Abstract
Background
Many HIV-infected children in Southern Africa have been started on antiretroviral therapy (ART), but loss to follow up (LTFU) can be substantial. We analyzed mortality in children retained in care and in all children starting ART, taking LTFU into account.
Patients and methods
Children who started ART before the age of 16 years in ten ART programs in South Africa, Malawi, Mozambique and Zimbabwe were included. Risk factors for death in the first year of ART were identified in Weibull models. A meta-analytic approach was used to estimate cumulative mortality at one year.
Results
8225 children (median age 49 months, median CD4 cell percent 11.6%) were included; 391 (4.8%) died and 523 (7.0%) were LTFU in the first year. Mortality at one year was 4.5% (95% CI 2.8–7.4%) in children remaining in care, but 8.7% (5.4–12.1%) at the program level, after taking mortality in children and LTFU into account. Factors associated with mortality in children remaining in care included age (adjusted hazard ratio [HR] 0.37; 95% CI 0.25–0.54 comparing ≥120 months with <18 months), CD4 cell percent (HR 0.56; 95% CI 0.39–0.78 comparing ≥ 20% with <10%), and clinical stage (HR 0.12; 95% CI 0.03–0.45 comparing WHO stage I with III/IV).
Conclusions
In children starting ART and remaining in care in Southern Africa mortality at one year is <5% but almost twice as high at the program level, when taking LTFU into account. Age, CD4 percentage and clinical stage are important predictors of mortality at the individual level.
Keywords: HIV, AIDS, ART, children, loss to follow-up, transfer-out, heterogeneity, mortality, adjustment, Southern Africa
INTRODUCTION
Antiretroviral therapy (ART) has become more widely available in resource-limited settings in recent years.1 Similar virological response and relative reductions in mortality compared to industrialized settings have been observed in adult patients.2;3 In sub-Saharan Africa mortality is, however, high in the first months of ART because many patients start therapy with advanced infection. Patients who start ART with less advanced disease have mortality rates that are comparable to the general population in the second year of ART.3;4 Loss to follow-up (LTFU) is a common, unfavorable outcome in ART programs in resource-limited settings.5;6 LTFU introduces bias in estimates of mortality at the level of the treatment program, i.e. mortality in all patients starting ART at a given site. Efforts to improve program retention by tracing all or a sample of patients LTFU have been introduced in some programs, and mortality in patients LTFU was found to be substantially higher than in patients retained in the program.7
African children are disproportionately affected by the HIV epidemic: an estimated 2.1 million children worldwide lived with HIV at the end of 2007; 90% of them in sub-Saharan Africa.8 Mortality and program retention of children starting ART are not well defined at present, with substantial heterogeneity between pediatric treatment programs.9–15 We examined early mortality and LFTU of HIV-infected children starting ART in ten sites participating in the International epidemiologic Databases to Evaluate AIDS in Southern Africa (IeDEA-SA). We estimated mortality and risk factors for death in children retained in care, and mortality at the program level, taking into account mortality among children LTFU.
PATIENTS AND METHODS
Study population and cohort characteristics
The IeDEA-SA collaboration includes programs from Botswana, Malawi, Mozambique, South Africa, Zimbabwe and Zambia (www.iedea-sa.org). IeDEA-SA is part of IeDEA, which includes similar networks in other regions of Africa, Latin America and the Caribbean, Asia and North America (www.hiv-iedea.org).16 Data are collected at each site as part of routine monitoring at baseline (ART initiation) and each follow-up visit, using standardized definitions. Data from the different sites are transferred to data centers at the Universities of Cape Town, South Africa or Bern, Switzerland, in a standardized format and merged at regular intervals. Information on each program is collected using standardized site assessments which are completed each year using the web-based World Health Organization (WHO) Data Collector tool.17 Descriptions on program characteristics are based on the most recent site assessment in 2008 and mainly reflect the situation towards the end of the period of data collection for this study.
Eligibility criteria and outcomes
All treatment-naïve children (age ≤ 16 years) who started ART after 1998 were included in the analysis. Enrolment of patients and selection into care vary between sites (see Table, Supplemental Digital Content 1). Small sites with less than 100 eligible children were excluded. Children were categorized into alive, dead, transferred out and LTFU. The primary outcome was death from all causes. A child was considered LTFU if the last visit was more than 6 months before the closure date for that site, with the closure date defined as the most recent visit date of each site recorded in the database. Only children who commenced ART at least 6 months prior to the closure of the site databases could therefore be LTFU. Weight and height measurements were converted to age- and sex-standardized z-scores using WHO growth reference standards.18;19 Clinical stage and immunological category were defined according to WHO guidelines.20
Statistical analysis
We used Weibull random-effects regression models to identify risk factors for death in children remaining in care. The following variables were considered: age (<18 months, 18–59 months, 60–119 months, ≥ 120 months), gender, CD4 percentage (<10%, 10% to < 20%, ≥ 20%), WHO clinical stage and year of starting ART. Missing values for CD4 percentage and WHO clinical stage were imputed using chained estimation with 10 imputation cycles.21 The same models were used to estimate cumulative mortality at one year for each program. Program-specific mortality estimates were then combined in random-effects meta-analysis.
We calculated a weighted average of the mortality observed in children remaining in care and mortality estimated for children LTFU.22 We obtained estimates of mortality in children lost to programs from a meta-analysis of studies that traced patients LTFU to ascertain their vital status.7 This study showed an inverse relation between mortality among those lost to programs and the rate of loss to follow-up (See Figure, Supplemental Digital Content 2). We used the mortality estimate that corresponded to the rate of loss to follow-up observed in a program to estimate overall, program-level mortality. For example, 0.8% of children were LTFU at year 1 in program C whereas 12.3% were lost in program F. We therefore assumed that 63.2% and 51.9%, respectively, of children LTFU had died (See Table, Supplemental Digital Content 3). 95% confidence intervals (CIs) for estimates of program-level mortality were obtained from Monte Carlo simulations.
We examined to what extent heterogeneity in program-level mortality might be explained by differences in prognostic factors at baseline. We adjusted analyses of children remaining in care for age, gender, WHO clinical stage, CD4 percentage and starting year of ART and applied a cohort-specific correction factor (adjusted mortality estimate divided by crude estimate) to estimates and 95% confidence intervals (CI) of program-level mortality. Adjusted estimates were centered and correspond to a hypothetical population that is typical for the primary care settings included in this study (age 59.7 months, 50.6% male, 53.4% in WHO stage III, CD4 cell percentage 12.6% and start of ART in 2005). Considering the young median age of the cohort we used CD4 percentage in all age groups and thus avoided using two separate measures for immune deficiency (percentage and count). All data were analyzed using Stata statistical software version 10 (College Station, Texas, USA).
Ethical Approval
All study sites had local institutional review board or ethics committee approval to collect data and participate in IeDEA-SA.
RESULTS
Characteristics of treatment programs
Ten treatment programs in four countries (South Africa, Malawi, Mozambique and Zimbabwe) were included in the present analysis. The Figure in Supplemental Digital Content 4 shows the location of sites and the Table in Supplemental Digital Content 1 the characteristics of programs. Four sites are based in Cape Town (South Africa), one each in Soweto, Johannesburg, and Durban (South Africa), one in Lilongwe (Malawi), one in Maputo (Mozambique) and one in Harare (Zimbabwe). Two of the ten programs were tertiary care sites, eight were public programs and three treated children only. All programs were located in urban areas and started ART according to clinical stage and CD4 percentage. At four sites ART in children younger than 12 months was initiated according to revised WHO guidelines23 towards the end of the period of data collection.
Patient characteristics
Table 1 summarizes patient characteristics at the start of ART: a total of 8225 HIV-infected children were analyzed. The three largest programs (sites B, D and G) contributed 4413 (53.7%) children; three sites with a total of 2153 (26.2%) patients were from outside South Africa. Median age was 49 months (range across sites 16 to 120 months), 2045 (24.9%) were younger than 18 months (range 2.7% to 53.4%), 4159 (50.6%) were male (range 45.8% to 55.3%), and median follow-up was 17.3 months (range 8.7 to 25.7 months). Median CD4 percent at start of ART ranged from 8% to 15% and the proportion of children with WHO clinical stage III/IV from 63.7% to 96.9%. The most frequent initial ART regimen was stavudine/lamivudine (d4T/3TC) in combination with efavirenz (EFV) [37.4%], nevirapine (NVP) [22.0%] or lopinavir/ritonavir (LPV/r) [18.1%].
Table 1.
Baseline characteristics and outcomes of children starting ART in each of ten treatment programs in Southern Africa.
| Characteristic | Site A | Site B | Site C* | Site D | Site E* | Site F | Site G | Site H | Site I† | Site J† | All sites |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n (% of total children) | 264 (3.2) | 1086 (13.2) | 266 (3.2) | 2202 (26.8) | 560 (6.8) | 764 (9.3) | 1125 (13.7) | 405 (4.9) | 854 (10.4) | 699 (8.5) | 8225 (100) |
| Age, median (IQR), mo | 120 (81–159) | 45 (16–86) | 48 (19–84) | 57 (22–92) | 45 (21–80) | 100 (58–143) | 39 (19–76) | 73 (33–109) | 16 (6–51) | 22 (9–60) | 49 (18–92) |
| Age group, n (%) | |||||||||||
| <18 mo | 7 (2.7) | 295 (27.2) | 65 (24.4) | 461 (20.9) | 115 (20.5) | 15 (2.0) | 266 (23.6) | 50 (12.3) | 456 (53.4) | 315 (45.1) | 2045 (24.9) |
| 18–59 mo | 43 (16.3) | 358 (32.9) | 93 (35.0) | 714 (32.4) | 232 (41.4) | 188 (24.6) | 478 (42.5) | 112 (27.7) | 216 (25.3) | 215 (30.7) | 2649 (32.2) |
| ≥60 mo | 214 (81.1) | 433 (39.9) | 108 (40.6) | 1027 (46.7) | 213 (38.1) | 561 (73.4) | 381 (33.9) | 243 (60) | 182 (21.3) | 169 (24.2) | 3531 (42.9) |
| Sex | |||||||||||
| Male, n (%) | 121 (45.8) | 539 (49.6) | 147 (55.3) | 1116 (50.7) | 290 (51.8) | 373 (48.8) | 583 (51.8) | 201 (49.6) | 433 (50.7) | 356 (50.9) | 4159 (50.6) |
| Weight-for-age z-score, n (%) | |||||||||||
| ≤−3 | 29/70 (41.4) | 86/367 (23.4) | NA | 162/612 (26.5) | 8/42 (19.1) | 23/104 (22.1) | 185/1038 (17.8) | 20/88 (22.7) | 246/824 (29.9) | 53/158 (33.5) | 812/3302 (24.6) |
| CD4 cell percentage, median (IQR) | NA | 12 (6–17) | NA | 10 (6–15) | 13 (9–19) | 11 (7–16) | 12 (8.5–17) | 8 (4–13) | 12 (7.6–19) | 15 (9–23) | 11.6 (7–17) |
| No. of observations | NA | 585 | NA | 1577 | 290 | 245 | 627 | 185 | 823 | 379 | 4711 |
| Imputed values, median (IQR) | 11 (6.4–16) ‡ | 13 (7.9–19) | 14 (8.8–19) ‡ | 11 (6.7–16) | 13 (9–19) | 12 (7.8–18) | 13 (9–19) | 11 (6–17) | 12 (7.6–19) | 15 (9.8–22) | 12.6 (7.8–18.0) |
| Viral load | |||||||||||
| Children with > 1Mio copies/ml, n (%) | NA | 55/487 (11.3) | 2/112 (1.8) | 316/1731 (18.3) | 57/343 (16.6) | NA | NA | 3/9 (33.3) | 235/759 (31.0) | 177/460 (38.5) | 59/3901 (21.7) |
| log10 copies/ml, median (IQR) | NA | 5.24 (4.70–5.70) | 5.04 (4.65–5.56) | 5.30 (4.72–5.87) | 5.15 (4.42–5.68) | NA | NA | 5.88 (5.54–6.04) | 5.66 (5.15–6.18) | 5.78 (5.20–6.25) | 5.40 (4.81–5.92) |
| Hemoglobin (g/dl) | |||||||||||
| <8, n (%) | 13/187 (7.0) | 10/179 (5.6) | 6/92 (6.5) | 14/288 (4.9) | 7/180 (3.9) | NA | 68/416 (16.4) | 24/243 (9.9) | 129/820 (15.7) | 62/468 (13.3) | 334/2874 (11.6) |
| WHO immunological classification | |||||||||||
| Advanced/Severe, n (%) | 128/152 (84.2) | 490/586(83.6) | NA | 1335/1587 (84.1) | 266/324 (82.1) | NA | 499/627 (79.6) | 191/211 (90.5) | 693/823 (84.2) | 311/398 (78.1) | 4153/5035 (82.5) |
| WHO clinical stages | |||||||||||
| III and IV, n (%) | NA | NA | 186/266 (69.9) | 968/1520 (63.7) | 422/560 (75.4) | 659/680 (96.9) | 773/995 (77.7) | NA | 735/835 (88.0) | 423/470 (90.0) | 4487/5725 (78.4) |
| Imputed values, n (%) | 202 (76.3) | 827 (76.2) | 186 (69.9) | 1481 (67.3) | 422 (75.4) | 726 (95.0) | 875 (77.8) | 314 (77.6) | 752 (88.1) | 613 (87.7) | 6398 (77.8) |
| TB treatment at start of ART, n (%) | NA | NA | NA | 534 (24.3) | 91 (16.3) | NA | 246 (21.9) | NA | NA | NA | NA |
| Outcomes & | |||||||||||
| Months of follow-up, median (IQR) | 14.9 (7.7–23.7) | 18.0 (5.6–30.7) | 25.7 (16.3–35.7) | 16.6 (6.4–28.7) | 24.3 (12.9–39.1) | 12.1 (3.3–28.8) | 24.4 (13.5–35.7) | 15.3 (5.4–26.1) | 8.7 (4.0–18.1) | 18.9 (6.1–33.5) | 17.3 (6.7–30.0) |
| Deaths, n (%) | 24 (9.1) | 49 (4.5) | 3 (1.1) | 113 (5.1) | 11 (2.0) | 29 (3.8) | 14 (1.2) | 24 (5.9) | 121 (14.2) | 67 (9.6) | 455 (5.5) |
| Loss to follow-up, n (%) # | 6/231 (2.6) | 125/965 (13.0) | 5/258 (1.9) | 176/1934 (9.1) | 37/536 (6.9) | 97/701 (13.8) | 155/1090 (14.2) | 13/346 (3.8) | 69/777 (8.9) | 34/644 (5.3) | 717/7482 (9.6) |
| Transfer-out, n (%) | 0 (0) | 66 (6.1) | 25 (9.4) | 143 (6.5) | 55 (9.8) | 174 (22.8) | 17 (1.5) | 33 (8.2) | 389 (45.6) | 274 (39.2) | 1176 (14.3) |
| Alive and in care, n (%) | 234 (88.6) | 846 (77.9) | 225 (84.6) | 1753 (79.6) | 455 (81.3) | 418 (54.7) | 935 (83.1) | 322 (79.5) | 273 (32.0) | 313 (44.8) | 5774 (70.2) |
| Others/unknown, n (%) | 0 (0) | 0 (0) | 8 (3.0) | 17 (0.8) | 2 (0.4) | 46 (6.0) | 4 (0.4) | 13 (3.2) | 2 (0.2) | 11 (1.6) | 103 (1.3) |
Community based program in South Africa;
Tertiary level of care;
All values imputed;
During the months of follow-up indicated;
Among children with >6 months of follow-up; NA, not available
Children treated in tertiary care programs were younger (median age 19 months versus 57 months, p<0.0001) and more likely to be in clinical stage III/IV (88.7% versus 75.3%, p<0.0001). Rates of transfer out were higher for tertiarycare programs: 42.7% versus 7.7% in other sites, p<0.0001. Overall 1176 (14.3%) had been transferred to another site (range 0%–45.6%). A total of 7482 eligible children with at least 6 months of follow-up could be included in calculations of the rate of LTFU: 717 children (9.6%) were LTFU (range across programs 1.9% to 14.2%).
Mortality at one year in children remaining in care and at the program level
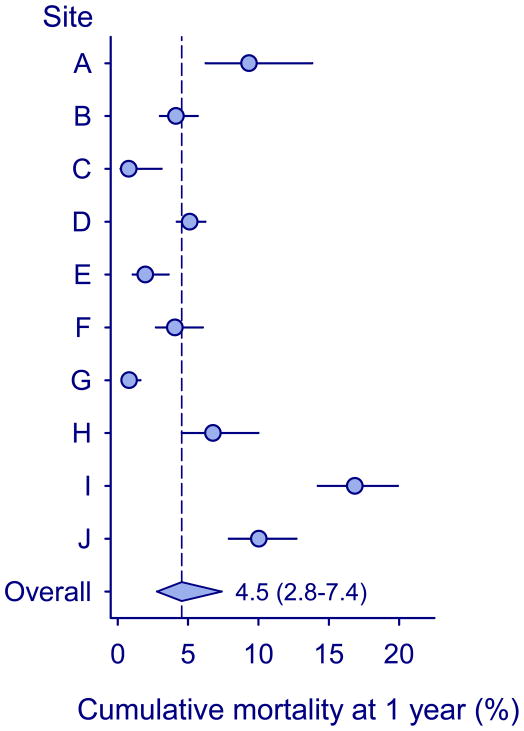
A total of 391 children (4.8%) died in the first year of ART while in care. Figure 1 shows cumulative mortality at one year by treatment program. Mortality was substantially higher in the first three months of ART compared to later months, but did not significantly change between 4 to 6 and 7 to 12 months after start of ART (Table 2). There was substantial heterogeneity between programs: mortality ranged from 0.8% (95% CI 0.2%–3.1%) in site C to 16.8% (95% CI 14.2%–19.9%) in site I. The combined mortality estimate for all programs from random-effects meta-analysis was 4.5% (95% CI 2.8%–7.4%) among children remaining in care (Figure 1). Mortality was higher in the tertiary care programs compared to the other sites (13.5% versus 4.1%, p=0.014). Table 2 shows baseline variables associated with the probability of death in children remaining in care from crude and adjusted Weibull models. Younger age, lower CD4 cell, more advanced clinical stage and starting ART in earlier calendar years were associated with higher mortality.
Figure 1. Cumulative mortality at one year in children starting ART and remaining in care in ten treatment programs in Southern Africa.
Circles show estimates from individual programs, horizontal lines indicate 95% confidence intervals, and the diamond shows the combined estimate from random-effects meta-analysis with 95% confidence intervals.
Table 2.
Hazard ratios for death in the first year of ART in children remaining in care in ten treatment programs in Southern Africa.
| Characteristic | Person years of follow-up (y) | No. of deaths | Unadjusted |
Adjusted |
||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value * | HR (95% CI) | p-Value * | |||
| Age (mo) | <0.0001 | <0.0001 | ||||
| <18 | 1328 | 204 | 1 | 1 | ||
| 18–59 | 2100 | 72 | 0.29 (0.22–0.38) | 0.29 (0.22–0.38) | ||
| 60–119 | 2000 | 68 | 0.27 (0.20–0.36) | 0.25 (0.19–0.34) | ||
| ≥ 120 | 759 | 47 | 0.42 0.29–0.61) | 0.37 (0.25–0.54) | ||
| Sex | 0.32 | 0.53 | ||||
| Male | 3158 | 188 | 1 | 1 | ||
| Female | 3028 | 203 | 1.11 (0.91–1.35) | 1.07 (0.87–1.30) | ||
| CD4 cell percentage | 0.0001 | <0.0001 | ||||
| < 10% | 2248 | 196 | 1 | 1 | ||
| 10% to <20% | 2809 | 121 | 0.52 (0.39–0.69) | 0.48 (0.36–0.64) | ||
| ≥ 20% | 1129 | 74 | 0.73 0.52–1.02) | 0.56 (0.39–0.78) | ||
| WHO clinical stages | <0.0001 | 0.0001 | ||||
| III and IV | 4637 | 356 | 1 | 1 | ||
| II | 1118 | 32 | 0.41 (0.26–0.66) | 0.46 0.29–0.74) | ||
| I | 431 | 3 | 0.10 (0.03–0.40) | 0.12 (0.03–0.45) | ||
| Starting year of ART | 0.49 | 0.0008 | ||||
| before 2006 | 3671 | 254 | 1 | 1 | ||
| after 2006 | 2515 | 137 | 0.93 (0.75–1.15) | 0.69 (0.55–0.86) | ||
| Time after starting ART | <0.0001 | |||||
| Month 1–3 | 92 | 273 | 1 | <0.0001 | 1 | |
| Month 4–6 | 333 | 80 | 0.13 (0.10–0.18) | 0.13 (0.10–0.18) | ||
| Month 7–12 | 5761 | 38 | 0.10 (0.08–0.13) | 0.10 (0.08–0.13) | ||
Models were adjusted for all variables listed in the table. Multiple imputation was used to deal with missing data for CD4 cell percent and clinical stage.
Abbreviations: HR, hazard ratios; ART, antiretroviral therapy
p-Values are from Wald tests.
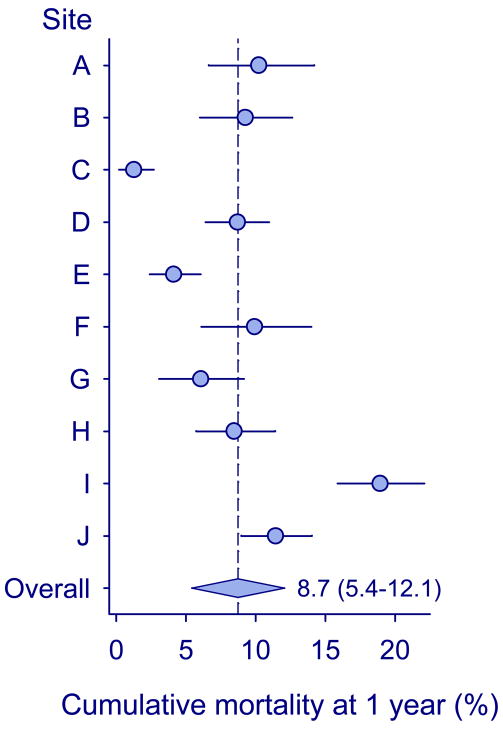
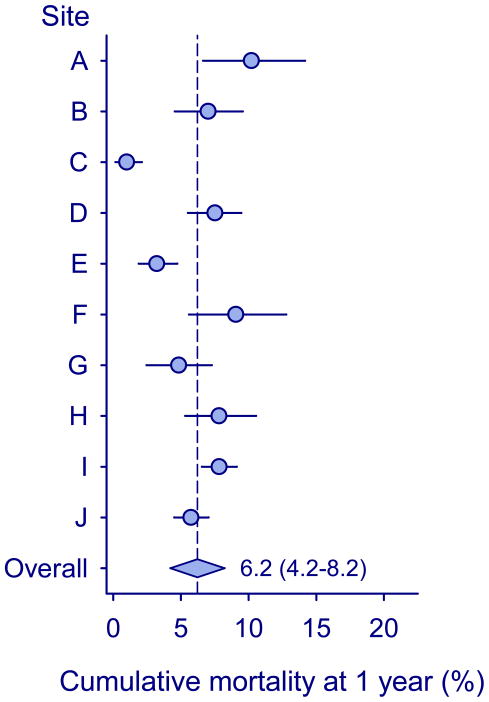
Figure 2 shows program-level mortality at one year, after taking mortality among children LTFU into account. Mortality ranged from 1.3% (95% 0.20%–2.7%) in program C to 18.9% (95% CI 15.9–22.1%) in program I. The combined estimate was 8.7% (95% CI 5.4%–12.1%). Finally, Figure 3 shows program-level mortality at one year after adjusting for age, gender, CD4 percentage, WHO clinical stage and year of starting ART. Mortality ranged from 1.0% (95% 0.2%–2.1%) in program C to 10.2% (95% CI 6.6%–14.2%) in program A. The higher mortality in tertiary care clinics was clearly explained by differences in prognostic factors, but some heterogeneity persisted due to lower mortality in children treated in two community programs in South Africa.
Figure 2. Cumulative mortality at one year at the level of the program in ten treatment programs in Southern Africa.
Circles show estimates from individual programs, horizontal lines indicate 95% confidence intervals, and the diamond shows the combined estimate from random-effects meta-analysis with 95% confidence intervals.
Figure 3. Cumulative mortality at one year at the level of the program in ten treatment programs in Southern Africa, adjusted to correspond to a hypothetical primary care population.
Circles show estimates from individual programs, horizontal lines indicate 95% confidence intervals, and the diamond shows the combined estimate from random-effects meta-analysis with 95% confidence intervals.
Sensitivity analysis
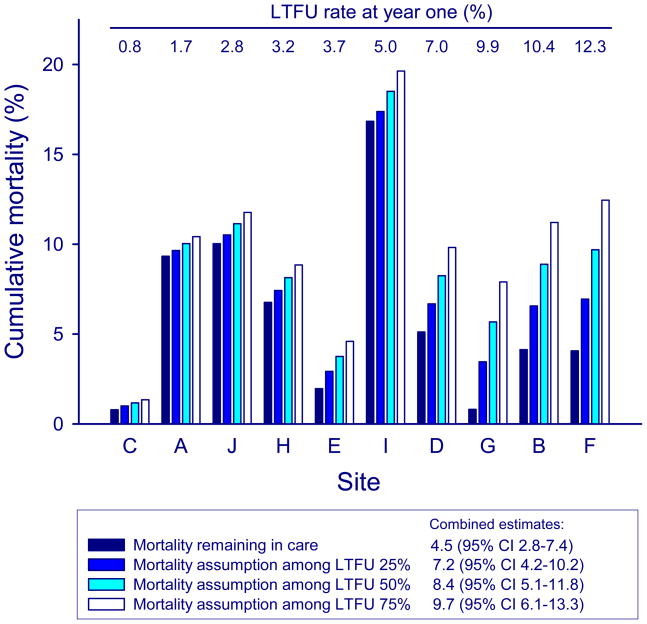
Figure 4 shows estimates of program-level mortality assuming different mortality rates among children LTFU. Taking LTFU up into account increased mortality estimates compared to mortality in children retained in care, with substantial changes being observed in programs with higher rates of LTFU. The combined estimates from random-effects meta-analysis were less sensitive to mortality assumptions: assuming that 25%, 50% or 75% of children LTFU had died resulted in combined estimates of mortality at one year of 7.2% (95% CI 4.2%–10.2%), 8.4% (95% CI 5.1%–11.8%) and 9.7% (95% CI 6.1%–13.3%), respectively.
Figure 4. Sensitivity analysis of mortality at one year after starting ART by treatment program, assuming mortality rates of 25%, 50% and 75% among children loss to follow-up (LTFU).
Sites are ordered by increasing rates of LTFU at one year (from left to right).
DISCUSSION
This collaborative analysis of ten pediatric treatment programs in four countries in Southern Africa found that one-year mortality in children starting ART varied across treatment programs, with heterogeneity partly explained by differences in prognostic factors at the start of ART, and differences in LTFU. Our study illustrates that ignoring LTFU may lead to substantial underestimation of mortality: mortality in children remaining in care overall was below 5% but was estimated to be about twice as high at the program level, when considering deaths in children LTFU.
We examined mortality and risk factors for death in children remaining in care, and then estimated program-level mortality. Previous analyses of mortality in patients starting ART have generally truncated (censored) follow-up time in patients LTFU, thus assuming that their mortality experience is comparable to similar patients remaining in care.12;24, 15 This assumption is problematic: many patients who are LTFU stop taking ART, and their mortality is high. In addition, patients may not return for a follow-up appointment because they have died. A meta-analysis of studies that traced adult patients LTFU found that in sub-Saharan Africa mortality in patients whose vital status could be ascertained was 46% (95% CI 39%–54%).7 Interestingly, there was an inverse relation between mortality among those LTFU and the rate of LTFU. We took this into account and used the mortality estimate that corresponded to the rate of LTFU observed in a given program. A recent analysis of patients LTFU in the rural Médecins sans Frontières (MSF) program in Chiradzulu, Malawi found that mortality among patients LTFU was similar for adults and children,25 thus supporting our use of estimates from studies in adults. Another study from Lilongwe, Malawi, described a mortality estimate of 33% (CI 20.4–48.4%) among children LTFU.26 The high early mortality in infants and young children means that adult estimates of mortality in those LTFU are not necessarily applicable to cohorts which include a large proportion of very young children.27;28 Our sensitivity analyses showed, however, that combined results were fairly robust to different mortality assumptions for children LTFU.
The context of LTFU in children is different and more complex compared to adults: children are dependent on caregivers, and child outcomes may be related to those of their parents. Even though caregivers are motivated to support children to adhere to ART, the caregiver may change frequently due to ill health, death or adult migration. In addition, unlike adult ART provision, most pediatric ART services remain located in larger hospitals in urban centers and the cost of transport and inconvenience of having to attend separate clinics for caregiver and child may contribute to LTFU.29–31 Interestingly, at one of the few family centered ART clinics, it has been shown that children whose caregiver is in HIV care at the same clinic experience better outcomes.32 The cost of pediatric drugs and difficulty of giving children large amounts of medicine which may be unpalatable are further obstacles to effectively remaining in care. 29;31;33 Furthermore, many caregivers are elderly grandparents for whom these obstacles may become a substantial barrier to continuing on treatment.28;31 Some of the children LTFU may have continued treatment at another clinic, perhaps a primary health care center closer to their home, without notifying the program where ART was started (“silent” transfer out).
Our study included children who were treated in ten ART programs in four countries in Southern Africa, including pediatric programs and programs mainly treating adults, and programs at tertiary and lower levels of care. These sites may not necessarily reflect the situation of this region as a whole: all sites were located in urban areas and cohorts from South Africa with a link to a research program predominated. However, this is one of the largest pooled analyses of children on ART ever done, and from one of the regions of the world most heavily affected by the HIV epidemic. Results should therefore be applicable to many other children on ART.
Few previous studies reported mortality among children starting ART: a pooled analysis from the Kids-ART-LINC collaboration on over 2000 children on ART in sub-Saharan Africa reported a probability of LTFU of 5.0% and a mortality of 6.0% at one year,9 which is slightly higher than the mortality estimate in children remaining in care in the present study. The number of children included was smaller than in the present study and, more importantly, the analysis did not take mortality among children LTFU into account. The estimate from the Kids-ART-LINC analysis is therefore probably an underestimate. A large program from Lusaka, Zambia, with 2398 children on ART showed a mortality rate of 6.6 deaths per 100 child-years, with an estimated cumulative mortality at one year (from the Kaplan-Meier curve) of 7.9%. Children LTFU were excluded from this analysis.10 A pediatric ART treatment program in Haïti indicated that 9% of children had died and 10% were lost after a median follow-up time of 20 months.34
The definition of LTFU was uniform across sites, but the period of six months chosen for this definition may be too short for some programs and too long for others. In the absence of dedicated studies on the appropriate definition of LTFU, this remains speculative. Follow-up of children who were transferred out was censored, thus assuming that their prognosis is the same as that of comparable children remaining in care at the site. Some children who were transferred out might, however, have a better or worse prognosis than the children remaining in a program. The proportion of children transferred out was particularly high in tertiary care, and these children will have been transferred to a lower level of care.
We observed differences between tertiary and other care programs in baseline characteristics as well as a higher mortality in tertiary care sites. The two tertiary programs are located in a region in the Republic of South Africa with a large pediatric ART network and a well functioning referral system; hence patients who are younger and represent the severe end of the pediatric HIV disease spectrum predominate at these sites. Statistical adjustment for prognostic factors showed that if children treated in tertiary centers were similar to the children followed in the other programs, particularly with regard to age and CD4 percentage, their mortality would be similar to that observed in the other programs. Indeed, young age is an important prognostic factor as shown in a recent collaborative analysis of 33 European cohorts, which demonstrated that mortality was almost double in children aged less than 2 years, compared to older children.27 Children treated at two township programs in South Africa3 had lower mortality and this was not explained by more favorable prognostic factors at baseline. These programs mainly treat adult patients and the children followed there may represent a selected group of children with a good prognosis. Other sources of heterogeneity might include different patterns of transfer out, different parental outcomes related to children’s outcome, incorrect assumptions on mortality in children LTFU, and incomplete or absent adjustment for other prognostic variables, for example exposure to antiretroviral drugs for preventing mother-to-child transmission. Furthermore, where programs do not have the capacity to commence ART quickly, mortality on ART may be lower due to survival bias among children who lived long enough to access treatment. The (non-HIV-related) background mortality in the different populations may also have played a role.4
Treatment programs should systematically trace patients LTFU where not yet done and record outcomes in these patients. Where such mechanisms are already in place as in most of the sites included in this study, capacities should be increased to intensify these activities. A modeling study based on a program in Côte d’Ivoire found that interventions that enhance program retention would both improve survival and be cost-effective by international criteria.35 An additional benefit is that program-level mortality can then be estimated by using double-sampling designs to account for mortality among children LTFU.36;37 For example, data from an outreach program have been used to adjust mortality estimates from an adult treatment program in Kenya.38;39 An important assumption made by this method is that a random sample of patients LTFU was selected and that the vital status of everyone from that sample was ascertained. These assumptions are not generally met: patients to be traced tend to be selected according to their assumed place of residence, excluding patients who live far from the treatment site, and typically about a third of patients cannot be located.7 Our approach is useful for sensitivity analysis in programs where no dedicated tracing studies are available, and to test the robustness of estimates based on the results of tracing activities.
In conclusion, our study indicates that mortality of HIV-infected children starting ART in Southern Africa is substantially higher than the mortality directly observed in treatment programs. A better understanding of outcomes in children LTFU, as well as children transferred out of programs is required, and the determinants of LTFU and transfer-out need to be defined. Dedicated field studies tracing children LTFU and studies linking data from treatment programs with routine mortality data from national death registries are of high priority. It is important both from a clinical and programmatic point of view to ensure capacity to trace this vulnerable patient group as soon as possible after a missed clinic visit.
Supplementary Material
Acknowledgments
We thank all children whose data were used in this study, and their caregivers. We also would like to thank all who contributed to recording and entering data as well as preparing and sending it to the IeDEA Southern Africa collaboration.
IeDEA-SA participating sites:
Margaret Pascoe, Newlands Clinic, Harare, Zimbabwe; Robin Wood, Gugulethu ART Program, Cape Town, South Africa; Harry Moultrie, University of Witwatersrand Paediatric HIV Clinics (Harriet Shezi Clinic, Chris Hani Baragwanath Hospital), Johannesburg, South Africa; Karl Technau, University of Witwatersrand Paediatric HIV Clinics (Rahima Moosa Mother and Child Hospital), Johannesburg, South Africa; Gilles van Cutsem, Khayelitsha ART Program, Médecins sans Frontières and University of Cape Town, Cape Town, South Africa; Paula Vaz, Paediatric Day Hospital, Maputo, Mozambique; Janet Giddy, McCord Hospital, University of Kwazulu Natal, Durban, South Africa; Brian Eley, Red Cross Children’s Hospital and the University of Cape Town, Cape Town, South Africa; Hans Prozesky, Tygerberg Academic Hospital, University of Stellenbosch, Cape Town, South Africa.
Footnotes
Previous presentation: These data were presented in part (“Mortality, loss to follow-up and transfer-out in pediatric ART programs in Southern Africa” [abstract WEPEB276]) at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; July 19–22, 2009; Cape Town, South Africa.
Potential conflict of interest: The authors declare that they have no conflict of interest.
Financial disclosure: This study was supported by the National Institute of Allergy and Infectious Diseases and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant 1 U01 AI069924–01). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Progress report 2008. Geneva: World Health Organization, UNAIDS, Unicef; 2008. Towards universal access. Scaling up priority HIV/AIDS interventions in the health sector. http://www.who.int/hiv/pub/towards_universal_access_report_2008.pdf. [Google Scholar]
- 2.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 3.Keiser O, Orrell C, Egger M, et al. Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoS Med. 2008;5:e148. doi: 10.1371/journal.pmed.0050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkhof MW, Boulle A, Weigel R, et al. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6:e1000066. doi: 10.1371/journal.pmed.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–67. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO; Geneva: 2007. Towards universal access by 2010: How WHO is working with countries to scale-up HIV prevention, treatment, care and support. http://www.who.int/hiv/mediacenter/universal_access_progres_report_en.pdf. [Google Scholar]
- 9.Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008;49:523–31. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 10.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 11.Kekitiinwa A, Lee KJ, Walker AS, et al. Differences in factors associated with initial growth, CD4, and viral load responses to ART in HIV-infected children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr. 2008;49:384–92. doi: 10.1097/QAI.0b013e31818cdef5. [DOI] [PubMed] [Google Scholar]
- 12.Sutcliffe CG, van Dijk JH, Bolton C, et al. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–89. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 13.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 14.Kekitiinwa A, Lee KJ, Walker AS, et al. Differences in factors associated with initial growth, CD4, and viral load responses to ART in HIV-infected children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr. 2008;49:384–92. doi: 10.1097/QAI.0b013e31818cdef5. [DOI] [PubMed] [Google Scholar]
- 15.Sutcliffe CG, van Dijk JH, Bolton C, et al. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–89. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 16.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007;36:294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Data Collector. https://extranet.who.int/datacol/WebHelp/DataCollector_OLH.htm.
- 18.World Health Organization. [Accessed February 2008];Growth reference data for 5–19 years. Available at http://www.who.int/growthref/en/
- 19.World Health Organization. [Accessed February 2008];WHO Child Growth Standards. Available at http://www.who.int/childgrowth/standards/en/
- 20.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access. [Accessed February 2008]; Available at www.who.int/hiv/pub/guidelines/WHOpaediatric.pdf. [PubMed]
- 21.Royston P. Multiple imputation of missing values: an update. Stata Journal. 2005;5:201241. [Google Scholar]
- 22.Egger M, Spycher B, Sidle J, et al. Correcting mortality for loss to follow up: a graphical approach applied to ART programmes in resource-limited settings. 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Cape Town. July 19–22, 2009; 2009. Abstract WEPED173. [Google Scholar]
- 23.World Health Organization. WHO antiretroviral therapy for infants and children 2008. Report of the WHO technical reference group, paediatric HIV/ART care guideline group meeting WHO headquarters; Geneva, Switzerland. [Accessed May 2009]. Available at http://www.who.int/hiv/pub/meetingreports/art_meeting_april2008/en/index.html. [Google Scholar]
- 24.Davies MA, Egger M, Keiser O, et al. Paediatric antiretroviral treatment programmes in sub-Saharan Africa: a review of published clinical studies. Afr J AIDS Res. 2009 doi: 10.2989/AJAR.2009.8.3.9.930. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukhuna B, McGuire M, Taremba K, et al. Outcomes of patient active tracing by lay community workers in a decentralized HIV care program of rural Malawi. 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Cape Town. July 19–22, 2009; 2009. Abstract WEPED195. [Google Scholar]
- 26.Weigel R, Hochgesang M, Brinkhof MW, et al. Outcomes and risk factors of patients lost to follow-up from antiretroviral treatment: a cross-sectional study from Lilongwe, Malawi. Submitted to J Acquir Immune Defic Syndr. 2010 [Google Scholar]
- 27.Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group. Response to combination antiretroviral therapy: variation by age. AIDS. 2008;22:1463–73. doi: 10.1097/QAD.0b013e3282f88d02. [DOI] [PubMed] [Google Scholar]
- 28.Davies MA, Keiser O, Technau K, et al. Outcomes of the South African National Antiretroviral Treatment Programme for children: the IeDEA Southern Africa collaboration. S Afr Med J. 2009;99:730–737. [PMC free article] [PubMed] [Google Scholar]
- 29.Vreeman RC, Wiehe SE, Ayaya SO, et al. Association of antiretroviral and clinic adherence with orphan status among HIV-infected children in Western Kenya. J Acquir Immune Defic Syndr. 2008;49:163–70. doi: 10.1097/QAI.0b013e318183a996. [DOI] [PubMed] [Google Scholar]
- 30.Eley B, Nuttall J. Antiretroviral therapy for children: challenges and opportunities. Ann Trop Paediatr. 2007;27:1–10. doi: 10.1179/146532807X170448. [DOI] [PubMed] [Google Scholar]
- 31.Meyers T, Moultrie H, Naidoo K, et al. Challenges to pediatric HIV care and treatment in South Africa. J Infect Dis. 2007;196 (Suppl 3):S474–S481. doi: 10.1086/521116. [DOI] [PubMed] [Google Scholar]
- 32.Reddi A, Leeper SC, Grobler AC, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr. 2007;7:13. doi: 10.1186/1471-2431-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies MA, Boulle A, Fakir T, et al. Adherence to antiretroviral therapy in young children in Cape Town, South Africa, measured by medication return and caregiver self-report: a prospective cohort study. BMC Pediatr. 2008;8:34. doi: 10.1186/1471-2431-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George E, Noel F, Bois G, et al. Antiretroviral therapy for HIV-1-infected children in Haiti. J Infect Dis. 2007;195:1411–18. doi: 10.1086/514823. [DOI] [PubMed] [Google Scholar]
- 35.Losina E, Toure H, Uhler LM, et al. Cost-effectiveness of preventing loss to follow-up in HIV treatment programs: a Cote d’Ivoire appraisal. PLoS Med. 2009;6:e1000173. doi: 10.1371/journal.pmed.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker SG, Wax Y, Patterson BH. Regression analysis of grouped survival data: informative censoring and double sampling. Biometrics. 1993;49:379–89. [PubMed] [Google Scholar]
- 37.Frangakis CE, Rubin DB. Addressing an idiosyncrasy in estimating survival curves using double sampling in the presence of self-selected right censoring. Biometrics. 2001;57:333–42. doi: 10.1111/j.0006-341x.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 38.Geng EH, Emenyonu N, Bwana MB, et al. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. 2008;300:506–7. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yiannoutsos CT, An MW, Frangakis CE, et al. Sampling-based approaches to improve estimation of mortality among patient dropouts: experience from a large PEPFAR-funded program in Western Kenya. PLoS One. 2008;3:e3843. doi: 10.1371/journal.pone.0003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.