Na+,K+-ATPase
Despite the widely known fact that low concentrations of extracellular free calcium can induce parathyroid hormone (PTH) secretion, the molecular mechanisms of such regulation and eventual calcium metabolism are not yet completely understood. The traditional view of calcium homeostasis is believed to be maintained by sensing changes in extracellular calcium in several distinct cell types, to stimulate the secretion of PTH, vitamin D and calcitonin, depending on the requirement of the body. These calciotropic hormones then act on the calcium-translocating cells of the kidney, bone and intestine to restore calcium balance [1]. New insights into calcium homeostasis were gained from a recent study by Imura et al.; the investigators proposed that low extracellular free calcium can induce PTH secretion through the klotho- and Na+,K+-adenosine triphosphatase (Na+,K+-ATPase)-dependent process [2].
Na+,K+-ATPase is a highly conserved integral membrane protein and is composed of two subunits, the alpha subunit (∼113 kDa) and a smaller beta subunit (∼35 kDa). The alpha subunit is mostly responsible for ion transport and catalytic activity, while the beta subunit regulates the catalytic activity of the alpha subunit [2].
Calcium and Na+,K+-ATPase activity
Intestinal absorption and renal reabsorption of calcium are delicately maintained for gain or loss of calcium, according to the needs of the body. Renal calcium transport is a complex process that takes place mostly in the distal and connecting tubular epithelial cells. Calcium from filtrated tubular fluid enters the epithelial cells through the luminal calcium channel TRPV5, which is transported across the cell in association with the calcium-binding protein, calbindin-D28k, and is eventually released into the bloodstream via the Na+/Ca2+-exchanger (NCX1) and the plasma membrane Ca2+-ATPase (PMCA1b) in the basolateral membrane. In the intestine, calcium absorption is regulated by the TRPV6 channel at the luminal side of the membrane, and further transported by a calcium-binding protein, calbindin-D9k, while PMCA1b helps in basolateral translocation [5,6].
Even though Na+,K+-ATPase is primarily involved in the cellular transport of Na+ and K+, the electrochemical gradient created across the cell membrane by such ionic translocation can also affect the transport process of calcium. In vitro experimental study, using isolated choroid plexus, has shown a rapid increase in the activity of Na+,K+-ATPase following incubation in low calcium-containing media, while the Na+,K+-ATPase activity was less following incubation in a high calcium-containing media. Moreover, the increased activity of Na+,K+-ATPase in low-calcium media was associated with the increased presence of Na+,K+-ATPase in the plasma membrane. Low calcium induces an increase of Na+,K+-ATPase of ∼12% at the plasma membrane. In contrast, high calcium led to a decrease of Na+,K+-ATPase of ∼8% at the plasma membrane. Such in vitro observations lead to the speculation that the Na+,K+-ATPase activity at the plasma membrane could be rapidly changed by the shift of calcium balance [2]. Interestingly, the amount or activity of Na+,K+-ATPase was not affected by the change in calcium concentration in the klotho-null mice. These findings led the investigators to suggest that klotho, a type 1 membrane protein, is an essential factor required for the rapid recruitment of Na+,K+-ATPase to the cell surface in response to the change in extracellular calcium concentration [2].
Klotho and Na+,K+-ATPase activity
Human klotho is a 5-exon gene and can generate two transcripts from this single gene. A full-length transcript of5.2 kb encodes to a 130 kDa membrane protein; once the short transmembrane domain is removed, this membrane form can be released into the circulation. An alternative mRNA splicing generates another transcript that encodes the N-terminal of klotho with a molecular weight of ∼65–70 kDa. Human klotho has ∼86% homology to mouse klotho and is mapped to chromosome 13q12 [7]. Klotho expression is observed in restrictive places and is predominantly present in tissues that regulate calcium homeostasis, including the distal convoluted tubules in the kidney, parathyroid gland and the epithelium of the choroid plexus in the brain [7].
Furthermore, klotho can regulate the Na+,K+-ATPase activity. Compared to wild-type mice, a significantly lower amount of the cell surface Na+,K+-ATPase was detected in the choroid plexus obtained from klotho-null mice. In vitro incubation of isolated choroid plexus in a buffer containing low calcium could increase secretion of klotho, whereas secretion was much less when exposed to a higher concentration of calcium. However, the in vitro ability of klotho to induce the Na+,K+-ATPase activity in choroid plexus in response to extracellular calcium concentration may not mimic in vivo physiological concentration, and its contribution to overall in vivo calcium homeostasis therefore needs an additional study.
Ex vivo organ-culture studies using thyroid and parathyroid glands exposed to low calcium-containing buffer can increase secretion of both klotho and PTH, suggesting a possible coordinated regulation of these molecules in response to extracellular calcium balance.
PTH and Na+,K+-ATPase activity
PTH is one of the most important hormones that control calcium homeostasis, by decreasing urinary calcium loss through stimulating calcium reabsorption, increasing the release of calcium from the bone through resorption and passively stimulating calcium absorption in the small intestine by facilitating vitamin-D synthesis in the kidney (Figure 1); what effect of PTH plays a dominant role depends on the body's overall need for calcium. Recent studies suggest involvement of klotho in renal calcium handling; klotho, by stabilizing TRPV5, facilitates calcium uptake in the kidney [8]. Furthermore, low calcium can induce secretion of klotho in the isolated parathyroid glands, similar to the choroid plexus [2]. However, in parathyroid glands obtained from klotho-null mice, PTH secretion in response to low calcium was ∼27% of the wild-type glands. In a similar line of study, ouabain, a specific inhibitor of Na+,K+-ATPase, can inhibit the secretion of PTH from the wild-type glands, but no such significant inhibitory effect of ouabain on PTH secretion was noted in the glands obtained from klotho-null mice. This result would suggest that klotho is an important mediator of Na+,K+-ATPase-dependent release of PTH. The reduced response of serum PTH in klotho-null mice is likely to be caused by the impaired Na+,K+-ATPase activity. It is however necessary to mention that impaired PTH release from the parathyroid glands of klotho-null mice could be influenced by intrinsic glandular microenvironments. Moreover, prolonged PTH suppression in klotho-null mice could be related to extremely high vitamin-D activities [9–12] and hypercalcaemia.
Fig. 1.
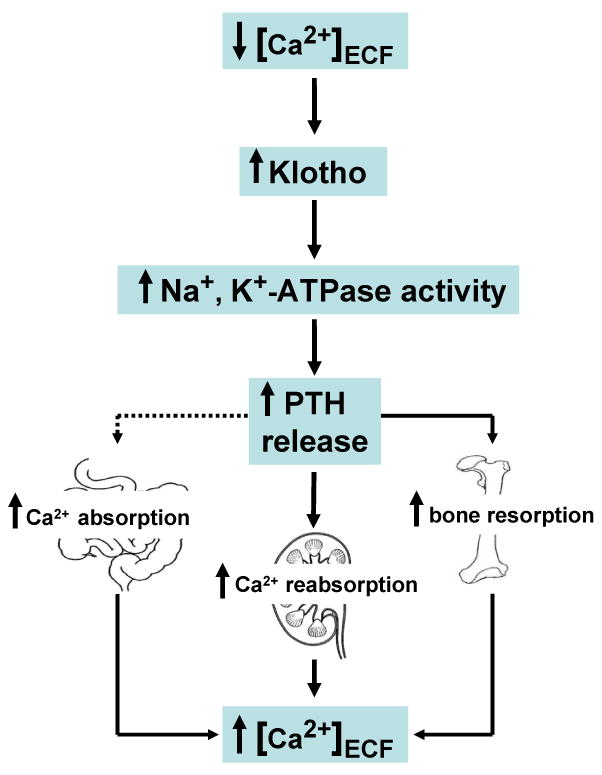
Simplified schematic outline of calcium regulation in response to low extracellular calcium concentration. Low extracellular calcium can coordinately induce klotho and Na+,K+-ATPase activity to increase the release of PTH to restore calcium balance by exerting direct effects on the kidney and bone [2].
The inter-relationship of vitamin D and klotho is a complex process. Administration of 1,25-(OH)2D3 could induce expression of klotho in the kidney [9], while expression of 1-alpha-hydroxylase gene and serum level of 1,25-(OH)2D3 is significantly higher in klotho-null mice [11]. Vitamin D could regulate klotho activities by inducing its production, and klotho could contribute to the negative regulatory circuit of active vitamin-D synthesis, possibly through suppressing 1-alpha-hydroxylase gene expression. Further studies are needed to determine how klotho-mediated release of PTH is affected by vitamin D.
How low calcium can influence the Na+,K+-ATPase activity in the parathyroid glands is an unsettled issue. It is proposed that when extracellular calcium is low, Na+,K+-ATPase is quickly recruited to the plasma membrane, in coordination with the secretion of klotho. An electrochemical gradient created by the increased Na+,K+-ATPase activity subsequently induces the release of PTH. Such possibilities are validated by the observation that disruption of klotho activity, either by genetic ablation from the mice or by administration of specific inhibitor of Na+,K+-ATPase, can suppress the regulated secretion of PTH. Since PTH release in response to changes in calcium concentration is an extremely rapid process, it will be of interest to know whether coordinated klotho and Na+,K+-ATPase activities are quick enough to completely explain such rapid release of PTH.
Conclusion
The molecular mechanism of the calcium-transport process across the cell membrane is an intense area of research. The observation that klotho can directly affect the Na+,K+-ATPase activity to increase the Na+ gradient, to drive the transepithelial calcium transport in coordination with various ion channels and transporters, partly explains calcium transport in the choroid plexus and the kidney [2]. However, this study also generated as many questions as it answered; for instance, how low extracellular calcium regulates the expression of klotho, and what role calcium sensors play in such a regulation. The scientific challenge and comprehensive understanding of calcium homeostasis lie in pursuing these unsolved questions. Finally, the role of klotho in influencing calcium homeostasis, directly through TRPV5 [8,13], and passively through fibroblast growth factor 23 (FGF23) [10,14–18] is known, but its interaction with Na+,K+-ATPase brings new insights into the complex regulation of the mineral-ion metabolism (Figure 1).
Supplementary Material
Acknowledgments
Part of the author's research work is supported by the grant (R01-DK077276–01A1) provided by NIH (NIDDK), and the institutional support from Harvard School of Dental Medicine, Boston, USA.
Footnotes
Conflict of interest statement. None declared.
Notes: Comment on: Imura A, Tsuji Y, Murata M et al. alpha-Klotho as a regulator of calcium homeostasis. Science 2007; 316: 1615–16180
References
- 1.Brown EM. Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab. 2007:3, 122–133. doi: 10.1038/ncpendmet0388. [CrossRef][Web of Science][Medline] [DOI] [PubMed] [Google Scholar]
- 2.Imura A, Tsuji Y, Murata M, et al. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 3.Ovchinnikov Yu A, Monastyrskaya GS, Broude NE, et al. Family of human Na+K+-ATPase genes. Structure of the gene for the catalytic subunit (alpha III-form) and its relationship with structural features of the protein. FEBS Lett. 1988:233, 87–94. doi: 10.1016/0014-5793(88)81361-9. [CrossRef][Web of Science][Medline] [DOI] [PubMed] [Google Scholar]
- 4.Gupta SP, Bagaria P, Kumaran S. A quantitative structure–activity relationship study on a series of Na+K+-ATPase inhibitors. J Enzyme Inhib Med Chem. 2004:19, 389–393. doi: 10.1080/1475636042000206437. [CrossRef][Web of Science][Medline] [DOI] [PubMed] [Google Scholar]
- 5.Schoeber JP, Hoenderop JG, Bindels RJ. Concerted action of associated proteins in the regulation of TRPV5 and TRPV6. Biochem Soc Trans. 2007:35, 115–119. doi: 10.1042/BST0350115. [CrossRef][Web of Science][Medline] [DOI] [PubMed] [Google Scholar]
- 6.Mensenkamp AR, Hoenderop JG, Bindels RJ. Recent advances in renal tubular calcium reabsorption. Curr Opin Nephrol Hypertens. 2006:15, 524–529. doi: 10.1097/01.mnh.0000242179.38739.fb. [Web of Science][Medline] [DOI] [PubMed] [Google Scholar]
- 7.Matsumura Y, Aizawa H, Shiraki-Iida T, et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998:242, 626–630. doi: 10.1006/bbrc.1997.8019. [CrossRef][Web of Science][Medline] [DOI] [PubMed] [Google Scholar]
- 8.Chang Q, Hoefs S, van der Kemp AW, et al. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 9.Tsujikawa H, Kurotaki Y, Fujimori T, et al. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 10.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and klotho mutant mice. Trends Mol Med. 2006:12, 298–305. doi: 10.1016/j.molmed.2006.05.002. [CrossRef][Web of Science][Medline] [DOI] [PubMed] [Google Scholar]
- 11.Yoshida T, Fujimori T, Nabeshima Y. Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology. 2002;143:683–689. doi: 10.1210/endo.143.2.8657. [Abstract/Free Full Text] [DOI] [PubMed] [Google Scholar]
- 12.Lanske B, Razzaque MS. Premature aging in klotho mutant mice: cause or consequence. Ageing Res Rev. 2007:6, 73–79. doi: 10.1016/j.arr.2007.02.002. [CrossRef][Medline] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topala CN, Bindels RJ, Hoenderop JG. Regulation of the epithelial calcium channel TRPV5 by extracellular factors. Curr Opin Nephrol Hypertens. 2007:16, 319–324. doi: 10.1097/MNH.0b013e3281c55f02. [CrossRef][Web of Science][Medline] [DOI] [PubMed] [Google Scholar]
- 14.Razzaque MS, Sitara D, Taguchi T, et al. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006:444, 770–774. doi: 10.1038/nature05315. [CrossRef][Medline] [DOI] [PubMed] [Google Scholar]
- 16.Razzaque MS, Lanske B. The emerging role of the fibroblast growth factor-23–klotho axis in renal regulation of phosphate homeostasis. J Endocrinol. 2007;194:1–10. doi: 10.1677/JOE-07-0095. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanske B, Razzaque MS. Mineral metabolism and aging: the fibroblast growth factor 23 enigma. Curr Opin Nephrol Hypertens. 2007:16, 311–318. doi: 10.1097/MNH.0b013e3281c55eca. [CrossRef][Medline] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetz R, Beenken A, Ibrahimi OA, et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [Abstract/Free Full Text] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


