Abstract
Objective
The primary aim of this pilot study was to identify structural and functional brain differences in older adults with self-reported disabling chronic low back pain (CLBP) compared with those who reported non-disabling CLBP.
Design
Cross-sectional.
Participants
Sixteen cognitively intact older adults, eight with disabling CLBP and eight with non-disabling. Exclusions were psychiatric or neurological disorders, substance abuse, opioid use, or diabetes mellitus.
Methods
Participants underwent: structural and functional brain MRI; neuropsychological assessment using the Repeatable Battery for the Assessment of Neuropsychological Status, Trail Making Tests A and B; and physical performance assessment using the Short Physical Performance Battery.
Results
In the disabled group there was significantly lower white matter (WM) integrity (P < 0.05) of the splenium of the corpus callosum. This group also demonstrated activation of the right medial prefrontal cortex at rest whereas the non-disabled demonstrated activation of the left lateral prefrontal cortex. Combined groups analysis revealed a strong positive correlation (rs = 0.80, P < 0.0002) between WM integrity of the left centrum semiovale with gait-speed. Secondary analysis revealed a strong negative correlation between total months of CLBP and WM integrity of the SCC (rs = −0.59, P < 0.02).
Conclusions
Brain structure and function is different in older adults with disabling CLBP compared to those with non-disabling CLBP. Deficits in brain morphology combining groups are associated with pain duration and poor physical function. Our findings suggest brain structure and function may play a key role in chronic-pain-related-disability and may be important treatment targets.
Introduction
Essential to successful aging is the maintenance of functional independence. Chronic low back pain (CLBP) poses a threat to successful aging as it is one of the most commonly reported chronic pain disorders in older adults and is associated with self-reported difficulty in performing functional tasks [1–3]. However, many older adults remain high functioning despite CLBP [3] and the underlying mechanism linking CLBP and disability is unknown.
Although low back pain is associated with self-reported disability in community-dwellingolder adults [4–9, 3], a number of studies have failed to demonstrate a relationship between low back pain and observed physical performance [3–4, 9]. Further, treatments that target the lumbar spine [10] including decompressive laminectomy [11] do not appear to improve physical performance, which is troubling given the robust relationship between physical performance and future disability [12–16]. Recent findings from our laboratory exploring the relationships among CLBP, brain structure, physical performance (PP), and neuropsychological performance (NP) in older adults suggest a new paradigm for understanding chronic-pain-related-disability that point toward a need for newly targeted treatments.
In our first study [17] we demonstrated that PP and NP were significantly impaired in older adults with CLBP compared to pain-free individuals and that there was a significant correlation between pain and physical performance in CLBP participants. However, the relationship between pain and physical performance was no longer significant when NP was taken into account. These findings suggest that either NP deficits may be a potential mediator of the relationship between pain and physical performance or that NP and PP share a common pathway within the brain. In our second study [18] comparing older adults with CLBP to pain-free individuals, we found CLBP participants performed significantly worse on digit span forward and had smaller regional brain volumes - left parietal lobe grey matter (GM) and middle cingulate white matter (WM). Taken together these findings suggest that there is a relationship between brain structure and function (as assessed by NP) with CLBP, raising the possibility that brain structure and function may play an important role in chronic-pain-related disability. Although altered brain structure and function has been associated with a number of chronic pain syndromes in younger adults [for a review see:19–20], our laboratory is the only one that has looked at brain structure in older adults. It is important to understand the mechanism by which CLBP causes functional limitation so that appropriately targeted treatments can be developed.
The primary aim of this pilot study is to identify structural and functional brain differences in older adults with self-reported disabling CLBP compared with those who report non-disabling CLBP. The secondary aim is to examine relationships among brain structure and function with neuropsychological function (NP) and physical performance (PP).
Methods
This study was approved by the University of Pittsburgh Internal Review Board and written informed consent was obtained at the time of enrollment.
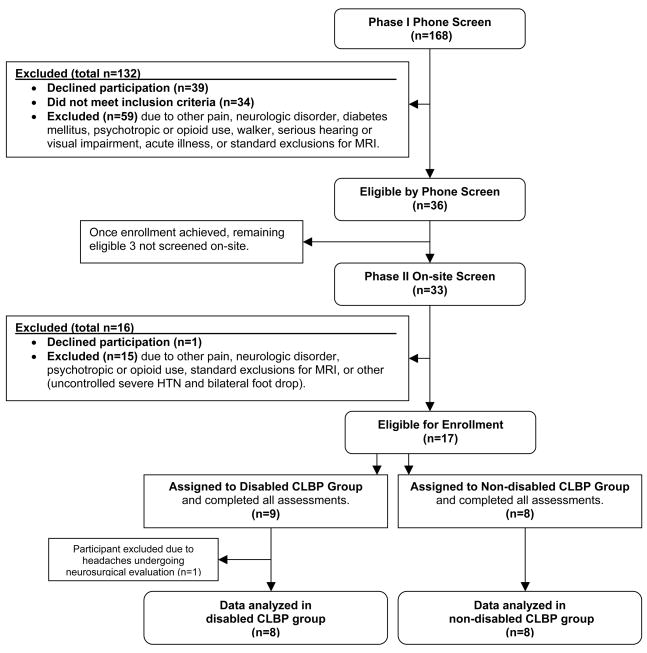
Screening and Enrollment of Participants (See flow diagram: Fig. 1 Screening and Enrollment Process)
Fig. 1.
Screening and Enrollment Process
Sixteen community-dwelling older adults (age ≥65) completed this study. Participants were recruited from the Pittsburgh Claude D. Pepper Older Americans Independence Center registry. A two-part screening process (telephone interview followed by on-site evaluation) was conducted to select candidates who reported CLBP every day or almost every day for ≥3 months of at least moderate intensity using the pain thermometer (a vertical verbal descriptor scale that has been validated in older adults) [21]. Moderate or greater intensity CLBP was required for study inclusion based on evidence from a large epidemiological study that demonstrated older adults with moderate or greater low back pain are significantly more likely to report functional impairment than older adults with less severe pain [3]. We required this level of pain for both disabled and non-disabled participants to avoid pain severity independently influencing brain structure and function. Participants were categorized into two groups: CLBP self-reported as (1) disabling (pain that necessitated cutting back on daily activities or resulted in being bed bound during some days of ≥6 weeks in the past 6 months) or (2) non-disabling (pain that had limited function for < 6 weeks over the past 6 months). We have shown that older adults can accurately recall the degree to which their low back pain has been disabling during the prior 6 months [22]. We operationally defined disabling versus non-disabling CLBP drawing upon the findings of Reid and colleagues [23]. In their prospective 18 month study it was found that older adults who reported having to cut back on daily activities or spend time in bed because of back pain during at least 4 of the 18 months experienced significantly greater decline in gait-speed than other participants.
In order to more specifically exam the relationship of CLBP with brain structure and function, individuals were excluded who experienced pain outside of the lower back of the same or greater frequency or of the same or greater intensity. Individuals were also excluded if they had disorders with known effects on brain structure or function (diabetes mellitus [24], depression [25], anxiety [26–27], post-traumatic stress disorder [28], multiple sclerosis, cerebral tumor, or a cerebrovascular accident), or were taking or had taken in the past 3 months prescription as well as over-the-counter pychotropics (such as St. John’s Wort), including those used for pain management. If an individual scored <24 on the Folstein Mini Mental State Examination (MMSE) [29–30] at the time of the onsite evaluation or if they had a disorder that could impact neuropsychological testing they were excluded (serious hearing or visual impairment, substance abuse, opioid use, history or evidence of traumatic brain injury with loss of consciousness, seizure disorder, or a diagnosis of dementia). Those who were mobility impaired were also excluded so as not to confound PP assessment (Parkinson’s disease or unable to ambulate without a walker or care). Finally, participants were excluded if they were experiencing an acute illness or had conditions that would render MRI performance unsafe (claustrophobia, metal objects in body including a pacemaker, or weight >250lbs). Though hypertension (HTN) is known to affect brain structure [31] this was not an exclusion for the current study. Because of its prevalence in older adults, having HTN as an exclusion would significantly impact our ability to generalize study findings.
Common reasons for exclusion were ineligible pain status (i.e. CLBP < everyday or almost everyday, CLBP < moderate intensity, or pain outside of the back > CLBP) and diabetes mellitus. Of the 168 older adults screened by phone, 36 were deemed eligible for onsite evaluation and 17 enrolled. One enrolled participant who completed the assessments was excluded and the data not analyzed due to chronic headaches that came under neurosurgical evaluation during the study.
Assessments
All participants underwent brain structural and functional MRI within two weeks of the NP and PP assessments. Before undergoing brain MRI, NP and PP testing, a history and physical exam was conducted in order to assure the participant was medically stable and met inclusion criteria. In addition the following data were collected: (1) demographic characteristics, including age, ethnicity, sex, economic, and educational status; pain intensity, using the McGill Pain Questionnaire Short Form [32]; co-morbidity, using the Cumulative Illness Rating Scale (CIRS) [33–34]; and, documentation of all regularly scheduled and as needed prescription medications as well as over the counter (OTC) medications including vitamins, supplements, herbals, and herbal teas.
Brain Imaging and Acquisition: Structural and Function
All images were acquired at the University of Pittsburgh’s Magnetic Resonance Research Center using a Siemens 3T Total Imaging Matrix (TIM) TRIO scanner. Each subject received 4 types of brain MRI scans: Localizer, Diffusion Tensor Imaging (DTI), high resolution anatomical imaging using Magnetization-Prepared Rapid Acquisition Gradient Echo (MPRAGE) acquisition, and resting state fMRI. The 3-plane T1 localizer was used for slice prescription and to ensure that the head was not tilted. DTI was collected using a single shot, spin echo, echo planar sequence with 12 directions with b values of 0 and 1000 (matrix = 128 × 128, TR = 5300ms, TE = 88ms, FOV = 256mm, axial slices = 28, thickness = 3mm, skip = 0 with four repetitions). For drawing grey and white matter Volumes of Interest (VOI), a high resolution 3D T1-weighted (MPRAGE) anatomical scan was collected (matrix = 256 × 256, TR = 2300ms, TE = 3.43ms, FOV = 25, axial slices = 160, thickness = 1.0 mm, and flip = 9). For the resting-state fMRI data, subjects were instructed to keep their eyes closed and rest during 5-minute acquisition block while echo planar imaging (EPI) was collected (matrix = 128 × 128, TR = 2000ms, TE = 34ms, 3 mm oblique axial slices, 28 slices, 2 mm × 2mm in-plane voxel dimensions).
Structural Neuroimaging Processing
Structural analysis included: (1) volumetric analysis of corpus callosum WM (genu, middle, and splenium) and superior parietal GM (these regions were selected based on our previous findings) [18]; and (2) Diffusion Tensor Imaging to assess WM microstructure of the corpus callosum (genu and splenium). The genu of the CC was included as previous studies have implicated prefrontal structural differences in younger patients with CLBP [35]. The splenium was chosen as we found a trend (p = 0.07) in poorer WM integrity of the SCC in CLBP subjects compared to pain free individuals (unpublished data) using a subset of participants from our previous study [18]. Using DTI we also examined (in both hemispheres) the posterior limb of the internal capsule and parietal peri-callosal WM near the sensory cortex (as these regions are associated with motor-sensory processing), and the centrum semiovale (as this is a major anterior to posterior WM tract). All structural MRI processing and analyses were conducted by an assessor masked to participant characteristics including group assignment (disabled vs. non-disabled). In order to ensure reliability of DTI analysis and volume measurements, a random 25% of the sample was measured by a second masked rater and a minimum inter-rater correlation coefficient of 0.85 was considered acceptable.
Structural Neuroimaging: Volumetric Analysis
The cerebellum and pons were masked and excluded so that all measures were based on supratentorial volume. After each brain was stripped and segmented into grey matter, white matter, and CSF using Statistical Parametric Mapping 2 (SPM2, http://www.fil.ion.ucl.ac.uk), the Volumes of Interest (VOI) were defined - WM volumes of the corpus callosum (genu, middle, and splenium) and superior parietal GM volumes. VOI analysis was conducted using a variation of manual tracing [36] using in-house software [37] implemented in Matlab (Mathworks, Sherborn, Mass., USA).
The CC WM VOI was defined by the methods described in our previous work [18, 37]. The CC VOI was defined in the sagittal plane. The mid-point was identified and then 20 slices on either side were included. We included only the middle 10mm as the edges can be difficult to determine. Superior parietal GM VOI were defined by first finding the most posterior portion of splenium of the corpus callosum (CC) at the mid-sagittal point, this being the most anterior boundary of the superior parietal lobe of each hemisphere as seen in the sagittal plane. The 20 slices moving posterior of this anterior boundary of the superior parietal lobe were selected for analysis.
To control for head size, total supratentorial brain volume (SBV) in cm3 was estimated using the methods of Benedetti et al. [39], but only including grey and white matter volumes as CSF segmented volumes were of poor quality. Each between groups volume comparison was based on the mean percentage of the VOI relative to the total SBV. For each VOI, group mean values were calculated and both Student t-test and Statterwaite unequal variance t-test was used for between group comparisons.
Structural Neuroimaging: Diffusion Tensor Imaging (DTI)
In a completely random state bulk water uniformly diffuses (isotropic). In the brain, WM restricts water molecule diffusion (anisotropic). DTI capitalizes on the effects of WM on water diffusion. The diffusion tensor estimates the orientation of the dominant direction of diffusion within a tissue voxel [41–42]. Fractional anisotropy (FA) is a scalar quantity of diffusion calculated for each voxel. The FA value describes the degree of anisotropy, from 0 (isotropic) to 1 (anisotropic) [40–42]. Lower FA values indicate less tissue structure and potential damage [43–46].
All DTI processing was completed using the diffusion tools in the FSL software package (FMRIB’s software library, http://www.fmrib.ox.ac.uk/fsl/). The data were corrected for head motion and eddy current distortion, and then FA maps were calculated. Volumes of interest were drawn on the b=0 DTI images to avoid using the dependent measure to define the dependent measure [47]. The DTI VOIs were defined using modified procedures of Sullivan [48] and Salat [49]. Drawn in the axial plane on two slices using MRICro (www.sph.sc.edu/comd/rorden/mricro.html), the DTI VOI’s were transferred to the FA map and values extracted. The following VOIs were examined: genu and splenium of the corpus callosum, right and left centrum semiovale, left and right posterior limb of the internal capsule, and left and right parietal peri-callosal WM near the sensory cortex. The splenium VOI’s were drawn on the two most superior slices to capture white matter connections of the parietal cortex, not the occipital cortex. For each VOI, group mean FA values were calculated and both Student t-test and Statterwaite unequal variance t-test used for between group comparisons.
Functional Neuroimaging: Resting State Functional MRI and the Default Mode Network
There is a growing body of evidence using fMRI that there is a spatiotemporally correlated resting-state functional network of the healthy brain - a default-mode network (DMN) – that is active during the resting state and attenuated during task performance [50–52, for a review see 53]. Functional connectivity is defined as the temporal correlation of activity between spatially disconnected areas. Functional connectivity is often investigated by correlating the time-course of a seed region with voxel-time-courses across the brain. The posterior cingulate cortex (PCC) has been shown to have consistently greater activity during resting state than during cognitive tasks and is the most often reported region of the DMN identified in healthy controls [52, 54]. Thus, the PCC is often used as a seed region to identify the DMN, as was done for this study.
All functional MRI processing and analyses were conducted by an assessor masked to participant characteristics using SPM2 (http://www.fil.ion.ucl.ac.uk) with standard parameters for motion correction, smoothing (8 mm FWHM Gaussian kernel), and normalization (co-registration of images to T1 ICBM template). A band-pass temporal filter with the cutoff frequencies of (.01 .1) Hertz was used to restrict signal to the DMN frequency band, and remove signal drift and high-frequency noise [55]. The left and right PCC from the Anatomical Automatic Labeling AAL atlas [56] was used as a seed region to generate a resting-state functional connectivity map for each individual, i.e., a map of correlation coefficients (r values) representing the strength of the correlation of that voxel with the PCC. Results were thresholded at 20 voxels (voxel-wise p < 0.0005). These individual resting-state connectivity maps were then compared between groups (disabled and non-disabled) in SPM using a 2-sample T-test. Montreal Neurologic Institute (MNI) coordinates were converted to Talairach space using Gingerale free-ware (http://brainmap.org, [57]) and Talairach Client freeware (www.talairach.org, [58]) was used to specify the grey matter center within and the Brodmann’s Areas (BA) associated with the Talairach space.
Neuropsychological Testing
In order not to fatigue participants the NP battery was limited to 45–60 minutes. In addition to the screening Folstein Mini-Mental State Exam (MMSE) [29,30] NP measures of enrolled participants included the National Adult Reading Test (NART) [59], the Repeatable Battery for the Assessment of Neuropsychological Status (RBANs) [60], Trail making test A and B, and Letter-Number Sequencing (LNS) from the Wechsler Adult Intelligence Scale-III. RBANS assesses a wide range of cognitive function (immediate and delayed memory, visuospatial ability, language, and attention) with norms for older adults [60]. Trail Making Test A is a measure of motor speed [61] and Trail Making Test B a measure of executive function [61] Letter-Number Sequencing also assesses executive function in addition to working memory [62].
Physical Performance
The Short Physical Performance Battery is reliable and well validated in older adults demonstrating strong predictive validity for physical disability and mortality [14, 63]. It consists of tests of balance (standing side-by-side, tandem stand, semi-tandem stand), gait-speed, and a timed standing chair rise. A summary score that is on a continuous scale is calculated for the SPPB. Gait-speed, a component of SPPB, is a reliable and valid measure in and of itself for predicting physical decline [13, 64] and has also been validated as a strong predictor of cognitive disability in older adults [65–66].
Statistical Analysis
Fisher’s Exact test was used for between groups comparisons of sex, ethnicity, economic and educational status. Student’s t-test was used for between groups comparison of age. Depending on whether the data were categorical or continuous and the distributional properties of the continuous data, independent samples t-test or Wilcoxon rank sum tests were used for between groups comparisons of other measures. To strengthen power, groups were combined for association analysis performed with Spearman’s correlation coefficient. We examined the association of the FA values (nine measures total) with the neuropsychological tests (the RBANS final score, Trails A time, and Trails B time), the Short Physical Performance Battery (single score), and gait-speed (single value). We also examined the association of the regional volumes (five measures total) and resting state subtraction map intensities (sixteen values) with the same NP and PP measures.
Results
Patient Characteristics
Participant characteristics and comparisons are displayed in Table 1. There is no statistical difference in age between the disabled (n=8, mean age 74.1) and non-disabled group (n=8, mean age 75.1) nor a statistical difference in ethnicity, sex, income, or education. In addition there is no statistical difference between groups in prevalence of HTN, total months of CLBP or months of everyday CLBP, co-morbidities (CIRS), MMSE, NART, RBANS, Trails (A and B), SPPB, or gait-speed. LNS was not analyzed due to missing data. Overall, participants were healthy and took few medications with the exception of multivitamins and over the counter (OTC) supplements. Given the small sample size, heterogeneity of the OTC’s, and the fact that prescription pharmaceutical use was rare, medication data were not further analyzed.
Table 1.
Participant characteristics.
| Variable | Disabling (n=8) | Non-Disabling (n=8) | p value |
|---|---|---|---|
| Age (mean, sd) | 74.1 (6.4) | 75.1 (7.3) | 0.77† |
| Ethnicity (white/black/Hispanic) | 7/1/0 | 5/3/0 | 0.60¶ |
| Sex (F/M) | 4/4 | 2/6 | 0.61¶ |
| Income (<$50K/>$50K) | 5/3 | 5/3 | 1.00¶ |
| Education | 0.46¶ | ||
| <12 years | n=0 | n=2 | |
| 12 years | n=1 | n=0 | |
| >12 years | n=7 | n=6 | |
| Hypertension | n=2 | n=1 | 1.00¶ |
| Total months CLBP (median, IQR) | 228 (7–804) | 240 (24–480) | 0.91§ |
| Total months everyday CLBP (median, IQR) | 84 (7–420) | 102 (4–180) | 0.95§ |
| CIRS (mean, sd) | 22 (2.1) | 22 (4.1) | 0.60† |
| MMSE (mean, sd) | 29 (0.92) | 28 (1.5) | 0.13† |
| NAART (mean, sd) | 112 (5.64) | 107 (10.9) | 0.30† |
| RBANS % (mean, sd) | 59.5 (30.4) | 57.3 (32.3) | 0.88† |
| Trails A (mean, sd) | 34.7(10.1) | 42.3(8.17) | 0.12† |
| Trails B (mean, sd) | 81.7(27.8) | 90.7(25.0) | 0.50† |
| SPPB (median, IQR) | 10 (8–12) | 10 (7–12) | 1.00§ |
| Gait speed m/s (median, IQR) | 1.0 (0.88–1.3) | 1.8 (0.57–1.3) | 1.00§ |
Statistics:
Student t-test (equal variances),
Fisher’s exact test, and
Wilcoxon rank.
Key: CIRS (Cumulative Illness Rating Scale); MMSE(Mini-Mental State Exam); NAART (North American Adult Reading Test); RBANS (Repeatable Battery for the Assessment of Neuropsychological Status); SPPB (Short Physical Performance Battery).
Primary aim
Though there are no differences in regional brain volumes between groups, there is significantly lower WM integrity (P < 0.05) of the splenium of the corpus callosum (SCC) in the disabled CLBP group (n=8) compared to non-disabled (n=8). (Fig. 2 and Table 2) The within-group resting-state fMRI showed the expected midline DMN pattern in both groups. The between group contrasts of resting-state fMRI demonstrated some significant differences (Fig. 3). The right medial prefrontal cortex showed higher functional connectivity in the disabled relative to the nondisabled group (degrees of freedom = 7, p < 0.01: MNI −6, 58, 22; Tal −6.69, 50.38, 28.76; Brodmann area 9, Fig 3); and the left lateral prefrontal cortex showed higher functional connectivity in the nondisabled relative to the disabled group (Fig. 3) (degrees of freedom = 7, p < 0.01: MNI 50, 0, 22; Tal 44.99, −3.92, 24.49; Brodmann area 6).
Fig 2.
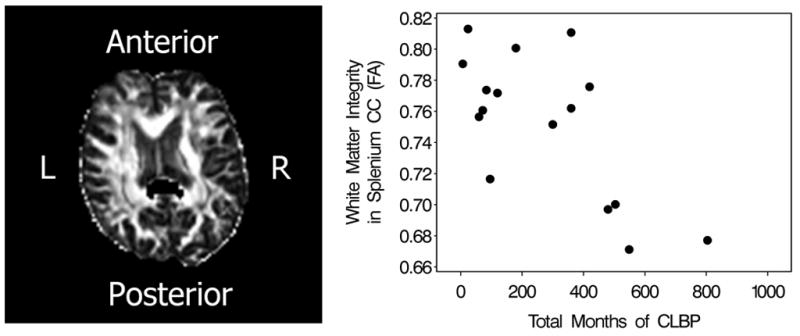
The disabled CLBP group demonstrates significantly lower white matter integrity of the splenium of the corpus callosum (p < 0.02) shown in black. (This is a single subject image in native space with eddy current and geometric distortions corrected.) Combined groups analysis demonstrates a strong negative correlation (rs = −0.59, P < 0.02) between the white matter integrity of the splenium of the corpus callosum and total months of CLBP.
Table 2.
Fractional Anisotropy (FA) between groups comparisons.
| Variable | Disabled | Non-disabled | T value | Pr > |t| |
|---|---|---|---|---|
| Mean (sd) | mean (sd) | |||
| GCCFA | 0.742(0.073) | 0.763(0.032) | −0.88 | 0.4077 |
| MCCFA | 0.659(0.037) | 0.654(0.037 | −1.01 | 0.3449 |
| SCCFA | 0.729(0.044) | 0.774(0.038) | −3.08 | 0.0179 |
| LPLICF | 0.619(0.088) | 0.625(0.036) | 1.46 | 0.1874 |
| RPLICFA | 0.628(0.027) | 0.634(0.041) | −0.05 | 0.9631 |
| LCSVFA | 0.338(0.023) | 0.348(0.024) | −1.11 | 0.3029 |
| RCSVFA | 0.343(0.024) | 0.333(0.025) | 0.12 | 0.9112 |
| LPPCFA | 0.328(0.057) | 0.345(0.057) | −1.37 | 0.2120 |
| RPPCF | 0.369(0.053) | 0.354(0.041) | −1.44 | 0.1937 |
XCCFA: GCCFA (genu), MCCFA (middle), and SCCFA (splenium) of the corpus callosum.
LPLICFA (left posterior limb internal capsule); RPLICFA (right posterior limb internal capsule)
LCSVFA (left centrum semiovale); RCSVFA (right centrum semiovale)
LPPCFA (left pericallosal parietal): RPPCFA (right pericallosal parietal)
Fig. 3.
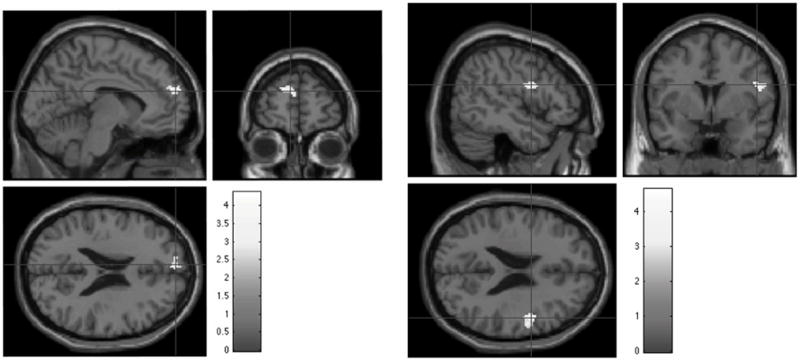
The resting state fMRI subtraction map for the disabled CLBP group–nondisabled (left sagittal, coronal, and axial image set) demonstrates activation of the right medial prefronal cortex (p < 0.01: MNI −6, 58, 22: Tal −6.69, 50.38, 28.76: Brodmann area 9) whereas the nondisabled-disabled subtraction map (right sagittal, coronal, and axial image set) demonstrates activation of left lateral prefrontal cortex at rest (p < 0.01: MNI 50, 0, 22: Tal 44.99, −3.92, 24.49: Brodmann area 6).
Secondary aims
Combined groups analysis reveals a strong positive correlation (rs 0.80, P < 0.0002) between higher WM integrity of the left centrum semiovale (LCS) and faster gait (Fig. 4) and a positive correlation between Trail Making Test A and left lateral prefrontal cortical activation at rest (rs = −0.5916, P < 0.02).
Fig. 4.
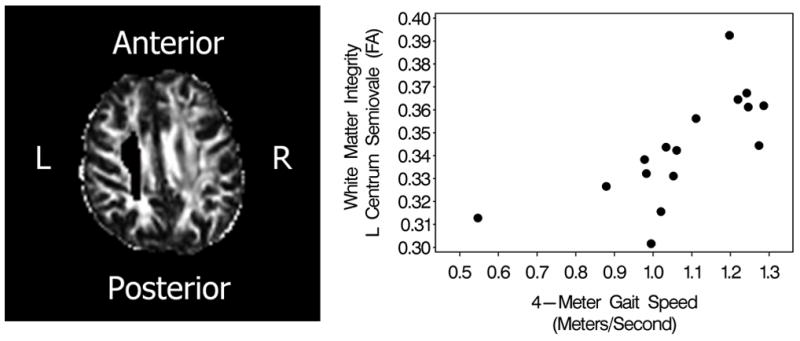
Gait-speed and white matter integrity of the left centrum semiovale (LCS) demonstrates a strong positive correlation (rs = 0.80, P < 0.0002). The LCS is shown to the left as a single subject image in native space with eddy current and geometric distortions corrected. Gait-speed is a sensitive marker and predictor of physical and cognitive disability as well as mortality in older adults (see text).
Post-hoc secondary analysis demonstrates a strong negative correlation (rs = −0.59, P < 0.02) between total months of CLBP and WM integrity of the splenium of the corpus callosum (Fig. 2) and a strong positive correlation between total months of CLBP as well as total months of everyday CLBP with the right centrum semiovale (rs = 0.51, P < 0.04 and rs = 0.64, P < 0.007 respectively).
Discussion
This is the first study in older adults with CLBP to examine differences in brain structure and function in those with self-reported disabling CLBP compared to those with non-disabling CLBP. We found deficits in WM integrity in posterior regions of the brain in those with disabling CLBP and between groups differences in the resting state DMN. Combined groups analyses demonstrated an association of WM structural deficits with poor physical function and pain duration.
Studies of younger adults found an association between GM volume deficits and pain duration [35, 67–68] supporting the hypothesis that brain-structural changes may be secondary to persistent pain. This study found poor WM integrity of the SCC in disabled CLBP participants and a strong negative correlation between total months of CLBP and SCC WM integrity. We also found a positive association between pain duration (total months of CLBP and total months of everyday CLBP) with WM integrity of the right centrum semiovale (rs = 0.51, P < 0.04 and rs = 0.64, P < 0.007 respectively).
Only one investigation has followed the longitudinal impact of chronic pain on brain structure using experimental pain [69]. The investigators found significantly increased GM in the midcingulate, somatosensory cortex, and parietal lobe in young males (mean age 26) who received 20 minutes of pain on 8 consecutive days. As evidenced by fMRI [70], these same subjects habituated to the pain with decreased activity in pain transmitting structures along with increased anterior cingulate cortex activity. If the response to repeated pain includes both decreased and increased activity, it is not surprising we found both decreased (SCC) and increased (right centrum semiovale) WM in association with pain duration. Whether disuse or overuse of neural resources for pain processing (or related emotional processing) over time leads to structural changes and subsequent disability cannot determined.
Our first study found parietal GM volume deficits comparing CLBP to pain-free participants [18]. In this study we found WM connectivity differences of the SCC (which projects to the parietal lobes) comparing disabled CLBP to non-disabled. The parietal lobe is important in pain processing [71–77] and parietal structural deficits have been found in other chronic pain syndromes [78–81]. The potential relationship between GM volume and WM connectivity was investigated in one chronic pain study. Using probabilistic tractography the investigators assessed WM connectivity to atrophic GM regions in complex regional pain syndrome (CRPS) participants compared to healthy controls [82]. They found WM tracts ipsilateral to the atrophic GM regions had reduced connections in long distance tracts and total number of connections.. The authors suggested that GM atrophy, perhaps due to the impact of chronic pain, could lead to WM reorganization. In addition, pain intensity and duration were associated with GM volume deficits.
The first study to associate CLBP with structural deficits found reduced GM in the DLPFC (and thalamus) in young adults with CLBP (mean age = 42.9) compared to pain-free individuals [35]. However, hypertension was not excluded and is associated with GM reductions, notably in the DLPFC [83]. Given the sensitivity of frontal regions to vascular disease [83] and the prevalence of HTN in older adults, it is an important confounder to consider in exploring relationships among chronic pain, disability, and brain structure.
Although our participants were not clinically depressed, our SCC findings should be considered in light of data that demonstrate an association between SCC atrophy and late-onset depression. Comparing healthy controls to older adults with early and late onset depression Ballmaier et al. [84] found depressed patients exhibited significant callosal thinning of the genu and splenium of the CC. Thinning of the genu was restricted to early onset depression, but both genu and splenium thinning were associated with late-onset depression. Given our findings of poorer WM integrity of the SCC in those with disabling CLBP, important questions arise: Is disabling chronic pain a somatic manifestation of late onset-depression or, if chronic pain is changing brain structure and function, is this predisposing older adults to late-onset depression and disability? These questions are important because there is a significant overlap in symptoms reported by older adults with chronic pain and depression [85] and because depressive symptoms are highly associated with CLBP that limits physical activity [9, 86–88]. Although we attempted to exclude current central nervous system disorders that could independently impact brain structure or function, we did not ask patients about past disorders such as depression or other types of psychological trauma. Clearly a longitudinal study is needed to more precisely determine the relationships among pain, changes in brain function and morphology, and disability.
This study found right MPFC activation (BA9) in disabled CLBP participants at rest and left LFC (BA6) activation in the non-disabled. Right frontal lateralization of pain processing has been proposed by a number of investigators [89–91] and observed in neuroimaging studies of acute and chronic pain [77, 92–94] as well as studies of rumination and retrieval of negative memories [95–96]. The prefrontal cortex is important in top-down inhibition of stimulus-provoked emotion [97–98], stimulus-independent emotional processing [99] and expectation [100]. It has been found in primates that parieto-frontal connections with BA6 [101–103] play an important role in voluntary movements. BA6 includes the supplementary motor area (SMA proper and pre-SMA [104]) and premotor area. The SMA influences planning, initiation, and coordination of complex movements [105–106]. A recent study of professional athletes found autobiographical negative emotions activated the MPFC while concomitantly suppressing premotor (BA6) and motor regions [107]. The authors suggested that the reciprocal relationship between the MPFC and premotor/motor regions is distinctive to self-referenced negative emotions in the setting of motor-planning. Studies have found that persons with chronic pain display recall bias for unpleasant and pain-related autobiographical memories [108–109]. It is possible the disabled group in this study devotes more of their neural resources to suppression of the emotional experience, memory, or expectation of CLBP, thus affecting motor-planning and response leading to disability, while the non-disabled group does not devote neural resources to negative emotion suppression and is better able to motor-plan. Other studies have found that suppression of negative emotions is particularly demanding and interferes with task performance [99, 110].
Although there were no differences in PP between disabled and non-disabled participants, combined groups analysis revealed a strong positive correlation (rs = 0.80, P < 0.0002) between WM integrity of the left centrum semiovale (LCS) and gait-speed (See Fig 4). A number of studies have found gait-speed deficits associated with brain structural abnormalities, principally increased WM hyperintensities [111–116] and a strong association between gait-speed [7, 117] and cognitive function deficits [16]. However none of these studies controlled for pain status. It is important to understand the contribution of chronic pain to mobility and cognitive impairment because in older adults, impaired mobility and cognitive function predict future disability [12, 16, 118–119], hospitalization [120], nursing home admission [63, 121], and mortality [63]. As our cohort was relatively healthy, our findings and the literature suggest chronic pain may contribute to brain structural changes leading to mobility disability, although the mechanism is unknown.
Limitations
The main limitation of our study is the small sample size. Although we did not have a pain-free control group, the finding of poorer WM integrity of the SCC in the disabled group is an interesting complement to our previous study, which found decreased volumes of the parietal lobe in CLBP participants compared to pain-free. However, the cross-sectional nature of this investigation prevents us from determining whether the brain differences in disabled compared to non-disabled participants are the result of chronic pain, a predisposition to the development of chronic pain or disability, or some other unidentified factor. Due to the number of assessments, the possibility of spurious results cannot be ruled out. Corrected P values in this exploratory study were not utilized as it was deemed the cost of missing a finding outweighed the value of using corrected P values.
Conclusion: Targeting the Brain in the Treatment of Pain
A fundamental principle of gerontology is that presentation of a new disease depends on the organ system made most vulnerable by previous changes [122]. Our research suggests the brain may be the vulnerable system leading to disability in older adults with CLBP. In support of this hypothesis are a number of studies that have demonstrated success using cognitive behavioral therapies to treat chronic pain in older adults [123–129]. Meditation not only modulates pain processing [129] but in older adults with CLBP decreases self-reported functional limitation [130]. In the study of professional athletes described above, cognitive behavioral intervention focused on performance success resulted in less activation of the MPFC, and restored activation of premotor and motor regions [98]. Taken together, these findings suggest that further investigation of brain structure and function in those with chronic disabling pain and the degree to which the changes observed are modifiable is imperative if we are to effectively treat chronic-pain-related disability in older adults.
Acknowledgments
The authors would like to generously thank the following people for their assistance and hard work in completing this study: Denise Davis at the University of Pittsburgh Medical Center, Presbyterian MR Research Center; Kate Dunfee and Meg Nable research assistants to Dr. Howard Aizenstein; and Dr. R. P. Detwiler. We would also like to thank Dr. Stephanie Studenski for sponsoring Neilly Buckalew under a National Institute of Health T32 training grant from the National Institute on Aging (NIA) and thank you to Dr. Amber Barnato of the CSTP and Dr. Michael Boninger of the Department of Physical Medicne and Rehabilitation for their added mentorship. We also extend our gratitude to the NIA for funding Ms. Buckalew’s training as well as to Dr. Arthur Levine, Senior Vice Chancellor for the Health Sciences and Dean of the School of Medicine at the University of Pittsburgh. This work was also supported by a grant from the University of Pittsburgh’s Claude Pepper Older Americans Independence Center.
References
- 1.Edmond SL, Felson DT. Prevalence of back symptoms in elders. J Rheumatol. 2000;27:220–225. [PubMed] [Google Scholar]
- 2.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clinics in Geriatric Medicine. 2001;17:417–31. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 3.Weiner DK, Haggerty CL, Kritchevsky SB, Harris T, Simonsick EM, Nevitt M, Newman A. How does low back pain impact physical function in independent, well-functioning older adults? Evidence from the Health ABC Cohort and implications for the future. Pain Med. 2003;4:311–20. doi: 10.1111/j.1526-4637.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- 4.Di Iorio A, Abate M, Guralnik JM, Bandinelli S, Cecchi F, Cherubini A, Corsonello A, Foschini N, Guglielmi M, Lauretani F, Volpato S, Abate G, Ferrucci L. From chronic low back pain to disability, a multifactorial mediated pathway: the InCHIANTI study. Spine. 2007;32:E809–15. doi: 10.1097/BRS.0b013e31815cd422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmond SL, Felson DT. Function and back symptoms in older adults. J Am Geriatr Soc. 2003;51:1702–1709. doi: 10.1046/j.1532-5415.2003.51553.x. [DOI] [PubMed] [Google Scholar]
- 6.Ettinger WH, Fried LP, Harris T, Shemanski L, Schulz R, Robbins J. Self-reported causes of physical disability in older people: the Cardiovascular Health Study. J Am Geriatr Soc. 1994;42:1035–1044. doi: 10.1111/j.1532-5415.1994.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 7.Inzitari M, Newman AB, Yaffe K, Boudreau R, de Rekeneire N, Shorr R, Harris TB, Rosano C. Gait Speed Predicts Decline in Attention and Psychomotor Speed in Older Adults: The Health Aging and Body Composition Study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leveille SG, Guralnik JM, Hochberg M, Hirsch R, Ferrucci L, Langlois J, Rantanen T, Ling S. Low back pain and disability in older women: independent association with difficulty but not inability to perform daily activities. J Gerontol Med Sci. 1999;54:M487–M493. doi: 10.1093/gerona/54.10.m487. [DOI] [PubMed] [Google Scholar]
- 9.Reid MC, Guo Z, Towle VR, Kerns RD, Concato J. Factors associated with pain-related disability among older male veterans receiving primary care. J Gerontol Med Sci. 2002;57:M727–M732. doi: 10.1093/gerona/57.11.m727. [DOI] [PubMed] [Google Scholar]
- 10.Weiner DK, Rudy TE, Glick RM, Boston JR, Lieber SJ, Morrow LA, Taylor S. Efficacy of percutaneous electrical nerve stimulation (PENS) for the treatment of chronic low back pain in older adults. J Am Geriatr Soc. 2003;51:599–608. doi: 10.1034/j.1600-0579.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H SPORT Investigators. Surgical versus nonsurgical therapy for lumbar spinal stenosis. NEJM. 2008;358:794. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill TM, Williams CS, Richardson ED, Tinetti ME. Impairments in physical performance and cognitive status as predisposing factors for functional dependence among nondisabled older persons. J Gerontol Med Sci. 1996;51:M283–M288. doi: 10.1093/gerona/51a.6.m283. [DOI] [PubMed] [Google Scholar]
- 13.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. NEJM. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onder G, Penninx BW, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:74–9. doi: 10.1093/gerona/60.1.74. [DOI] [PubMed] [Google Scholar]
- 16.Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56:1618–1625. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 2006;7:60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 18.Buckalew N, Haut MW, Morrow L, Weiner D. Chronic pain is associated with brain volume loss in older adults: preliminary evidence. Pain Med. 2008;9:240–8. doi: 10.1111/j.1526-4637.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- 19.May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis 20. Neurophysiol Clin. 2000;30:263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 21.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: What is moderate pain in millimeters? Pain. 1997;72:95–7. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 22.Weiner D, Pieper C, McConnell E, Martinez S, Keefe F. Pain measurement in elders with chronic low back pain: traditional and alternative approaches. Pain. 1996;67:461–7. doi: 10.1016/0304-3959(96)03150-8. [DOI] [PubMed] [Google Scholar]
- 23.Reid MC, Williams CS, Gill TM. Back pain and decline in lower extremity physical function among community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 2005;60:793–7. doi: 10.1093/gerona/60.6.793. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt R, Launer LJ, Nilsson LG, Pajak A, Sans S, Berger K, Breteler MM, de Ridder M, Dufouil C, Fuhrer R, Giampaoli S, Hofman A. Magnetic resonance imaging of the brain in diabetes: the cardiovascular determinant of dementia (CASCADE) study. Diabetes. 2004;53:687–92. doi: 10.2337/diabetes.53.3.687. [DOI] [PubMed] [Google Scholar]
- 25.Taylor WD, MacFall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, Krishnan JR. Greater lesion volumes in elderly depressed subjects than in control subjects. Psychiatry Research. 2005;139:1–7. doi: 10.1016/j.pscychresns.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Hayano F, Nakamura M, Asami T, Uehara K, Yoshida T, Roppongi T, Otsuka T, Inoue T, Hirayasu Y. Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry Clin Neurosci. 2009;63:266–76. doi: 10.1111/j.1440-1819.2009.01960.x. [DOI] [PubMed] [Google Scholar]
- 27.Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 2009;34:296–302. [PMC free article] [PubMed] [Google Scholar]
- 28.Villarreal G, Hamilton DA, Graham DP, Driscoll I, Qualls C, Petropoulos H, Brooks WM. Reduced area of the corpus callosum in posttraumatic stress disorder. Psychiatry Res Neuroimaging. 2004;131:227–35. doi: 10.1016/j.pscychresns.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Bleecker ML, Bolla-Wilson K, Kawas C, Agnew J. Age-specific norms for the Mini-Mental State Exam. Neurology. 1988;38(10):1565–8. doi: 10.1212/wnl.38.10.1565. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein S, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience. 2003;117:1169–80. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 32.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 33.Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995;43:130–7. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 34.Linn BS, Linn MW, Gurel L. Cumulative Illness Rating Scale. J Am Geriatr Soc. 1968;16:622–26. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 35.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. The Journal of Neuroscience. 2004;24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tisserand DJ, Pruessner JC, Argita EJS, van Boxtel MPJ, Evans AC, Jolles J, Uylings HBM. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–69. [PubMed] [Google Scholar]
- 37.Haut MW, Kuwabara H, Ducatman AM, Hatfield G, Parsons MW, Scott A, Parsons E, Morrow LA. Corpus callosum volume in railroad workers with chronic exposure to solvents. J Occup Environ Med. 2006;48:615–24. doi: 10.1097/01.jom.0000205211.67120.23. [DOI] [PubMed] [Google Scholar]
- 38.Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–82. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 39.Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, Filippi M. Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology. 2006;66:535–9. doi: 10.1212/01.wnl.0000198510.73363.c6. [DOI] [PubMed] [Google Scholar]
- 40.Basser P, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. JMR B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 41.Basser P, Mattiello J, Le Bihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson. 1994;103:47–54. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 42.Basser P, Mattiello J, Le Bihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–67. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix L, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–85. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 44.Sorensen AG, Wu O, Copen WA, Davis TL, Gonzalez RG, Koroshetz WJ, Reese TG, Rosen BR, Wedeen VJ, Weisskoff RM. Human acute cerebral ischemic: detection of changes in water diffusion anisotropy by using MR imaging. Radiology. 1999;212:785–92. doi: 10.1148/radiology.212.3.r99se24785. [DOI] [PubMed] [Google Scholar]
- 45.Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J. Diffusion tensor imaging detects early wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage. 2004;22:1767–74. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 46.Werring D, Toosy A, Clark C, Parker G, Barker G, Miller D, Thompson AJ. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Pschiat. 2000;69:269–72. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–68. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan EV, Adalsteinsson E, Hedehus J, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. NeuroReport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- 49.Salat DH, Tuch DS, Greve DN, van der Kouwe AJW, Hevelone ND, Saleta AK, Rosen BR, Fischl B, Corkin S, Rosas H, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging. 2005;26:1215–27. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Buckner R, Vincent J. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–6. doi: 10.1016/j.neuroimage.2007.01.010. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 51.Raichle M, Snyder A. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–90. doi: 10.1016/j.neuroimage.2007.02.041. discussion 97–9. [DOI] [PubMed] [Google Scholar]
- 52.Raichle M, MacLeod A, Snyder A, Powers W, Gusnard A, Shulman L. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Auer D. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the ‘resting’ brain. Magn Reson Imaging. 2008;26:055–64. doi: 10.1016/j.mri.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–98. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 55.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–32. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 56.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 57.Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–64. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crawford J. Current and premorbid intelligence measures in neuropsychological assessment. In: Crawford JR, Parker DM, McKinlay WW, editors. A Handbook of Neuropsychological Assessment. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. pp. 21–50. [Google Scholar]
- 60.Randolph C, Tierney M, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–9. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 61.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Archives of Clinical Neuropsychology. 2004;19:203–14. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 62.Lemay S, Bedard MA, Rouleau I, Tremblay PL. Practice effect and test-retest reliability of attentional and executive tests in middle-aged to elderly subjects. Clin Neuropsychol. 2004;18:284–302. doi: 10.1080/13854040490501718. [DOI] [PubMed] [Google Scholar]
- 63.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol Med Sci. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 64.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, Fox M, Guralnik JM. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 65.Inzitari M, Newman AB, Yaffe K, Boudreau R, de Rekeneire N, Shorr R, Harris TB, Rosano C. Gait Speed Predicts Decline in Attention and Psychomotor Speed in Older Adults: The Health Aging and Body Composition Study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosano C, Simonsick EM, Harris TB, Kritchevsky SB, Brach J, Visser M, Yaffe K, Newman ABftHACRG. Association between physical and cognitive function in healthy elderly: the Health, Aging and Body Composition Study. Neuroepidemiology. 2005;24:8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- 67.Schmidt-Wilcke T, Leinisch E, Straube A, Kampfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- 68.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teutsch S, Herken W, Bingel U, Schoell E, May A. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage. 2008;42:845–9. doi: 10.1016/j.neuroimage.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 70.Bingel U, Schoell E, Herken W, Buchel C, May A. Habituation to painful stimulation involves the antinociceptive system. Pain. 2007;131:21–30. doi: 10.1016/j.pain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 71.Albanese MC, Duerden EG, Rainville P, Duncan GH. Memory traces of pain in human cortex. J Neurosci. 2007;27:4612–20. doi: 10.1523/JNEUROSCI.0695-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duncan GH, Albanese MC. Is there a role for the parietal lobes in the perception of pain? Adv Neurol. 2003;93:69–86. [PubMed] [Google Scholar]
- 73.Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J Neurosci. 2000;20:7438–45. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain: A review and meta-analysis. Neurophysiol Clin. 2000;30:263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 75.Apkarian AV, Darbar A, Krauss BR, Gelnar PA, Szeverenyi NM. Differentiating cortical areas related to pain perception from stimulus identification: Temporal analysis of fMRI activity. J Neurophysiol. 1999;81:2956–63. doi: 10.1152/jn.1999.81.6.2956. [DOI] [PubMed] [Google Scholar]
- 76.Casey KL, Minoshima S, Berger KL, et al. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–7. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- 77.Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–8. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- 78.Kuchinad A, Schweinhardt P, Seminowicz DA, et al. Accelerated brain gray matter loss in fibromyalgia patients: Premature aging of the brain? J Neurosci. 2007;27(15):4004–7. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmidt-Wilcke T, Leinisch E, Straube A, et al. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65(9):1483–6. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- 80.Peyron R, Garcia-Larrea L, Gregoire MC, et al. Parietal and cingulate processes in central pain. A combined positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) study of an unusual case. Pain. 2000;84:77–87. doi: 10.1016/S0304-3959(99)00190-6. [DOI] [PubMed] [Google Scholar]
- 81.Schmahmann JD, Leifer D. Parietal pseudothalamic pain syndrome. Clinical features and anatomic correlates. Arch Neurol. 1992;49:1032–7. doi: 10.1001/archneur.1992.00530340048017. [DOI] [PubMed] [Google Scholar]
- 82.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–81. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience. 2003;117:1169–80. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 84.Ballmaier M, Kumar A, Elderkin-Thompson V, Narr KL, Luders E, Thompson PM, Hojatkashani C, Pham D, Heinz A, Toga AW. Mapping callosal morphology in early-and late-onset elderly depression: an index of distinct changes in cortical connectivity. Neuropsychopharmacology. 2008;33:1528–36. doi: 10.1038/sj.npp.1301538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karp JF, Rudy TE, Weiner DK. Persistent Pain Biases Item Response on the Geriatric Depression Scale: Preliminary Evidence for Validity of the GDS-PAIN. Pain Medicine. 2008;9:33–43. doi: 10.1111/j.1526-4637.2007.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartvigsen J, Frederiksen H, Christensen K. Physical and mental function and incident low back pain in seniors: a population-based two-year prospective study of 1387 Danish Twins aged 70 to 100 years. Spine. 2006;31:1628–32. doi: 10.1097/01.brs.0000222021.00531.ea. [DOI] [PubMed] [Google Scholar]
- 87.Reid MC, Williams CS, Concato J, Tinetti ME, Gill TM. Depressive symptoms as a risk factor for disabling back pain in community-dwelling older persons. J Am Geriatr Soc. 2003;51:1710–7. doi: 10.1046/j.1532-5415.2003.51554.x. [DOI] [PubMed] [Google Scholar]
- 88.McNamara P, Stavitsky K, Harris E, Szent-Imrey O, Durso R. Mood, side of motor symptom onset and pain complaints in Parkinson’s disease. Int J Geriatr Psychiatry. 2009 doi: 10.1002/gps.2374. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 89.Hari R, Portin K, Kettenmann B, Jousmaki V, Kobal G. Right hemisphere preponderance of responses to painful CO2 stimulation of the human nasal mucosa. Pain. 1997;72:145–51. doi: 10.1016/s0304-3959(97)00023-7. [DOI] [PubMed] [Google Scholar]
- 90.Hsieh JC, Hannerz J, Ingvar M. Right lateralized central processing for pain of nitroglycerin-induced cluster headache. Pain. 1996;67:59–68. doi: 10.1016/0304-3959(96)03066-7. [DOI] [PubMed] [Google Scholar]
- 91.Neri M, Agazzani E. Aging and right-left asymmetry in experimental pain measurement. Pain. 1984;19:43–48. doi: 10.1016/0304-3959(84)90063-0. [DOI] [PubMed] [Google Scholar]
- 92.Derbyshire SW, Jones AK. Cerebral responses to a continual tonic pain stimulus measured using positron emission tomography. Pain. 1998;76:127–35. doi: 10.1016/s0304-3959(98)00034-7. [DOI] [PubMed] [Google Scholar]
- 93.Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995;63:225–36. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- 94.Iadarola MJ, Berman KF, Zeffiro TA, Byas-Smith MG, Gracely RH, Max MB, Bennett GJ. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain. 1998;121:931–47. doi: 10.1093/brain/121.5.931. [DOI] [PubMed] [Google Scholar]
- 95.Edwards-Lee TA, Saul R. Neuropsychiatry of the right frontal lobe. In: Miller BL, Cummings L, editors. The human frontal lobes: Functions and disorders. New York: The Guilford Press; 1991. pp. 304–320. [Google Scholar]
- 96.Schiffer F, Teicher MH, Papanicolaou AC. Evoked potential evidence for right brain activity during recall of traumatic memories. Journal of Neuropsychiatry. 1995;7:169–175. doi: 10.1176/jnp.7.2.169. [DOI] [PubMed] [Google Scholar]
- 97.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 99.Sommer M, Dohnel K, Sodian B, Meinhardt J, Thoermer C, Hajak G. Neural correlates of true and false belief reasoning. Neuroimage. 2007;35:1378–84. doi: 10.1016/j.neuroimage.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 100.Bermpohl F, Pascual-Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, Alsop D, Schlaug G, Northoff G. Attentional modulation of emotional stimulus processing: an fMRI study using emotional expectancy. Hum Brain Mapp. 2006;27:662–77. doi: 10.1002/hbm.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Bio. 2004;174:I–VIII. 1–89. doi: 10.1007/978-3-642-18910-4. [DOI] [PubMed] [Google Scholar]
- 102.Porter R. The Kugelberg lecture. Brain mechanisms of voluntary motor commands: a review. Electroencephalogr Clin Neurophysiol. 1990;76:282–93. doi: 10.1016/0013-4694(90)90029-j. [DOI] [PubMed] [Google Scholar]
- 103.Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 104.Matsuzaka Y, Aizawa H, Tanji J. A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: neuronal activity during a learned motor task. J Neurophysiol. 1992;68:653–62. doi: 10.1152/jn.1992.68.3.653. [DOI] [PubMed] [Google Scholar]
- 105.Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- 106.Shima K, Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. J Neurophysiol. 1998;80:3247–3260. doi: 10.1152/jn.1998.80.6.3247. [DOI] [PubMed] [Google Scholar]
- 107.Davis H, IV, Liotti M, Ngan ET, Woodward TS, Van Snellenberg JX, van Anders SM, Smith A, Mayberg HS. fMRI BOLD Signal Changes in Elite Swimmers While Viewing Videos of Personal Failure. Brain Imaging and Behavior. 2008;2:84–93. [Google Scholar]
- 108.Gamsa A. The role of psychological factors in chronic pain. A half century of study. Pain. 1994;57:5–15. doi: 10.1016/0304-3959(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 109.Wright J, Morley S. Autobiographical memory and chronic pain. British Journal of Clinical Psychology. 1995;34:255–265. doi: 10.1111/j.2044-8260.1995.tb01460.x. [DOI] [PubMed] [Google Scholar]
- 110.Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–40. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosano C, Branch J, Longstreth WT, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in higher-functioning older adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 112.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- 113.Sachdev PS, Wen W, Christensen H, Jorm AF. White matter hyperintensities are related to physical disability and poor motor function. J Neurol Neurosurg Psychiatry. 2005;76:362–367. doi: 10.1136/jnnp.2004.042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wolfson L, Wei X, Hall CB, Panzer V, Wakefield D, Benson RR, Schmidt JA, Warfield SK, Guttmann CR. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005;232:23–27. doi: 10.1016/j.jns.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 115.Starr JM, Leaper SA, Murray AD, Lemmon HA, Staff RT, Deary IJ, Whalley LJ. Brain white matter lesions detected by magnetic resonance [correction of resonance] imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74:94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Whitman GT, Tang Y, Lin A, Baloh RW. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57:990–994. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- 117.Atkinson HH, Rosano C, Simonsick EM, Williamson JD, Davis C, Ambrosius WT, Rapp SR, Cesari M, Newman AB, Harris TB, Rubin SM, Yaffe K, Satterfield S, Kritchevsky SB. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62:844–50. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 118.Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62:1134–41. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Royall DR, Chiodo LK, Mouton C, Polk MJ. Cognitive predictors of mortality in elderly retirees: Results from the freedom house study. Am J Geriatr Psychiatry. 2007;15:243–251. doi: 10.1097/01.JGP.0000240824.84867.02. [DOI] [PubMed] [Google Scholar]
- 120.Lavery LL, Starenchak SM, Flynn WB, Stoeff MA, Schaffner R, Newman AB. The clock drawing test is an independent predictor of incident use of 24-hour care in a retirement community. J Gerontol A Biol Sci Med Sci. 2005;60A:928–932. doi: 10.1093/gerona/60.7.928. [DOI] [PubMed] [Google Scholar]
- 121.Pennix BWJH, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol Med Sci. 2000;55A:M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 122.Resnick NM, Marcantonio ER. How should clinical care of the aged differ? Lancet. 1997;350:1157–58. doi: 10.1016/S0140-6736(05)63817-2. [DOI] [PubMed] [Google Scholar]
- 123.Lunde LH, Nordhus IH, Pallesen S. The effectiveness of cognitive and behavioural treatment of chronic pain in the elderly: a quantitative review. J Clin Psychol Med Settings. 2009;16:254–62. doi: 10.1007/s10880-009-9162-y. [DOI] [PubMed] [Google Scholar]
- 124.Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7:261–271. doi: 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 125.Ostelo RW, van Tulder MW, Vlaeyen JW, Linton SJ, Morley SJ, Assendelft WJ. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev. 2005;(1):CD002014. doi: 10.1002/14651858.CD002014.pub2. [DOI] [PubMed] [Google Scholar]
- 126.Reid MC, Otis J, Barry LC, Kerns RD. Cognitive-behavioral therapy for chronic low back pain in older persons: a preliminary study. Pain Med. 2003;4:223–30. doi: 10.1046/j.1526-4637.2003.03030.x. [DOI] [PubMed] [Google Scholar]
- 127.Middaugh SJ, Pawlick K. Biofeedback and behavioral treatment of persistent pain in the older adult: a review and a study. Appl Psychophysiol Biofeedback. 2002;27:185–202. doi: 10.1023/a:1016208128254. [DOI] [PubMed] [Google Scholar]
- 128.Cipher DJ, Fernandez E, Clifford AP. Cost-effectiveness and health care utilization in a multidisciplinary pain center: Comparison of three treatment groups. J Clin Psychol Med Settings. 2001;8:237–244. [Google Scholar]
- 129.Orme-Johnson DW, Schneider RH, Son YD, Nidich S, Cho ZH. Neuroimaging of meditation’s effect on brain reactivity to pain. Neuroreport. 2006;17:1359–63. doi: 10.1097/01.wnr.0000233094.67289.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: A randomized controlled pilot study. Pain. 2008;134:310–319. doi: 10.1016/j.pain.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]