Abstract
Introduction
Airway secretions possess intrinsic antimicrobial properties that contribute to the innate host defense of the respiratory tract. These microbicidal capabilities have largely been attributed to the presence of antibacterial polypeptides. However, recent investigation has demonstrated that host-derived lipids including cholesteryl esters also exhibit antimicrobial properties. The purpose of this study was to determine whether sinus secretions contain such antimicrobial lipids and to compare the lipid composition in patients with and without chronic rhinosinusitis (CRS).
Methods
Maxillary sinus fluid was obtained via antral lavage from subjects with (7) and without (9) a history of CRS. Following specimen collection, total lipid was extracted according to Bligh & Dyer and lipid profiles were obtained by reverse phase HPLC on an amide-embedded C18 column. In addition, the neutrophil specific antimicrobial peptides HNP1-3 were quantified by immunoblotting.
Results
Lipids were identified in the maxillary sinus secretions of patients with and without CRS including cholesteryl esters. However, levels of lipid composition differed between the two groups with CRS patients exhibiting greater amounts of all classes of lipids; reaching over 10-fold higher concentration when compared to nonCRS patients. This increase was independent of HNP1-3 content.
Conclusions
Sinus secretions of patients with CRS appear to demonstrate elevated levels of antimicrobial lipids compared to controls independent from neutrophil influx. This upregulation suggests that host-derived lipids act as mediators of mucosal immunity in CRS. Further study is necessary to determine if such antimicrobial lipids function alone or synergistically with antibacterial peptides in conferring such inherent microbicidal properties.
INTRODUCTION
The innate immune system has been increasingly found to play a role in the pathogenesis of airway inflammatory disease including asthma and chronic rhinosinusitis (CRS). This inherent host response provides initial protection of mucosal surfaces against infection; utilizing multiple effector molecules to achieve early recognition of microbial pathogens, preliminary bactericidal activity, and activation of the adaptive immune response.1–4 Toll-like receptors, surfactant proteins, and antimicrobial peptides represent significant mediators of this intrinsic defense mechanism, with the latter believed to be predominantly responsible for the innate microbicidal properties of airway surface fluid (ASF) including nasal and bronchoalveolar secretions. 5–10 The cathelicidin LL-37 and defensins comprise the majority of these antimicrobial polypeptides and have been found to exhibit broad spectrum antibacterial activity. 1–2,10
Cathelicidins are prepropeptides that are cleaved to produce a distinct C-terminal peptide. 11 In humans only one cathelicidin has been described, LL-37, which possesses not only antimicrobial capabilities but also immunomodulatory activities. 11 Human defensins are small cationic peptides of 29–40 AA containing 6 cysteine residues linked by 3 disulfide bonds. 1,2 The defensins have been subdivided into 2 categories according to their distinguishing molecular structure characteristics: the alpha and beta defensins. 1,2 Human neutrophil peptides 1–4 are alpha defensins located in neutrophils and are associated with inflammation. 1,2 While HNP4 is present only in minute amounts, HNP1-3 are abundantly expressed. 1,2 HNP1-3 differ only by a single amino acid and polyclonal antibodies of HNP 1- 3 are cross reactive. 1,2 Beta defensins are predominant on mucosal epithelial surfaces i.e. skin, lungs, gastrointestinal tract. 1,2 The presence of antibacterial peptides in ASF and their contributions to intrinsic mucosal immunity of the respiratory tract have been well established. 1,2
However, recent studies indicate that host-derived lipids, particularly cholesteryl esters, also possess antimicrobial properties and may constitute a novel defense component of the innate immune system. 13,14 Both cholesteryl linoleate and arachidonate demonstrate microbicidal effects against various microbes in vitro, including Pseudomonas aeruginosa. 14 In addition, antibacterial lipids have been known to participate in the inherent host defense of the skin and play a pivotal role in the protection of neonates from infection thru their presence in breast milk and the vernix caseosa (the newborn coating). 15–23 In the upper airway, nasal secretions have been reported to contain all major classes of lipids as well as lipoproteins. 14 Selective removal of lipids from nasal fluid has been described to cause diminished antibacterial activity. Conversely, their reintroduction into lipid depleted nasal fluid partially restores its bactericidal properties. 14
Although a lipid mediated component of the innate immune system is still an emerging concept, such findings suggest that antimicrobial lipids may contribute to the instrinsic host defense of the upper airway and potentially the paranasal sinuses. The purpose of this study was to determine if sinus secretions contain such antimicrobial lipids, and if the lipid composition differed in patients with and without CRS. In addition, HNP1-3 levels of CRS and nonCRS patients were analyzed to compare defensin and antimicrobial lipid response to infection and to ascertain whether any antimicrobial lipid production was indigenous to the epithelia or secondary to neutrophil delivery during inflammation.
MATERIALS AND METHODS
Specimen collection
Maxillary sinus fluid was obtained from 9 subjects without a history of sinus disease via antral lavage through the canine fossa under institutional board review approval. All patients denied having any previous history of sinus infection and showed no radiographic evidence of sinus pathology either on CT scan or MRI. Similar specimens were also procured from 7 subjects who fulfilled the diagnostic criteria for CRS as defined by the 2007 American Academy of Otolaryngology-Head and Neck Surgery multispecialty panel clinical practice guidelines. (Rosenfeld et al) Nasal endoscopy revealed either mucopurulent discharge and/or edema of the middle meatal mucosa in these patients. CT demonstrated either partial or complete opacification of the maxillary sinus from which the specimen was obtained. All the CRS patients were treated with at least a 3 week course of antibiotics in conjunction with saline rinses and steroid therapy and were considered for FESSbecause they continued to have persistent symptoms along with abnormal nasal endoscopic and CT findings. None of the 7 CRS patients had asthma, nasal polyposis, or undergone previous sinus surgery. No perioperative antibiotics were administered. Intraoperatively, the maxillary sinuses from which the specimens were acquired demonstrated mucopurulent secretions with edema of the mucosa lining. Cultures obtained from the maxillary sinuses tested were positive for Staphylococcus aureus (4), Pseudomonas aeruginosa (2), and Klebsiella pneumoniae (1), respectively. Pathology reports from the maxillary sinus tissue resected all showed evidence of chronic inflammation. Antral lavage was performed immediately prior to functional endoscopic sinus surgery. Following specimen collection samples were centrifuged to eliminate cellular material and the resulting supernatants were stored at −70°C. Among both groups some sinus washes appeared to be tinted with blood.
Lipid Standards
Lipid standards were purchased from Sigma-Aldrich. These were dissolved in dicholormethane in glass vials, overlaid with nitrogen gas, and stored at −20°C. Free fatty acids used were as follows: myristic acid (C14:0, defining the number of C atoms to the number of double bonds), palmitic acid (C16:0), heptadecanoic acid (C17:0, plant fatty acid absent in humans and used as internal standard), stearic acid (C18:0), and docosahexaenoic acid (C22:6). The glycerolipid used was tripalmitic acid. Sterols used include cholesterol, and the cholesteryl esters were cholesteryl palmitate, cholesteryl stearate, cholesteryl linoleate, and cholesteryl arachidonate.
Sinus fluid processing
Sinus fluid was processed similarly to the method used by Do et al 2008. 14 Briefly, sinus fluid was heat inactivated for 10 minutes at 60°C and then sonicated 3 times for 10 seconds using a Fisher Dismembrator (Fisher Scientific International, Hampton, NH) at power setting 2. The samples were then centrifuged for 3 minutes at 50 × g (15°C), and the supernatants were aspirated and further centrifuged for 30 minutes at 16,100 × g (4°C). The resulting supernatants were stored under N2 gas at −20°C until further use for lipid extraction, total protein, and HNP1-3 quantification.
Lipid Extraction
Lipid extractions were based on the Bligh & Dyer method. 24 Briefly, 1.25 ml of methanol and 0.625 ml of chloroform were added to 0.5 ml of sample, flushed with N2 gas, and vigorously vortexed for 1 min. Then, 0.625 ml of chloroform were added, the sample flushed with N2 gas, and vortexed for 1 min. The procedure was repeated with 0.625 ml of dH2O. Samples were then incubated on a rotary shaker for 20 min at power setting 6 (Lab-Line Maxi Rotator, Barnstead International, Dubuque, Iowa) and centrifuged (652 × g, 15°C, 15 min). The lower phase containing lipids was collected and a volume of chloroform equal to the volume of lower phase was added to the upper (aqueous) phase to repeat the lipid extraction. The chloroform layers from both steps were combined and the organic solvent was removed under a gentle stream of N2 gas at 37°C. To control for lipid extraction efficiency, heptadecanoic acid was added (15 μg) to all samples prior to lipid extraction. For pooled samples, 0.5 ml volumes were extracted, while for individual samples typically 150 μl were extracted.
Reverse-phase HPLC
Separation and quantification of lipids was performed with a low-pressure quarternary gradient system (Summit HPLC System, Dionex Corporation, Sunyvale, CA with Dionex PCS1Chromeleon software) on a reverse phase column [Dionex Polar Advantage 2, particle size 3 μm, 150 mm length × 2.1 mm ID, pre-equilibrated in acetonitrile (solvent A), reagent grade alcohol (solvent B), water (solvent C), 68.6/16.4/15; column temperature 25°C] with evaporative light scattering detection (ELSD 800, Alltech Associates, Inc., Deerfield, IL, operated at 45°C, 1.9 bar N2). The gradient was as follows: from 0 to 5 minutes: water: 15% to 0%, alcohol: 16.4% to 19.4%, acetonitrile: 68.6% to 80.6%; from 5 to 6.1 minutes: alcohol: 19.4% to 50%; ACN: 80.6% to 50%; from 6.2 to 33.1 minutes: alcohol: 50%, ACN: 50%. Lipid extracts were resuspended in 10 μl dichloromethane and the entire aliquot was injected and eluted (0.307 ml/min). Response curves were established for the sterols and cholesteryl linoleate. All solvents used were HPLC grade.
Total protein quantification
NanoDrop ND-1000 (NanoDrop Products, Wilmington, DE) was used to quantify the total proteins according to the manufacturer’s instructions. The sample volume measured was 2 μl. Individual samples were measured twice and averages were calculated. PBS was used as blank. From the NanoDrop software, the A280 application was used to determine the total protein concentration.
HNP1-3 quantification
To quantify HNP1-3 in individual samples, 10 μl each were subjected to AU-PAGE followed by Western Immunoblotting with polyclonal rabbit anti HNP1-3 (1:400; kindly provided by Tomas Ganz, UCLA), alkaline phosphatase-conjugated goat-anti rabbit polyclonal antibodies (PIERCE Biotechnology, Inc., Rockfort, IL, 1:2000) and a BCIP/NBT colorimetric detection system (Research Products International Corp., Mount Prospect, IL) as described previously.25 Developed blots were scanned and sample HNP1-3 concentrations were calculated based on a standard curve derived from purified HNP-2 peptide (kindly provided from Tomas Ganz, UCLA) using the Versadoc imaging system and QuantityOne software (BioRad, Hercules, CA). Samples which did not reveal any bands for HNP1-3 were subjected to the more sensitive dot blot to confirm absence of HNP1-3 following a protocol described in Shen et al., 2005, but using the polyclonal rabbit anti-HNP1-3, standard HNP-2 peptide and imaging analysis as described above. 26
Data analysis
Data analysis was conducted with SPSS version 16.0. The data values for most of the variables assessed spanned a range of several orders of magnitude (for example nonpolar lipids ranged from a minimum of 135 mV×min in one patient to maximum of 159359 mV×min in another). Therefore, to control for heteroskedasticity among treatment groups, a variance stabilizing logarithmic transformation, log10(1+X), was used. Dependent variables were analyzed using a two-factor analysis of variance with disease (nonCRS vs. CRS) and sample appearance (clear vs. tinted) as the main factors. Graphs were prepared with SigmaPlot version 9.0.
RESULTS
Nonpolar lipids (NPL), cholesteryl esters, are elevated in sinus secretions of CRS patients
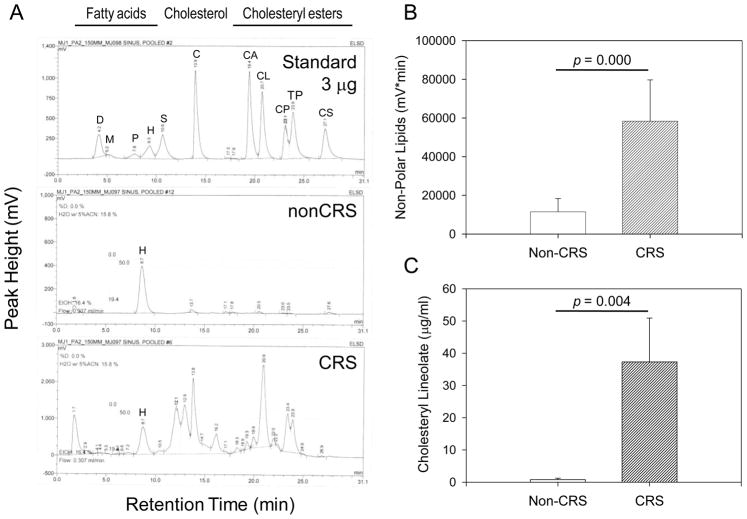
Maxillary sinus secretions acquired from nonCRS and CRS patients were subjected to lipid analysis using HPLC as described above. Corresponding representative chromatograms are shown in Figure 1A. While nonCRS samples contained minimal amounts of all classes of lipids in both individual and pooled specimens; a marked elevation of NPL was evident, particularly with respect to cholesteryl esters, in maxillary sinus secretions from CRS patients. Among the cholesteryl esters, the most prominent peak was demonstrated for cholesteryl linoleate. Comparison of total NPL (Figure 1B) and cholesteryl linoleate levels (Figure 1C) in nonCRS and CRS specimens revealed at least 10 –fold and 5-fold increases in the CRS samples, respectively (p = 0.000 for NPL and 0.004 for cholesteryl linoleate in univariate ANOVA). Cholesteryl ester levels reached over 30 μg/ml concentrations in CRS patients. This finding suggests that antimicrobial lipid production is inducible in response to infection.
Figure 1.
Nonpolar Lipids including cholesteryl esters are increased in chronic rhinosinusitis. (A): Representative HPLC chromatograms from standard lipids (Standard, 3 μg each) and lipid extracts prepared from 0.5 ml pooled sinus samples obtained from patients with (CRS) and without (nonCRS) chronic rhinosinusitis respectively. D: docosahexaenoic acid, M: myristic acid, P: palmitic acid, H: heptadecanoic acid (internal standard), S: stearic acid; C: cholesterol; CA: cholesteryl arachidonate, CL: cholesteryl linoleate, CP: cholesteryl palmitate, TP: tri-palmitin, CS: cholesteryl stearate. (B): Quantification of nonpolar lipids consisting of cholesterol, cholesteryl esters and triglycerides. Shown are the means + SEM of the area under the curves of the HPLC chromatograms expressed as mV × min for nonCRS and CRS specimens. (C): Cholesteryl linoleate concentrations shown in (B) were calculated according to the equation derived from standard curves. Shown are means + SEM, n =5 for nonCRS and n = 7 for CRS. Statistical significance was calculated by univariate ANOVA.
Upregulation of antimicrobial lipids is independent of HNP amplification
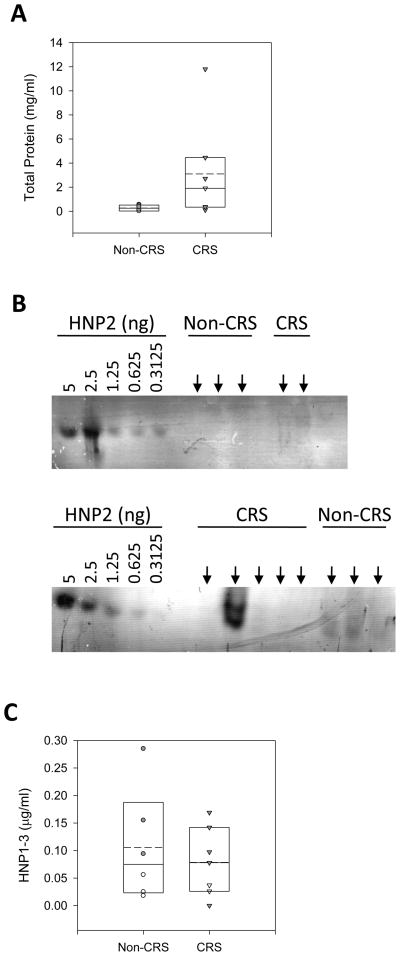
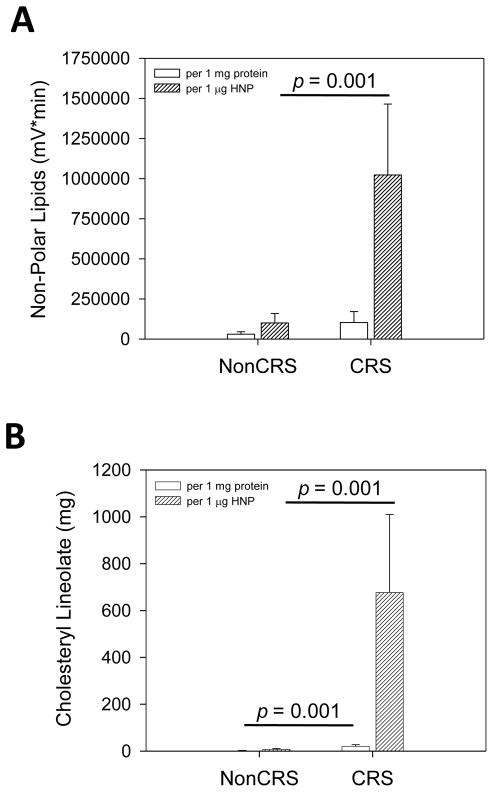
A hallmark of inflammation is neutrophil influx accompanied by elevated human neutrophil peptide levels. To further corroborate that the observed increased lipid levels in CRS patients are a product of mucosal epithelial cells and not simply a by-product of inflammation contributed by neutrophils or blood contamination acquired during sample aspiration, we quantified HNP1-3 of sinus secretions from nonCRS and CRS patients. In addition, we determined total protein concentrations to address possible variations in sample density. Although the mean total protein (Figure 2A) and HNP1-3 (Figure 2B, representative Western immunoblot, and Figure 2C) content were not statistically significantly elevated in CRS samples when compared to nonCRS specimens, antimicrobial lipids were found to be significantly higher in CRS samples (Figure 3) when adjusted to both total protein and HNP concentrations. The nonpolar lipids showed a statistically significant increase in CRS patients when compared to nonCRS patients following adjustment to HNP1-3 content (p = 0.001 in univariate ANOVA). Both nonpolar lipids and cholesteryl linoleate were also significantly increased in CRS samples when adjusted to HNP1-3 (p = 0.001 for both in univariate ANOVA). This finding suggests that antibiotic lipid synthesis is independent from HNP upregulation and potentially indigenous to the epithelia rather than exclusively delivered by neutrophil infiltration during the inflammatory process.
Figure 2.
Quantification of total protein and HNP1-3 in sinus samples. (A): Total protein concentrations from nonCRS and CRS patients are depicted. Shown are individual measurements and a summary box plot. For the box plot the boundary of the box closest to and farthest from zero indicates the 25th and 95th percentile, respectively; the straight line and the dashed line within the box indicate the median and the mean, respectively. The error bar above the box indicates the 90th percentile. Whereby sinus washes tinted with blood are indicated by closed symbols. (B) Representative Western immunoblots for HNP1-3 quanitfication. Purified HNP2 standard peptide and 10 μl of each sinus sample (arrows) were subjected to AU-PAGE followed by Western immunoblotting probing for HNP1-3 with a polyclonal antibody. top: washes that appeared clear; bottom: washes that appeared tinted with blood. (C) Quantification of HNP1-3 was accomplished with VersaDoc imaging and QuantityOne software. Shown are individual HNP1-3 concentrations and a summary box plot (see panel A). For nonCRS, n = 6, and for CRS, n = 7. Sinus washes tinted with blood are indicated by closed symbols.
Figure 3.
Nonpolar lipids including cholesteryl esters are increased in sinus samples from CRS patients independent from total protein and HNP1-3 levels. Nonpolar lipid contents expressed as mV×min (A) and cholesteryl linoleate concentrations (B) in lipid extracts from individual nonCRS and CRS samples were calculated per 1 mg total protein (n = 5 for nonCRS and n = 7 for CRS samples) and 1 μg HNP1-3 (n = 5 for nonCRS and n = 6 for CRS samples). Means + S.E.M. are shown. Statistical significance was calculated with univariate ANOVA.
DISCUSSION
The innate immune system plays a pivotal role in the initial defense of mucosal surfaces of the respiratory tract. Toll-like receptors (TLR), surfactant proteins, and antimicrobial peptides represent distinct, integral components of the intrinsic host response and their respective contributions have been well delineated. 1,2,6,7,9,10 However, recent studies have shown that host-derived lipids may also contribute to the nascent protection of mucosal surfaces against infection.
In the skin, antimicrobial lipids have already been documented to assist in combating microbial infiltration. Free fatty acids in the stratum corneum possess antimicrobial activity against S. aureus, S. pyogenes, and S. epidermidis. 15 Mutations in the enzyme responsible for the synthesis of oleic and palmitoleic acids have resulted in failure to clear gram positive bacterial skin infections in murine models. 27 Antimicrobial lipids also serve an integral role in the protection of neonates against infection. 17–23 Lipids present in breast milk (sphingolipids, triacylglycerides) are hydrolyzed by lipases in the gastrointestinal tract into breakdown products (medium chain saturated, long chain unsaturated fatty acids, monoglycerides) which exhibit innate antibacterial activity against E. coli, C. jejuni, L. monocytogenes, and S. enteritidis that are believed to help reduce the incidence of gastrointestinal infection in infants. 17–23,28 In addition, free fatty acids (palmitic and linolenic) in the vernix caseosa (the newborn coating) have been found to exert antimicrobial activity against B. megaterium which is synergistic with the antibacterial actions of the cathelicidin LL-37. 16
Antimicrobial lipids may also play a role in the intrinsic host defense of the airway. Both bronchoalveolar lavage fluid (BALF) and nasal fluid contain all major classes of lipids, with cholesterol and cholesteryl esters being the most predominant nonpolar lipids. 14 In addition, cholesteryl linoleate (CL) and cholesteryl arachidonate (CA) have been described to exert dose dependent bactericidal and bacteriostatic activity respectively against P. aeruginosa at concentrations similar to that seen physiologically in nasal secretions. 14 Removal of such nonpolar antimicrobial lipids from nasal fluid (while taking care to still maintain the same antimicrobial peptide content) diminished its bactericidal activity against P. aeruginosa; while reintroduction of lipid extracts into lipid depleted nasal secretions resulted in partial restoration of its microbicidal properties. 14
No previous studies in the literature to the best of our knowledge have evaluated sinus secretions for the presence of antimicrobial lipids. We hypothesized that antibacterial lipids, due to their potential role as mediators of innate immunity, would be upregulated in sinus secretions in response to infection. HPLC analysis of maxillary sinus fluid in both CRS and nonCRS patients revealed a lipid profile that was comparable to nasal secretions. All major lipid classes were evident, including more polar fatty acids and non-polar cholesterol and cholesteryl esters. However, the amounts of lipids varied dramatically between the CRS and nonCRS patients.
Low levels of all lipids were detected in sinus secretions of nonCRS patients when examined collectively. In contrast, when the sinus secretions of CRS patients were analyzed, marked upregulation of nonpolar lipids was evident both in individual and pooled samples. This amplification was statistically significant for cholesteryl linoleate when controlled for the total protein content. Moreover, cholesteryl linoleate reached concentrations above that needed for antibacterial activity,14 consistent with a role of cholesteryl linoleate as an effector molecule of mucosal innate defense. Fatty acids also appeared to be upregulated in CRS patients. However, since fatty acids (unlike cholesteryl esters) are also found in bacteria, it is unclear whether this increase was secondary to microbial infiltration or from the epithelia itself.
This enhanced response of antimicrobial lipids, especially cholesteryl linoleate, is similar to what has been observed in the behavior of other intrinsic defense molecules in the context of infection. Multiple mediators of innate immunity have been found to be upregulated in CRS. 7, 29–33 Toll-like receptor 2 mRNA expression, defensins, namely human defensins 1–3, HBD1-2, and the cathelicidin LL37, have been reported to be elevated in CRS patients when compared to controls. 7, 29–33 Though some studies suggest that the induction of these innate immune factors is also found when polyps are present, others have found decreased levels of these factors (Lane et al., 2006; Ramanathan M et al. 2008). However, none of our patients had polyps. In our study, we did not detect significantly higher mean levels of HNP1-3 in maxillary sinus secretions of CRS patients versus controls possibly due to blood contamination.
However, the degree of upregulation of antimicrobial lipids seen in CRS specimens was even greater than that observed for HNP1-3. When nonpolar lipid amounts for nonCRS and CRS samples were adjusted to the total protein concentration and HNP1-3 levels of each group, there was a pronounced increase in the nonpolar lipid:protein and a statistically significant increase in the cholesteryl linoleate: protein, nonpolar lipid:HNP and cholesteryl linoleate: HNP ratios in CRS patients. This finding suggests that antibiotic lipid production is (1) inducible similar to the defensin response and (2) potentially originates from the epithelia itself rather than being exclusively delivered to the site of infection by inflammatory cells i.e. neutrophils. Murine models have illustrated that synthesis of certain skin antimicrobial lipids are TLR responsive, corroborating the concept that production of antibacterial lipids in the upper airways is an inducible reaction and part of the innate immune response. 16
Abnormalities in antimicrobial lipids have been previously implicated in the development of other inflammatory diseases. Atopic dermatitis and dermal S. aureus colonization has been associated with decreased levels of sphingosine in the stratum corneum. 34 Irregularities in fatty acid metabolism has been reported in cystic fibrosis (CF) with diminished amounts of linoleic acid and docosahexaenoic acid (DA) seen in the saliva and serum. 35–37 Dietary supplementation of DA has been reported to ameliorate the clinical condition of CF patients. 38 Aberrancies in antibacterial cholesteryl esters may play a role in the pathogenesis of CRS as well. Antimicrobial lipid responses may vary in the sinus epithelia of healthy subjects versus CRS patients in response to bacterial challenge, allowing microbial colonization in the latter. Additional research is warranted to elucidate and compare cholesteryl ester production of sinus mucosa in CRS and nonCRS patients to corroborate this hypothesis.
The precise bactericidal mechanism of antimicrobial lipids is still under investigation. Membrane disruption from insertion of hydrophobic chains into the lipid bilayer similar to what is seen in defensins has been the primary hypothesis proposed. 39 Antimicrobial lipids also work in concert with antibacterial peptides to eliminate offending pathogens. DA, palmitic acid, and cholesteryl linoleate, have been described to act synergistically with lysozyme, LL-37, and HNP-2, respectively, in terms of their microbicidal effects. 14,16,39 However, it is unclear what the relative contributions of antimicrobial lipids and antibacterial peptides are to mucosal host defense. Further investigation is needed to delineate the complex interactions of antimicrobial lipids with other effectors of the innate immune response in providing comprehensive nascent protection against microbial invasion.
CONCLUSION
The concept of a host-derived lipid mediated component of innate immunity is an emerging phenomenon. Endogenous antibiotic lipids have already been determined to contribute to the intrinsic defense of the skin, neonate, and the upper and lower airways. Their dramatic upregulation in patients with CRS independent from HNP amplification suggests that antibacterial lipids, particularly cholesteryl esters, also play a role in the inherent mucosal host resistance and microbial pathogenesis of this disease. These findings support the contribution of a novel, distinct, lipid based antimicrobial effector pathway in the innate immune response to infection. However, further investigation is necessary to determine the relationship between such antimicrobial lipids and other mediators of mucosal immunity in the context of CRS.
Acknowledgments
This study was funded by NIH grants 1P20 MD001824 RIMI and R25 GM61331 MBRS-RISE.
Footnotes
Presented at the American Rhinologic Society Meeting, Rhinology World, Philadelphia, PA April 17, 2009
References
- 1.Ganz T. Defensins: Antimicrobial peptides of innate immunity. Nature. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 2.Ganz T. Antimicrobial polypeptides. J Leukoc Biol. 2004;75:34–38. doi: 10.1189/jlb.0403150. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Janeway C., Jr Innate immunity. N Eng J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R, Janeway C., Jr Innate immune recognition: Mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 5.Ooi EH, Wormald PJ, Tan LW. Innate immunity in the paranasal sinuses: A review of nasal host defenses. Am J Rhin. 2008;22:13–19. doi: 10.2500/ajr.2008.22.3127. [DOI] [PubMed] [Google Scholar]
- 6.Mccormack F. New concepts in collectin- mediated host defense at the air-liquid interface of the lung. Respirology. 2006;11:s7–s10. doi: 10.1111/j.1440-1843.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- 7.Vandermeer J, Sha Q, Kane AP, et al. Innate immunity of the sinonasal cavity: Expression of messenger RNA for complement cascade components and toll-like receptors. Arch Otolaryngol Head Neck Surg. 2004;130:1374–1380. doi: 10.1001/archotol.130.12.1374. [DOI] [PubMed] [Google Scholar]
- 8.Singh PK, Tack BF, McCray PB, et al. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 9.Cole AM, Dewan P, Ganz Innate antimicrobial activity of nasal secretions. Infect and Immun. 1999;67:3267–3275. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole AM, Liao J, Stuchlik O, et al. Cationic polypeptides are required for antibacterial activity of human airway fluid. J Immunol. 2002;169:6985–6991. doi: 10.4049/jimmunol.169.12.6985. [DOI] [PubMed] [Google Scholar]
- 11.Nijnik A, Hancock RE. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr Opin Hematol. 2009 Jan;16(1):41–7. doi: 10.1097/moh.0b013e32831ac517. Review. [DOI] [PubMed] [Google Scholar]
- 12.Herr C, Shaykhiev R, Bals R. The role of cathelicidin and defensins in pulmonary inflammatory diseases. Expert Opin Biol Ther. 2007 Sep;7(9):1449–61. doi: 10.1517/14712598.7.9.1449. Review. [DOI] [PubMed] [Google Scholar]
- 13.Thormar H, Hilmarsson H. The role of microbicidal lipids in host defense against pathogens and their potential as therapeutic agents. Chem Phys Lipids. 2007;150:1–11. doi: 10.1016/j.chemphyslip.2007.06.220. [DOI] [PubMed] [Google Scholar]
- 14.Do TQ, Moshkani S, Castillo P, et al. Lipids including cholesteryl linoleate and cholesteryl arachidonate contribute to the inherent antibacterial activity of human nasal fluid. Journal of Immunology. 2008;181:4177–4187. doi: 10.4049/jimmunol.181.6.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake DR, Brogden KA, Dawson DV, et al. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Tollin M, Bergsson G, Kai-Larsen Y, et al. Vernix caseosa as a multi-component defence system based on polypeptides, lipids, and their interactions. Cell Mol Lif Sci. 2005;62:2390–2399. doi: 10.1007/s00018-005-5260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thormar H, Isaacs CE, Brown HR, et al. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob Agents Chemother. 31:27–31. doi: 10.1128/aac.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaccs CE, Kashyap S, Heird WC, et al. Antiviral and antibacterial lipids in human milk and infant formula feeds. Archives of Disease in Childhood. 1990;65:861–864. doi: 10.1136/adc.65.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isaacs CE, Litov RE, Marie P, Thormar H. Addition of lipases to infant formulas produces antiviral and and antibacterial activity. J Nutr Biochem. 1992;3:304–8. [Google Scholar]
- 20.Isaacs CE, Litov RE, Thormar H. Antimicrobial activity of lipids added to human milk, infant formulas, and bovine mild. J Nutr Biochem. 1995;6:362–366. doi: 10.1016/0955-2863(95)80003-u. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs CE. Antimicrobial function of milk lipids. Adv Nutr Res. 2001;10:271–85. doi: 10.1007/978-1-4615-0661-4_13. [DOI] [PubMed] [Google Scholar]
- 22.Sun CQ, O’Connor CJ, Roberton Am. The antimicrobial properties of milkfat after partial hydrolysis by calf pregastric lipase. Chem Biol Interact. 2002;140:185–8. doi: 10.1016/s0009-2797(02)00016-9. [DOI] [PubMed] [Google Scholar]
- 23.Sprong RC, Hulstein MF, van der Meer R. Bactericidal activities of milk lipids. Antimicrob Agents Chemother. 2001;45:1298–1301. doi: 10.1128/AAC.45.4.1298-1301.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–918. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 25.Porter E, Yang H, Yavagal S, et al. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect Immun. 2005;73:4823–33. doi: 10.1128/IAI.73.8.4823-4833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen B, Porter EM, Reynoso E, et al. Human defensin 5 expression in intestinal metaplasia of the upper gastrointestinal tract. J Clin Pathol. 2005;58:687–694. doi: 10.1136/jcp.2004.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgel P, Crozat K, Lauth X, et al. A toll-like receptor 2- responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infection and Immunity. 2005;73:4512–4521. doi: 10.1128/IAI.73.8.4512-4521.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.German JB, Dillard CJ. Composition, structure, and absorption of milk lipids: a source of energy, fat soluble nutrients and bioactive molecules. Critical Reviews in Food Science and Nutrition. 2006;46:57–92. doi: 10.1080/10408690590957098. [DOI] [PubMed] [Google Scholar]
- 29.Psaltis AJ, Bruhn MA, Tan LW, et al. Nasal mucosa expression of lactoferrin in patients with chronic rhinosinusitis. Laryngoscope. 2007;117:2030–2035. doi: 10.1097/MLG.0b013e31812e01ab. [DOI] [PubMed] [Google Scholar]
- 30.Ooi EH, Wormald PJ, Carney AS, et al. Human cathelicidin antimicrobial peptide is upregulated in the eosinophilic mucus subgroup of chronic rhinosinusitis patients. Am J Rhinol. 2007;21:395–401. doi: 10.2500/ajr.2007.21.3048. [DOI] [PubMed] [Google Scholar]
- 31.Kim ST, Cha HE, Kim DY, et al. Antimicrobial peptide LL-37 is upregulated in chronic nasal inflammatory disease. Acta Otolaryngol. 2003;123:81–85. doi: 10.1080/0036554021000028089. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Kim JE, Lim HH, et al. Antimicrobial defensin peptides of the human nasal mucosa. Ann Otol Rhinol Laryngol. 2002;111:135–141. doi: 10.1177/000348940211100205. [DOI] [PubMed] [Google Scholar]
- 33.Carothers DG, Graham SM, Jia HP, et al. Production of beta defensin antimicrobial peptides by maxillary sinus mucosa. Am J Rhinol. 2001;15:175–179. doi: 10.2500/105065801779954238. [DOI] [PubMed] [Google Scholar]
- 34.Arikawa J, Ishibashi M, Kawashima M, et al. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by staphylococcus aureus. J Invest Dermatol. 2002;119:433–439. doi: 10.1046/j.1523-1747.2002.01846.x. [DOI] [PubMed] [Google Scholar]
- 35.Freedman SD, Blanco PG, Zaman MM, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Eng J Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 36.Strandvik B, Gronowitz E, Enlund F, et al. Essential fatty acid deficiency in relation to genotype in patients with cystic fibrosis. J Pediatr. 2001;139:650–655. doi: 10.1067/mpd.2001.118890. [DOI] [PubMed] [Google Scholar]
- 37.Keen C, Olin AC, Edentoft A, et al. Airway nitric oxide in patients with cystic fibrosis is associated with pancreatic function, pseudomonas infection, and polyunsaturated fatty acids. Chest. 2007;131:1857–1864. doi: 10.1378/chest.06-2635. [DOI] [PubMed] [Google Scholar]
- 38.Coste TC, Armand M, Lebacq P, et al. An overview of monitoring and supplementation of omega 3 fatty acids in cystic fibrosis. Clin Biochem. 2007;40:511–520. doi: 10.1016/j.clinbiochem.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Martinez JG, Waldon M, Huang Q, et al. Membrane-targeted synergistic activity of docosahexaenoic acid and lysozyme against pseudomonas aeruginosa. Biochem J. 2009;419:193–200. doi: 10.1042/BJ20081505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenfeld R, Andes D, Bhattacharyya N, et al. Clinical practice guidelines: Adult sinusitis. Otolaryngol Head Neck Surg. 2007;137 (3 suppl):S1–S31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 41.Ramanathan M, Jr, Lee WK, Dubin MG, et al. Sinonasal epithelial expression of toll-like receptor 9 is decreased in chronic rhinosinusitis with polyps. Am J Rhinol. 2007;21:110–116. doi: 10.2500/ajr.2007.21.2997. [DOI] [PubMed] [Google Scholar]
- 42.Ramnathan M, Jr, Lee WK, Spannhake EW, et al. Th2 cytokines associated with chronic rhinosinusitis with polyps down-regulate the antmicorbial immunefunction of human sinonasal epithelial cells. Am J Rhinol. 2008;22:115–121. doi: 10.2500/ajr.2008.22.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lane A, Truong-Tran QA, Schleimer RP, et al. Altered expression of genes associated with innate immunity and inflammation in recalciferant rhinosinusitis with polyps. Am J Rhinol. 2006;20:138–144. [PMC free article] [PubMed] [Google Scholar]