (~20 h) of cells in medium supplemented with maximum permissive concentrations of various metals. At these biologically significant metal levels, elevated activity was detected in response to CdII and, to a lesser extent, PbII but no other metals (Fig. 6A). Furthermore, in the absence of added metal ions, elevated β-galactosidase activity was detected in cells containing an analogous construct in which codon-16 within the cmtRSc coding region was converted to a stop codon (Fig. 6B), confirming that CmtRSc acts negatively toward expression.
Figure 6. CmtRSc responds to CdII and PbII in an actinomycete host.
(A) β-galactosidase activity measured in M. smegmatis mc2155 containing cmtRSc and its operator-promoter region fused to lacZ following growth in LB medium with no metal supplement or with maximum permissive concentrations of ZnII (100 μM), CoII (200 μM), NiII (500 μM), CdII (7.5 μM), CuII (500 μM), PbII (3.75 μM), AsIII(20 μM), or HgII (0.025 μM). (B) β-galactosidase activity in cells containing wild-type CmtRSc (WT) or the stop codon derivative (R16*) following growth in LB medium with no metal supplement (black) or maximum permissive concentrations of CdII (gray) or PbII (white).
CmtRSc senses CdII and PbII using α4C sites
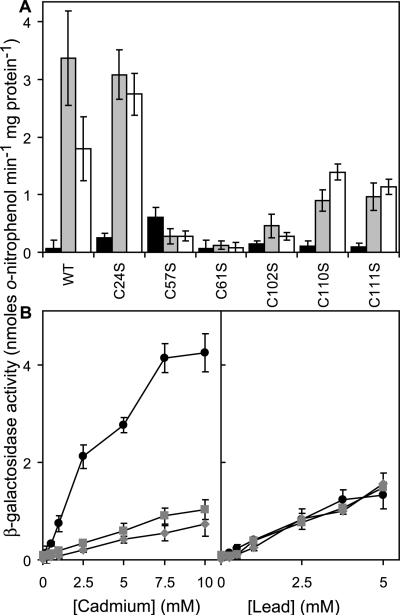
The previously characterized CdII binding α4C sensing sites of CmtRMtb involve Cys102 from the C-terminal region of one subunit in association with Cys57 and Cys61 from helix αR of the other subunit (6–8) (see Fig. 1B). Comparison of the amino acid sequences of CmtRSc and related ArsR family sensors reveals that the α4C ligands are completely conserved in CmtRSc and CmtRMtb, while CmtRSc lacks residues corresponding to other previously defined ArsR family metal-binding motifs (1, 10). Substitution of the α4C cysteines (Cys57, Cys61 and Cys102) in CmtRSc with serines created functional repressors that mediated low expression of lacZ in cells grown with no metal supplement, but repression was not alleviated at CdII concentrations that caused loss of repression by wild-type CmtRSc (Fig. 7A). In addition, no alleviation of repression was observed in the presence of maximum permissive concentrations of PbII (Fig. 7A). Hence, consistent with findings for CmtRMtb (6), Cys57, Cys61 and Cys102 at α4C are obligatory for both CdII and PbII recognition by CmtRSc in vivo and likely contribute toward a single common metal-binding site, metal site 1.
Figure 7. Metal sensing ligands of CmtRSc.
(A) β-galactosidase activity measured in M. smegmatis mc2155 containing wild-type CmtRSc (WT) or derivatives with indicated cysteine to serine codon substitutions, following growth in LB medium with no metal supplement (black) or maximum permissive concentrations of CdII (gray) or PbII (white). (B) β-galactosidase activity in cells containing wild-type CmtRSc (black circles) or the C110S (gray squares) and C111S derivatives (gray diamonds) grown in LB with up to inhibitory concentrations of CdII or PbII. Data points represent the mean (± SE) for three independent experiments, each performed in triplicate.
CdII, but not PbII, sensing by CmtRSc involves an additional pair of C-terminal cysteines
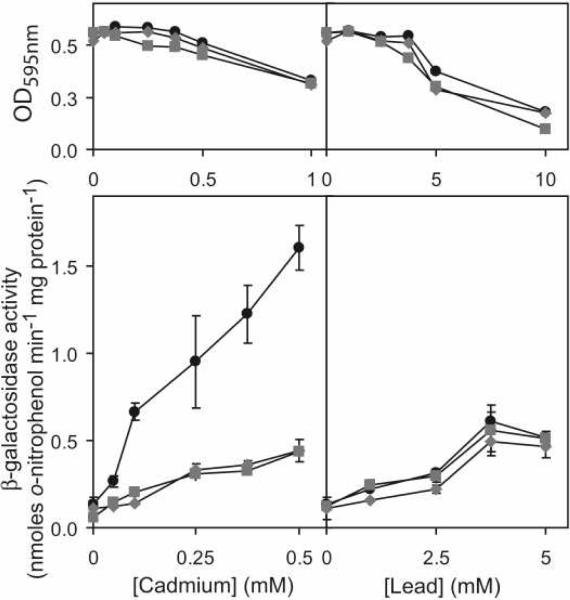
As discussed above, in addition to Cys57, Cys61 and Cys102 at α4C, CmtRSc possesses three further cysteines (Cys24 found in both CmtRs, and Cys110 and Cys111 which are unique to CmtRSc, Fig. 1C). In the absence of added CdII or PbII, β-galactosidase activity remained low in cells containing Ser substitutions of Cys24, Cys110 or Cys111, consistent with retention of repressor function (Fig. 7A). However, although inducer recognition was clearly retained in these cells, CdII-mediated derepression was substantially reduced in cells containing the single Cys110 and Cys111 substitution mutants (Fig. 7A). Indeed, when expression from the cmtR operator-promoter was examined in cells exposed to a range of CdII and PbII concentrations, CdII-responsiveness was significantly impaired for the C110S and C111S mutants at all cadmium concentrations up to inhibitory levels, whereas PbII-responsiveness appeared unaffected (Fig. 7B). Equivalent findings were also obtained using minimal (Sauton) medium (Fig. 8) which reveal no significant reduction in the magnitude of derepression by PbII in the C110S and C111S mutants, compared to wild-type CmtRSc, whereas CdII-responsiveness is substantially reduced. These results, when considered in the context of the metal binding properties of wild-type and C110G/C111S CmtRSc, reveal that coordination to metal site 2 in CmtRSc is required for full transcriptional derepression by CdII in the cell. In contrast, PbII is a less potent inducer of cmt-lacZ expression in vivo and does not require metal site 2 for this activity.
Figure 8. Metal sensing ligands of CmtRSc as determined on a chemically defined minimal medium.
Top panel, Cell viability of M. smegmatis mc2155 containing wild-type CmtRSc (black circles), C110S (gray squares) and C111S derivatives (gray diamonds) grown on minimal Sauton medium (containing 2.9 mM phosphate) as a function of total added CdII (left) or PbII (right). Lower panel, β-galactosidase activity in cells containing wild-type CmtRSc (black circles), C110S (gray squares) or C111S CmtRSc (gray diamonds) grown in minimal media up to inhibitory concentrations of CdII or PbII. Data points represent the mean (± SE) for three independent experiments, each performed in triplicate.
DISCUSSION
CmtRSc (SCO0875) is a M. tuberculosis CmtR homolog in S. coelicolor that shares 52% sequence identity with CmtRMtb. S. coelicolor belongs to the same taxonomic order (Actinomycetales) as the causative agents of tuberculosis and leprosy (M. tuberculosis and M. leprae), but is a soil-dwelling bacterium that may more often encounter heavy metal polluted environments (14). However, M. tuberculosis is also suggested to encounter heavy metal ions such as CdII due to their accumulation in alveolar macrophages as a result of air pollution and cigarette smoking (9). As a result, metal-specific regulatory machinery is required to manage the intracellular concentrations of these ions (1). M. tuberculosis CmtR is the founding member of a subfamily of winged helical DNA-binding repressors from the ArsR metal-sensing family that employ characteristic α4C metal sites that respond selectively to CdII and PbII (6–8). A search of CmtRMtb homologs in S. coelicolor identified two nearly identical open reading frames (locus tags SCO0875 and SCO3522) that appear to encode a CmtRSc that differ by just ten amino acids, and immediately upstream of ORFs that encode what are predicted to be nearly identical cation diffusion facilitator (CDF) family heavy metal transporters. The presence of what would appear to be two functionally redundant operons may well reflect the need for S. coelicolor to rapidly sense and efflux (or otherwise detoxify) any CdII and PbII encountered in the environment.
In this study, we chose the gene encoded by locus tag SCO0875 as representative of CmtRSc for detailed study and comparison with CmtRMtb. We were particularly intrigued by the presence of two C-terminal vicinal cysteines in CmtRSc, Cys110 and Cys111, both of which are conserved in SCO0875 and SCO3522. Substitution of these residues with non-liganding residues was expected to yield a regulator that behaves much like wild-type CmtRMtb. In vitro metal titration experiments confirm this, but also reveal a second metal site in CmtRSc which is formed by Cys-S− coordination bonds donated by Cys110, Cys111 and a third as yet unidentified ligand. Cys24, which is at least partly exposed to solvent in the solution structure of CdII-bound CmtR (Fig. 1B) (8) could readily complete an S3 or S3(O) coordination complex. Since the solution structural model of CdII-bound CmtR ends at Arg106, this suggests that the C-terminal tail is highly flexible; if so, inspection of this structure suggests that the C-terminal Cys110/Cys111 pair of one subunit could potentially come in close physical proximity of Cys24 in the α2 helix of the same subunit by crossing over the “front” of the molecule in the orientation shown in Fig. 1B. In this case however Cys24 can not function as a key regulatory residue since in vivo β-galactosidase assays reveal that substitution of Cys24 does not influence CdII responsiveness of CmtRSc in contrast to Cys110 and Cys111; a similar finding characterizes the primary α4C metal site in C24S CmtRMtb (6). Cys24 may still complete the coordination structure of metal site 2, but it may simply increase the affinity of the site for CdII rather than function as an allosteric residue (23), much like that found previously for His100 in the ZnII sensor S. aureus CzrA (42) and the Cys7 and Cys58 of the S4 CdII sensor S. aureus pI258 CadC (12). Other non-thiolate possibilities for the third ligand are of course formally possible, but would not be compatible with the absorption spectroscopy of the CdII and PbII complexes (12). Detailed structural or 113Cd NMR studies (7, 12, 32) will be required to further elucidate the coordination structure of metal site 2 in CmtRSc.
CmtRSc displays an in vivo metal specificity for CdII and PbII that is identical to that of CmtRMtb in the heterologous host M. smegmatis; given the similarity of these two bacterial species, these findings suggest that CmtRSc is a bona fide CdII/PbII sensor in S. coelicolor. Although both CmtRSc and CmtRMtb share the same primary allosteric α4C metal site with the HgII sensor MerR in Streptomyces lividans (43), neither CmtR senses HgII in M. smegmatis (7). In addition, neither CmtRSc- nor CmtRMtb-repressed transcription is inducible by ZnII (7), despite the fact that each retains the ability to bind ZnII in vitro (7). Little is known about how Streptomyces spp. handle HgII toxicity and how this might differ from Mycobacteria; as a result, it is formally possible that in addition to detecting CdII and PbII, CmtRSc may detect HgII in the native host. A more important issue is how CmtRs sense CdII and PbII over ZnII in the cell. Although absolute metal affinity of the sensing sites may not fully explain metal selectivity in the cell, this simple explanation may be at least partially operative here. Both proteins have ZnII affinity ≤ 1010 M−1, which is three orders less than the affinity for CdII (Fig. 3) (7) which itself is comparable to that previously measured KCd for the CdII/PbII sensor CadC (27). Furthermore, a KZn ≤1010 M−1 may not be sufficient to be detected in cells by CmtR, since the typical bacterial ZnII sensor is characterized by an equilibrium affinity for ZnII in the 1012–1015 M−1 range. In fact, a protein encoded by locus tag SCO6459 is a strong candidate α5 site zinc sensor in S. coelicolor that may be poised to detect weakly chelated ZnII buffered in the 10−12 to 10−15 M range (29, 42, 44, 45). This ensures that both cmtRSc-sco0874 and sco3522-sco3521 operons will be induced by CdII and PbII and not by ZnII so that these toxic metals can be selectively effluxed from the cytosol.
The primary α4C metal site 1 is necessary for both CdII and PbII detection in the cell, but is not sufficient for full CdII responsiveness, which clearly requires coordination by metal site 2 (Figs. 7–8). The evolutionary advantage of this to S. coelicolor is unknown, but may reflect the possibility that CdII salts and low molecular weight complexes are far more bioavailable (soluble) in soils than PbII complexes (which may be readily precipitated) and thus must be more efficiently detoxified. Clearly, CdII is a stronger inducer of CmtRSc-mediated transcriptional derepression in M. smegmatis than is PbII over a similar concentration range of added metal, and most of this difference can be traced to CdII occupancy of metal site 2 (Figs. 6–7). PbII, being the larger cation, may well fail to induce the same change in homodimer structure and/or dynamics that has been shown to be important in CmtRMtb (8) and other ArsR family sensors (46). Alternatively, the bioavailable concentration of PbII achievable in M. smegmatis at the maximum permissive concentration of this cation (≈5 mM; Figs. 7–8) may not high enough to fill metal site 2 in the M. smegmatis cytosol; as a result, CmtRSc is a poorer PbII sensor in vivo. In any case, filling both metal sites in CmtRSc may well stabilize the allosterically inhibited low-affinity DNA binding state beyond that which can be achieved by filling the pair of primary α4C metal sites alone. Indeed, previous quantitative DNA binding experiments reveal that CdII is a relative poorer allosteric negative inhibitor of CmtRMtb binding to DNA relative to other ArsR-family metalloregulatory proteins (1, 11); this suggests that CdII binding to metal site 2 may further enhance allosteric regulation of DNA binding in a way that leads to increased transcription derepression in the cell. Alternatively or in addition, the second high affinity (KCd≥1012 M−1) cadmium binding site on CmtRSc may also simply allow S. coelicolor to sequester (chelate) more CdII under conditions of CdII stress once CmtRSc is dissociated from its DNA operator. Understanding the structural and physiological role of metal site 2 in CmtRSc requires further investigation.
CmtRSc is not the first ArsR family regulator with two structurally distinct pairs of metalloregulatory sites per dimer. Other examples include the CdII/PbII sensor pI258 CadC (12) and CuI sensor O. brevis BxmR (11). CadC employs four conserved Cys in CdII, CoII and BiIII binding while adopting an S3 coordination complex with PbII in its primary α3N metal site; ZnII binding to a pair of C-terminal interhelical α5 sites is functionally silent in CadCs, while other CadCs and α3N-type sensors have dispensed with this site entirely (12, 47). In BxmR, the α3N site is a major sensing site for CdII and CuI, the latter of which forms a binuclear CuI2S4 cluster (11); a C-terminal α5 site has evolved exclusively for ZnII sensing. Thus, in the case of BxmR, the presence of two distinct metal sites relaxes the metal selectivity of this particular sensor. In CmtRSc, the impact of metal site 2 on CmtRSc function largely parallels the situation in CadCs, where the secondary site does not change the metal specificity profile of the primary metal site (12, 48), but unlike in CadCs, enhances its effectiveness in the cell.
Finally, CmtRSc is the first ArsR family metal sensor to be characterized from S. coelicolor and is shown here to possess both a metal sensing site anticipated on the basis of its classification as an CmtR-like α4C PbII/CdII sensor, as well as a second novel metalloregulatory site not found in other CmtRs. In fact, the presence of consecutive or vicinal Cys residues in what are predicted to be flexible or unstructured N-terminal or C-terminal “tails” like that in metal site 2 in CmtRSc, have previously been shown to function as AsIII ligands in two arsenic-specific ArsR family proteins recently characterized (49, 50); given this, it seemed possible that Cys110 and/or Cys111 might confer a AsIII sensing function on the PbII/CdII sensing CmtR. Our data reveal that this is clearly not the case, at least as measured by transcriptional derepression in M. smegmatis (Fig. 6). These findings are consistent with the idea that ArsR family sensors may well be readily classified into subfamilies of paralogs that detect distinct cellular inducers solely on the basis of well-characterized primary structural motifs, rather than global sequence similarity (1, 10). However, these core characteristics can be modulated by additional or altogether novel metal sites capable of tuning inducer responsiveness appropriate for the microenvironmental niche in which an organism resides (11, 49).
Acknowledgments
This work was supported, in whole or in part, by grants from the National Institutes of Health (GM042569 to D. P. G.) and the Biotechnology and Biological Sciences Research Council (BBSRC) (BB/G010765/1 to J. S. C.)
Footnotes
Abbreviations used are: DTNB, 5,5'-dithiobis(2-nitrobenzoic acid); LMCT, ligand-to-metal charge transfer; O/P, operator/promoter.
REFERENCES
- 1.Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009;109:4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nies DH. Heavy metal-resistant bacteria as extremophiles: molecular physiology and biotechnological use of Ralstonia sp. CH34. Extremophiles. 2000;4:77–82. doi: 10.1007/s007920050140. [DOI] [PubMed] [Google Scholar]
- 3.Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, Dolganov G, Efron B, Butcher PD, Nathan C, Schoolnik GK. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J Exp Med. 2003;198:693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne JC, ter Horst MA, Godwin HA. Lead fingers: Pb2+ binding to structural zinc-binding domains determined directly by monitoring lead-thiolate charge-transfer bands. J. Am. Chem. Soc. 1999;121:6850–6855. [Google Scholar]
- 5.Warren MJ, Cooper JB, Wood SP, Shoolingin-Jordan PM. Lead poisoning, haem synthesis and 5-aminolaevulinic acid dehydratase. Trends Biochem Sci. 1998;23:217–221. doi: 10.1016/s0968-0004(98)01219-5. [DOI] [PubMed] [Google Scholar]
- 6.Cavet JS, Graham AI, Meng W, Robinson NJ. A cadmium-lead-sensing ArsR-SmtB repressor with novel sensory sites. Complementary metal discrimination by NmtR and CmtR in a common cytosol. J. Biol. Chem. 2003;278:44560–44566. doi: 10.1074/jbc.M307877200. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Hemmingsen L, Giedroc DP. Structural and functional characterization of Mycobacterium tuberculosis CmtR, a PbII/CdII-sensing SmtB/ArsR metalloregulatory repressor. Biochemistry. 2005;44:8976–8988. doi: 10.1021/bi050094v. [DOI] [PubMed] [Google Scholar]
- 8.Banci L, Bertini I, Cantini F, Ciofi-Baffoni S, Cavet JS, Dennison C, Graham AI, Harvie DR, Robinson NJ. NMR structural analysis of cadmium sensing by winged helix repressor CmtR. J. Biol. Chem. 2007;282:30181–30188. doi: 10.1074/jbc.M701119200. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan S, Kumar A, Singhal A, Tyagi JS, Krishna Prasad H. CmtR, a cadmium-sensing ArsR-SmtB repressor, cooperatively interacts with multiple operator sites to autorepress its transcription in Mycobacterium tuberculosis. FEBS J. 2009;276:3428–3439. doi: 10.1111/j.1742-4658.2009.07066.x. [DOI] [PubMed] [Google Scholar]
- 10.Campbell DR, Chapman KE, Waldron KJ, Tottey S, Kendall S, Cavallaro G, Andreini C, Hinds J, Stoker NG, Robinson NJ, Cavet JS. Mycobacterial cells have dual nickel-cobalt sensors: sequence relationships and metal sites of metal-responsive repressors are not congruent. J. Biol. Chem. 2007;282:32298–32310. doi: 10.1074/jbc.M703451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu T, Chen X, Ma Z, Shokes J, Hemmingsen L, Scott RA, Giedroc DP. A Cu(I)-sensing ArsR family metal sensor protein with a relaxed metal selectivity profile. Biochemistry. 2008;47:10564–10575. doi: 10.1021/bi801313y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busenlehner LS, Weng TC, Penner-Hahn JE, Giedroc DP. Elucidation of primary (α3N) and vestigial (α5) heavy metal-binding sites in Staphylococcus aureus pI258 CadC: evolutionary implications for metal ion selectivity of ArsR/SmtB metal sensor proteins. J. Mol. Biol. 2002;319:685–701. doi: 10.1016/S0022-2836(02)00299-1. [DOI] [PubMed] [Google Scholar]
- 13.Ye J, Kandegedara A, Martin P, Rosen BP. Crystal structure of the Staphylococcus aureus pI258 CadC Cd(II)/Pb(II)/Zn(II)-responsive repressor. J. Bacteriol. 2005;187:4214–4221. doi: 10.1128/JB.187.12.4214-4221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O'Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2) Nature. 2002;417:141–147. doi: 10.1038/417141a. [DOI] [PubMed] [Google Scholar]
- 15.Borodina I, Krabben P, Nielsen J. Genome-scale analysis of Streptomyces coelicolor A3(2) metabolism. Genome Res. 2005;15:820–829. doi: 10.1101/gr.3364705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner D, Maser J, Lai B, Cai Z, Barry CE, 3rd, Honer Zu Bentrup K, Russell DG, Bermudez LE. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell's endosomal system. J. Immunol. 2005;174:1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- 17.Collins HL. Withholding iron as a cellular defence mechanism--friend or foe? Eur. J. Immunol. 2008;38:1803–1806. doi: 10.1002/eji.200838505. [DOI] [PubMed] [Google Scholar]
- 18.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. The Journal of biological chemistry. 2009;284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Techau ME, Valdez-Taubas J, Popoff JF, Francis R, Seaman M, Blackwell JM. Evolution of differences in transport function in Slc11a family members. J Biol Chem. 2007;282:35646–35656. doi: 10.1074/jbc.M707057200. [DOI] [PubMed] [Google Scholar]
- 20.An YJ, Ahn BE, Han AR, Kim HM, Chung KM, Shin JH, Cho YB, Roe JH, Cha SS. Structural basis for the specialization of Nur, a nickel-specific Fur homolog, in metal sensing and DNA recognition. Nucleic Acids Res. 2009:3442–3451. doi: 10.1093/nar/gkp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn BE, Cha J, Lee EJ, Han AR, Thompson CJ, Roe JH. Nur, a nickel-responsive regulator of the Fur family, regulates superoxide dismutases and nickel transport in Streptomyces coelicolor. Mol. Microbiol. 2006;59:1848–1858. doi: 10.1111/j.1365-2958.2006.05065.x. [DOI] [PubMed] [Google Scholar]
- 22.Shin JH, Oh SY, Kim SJ, Roe JH. The zinc-responsive regulator Zur controls a zinc uptake system and some ribosomal proteins in Streptomyces coelicolor A3(2) J Bacteriol. 2007;189:4070–4077. doi: 10.1128/JB.01851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giedroc DP, Arunkumar AI. Metal sensor proteins: nature's metalloregulated allosteric switches. Dalton Trans. 2007;29:3107–3120. doi: 10.1039/b706769k. [DOI] [PubMed] [Google Scholar]
- 24.Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics. 2007;8:107. doi: 10.1186/1471-2164-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo J, Giedroc DP. Zinc site redesign in T4 gene 32 protein: structure and stability of cobalt(II) complexes formed by wild-type and metal ligand substitution mutants. Biochemistry. 1997;36:730–742. doi: 10.1021/bi9617769. [DOI] [PubMed] [Google Scholar]
- 26.Tan X, Kagiampakis I, Surovtsev IV, Demeler B, Lindahl PA. Nickel-dependent oligomerization of the alpha subunit of acetyl-coenzyme a synthase/carbon monoxide dehydrogenase. Biochemistry. 2007;46:11606–11613. doi: 10.1021/bi7014663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busenlehner LS, Cosper NJ, Scott RA, Rosen BP, Wong MD, Giedroc DP. Spectroscopic properties of the metalloregulatory Cd(II) and Pb(II) sites of S. aureus pI258 CadC. Biochemistry. 2001;40:4426–4436. doi: 10.1021/bi010006g. [DOI] [PubMed] [Google Scholar]
- 28.Martell AE, Smith RM. Critical Stability Constants. Plenum Press; New York: 1979–1989. [Google Scholar]
- 29.VanZile ML, Chen X, Giedroc DP. Structural characterization of distinct α3N and α5 metal sites in the cyanobacterial zinc sensor SmtB. Biochemistry. 2002;41:9765–9775. doi: 10.1021/bi0201771. [DOI] [PubMed] [Google Scholar]
- 30.Timm J, Lim EM, Gicquel B. Escherichia coli-mycobacteria shuttle vectors for operon and gene fusions to lacZ: the pJEM series. Journal of bacteriology. 1994;176:6749–6753. doi: 10.1128/jb.176.21.6749-6753.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavet JS, Meng W, Pennella MA, Appelhoff RJ, Giedroc DP, Robinson NJ. A nickel-cobalt-sensing ArsR-SmtB family repressor. Contributions of cytosol and effector binding sites to metal selectivity. J Biol Chem. 2002;277:38441–38448. doi: 10.1074/jbc.M207677200. [DOI] [PubMed] [Google Scholar]
- 32.Matzapetakis M, Farrer BT, Weng TC, Hemmingsen L, Penner-Hahn JE, Pecoraro VL. Comparison of the binding of cadmium(II), mercury(II), and arsenic(III) to the de novo designed peptides TRI L12C and TRI L16C. J. Am. Chem. Soc. 2002;124:8042–8054. doi: 10.1021/ja017520u. [DOI] [PubMed] [Google Scholar]
- 33.Pountney DL, Tiwari RP, Egan JB. Metal- and DNA-binding properties and mutational analysis of the transcription activating factor, B, of coliphage 186: a prokaryotic C4 zinc-finger protein. Protein Sci. 1997;6:892–902. doi: 10.1002/pro.5560060416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henehan CJ, Pountney DL, Zerbe O, Vasak M. Identification of cysteine ligands in metalloproteins using optical and NMR spectroscopy: cadmium-substituted rubredoxin as a model [Cd(CysS)4]2- center. Protein Sci. 1993;2:1756–1764. doi: 10.1002/pro.5560021019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claudio ES, Magyar JS, Godwin HA. Prog. Inorg. Chem. 2003;51:1–144. [Google Scholar]
- 36.Magyar JS, Weng TC, Stern CM, Dye DF, Rous BW, Payne JC, Bridgewater BM, Mijovilovich A, Parkin G, Zaleski JM, Penner-Hahn JE, Godwin HA. Reexamination of lead(II) coordination preferences in sulfur-rich sites: implications for a critical mechanism of lead poisoning. J Am Chem Soc. 2005;127:9495–9505. doi: 10.1021/ja0424530. [DOI] [PubMed] [Google Scholar]
- 37.Gamelin DRR, D. W., Hay MT, Houser RP, Mulder TC, Canters GW, de Vries S, Tolman WB, Lu Y, Solomon EI. Spectroscopy of mixed-valence CuA-type centers: Ligand-field control of ground-state properties related to electron transfer. J. Am. Chem. Soc. 1998;120:5246–5263. [Google Scholar]
- 38.Basumallick LG, S. D., Randall DW, Hedman B, Hodgson KO, Fujisawa K, Solomon EI. Spectroscopic comparison of the five-coordinate [Cu(SMeIm)(HB(3,5-iPr2pz)3)] with the four-coordinate [Cu(SCPh3)(HB(3,5-iPr2pz)3)]: effect of coordination number increase on a blue copper type site. Inorg. Chim. Acta. 2002;337:357–365. [Google Scholar]
- 39.Lever ABP. J. Chem. Educ. 1974;51:612–616. [Google Scholar]
- 40.Hernandez-Abanto SM, Woolwine SC, Jain SK, Bishai WR. Tetracycline-inducible gene expression in mycobacteria within an animal host using modified Streptomyces tcp830 regulatory elements. Arch Microbiol. 2006;186:459–464. doi: 10.1007/s00203-006-0160-2. [DOI] [PubMed] [Google Scholar]
- 41.Raghunand TR, Bishai WR. Mapping essential domains of Mycobacterium smegmatis WhmD: insights into WhiB structure and function. J Bacteriol. 2006;188:6966–6976. doi: 10.1128/JB.00384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pennella MA, Arunkumar AI, Giedroc DP. Individual metal ligands play distinct functional roles in the zinc sensor Staphylococcus aureus CzrA. J. Mol. Biol. 2006;356:1124–1136. doi: 10.1016/j.jmb.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Rother D, Mattes R, Altenbuchner J. Purification and characterization of MerR, the regulator of the broad-spectrum mercury resistance genes in Streptomyces lividans 1326. Mol Gen Genet. 1999;262:154–162. doi: 10.1007/s004380051070. [DOI] [PubMed] [Google Scholar]
- 44.Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 45.Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol. 2009;7:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 46.Arunkumar AI, Campanello GC, Giedroc DP. Solution structure of a paradigm ArsR family zinc sensor in the DNA-bound state. Proc Natl Acad Sci USA. 2009;106:18177–18182. doi: 10.1073/pnas.0905558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu T, Golden JW, Giedroc DP. A zinc(II)/lead(II)/cadmium(II)-inducible operon from the cyanobacterium Anabaena is regulated by AztR, an α3N ArsR/SmtB metalloregulator. Biochemistry. 2005;44:8673–8683. doi: 10.1021/bi050450+. [DOI] [PubMed] [Google Scholar]
- 48.Kandegedara A, Thiyagarajan S, Kondapalli KC, Stemmler TL, Rosen BP. Role of bound Zn(II) in the CadC Cd(II)/Pb(II)/Zn(II)-responsive repressor. J. Biol. Chem. 2009;284:14958–14965. doi: 10.1074/jbc.M809179200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ordoñez E, Thiyagarajan S, Cook JD, Stemmler TL, Gil JA, Mateos LM, Rosen BP. Evolution of metal(loid) binding sites in transcriptional regulators. J. Biol. Chem. 2008;283:25706–25714. doi: 10.1074/jbc.M803209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin J, Fu HL, Ye J, Bencze KZ, Stemmler TL, Rawlings DE, Rosen BP. Convergent evolution of a new arsenic binding site in the ArsR/SmtB family of metalloregulators. J. Biol. Chem. 2007;282:34346–34355. doi: 10.1074/jbc.M706565200. [DOI] [PMC free article] [PubMed] [Google Scholar]