Abstract
Despite their synthetic significance there is a general lack of asymmetric vinylogous aldol reactions that tolerate variations of both the silyloxy furans and aldehydes. We have developed a new chiral organic catalyst based on a carboxylate-ammonium salt prepared form a thiourea-amine and a carboxylic acid. This new catalyst enabled us to develop an efficient asymmetric vinylogous aldol reaction of unprecedented scope with respect to both 2-trimethylsilyloxy furans and aldehydes.
Chiral butenolides are common structural subunit in natural products1 and provide valuable chiral building block for the asymmetric synthesis of biologically active compound.2 Owing to their synthetic significance, the development of efficient catalytic methods for the generation of optically active butenolides has attracted considerable attention.3 In particular asymmetric vinylogous aldol reactions of 2-silyloxy furan and prochiral carbonyl compounds have been investigated with both chiral metal and organic catalysts.4 Significant progress has also been made in the promotion of catalytic asymmetric vinylogous aldol reactions between dihydrofuranone and aldehydes.5 Nonetheless, the development of such asymmetric vinylogous aldol reactions that afford useful level of enantioselectivity and diastereoselectivity with both aryl and alkyl aldehydes still represents a challenging task. Moreover there is a general lack of catalytic methods that tolerate variations of both the silyloxy furans and aldehydes. For example, there are only two examples of anti-selective, highly enantioselective vinylogous aldol reactions of silyloxy furans and aliphatic aldehydes, which are achieved with a chiral Cu-bisoxazoline complex and chiral phase transfer catalyst, respectively.4b,4g However, the former affords useful enantioselectivity and diastereoselectivity for only benzyloxyacetaldehyde while the latter for only the 4-methyl 2-silyloxy furan. Herein, we report the design and development of a chiral organic catalyst system that effectively promotes anti-selective asymmetric vinylogous aldol reactions of various 2-trimethylsilyloxyfurans with aryl, alkenyl and alkyl aldehydes.
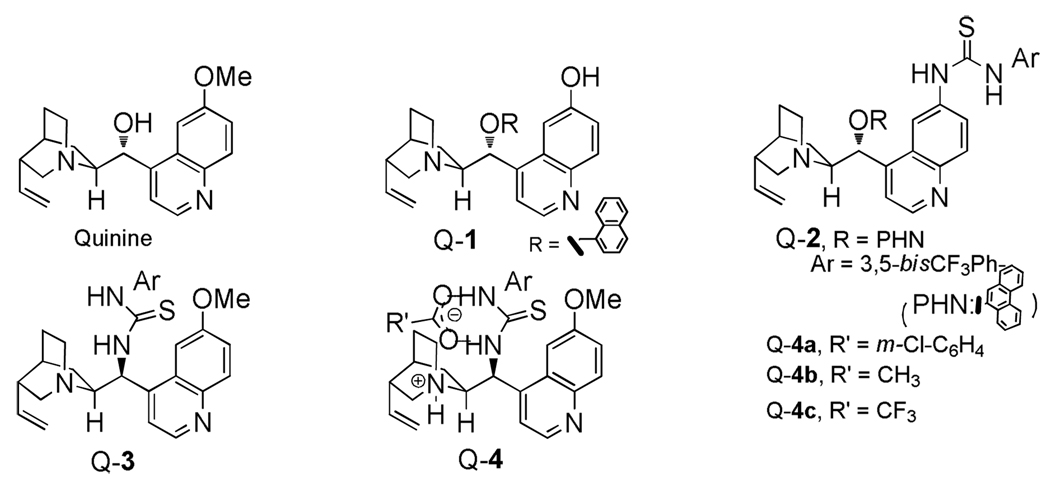
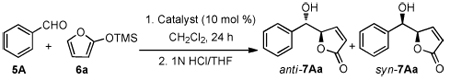
Earlier we reported a highly enantioselective addition of TMSCN to α-acetal ketones with cinchona alkaloids as monofunctional chiral Lewis base catalysts.6 Recently, modified cinchona alkaloids bearing various hydrogen bond donors have been shown by us and others as efficient bifunctional chiral organic catalysts with a broad range of asymmetric reactions, including 1,2-additions to carbonyls.7 These precedents prompted us to explore 6 ′-OH, 6′- and 9-thiourea cinchona alkaloids as bifunctional catalysts for the vinylogous aldol reactions of 2-trimethylsilyloxyfuran (6a) and benzylaldehyde (5A). As summarized in Table 1 quinine and 6′-OH cinchona alkaloids Q-1 gave very poor conversion (entries 1 & 2). On the other hand, the thiourea catalysts Q-2 and Q-3 were found to be more active. However, even the better catalyst Q-3 provided only a modest enantioselectivity.
Table 1.
Vinylogous Aldol Reaction with Cinchona Alkaloids
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| entry | Catalyst | T(°c) | % Convb | drb (anti:syn) |
% ee (anti)c |
enry | Catalyst | T(°c) | % Convb | drb (anti:syn) |
% ee (anti)c |
| 1 | Quinine | 23 | ~8 | ND | - | 5 | Q-4a | 23 | 64 | 74:26 | 79 |
| 2 | Q-1 | 23 | ~5 | ND | - | 6 | Q-4b | 23 | 68 | 69:31 | 70 |
| 3 | Q-2 | 23 | 86 | 59:41 | −26 | 7 | Q-4c | 23 | 77 | 81:19 | 88 |
| 4 | Q-3 | 23 | 54 | 63:37 | 73 | 8d | Q-4c | −20 | 96 | 95:5 | 95 |
Unless noted, reactions were performed with 0.1 mmol of 5A and 0.15 mmol of 6a in 0.2 ml of CH2Cl2 with 10 mol% of catalyst.
Determined by 1H NMR analysis.
Determined by HPLC analysis.
Reaction was performed in 0.1 ml of Et2O/CH2Cl2 (1/1) for 96 h.
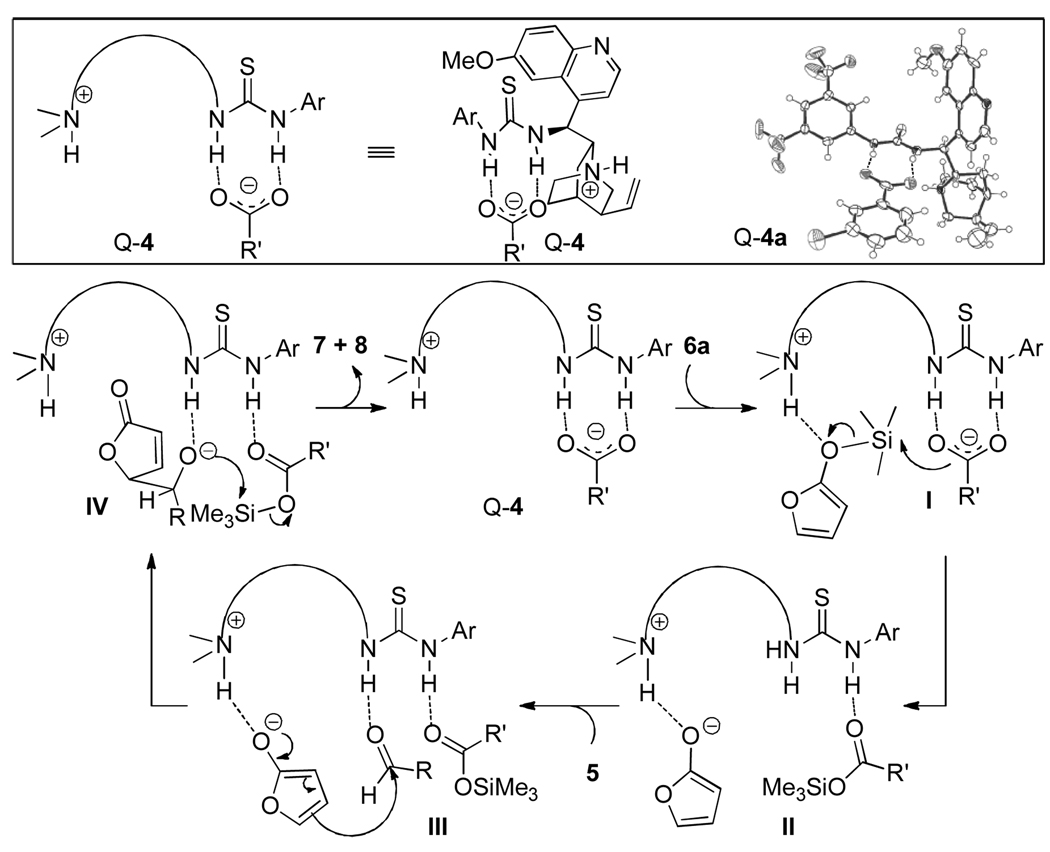
We recently determined the structure by X-ray crystallography of a carylate ammonium salt Q-4a, which was derived from a solution of Q-3 and m-chlorobenzoic acid in methanol (Scheme 1). As expected the quinuclidine nitrogen was protonated. Interestingly, the carboxylate was found to bind to the thiourea moiety through hydrogen-bonding interactions8 instead of to form a tight ion pair with the quinuclidinium. We envisaged a catalytic cycle for a Q-4a-promoted vinylogous aldol reaction (Scheme 1). Presumably, the hydrogen-bonded carboxylate could react with silyloxy furan 6a to form the corresponding trimethylsilylester and the 2-furoxy anion while releasing a thiourea-NH that could serve as a hydrogen bond donor to activate aldehyde 5A (Intermediates I & II). With the 2-furoxy anion and aldehyde 5A interacting with the protonated quinuclidine and thiourea-NH, respectively, the reaction between the two reactants might proceed in a highly diastereoselective and enantioselective fashion (Intermediate III). Importantly, the silyl transfer step should be facile in light of the proximity of the trimethylsilylester and the aldolate in intermediate IV. In this proposed catalytic cycle the carboxylate is postulated to serve a dual-role; activating the silyloxy furan 6a and facilitating the silyl transfer from 6a to the aldolate product. It should be noted that the second function was also speculated to be responsible for the beneficial effect of the carboxylate ligand on asymmetric aldol9 and vinylogous aldol reactions10 mediated by metal-based chiral Lewis acids.
Scheme 1.
Plausible Catalytic Cycle for Vinylogous Aldol Reaction
We were pleased to find that the salt Q-4a, prepared by simply mixing Q-3 and m-chloro benzoic acid in a 1:1 ratio, furnished improved activity and selectivity over those by Q-3 under identical conditions (entry 4 vs 5, Table 1). Upon investigations of various carboxylic acids we found the reaction occurred in a highly diastereo- and enantioselective fashion with the trifluoroacetic acid-derived salt Q-4c (Table 1, entry 7). A further improved reaction was achieved at −20°C in Et2O:CH2Cl2 (1:1), affording the anti-adduct 7Aa in 95/5 dr and 95% ee.
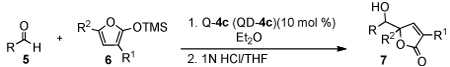
Under the optimized condition the Q-4c catalyzed reaction of 6a and various aldehydes (5A-P) were investigated. Aryl and heteroaryl aldehydes (5A-K) were converted into the corresponding anti-adduct 7 in 84/16 to 95/5 dr, 91–95% ee and 71–94% yield. Useful diastereoselectivity and enantioselectivity could be attained with alkenyl (5L) and, most significantly, alkyl aldehydes (5M-P). These results constitute a significant progress for asymmetric vinylogous aldol reactions of silyloxy furans and this class of synthetically useful but highly challenging aldehydes. We also investigated the reaction with various substituted 2-trimethylsilyloxy furan (6b-e). Remarkably, the catalyst 4c even afforded useful selectivity for reactions of sterically hindered 5-substituted-2-trimethylsilyloxy furans 6c-e, which generate chiral adducts bearing adjacent tertiary-quaternary centers.
In summary, we have developed a readily accessible and efficient organic catalyst based on a carboxylate ammonium salt prepared by mixing a thiourea-amine and a carboxylic acid. This new catalyst enabled us to develop an efficient asymmetric vinylogous aldol reaction of 2-trimethylsilyloxy furans and aldehydes of unprecedented scope with respect to both reactants.11 As a broad range of both chiral amine-ureas and carboxylic acids are readily available, such chiral salts provide a new class of easily tunable chiral catalysts. Finally, the cooperative and multifunctional catalysis by these chiral salts designed for the promotion of the asymmetric vinylogous aldol reactions should in principle be applicable to a range of other asymmetric reactions.
Supplementary Material
Figure 1.
The structure of Cinchona Alkaloid Catalysts
Table 2.
Reactions of 5 and 6a with Q-4c (QD-4c).
| entrya | 5 | R | T(°C) | yield/%b | drc (anti:syn) |
% ee (anti)d |
|---|---|---|---|---|---|---|
| 1 | 5A | Ph | −20 | 94 (91) | 95:5 (91:9) | 95 (91)e |
| 2 | 5B | 4-F-C6H4 | −20 | 95 | 96:4 | 95 |
| 3 | 5C | 4-CF3-C6H4 | −20 | 96 | 96:4 | 93 |
| 4 | 5D | 4-Cl-C6H4 | −20 | 93 | 94:6 | 93 |
| 5f | 5E | 4-Br-C6H4 | −20 | 97 (98) | 96:4 (94:6) | 94 (92)e |
| 6 | 5F | 4-Me-C6H4 | −10 | 75 | 94:6 | 90 |
| 7 | 5G | 3-MeO-C6H4 | −50 | 96 | 95:5 | 95 |
| 8 | 5H | −20 | 98 (92) | 95:5 (91:9) | 95 (92)e | |
| 9 | 5I | −20 | 71 | 84:16 | 93 | |
| 10 | 5J | −10 | 78 | 92:8 | 91 | |
| 11 | 5K | −30 | 98 | 90:10 | 93 | |
| 12 | 5L | −20 | 74(60) g | 81:19 | 86 | |
| 13h | 5M | CH3 | −50 | 76 | 72:28 | 93 |
| 14 | 5N | −10 | 51 | 73:27 | 88 | |
| 15h | 5O | CH3(CH2)5CH2 | 23 | 64 | 82:18 | 80 |
| 16i | 5P | c-C6H11 | 0 | 47(37)g | 78:22 | 84 |
Unless noted, reactions were performed with 0.25 mmol of 5, 0.37 mmol of 6a and 10 mol% of Q-4c in 0.25 ml of solvent.
Unless noted, isolated yield of vinylogous aldol adduct 7 with ratio of anti/syn diastereomers indicated.
Determined by 1H NMR analysis of crude reaction mixture.
Determined by HPLC analysis.
The results in parentheses were obtained with QD-4c; see SI for details.
The absolute configuration of the aldol adduct anti-7Ea was established by X-ray crystallographic analysis (see SI).
Isolated yield of pure anti-7.
Reaction was run with 1.25 mmol of 5M and 0.25 mmol of 6a and 10 mol% of Q-4c in 0.25 mL of solvent.
Reaction was performed with 20 mol% of Q-4c.
Table 3.
Reaction with Substituted 2-Silyloxy Furan.
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| entrya | 5 | R | 6 | R1 | R2 | T(°C) | Yieldb (%) |
drc | % eed (major) |
| 1 | 5A | Ph | 6b | Me | H | 0 | 75 | 93:7e | 94 |
| 2 | 5N | PhCH2CH2 | 6b | Me | H | 0 | 62 | 80:20e | 87 |
| 3f | 5A | Ph | 6c | Me | Me | rt | 77 | 95:5g | 85 |
| 4 | 5A | Ph | 6d | H | CH3cH2 | −10 | 65 | 95:5g | 81 |
| 5 | 5A | Ph | 6b | H | CH2CH=CH2 | −10 | 70 | 96:4g | 84 |
Unless noted, reactions were run with 0.1 mmol of aldehyde, 0.15 mmol of TMSOF and 10 mol% of Q-4c in 0.1 ml of Et2O.
Isolated yield of vinylgour aldol adduct 7 with ratio of anti/syn diastereomers indicated in the supporting information (SI).
Determined by 1H NMR analysis of crude reaction mixture.
ee of major diastereomer as determined by HPLC analysis.
Radio of anti/syn diasteromers.
Reaction was run with 10 mol% of QD-4c (see SI).
anti/syn diasteremers not determined.
ACKNOWLEDGMENT
We are grateful for the generous financial support from National Institutes of Health (GM-61591).
Footnotes
SUPPORTING INFORMATION: Experimental procedures and characterization of the products. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Carmen Zafra-Polo M, Figadère B, Gallardo T, Tormo J, Cortes D. Phytochemistry. 1998;48:1087. [Google Scholar]
- 2.(a) de March P, Figueredo M, Font J, Raya J, Alvarez-Larena A, Piniella JF. J. Org. Chem. 2003;68:2437. doi: 10.1021/jo026705w. [DOI] [PubMed] [Google Scholar]; (b) Gao S, Wang Q, Chen C. J. Am. Chem. Soc. 2009;131:1410. doi: 10.1021/ja808110d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yoshimitsu T, Makino T, Nagaoka H. J. Org. Chem. 2004;69:1993. doi: 10.1021/jo0303721. [DOI] [PubMed] [Google Scholar]
- 3.For reviews of vinylogous aldol reactions, see: Casiraghi G, Zanardi F, Appendino G, Rassu G. Chem. Rev. 2000;100:1929. doi: 10.1021/cr990247i. Denmark SE, Jr, J.R.H, Beutner GL. Angew. Chem. Int. Ed. 2005;44:4682. doi: 10.1002/anie.200462338. Kalesse M. Top. Curr. Chem. 2005;244:43.
- 4.For enantioselective vinylogous aldol reactions of 2-silyloxyfurans, see: Szlosek M, Franck X, Figadere B, Cave A. J. Org. Chem. 1998;63:5169. Evans DA, Kozlowski MC, Murry JA, Burgey CS, Campos KR, Connell BT, Staples RJ. J. Am. Chem. Soc. 1999;121:669. Szlosek M, Figadère B. Angew. Chem. Int. Ed. 2000;39:1799. doi: 10.1002/(sici)1521-3773(20000515)39:10<1799::aid-anie1799>3.0.co;2-z. Matsuoka Y, Irie R, Katsuki T. Chem. Lett. 2003;32:584. Onitsuka S, Matsuoka Y, Irie R, Katsuki T. Chem. Lett. 2003;32:974. Nagao H, Yamane Y, Mukaiyama T. Chem. Lett. 2007;36:8. doi: 10.1002/asia.200600228. Palombi L, Acocella MR, Celenta N, Massa A, Villano R, Scettri A. Tetrahedron Asymmetry. 2006;17:3300. Sedelmeier J, Hammerer T, Bolm C. Org. Lett. 2008;10:917. doi: 10.1021/ol703065x.
- 5.Ube H, Shimada N, Terada M. Angew. Chem. Int. Ed. 2010;49:1858. doi: 10.1002/anie.200906647. [DOI] [PubMed] [Google Scholar]
- 6.Tian S-K, Hong R, Deng L. J. Am. Chem. Soc. 2003;125:9900. doi: 10.1021/ja036222p. [DOI] [PubMed] [Google Scholar]
- 7.For recent reviews, see Doyle AG, Jacobsen EN. Chem. Rev. 2007;107:5713. doi: 10.1021/cr068373r. Wang Y, Deng L.Ojima I.Asymmetric Acid-Base Bifunctional Catalysis with Organic Molecules Catalytic Asymmetric Synthesis 2010Hoboken, New Jersey: John Wiley & Sons Inc.59 For a report, see Li H, Wang B, Deng L. J. Am. Chem. Soc. 2004;126:9906. doi: 10.1021/ja047281l.
- 8.Fan E, Van Arman SA, Kincaid S, Hamilton AD. J. Am. Chem. Soc. 1993;115:369. [Google Scholar]
- 9.(a) Furuta K, Maruyama T, Yamamoto H. J. Am. Chem. Soc. 1991;113:1041. [Google Scholar]; (b) Parmee ER, Tempkin O, Masamune S, Abiko A. J. Am. Chem. Soc. 1991;113:9365. [Google Scholar]
- 10.(a) Carreira EM, Singer RA, Lee W. J. Am. Chem. Soc. 1994;116:8837. [Google Scholar]; (b) Kruger J, Carreira EM. J. Am. Chem. Soc. 1998;120:837. [Google Scholar]
- 11.A recent report confirmed that cinchona alkaloid 3 by itself is not an efficient catalyst for vinylogous aldol reaction of 2-trimethylsilyloxy furans: Zhu N, Ma B-C, Zhang Y, Wang W. Adv. Synth. Catal. 2010;352:1291.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.