Abstract
Aging and Alzheimer’s disease (AD) are both characterized by memory impairments and sleep changes. We investigated the potential link between these disturbances, focusing on sleep spindles, involved in memory consolidation. Two episodic memory tasks were given to young and old healthy subjects, as well as to AD patients. Post-learning sleep was recorded. Sleep spindles were globally reduced in aging and AD. AD patients also exhibited a further decrease in fast spindles. Besides, mean intensity of fast spindles was positively correlated, in AD patients, with immediate recall performance. Our results are the first report of a specific decrease in fast spindles in AD, associated with learning abilities. They also give further hints for a functional differentiation between slow and fast spindles.
Keywords: Adult; Age Factors; Aged; Aged, 80 and over; Alzheimer Disease; physiopathology; psychology; Analysis of Variance; Cognition; physiology; Female; Humans; Learning; physiology; Male; Memory; physiology; Mental Recall; physiology; Polysomnography; methods; Sleep; physiology; Sleep Stages; physiology; Sleep, REM; physiology
Keywords: slow spindles, fast spindles, aging, cognitive abilities, stage 2 sleep
Introduction
The formation of long-term memories requires a process of consolidation, which is facilitated by sleep. In the case of hippocampus-dependent declarative memories, this process appears to benefit mainly from slow-wave sleep (SWS) [1]. Recently, the focus has also been put on stage 2 sleep and more precisely on sleep spindles. Several studies have reported an increase in spindle density after verbal learning [2,3], a link between spindle activity and overnight change in declarative memory performance [4], or more general and trait-like relationships between spindle activity and measures of learning [2] or cognitive abilities [5].
Aging and Alzheimer’s disease (AD) are characterized by episodic memory impairment and changes in global sleep architecture [6]. Although modifications in the amount of sleep stages and decreases in sleep spindles are now well documented in both cases, the potential aftermaths of such changes on memory consolidation have received little attention. Mazzoni et al. [7] investigated the importance of sleep structure in aged adults and found the duration of sleep cycles, but not the amount of NREM, SWS or REM sleep, to be positively associated with morning recall performance on a declarative memory task. Backhaus et al. [8] provided evidence of a decline in sleep-dependent declarative memory consolidation in aging, associated with a decrease in SWS. Nevertheless, to the best of our knowledge, no study directly addressed this question in AD.
This study aimed at investigating, in aging and AD, the potential link between episodic memory impairment and sleep changes, especially sleep spindles, in subjects free of any cholinergic medication. As recent data indicate that fast spindles may be preferentially involved in cognitive re-processing [5,9], we also investigated the potential differential effect of slow and fast spindles on memory consolidation.
Methods
Subjects
14 AD patients (5 men, age: 76.9 ± 4.1 years) with a MMSE score [10] of 21 or higher (24.9 ± 2) participated in this study. They were recruited through a memory clinic and selected on the basis of a neurological examination and a neuropsychological assessment, using standard criteria for probable AD [11]. Structural imaging (MRI) showed no focal abnormality. At the time of the study, none of the patients was being or had been treated with cholinergic medications. None of them suffered from sleep disorders such as periodic limb movements disorder or sleep apnoea.
14 healthy elderly subjects (5 men; age: 75.1 ± 4.6 years), paired according to their level of education with AD patients, also participated in this study. They were recruited in clubs for retired people, after an interview with a neurologist. They had no medical, neurological, vascular, sleep or psychiatric disorders. Their mean MMSE score was 29.4 (± 0.9). They were free of any medication that could influence sleep and memory.
Lastly, we also recruited 14 healthy young subjects (7 men; age: 23.4 ± 3.1 years). None of them had history of medical, psychiatric or sleep disorders, nor disturbances of their sleep-wake- cycles during the last six weeks.
All subjects were right-handed, native French speakers and gave their written consent to the study after detailed information was provided to them. The study was done in-line with the Declaration of Helsinki following approval by the Regional Ethics Committee.
Memory task(s)
Episodic memory was assessed using a task derived from Grober and Buschke’s procedure [12], consisting in learning a series of 15 words in successive trials. The subject was first asked to process each word in depth by pointing out and reading aloud the item belonging to the semantic category given by the examiner. Then, in order to ensure the actual carrying-out of deep encoding, a task of immediate cued recall was given every three words. This procedure was repeated throughout the whole list. If the subject failed in the immediate recall task, he was reminded of the word and again requested to recall it in response to its cue. Immediately following the processing of the 15 words, retrieval was assessed with a cued recall task (using the same cues). The originality of the procedure used here lies in the fact that we used 3 consecutive trials in Young subjects and 5 for the two other groups, to account for their known encoding difficulties. This was done to have almost the same starting performance before sleep in each group and to avoid, as much as possible, floor effects when retesting AD patients the day after. A delayed cued recall and recognition were conducted after a night of sleep.
Episodic memory was also assessed with a 12-item Story Recall task taken from the BEM-144 memory battery [13]. In the evening, the experimenter gave a single reading of the story, and then the patients and subjects had to freely recall the story. In the morning, about one hour after awakening, a second free recall task was proposed.
These two tasks were chosen to contrast the depth of encoding (deeper for the Grober and Buschke’s task).
Sleep recordings and spindle detection
Sleep was assessed, following an adaptation night,, by standard polysomnography and scored using a validated staging algorithm [14] according to standard criteria [15].
Total sleep time, sleep onset latency, sleep efficiency, and the time and percentage of time spent in each sleep stage were determined. Sleep spindles were detected automatically using the central electrodes (C3/C4), rereferenced to contralateral mastoids, based on the following criteria: frequency range of 11–16 Hz, amplitude > 12μV, and duration: 0.3–2 s [14]. The applied algorithm also provides features such as the duration, amplitude and frequency. Therefore, it allows to distinguish slow (11–13 Hz) from fast spindles (13–15 Hz) [16,17] and to calculate the mean (and mean weighted) intensity (duration × amplitude) of spindle events. Indeed, spindle activity (duration × mean amplitude) is generally more sensitive than the number or density of sleep spindles [4].
Statistical analyses
Neuropsychological and sleep data (global measures and amounts of sleep stages) were compared using unpaired Student t tests. Then, we conducted independent analyses of variance (ANOVAs) on each spindle measure (mean number, intensity and weighted intensity). In order to not confound the effect of age and AD, we compared in different analyses, Old subjects to AD patients, and Old subjects to Young ones.
Correlations analyses between neuropsychological data and sleep stages or spindles were conducted using Pearson’s test.
Results
Episodic memory was globally preserved in Old subjects contrasting with the impairment observed for both tasks and almost all measures in AD patients (Table 1).
Table 1.
Episodic memory performance in the three groups of subjects.
| Young (n = 14)• | Old (n = 14) | AD (n = 14) | |
|---|---|---|---|
| Grober & Buschke task | |||
| Last immediate cued recall (max 15, evening) | 15 ± 0 | 15 ± 0 | 12 ± 2.5◦◦ |
| Delayed cued recall (max 15, morning) | 15 ± 0 | 14.8 ± 0.6 | 10.2 ± 3.8◦◦◦ |
| Forgetting rate (%) | 0 ± 0 | −1.6 ± 4.45 | 20.2 ± 17.6◦◦◦ |
| Morning – Evening performance | 0 ± 0 | −0.2 ± 0.6 | −2.2 ± 1.8◦◦◦ |
| Correct recognitions (max 15) | 15 ± 0 | 14.8 ± 0.4 | 12.8 ± 2.3◦◦ |
| False recognitions | 0.08 ± 0.3 | 0.1 ± 0.4 | 6.5 ± 4.9◦◦◦ |
| Story recall task | |||
| Immediate free recall (max 12, evening) | 10 ± 1.5 | 8.7 ± 1.9t | 2.9 ± 1.2◦◦◦ |
| Delayed free recall (max 12, morning) | 8.6 ± 1.9 | 7.1 ± 1.7* | 0.6 ± 1.1◦◦◦ |
| Forgetting rate (%) | 14.4 ± 14.1 | 17.2 ± 15.7 | 82.1 ± 30.7◦◦◦ |
| Morning – Evening performance | −1.4 ± 1.2 | −1.6 ± 1.4 | −2.3 ± 1.1 |
Displayed are means ± SD and results from statistical groups comparisons (unpaired Student t tests). Old vs Young comparison:
<.05;
p<.07;
AD vs Old comparison:
p<.01;
p<.001.
Last immediate cued recall of the Grober and Buschke’s task as well as immediate free recall of the Story task, were proposed in the evening, before sleep. “Morning – Evening” performance corresponds to the difference of scores on the delayed minus immediate recall scores. Forgetting rate was calculated as follows: ((Immediate recall − delayed recall)/immediate recall) × 100.
One young subject has been excluded from the analyses due to abnormal proportion of false recognitions (> 2.5 SD of the mean performance of the Young group).
Age-related changes in sleep architecture consisted in a significant decrease in total sleep time sleep efficiency and increased number of awakenings. Besides, Old subjects also show a decrease in the amount of SWS and REM sleep contrasting with an increase in light sleep (Table 2). Comparing AD patients to Old subjects did not reveal any significant difference in sleep architecture (Table 2).
Table 2.
Global sleep measures and sleep architecture.
| Young (n = 14) | Old (n = 14) | AD (n = 14) | |
|---|---|---|---|
| Global measures of sleep | |||
| SPT | 477 ± 37.5 | 446.6 ± 60.7 | 489.3 ± 80.1 |
| TST | 452.2 ± 41.7 | 387.3 ± 65.7** | 393.6 ± 101 |
| Sleep latency (min) | 14.4 ± 11.3 | 19.6 ± 20.5 | 30.6 ± 32.9 |
| REM sleep latency (min) | 89.8 ± 33.1 | 96.9 ± 49.1 | 115.5 ± 76.2 |
| Sleep efficiency (%) | 90.9 ± 3.7 | 78.5 ± 11*** | 73.6 ± 13.4 |
| Awakenings | 10.4 ± 5.2 | 22.4 ± 12** | 23.9 ± 12.1 |
| Wake after sleep onset (min) | 26.1 ± 10.5 | 85.8 ± 42.9*** | 103.5 ± 59.2 |
| Sleep architecture (% TST) | |||
| Stage 1 NREM | 6.6 ± 2.7 | 12.4 ± 5.2*** | 13.9 ± 9 |
| Stage 2 NREM | 52 ± 7.2 | 61.2 ± 4.4*** | 55.8 ± 12 |
| SWS | 23.1 ± 8.1 | 14.1 ± 6.6** | 15.8 ± 12.6 |
| REM sleep | 18.3 ± 4 | 12.3 ± 5.5** | 14.5 ± 6.4 |
Displayed are means ± SD and results from statistical groups comparisons (unpaired Student t tests). Old/Young comparison:
p<.01;
p<.001.
Our data revealed age-related changes in spindle activity. To sum up, despite an increase in stage 2 sleep (Table 2), a significant decrease in the total number, mean intensity and mean weighted intensity of sleep spindles is observed in the group of Old subjects (Table 3). This difference affects both slow and fast spindles which are fewer in Old than in Young subjects (Slow spindles: Old = 314.2 ± 350.1 vs Young = 1210 ± 823.5, p<.01; Fast spindles: Old = 872.8 ± 698.4 vs Young = 1792.7 ± 893.2, p<.01).
Table 3.
Age and AD related changes in spindle activity.
| Age- and AD-related changes1 | ||||
|---|---|---|---|---|
| Old vs Young | AD vs OLD | |||
| F(1,25) | p value | F(1,25) | p value | |
| Mean number all spindles | 19.4 | <.001 | 2.7 | ns |
| Mean intensity (μV) | 44.4 | <.001 | <1 | ns |
| Mean weighted intensity | 21.5 | <.001 | 1.9 | ns |
| Group × Spindle type2 | ||||
| Number of spindles | ||||
| Group effect | 19.4 | <.001 | 2.7 | ns |
| Spindle type effect | 9 | <.01 | 19.6 | <.001 |
| Group × spindle type | <1 | ns | 4 | <.05 |
| Mean spindle intensity | ||||
| Group effect | 41.7 | <.001 | <1 | ns |
| Spindle type effect | <1 | ns | 7.4 | <.05 |
| Group × spindle type | <1 | ns | 1.1 | ns |
| Mean weighted intensity | ||||
| Group effect | 21.5 | <.001 | 1.9 | ns |
| Spindle type effect | 4.4 | <0.05 | 15.3 | <.001 |
| Group × spindle type | <1 | ns | 2.7 | ns |
Mean weighted intensity refers to the mean number of spindles × their mean intensity. In order to detect all possible spindles events the spindle algorithm was set to a high sensitivity level (d = 0.8). Based on previous analyses, the frequency that best separates slow from fast spindles is about 13 Hz [16,17]. Consequently, slow and fast sleep spindles were defined as having a frequency between 11–13 Hz and 13–15 Hz respectively. Results of spindles analyses are reported using data recorded on C3 electrode.
One-way ANOVAs;
Two-way ANOVAs. One old subject has been excluded due to spindles values abnormally different from those of the mean group of elderly subjects (values different from ± 2.5 SD).
As for changes in spindle activity associated with mild AD, AD patients do not show any significant decrease in the number of total sleep spindles, nor of spindle intensity if slow and fast spindles are not distinguished. However, distinguishing between slow and fast spindles, revealed a significant reduction in specifically the number of fast spindles in AD patients (number of fast spindles in AD: 420.9 ± 571.7, p<.02; Table 3).
As Young and Old subjects exhibited ceiling effects in both tasks, correlations were searched only in the AD group. No systematic relationship between memory performance and sleep stages could be evidenced, whatever the memory task considered (data not shown). However, performance on immediate recall in the Story task positively correlated with the mean intensity of all (r = 0.58, p<0.03) and fast spindles (r = 0.62, p<0.02; Figure 1). No significant correlation was observed for the Grober and Buschke’s task.
Figure 1. Significant correlations between spindle intensity and immediate free recall performance on the story recall task, in AD patients.
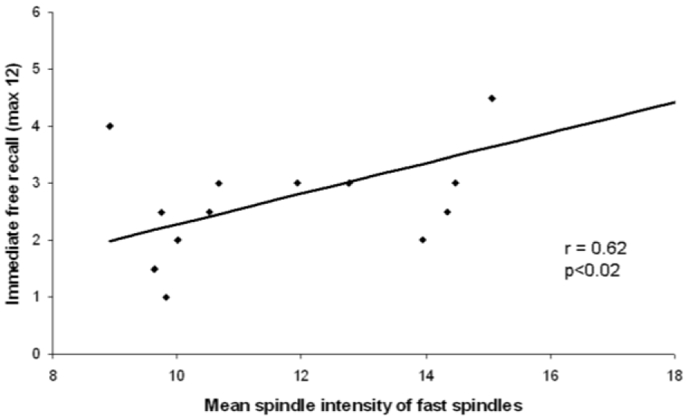
Scatter plot representing the significant and positive correlations between spindle intensity of fast spindles and immediate free recall performance on the story recall task.
Discussion
Our data revealed a global reduction in the number, intensity and weighted intensity of spindles in aging and AD. We show for the first time that a more pronounced fast spindles decrease became evident in AD patients as compared to Old controls. Since the thalamus is known to be involved in sleep spindles generation [18], its atrophy usually observed in AD [19] could explain the global decrease in spindles. Furthermore, in a recent combined EEG and fMRI study, Schabus et al. [17] showed that fast spindles recruited various cortical areas, among which is the hippocampus. Sleep-related memory consolidation is supposed to occur through a dialogue between the hippocampus and neocortical areas, in which sleep spindles appear to play an important role [1]. The hippocampus being the earliest and most severely affected region in AD [19,20], sleep-related consolidation could be impaired in AD. In addition, the hippocampal atrophy could explain the selective changes in fast spindles reported here. This result is also particularly interesting since recent studies suggest that fast spindles may be preferentially involved in memory consolidation [5,9].
In addition to the specific decrease in fast sleep spindles, we also report a positive correlation between mean intensity of fast spindles and performance in an immediate retrieval task. This result is reminiscent of studies who reported associations between spindle intensity and memory improvement [3,4] or general measures of learning abilities [5,21]. Our correlation suggests that higher-performing patients have fast spindles with the highest intensity during sleep. It also means that subjects having generally more spindles are probably better on some memory tasks because spindles might reflect general network properties (i.e., more efficient thalamo-cortical networks simply generating more spindles during sleep). In other words, this relationship might reflect the efficiency of information processing that relies upon thalamo-cortical communication [21]. No significant correlation was observed with delayed performance or with “Morning-to-Evening” performance, as previously reported [4]. This can be explained by the fact that items which should be recalled the next day (delayed recall) already tended to be lost before sleep in AD. Thus, practically patients had nothing to loose or to consolidate during sleep (e.g., less than 3 items for the Story task). It is remarkable that after a relatively elaborate encoding in the Grober and Buschke’s task, AD patients only “lost” 2 more items during subsequent sleep. It could be argued that AD patients have problems in encoding, but the delayed recall of the Grober and Buschke’s task suggests that the consolidation is, at least partially, preserved provided that information has been encoded deeply before sleep (i.e., several repetitions of each item). The fact that AD patients present morphologic alterations in the hippocampus and significant changes in sleep spindles, known to be correlated with learning abilities may account for these decrements in learning or encoding ability.
Conclusion
We demonstrated a specific reduction of fast spindles in the early stages of AD. In addition, the intensity of these fast spindles correlated with episodic memory performance on an immediate free recall task. Although the first of its kind and requiring replication, these results are reminiscent of earlier findings reporting an association between sleep spindles and general learning abilities in healthy subjects. Here we extend these findings to AD patients and give further hints pointing to functional differentiations and significance of slow and fast spindles.
Acknowledgments
GR was supported by the France Alzheimer association, MS by an Erwin Schrödinger fellowship of the Austrian Science Fund (FWF; J2470-B02).
Footnotes
Competing interests. The authors have declared that no competing interests exist
References
- 1.Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 2.Gais S, Mölle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Schabus M, Gruber G, Parapatics S, Sauter C, Klosch G, Anderer P, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–1485. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 5.Bodizs R, Kis T, Lazar AS, Havran L, Rigo P, Clemens Z, et al. Prediction of general mental ability based on neural oscillation measures of sleep. J Sleep Res. 14:285–292. doi: 10.1111/j.1365-2869.2005.00472.x. [DOI] [PubMed] [Google Scholar]
- 6.Petit D, Gagnon JF, Fantini ML, Ferini-Strambi L, Montplaisir J. Sleep and quantitative EEG in neurodegenerative disorders. J Psychosom Res. 2004;56:487–496. doi: 10.1016/j.jpsychores.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Mazzoni G, Gori S, Formicola G, Gneri C, Massetani R, Murri L, et al. Word recall correlates with sleep cycles in elderly subjects. J Sleep Res. 1999;8:185–188. doi: 10.1046/j.1365-2869.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- 8.Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14:336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruber G, Anderer P, Parapatics S, Saletu B, Miazhynskaia T, Schabus M, et al. Differential effects of fast and slow spindles in early and late sleep on the consolidation of an explicit memory task. J Sleep Res. 2006;15:488. [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 12.Grober E, Buschke H. Genuine memory deficits in dementia. Developmental Neuropsychology. 1987;3:13–36. [Google Scholar]
- 13.Signoret J-L. Batterie d’efficience mnésique 144. Elsevier; Paris: 1991. [Google Scholar]
- 14.Anderer P, Gruber G, Parapatics S, Woertz M, Miazhynskaia T, Klösch G, et al. An E-health solution for automatic sleep classification according to Rechtschaffen and Kales: validation study of the Somnolyzer 24×7 utilizing the Siesta database. Neuropsychobiology. 2005;51:115–133. doi: 10.1159/000085205. [DOI] [PubMed] [Google Scholar]
- 15.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Brain Information Service, University of California; Los Angeles: 1968. [Google Scholar]
- 16.Anderer P, Klosch G, Gruber G, Trenker E, Pascual-Marqui RD, Zeitlhofer J, et al. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience. 2001;103:581–592. doi: 10.1016/s0306-4522(01)00028-8. [DOI] [PubMed] [Google Scholar]
- 17.Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2007;104:13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 19.Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 20.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 21.Fogel SM, Nader R, Cote KA, Smith CT. Sleep spindles and learning potential. Behav Neurosci. 2007;121:1–10. doi: 10.1037/0735-7044.121.1.1. [DOI] [PubMed] [Google Scholar]


