Abstract
Genome stability is essential for normal foetal growth and development. To date, genome stability in human lymphocytes has not been studied in relation to late pregnancy diseases, such as pre-eclampsia (PE) and intrauterine growth restriction (IUGR), which can be life-threatening to mother and baby and together affect >10% of pregnancies. We performed a prospective cohort study investigating the association of maternal chromosomal damage in mid-pregnancy (20 weeks gestation) with pregnancy outcomes. Chromosome damage was measured using the cytokinesis-block micronucleus cytome (CBMNcyt) assay in peripheral blood lymphocytes. The odds ratio for PE and/or IUGR in a mixed cohort of low- and high-risk pregnancies (N = 136) and a cohort of only high-risk pregnancies (N = 91) was 15.97 (P = 0.001) and 17.85 (P = 0.007), respectively, if the frequency of lymphocytes with micronuclei (MN) at 20 weeks gestation was greater than the mean + 2 SDs of the cohort. These results suggest that the presence of lymphocyte MN is significantly increased in women who develop PE and/or IUGR before the clinical signs or symptoms appear relative to women with normal pregnancy outcomes. The CBMNcyt assay may provide a new approach for the early detection of women at risk of developing these late pregnancy diseases and for biomonitoring the efficacy of interventions to reduce DNA damage, which may in turn ameliorate pregnancy outcome.
Introduction
Genome damage can influence virtually any cellular process due to its ability to affect gene dosage, level of gene expression and subsequent gene products (1). As genome damage increases, so too does the risk for developmental and degenerative disease, which in turn is dependent on nutritional status and genetic polymorphisms that determine susceptibility (2). During foetal development, rapid cell proliferation, active gene transcription and a high rate of DNA metabolism can increase genotoxic insult susceptibility (1). Genome damage can occur via a variety of mechanisms, including point mutations, base modifications, chromosome breakage, chromosome rearrangement and chromosome loss or gain (3). Increased DNA damage at the molecular and chromosomal level has been associated with infertility (4,5), recurrent miscarriage (6), developmental defects (7) and childhood or adult cancer (8).
Although it has long been acknowledged that both male and female partners contribute to human reproductive success (9,10), the effect of maternal DNA damage on reproductive success has received less attention. It is not known how maternal DNA damage affects late onset pregnancy diseases, particularly those associated with abnormal placental development. Abnormal placental development leading to restricted maternal–foetal circulation could impair development of the foetus by restricting exchange of nutrients and wastes, a condition known as uteroplacental insufficiency (UPI). UPI is characterised by impaired trophoblast invasion of the decidua and an inadequate response of the uterine arteries in their physiological transformation to allow for greater blood flow necessary for foetal development (11). The clinical phenotypes associated with UPI include pre-eclampsia (PE) and intrauterine growth restriction (IUGR).
PE is a multisystem disorder of unknown cause and is unique to human pregnancy. It affects 5–10% of pregnancies and is a leading cause of maternal mortality and morbidity (12). Pathophysiological processes underlying PE are complex and include oxidative stress, placental ischemia, maternal–foetal immune maladaptation and genetic factors (such as polymorphisms in folate metabolism genes) (13). IUGR occurs when a foetus does not reach its full growth potential (14). Worldwide, it is the second leading cause of perinatal morbidity and mortality (15). The incidence of IUGR is estimated to be 8% in the developed world (16). Birthweight, especially when adjusted for gestational age, is a key indicator of a baby’s health status and also of its future health as an adult. Growth-restricted infants are at risk for multiple morbidities, including cardiovascular disease, hypertension, stroke and type 2 diabetes (17,18).
Factors that may contribute to the different clinical phenotypes observed in women with either PE or IUGR include obesity (19), insulin resistance (20), genetic or acquired thrombophilias (21), hyperhomocysteinemia (22) and substance abuse (23,24). Furthermore, increased DNA damage is associated with many of these factors (25–28). It is plausible that systemic DNA damage in pregnancy may be a common fundamental pathology of PE and IUGR because it may diminish the replicative capacity of trophoblasts and thus impair their invasion of the decidua and uterine spiral arteries associated with these pregnancy diseases.
Analysis of DNA damage in peripheral blood lymphocytes at the molecular and chromosomal level has been accepted as a technique for the biological monitoring of genotoxic events in somatic cells since the early 1970s (29). Lymphocytes circulate continuously throughout the body experiencing physiological and nutrient imbalances within tissues. Thus, DNA damage in these cells provides an integrated estimate of inherited and/or acquired genomic instability (30).
Chromosomal DNA damage in the form of micronuclei (MN) in human peripheral lymphocytes measured using the cytokinesis-block micronucleus cytome (CBMNcyt) assay is recognised as a valuable biomarker to study the genotoxic effects of environmental, genetic and dietary factors and has been validated and internationally standardised (31–33). The CBMNcyt assay is the most robust, as well as commonly used, method for measuring MN in cultured human lymphocytes because scoring is specifically restricted to once-divided cells, which are the cells that can express MN originating from lagging whole chromosomes or chromosome fragments during mitosis. These cells are recognised by their binucleated (BN) appearance after the inhibition of cytokinesis by cytochalasin-B (34). Restricting scoring of MN to binucleate cells prevents confounding effects caused by differences in cell division kinetics between subjects. The CBMNcyt assay is also used to measure other complementary biomarkers of genomic instability such as nucleoplasmic bridges (NPBs) which originate from dicentric chromosomes caused by mis-repair of DNA breaks or telomere end-fusions and nuclear buds (NBUDs), which are indicative of gene amplification (35).
Previous studies have shown that a high frequency of MN in lymphocytes, or metaphase chromosome aberrations from which they originate, is predictive of an increased risk of both cancer (35–37) and cardiovascular disease (38) and has been associated with neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease (39,40). Using the CBMNcyt assay, two previous studies have shown that chromosomal damage, as assessed by MN frequency, is increased in infertile couples and in those who have experienced recurrent miscarriage compared with fertile couples (5,41). These results suggest that MN may be a useful prognostic marker of a successful pregnancy, as they provide an index of the genomic instability of the parental and/or foetal tissues, either because of inherited factors predisposing to genomic instability or because of nutritional inadequacies or environmental exposure to genotoxic factors causing genome damage. Furthermore, a study by Levario-Carillo et al. (42) evaluated the formation of MN in umbilical cord blood and demonstrated that the frequency of MN in mothers was correlated with that in their babies, supporting the validity of using MN frequency in maternal lymphocytes to estimate genotoxic risk in foetal tissue.
Currently, women at risk of pregnancy complications are identified on the basis of epidemiological and clinical risk factors. There is no reliable test available early enough in pregnancy to identify women at risk for developing these disorders to permit preventative interventions. A better understanding of the mechanisms underlying abnormal placental development leading to UPI, associated with PE and IUGR, will not only improve the identification of those at risk but may also allow more effective prevention of these important pathologies.
The aim of this study was to investigate the potential association of chromosomal DNA damage with the pathophysiology of late pregnancy diseases including PE and IUGR and to test the following specific hypotheses:
DNA damage in lymphocytes at 20 weeks gestation is increased in women who are at high risk for developing adverse pregnancy outcomes compared with women who are at low risk of adverse pregnancy outcomes.
DNA damage in lymphocytes at 20 weeks gestation predicts PE and/or IUGR in a mixed cohort of women and among those women classified as at high risk for developing adverse pregnancy outcomes.
Methods
Study design
This was a prospective cohort study conducted at Commonwealth Scientific and Industrial Research Organisation Food and Nutritional Sciences and at the Women’s and Children’s Hospital (WCH), Adelaide, South Australia, Australia. The study was approved by the Human Experimentation and Research Ethics Committees of both institutions. Pregnant volunteers were enrolled in early pregnancy (before 20 weeks gestation), after obtaining informed consent. A fasting blood sample was collected and body mass index (BMI: kilogram per square meter) was determined at 20 weeks gestation. Information was collected on obstetric and medical history as well as socioeconomic and lifestyle factors. Pregnancy outcome data were collected after delivery and classified by obstetricians at the WCH using strict criteria (as described below).
Patient selection criteria
Inclusion criteria.
Women attending the high-risk pregnancy clinic at the WCH in 2004–2006 were invited to participate in this study. Low-risk women who did not have previous obstetric complications were recruited prior to routine morphology scans at 18–20 weeks gestation during 2005.
Exclusion criteria.
Exclusion criteria included any maternal or foetal condition requiring termination of pregnancy, any known major foetal anomaly or foetal demise, multifoetal pregnancy, any medical disorder requiring systemic steroids, pre-existing renal disease (serum creatinine >100 μmol/l) and inability to give informed consent.
Patient classification
Women were classified as ‘high risk’ (i.e. at high risk of developing an adverse pregnancy outcome) based on a variety of historical risk factors, previous PE/eclampsia, early-onset IUGR (<34 weeks gestation and birthweight <10th centile), placental abruption, preterm birth <34 weeks gestation, history of recurrent pregnancy loss (three or more miscarriages) and previous foetal demise. Women were classified as ‘low risk’ (i.e. at low risk of developing an adverse pregnancy outcome) if they were healthy women without any known pre-existing medical disorder, had had one or more previous normal pregnancies (delivery >37 weeks gestation, customised birthweight >10th centile, no hypertension) and had had no abnormal pregnancies.
Clinical diagnosis of pregnancy outcome
PE and gestational hypertension were defined according to the criteria of the Australasian Society for the Study of Hypertension in Pregnancy (43). Customised growth centiles adjusting for maternal height, weight, parity, ethnic group and foetal sex were used to identify birthweights for gestational age. The customised centile calculator used was GROW centile v 5.1.2006. (downloaded from the Gestation Network, www.gestation.net) (44). IUGR was defined using sonographic criteria of estimated foetal weight below the 10th centile, using appropriate population-based growth charts. After an early dating scan had established the gestational age, sonographic growth curves, using Australian Society for Ultrasound in Medicine biometric charts, were used in the diagnosis of IUGR; reduction of foetal growth as observed on serial ultrasound scans was used as a criterion for diagnosis. IUGR classification by ultrasound was defined as a serial tapering of growth in abdominal circumference and estimated foetal weight.
CBMNcyt assay
The lymphocyte CBMNcyt assay using whole blood cultures was performed as described by Fenech (32). In this assay, lymphocytes are cultured to allow them to complete nuclear division to allow expression of DNA damage as MN, NPB or NBUD at the binucleate stage at which they are accumulated by inducing a cytokinesis-block using cytochalasin-B (35,37–39).
Venous blood was collected in lithium heparin tubes. Whole blood cultures of lymphocytes were prepared in RPMI 1640 (Sigma, Sydney, Australia) containing 10% fetal bovine serum (Trace Scientific, Melbourne, Australia) 2 mM L-glutamine (Sigma), 1 mM sodium pyruvate (Trace Scientific) as previously described (35) and kept at 37°C and 5% CO2 in a humidified incubator. Duplicate cultures were set-up for each subject. Cytochalasin-B was added after 44 h of culture and cells were harvested 28 h later and then fixed and stained using Diff-Quik (Lab-Aids, North Narrabeen, New South Wales, Australia). The CBMNcyt assay slides were labelled with the patient code number and scored by a single scorer (D.L.F.F.) who was not aware of the risk group and the eventual pregnancy outcome of the participants when the slides were scored. Frequency of BN cells with one or more micronuclei (MN-BN), nucleoplasmic bridges (NPB-BN) or nuclear buds (NBUD-BN) was determined by scoring 1000 BN cells from each duplicate culture using established scoring criteria (35,45). Coefficient of variation of duplicate measurements of MN-BN, NPB-BN and NBUD-BN was 11.1, 21.5 and 17.9%, respectively.
Study design, power calculations and statistics
The study was designed so that the smallest difference in the frequency of MN-BN lymphocytes that could be detected with P <0.05 and 80% power would be 5.8 MN-BN per 1000 BN cells, assuming a subject group size of N = 25. The power calculation was based on the expected SD of 7.1 for the frequency of MN-BN cells per 1000 BN cells observed in previous population studies of MN-BN frequency in females within the age range of study participants (46). A difference of 5.8 MN-BN cells per 1000 BN cells was considered to be pathologically significant because it is a difference of MN-BN frequency similar to that induced by exposure to 10 cGy of ionising radiation (47), which is five times greater than the safety limit of exposure for non-radiation workers and which is associated with increased significant risk of developmental abnormalities and cancer (48).
The association between MN-BN frequency and continuous variables that could be measured accurately (i.e. age and BMI) was determined by the non-parametric Spearman’s correlation test, given that MN-BN frequency was not normally distributed as determined by the D’Agostino and Pearson normality test. Because MN-BN was positively and significantly correlated with age (r = 0.306, P = 0.003), all the MN-BN data were age-adjusted using the slope of the regression line and the following equation as described previously (26):
In this equation A = age, S = slope of the regression line, MN-BN = the actual measured MN-BN frequency, MN-BN33y = the age-adjusted MN-BN frequency for age of 33 years (the mean age of the total cohort).
Because non-smokers had an age-adjusted MN-BN frequency that was 70.3% that of smokers, all the smokers’ age-adjusted MN-BN frequency were also further adjusted by a factor of 0.703 to eliminate mathematically the estimated contributing effect of smoking. All the age- and smoking-adjusted MN-BN frequency data were also log-transformed prior to further analysis because the distribution of the non-transformed data within cohorts and sub-groups was not Gaussian. The log-transformed data had a normal distribution as confirmed by the D’Agostino and Pearson normality test. Final comparisons of age- and smoking-adjusted MN-BN frequency were performed using log-transformed data and parametric tests of significance. The association of MN-BN, NPB-BN and NBUD-BN frequency with risk groups and pregnancy outcome groups was also analysed using multiple regression (logistic and multinomial, respectively) analysis to take into account any effects of other factors such as age, smoking and BMI and their interactions.
Sensitivity, specificity, positive and negative predictive values, odds ratios (ORs) and likelihood ratios for a specific threshold value of MN-BN cell frequency were determined for prediction of being a pre-eclamptic and/or an IUGR case. Results are reported as mean ± standard error (SE). P values <0.05 were considered statistically significant. All statistical tests were performed using GraphPad PRISM 4.0 (GraphPad, San Diego, CA, USA) with the exception of multiple regression analyses, which were performed using SPSS for WINDOWS (version 17.0, SPSS Inc, Chicago, IL).
Results
Pregnancy outcomes in the investigated cohorts
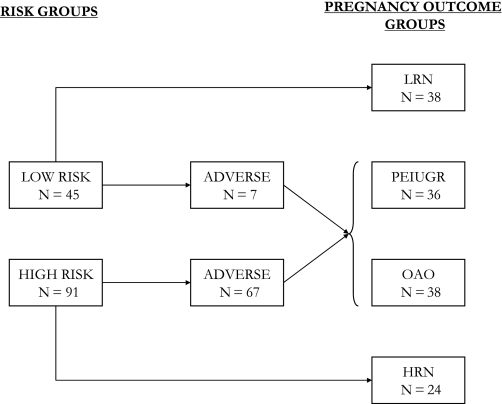
A total of 136 pregnant women, comprising 45 healthy women classified as low risk and 91 women who were classified as high risk for an adverse pregnancy outcome, were recruited into the study. A total of 95.1% of the low-risk group and 94.3% of the high-risk group were Caucasian. From the low-risk group, 38 (84%) had normal pregnancy outcomes (no clinical complications) and were then allocated into the low-risk normal (LRN) group that was considered the ‘healthy’ reference group for comparisons with other groups. Twenty-four (26%) of the women from the high-risk pregnancy outcome group had a normal pregnancy (no clinical complications); these were allocated into the high-risk normal (HRN) group (Figure 1).
Fig. 1.
Distribution of subjects amongst low- and high-risk groups and their subsequent pregnancy outcomes. Low risk, healthy women with low risk of developing pregnancy complications; high risk, women with high risk of developing pregnancy complications; Low risk normal (LRN), pregnancies with clinically normal outcomes from the low-risk group; High risk normal (HRN), pregnancies with clinically normal outcomes from the high-risk group; adverse, pregnancies with adverse outcome; PEIUGR, PE and/or IUGR outcome; OAO, other adverse outcome i.e. other than PE or IUGR, e.g. gestational hypertension and gestational diabetes.
Seven (15%) of the low-risk women and 67 (74%) of the high-risk women had an adverse pregnancy outcome. The adverse outcomes included 21 cases of IUGR and 15 cases of PE (including cases with both PE and IUGR, N = 5). The other adverse outcomes (OAOs) (N = 38) included 14 cases with gestational hypertension, 14 with preterm birth (varied causes), 9 with gestational diabetes and 1 placental abruption. The pregnancy outcomes are summarised in Figure 1. PE and IUGR were grouped together based on the possible common aetiology and also as some of the women developed both conditions, PEIUGR (N = 36). The pregnancies with OAOs were grouped as one under the grouping called OAO (N = 38).
Age, BMI, smoking status and CBMNcyt DNA damage biomarkers in study groups
Women classified as having a high risk pregnancy were older, overweight and were more likely to smoke cigarettes at 20 weeks gestation compared to the low risk pregnancy group (Tables 1 and 2).
Table I.
Comparison of age, BMI, smoking status and CBMNcyt assay DNA damage biomarkers between low- and high-risk pregnancy groups
| Age (years)a | BMI (kg/M2)a | % Smokers | MN-BN (‰)a | NPB-BN (‰)a | NBUD-BN (‰)a | |
| Low risk (N = 45) | 30.9 ± 0.7 | 26.4 ± 0.6 | 6.6 | 16.0 ± 0.8 | 7.4 ± 1.0 | 24.4 ± 2.4 |
| High risk (N = 91) | 33.9 ± 0.8 | 29.6 ± 0.8 | 19.8 | 22.8 ± 1.2 | 6.5 ± 0.4 | 28.4 ± 2.5 |
| P | 0.014b | 0.009b | 0.046c | 0.006d | 0.298d | 0.244d |
Mean ± 1 SE.
Unpaired Student’s t-test.
Fisher exact test.
Logistic regression analysis using age, BMI and smoking as covariates.
Table II.
Comparison of age, BMI and smoking status and CBMNcyt assay DNA damage biomarkers among pregnancy outcome groups
| Age (years)a | BMI (kg/M2)a | % Smokers | MN-BN (‰)a | NPB-BN (‰)a | NBUD-BN (‰)a | |
| LRN (N = 38) | 30.8 ± 0.7 | 26.6 ± 0.7 | 5.2 | 16.0 ± 0.9 | 7.3 ± 0.9 | 25.7 ± 2.8 |
| HRN (N = 24) | 36.2 ± 1.4 | 28.9 ± 1.1 | 12.5 | 20.7 ± 1.9 | 5.5 ± 0.5 | 25.0 ± 4.2 |
| OAO (N = 38) | 33.3 ± 1.2 | 29.3 ± 1.2 | 10.5 | 18.7 ± 1.2 | 7.2 ± 1.1 | 23.6 ± 4.4 |
| PEIUGR (N = 36) | 32.6 ± 1.3 | 29.5 ± 1.3 | 33.3 | 27.1 ± 2.3 | 6.8 ± 0.6 | 33.6 ± 3.4 |
| P | 0.027b | 0.209b | 0.005c | 0.001d | 0.628d | 0.134d |
LRN, pregnancies with clinically normal outcomes from the low risk group; HRN, pregnancies with clinically normal outcomes from the high-risk group; PEIUGR, PE and/or IUGR outcome; OAO, other adverse outcome, i.e. other than PE or IUGR such as gestational hypertension and gestational diabetes.
Mean ± 1 SE.
One-way ANOVA.
Fisher exact test.
Multinomial regression analysis performed using reference category: LRN (low risk normal) and P value in relation to PEIUGR with age, BMI and smoking as covariates.
When classified according to pregnancy outcome (i.e. LRN, HRN, OAO and PEIUGR) (Table 2), it was evident that there were significant differences between groups with respect to age and smoking status but not with respect to BMI.
With respect to the CBMNcyt DNA damage biomarkers, it was evident that frequency of MN-BN was significantly greater in the high-risk pregnancy group relative to the low-risk pregnancy group by 71.3 % (P = 0.006) but there were no differences in the frequency of NPB-BN and NBUD-BN (Table 1). When comparing the pregnancy outcome groups, it was evident that MN-BN frequency was highest in the PEIUGR group and significantly so compared to the LRN group (P = 0.001) (Table 2); there were no other significant differences between these groups with respect to MN-BN, NPB-BN and NBUD-BN frequency in lymphocytes. The DNA damage biomarker comparisons were performed using multiple regression analysis as explained previously.
Correlation of age, BMI and smoking status with frequency of MN-BN
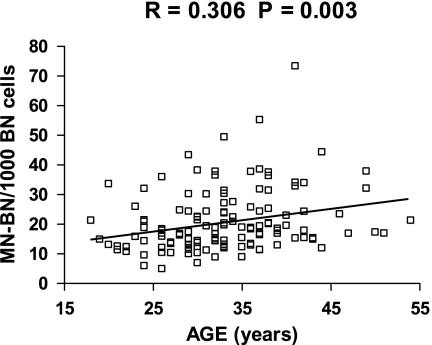
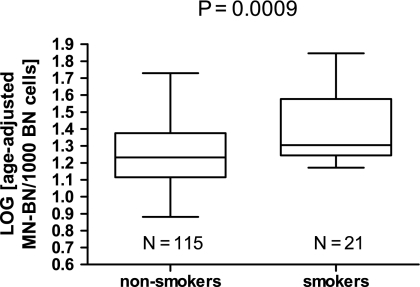
Because of the significant differences between groups with respect to MN-BN frequency, we explored the impact of age, smoking and BMI on this DNA damage biomarker in more detail Figures 2 and 3. BMI was not significantly correlated with MN-BN frequency (r = 0.054, P = 0.533) and therefore no adjustment for BMI was made in subsequent analyses. Age was positively correlated with MN-BN frequency (r = 0.306, P = 0.003; Figure 2), indicating that older women had increased DNA damage. Consequently MN-BN frequency was adjusted for age in all subsequent analyses, using the slope of the regression as correction factor as explained in the Materials and methods. Women who did not smoke cigarettes had significantly lower DNA damage (age-adjusted MN-BN frequency, mean ± SE: 19.3 ± 0.8) compared with women who smoked (age-adjusted MN-BN frequency, mean ± SE: 27.5 ± 3.1, P = 0.001). These differences between smokers and non-smokers were also evident after comparing log-transformed data (Figure 3).
Fig. 2.
Relationship between MN-BN frequency in lymphocytes and age in the total cohort. The slope of the regression line is 0.3823 (P = 0.0025); N = 136.
Fig. 3.
Box and whiskers plot of comparison of log-transformed age-adjusted MN-BN frequency between smokers and non-smokers. The horizontal line within each box is the median and the lower and higher horizontal edges of each box are the 25th and 75th percentile, respectively; the ends of the error bars or ‘whiskers’ represent the lowest and highest value in the data distribution. The P-value was calculated using Student’s t-test.
The ratio of non-transformed age-adjusted MN-BN frequency in smokers relative to that of smokers was 0.703. This factor was used to adjust age-adjusted MN-BN frequency of smokers for smoking effect in subsequent analyses. Because age- and smoking-adjusted MN-BN frequency was not normally distributed within groups, these data were log-transformed prior to comparing groups (see below).
MN-BN frequency in the risk groups and pregnancy outcome groups
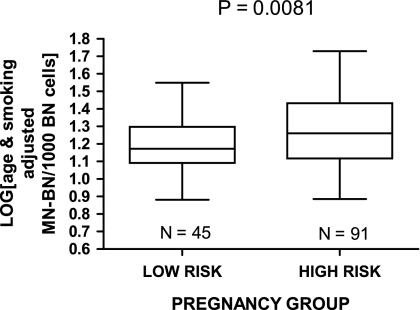
The age- and smoking-adjusted MN-BN frequency at 20 weeks gestation was 25% higher in the high-risk women compared to the low-risk women with a P value of 0.0081 for the log-transformed data (Figures 4 and 5).
Fig. 4.
MN-BN frequency (log-transformed, age- and smoking-adjusted) in the low-risk and high-risk pregnancy groups.
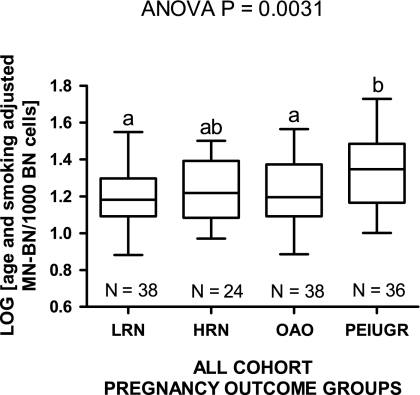
Fig. 5.
Log-transformed age- and gender-adjusted MN-BN frequency data for the low risk normal (LRN), high risk normal (HRN), other adverse outcome (OAO) and PEIUGR pregnancy outcome groups. Groups not sharing the same letter are significantly different from each other.
Age- and smoking-adjusted MN-BN frequency also differed between pregnancy outcome groups, being lowest in women classified as low risk with normal pregnancy outcomes (LRN) group (16.6‰) and highest in the PEIUGR group (24.2‰). These differences were significant between the PEIUGR group and the LRN pregnancy group (P < 0.01) as well as the OAO group (P < 0.05) for the log-transformed data (Figure 5).
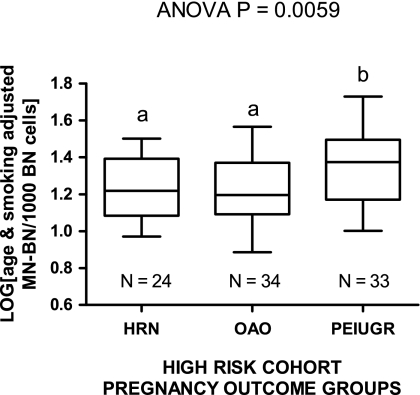
MN-BN frequency in pregnancy outcome groups from the high-risk cohort
We also tested the association of age- and smoking-adjusted MN-BN frequency with pregnancy outcome within the high-risk cohort because it is of particular clinical relevance to be able to predict outcomes within this group. Age- and smoking-adjusted MN-BN frequency differed between pregnancy outcome groups within the high-risk cohort, being lowest in women classified as high risk with normal pregnancy outcomes (HRN) and OAO group and highest in the PEIUGR group. The result for the PEIUGR group was higher than those for the HRN and OAO groups (P < 0.05 and P < 0.01, respectively) when comparing log-transformed age- and smoking-adjusted MN-BN frequency data (Figure 6). Furthermore, we also compared the log-transformed age-adjusted MN-BN frequency between groups in non-smokers only to completely exclude smoking as a confounding variable and found that the age-adjusted MN-BN frequency in non-smokers was also significantly higher in the PEIUGR group relative to non-smokers in the HRN and OAO groups (analysis of variance P = 0.0081) and that there was no difference between the non-smoker HRN and OAO groups (data not shown); Figure 6.
Fig. 6.
Log-transformed age- and smoking-adjusted MN-BN frequency data for the high risk normal (HRN), other adverse outcome (OAO) and PEIUGR pregnancy outcome groups within the high-risk cohort. Data include results of non-smokers and smokers. Groups not sharing the same letter are significantly different from each other.
MN-BN frequency as a risk marker for PE and/or IUGR
The data of the total cohort (i.e. the combined low- and high-risk groups) were analysed to determine the potential of the lymphocyte age- and smoking-adjusted MN-BN frequency index as a predictive marker of PE and/or IUGR by calculating the ORs, likelihood ratios, sensitivity, specificity and positive and negative predictive values. The chosen cut-off values for these calculations were the mean (19.3‰), the mean + 1 SD (28.0‰) and the mean + 2 SD (36.7‰) of the age- and smoking-adjusted MN-BN frequency of the entire cohort. Based on these results, pregnant women with an age- and smoking-adjusted MN-BN frequency (at 20 weeks gestation) >19.3‰, 28.0‰ or 36.7% had an OR of 3.23 (P = 0.003), 3.56 (P = 0.006) and 15.9 (P = 0.001), respectively, for eventually developing PE and/or IUGR within the same pregnancy Table 3.
Table III.
Summary data describing the odds of prospective risk for becoming a PEIUGR case
| Prospective Odds of PEIUGR for all cohort |
Prospective Odds of PEIUGR for high risk cohort only |
|||||
| MN-BN ‰ | MN-BN ‰ | MN-BN ‰ | MN-BN ‰ | MN-BN ‰ | MN-BN ‰ | |
| >19.3a | >28.0b | >36.7c | >20.7a | >30.3b | >39.1c | |
| Chi-square P value (two tailed) | 0.0031 | 0.0063 | 0.0012 | 0.0121 | 0.0162 | 0.0067 |
| OR (95% CI) | 3.23 (1.46–7.17) | 3.56 (1.38–9.17) | 15.97 (1.79–142.00) | 3.08 (1.26–7.51) | 3.77 (1.22–11.61) | 17.85 (0.93–343.0) |
| Sensitivity | 0.60 | 0.31 | 0.14 | 0.59 | 0.30 | 0.12 |
| Specificity | 0.68 | 0.89 | 0.99 | 0.68 | 0.90 | 1.00 |
| Positive predictive value | 0.40 | 0.50 | 0.83 | 0.50 | 0.62 | 1.00 |
| Negative predictive value | 0.83 | 0.78 | 0.76 | 0.75 | 0.69 | 0.67 |
| Likelihood ratio | 1.89 | 2.78 | 13.89 | 1.84 | 2.93 | >15.00 |
Cohort mean of age and smoking adjusted MN-BN frequency per 1000 BN cells.
Cohort mean + 1 SD of age and smoking adjusted MN-BN frequency per 1000 BN cells.
Cohort mean + 2 SD of age and smoking adjusted MN-BN frequency per 1000 BN cells.
Using a similar analysis, but restricted to the high risk cohort, and using age- and smoking-adjusted MN-BN frequency cut-offs based on the mean (20.7‰), the mean + 1 SD (30.3‰) and the mean + 2 SD (39.1‰) of the high-risk cohort, we calculated the strength of the prospective association of the MN-BN biomarker with PE and/or IUGR risk within the high-risk group (Table 3). Based on these results, pregnancies with an age- and smoking-adjusted MN-BN frequency >20.7, 30.3 and 39.1‰ had an OR of 3.08 (P = 0.012), 3.77 (P = 0.016) or 17.85 (P = 0.007) for developing PE and/or IUGR within the same pregnancy.
The highest sensitivity of the MN-BN frequency biomarker for predicting a PE and/or IUGR outcome was 0.59–0.60 for values greater than the cohort mean with an accompanying specificity of 0.68. Specificity was high (0.99–1.00) for MN-BN frequency values greater than the cohort mean + 2 SD but the corresponding sensitivity was low (0.12–0.14).
Discussion
This study is the first to investigate the association between chromosomal DNA damage measured using the CBMN assay and pregnancy outcome, showing that increased DNA damage in maternal peripheral lymphocytes at 20 weeks gestation (mid-pregnancy) is associated prospectively with PE and IUGR. When genome damage increased >36.7 MN-BN per 1000 BN cells, the OR of developing PE and/or IUGR was 15.97 in the whole cohort. However, although the specificity of the MN-BN biomarker is high at this cut-off, the sensitivity is low which means that this biomarker will correctly identify 99% of pregnancies that will not develop PEIUGR but only capture 14% of pregnancies that will develop PEIUGR. The positive and negative predictive value suggests that 83% of pregnancies with a positive test and 76% of pregnancies with a negative test will be correctly diagnosed. The likelihood ratio (13.9) was >10 which is the minimum ratio value considered to provide strong evidence of a clinically useful diagnostic in most circumstances (49).
The discriminatory power of the MN-BN test may be enhanced by using centromere probes to distinguish between MN originating from chromosome fragments and those due to whole chromosome loss (46), assuming that one of these mechanisms is the predominant cause of PE or IUGR. Another approach could be by combination with other risk markers such as plasma homocysteine (50) or folate metabolism genotype (45). The lack of difference between groups in NPB, which are caused by telomere end fusions or mis-repair of chromosome breaks suggest that the increase in MN formation was not related to these mechanisms. The alternative mechanism for MN formation is whole chromosome loss which can be caused by defects in chromosome segregation machinery, such as deficiencies in the cell cycle controlling genes (e.g. hCDC4 and BUBR1 (51,52)), mitotic spindle failure, defects in kinetochore proteins (31) or hypomethylation of centromeric DNA, which leads to fragility and despiralisation of the centromeres and increased loss of chromosomes 1,9 and 16 (53). Aneuploidy resulting from chromosome malsegregation produces permanent genomic changes in cells and such changes can alter gene expression (54). Aneuploidy has been associated with infertility (55), developmental defects in the foetus (56) and leukaemia (8).
MN provide a reliable measure of an individual’s genome stability and have been shown to correlate with DNA damage in cells in other tissues of the body (3,31,57). As lymphocytes circulate around the body, they experience physiologic and nutrient imbalances within tissues: thus DNA damage in these cells may provide an integrated estimate of inherited and/or acquired genomic instability. During early placentation, natural killer cells in the uterus accumulate as a dense infiltrate around the invading trophoblast cells and affect both trophoblast invasion and vascular changes in the maternal placental bed (12). Hypothetically, DNA damage in natural killer cells and cytotrophoblasts may adversely affect their function, viability and proliferation, and thus modify the placentation process and increase the risk of PE and/or IUGR. Increased genome instability in lymphocytes may reflect a systemic problem that could be observed in other tissues, given the recently reported associations of MN-BN frequency in lymphocytes with hypertension (58), cardiovascular disease (38,59) and cancer risk (60,61). Furthermore, a previous study showed that MN frequency and aneuploidy were marginally increased in lymphocytes in women with polycystic ovary syndrome and were also correlated with insulin resistance (62). The results of the present study demonstrate that women who develop late pregnancy diseases are more likely to have abnormally high chromosomal damage in their lymphocytes. If such events also occurred in trophoblasts (placental cells), it is possible that altered gene dosage due to DNA damage (MN formation) and resulting abnormal gene expression might affect trophoblast cell phenotype and proliferative potential causing abnormal placentation, more specifically influencing invasion and modification of maternal spiral arteries and ultimately reducing uteroplacental circulation.
Base-line genome damage biomarker frequencies provide an index of accumulated genetic damage that has occurred during the life span of the lymphocytes (half-life ∼6 months (30)), as well as being a marker of inherited or acquired defects in genome maintenance. The mean value of MN-BN in women in the high-risk pregnancy group was higher than that of the low-risk group at 20 weeks gestation, suggesting that such women have increased genetic susceptibility to genotoxic stress, or that they are excessively exposed to environmental genotoxins, or that they have deficiencies in specific dietary factors that increase susceptibility to genome instability (2). Other studies have shown mean MN-BN from lymphocytes of healthy, non-smoking and non-pregnant women to be 9.3‰ when aged between 20 and 29 years, 14.4‰ when aged between 30 and 39 years and 25.6‰ when aged between 40 and 49 years per 1000 BN cells (33,46,63), which is comparable to the range of MN-BN frequencies reported in our cohort. In the present study, age, BMI and the prevalence of smoking were increased in the high-risk subjects when compared with low-risk subjects and controls. However, only age and smoking status were associated with increased MN-BN frequency, consistent with other previously published studies (33,46,63). A meta-analysis of published studies showed that smokers do not usually experience an increase in micronucleus frequency in lymphocytes except when there is an interaction with occupational genotoxin exposure particularly in heavy smokers (64). It is therefore possible that our observation of a smoking effect might be due to an interaction with another factor that is directly or indirectly genotoxic. A plausible hypothesis could be an interaction between relatively heavy smoking and the potential pro-inflammatory condition in pregnancy (65). However, we have no biomarker data to support this possibility, which is a limitation of this pilot study and will require attention in future larger studies. The only other known study to measure MN in maternal lymphocytes during pregnancy was performed at 7–8 weeks gestation to test the effect of gestogens, in the form of hormonal substitution therapy, on pregnancy outcome (66). The results of this study demonstrated that increased therapeutic doses of gestogen were associated with an increase in DNA damage (MN frequency).
Maternal diet is one of the major environmental factors influencing the foetal developmental process and supports the high rates of cellular proliferation and DNA replication that take place during this critical stage of life (67). Diet is also a key factor in determining genomic stability as it affects all relevant developmental pathways, i.e. activation/detoxification of chemicals preventing DNA oxidation, DNA repair, apoptosis and DNA synthesis (68). Future studies will need to focus on whether or not increased MN frequency in patients with PE and/or IUGR is due to deficiency in micronutrients that affect chromosome maintenance and chromosome segregation (2,69). Likely candidates are folate and vitamin B12, deficiency of which can cause DNA hypomethylation, a defect that leads to chromosome loss and malsegregation, pathological events which are also observed when DNA methyltransferase activity is diminished as shown in studies of ICF (Immunodeficiency, Centromere instability and Facial anomalies) syndrome (53). Another mechanism that could explain the increased level of MN in PE is placental oxidative stress, which is one of the key mechanisms in the pathogenesis of this disease (70). Placental oxidative stress may be due to deficient spiral artery remodelling leading to ischemia/reperfusion injury, which triggers an inflammatory response and the generation of reactive oxygen species, which can induce chromosome breaks and MN (65).
Emerging evidence suggests that use of micronutrient-containing prenatal vitamins before and during pregnancy may be associated with reductions in the risk of congenital defects, pre-term delivery, low-infant birthweight and PE (71). A predictive biomarker, such as MN-BN frequency in the lymphocyte CBMNcyt assay, may be useful in studying the effects on genome stability of micronutrient supplementation recommendations on an individual basis. There are ∼40 essential micronutrients required in the human diet (72), many of which are crucial in determining genomic stability. This suggests the need for further studies that also account for effect of dietary factors, including polymorphisms in genes involved in DNA synthesis and repair, such as those involved in one carbon metabolism (45,73,74), when investigating the relationship between chromosome damage and pregnancy outcome. Another possible cause of increased MN frequency may be excessive exposure to a variety of environmental genotoxins, such as polycyclic hydrocarbons (75), ionising radiation (47,76), and the carcinogenic metabolite of alcohol acetaldehyde (27,77) .
In this study, we did not test the relationship of lymphocyte DNA damage with that of the placenta or foetus. However, the plausibility of an association between maternal DNA damage and transplacental DNA damage in the foetus is supported by the observations of increased MN and DNA adducts in maternal peripheral blood lymphocytes and cord blood lymphocytes in various environmental genotoxin exposure situations (42,78,79).
Weaknesses of the present study include the relatively small sample size and therefore the need to verify these observations in a second independent and larger cohort. The small sample size also precluded the possibility of obtaining reliable estimates of the effect of genotype and haplotypes for common polymorphisms of DNA repair and folate metabolism genes that are associated with altered MN frequency in lymphocytes (80) even though such effects on MN-BN frequency tend to be smaller than the differences observed between the high-risk group and the PEIUGR groups relative to control referent groups. As indicated above, direct mechanistic information on the origin of the excess MN in the high risk and PEIUGR groups could be obtained using centromeric probes, which would allow MN originating from whole chromosomes to be distinguished from those originating from acentric fragments (81,82). Ideally future studies might also measure DNA damage directly in trophoblasts and verify that DNA damage measured in lymphocytes is correlated with DNA damage in the placenta. Furthermore, studies in larger cohorts that do not discriminate participants based on pregnancy risk status are required to obtain a more objective assessment of the practical use of the MN-BN index as a predictive marker for late onset pregnancy diseases in the routine clinical setting.
In conclusion, this study shows that chromosomal DNA damage in peripheral blood lymphocytes is increased in women who develop PE and/or IUGR. Further progress in this field is desirable because it is becoming increasingly evident that a reduced rate of DNA damage may help couples to become pregnant naturally as well as promoting the health of both mother and her baby.
Funding
Channel 7 Children’s Research Foundation of SA Inc; National Institute of Health (USA); Cooperative Research Centre for Diagnostics.
Acknowledgments
We thank the staff at the South Australian Women’s and Children’s Hospital, especially Denise Healy and Michelle Cox, for assistance with recruiting and patient care, as well as Dr Colin Storey for the use of his laboratory. At the Commonwealth Scientific and Industrial Research Organisation Nutritional Genomics and Genome Health laboratory, we acknowledge Carolyn Salisbury and Sasja Beetstra for teaching D.L.F.F. how to perform the cytokinesis block micronucleus assay. We are also grateful to all subjects for donating their time and blood for this study.
Conflict of interest statement: The funding bodies had no involvement in the study design, in the collection, analysis and interpretation of the data, in the writing of the report or in the decision to submit the paper for publication.
References
- 1.Vinson RK, Hales BF. DNA repair during organogenesis. Mutat. Res. 2002;509:79–91. doi: 10.1016/s0027-5107(02)00223-3. [DOI] [PubMed] [Google Scholar]
- 2.Fenech M. Genome health nutrigenomics and nutrigenetics—diagnosis and nutritional treatment of genome damage on an individual basis. Food Chem. Toxicol. 2007;46:1365–1370. doi: 10.1016/j.fct.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Fenech M. The Genome Health Clinic and Genome Health Nutrigenomics concepts: diagnosis and nutritional treatment of genome and epigenome damage on an individual basis. Mutagenesis. 2005;20:255–269. doi: 10.1093/mutage/gei040. [DOI] [PubMed] [Google Scholar]
- 4.Sankoff D, Deneault M, Turbis P, Allen C. Chromosomal distributions of breakpoints in cancer, infertility, and evolution. Theor. Popul. Biol. 2002;61:497–501. doi: 10.1006/tpbi.2002.1599. [DOI] [PubMed] [Google Scholar]
- 5.Trkova M, Kapras J, Bobkova K, Stankova J, Mejsnarova B. Increased micronuclei frequencies in couples with reproductive failure. Reprod. Toxicol. 2000;14:331–335. doi: 10.1016/s0890-6238(00)00087-3. [DOI] [PubMed] [Google Scholar]
- 6.Warren JE, Silver RM. Genetics of pregnancy loss. Clin. Obstet. Gynecol. 2008;51:84–95. doi: 10.1097/GRF.0b013e318161719c. [DOI] [PubMed] [Google Scholar]
- 7.Morawiec Z, Janik K, Kowalski M, Stetkiewicz T, Szaflik J, Morawiec-Bajda A, Sobczuk A, Blasiak J. DNA damage and repair in children with Down's syndrome. Mutat. Res. 2008;637:118–123. doi: 10.1016/j.mrfmmm.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Izraeli S. Perspective: chromosomal aneuploidy in leukemia—lessons from down syndrome. Hematol. Oncol. 2006;24:3–6. doi: 10.1002/hon.758. [DOI] [PubMed] [Google Scholar]
- 9.Aitken RJ, Skakkebaek NE, Roman SD. Male reproductive health and the environment. Med. J. Aust. 2006;185:414–415. doi: 10.5694/j.1326-5377.2006.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 10.Aitken RJ, De Iuliis GN, McLachlan RI. Biological and clinical significance of DNA damage in the male germ line. Int. J. Androl. 2009;32:46–56. doi: 10.1111/j.1365-2605.2008.00943.x. [DOI] [PubMed] [Google Scholar]
- 11.Khong TY. The Robertson-Brosens-Dixon hypothesis: evidence for the role of haemochorial placentation in pregnancy success. Br. J. Obstet. Gynaecol. 1991;98:1195–1199. doi: 10.1111/j.1471-0528.1991.tb15387.x. [DOI] [PubMed] [Google Scholar]
- 12.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 13.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am. J. Obstet. Gynecol. 1998;179:1359–1375. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 14.Sizonenko SV, Borradori-Tolsa C, Bauthay DM, Lodygensky G, Lazeyras F, Huppi P. Impact of intrauterine growth restriction and glucocorticoids on brain development: insights using advanced magnetic resonance imaging. Mol. Cell. Endocrinol. 2006;254–255:163–171. doi: 10.1016/j.mce.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am. J. Obstet. Gynecol. 2000;182:198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- 16.Prada JA, Tsang RC. Biological mechanisms of environmentally induced causes of IUGR. Eur. J. Clin. Nutr. 1998;52(Suppl 1):S21–S27. discussion, S27–S28. [PubMed] [Google Scholar]
- 17.Baserga M, Hale MA, Wang ZM, Yu X, Callaway CW, McKnight RA, Lane RH. Uteroplacental insufficiency alters nephrogenesis and downregulates cyclooxygenase-2 expression in a model of IUGR with adult-onset hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1943–R1955. doi: 10.1152/ajpregu.00558.2006. [DOI] [PubMed] [Google Scholar]
- 18.Barker DJ. The developmental origins of well-being. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359:1359–1366. doi: 10.1098/rstb.2004.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frederick IO, Rudra CB, Miller RS, Foster JC, Williams MA. Adult weight change, weight cycling, and prepregnancy obesity in relation to risk of preeclampsia. Epidemiology. 2006;17:428–434. doi: 10.1097/01.ede.0000221028.33245.0b. [DOI] [PubMed] [Google Scholar]
- 20.Moran C, Sandoval T, Duque X, Gonzalez S, Moran S, Bermudez JA. Increased insulin levels independent of gestational overweight in women with preeclampsia. Arch. Med. Res. 2006;37:749–754. doi: 10.1016/j.arcmed.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Sibai BM. Imitators of severe preeclampsia. Obstet. Gynecol. 2007;109:956–966. doi: 10.1097/01.AOG.0000258281.22296.de. [DOI] [PubMed] [Google Scholar]
- 22.Makedos G, Papanicolaou A, Hitoglou A, Kalogiannidis I, Makedos A, Vrazioti V, Goutzioulis M. Homocysteine, folic acid and B12 serum levels in pregnancy complicated with preeclampsia. Arch. Gynecol. Obstet. 2007;275:121–124. doi: 10.1007/s00404-006-0223-2. [DOI] [PubMed] [Google Scholar]
- 23.Kennare R, Heard A, Chan A. Substance use during pregnancy: risk factors and obstetric and perinatal outcomes in South Australia. Aust. N. Z. J. Obstet. Gynaecol. 2005;45:220–225. doi: 10.1111/j.1479-828X.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- 24.Shankaran S, Lester BM, Das A, Bauer CR, Bada HS, Lagasse L, Higgins R. Impact of maternal substance use during pregnancy on childhood outcome. Semin. Fetal. Neonatal. Med. 2007;12:143–150. doi: 10.1016/j.siny.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demirbag R, Yilmaz R, Gur M, Celik H, Guzel S, Selek S, Kocyigit A. DNA damage in metabolic syndrome and its association with antioxidative and oxidative measurements. Int. J. Clin. Pract. 2006;60:1187–1193. doi: 10.1111/j.1742-1241.2006.01042.x. [DOI] [PubMed] [Google Scholar]
- 26.Fenech M, Aitken C, Rinaldi J. Folate, vitamin B12, homocysteine status and DNA damage in young Australian adults. Carcinogenesis. 1998;19:1163–1171. doi: 10.1093/carcin/19.7.1163. [DOI] [PubMed] [Google Scholar]
- 27.Maffei F, Fimognari C, Castelli E, Stefanini GF, Forti GC, Hrelia P. Increased cytogenetic damage detected by FISH analysis on micronuclei in peripheral lymphocytes from alcoholics. Mutagenesis. 2000;15:517–523. doi: 10.1093/mutage/15.6.517. [DOI] [PubMed] [Google Scholar]
- 28.Reddy Thavanati PK, Kanala KR, de Dios AE, Cantu Garza JM. Age-related correlation between antioxidant enzymes and DNA damage with smoking and body mass index. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:360–364. doi: 10.1093/gerona/63.4.360. [DOI] [PubMed] [Google Scholar]
- 29.Srb V, Puza V. [Methods for the analysis of human chromosome aberrations (author's transl)] Cas. Lek. Cesk. 1975;114:231–236. [PubMed] [Google Scholar]
- 30.International Atomic Energy Agency. Biological dosimetry: chromosomal aberrations analysis for dose assessment. 2001. Technical Report Series No. 405. International Atomic Energy Agency, Vienna, Austria. [Google Scholar]
- 31.Albertini RJ, Anderson D, Douglas GR, et al. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. International Programme on Chemical Safety. Mutat. Res. 2000;463:111–172. doi: 10.1016/s1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- 32.Fenech M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007;2:1084–1104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- 33.Bonassi S, Fenech M, Lando C, et al. HUman MicroNucleus project: international database comparison for results with the cytokinesis-block micronucleus assay in human lymphocytes: I. Effect of laboratory protocol, scoring criteria, and host factors on the frequency of micronuclei. Environ. Mol. Mutagen. 2001;37:31–45. [PubMed] [Google Scholar]
- 34.Fenech M. Cytokinesis-block micronucleus assay evolves into a “cytome” assay of chromosomal instability, mitotic dysfunction and cell death. Mutat. Res. 2006;600:58–66. doi: 10.1016/j.mrfmmm.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 35.Bonassi S, Ugolini D, Kirsch-Volders M, Stromberg U, Vermeulen R, Tucker JD. Human population studies with cytogenetic biomarkers: review of the literature and future prospectives. Environ. Mol. Mutagen. 2005;45:258–270. doi: 10.1002/em.20115. [DOI] [PubMed] [Google Scholar]
- 36.Bonassi S, Hagmar L, Stromberg U, et al. Chromosomal aberrations in lymphocytes predict human cancer independently of exposure to carcinogens. European Study Group on Cytogenetic Biomarkers and Health. Cancer Res. 2000;60:1619–1625. [PubMed] [Google Scholar]
- 37.Rossner P, Boffetta P, Ceppi M, Bonassi S, Smerhovsky Z, Landa K, Juzova D, Sram RJ. Chromosomal aberrations in lymphocytes of healthy subjects and risk of cancer. Environ. Health Perspect. 2005;113:517–520. doi: 10.1289/ehp.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Federici C, Botto N, Manfredi S, Rizza A, Del Fiandra M, Andreassi MG. Relation of increased chromosomal damage to future adverse cardiac events in patients with known coronary artery disease. Am. J. Cardiol. 2008;102:1296–1300. doi: 10.1016/j.amjcard.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Migliore L, Botto N, Scarpato R, Petrozzi L, Cipriani G, Bonuccelli U. Preferential occurrence of chromosome 21 malsegregation in peripheral blood lymphocytes of Alzheimer disease patients. Cytogenet. Cell Genet. 1999;87:41–46. doi: 10.1159/000015389. [DOI] [PubMed] [Google Scholar]
- 40.Migliore L, Scarpato R, Coppede F, Petrozzi L, Bonuccelli U, Rodilla V. Chromosome and oxidative damage biomarkers in lymphocytes of Parkinson's disease patients. Int. J. Hyg. Environ. Health. 2001;204:61–66. doi: 10.1078/1438-4639-00074. [DOI] [PubMed] [Google Scholar]
- 41.Govindaiah V, Naushad SM, Prabhakara K, Krishna PC, Radha Rama Devi A. Association of parental hyperhomocysteinemia and C677T Methylene tetrahydrofolate reductase (MTHFR) polymorphism with recurrent pregnancy loss. Clin. Biochem. 2008;42:380–386. doi: 10.1016/j.clinbiochem.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Levario-Carrillo M, Sordo M, Rocha F, Gonzalez-Horta C, Amato D, Ostrosky-Wegman P. Micronucleus frequency in human umbilical cord lymphocytes. Mutat. Res. 2005;586:68–75. doi: 10.1016/j.mrgentox.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, Oats J, Peek MJ, Rowan JA, Walters BN. The detection, investigation and management of hypertension in pregnancy: full consensus statement. Aust. N. Z. J. Obstet. Gynaecol. 2000;40:139–155. doi: 10.1111/j.1479-828x.2000.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 44.Gestation Network. Birthweight Centile Calculator, Perinatal Institute. 2007. www.gestation.net (accessed, 2007) [Google Scholar]
- 45.Furness DL, Fenech MF, Khong YT, Romero R, Dekker GA. One-carbon metabolism enzyme polymorphisms and uteroplacental insufficiency. Am. J. Obstet. Gynecol. 2008;199:e271–e278. doi: 10.1016/j.ajog.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Fenech M. Important variables that influence base-line micronucleus frequency in cytokinesis-blocked lymphocytes-a biomarker for DNA damage in human populations. Mutat. Res. 1998;404:155–165. doi: 10.1016/s0027-5107(98)00109-2. [DOI] [PubMed] [Google Scholar]
- 47.Fenech M, Morley AA. Cytokinesis-block micronucleus method in human lymphocytes: effect of in vivo ageing and low dose X-irradiation. Mutat. Res. 1986;161:193–198. doi: 10.1016/0027-5107(86)90010-2. [DOI] [PubMed] [Google Scholar]
- 48.Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc. Natl Acad. Sci. USA. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. 2004;329:168–169. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dekker GA, de Vries JI, Doelitzsch PM, Huijgens PC, von Blomberg BM, Jakobs C, van Geijn HP. Underlying disorders associated with severe early-onset preeclampsia. Am. J. Obstet. Gynecol. 1995;173:1042–1048. doi: 10.1016/0002-9378(95)91324-6. [DOI] [PubMed] [Google Scholar]
- 51.Shichiri M, Yoshinaga K, Hisatomi H, Sugihara K, Hirata Y. Genetic and epigenetic inactivation of mitotic checkpoint genes hBUB1 and hBUBR1 and their relationship to survival. Cancer Res. 2002;62:13–17. [PubMed] [Google Scholar]
- 52.Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- 53.Xu GL, Bestor TH, Bourc'his D, et al. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature. 1999;402:187–191. doi: 10.1038/46052. [DOI] [PubMed] [Google Scholar]
- 54.Kaushal D, Contos JJ, Treuner K, et al. Alteration of gene expression by chromosome loss in the postnatal mouse brain. J. Neurosci. 2003;23:5599–5606. doi: 10.1523/JNEUROSCI.23-13-05599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubio C, Pehlivan T, Rodrigo L, Simon C, Remohi J, Pellicer A. Embryo aneuploidy screening for unexplained recurrent miscarriage: a minireview. Am. J. Reprod. Immunol. 2005;53:159–165. doi: 10.1111/j.1600-0897.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 56.Bianco K, Caughey AB, Shaffer BL, Davis R, Norton ME. History of miscarriage and increased incidence of fetal aneuploidy in subsequent pregnancy. Obstet. Gynecol. 2006;107:1098–1102. doi: 10.1097/01.AOG.0000215560.86673.22. [DOI] [PubMed] [Google Scholar]
- 57.Fenech M. Chromosomal biomarkers of genomic instability relevant to cancer. Drug Discov. Today. 2002;7:1128–1137. doi: 10.1016/s1359-6446(02)02502-3. [DOI] [PubMed] [Google Scholar]
- 58.Schupp N, Schmid U, Rutkowski P, Lakner U, Kanase N, Heidland A, Stopper H. Angiotensin II-induced genomic damage in renal cells can be prevented by angiotensin II type 1 receptor blockage or radical scavenging. Am. J. Physiol. Renal. Physiol. 2007;292:F1427–F1434. doi: 10.1152/ajprenal.00458.2006. [DOI] [PubMed] [Google Scholar]
- 59.Botto N, Rizza A, Colombo MG, Mazzone AM, Manfredi S, Masetti S, Clerico A, Biagini A, Andreassi MG. Evidence for DNA damage in patients with coronary artery disease. Mutat. Res. 2001;493:23–30. doi: 10.1016/s1383-5718(01)00162-0. [DOI] [PubMed] [Google Scholar]
- 60.Bonassi S, Znaor A, Ceppi M, et al. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2007;28:625–631. doi: 10.1093/carcin/bgl177. [DOI] [PubMed] [Google Scholar]
- 61.Murgia E, Ballardin M, Bonassi S, Rossi AM, Barale R. Validation of micronuclei frequency in peripheral blood lymphocytes as early cancer risk biomarker in a nested case-control study. Mutat. Res. 2008;639:27–34. doi: 10.1016/j.mrfmmm.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 62.Moran LJ, Noakes M, Clifton PM, Norman RJ, Fenech MF. Genome instability is increased in lymphocytes of women with polycystic ovary syndrome and is correlated with insulin resistance. Mutat. Res. 2008;639:55–63. doi: 10.1016/j.mrfmmm.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Di Giorgio C, De Meo MP, Laget M, Guiraud H, Botta A, Dumenil G. The micronucleus assay in human lymphocytes: screening for inter-individual variability and application to biomonitoring. Carcinogenesis. 1994;15:313–317. doi: 10.1093/carcin/15.2.313. [DOI] [PubMed] [Google Scholar]
- 64.Bonassi S, Neri M, Lando C, et al. Effect of smoking habit on the frequency of micronuclei in human lymphocytes: results from the Human MicroNucleus project. Mutat. Res. 2003;543:155–166. doi: 10.1016/s1383-5742(03)00013-9. [DOI] [PubMed] [Google Scholar]
- 65.Cindrova-Davies T. Gabor Than Award Lecture 2008: pre-eclampsia—from placental oxidative stress to maternal endothelial dysfunction. Placenta. 2009;30(Suppl A):S55–S65. doi: 10.1016/j.placenta.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 66.Milosevic-Ethordevic O, Grujicic D, Marinkovic D, Arsenijevic S, Bankovic S. Effect of various doses of gestogens on micronuclei frequency in human peripheral blood lymphocytes of pregnant women. Hum. Reprod. 2003;18:433–436. doi: 10.1093/humrep/deg068. [DOI] [PubMed] [Google Scholar]
- 67.Maloney CA, Rees WD. Gene-nutrient interactions during fetal development. Reproduction. 2005;130:401–410. doi: 10.1530/rep.1.00523. [DOI] [PubMed] [Google Scholar]
- 68.Fenech M. Recommended dietary allowances (RDAs) for genomic stability. Mutat. Res. 2001;480-481:51–54. doi: 10.1016/s0027-5107(01)00168-3. [DOI] [PubMed] [Google Scholar]
- 69.Fenech M, Baghurst P, Luderer W, Turner J, Record S, Ceppi M, Bonassi S. Low intake of calcium, folate, nicotinic acid, vitamin E, retinol, beta-carotene and high intake of pantothenic acid, biotin and riboflavin are significantly associated with increased genome instability–results from a dietary intake and micronucleus index survey in South Australia. Carcinogenesis. 2005;26:991–999. doi: 10.1093/carcin/bgi042. [DOI] [PubMed] [Google Scholar]
- 70.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am. J. Obstet. Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 71.Scholl TO. Maternal nutrition before and during pregnancy. Nestle Nutr. Workshop Ser Pediatr. Program. 2008;61:79–89. doi: 10.1159/000113172. [DOI] [PubMed] [Google Scholar]
- 72.Fletcher RH, Fairfield KM. Vitamins for chronic disease prevention in adults: clinical applications. JAMA. 2002;287:3127–3129. doi: 10.1001/jama.287.23.3127. [DOI] [PubMed] [Google Scholar]
- 73.Umegaki K, Fenech M. Cytokinesis-block micronucleus assay in WIL2-NS cells: a sensitive system to detect chromosomal damage induced by reactive oxygen species and activated human neutrophils. Mutagenesis. 2000;15:261–269. doi: 10.1093/mutage/15.3.261. [DOI] [PubMed] [Google Scholar]
- 74.Botto N, Andreassi MG, Manfredi S, Masetti S, Cocci F, Colombo MG, Storti S, Rizza A, Biagini A. Genetic polymorphisms in folate and homocysteine metabolism as risk factors for DNA damage. Eur. J. Hum. Genet. 2003;11:671–678. doi: 10.1038/sj.ejhg.5201024. [DOI] [PubMed] [Google Scholar]
- 75.Roubicek DA, Gutierrez-Castillo ME, Sordo M, Cebrian-Garcia ME, Ostrosky-Wegman P. Micronuclei induced by airborne particulate matter from Mexico City. Mutat. Res. 2007;631:9–15. doi: 10.1016/j.mrgentox.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Song EY, Rizvi SM, Qu CF, Raja C, Yuen J, Li Y, Morgenstern A, Apostolidis C, Allen BJ. The cytokinesis-block micronucleus assay as a biological dosimeter for targeted alpha therapy. Phys. Med. Biol. 2008;53:319–328. doi: 10.1088/0031-9155/53/2/001. [DOI] [PubMed] [Google Scholar]
- 77.Ishikawa H, Ishikawa T, Yamamoto H, Fukao A, Yokoyama K. Genotoxic effects of alcohol in human peripheral lymphocytes modulated by ADH1B and ALDH2 gene polymorphisms. Mutat. Res. 2007;615:134–142. doi: 10.1016/j.mrfmmm.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 78.Pedersen M, Wichmann J, Autrup H, et al. Increased micronuclei and bulky DNA adducts in cord blood after maternal exposures to traffic-related air pollution. Environ. Res. 2009;109:1012–1020. doi: 10.1016/j.envres.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Arab K, Pedersen M, Nair J, Meerang M, Knudsen LE, Bartsch H. Typical signature of DNA damage in white blood cells: a pilot study on etheno adducts in Danish mother-newborn child pairs. Carcinogenesis. 2009;30:282–285. doi: 10.1093/carcin/bgn264. [DOI] [PubMed] [Google Scholar]
- 80.Dhillon V, Thomas P, Fenech M. Effect of common polymorphisms in folate uptake and metabolism genes on frequency of micronucleated lymphocytes in a South Australian cohort. Mutat. Res. 2009;665:1–6. doi: 10.1016/j.mrfmmm.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 81.Touil N, Elhajouji A, Thierens H, Kirsch-Volders M. Analysis of chromosome loss and chromosome segregation in cytokinesis-blocked human lymphocytes: non-disjunction is the prevalent mistake in chromosome segregation produced by low dose exposure to ionizing radiation. Mutagenesis. 2000;15:1–7. doi: 10.1093/mutage/15.1.1. [DOI] [PubMed] [Google Scholar]
- 82.Lindberg HK, Wang X, Jarventaus H, Falck GC, Norppa H, Fenech M. Origin of nuclear buds and micronuclei in normal and folate-deprived human lymphocytes. Mutat. Res. 2007;617:33–45. doi: 10.1016/j.mrfmmm.2006.12.002. [DOI] [PubMed] [Google Scholar]