Abstract
In utero alcohol exposure can lead to fetal alcohol spectrum disorders, characterized by cognitive and behavioral deficits. In vivo and in vitro studies have shown that ethanol alters neuronal development. We have recently shown that stimulation of M3 muscarinic receptors in astrocytes increases the synthesis and release of fibronectin, laminin, and plasminogen activator inhibitor-1, causing neurite outgrowth in hippocampal neurons. As M3 muscarinic receptor signaling in astroglial cells is strongly inhibited by ethanol, we hypothesized that ethanol may also inhibit neuritogenesis in hippocampal neurons induced by carbachol-stimulated astrocytes. In the present study we report that the effect of carbachol-stimulated astrocytes on hippocampal neuron neurite outgrowth was inhibited in a concentration-dependent manner (25–100 mM) by ethanol. This effect was due to the inhibition of the release of fibronectin, laminin, and plasminogen activator inhibitor-1. Similar effects on neuritogenesis and on the release of astrocyte extracellular proteins were observed after the incubation of astrocytes with carbachol in the presence of 1-butanol, another short-chain alcohol that, like ethanol, is a competitive substrate for phospholipase D, but not by tert-butanol, its analogue that is not a substrate for this enzyme. This study identifies a potential novel mechanism involved in the developmental effects of ethanol mediated by the interaction of ethanol with cell signaling in astrocytes, leading to an impairment in neuron-astrocyte communication.
Keywords: Ethanol, astrocytes, neurite outgrowth
Introduction
In utero alcohol exposure can lead to Fetal Alcohol Spectrum Disorders (FASD), characterized by growth retardation, birth defects, and neurodevelopmental abnormalities, leading to long-lasting cognitive and behavioral deficits (Bertrand et al. 2005; Jones and Smith 1975). The neurodevelopmental effects of ethanol are considered the most severe consequence of in utero alcohol exposure. Prenatal alcohol exposure has been shown to affect the development of different brain regions including the hippocampus, whose role in learning and memory is well characterized (Berman and Hannigan 2000; Mattson et al. 2001). The mechanisms involved in the cognitive and behavioral effects of ethanol exposure during gestation remain elusive; their understanding may lead to the development of novel strategies for the treatment and prevention of FASD.
Alcohol exposure affects neuronal development both in vivo and in vitro, although opposite effects of ethanol have been reported, with some studies indicating that ethanol increases the elongation of neuritis, and others showing an inhibitory effect (Bearer et al. 1999; Bingham et al. 2004; Messing et al. 1991; Miller 1997; Miller and al-Rabiai 1994; Saunders et al. 1995). Neurite outgrowth occurs in a strictly regulated fashion and is orchestrated by molecules present in the extracellular space such as extracellular matrix proteins, cell adhesion molecules, and soluble factors, that can promote or inhibit the growth of the neurite; many of these factors are produced by glial cells (Kiryushko et al. 2004).
Glial cells exert a profound effect on neuronal development (Booth et al. 2000; Chotard and Salecker 2004; Pfrieger and Barres 1997; Richards 2002); it has also been recently reported that ethanol may interfere with the ability of astrocytes to induce neurite outgrowth (Chen and Charness 2008; Pascual and Guerri 2007).
There is substantial evidence that acetylcholine may play an important role in brain development. Components of the cholinergic system, including choline acetyltransferase and acetylcholine receptors, are present prenatally long before the appearance of synapses (Armstrong et al. 1987; Daubas et al. 1990; Schambra et al. 1989). Acetylcholine may have non-transmitter effects during development, as it can regulate morphogenic cell movements during gastrulation (Buznikov 1984; Laasberg 1990; Schmidt 1981), glial cell proliferation (Lauder and Schambra 1999; Weiss et al. 1998), and neuronal differentiation and survival in the developing central nervous system (Lipton and Kater 1989; Nguyen et al. 2001). Furthermore, acetylcholine levels are high in the neonatal rat brain (80–90% of adult-values), and muscarinic receptor-activated signal transduction systems [notably phospholipase C and D (PLD)] are greatly enhanced in the neonatal rat brain compared to adults, despite a lower receptor density (Balduini et al. 1987). Developing neurons may fire action potentials and trigger acetylcholine secretion from the axonal growth cone while the axon is still growing; neuron-released acetylcholine contributes to further axonal growth and formation of synaptic contacts (Xie and Poo 1986; Yao et al. 2000). Astrocytes may be another possible source of acetylcholine in the developing brain, as it has been shown that, in culture, astrocytes indeed release acetylcholine (Wessler et al. 1997).
We have recently reported that the stimulation of muscarinic receptors in astrocytes induces neuritogenesis in hippocampal neurons (Guizzetti et al. 2008). This effect is due to the stimulation of M3 muscarinic receptors and is mediated by the release, by astrocytes, of fibronectin and laminin, two extracellular matrix proteins involved in neuronal development, and of plasminogen activator inhibitor 1 (PAI-1), a component of the plasminogen activator system which inhibits the formation of plasmin, a potent proteolytic enzyme involved in the degradation of several extracellular matrix proteins, including fibronectin and laminin (Irigoyen et al. 1999).
Our previous work characterized the signaling pathways activated by M3 muscarinic receptors in astrocytes (Costa et al. 2004). Furthermore, we found that muscarinic signaling is strongly inhibited by relevant concentrations (25–100 mM) of ethanol; ethanol interferes with phospholipase D (PLD) signaling, leading to inhibition in the production of the PLD product phosphatidic acid (PA), and down-stream activation of PKC ζ, p70S6 kinase and NF-κB (Costa et al. 2004).
As ethanol potently inhibits muscarinic receptor signaling in astrocytes, we hypothesized that it may also inhibit carbachol-stimulated astrocyte-induced neuritogenesis in hippocampal pyramidal neurons by inhibiting the release of fibronectin, laminin, and PAI-1, and that this effect may be mediated by the inhibition of PLD signaling in astrocytes. This hypothesis was confirmed by our finding presented in this paper.
Inhibition of neuronal development by ethanol through the inhibition of the release of neuritogenic factors by astrocytes represents a novel mechanism that may contribute to the deleterious effects of ethanol in the developing brain.
Materials and methods
Materials
Transmembrane inserts and tissue cultures vessels were from Corning (Acton, MA) or BD Bioscience (Bedford, MA); βIII-tubulin antibody was from Chemicon International (Tamecula, CA). The ELISA kit for the determination of PAI-1 in the culture medium was purchased from DiaPharma (West Chester, OH). Glass coverslips were from Fisher Scientific (Federal Way, WA). Alexa fluor-594 and Alexa fluor-555 secondary antibodies, Hoechst 33342, tissue culture media, serum, and B27 supplements were from Invitrogen (Carlsbad, CA), while kits for ethanol determination were purchased from r-Biopharm (Marshall, MI). All other chemicals and antibodies were from Sigma Chemical Co. (St. Louis, MO). Time-pregnant Sprague-Dawley rats were purchased from Charles River (Wilmington, MA).
Hippocampal neuron cultures
Hippocampal neurons from rat E21 fetuses were prepared as previously described (Guizzetti et al. 2008). For quantitative morphological analysis of neurite outgrowth, neurons (1×104 cells/coverslip) were plated in glass coverslips, coated overnight with 100 μg/ml poly-D-lysine to support neuron attachment, to which 3–4 beads of paraffin were previously affixed. After neuron attachment, the glass coverslips were inverted in 24-well plates above the astrocyte monolayer (the paraffin drops preventing neuron-astrocyte contact), as previously described (Guizzetti et al. 2008).
Astrocyte cultures
Cortical astrocyte cultures from rat E21 fetuses were prepared as previously described (Guizzetti et al. 1996). After nine days in culture, astrocytes were plated in 24 well plates for astrocyte-neuron co-culture experiments and for ELISA assays (2.5×105cells/well), on glass coverslips (2.5×105 cells/coverslip) for immunocytochemistry experiments, or in 100 mm dishes for Western blot analysis (2×106 cells/dish); 4 days later, cells were serum-deprived for 24 h followed by treatments.
Treatments of astrocyte/neuron co-cultures
Astrocytes were treated with carbachol, ethanol, and/or butanols for 24 hours. After treatment washed out, neurons were added to the astrocyte monolayer for an additional 24 h. Each plate was treated with only one concentration of ethanol, and cells treated with different alcohols or without alcohols (controls) were plated in separate plates as alcohol redistributes uniformly in all the wells within the same plate over the course of the incubation. To minimize alcohol evaporation from 24-well plates, water containing the same alcohol concentration present in the treatments was added in the inter-well spaces and to all empty wells, and Parafilm was wrapped around the two long sides of the plates closing part of the space between the lid and the dish without stopping the air exchange. We found that in these conditions ethanol loss does not exceed 15%, cells do not suffer from lack of oxygen, and no pH changes occur. In our hands, this method of ethanol exposure is comparable to the use of sealed chambers (also in use in our laboratory) for treatments up to 24 h (Guizzetti and Costa 1996; 2002; VanDeMark et al. 2009). Ethanol concentrations at the beginning and the end of the incubation were determined in some experiments using a commercially available kit.
Assay for neurite protein quantification
Neurite extension was assessed spectrophotometrically following a previously described method (Guizzetti et al. 2008). Hippocampal neurons were plated on inserts with a 3 μm pore membrane at their base fitting the wells of 24-well plates (2.5×105 cells/coverslip). The inserts were coated with poly-D-lysine and placed into wells containing a monolayer of astrocytes. During the following 24 h incubation, neurons extended their neurites through the microporous membrane to the lower chamber. At the end of the incubation, membrane inserts were then removed, neurons were fixed in methanol and neurites were stained with a cresyl violet solution (0.09%). Cell bodies were removed from the top of the membrane using a cotton swab, and the dye was extracted from the neurites in the underside of the porous inserts and quantified at 562 nm on a spectrophotometer using a SPECTRAmax® PLUS microplate spectrophotometer. Because cell bodies are separated from neurites by the porous membrane, they are easily removed from the top of the membrane while leaving undisturbed the neurites on the lower side of the membrane.
Quantitative morphological analysis of neurite outgrowth
At the end of each incubation, neurons plated in coverslips were fixed in 4% formaldehyde, permeabilized in 0.2% Triton X-100, labeled with an anti-βIII-tubulin antibody followed by a Alexa fluor-555 secondary antibody, and mounted on microscope slides. The analysis was carried out blind. Neurons whose processes were intermingled with those of neighboring cells were excluded from the analysis. Neurite length was measured from the point of emergence at the cell body to the tip of each segment. In each experiment, three coverslips per treatment were analyzed. Only pyramidal neurons (i.e. neurons with an oval or pyramidal cell body and a multipolar morphology) were selected for analysis. Images were obtained with a NIKON Labphot 2A microscope and projected to a Spot RT Slider cooled CCD digital camera. Quantification of the length of the neurites was carried out using a MetaMorph 6.1 analysis software as previously reported (Guizzetti et al. 2008). The neurite had to meet the following requirements: it must emerge from an isolated cell (not a clump of cells), it must not contact other cells or neurites, and it must be at least as long as the diameter of the cell body.
Astrocyte Immunocytochemistry
At the end of each incubation, astrocytes, plated on glass coverslips, were fixed in 4% paraformaldheyde for 3 min without permeabilization, for immunostaining of extracellular membrane proteins. After blocking non-specific binding sites with 10% goat serum, astrocytes were incubated with anti-fibronectin or anti-laminin-1 antibodies followed by the Alexa-Fluor 594 secondary antibody. Nuclei were stained with Hoechst 33342 (5 μg/ml) for 5 min at room temperature. Coverslips were then mounted on microscope slides and analyzed with a Zeiss Meta confocal microscope (Keck Imaging Center, University of Washington). Images from 21 plans 0.5 μm thick were acquired per each field; the total stack size was 11 μm. Fluorescence intensities were quantified using a MetaMorph software as the sum of the integrated intensities for each plane, as previously reported (Guizzetti et al. 2008).
ELISA
Astrocytes plated in 24 well plates (2×105 cells/well) were treated in triplicate; the medium from the three wells was then pooled and concentrated 10× using iCON centrifuge filter (Pierce; 9kDa cut-off). The amount of PAI-1 in the concentrated medium was determined by ELISA using a kit purchased from DiaPharma (West Chester, OH) following the directions of the manufacturer, as previously described (Guizzetti et al. 2008).
Western Blot Analysis
Western blot analysis of astrocyte-conditioned medium and cell lysate after treatments was carried out as previously described (Guizzetti et al. 2008).
Statistical Analysis
Each experiment was carried out at least three times. In the morphometric analyses at least twenty cells per treatment were analyzed in each experiment and results from 2 or 3 experiments were pooled. At least 60 cells per treatment were measured (n ≥ 60). All statistical tests were carried out using KaleidaGraph 4.0. Unless otherwise stated, for comparing multiple treatments to controls, one–way ANOVA followed by Dunnett's test was used to determine significant differences. Values are expressed as mean ± S.E.M.
Results
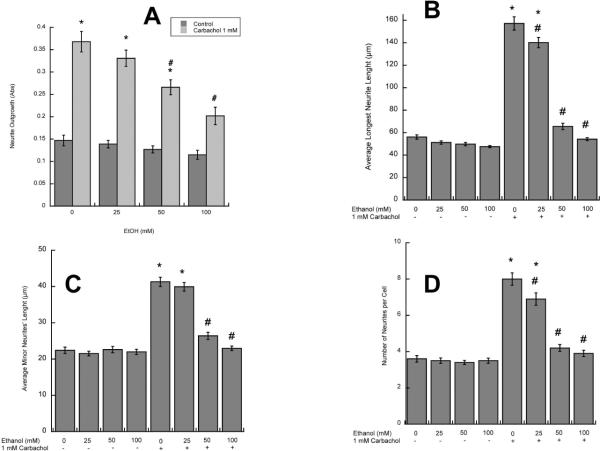
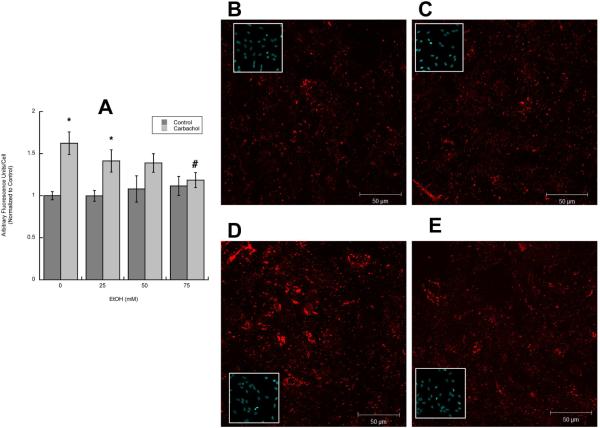
We have previously reported that stimulation of muscarinic receptors in astrocytes induces neurite outgrowth in hippocampal pyramidal neurons (Guizzetti et al. 2008). As ethanol inhibits muscarinic receptor signaling in astrocytes (Costa et al. 2004), we hypothesized that this may result in inhibition of neurite outgrowth induced by carbachol-stimulated astrocytes. To verify this hypothesis, rat cortical astrocytes were treated with the cholinergic agonist carbachol in the presence and in the absence of 25–100 mM ethanol for 24h, followed by treatment washout. Fetal hippocampal neurons were plated in porous transwell cell culture inserts and placed into the 24-well plates containing untreated or carbachol- and/or ethanol-pre-treated astrocytes for 24 h. Proteins from the neurites growing on the underside of the porous membrane were quantified spectrophotometrically as previously described (Guizzetti et al. 2008). The co-incubation of astrocytes with carbachol and ethanol resulted in a concentration-dependent inhibition of neuritogenesis induced by carbachol-stimulated astrocytes (Fig. 1 A).
Figure 1. Effect of ethanol on carbachol-treated astrocyte-induced hippocampal neuron neurite outgrowth.
A: Hippocampal neurons were incubated in the presence of astrocytes previously treated with 1 mM carbachol and/or 25, 50, or 100 mM ethanol. (A): Neurite proteins were quantified spectrophotometrically as described in Methods (n=6). (B, C, D): Morphometric analysis was carried out in hippocampal neurons immunostained with a neuron-specific βIII-tubulin antibody. Pictures were taken with a digital camera attached to a fluorescence microscope. Quantifications of the length of the longest neurite (A) and the length of minor neurites (B) were carried out using the software MetaMorph. C: number of processes per cell. The results (mean +/− S.E.) derive from the measurements of 60–80 cells per treatment. Representative fields of hippocampal neurons co-cultured with control (D), carbachol-treated (E) or carbachol- and 50 mM ethanol-treated (F) astrocytes are also shown.
*: p<0.05 vs control; #: p<0.05 vs carbachol by the Dunnett post-hoc test.
Since the described method does not provide information about neuronal morphology, further morphometric studies were carried out, to characterize the effect of carbachol- and ethanol-treated astrocytes on neurite outgrowth (see Methods). The length of the longest neurite (the axon or the extension that is going to develop into an axon) and the length of minor neuritis were measured in βIII-tubulin-immunolabeled neurons using Metamorph analysis software. We have previously reported that carbachol-treated astrocytes induce a three-fold increase in the length of the longest neurite, and a two-fold increase in minor neurite length (Guizzetti et al. 2008). Ethanol, starting at 25 mM, inhibited carbachol-treated astrocyte-induced longest neurite and minor neurites' length (Fig. 1 B, C). Carbachol also increased the number of neurites per cell, and ethanol inhibited also this effect in a concentration-dependent manner (Fig. 1 D). Fig. 1 E, F, G shows representative neurons exposed to control, carbachol-, and carbachol plus 50 mM ethanol-treated astrocytes, respectively.
Neurite outgrowth in CNS neurons is primarily dependent on extracellular matrix proteins that are produced and released by glial cells. Two of these proteins, fibronectin and laminin, play a pivotal role in neuronal differentiation mediated by astrocytes (Martinez and Gomes 2002; Tom et al. 2004). We have previously shown that carbachol stimulation increases the levels and release of these extracellular matrix proteins in astrocytes, and that the upregulation of fibronectin and, to a lesser extent laminin, is involved in the neuritogenic effect of carbachol-stimulated astrocytes (Guizzetti et al. 2008).
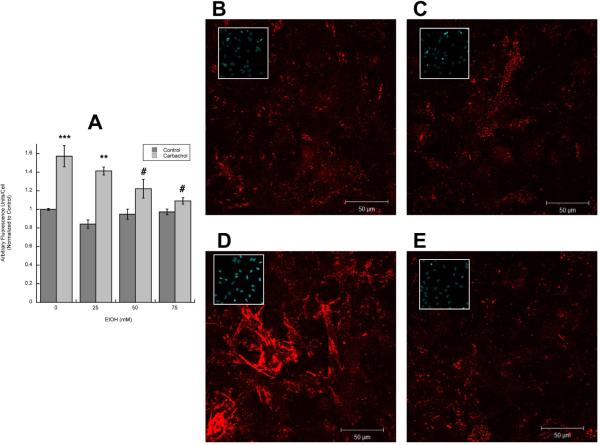
To determine whether ethanol inhibits fibronectin and laminin release from astrocytes, after treatment with 1 mM carbachol and/or ethanol (25–75 mM) for 24 h, astrocytes were fixed, but not permeabilized, and fibronectin and laminin-1 in the extracellular matrix were immunolabeled and visualized using fluorescence confocal microscopy. Fields containing a similar number of cells were quantified for fibronectin and laminin fluorescence and normalized for cell number. Ethanol alone did not affect the basal levels of laminin or fibronectin, while it inhibited the effect of carbachol on the release of both proteins in a concentration-dependent manner (Figs. 2 A, 3 A). Representative fields of extracellular fibronectin and laminin levels in control, ethanol-, carbachol-, and carbachol plus ethanol-treated astrocytes are shown in Figs. 2 B, C, D, E and 3 B, C, D, E respectively. Inset pictures show the cell nuclei (stained by cell-permeable Hoechst) in each field.
Figure 2. Effect of ethanol on carbachol-induced increase in extracellular fibronectin.
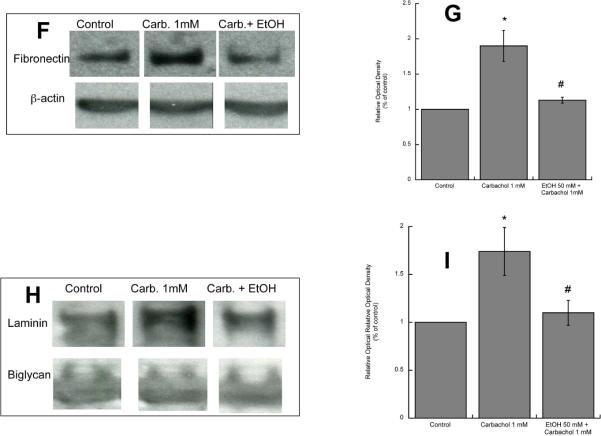
A: Astrocytes were stimulated for 24 h with 1 mM carbachol alone or in the presence of 25, 50, or 75 mM ethanol. After immunolabeling with a fibronectin antibody, cells were analyzed by confocal microscopy. Average fibronectin immunofluorescence per cell was quantified as described in “Methods”. *: p<0.05, vs. control; #: p<0.05 vs. carbachol by the Dunnett post hoc test (n=6). Representative fields of astrocytes treated with serum-free medium (Control; B); 50 mM ethanol (C); 1 mM carbachol (D); and 1 mM carbachol in the presence of 50 mM ethanol (E) are shown.
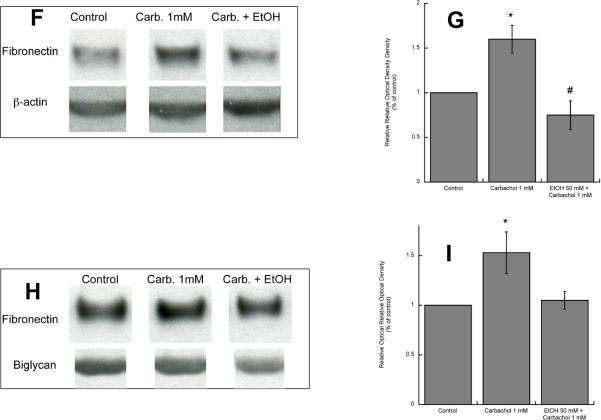
(F, G,): Western blot analysis was carried out in the cell lysate of astrocytes treated for 24 h with 1 mM carbachol in the presence or absence of 50 mM ethanol. After protein transfer, membranes were labeled with a fibronectin (F, upper blot) or β-actin (F, lower blot) antibody. (G): Densitometric analysis of cellular levels of fibronectin normalized to β-actin is shown. (H, I): Proteins present in astrocyte-conditioned medium were separated by electrophoresis, transferred to PVDF membranes, and labeled with a fibronectin (H, upper blot) of byglican (H, lower blot) antibody and detected by Western blot. (I): Densitometric analysis of fibronectin in astrocyte-conditioned medium normalized to byglican is shown. *, p<0.05 vs. control; #, p<0.05 vs. carbachol (n=3).
Figure 3. Effect of ethanol on carbachol-induced increase in extracellular laminin.
A: Astrocytes were stimulated for 24 h with 1 mM carbachol alone or in the presence of 25, 50, or 75 mM ethanol. After immunolabeling with a laminin-1 antibody, cells were analyzed by confocal microscopy. Average laminin immunofluorescence per cell was quantified as described in “Methods”. *: p<0.05, vs. control; #: p<0.05 vs. carbachol by the Dunnett post hoc test (n=6). Representative fields of astrocytes treated with serum-free medium (Control; B); 50 mM ethanol (C); 1 mM carbachol (D); and 1 mM carbachol in the presence of 50 mM ethanol (E) are shown. (F, G,): Western blot analysis was carried out in the cell lysate of astrocytes treated for 24 h with 1 mM carbachol in the presence or absence of 50 mM ethanol. After protein transfer, membranes were labeled with a laminin (F, upper blot) or β-actin (F, lower blot) antibody. (G): Densitometric analysis of cellular levels of laminin normalized to β-actin is shown. (H, I): Proteins present in astrocyte-conditioned medium were separated by electrophoresis, transferred to PVDF membranes, and labeled with a laminin (H, upper blot) of byglican (H, lower blot) antibody and detected by Western blot. (I): Densitometric analysis of laminin in astrocyte-conditioned medium normalized to byglican is shown. *, p<0.05 vs. control; #, p<0.05 vs. carbachol (n=3).
We also found that ethanol inhibited carbachol-induced increase in fibronectin and laminin levels and release by Western blot analysis of astrocyte lysate and astrocyte-condioned medium (Figs. 2 F, G, H, I; 3 F, G, H, I). The levels of fibronectin and laminin-1 present in astrocyte-conditioned medium were normalized for biglycan, a proteoglycan whose release by astrocytes is not affected by carbachol and ethanol treatments (Guizzetti et al. 2008).
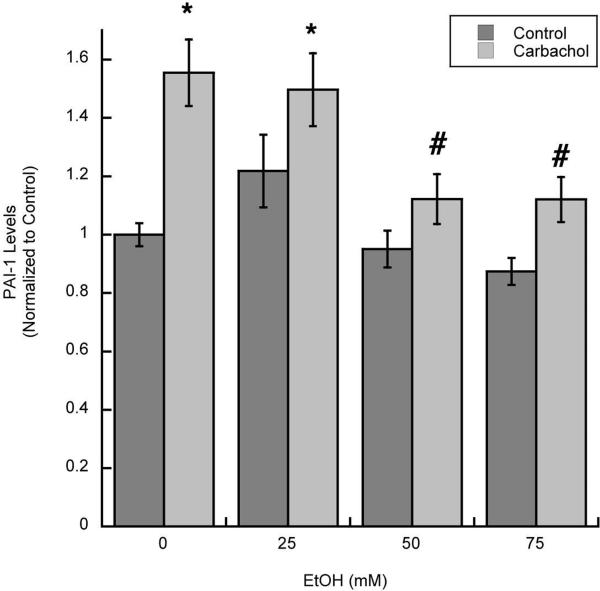
We have also previously shown that the upregulation of fibronectin and laminin by carbachol is in part mediated by the inhibition of their degradation. Indeed, the release by astrocytes of Plasminogen Activator Inhibitor-1 (PAI-1), which inhibits the generation of the proteolytic enzyme plasmin (Irigoyen et al. 1999), was increased by carbachol treatment (Guizzetti et al. 2008). We found that ethanol (25–75 mM) also inhibited the release of PAI-1 from astrocytes induced by carbachol (Fig. 4).
Figure 4. Effect of ethanol on the release of PAI-1 from carbachol-treated astrocytes.
Media from astrocytes stimulated with 1 mM carbachol alone or in the presence of 25, 50, or 75 mM ethanol were collected and analyzed for PAI-1 levels by ELISA. *: p<0.05 vs. control; #: p<0.05 vs. carbachol by the Dunnett post hoc test; n=6.
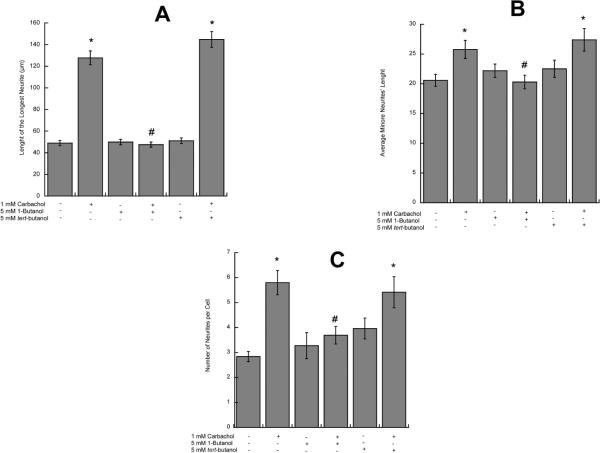
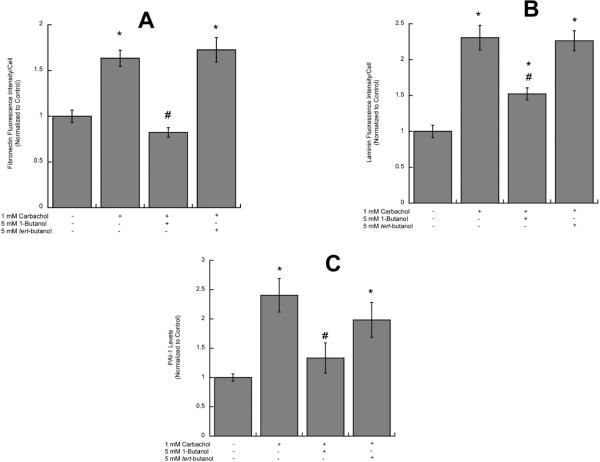
Carbachol activates PLD in astrocytes and ethanol, a competitive substrate for PLD, inhibits the formation of PA, the physiological product of PLD (Guizzetti et al. 2004). The inhibition of PLD signaling is not unique to ethanol, as other short-chains primary alcohols (namely 1-butanol and 1-propanol), but not their secondary or tertiary analogues, are also competitive substrates for PLD. We tested the effect of 1-butanol and its tertiary analogue tert-butanol on carbachol-induced neurite outgrowth, and laminin, fibronectin and PAI-1 upregulation. 1-Butanol, but not tert-butanol, inhibited longest and minor neurite outgrowth and the increase in the number of processes per neuron induced by carbachol-stimulated astrocytes (Fig. 5 A, B, C). In addition, 1-butanol, but not tert-butanol, also inhibited carbachol-induced fibronectin (Fig. 6 A), laminin (Fig. 6 B) and PAI-1 (Fig. 6 C) release, suggesting that ethanol-induced inhibition of PLD signaling may be the mechanism involved in the inhibition of carbachol-induced neuritogenesis. To further test the hypothesis that ethanol, by inhibiting PLD-mediated PA formation, inhibits the release of neuritogenic factors from astrocytes, we investigated the effect of exogenous PA (200 μM) and ethanol on laminin levels in astrocytes by Western blot analysis. Indeed, PA upregulated laminin levels 2.21 (±0.25) folds above control; ethanol (75 mM) alone did not affect laminin levels (1.077 ± 0.28 of the control levels); when ethanol and PA were co-incubated, ethanol did not inhibit the effect of PA (3.1 folds above control ± 0.63), supporting the hypothesis that ethanol's primary target is upstream of PA.
Figure 5. Effect of 1-butanol and tert-butanol on carbachol-treated astrocytes-induced hippocampal neuron neuritogenesis.
Hippocampal neurons were incubated in the presence of astrocytes previously treated with 1 mM carbachol, 1-butanol (5 mM), and/or tert-butanol (5 mM) for 24 h. Morphometric analysis was carried out in neurons fixed and immunostained with a neuron-specific βIII-tubulin antibody. Pictures were taken with a digital camera attached to a fluorescence microscope. Quantifications of the length of the longest neurite (A) and the length of minor neurites (B) were carried out using the software MetaMorph. C: number of processes per cell. The results (mean +/− S.E.) derive from the measurements of 50–70 cells per treatment. *: p<0.05 vs control; #: p<0.05 vs carbachol by the Dunnett post-hoc test.
Figure 6. Effect of 1-butanol and tert-butanol on carbachol-induced increase in extracellular fibronectin, laminin and PAI-1.
Astrocytes were stimulated for 24 h with 1 mM carbachol alone or in the presence of 1-butanol (5 mM) or tert-butanol (5 mM). After 24 h, astrocytes were fixed and immunolabeled with a fibronectin (A) or a laminin (B) antibodies followed by a fluorescent secondary antibody and analyzed by confocal microscopy. A: Average fibronectin immunofluorescence per cell. B: Average laminin immunofluorescence per cell. C: Media from astrocytes stimulated with 1 mM carbachol alone or in the presence of 1-butanol (5 mM) or tert-butanol (5 mM) were collected and analyzed for PAI-1 levels by ELISA. *: p<0.05, vs. control; #: p<0.05 vs. carbachol by the Dunnett post hoc test (n=6).
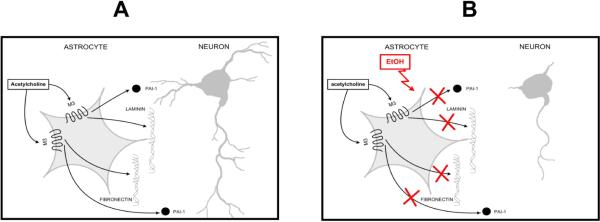
Fig. 7 summarizes our findings; we have previously shown that acetylcholine, which is present in the developing brain before synapses are formed, stimulates M3 muscarinic receptors in astrocytes and triggers the release of fibronectin, laminin, and PAI-1, which accelerate neuronal development (Fig. 7 A) (Guizzetti et al. 2008). Ethanol, by inhibiting muscarinic signaling, prevents and astrocyte-mediated neuritogenesis (Fig. 7 B).
Figure 7. Proposed model for astrocyte-neuron interaction and its inhibition by ethanol.
Acetylcholine, present in the developing brain, stimulates M3 muscrinic receptors in astrocytes causing the release of factors that trigger neuritogenesis (A). Ethanol, by inhibiting muscarinic signaling, inhibits the release of factors from astrocytes and, consequently, prevents neurite outgrowth (B).
Discussion
Maternal alcohol consumption during pregnancy can produce a variety of central nervous system abnormalities resulting in a broad spectrum of cognitive and behavioral impairments that constitute the most severe and long-lasting effects observed in FASD (Kelly et al. 2000; Mattson and Riley 1998; Streissguth et al. 1980).
A plethora of studies aimed at exploring the mechanisms by which ethanol alters brain development have focused on neuronal susceptibility. While it is generally accepted that alcohol exposure affects neuronal development in vivo, a review of the literature reveals that contradictory effects of ethanol have been reported, with some studies indicating that ethanol increases the elongation of neuritis, and other showing an inhibitory effect of alcohol. For instance, an increase in axonal growth in the corpus callosum (an area consisting mostly of axonal projections) was observed in non-human primates (Miller et al. 1999). In contrast, other in vivo animal models have shown decreased callosal size with prenatal ethanol exposure (Moreland et al. 2002), similarly to what has been observed clinically in Fetal Alcohol Syndrome (FAS) children (Coulter et al. 1993; Riley et al. 1995; Sowell et al. 2001).
The effect of ethanol on neurite outgrowth has been investigated also in several in vitro systems and again, both inhibitory and stimulatory effects have been reported (Bearer et al. 1999; Bingham et al. 2004; Messing et al. 1991; Saunders et al. 1995). It is likely that the conflicting results reported in different studies reflect the heterogeneity of brain regions, as suggested by the observation that ethanol inhibits neurite outgrowth stimulated by L1CAM in cerebellar granule neurons but not in cortical neurons (Bearer et al. 1999; Hoffman et al. 2008). Furthermore, the developing brain undergoes continuous and rapid changes; therefore, the timing of ethanol exposure plays a pivotal role in the type of response ethanol may trigger.
Recently, increasing attention has been devoted to the susceptibility of glial cells to ethanol (Guerri et al. 2001). Several studies have shown that ethanol, both in vivo and in vitro, can significantly affect glial cells. Abnormalities in glial cell development are indeed suspected of contributing to the adverse effects of ethanol on the developing brain. Evidence exists of abnormal glial migration in humans with FAS, as well as in primates and rats exposed to ethanol during development (Miller and Robertson 1993). A reduction in glial cell number has been reported in rat models of FAS (Miller and Potempa 1990; Perez-Torrero et al. 1997). Hypoplasia of the corpus callosum and anterior commissure in FAS may indeed be ascribed to an effect of ethanol on glia, as neuroglial cells are involved in the formation of these two areas (Riley et al. 1995). The finding that microencephaly is strongly associated with ethanol exposure during the brain growth spurt (Samson 1986), a period characterized by rapid glial cell proliferation and maturation, also suggests a potential effect of ethanol on the proliferation, growth and maturation of glia.
The question of how ethanol, by affecting glial cells, may affect neuronal development has only recently been raised. Two recent studies have investigated the effect of ethanol on astrocyte-or astrocyte-released factor-induced neuritogenesis, and have reported that neuritogenesis is inhibited in cortical neurons grown in the presence of astrocytes prepared from rats prenatally exposed to ethanol (Pascual and Guerri 2007), and that ethanol inhibits axonal growth of cerebellar neurons induced by the active fragment of the astrocyte-released activity-dependent neuroprotective protein (Chen and Charness 2008).
We and others have been pursuing the hypothesis that acetylcholine, through the activation of muscarinic receptors, may play an important role in brain development, and particularly in neuronal maturation (Costa 1993; Lauder and Schambra 1999). We have recently identified a novel mechanism of glia-neuron communication involved in brain development: activation of muscarinic receptors in astrocytes induces neuritogenesis in neurons through the release of extracellular matrix proteins and modulators of protease activity (Guizzetti et al. 2008; Moore et al. 2009). A role for acetylcholine during development is suggested by the presence of choline acetyltransferase and acetylcholine receptors long before synaptogenesis (Armstrong et al. 1987; Daubas et al. 1990; Schambra et al. 1989; Schlumpf et al. 1991). The direct release of acetylcholine from growing axons during brain development was demonstrated in Xenopus laevis and Drosphila melanogaster (Sun and Poo 1987; Xie and Poo 1986; Yao et al. 2000); acetylcholine can be released by the same astrocytes in vitro (Wessler et al. 1997); furthermore, cultured rat motoneurons synthesize and release acetylcholine even in the absence of a synaptic contact with a muscular target (Limozin et al. 1997). We therefore hypothesized that autocrine and paracrine acetylcholine may signal astrocytes to secrete factors promoting further neuronal development.
Previous research in our laboratory had shown that the signaling activated by muscarinic receptors in astrocytes is inhibited by ethanol (Costa et al. 2004); the hypothesis investigated in this study was thus that ethanol may inhibit neuronal development by interfering with muscarinic receptor signaling in astrocytes.
Ethanol was found to inhibit the effect of carbachol-stimulated astrocytes on hippocampal neuron neurite outgrowth in a concentration-dependent manner. Similar effects were also observed after the incubation of astrocytes with carbachol in the presence of 1-butanol, but not tert-butanol, suggesting that inhibition of muscarinic signaling by ethanol at the level of PLD is responsible for ethanol-induced inhibition of astrocyte-mediated neuritogenesis. Indeed, ethanol and other short-chain primary alcohols, such as 1-butanol, are known to compete with water as substrates for PLD, whereby PA production is inhibited and phosphatidylethanol or phosphatidyl 1-butanol are formed instead by a transphosphatidylation reaction; in contrast, secondary or tertiary alcohols (such as tert-butanol) are poor substrates for PLD (Gustavsson 1995; Seidler et al. 1996). We had previously shown that in an astrocytoma cell line, ethanol and 1-butanol reduce the formation of PA and increase the formation of phosphatidylethanol and phosphatidyl 1-butanol, respectively, at concentrations similar to those used in this study (Guizzetti et al. 2004). The incubation of astrocytes with exogenous PA increased laminin levels and this effect was not inhibited by ethanol, further confirming that ethanol may inhibit the formation of PA stimulated by muscarinic receptors, and that this effect may be involved in the inhibition of the secretion of neuritogenic factors.
We have previously reported that the stimulation of neuritogenesis by carbachol-stimulated astrocytes is mediated, at least in part, by the release by astrocytes of factors involved in neuronal development, namely fibronectin, laminin, and PAI-1 (Guizzetti et al. 2008). Ethanol, as well as 1-butanol, but not tert-butanol, inhibited the extracellular levels of fibronectin, laminin, and PAI-1; this is consistent with our hypothesis that ethanol, by inhibiting PLD signaling, inhibits the upregulation of astrocyte-secreted proteins involved in neuronal development.
The alcohol concentrations used in this study (25, 50, 75, and 100 mM) corresponding to 0.115, 0.23, 0.35, and 0.46 g/dl, respectively, are found in the blood of individuals after moderate to high ethanol intake, and are in the range of concentrations recommended for in vitro studies (Deitrich and Harris 1996).
Altogether, our previous results suggested that cholinergic stimulation of astrocytes enacts a cascade of events that culminates with the release of factors, including laminin, fibronectin and PAI-1, that contribute to the creation of an extracellular environment favorable to neuronal development, leading to neurite outgrowth and axonal differentiation in hippocampal neurons (Guizzetti et al. 2008) (Fig. 7 A). By inhibiting muscarinic signaling in astrocytes, ethanol prevents the release of these factors, and thus prevents neuronal differentiation and neurite elongation (Fig. 7 B). This newly identified mechanism may be involved in some of the neurodevelopmental effects that constitute the most severe and long-lasting consequences of in utero alcohol exposure. The involvement of the cholinergic system and of PLD in this effect may also provide a mechanistic explanation for the observation that the administration of choline (a precursor of acetylcholine and phosphatidylcholine) appears to improve some of the learning deficits associated with in utero alcohol exposure (Ryan et al. 2008).
Acknowledgements
This work was supported in part by grants AA-08154, ES-07032 and ES-07033 from the National Institutes of Health. We thank Ms. Khoi Dao for her assistance with some of the experiments, and Mr. Jeff Frkonja for the graphic design of Fig. 7.
References
- Armstrong DM, Bruce G, Hersh LB, Gage FH. Development of cholinergic neurons in the septal/diagonal band complex of the rat. Brain Res. 1987;433(2):249–56. doi: 10.1016/0165-3806(87)90028-9. [DOI] [PubMed] [Google Scholar]
- Balduini W, Murphy SD, Costa LG. Developmental changes in muscarinic receptor-stimulated phosphoinositide metabolism in rat brain. J Pharmacol Exp Ther. 1987;241(2):421–7. [PubMed] [Google Scholar]
- Bearer CF, Swick AR, O'Riordan MA, Cheng G. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. J Biol Chem. 1999;274(19):13264–70. doi: 10.1074/jbc.274.19.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10(1):94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Floyd LL, Weber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR Recomm Rep. 2005;54(RR-11):1–14. [PubMed] [Google Scholar]
- Bingham SM, Mudd LM, Lopez TF, Montague JR. Effects of ethanol on cultured embryonic neurons from the cerebral cortex of the rat. Alcohol. 2004;32(2):129–35. doi: 10.1016/j.alcohol.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Booth GE, Kinrade EF, Hidalgo A. Glia maintain follower neuron survival during Drosophila CNS development. Development. 2000;127(2):237–44. doi: 10.1242/dev.127.2.237. [DOI] [PubMed] [Google Scholar]
- Buznikov GA. The action of neurotransmitters and related substances on early embryogenesis. Pharmacol Ther. 1984;25(1):23–59. doi: 10.1016/0163-7258(84)90023-8. [DOI] [PubMed] [Google Scholar]
- Chen S, Charness ME. Ethanol inhibits neuronal differentiation by disrupting activity-dependent neuroprotective protein signaling. Proc Natl Acad Sci U S A. 2008;105(50):19962–7. doi: 10.1073/pnas.0807758105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotard C, Salecker I. Neurons and glia: team players in axon guidance. Trends Neurosci. 2004;27(11):655–61. doi: 10.1016/j.tins.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Costa LG. Muscarinic receptors and the developing nervous system. In: Zagon I, McLaughlin P, editors. Receptors in the developing nervous system. Chapman and Hall; London: 1993. pp. 21–42. [Google Scholar]
- Costa LG, Vitalone A, Guizzetti M. Signal transduction mechanisms involved in the antiproliferative effects of ethanol in glial cells. Toxicol Lett. 2004;149(1–3):67–73. doi: 10.1016/j.toxlet.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Leech RW, Schaefer GB, Scheithauer BW, Brumback RA. Midline cerebral dysgenesis, dysfunction of the hypothalamic-pituitary axis, and fetal alcohol effects. Arch Neurol. 1993;50(7):771–5. doi: 10.1001/archneur.1993.00540070083022. [DOI] [PubMed] [Google Scholar]
- Daubas P, Devillers-Thiery A, Geoffroy B, Martinez S, Bessis A, Changeux JP. Differential expression of the neuronal acetylcholine receptor alpha 2 subunit gene during chick brain development. Neuron. 1990;5(1):49–60. doi: 10.1016/0896-6273(90)90032-b. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Harris RA. How much alcohol should I use in my experiments? Alcohol Clin Exp Res. 1996;20(1):1–2. doi: 10.1111/j.1530-0277.1996.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Guerri C, Pascual M, Renau-Piqueras J. Glia and fetal alcohol syndrome. Neurotoxicology. 2001;22(5):593–9. doi: 10.1016/s0161-813x(01)00037-7. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Inhibition of muscarinic receptor-stimulated glial cell proliferation by ethanol. J Neurochem. 1996;67:2236–2245. doi: 10.1046/j.1471-4159.1996.67062236.x. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa LG. Effect of ethanol on protein kinase C ζ and p70S6 kinase activation by carbachol: a possible mechanism for ethanol-induced inhibition of glial cell proliferation. J Neurochem. 2002;82(1):38–46. doi: 10.1046/j.1471-4159.2002.00942.x. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur J Pharmacol. 1996;297:265–273. doi: 10.1016/0014-2999(95)00746-6. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Moore NH, Giordano G, Costa LG. Modulation of neuritogenesis by astrocyte muscarinic receptors. J Biol Chem. 2008;283(46):31884–97. doi: 10.1074/jbc.M801316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizzetti M, Thompson BD, Kim Y, VanDeMark K, Costa LG. Role of phospholipase D signaling in ethanol-induced inhibition of carbachol-stimulated DNA synthesis of 1321N1 astrocytoma cells. J Neurochem. 2004;90(3):646–53. doi: 10.1111/j.1471-4159.2004.02541.x. [DOI] [PubMed] [Google Scholar]
- Gustavsson L. ESBRA 1994 Award Lecture. Phosphatidylethanol formation: specific effects of ethanol mediated via phospholipase D. Alcohol Alcohol. 1995;30(4):391–406. [PubMed] [Google Scholar]
- Hoffman EJ, Mintz CD, Wang S, McNickle DG, Salton SR, Benson DL. Effects of ethanol on axon outgrowth and branching in developing rat cortical neurons. Neuroscience. 2008;157(3):556–65. doi: 10.1016/j.neuroscience.2008.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irigoyen JP, Munoz-Canoves P, Montero L, Koziczak M, Nagamine Y. The plasminogen activator system: biology and regulation. Cell Mol Life Sci. 1999;56(1–2):104–32. doi: 10.1007/PL00000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. The fetal alcohol syndrome. Teratology. 1975;12(1):1–10. doi: 10.1002/tera.1420120102. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22(2):143–9. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryushko D, Berezin V, Bock E. Regulators of neurite outgrowth: role of cell adhesion molecules. Ann N Y Acad Sci. 2004;1014:140–54. doi: 10.1196/annals.1294.015. [DOI] [PubMed] [Google Scholar]
- Laasberg T. Ca2(+)-mobilizing receptors of gastrulating chick embryo. Comp Biochem Physiol C. 1990;97(1):9–12. doi: 10.1016/0742-8413(90)90164-5. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Schambra UB. Morphogenetic roles of acetylcholine. Environ Health Perspect. 1999;107(Suppl 1):65–9. doi: 10.1289/ehp.99107s165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limozin L, Pelce P, Savtchenko L, Chamoin MC, Ternaux JP. Predicted acetylcholine layer around embryonic motoneurones. Neuroreport. 1997;8(13):2957–60. doi: 10.1097/00001756-199709080-00030. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Kater SB. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival. Trends Neurosci. 1989;12(7):265–70. doi: 10.1016/0166-2236(89)90026-x. [DOI] [PubMed] [Google Scholar]
- Martinez R, Gomes FC. Neuritogenesis induced by thyroid hormone-treated astrocytes is mediated by epidermal growth factor/mitogen-activated protein kinase-phosphatidylinositol 3-kinase pathways and involves modulation of extracellular matrix proteins. J Biol Chem. 2002;277(51):49311–8. doi: 10.1074/jbc.M209284200. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22(2):279–94. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25(3):185–91. [PMC free article] [PubMed] [Google Scholar]
- Messing RO, Henteleff M, Park JJ. Ethanol enhances growth factor-induced neurite formation in PC12 cells. Brain Res. 1991;565(2):301–11. doi: 10.1016/0006-8993(91)91662-k. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effects of prenatal exposure to ethanol on callosal projection neurons in rat somatosensory cortex. Brain Res. 1997;766(1–2):121–8. doi: 10.1016/s0006-8993(97)00533-7. [DOI] [PubMed] [Google Scholar]
- Miller MW, al-Rabiai S. Effects of prenatal exposure to ethanol on the number of axons in the pyramidal tract of the rat. Alcohol Clin Exp Res. 1994;18(2):346–54. doi: 10.1111/j.1530-0277.1994.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Astley SJ, Clarren SK. Number of axons in the corpus callosum of the mature macaca nemestrina: increases caused by prenatal exposure to ethanol. J Comp Neurol. 1999;412(1):123–31. [PubMed] [Google Scholar]
- Miller MW, Potempa G. Numbers of neurons and glia in mature rat somatosensory cortex: effects of prenatal exposure to ethanol. J Comp Neurol. 1990;293(1):92–102. doi: 10.1002/cne.902930108. [DOI] [PubMed] [Google Scholar]
- Miller MW, Robertson S. Prenatal exposure to ethanol alters the postnatal development and transformation of radial glia to astrocytes in the cortex. J Comp Neurol. 1993;337(2):253–66. doi: 10.1002/cne.903370206. [DOI] [PubMed] [Google Scholar]
- Moore NH, Costa LG, Shaffer SA, Goodlett DR, Guizzetti M. Shotgun proteomics implicates extracellular matrix proteins and protease systems in neuronal development induced by astrocyte cholinergic stimulation. J Neurochem. 2009;108(4):891–908. doi: 10.1111/j.1471-4159.2008.05836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland N, La Grange L, Montoya R. Impact of in utero exposure to EtOH on corpus callosum development and paw preference in rats: protective effects of silymarin. BMC Complement Altern Med. 2002;2:10. doi: 10.1186/1472-6882-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305(2):187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- Pascual M, Guerri C. The peptide NAP promotes neuronal growth and differentiation through extracellular signal-regulated protein kinase and Akt pathways, and protects neurons co-cultured with astrocytes damaged by ethanol. J Neurochem. 2007;103(2):557–68. doi: 10.1111/j.1471-4159.2007.04761.x. [DOI] [PubMed] [Google Scholar]
- Perez-Torrero E, Duran P, Granados L, Gutierez-Ospina G, Cintra L, Diaz-Cintra S. Effects of acute prenatal ethanol exposure on Bergmann glia cells early postnatal development. Brain Res. 1997;746(1–2):305–8. doi: 10.1016/s0006-8993(96)01235-8. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277(5332):1684–7. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Richards LJ. Axonal pathfinding mechanisms at the cortical midline and in the development of the corpus callosum. Braz J Med Biol Res. 2002;35(12):1431–9. doi: 10.1590/s0100-879x2002001200004. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19(5):1198–202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson H. Microenchephaly and fetal alcohol syndrome: human and animal studies. In: West J, editor. Alcohol and brain development. Oxford Press; Oxford: 1986. pp. 167–183. [Google Scholar]
- Saunders DE, Zajac CS, Wappler NL. Alcohol inhibits neurite extension and increases N-myc and c-myc proteins. Alcohol. 1995;12(5):475–83. doi: 10.1016/0741-8329(95)00034-o. [DOI] [PubMed] [Google Scholar]
- Schambra UB, Sulik KK, Petrusz P, Lauder JM. Ontogeny of cholinergic neurons in the mouse forebrain. J Comp Neurol. 1989;288(1):101–22. doi: 10.1002/cne.902880109. [DOI] [PubMed] [Google Scholar]
- Schlumpf M, Palacios JM, Cortes R, Lichtensteiger W. Regional development of muscarinic cholinergic binding sites in the prenatal rat brain. Neuroscience. 1991;45(2):347–57. doi: 10.1016/0306-4522(91)90232-d. [DOI] [PubMed] [Google Scholar]
- Schmidt H. Muscarinic acetylcholine receptor in chick limb bud during morphogeneis. Histochemistry. 1981;71(1):89–98. doi: 10.1007/BF00592573. [DOI] [PubMed] [Google Scholar]
- Seidler L, Kaszkin M, Kinzel V. Primary alcohols and phosphatidylcholine metabolism in rat brain synaptosomal membranes via phospholipase D. Pharmacol Toxicol. 1996;78(4):249–53. doi: 10.1111/j.1600-0773.1996.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001;57(2):235–44. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Landesman-Dwyer S, Martin JC, Smith DW. Teratogenic effects of alcohol in humans and laboratory animals. Science. 1980;209(4454):353–61. doi: 10.1126/science.6992275. [DOI] [PubMed] [Google Scholar]
- Sun YA, Poo MM. Evoked release of acetylcholine from the growing embryonic neuron. Proc Natl Acad Sci U S A. 1987;84(8):2540–4. doi: 10.1073/pnas.84.8.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Doller CM, Malouf AT, Silver J. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J Neurosci. 2004;24(42):9282–90. doi: 10.1523/JNEUROSCI.2120-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDeMark KL, Guizzetti M, Giordano G, Costa LG. Ethanol inhibits muscarinic receptor-induced axonal growth in rat hippocampal neurons. Alcohol Clin Exp Res. 2009;33:1945–1955. doi: 10.1111/j.1530-0277.2009.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ER, Maness P, Lauder JM. Why do neurotransmitters act like growth factors? Perspect Dev Neurobiol. 1998;5(4):323–35. [PubMed] [Google Scholar]
- Wessler I, Reinheimer T, Klapproth H, Schneider FJ, Racke K, Hammer R. Mammalian glial cells in culture synthesize acetylcholine. Naunyn Schmiedebergs Arch Pharmacol. 1997;356(5):694–7. doi: 10.1007/pl00005107. [DOI] [PubMed] [Google Scholar]
- Xie ZP, Poo MM. Initial events in the formation of neuromuscular synapse: rapid induction of acetylcholine release from embryonic neuron. Proc Natl Acad Sci U S A. 1986;83(18):7069–73. doi: 10.1073/pnas.83.18.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao WD, Rusch J, Poo M, Wu CF. Spontaneous acetylcholine secretion from developing growth cones of Drosophila central neurons in culture: effects of cAMP-pathway mutations. J Neurosci. 2000;20(7):2626–37. doi: 10.1523/JNEUROSCI.20-07-02626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]