Abstract
The molecular interaction of fibroblast growth factor 23 (FGF23) and klotho is essential for physiologic regulation of phosphate balance. In the absence of klotho, the FGF23 protein cannot exert its physiologic functions, as demonstrated by in vivo mouse genetic studies. Bioactive FGF23 protein loses its phosphate lowering effects in genetically modified mice with no klotho activity. The FGF23–klotho system not only affects phosphate homeostasis but can also influence parathyroid hormone (PTH) and vitamin D activities. Dysregulation of the FGF23–klotho system is noted in a number of human acquired and genetic diseases, including chronic kidney disease. Vitamin D is a strong inducer of both FGF23 and klotho expression, while FGF23 can suppress the renal expression of 1α(OH)ase to reduce 1,25(OH)2D activity. An understanding of the complex interactions of phosphate, vitamin D and PTH with the FGF23–klotho system has paved the way to explore the therapeutic benefits of modulating the FGF23–klotho system in diseases associated with abnormal mineral ion balance. The patent (WO2009095372) under discussion proposes using fusion polypeptides to manipulate the FGF23–klotho system.
1. FGF23
FGF23 was initially identified as a mutated gene causing renal phosphate wasting in patients with autosomal-dominant hypophosphatemic rickets, as well as a causative factor in tumor-induced osteomalasia [1–3]. Fibroblast growth factor 23 (FGF23) is a ~30 kDa protein that can be proteolytically processed into smaller N-terminal (~ 18 kDa) and C-terminal (~ 12 kDa) fragments. Recent studies have found that the C-terminal tail of FGF23 can act as an endogenous inhibitor of full length FGF23 [4]. FGF23-mediated renal phosphate wasting can be alleviated by the C-terminal tail of FGF23 [4]. FGF23 can also influence systemic vitamin D activity by suppressing the renal expression of 1α(OH)ase [5]. FGF23-overproducing mice have shown an increased renal excretion of phosphate, leading to hypophosphatemia. In accord with the experimental studies, mutations of the PHEX (a phosphate-regulating gene that is homologous to the endopeptidases of the X-chromosome) gene in patients with X-linked hypophosphatemia (XLH) lead to increased production of bioactive FGF23, causing excessive urinary phosphate loss and the development of rickets [6]. In contrast to XLH patients, reduced activity of FGF23 in familial tumoral calcinosis patients due to mutations in the human FGF23 gene usually develops hyperphosphatemia and ectopic calcification [7]. As mentioned, in patients with chronic kidney disease (CKD), FGF23–klotho-mediated systemic phosphate metabolism is severely impaired. Patients with CKD have increased serum levels of FGF23 and reduced renal expression of klotho [8,9]. The exact reasons for high serum levels of FGF23 in patients with CKD are not yet clear, but it appears likely that decreased renal clearance of FGF23 and compensatory response to the hyperphosphatemia may contribute to such imbalance. Furthermore, FGF23 resistance in patients with CKD may be due to reduced renal expression of FGF receptors and klotho.
2. Klotho
Klotho is a type 1 membrane protein that is mainly expressed in the distal convoluted tubules of the kidney, the parathyroid gland and the choroid plexus in the brain. Klotho can be detected in the serum as a ‘secreted form’ when the short transmembrane domain is removed. A disintegrin and metalloproteinases (ADAM)-10 and ADAM-17 can cleave klotho from the plasma membrane to induce klotho shedding [10]. The physical, morphological, biochemical and molecular changes in klotho knockout mice are identical to Fgf23 knockout mice [11]. Both klotho and Fgf23 knockout mice have shown increased renal expression of sodium-phosphate (Na-Pi)-2a and Na-Pi-2c co-transporter proteins with concomitant hyperphosphatemia [12]. Additionally, both klotho and Fgf23 knockout mice have increased expression of 1α(OH)ase in the kidney, with elevated serum levels of 1,25(OH)2D [12]. The generation of klotho-1α(OH)ase or Fgf23-1α(OH)ase double knockout mice also showed comparable phenotypes [13–15]. Finally, the overall phenotypes of klotho or Fgf23 single knockout mice are similar to those of Fgf23–klotho double knockout mice [12]. The indistinguishable phenotypes of these two mutant mice led to the identification of klotho as an essential component in FGF23 signaling pathways, as well as the fact that a limited number of molecules form the biological network to coordinately regulate mineral ion balance (Figure 1) [16,17].
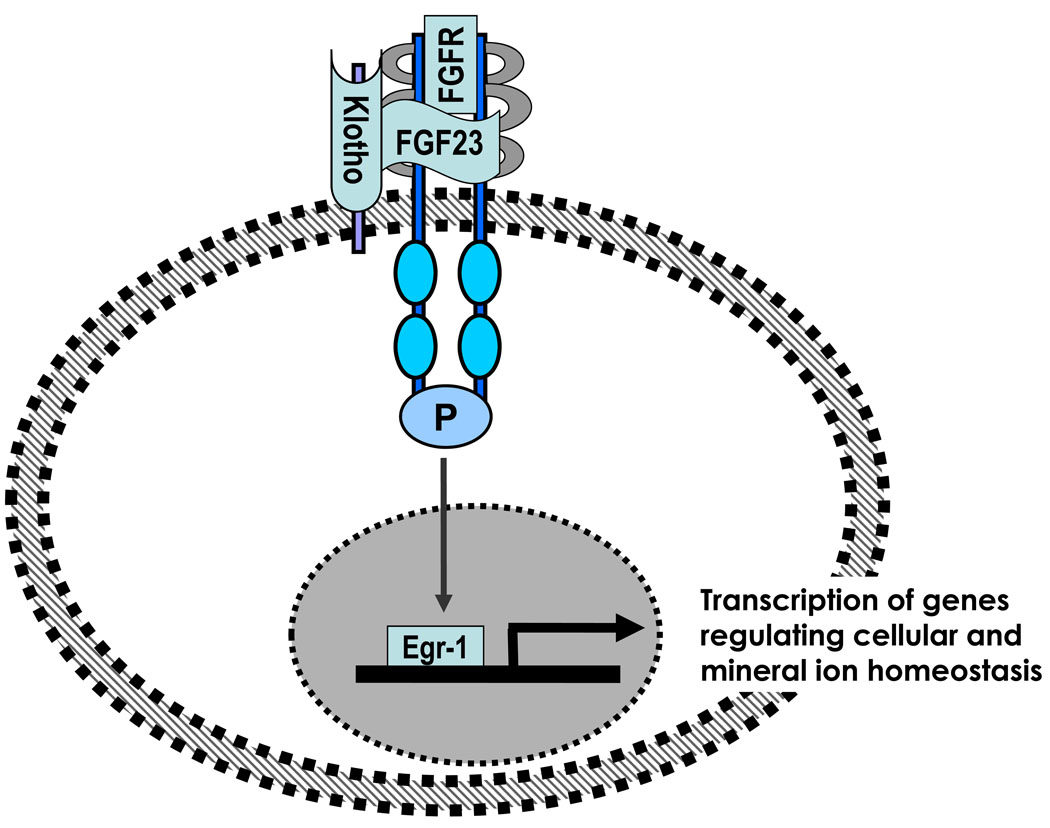
Figure 1.
Simplified schematic outline showing how FGF23, klotho and the receptor form a complex to generate downstream signaling events to induce the transcription of genes regulating mineral ion metabolism.
3. FGF23 signaling
FGF23 can bind to multiple FGF receptors, including FGFR1c, FGFR3c and FGFR4 [18–20]. Subsequent studies, however, have claimed that FGFR1 is the main receptor through which FGF23 exerts its effects in vivo [21,22]. In vitro studies have shown that FGF23 has a much higher affinity towards FGF receptors in the presence of klotho. The binding of the FGF23–klotho–FGF receptor complex can generate a downstream signaling network to induce the transcriptional activation of relevant genes [5,19,20,23,24]. It is important to note that FGF receptors are tyrosine kinase receptors. This signal transduction is usually conducted through autophosphorylation of FGF receptors, phosphorylation of FGF receptor substrate 2 and extracellular signal-regulated protein kinase 1/2 (ERK1/2), which can eventually activate early growth response-1. It is worth mentioning that the role of circulating klotho in FGF23-mediated regulation of phosphate metabolism is not yet clearly defined, and further studies are needed to examine whether circulating klotho can facilitate FGF receptor activation by FGF23, in vivo.
The phosphate lowering effects of FGF23 are partly mediated through the reduced activity of NaPi-2a in the proximal tubular epithelial cells. The essential in vivo role of klotho in the FGF23-mediated regulation of phosphate homeostasis has been shown by mouse genetic studies [12,25]. For instance, bioactive FGF23 injection into either wild-type or Fgf23 knockout mice resulted in a significant reduction of serum phosphate levels [12], whereas the injection of bioactive FGF23 protein into either klotho single knockout mice or Fgf23–klotho double knockout mice failed to reduce serum phosphate levels [12], implying that in the absence of klotho, FGF23 is unable to control phosphate homeostasis [12,25]. Similarly, phex-mutated mice have an increased serum accumulation of FGF23, causing excessive urinary phosphate loss and the development of severe hypophosphatemia [25]. Genetic inactivation of klotho in phex-mutated mice resulted in hyperphosphatemia in phex/klotho double mutant mice, even though the serum levels of FGF23 were high in double mutant mice [25], again suggesting the in vivo importance of klotho in FGF23-mediated phosphate metabolism [17,26] and implying that klotho may be a potential therapeutic target to manipulate FGF23-associated hypophosphatemic diseases [17,27]. Importantly, manipulation of the FGF23–klotho axis can also affect vitamin D homeostasis. The patent under review describes methods of generation of klotho–FGF23 fusion polypeptides and their utility for therapeutic use.
4. Patent WO2009095372
The patent describes the generation of fusion polypeptides containing at least one extracellular subdomain of the klotho protein that is connected to the N terminus of FGF by a polypeptide linker. The fusion polypeptides are claimed to bind with FGF receptors to influence downstream signaling events. Multiple batches of fusion polypeptides were generated using α- and β-klotho. Different variants of FGF23 were connected to α-klotho, while FGF19 and FGF21 were connected to β-klotho. Mostly, amino-acid linkers or chemical linkers were used to connect klotho and FGF. The biological activity of the α-klotho fusion polypeptide was evaluated based on its ability to bind with FGF23 receptors and subsequently induce Egr-1 expression. Treating myoblasts (C2C12 cells) with klotho–FGF23 fusion polypeptides has been shown to activate signaling molecules, including the phosphorylation of p70S6K and ERK, and to increase the diameter of myotubes. It is, however, not clear how the bioactivity of klotho–FGF19 or the 21 fusion polypeptides were determined.
The inventors of the patent provide a long list of human disorders that they believe may benefit from pharmaceutical compositions of their fusion polypeptides without providing enough scientific rationale. For instance, klotho–FGF23 fusion polypeptides are suggested to be of pharmacological importance to treat age-related conditions that can induce sarcopenia, skin atrophy, muscle wasting, brain atrophy, atherosclerosis, arteriosclerosis, pulmonary emphysema, osteoporosis, osteoarthritis, immunologic incompetence, hypertension, dementia, Huntington's disease, Alzheimer's disease, cataracts, age-related macular degeneration, prostate cancer, stroke, memory loss, wrinkles, impaired kidney function and age-related hearing loss. Similarly, the patent proposes the possible utility of the fusion polypeptides in preventing hyperphosphatemia, calcinosis and CKD. The patent also identifies metabolic and malignant diseases, including diabetes and breast cancer, where the generated fusion polypeptides may be of pharmacological use without providing convincing scientific evidences for such claims.
5. Expert opinion
Patients with advanced stages of CKD have elevated serum levels of FGF23 [8]. Any toxic effects of FGF23 in CKD patients with non-klotho expressing tissues would suggest a klotho-independent process, and the existence of such a process remains to be demonstrated [27]. Importantly, the klotho gene is mainly detected in the kidney, parathyroid gland and choroid plexus, and restricted expression of klotho provides tissue-specificity to the action of FGF23. Moreover, the expression of klotho is reduced in patients with CKD [9]. Therefore, the rationale of designing a therapeutic strategy to reduce serum FGF23 levels in CKD patients, at this stage, is not scientifically justified. It is, however, worth mentioning that elevated serum FGF23 levels in patients with CKD are full-length and bioactive proteins [8,28]. Recent identification of C-terminal FGF23 as an endogenous inhibitor of bioactive full-length FGF23 provides a realistic approach to neutralize the effects of FGF23 using small molecule peptides [4]. It will be interesting to know whether the generated fusion polypeptides of the discussed patent can exert similar neutralizing effects that manipulate FGF23 bioactivity. Parathyroid gland is one of the target organs for FGF23, but in patients with CKD, FGF23 appears to develop resistance [29], and the potential effects of generated fusion polypeptides on parathyroid function will be an interesting area to explore.
Another potential use of the generated fusion polypeptides in this patent is in the treatment of ageing, based on the observation that genetic inactivation of klotho function can induce accelerated ageing phenotypes in mice [11]. Subsequent studies, however, have convincingly shown that premature aging-like phenotypes in klotho ablated mice are caused by ‘phosphate toxicity’, due to the inability of FGF23 to lower serum phosphate levels in the absence of klotho activity. More importantly, when serum phosphate levels are lowered in klotho knockout mice, most of the premature ageing-like phenotypes are reversed, which include, but are not limited to, prevention of the occurrence of atherosclerosis, vascular calcifications, amelioration of soft tissue atrophy, reduction of emphysema and regaining fertility. The resultant effect in these cases is extended survival of klotho knockout mice with lower serum phosphate levels [14,25,30]. It will be interesting to know how the generated klotho–FGF fusion polypeptides of the patent under review can be used to manipulate the ageing process when klotho is not primarily driving it [31,32]. Similarly, the investigators consider use of the generated fusion polypeptides in malignant diseases worthy of patent protection; however, the data provided in the patent and other existing information do not sufficiently justify such a use.
One of the important areas where the patent may have a helpful contribution is the availability of klotho–FGF23 fusion polypeptides to the scientific community. Commercial accessibility of such fusion polypeptides may provide investigators with tools to study the biological activity of the koltho–FGF23 axis in more depth and detail. The current limited accessibility to bioactive proteins has significantly impaired research activity. Whether such studies will eventually lead to the discovery of novel therapeutic options to minimize the damage caused by abnormal mineral ion metabolism is a clinically important question that should be addressed. Similar applicability also exists for FGF19 and 21 fusion polypeptides, particularly in the study of metabolic diseases.
Finally, fine tuning of the effects of clinical therapy to decrease drug toxicity, suppress disease pathology and reduce disease burden is desirable, and whether manipulation of the FGF–klotho axis can help in achieving such a desirable outcome is not yet clear, but worth exploring [16,17,33]. Nevertheless, it remains to be demonstrated whether artificial manipulation of the FGF–klotho axis has a negative impact on normal cellular homeostasis and/or systemic organ function.
Supplementary Material
Acknowledgements
The author wishes to thank Drs. Teruyo Nakatani and Mutsuko Ohnishi of Harvard School of Dental Medicine, Boston for performing mouse genetics studies which are partly supported by a grant (R01-DK077276) from NIDDK and institutional supports from the Harvard School of Dental Medicine in Boston, USA.
Footnotes
Declaration of interest
The author states no conflict of interest and has received no payment in preparation of this manuscript.
Bibliography
- 1.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 2.ADHR_Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. The ADHR Consortium. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 3.Shimada T, Mizutani S, Muto T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetz R, Nakada Y, Hu MC. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci USA. 2010;107:407–412. doi: 10.1073/pnas.0902006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medici D, Razzaque MS, Deluca S. FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. J Cell Biol. 2008;182:459–465. doi: 10.1083/jcb.200803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson KB, Zahradnik R, Larsson T. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 7.Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 8.Fliser D, Kollerits B, Neyer U. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 9.Koh N, Fujimori T, Nishiguchi S. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 10.Chen CD, Podvin S, Gillespie E. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuro-o M, Matsumura Y, Aizawa H. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 12.Nakatani T, Bara S, Ohnishi M. In vivo genetic evidence of klotho-dependent functions of FGF23 in regulation of systemic phosphate homeostasis. Faseb J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razzaque MS, Sitara D, Taguchi T. Premature ageing-like phenotype in fibroblast growth factor 23 null mice is a vitamin-D mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razzaque MS. The FGF23 Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razzaque MS. FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? Am J Physiol Renal Physiol. 2009;296:F470–F476. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Kurosu H, Ogawa Y, Miyoshi M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urakawa I, Yamazaki Y, Shimada T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 21.Gattineni J, Bates C, Twombley K. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.90742.2008. doi: 10.1152/ajprenal.90742.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Vierthaler L, Tang W. FGFR3 and FGFR4 do not mediate renal effects of FGF23. J Am Soc Nephrol. 2008;19:2342–2350. doi: 10.1681/ASN.2007121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goetz R, Beenken A, Ibrahimi OA. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- 25.Nakatani T, Ohnishi M, Razzaque MS. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. Faseb J. 2009;23:3702–3711. doi: 10.1096/fj.08-123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razzaque MS, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol. 2007;194:1–10. doi: 10.1677/JOE-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razzaque MS. Does FGF23 toxicity influence the outcome of chronic kidney disease? Nephrol Dial Transplant. 2009;24:4–7. doi: 10.1093/ndt/gfn620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada T, Urakawa I, Isakova T. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2009-1603. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lafage-Proust MH. Does the downregulation of the FGF23 signaling pathway in hyperplastic parathyroid glands contribute to refractory secondary hyperparathyroidism in CKD patients? Kidney Int. 2010;77:390–392. doi: 10.1038/ki.2009.512. [DOI] [PubMed] [Google Scholar]
- 30.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. In vivo genetic evidence for suppressing vascular and soft tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin-D levels. Circ Cardiovasc Genet. 2009;2:583–590. doi: 10.1161/CIRCGENETICS.108.847814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanske B, Razzaque MS. Premature aging in klotho mutant mice: cause or consequence? Ageing Res Rev. 2007;6:73–79. doi: 10.1016/j.arr.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanske B, Razzaque MS. Mineral metabolism and aging: the fibroblast growth factor 23 enigma. Curr Opin Nephrol Hypertens. 2007;16:311–318. doi: 10.1097/MNH.0b013e3281c55eca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Razzaque MS. Can fibroblast growth factor 23 fine-tune therapies for diseases of abnormal mineral ion metabolism? Nat Clin Pract Endocrinol Metab. 2007;3:788–789. doi: 10.1038/ncpendmet0667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.