Abstract
Rotaviruses (RVs) cause severe gastroenteritis in infants and young children; yet, several strains have been isolated from newborns showing no signs of clinical illness. Two of these neonatal strains, RV3 (G3P[6]) and 116E (G9P[11]), are currently being developed as live-attenuated vaccines. In this study, we sequenced the eleven-segmented double-stranded RNA genomes of cell culture-adapted RV3 and 116E and compared their genes and protein products to those of other RVs. Using amino acid alignments and structural predictions, we identified residues of RV3 or 116E that may contribute to attenuation or influence vaccine efficacy. We also discovered residues of the VP4 attachment protein that correlate with the capacity of some P[6] strains, including RV3, to infect newborns versus older infants. The results of this study enhance our understanding of the molecular determinants of RV3 and 116E attenuation and are expected to aid in the ongoing development of these vaccine candidates.
Keywords: rotavirus, neonatal, vaccine, attenuated, genome, RV3, 116E
Introduction
Group A rotaviruses (RVs) are important pathogens that cause acute, dehydrating gastroenteritis in infants and young children. The burden of disease is severe, particularly in developing countries where RV infections lead to more than 500,000 deaths annually (Parashar et al., 2006). RVs are non-enveloped, triple-layered icosahedral particles that enclose an eleven-segmented, double-stranded (ds) RNA genome (Pesavento et al., 2006). Together, the genome codes for six structural proteins (VP1-VP4, VP6, and VP7) and five or six non-structural proteins (NSP1-NSP5, and sometimes NSP6) (Estes and Kapikian, 2007). Individual RV strains have traditionally been classified into serotypes based on the antibody responses generated against the outermost structural proteins VP7 (G-serotypes) and VP4 (P-serotypes) (Estes and Kapikian, 2007). Due to the ease of sequencing, RVs are now classified into G/P-genotypes based on the relatedness of the genes encoding VP7 and VP4 (Estes and Kapikian, 2007; Matthijnssens et al., 2008a). Molecular sequencing of RVs has also led to the development of a classification system for the internal genes (i.e., dsRNA segments encoding proteins other than VP7 and VP4). In this system, each internal gene is assigned a particular genotype based on established nucleotide identity cut-off percentages (Matthijnssens et al., 2008a; Matthijnssens et al., 2008b). Now, the acronym Gx-P[x]-Ix-Rx-Cx-Mx-Ax-Nx-Tx-Ex-Hx is used to classify the VP7-VP4-VP6-VP1-VP2-VP3-NSP1-NSP2-NSP3-NSP4-NSP5/6-encoding segments. The majority of human RVs sequenced to date contain either genotype 1 (Wa-like) or genotype 2 (DS-1-like) internal genes. However, reassortment events can lead to human strains containing dsRNA segments with other genotypes.
Although the mechanism by which RV infection elicits immunological protection is not fully understood, G/P-type-specific neutralizing antibodies have been shown to play an important role (Ward, 2003). Strains with G/P-type combinations of G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8] are the most prevalent causes of disease in humans worldwide, and thus, are targets of the two currently licensed RV vaccines (Santos and Hoshino, 2005). RotaTeq (Merck) contains five live-attenuated, reassortant viruses with human VP7 (G1, G2, G3 and G4) and VP4 (P[8]) genes in a predominantly bovine RV background (strain WC3) (Matthijnssens et al., 2010a; Ciarlet and Schodel, 2009; Heaton and Ciarlet, 2007). In contrast, Rotarix (GlaxoSmithKline) is a live-attenuated, G1P[8] human RV (strain 89-12) containing genotype 1 internal genes (Ward, 2003). Both vaccines have proven safe and effective at protecting against severe diarrheal disease in industrialized countries and Latin America (Ruiz-Palacios et al., 2006; Vesikari et al., 2006; Ward, 2003). However, the efficacy of RotaTeq and Rotarix in developing countries is expected to be reduced, which may be related to viral serotype diversity among other factors (Madhi et al., 2010; Tate et al., 2010). Additionally, the high monetary cost of these current vaccines may limit their availability in regions of the world where they are most needed. As a result, there is a global health initiative to develop new RV vaccines that can be manufactured on-site at a lower cost. Two vaccine candidates being considered are the live-attenuated human strains RV3 (G3P[6]) and 116E (G9P[11]).
RV3 and 116E were isolated from asymptomatic newborns (<28 days old) in hospital neonatal units in Australia (1977) and India (1985), respectively (Albert et al., 1987a; Glass et al., 2005). Follow-up studies showed that the RV3- or 116E-infected newborns did not experience episodes of severe diarrhea later in life when compared with uninfected individuals (Bhan et al., 1993; Bishop et al., 1983). This observation suggested that these attenuated strains might protect against subsequent, symptomatic RV infection, providing rationale for their development as vaccines. Clinical testing with cell culture-adapted RV3 and 116E demonstrates that they are safe, attenuated, and immunogenic in all age groups, and that they replicate well in the infant gut (Barnes et al., 1997; Barnes et al., 2002; Bhandari et al., 2006; Bhandari et al., 2009). Moreover, phase II trials show that RV3 partially protected infants against severe diarrhea during successive winter months (Barnes et al., 2002). Studies are ongoing to determine whether RV3 and 116E elicit broadly-protective immune responses against heterotypic strains, or whether they will be limited to preventing infections with G/P-type-matched strains. Because they are being considered as vaccines, there is also great interest in elucidating the molecular basis for the attenuated, neonatal phenotypes of RV3 and 116E. Towards this goal, a few dsRNA segments have been sequenced and analyzed for these viruses (Cunliffe et al., 1997; Das et al., 1993; Gentsch et al., 1993; Kirkwood et al., 1996; Palombo and Bishop, 1994b). Nonetheless, in the absence of complete RV3 and 116E genome sequences, it is impossible to fully identify attenuation and neonatal infection determinants.
In this study, we deduced the nucleotide sequences of the open-reading frames (ORFs) for each of the eleven dsRNA genome segments of cell culture-adapted RV3 and 116E viruses. The segments were classified into genotypes according to the nucleotide identity cut-off percentages established by the Rotavirus Classification Working Group (RCWG). The genetic relatedness of RV3 and 116E genome segments to those of other human and animal RVs was determined using phylogenetic analyses. By performing amino acid alignments of the deduced proteins, we identified residues of RV3 or 116E that may contribute to their attenuation. Additionally, the three-dimensional locations of VP7 residues specific to RV3 or 116E in comparison to serotype-matched strains were mapped onto a high-resolution structure of the glycoprotein trimer. This analysis revealed changes that could influence the efficacy of RV3 or 116E as vaccines. Equally important, using amino acid alignments we found that the VP4 attachment proteins of P[6] strains isolated from neonates, such as RV3, differed at several positions compared with P[6] RVs isolated from older infants. While these changes do not correlate with disease outcome, several of them localize to the protein surface and may influence viral entry into cells of the neonatal gut. Together, the results presented in this study are important for the continued development of RV3- and 116E-based vaccines.
Results
Genotype classification of RV3 and 116E genes
The ORF nucleotide sequences for the eleven genome segments of cell culture-adapted RV3 and 116E strains were determined. The sequences deduced in this study are either identical or show a few changes from those that are already in GenBank for RV3 (VP4, VP6, and NSP4 genes) and 116E (VP4, VP6, VP7, NSP1, and NSP4 genes) (Table S1) (Cunliffe et al., 1997; Das et al., 1993; Gentsch et al., 1993; Kirkwood et al., 1996; Palombo and Bishop, 1994b). Genotypes were assigned for each genome segment based on the nucleotide percent identity cut-off values defined by the RCWG and by submission to RotaC (http://rotac.regatools.be) (Matthijnssens et al., 2008a; Matthijnssens et al., 2008b; Maes et al., 2009).
Our analyses show that RV3 can be classified as a G3P[6] strain, which is consistent with previous reports (Albert et al., 1987a; Kirkwood et al., 1996). Specifically, RV3 VP7 shares 97% nucleotide identity with the G3 human strain P (98% amino acid identity), and RV3 VP4 shares 98% nucleotide identity with the P[6] human strain ST3 (96% amino acid identity) (Tables 1 and 2). Also, like previous reports, our results show that 116E is a G9P[11] strain, with a VP4 that is very similar to those of several bovine RVs (Das et al., 1994; Das et al., 1993; Gentsch et al., 1993). In particular, 116E VP7 shares 89% nucleotide identity with the G9 human strain Wi61 (94% amino acid identity), while 116E VP4 shares 91% nucleotide identity with the P[11] bovine strain B223 (94% amino acid identity) (Tables 1 and 2). Moreover, we found that the nine internal genes of RV3 and 116E show percent nucleotide identity values above the cut-offs when compared with the genotype 1 prototype strain Wa (Table 3). Thus, RV3 and 116E have genome constellations of G3-P[6]-I1-R1-C1-M1-A1-N1-T1-E1-H1 and G9-P[11]-I1-R1-C1-M1-A1-N1-T1-E1-H1, respectively.
Table 1. Sequence-based G-typing of RV3 and 116E VP7.
| RV3 | 116E | ||||
|---|---|---|---|---|---|
| G-type | Strain | %NTa | %AAb | %NT | %AA |
| G1 | Wa | 75 | 83 | 75 | 79 |
| G2 | DS-1 | 73 | 75 | 74 | 76 |
| G3 | P | 97 | 98 | 79 | 85 |
| G4 | ST3 | 74 | 76 | 75 | 78 |
| G5 | IAL28 | 77 | 86 | 78 | 81 |
| G6 | Se584 | 77 | 86 | 77 | 83 |
| G8 | 69M | 75 | 83 | 75 | 82 |
| G9 | Wi61 | 79 | 86 | 89 | 94 |
| G10 | A64 | 75 | 83 | 75 | 81 |
| G12 | L26 | 74 | 80 | 76 | 82 |
abbreviation: percent nucleotide identity (%NT)
abbreviation: percent amino acid identity (%AA)
Table 2. Sequence-based P-typing of RV3 and 116E VP4.
| RV3 | 116E | ||||
|---|---|---|---|---|---|
| P-type | Strain | %NTa | %AAb | %NT | %AA |
| P[1] | RF | 69 | 73 | 60 | 59 |
| P[2] | SA11 | 71 | 74 | 59 | 58 |
| P[4] | DS-1 | 74 | 76 | 60 | 58 |
| P[5] | UK | 66 | 68 | 59 | 58 |
| P[6] | ST3 | 98 | 96 | 59 | 56 |
| P[8] | Wa | 74 | 77 | 59 | 58 |
| P[9] | AU-1 | 64 | 66 | 59 | 55 |
| P[10] | 69M | 70 | 75 | 61 | 59 |
| P[11] | B223 | 58 | 56 | 91 | 94 |
abbreviation: percent nucleotide identity (%NT)
abbreviation: percent amino acid identity (%AA)
Table 3. Genotyping of RV3 and 116E Internal Genes.
| RV3 v. Wa | 116E v. Wa | ||||
|---|---|---|---|---|---|
| Gene | % cut-offa | %NTb | %AAc | %NT | %AA |
| VP1 | 83 | 96 | 98 | 96 | 99 |
| VP2 | 84 | 94 | 97 | 94 | 98 |
| VP3 | 81 | 98 | 99 | 89 | 92 |
| VP6 | 85 | 90 | 98 | 91 | 98 |
| NSP1 | 79 | 85 | 83 | 89 | 88 |
| NSP2 | 85 | 91 | 95 | 92 | 96 |
| NSP3 | 85 | 97 | 97 | 89 | 90 |
| NSP4 | 85 | 98 | 96 | 96 | 97 |
| NSP5 | 91 | 93 | 93 | 95 | 96 |
percent nucleotide identity cut-off values for genotyping
abbreviation: percent nucleotide identity (%NT)
abbreviation: percent amino acid identity (%AA)
Phylogenetic relationship of RV3 and 116E to other strains
To further investigate the genetic relatedness of RV3 and 116E VP7 and VP4 genes with those of other G/P-type-matched strains, we constructed phylogenetic trees using the ORF sequences and the neighbor-joining method (Fig. 1A and B). Strain DS-1 (G2P[4]) was included as an out-group in the analyses. The results show that RV3 VP7 clustered tightly with human G3 VP7s and away from those of animal G3 strains (Fig. 1A). The VP7s of G9 strains have been previously defined into at least three phylogenetic lineages (1, 2, and 3) (Cao et al., 2008; Martinez-Laso et al., 2010). We found that 116E VP7, which is the only representative of lineage 2, clustered with lineage 1 VP7s and was distinct from lineage 3 VP7s (Fig. 1A). For VP4, we found that RV3 segregated with other human P[6] strains and away from the porcine P[6] strain Gottfried (Fig. 1B). Yet, interestingly, RV3 VP4 was part of a subcluster of P[6] VP4s belonging to human strains that were isolated from newborns (Fig. 1B). This neonatal P[6] VP4 lineage was separate from human RVs isolated from infants or children (>28 days old) and contained both symptomatic (strain 12/85) and asymptomatic (strains M37, 1076, NnB1, and ST3) P[6] strains (Fig. 1B). As reported previously, 116E VP4 showed a close phylogenetic relationship to VP4s of the human neonatal strains I321 (asymptomatic) and N155 (symptomatic), as well as to the P[11] VP4 proteins of several bovine RVs (Fig. 1B) (Gentsch et al., 1993; Glass et al., 2005). This result is consistent with the notion that 116E, I321, and N155 contain bovine RV VP4 genes.
Fig. 1.
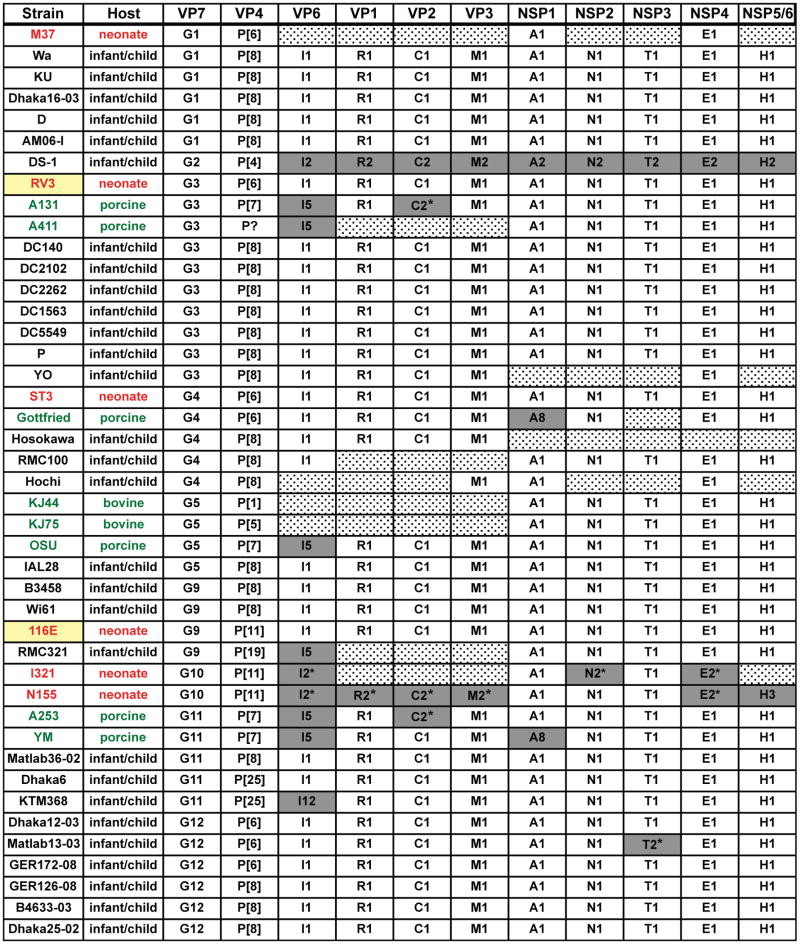
Phylogenetic relationships of RV3 and 116E genome segments to those of other RVs. The neighbor-joining trees were constructed using the individual ORF nucleotide sequences for each isolate and are out-group rooted to DS-1 for purposes of clarity. Horizontal branch lengths are drawn to scale (nucleotide substitutions per base), and bootstrap values are shown as percentages for key nodes. Strain names are colored accordingly: animal strains (green), human neonatal strains (red), and human strains isolated from older infants/children (black). Strains RV3 and 116E are highlighted in yellow.
We next sought to determine the phylogenetic relationships of the RV3 and 116E genotype 1 internal genes to those of other RVs. Although genotype 1 genes are predominantly found in human RVs that cause disease in young children (i.e., human Wa-like strains), several porcine and two bovine strains also contain dsRNA segments classified as genotype 1 (Matthijnssens et al., 2008a). These porcine RV-like genotype 1 genes generally cluster distantly from the human RV genotype 1 genes in phylogenetic trees, suggesting that they comprise distinct evolutionary lineages (Matthijnssens et al., 2008a). For VP6, the porcine RV lineage was separate enough to warrant its assignment as a different genotype I5 (Matthijnssens et al., 2008a; Matthijnssens et al., 2008b). In this study, we created neighbor-joining phylogenetic trees using the internal gene ORF sequences of human, porcine, and bovine RVs whose genomes contain several genotype 1 genes (Fig 1C-K). Figure 2 summarizes the genome constellations of the viruses analyzed. Strain DS-1 (genotype 2) was included in the trees as an out-group.
Fig. 2.
Genome constellations of several human and animal RVs. The schematic illustrates the genotype of each genome segment for several RV strains. The strain name is listed to the left of the corresponding genome constellation, and the protein encoded by each gene is listed at the top. RV strain names are colored accordingly: animal strains (green), human neonatal strains (red), and human strains isolated from older infants/children (black). Strains RV3 and 116E are highlighted in yellow. All genotype 1 genes are shown as white boxes, non-genotype 1 genes are shown as grey boxes, and dotted boxes indicated genome segments in which no or only partial ORF sequences are available. Asterisks (*) indicate sequences that were omitted from the phylogenetic trees for purposes of clarity.
These results show that most of the RV3 and 116E internal genes are quite similar to those of prototypic human Wa-like strains and are more distantly related to porcine RV-like genes. Interestingly, seven of the nine RV3 internal genes (VP1, VP2, VP6, NSP1, NSP3, NSP4, and NSP5/6) clustered tightly with those of ST3, a G4P[6] strain that was also isolated from an asymptomatic neonate (England, 1975) (Chrystie et al., 1975; Wyatt et al., 1983) (Fig. 1C-E, G, I and J). RV3 VP3 and NSP2 genes did not cluster with ST3, but did show close relationships to genes of other human strains (Fig. 1F, H, and K). The 116E VP1, VP2, VP6, and NSP1-5/6 genes segregated phylogenetically with those of prototypic human Wa-like RVs and away from porcine RV-like genes (Fig 1C-E, G-K). However, 116E NSP4 formed a separate branch, located just off the main group of human RV genes (Fig. 1J). Most strikingly, 116E VP3 did not group with any other known human or animal genotype 1 genes (Fig. 1F). Using BLAST, we found that the VP3 gene of 116E is most closely related that of strain Wa; yet, these genes only share 89% nucleotide identity (Table 3). While it is not possible to determine the ancestral origin of 116E VP3, the branching pattern is consistent with a reassortment event. A similar pattern was seen with several genes from the G11 human strains Matlab36-02, KTM368, and Dhaka6 (Matthijnssens et al., 2010b). Together, these phylogenetic analyses indicate that all of the RV3 genome segments are most likely of human Wa-like RV origin. These results also suggests that 116E may be a multi-gene reassortant, containing a bovine RV VP4 gene and a VP3 gene of unknown ancestry in a human Wa-like RV background.
Atypical residues of RV3 and 116E proteins
To gain insight into possible attenuation determinants for RV3 and 116E, we created alignments using the deduced amino acid sequences. We found that RV3 proteins exhibit several amino acid changes not yet documented in RVs whose sequences are available in GenBank (Table 4). Specifically, each internal gene protein had one to three amino acid differences when compared with other known RV strains; many of these changes occurred at invariable sites in the protein sequence (Table 4 and data not shown). Of particular interest are the two amino acid changes (Y85C and G162V) in NSP4, a viral nonstructural protein that can function as an enterotoxin (Estes and Kapikian, 2007). Though both changes are outside of the putative toxin domain (NSP4 residues ∼114-135), the Y85C change is non-conservative and may influence the function of this protein (Estes and Kapikian, 2007). Neither of these changes are seen in a RV3 NSP4 sequence determined from the primary stool specimen, indicating have been acquired during cell culture passage (Kirkwood et al., 1996) (Table S1). At 92 amino acids, the length of the putative NSP6 protein of RV3 is identical to that of strain Wa, but contained three changes compared with known strains (Table 4).
Table 4. Atypical Residues of RV3 Proteins.
| Structural Proteins | Non-structural Proteins | ||
|---|---|---|---|
| VP1 | I732V | NSP1 | L138V |
| V741I | C/Y/Q394W | ||
| VP2 | S606G | NSP2 | A243V |
| M672T | T244A | ||
| D/E256N | |||
| VP3 | N139D | ||
| N686S | NSP3 | T137S | |
| VP6 | E/D86K | NSP4 | Y85C |
| G162V | |||
| VP7a,b | S97A (7-1A, A) | ||
| Q280R | NSP5 | E46G | |
| VP4c | none | NSP6 | K27E |
| S30L | |||
| R63K | |||
compared to human G3 RV sequences in GenBank
VP7 antigenic domain or region as defined in (Matthijnssens et. al., 2010c) is indicated in parentheses next to associated residue
compared to P[6] RV sequences in GenBank
Consistent with the phylogenetic analysis using nucleotide sequences, we found that RV3 VP7 is nearly identical at the amino acid level to other human RV G3 VP7 proteins. We did discover two residues of RV3 VP7 that are not seen in most other G3 proteins from human RVs for which sequences are available in GenBank (Table 4 and Fig. 3A). Specifically, RV3 VP7 has alanine and arginine at positions 97 and 280, respectively, while nearly every other human RV G3 VP7 protein shows serine and glutamine at these locations (Fig. 3A). By mapping these residues onto the three-dimensional structure of the VP7 trimer, we saw that the S97A change is located near antigenic domain 7-1A (classic antigenic region A) (Fig. 3B) (Aoki et al., 2009; Matthijnssens et al., 2010c). In contrast, the Q280R change is buried underneath the VP7 trimer, likely at the VP6 interface (data not shown). For RV3 VP4, all residues are represented in other human P[6] RV strains (Table 4).
Fig. 3.
Surface-exposed VP7 amino acids unique to RV3 or 116E. (A) Architecture of a RV virion (modified with permission from B.V.V. Prasad), showing the positions of VP7 and VP4. (B and C) Three-dimensional locations of amino acid changes in RV3 or 116E. In both images, a surface representation of the VP7 trimer crystal structure (PDB 3FMG) is shown. Residues comprising the putative neutralization domains have been colored as follows: 7-1A (red), 7-1B (salmon), and 7-2 (purple). Amino acids unique to RV3 or 116E, not represented in any other G-type-matched strains, are shown in cyan and are labeled for a single monomer of the trimer.
The amino acid alignments revealed that many of the 116E proteins exhibit numerous residues not yet seen in other RVs whose sequences are in GenBank (Table 5). Of the 116E internal gene proteins, VP2, VP3, and NSP1 exhibit the largest number of changes (4, 9, and 10 residues, respectively) (Table 5). The changes in NSP1 are interesting in light of the attenuated phenotype of 116E, as this nonstructural protein can function as an interferon antagonist (Barro and Patton, 2005). Moreover, VP1, VP6, NSP3, and NSP5 each show a single residue differing for 116E compared with all other RV strains (Table 5). The 116E NSP6 protein is predicted to be 92 amino acids long, like that of RV3, and shows two changes compared with known strains (Table 5). We found no amino acid residues that were unique to 116E NSP2 and NSP4. The fact that 116E NSP4 did not show any amino acids changes was surprising, given its distant phylogenetic relationship to human RV genes at the nucleotide level.
Table 5. Atypical Residues of 116E Proteins.
| Structural Proteins | Non-structural Proteins | ||||
|---|---|---|---|---|---|
| VP1 | G95V | VP7a,b | L26V | NSP1 | D94N |
| L41I | N109T | ||||
| VP2 | Q/L123R | I43V | Q203R | ||
| Q180H | L57V | H237Y | |||
| E197D | T87I (7-1A, A) | Q/K292R | |||
| H850Y | D100G (7-1A, A, B) | K296E | |||
| L133F (B) | H319R | ||||
| VP3 | D83N | M142V | I444V | ||
| N139K | D145N (7-2, B) | R451T | |||
| A184T | T171A | Y478S | |||
| A395T | D179N | ||||
| K/N480Q | S221N (7-1B, B, C) | NSP2 | none | ||
| K496R | A278T | ||||
| K/T687I | NSP3 | S280A | |||
| K704E | VP4c | S73K | |||
| T807M | N116S | NSP4 | none | ||
| T118V | |||||
| VP6 | A/V101S | T136A | NSP5 | T/N113S | |
| R138K | |||||
| T148A | NSP6 | T49M | |||
| Y156F | R56L | ||||
| T181A | |||||
| G221R | |||||
| M226I | |||||
| I241V | |||||
| V309I | |||||
| N326D | |||||
| I402V | |||||
| I651M | |||||
| E675D | |||||
| V698M | |||||
compared to human G9 RV sequences in GenBank
VP7 antigenic domain or region as defined in (Matthijnssens et. al., 2010c) is indicated in parentheses next to associated residue
compared to P[11] RV sequences in GenBank
Amino acid alignments of 116E VP7 with other G9 sequences showed that its glycoprotein has several atypical residues. In particular, we found 14 amino acids in 116E VP7 (positions 26, 41, 43, 57, 68, 87, 100, 133, 142, 145, 171, 179, 221, and 278) that differ for the majority of G9 VP7s (Table 5). Most of these changes are buried, but T87I, D100G, D145N, T171A, and S221N are located on the surface of VP7 in proximity to potential sites of antibody binding (Table 5 and Fig. 3C) (Aoki et al., 2009; Matthijnssens et al., 2010c). Likewise, 116E VP4 contains 17 amino acid changes when compared to other human and animal P[11] proteins (Table 5).
P[6] VP4 residues that correlate with neonatal infection
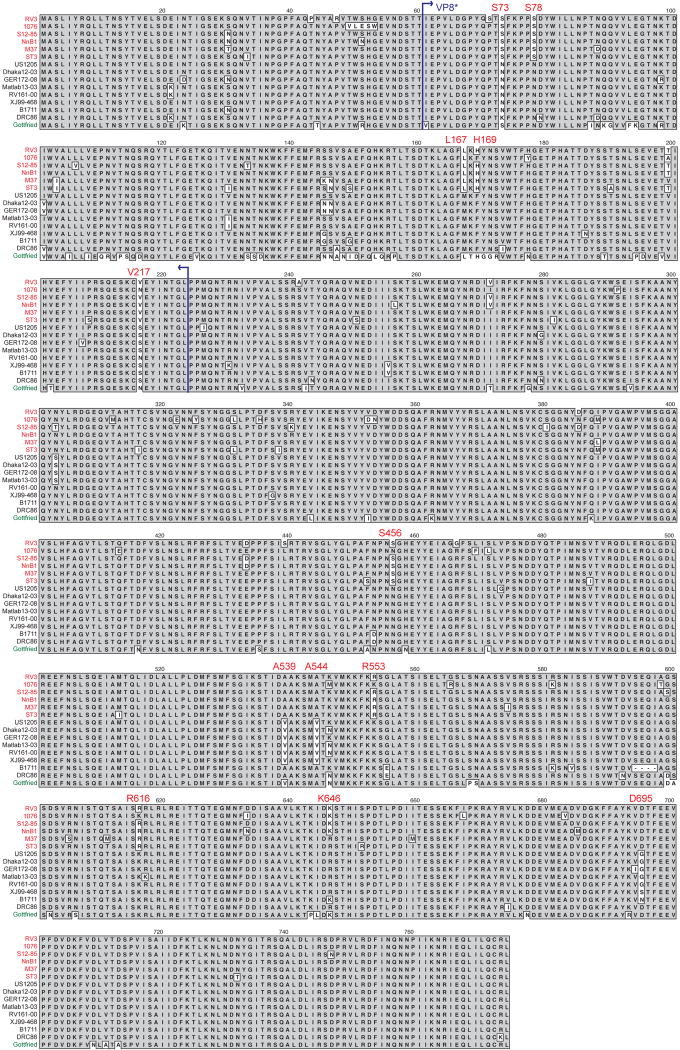
Phylogenetic analysis at the nucleotide level suggested that the P[6] VP4 genes of neonatal RVs, such as RV3, are genetically-divergent from the P[6] VP4 gene of strains isolated from infants and children (>28 days old). In agreement, using amino acid alignments, we discovered that the P[6] VP4 proteins of neonatal RVs differed at several positions (73, 78, 167, 169, 217, 456, 539, 544, 553, 616, 646, and 695) compared with those of non-neonatal strains (Fig. 4). While the correlation at each position was not absolute, the observation that P[6] VP4 proteins of RVs isolated from newborns generally vary from those of other P[6] human RVs was surprising. Importantly, because strains that cause symptomatic (strain 12/85) and asymptomatic (strains RV3, M37, 1076, NnB1, and ST3) infections are conserved at these sites, we predict that they do not contribute to virulence. Instead, we hypothesize that some or all of these amino acid changes confer P[6] strains with the enhanced capacity to infect newborns. By mapping these neonatal RV-specific VP4 differences onto the three-dimensional structure of the attachment protein, we found that six of them (S73, S78, L167, H169, T199, and V217) are located at the basal surface of VP8*, which is the trypsin-activated distal domain of VP4 (Fig. 5) (Dormitzer et al., 2004; Dormitzer et al., 2002). It is possible that these residues allow P[6] RVs to adhere more efficiently to cell surface receptors in the neonatal gut, thereby aiding in viral entry.
Fig. 4.
Alignment of P[6] VP4 showing residues that correlate with neonatal infection. The VP4 amino acid sequences of several representative P[6] strains are shown. The strain names are to the left of each sequence and are colored according to Fig. 1. Grey shading indicates conservation of amino acid identity. Residues associated with most P[6] strains isolated from neonates are labeled. Blue arrows show the region of VP4 comprising the VP8* core.
Fig. 5.
Location of surface-exposed P[6] VP8* residues that correlate with neonatal infection. The left image shows a surface representation of the VP4 crystal structure (PDB 1KQR). A white box defines the position of VP8*. The right images show the VP8* core from two different viewpoints (front or back). The front viewpoint is rotated 90° to the right compared with the image in the white box. The back viewpoint is rotated 180° to the left compared with the front. Residues comprising the putative neutralization domains of VP8* have been colored as follows: pink (8-1), salmon (8-2), purple (8-3) and green (8-4). Residues that correlate with the capacity of P[6] strains to infect neonates are shown in cyan.
Discussion
In many developing countries, RV infections are a leading cause of illness and death among children less than 2 years of age (Parashar et al., 2006). It is unclear whether the currently available RV vaccines will be effective and affordable in these regions of the world (Tate et al, 2010). Consequently, new vaccines are being generated, several of which are based on live-attenuated strains isolated from newborn infants. Two neonatal RV strains, RV3 and 116E, were adapted to growth in culture by multiple passages in primary African green monkey kidney (AGMK) cells (Palombo and Bishop, 1994; Das et al., 1993). Derivatives of these cell culture adapted versions of RV3 and 116E have undergone several phases of clinical testing and were proven safe and immunogenic (Barnes et al., 1997; Barnes et al., 2002; Bhandari et al., 2006; Bhandari et al., 2009). Prior to this study, the genome sequences of RV3 and 116E had not been determined, making it difficult to fully identify factors contributing to their inherent attenuation. The results described in this report help close this gap in knowledge by revealing amino acid atypical residues of proteins encoded by these viruses. These changes are not only predicted to influence virulence, but also impact neonatal host infection and the efficacy of RV3- or 116E-based vaccines. Still, it is important to note that the RV3 and 116E strains sequenced in this study are not the actual vaccine preparations. Also, the VP4, VP6, and NSP4 sequences of the original RV3 stool isolate that are available in GenBank show changes compared with the sequences we determined (Table S1) (Palombo et al., 1994; Kirkwood et al., 1996). We think it is possible that some of these changes resulted from adaptation to growth in vitro. For 116E there is no available sequence information of the original stool material. However, the 116E VP6, NSP1, and NSP4 sequences that we determined in this study exactly match those in GenBank for the culture-adapted strain (Table S1) (Cunliffe et al., 1997). For 116E VP7 and VP4, we found a few nucleotide and amino acid changes compared to the sequences in GenBank (Das et al., 1993; Gentsch et. al., 1993) (Table S1). Because we passaged 116E six additional times in AGMK cells (see Material and Methods), it is possible that these differences could be attributed to evolution of the virus in culture. The manufacturers have not released the sequences of the RV3 or 116E vaccine preparations; thus, this study is the first to describe the complete genetic characteristics of these important cell culture-adapted strains.
Why are strains RV3 and 116E attenuated?
RV infections of newborns are uncommonly documented, but are generally asymptomatic. Factors such as the prevalence of maternal antibodies and immature gut physiology likely contribute to the lack of diarrhea in neonates (Haffejee, 1991). However, some of the strains isolated from newborns also seem attenuated in older infants and children, demonstrating that they are phenotypically different from pathogenic RVs. The complete genome sequence of only one asymptomatic neonatal strain, ST3 (G4P[6]), has been reported to date, but partial genome sequences exist for M37 (G1P[6]) and I321 (G10P[11]) (Dunn et al., 1994; Heiman et al., 2008; Kirkwood and Palombo, 1997; Palombo and Bishop, 1994a; Rao et al., 1995). Strains M37 and I321 were originally considered as vaccine candidates, along with RV3 and 116E, prior to studies showing that they are poorly immunogenic or do not protect against RV disease (Bhandari et al., 2006; Perez-Schael et al., 1994; Vesikari et al., 1991). While the evolutionary origin of M37 is unknown, I321 is thought to have derived from an inter-species transmission of a bovine RV to a human, providing a basis for its lack of virulence (Sukumaran et al., 1992). On the contrary, the attenuating determinants of ST3, RV3, and 116E seem subtler, as the vast majority of their genes are of human RV origin.
The atypical G/P-type combinations seen in RV3 and 116E may contribute, at least in part, to their attenuated phenotypes. Specifically, RV3 is a G3P[6] strain and, although G3 strains readily circulate in the human population, they are usually combined with P[8] VP4 proteins (Albert et al., 1987a; Kirkwood et al., 1996). Human RVs with P[6] specificity are more rare, but can be seen with multiple G-types (Matthijnssens et al., 2009). Interestingly, many P[6] strains have been isolated from neonates with both symptomatic and asymptomatic infections, but there does not seem to be a clear correlation with residues of VP4 and clinical illness (Hoshino et al., 2003; Santos et al., 1994). Two RV3 internal genes (encoding VP6 and NSP4) had been sequenced previously, revealing that they are similar to common Wa-like human strains (Kirkwood et al., 1996; Palombo and Bishop, 1994b). Our results extend these findings and show that all of the internal RV3 genes are classified as genotype 1. Despite their phylogenetic similarities to pathogenic RV isolates, we found unique amino acid changes in every RV3 internal gene protein. We hypothesize that some of these point mutations contribute to the attenuation of this strain.
In contrast to RV3, which seems to have human RV-like VP7 and VP4 proteins, 116E is a G9 strain with a P[11] VP4 that is very similar to that of several bovine RVs (Das et al., 1994; Das et al., 1993; Gentsch et al., 1993). The only other known P[11] human strains were also isolated from neonates, suggesting that, like P[6] RVs, those with P[11]-specificity may have an enhanced capacity to replicate in the newborn gut. Previous sequence and RNA hybridization studies showed that 116E contains Wa-like internal genes (Cunliffe et al., 1997; Das et al., 1993). Our results are consistent with these earlier ones, but also indicate that many of the internal genes/proteins of 116E are unusual compared with other genotype 1 genes/proteins. Most notably, the gene of the 116E RNA capping enzyme, VP3, is distantly related to human and animal RV genotype 1 genes at the nucleotide level and shows 9 amino acid changes not represented in any known RV sequence. It is possible that 116E picked up its VP3 gene by reassorting with an unidentified Wa-like RV ancestor. The changes in the other 116E proteins (e.g., the VP2 inner capsid) might reflect co-evolution driven by the requirement to maintain VP3 interactions during viral replication. Alternatively, 116E VP3 could have accumulated point mutations via genetic drift; however, the pressures that would have selected for such changes in this enzyme are not obvious. Based on the available data, we speculate that the ancestor of 116E may have been a human G9P[8] strain that acquired a bovine P[11] VP4 gene and a VP3 gene from an unknown RV during separate reassortment events. Because VP3 has been shown to be a virulence determinant, it is possible that this gene alone is responsible for the attenuation of 116E (Hoshino et al., 1995). However, we think it is more likely that the combination of changes in the 116E genome is responsible for its avirulent phenotype. Of particular interest are the amino acid changes at highly conserved positions of the interferon-antagonist NSP1 protein of 116E (Barro and Patton, 2005).
Does VP4 confer some RVs an enhanced capacity to infect newborns?
A surprising result of this study was the apparent relationship between VP4 sequence and neonatal infection. The phylogenetic clustering pattern we found is in agreement with analysis done on the VP8* gene region of neonatal P[6] strains isolated in Brazil (Mascarenhas et al., 2007). Using amino acid alignments, we found that the VP4 proteins of neonatal P[6] RVs tend to differ at several positions compared with P[6] strains isolated from older infants for children. However, the correlation was not absolute; there are examples of P[6] strains isolated from children that show neonatal RV-like amino acids and vice-versa. Because P[6] strains that cause symptomatic (strain 12/85) and asymptomatic (strains RV3, M37, 1076, NnB1, and ST3) infections are conserved at these sites, they probably do not contribute to virulence (Santos et al., 1994). However, these amino acid changes may confer some P[6] strains with the enhanced capacity to infect newborns. We found that six of them (S73, S78, L167, H169, T199, and V217) are located at the basal surface of the VP8* core, which is exposed upon trypsin cleavage of the VP4 spike. The location of these changes outside putative neutralization domains suggests that do not merely allow P[6] strains to evade maternal antibodies. Instead, we hypothesize that the changes help these viruses adhere more efficiently to cell surface receptors in the neonatal gut. Interestingly, P[11] strains (including 116E, I321, and N155) also show serines at positions 73 and 78. Thus, these two sites might be of particular importance in determining whether a RV can establish an infection in a newborn. Treatment of RV3 (P[6]) or 116E (P[11]) with neuraminidase does not alter infectivity in cell culture, suggesting attachment is sialic-acid independent (Ciarlet et al., 2002). Likewise, the VP4 proteins of all known P[6] and P[11] strains do not conserve the sialic acid-binding residues of strain RRV (Matthjnssens et al., 2010c). These observations indicate that the VP4 amino acid changes identified in this study may confer binding to alternative carbohydrate molecules or to proteins present on the surface of neonatal enterocytes.
Will RV3 and 116E protect against RV disease?
The most significant question that remains regarding monovalent vaccines, such as RV3 (G3P[6]) and 116E (G9P[11]), is whether they will be effective at protecting against disease brought on by heterologous serotype strains (G1, G2, G4, P[4], P[8], etc.). The clinical efficacy of RV3, even in communities where G1 and G2 strains dominated, provides indirect evidence of cross-protection with this vaccine candidate (Bishop et al., 1983). RV3 VP7 shows a single amino acid difference (S97A) located near epitope 7-1A when compared with common human G3 RVs; yet, we do not expect this conservative change to dramatically influence neutralization. In contrast, the VP7 protein of 116E is quite divergent from most other human G9 VP7s and contains numerous amino changes located on the protein surface. Because of the number of changes, we think that 116E might be less effective at producing antibodies capable of protecting against circulating G9 strains. In support of this notion, guinea pig hyper-immune serum raised against 116E VP7 failed to robustly neutralize several human G9 RVs in plaque reduction assays (Cao et al., 2008). In fact, G9 strains with VP7 proteins belonging to phylogenetic lineage 1 may be more suitable for vaccines, as they more broadly cross-neutralize other G9 strains in vitro (Hoshino et al., 2004). However, studies in animal models have shown that CD4 and CD8 T cell responses can contribute to serotype-independent protection for RVs (Ward, 2003). If this is the case, vaccines that are based on human Wa-like strains, such as RV3 and 116E, may be quite successful. The findings from this study are expected to aid future research into the true protective efficacy of RV3 and 116E.
Materials and Methods
Viruses and RNA isolation
RV3 was amplified from the original stool specimen in primary AGMK cells (30 passages) and was triple-plaque purified during passages 19 to 23 (Palombo and Bishop, 1994). The virus was then grown in monkey kidney (MA104) cells for >15 passages. An aliquot this cell culture-adapted RV3 strain was generously provided to us by Carl Kirkwood (Melbourne Children's Research Institute). Our laboratory further amplified the virus (one passage) in MA104 cells and used the clarified infected cell culture supernatant for RNA extraction and sequencing.
116E was amplified from the original stool specimen in primary AGMK cells (2 passages), grown in MA104 cells (8 passages), and plaque purified twice in MA104 cells (Das et. al, 1993). An aliquot this cell culture-adapted 116E strain was sent to Taka Hoshino (National Institutes of Health) from Jon Gentsch (Centers for Disease Control). In the Hoshino laboratory, the virus was passaged six times in primary AGMK cells, and an aliquot of the cell culture supernatant was given to our laboratory for RNA extraction and sequencing.
For both viruses, RNA was extracted from the clarified supernatant using TRIzol-LS as described by the manufacturer's protocol (Invitrogen). RNA was denatured in 50% dimethyl sulfoxide for ten minutes at 94°C and then used as template for RT-PCR.
RT-PCR and sequencing
Denatured RNA was used as template for RT-PCR to amplify cDNA fragments corresponding to regions of RV3 or 116E gene ORFs. For 116E genome segments 1, 2, and 3, SuperScript II RT and Accuprime Pfx Supermix were used according to the manufacturer's protocols (both Invitrogen). For 116E segments 4, 5, 6, 7, 8, 9, 10 and 11 and all eleven RV3 segments, the SuperScript One-Step system (Invitrogen) was used according to the manufacturer's protocol. Primers are listed in supplemental materials (Table S2).
The RT-PCR reactions were electrophoresed in a 1% agarose gel, and specific cDNA products were excised and purified using a QIAquick gel extraction kit (Qiagen). The purified cDNAs were then sequenced with an ABI Prism BigDye v3.1 terminator cycle sequencing kit (Applied Biosystems Group). The dye terminator was removed using Performa DTR (Edge Biosystems) and sequences were obtained with a 3730 DNA Analyzer (Applied Biosystems). The sequence files were assembled and verified using Sequencher 4.7 (Gene Codes Corporation). Nucleotide sequences generated in this study have been deposited into GenBank with the following accession numbers: FJ998270-FJ998280 (RV3) and FJ361201-FJ361211 (116E). Previously determined sequences for these two strains have the following GenBank accession numbers: U16299 (RV3 VP4); U04741 (RV3 VP6); U42628 (RV3 NSP4); L14072 (116E VP7); L07934 (116E VP4); U85998 (116E VP6); U85999 (116E NSP1); and U78558 (116E NSP4)
Phylogenetic analyses, amino acid alignments, and structure-based predictions
Phylogenetic trees were generated in MacVector 8.1.2 (Accelrys) using the neighbor-joining method (1000 bootstrap repetitions) and the Kimura-2 correction parameter. Amino acid alignments were constructed with MacVector 8.1.2. using ClustalW, BLOSUM Series, with the defaults set (open gap penalty of 10.0, extended gap penalty of 0.05, and delay divergence of 40%). Structural analysis of VP7 (PBD 3FMG), VP8* (PDB 1KQR), and VP5* (PDB 2B4H) was performed using UCSF Chimera-Molecular Molecular Modeling System (Petterson, 2004). GenBank accession numbers for all RV sequences used in this study are included in the supplemental materials (Table S3).
Supplementary Material
Acknowledgments
We would like to thank Carl Kirkwood and Taka Hoshino for providing the viruses sequenced in this study and for reviewing the manuscript. We also thank Kristen Guglielmi, Jelle Matthijnssens, and Al Kapikian for important scientific and editorial comments. This study was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert MJ, Unicomb LE, Barnes GL, Bishop RF. Cultivation and characterization of rotavirus strains infecting newborn babies in Melbourne, Australia, from 1975 to 1979. J Clin Microbiol. 1987a;25(9):1635–40. doi: 10.1128/jcm.25.9.1635-1640.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MJ, Unicomb LE, Tzipori SR, Bishop RF. Isolation and serotyping of animal rotaviruses and antigenic comparison with human rotaviruses. Brief report. Arch Virol. 1987b;93(1-2):123–30. doi: 10.1007/BF01313898. [DOI] [PubMed] [Google Scholar]
- Aoki ST, Settembre EC, Trask SD, Greenberg HB, Harrison SC, Dormitzer PR. Structure of rotavirus outer-layer protein VP7 bound with a neutralizing Fab. Science. 2009;324(5933):1444–7. doi: 10.1126/science.1170481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M, Patton JT, McDonald SM. Culturing, storage, and quantification of rotaviruses. Curr Protoc Microbiol. 2009;Chapter 15 doi: 10.1002/9780471729259.mc15c03s15. Unit 15C 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GL, Lund JS, Adams L, Mora A, Mitchell SV, Caples A, Bishop RF. Phase 1 trial of a candidate rotavirus vaccine (RV3) derived from a human neonate. J Paediatr Child Health. 1997;33(4):300–4. doi: 10.1111/j.1440-1754.1997.tb01604.x. [DOI] [PubMed] [Google Scholar]
- Barnes GL, Lund JS, Mitchell SV, De Bruyn L, Piggford L, Smith AL, Furmedge J, Masendycz PJ, Bugg HC, Bogdanovic-Sakran N, Carlin JB, Bishop RF. Early phase II trial of human rotavirus vaccine candidate RV3. Vaccine. 2002;20(23-24):2950–6. doi: 10.1016/s0264-410x(02)00235-9. [DOI] [PubMed] [Google Scholar]
- Barro M, Patton JT. Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3. Proc Nat Acad Sci USA. 2005;102(11):4114–9. doi: 10.1073/pnas.0408376102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan MK, Lew JF, Sazawal S, Das BK, Gentsch JR, Glass RI. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhea. J Infect Dis. 1993;168(2):282–7. doi: 10.1093/infdis/168.2.282. [DOI] [PubMed] [Google Scholar]
- Bhandari N, Sharma P, Glass RI, Ray P, Greenberg H, Taneja S, Saksena M, Rao CD, Gentsch JR, Parashar U, Maldonado Y, Ward RL, Bhan MK. Safety and immunogenicity of two live attenuated human rotavirus vaccine candidates, 116E and I321, in infants: results of a randomised controlled trial. Vaccine. 2006;24(31-32):5817–23. doi: 10.1016/j.vaccine.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bhandari N, Sharma P, Taneja S, Kumar T, Rongsen-Chandola T, Appaiahgari MB, Mishra A, Singh S, Vrati S. A dose-escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 2009;200(3):421–9. doi: 10.1086/600104. [DOI] [PubMed] [Google Scholar]
- Bishop RF, Barnes GL, Cipriani E, Lund JS. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983;309(2):72–6. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- Cao D, Santos N, Jones RW, Tatsumi M, Gentsch JR, Hoshino Y. The VP7 genes of two G9 rotaviruses isolated in 1980 from diarrheal stool samples collected in Washington, DC, are unique molecularly and serotypically. J Virol. 2008;82(8):4175–9. doi: 10.1128/JVI.02537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrystie IL, Totterdell B, Baker MJ, Scopes JW, Banatvala JE. Letter: Rotavirus infections in a maternity unit. Lancet. 1975;2(7924):79. doi: 10.1016/s0140-6736(75)90525-5. [DOI] [PubMed] [Google Scholar]
- Ciarlet M, Crawford SE, Cheng E, Blutt SE, Rice DA, Bergelson JM, Estes MK. VLA-2 (alpha2beta1) integrin promotes rotavirus entry into cells but is not necessary for rotavirus attachment. J Virol. 2002;76(3):1109–23. doi: 10.1128/JVI.76.3.1109-1123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarlet M, Schodel F. Development of a rotavirus vaccine: clinical safety, immunogenicity, and efficacy of the pentavalent rotavirus vaccine, RotaTeq. Vaccine. 2009;27 6:G72–81. doi: 10.1016/j.vaccine.2009.09.107. [DOI] [PubMed] [Google Scholar]
- Cunliffe NA, Das BK, Ramachandran M, Bhan MK, Glass RI, Gentsch JR. Sequence analysis demonstrates that VP6, NSP1 and NSP4 genes of Indian neonatal rotavirus strain 116E are of human origin. Virus Genes. 1997;15(1):39–44. doi: 10.1023/a:1007958914141. [DOI] [PubMed] [Google Scholar]
- Das BK, Gentsch JR, Cicirello HG, Woods PA, Gupta A, Ramachandran M, Kumar R, Bhan MK, Glass RI. Characterization of rotavirus strains from newborns in New Delhi, India. J Clin Microbiol. 1994;32(7):1820–2. doi: 10.1128/jcm.32.7.1820-1822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das BK, Gentsch JR, Hoshino Y, Ishida S, Nakagomi O, Bhan MK, Kumar R, Glass RI. Characterization of the G serotype and genogroup of New Delhi newborn rotavirus strain 116E. Virology. 1993;197(1):99–107. doi: 10.1006/viro.1993.1570. [DOI] [PubMed] [Google Scholar]
- Dormitzer PR, Nason EB, Prasad BV, Harrison SC. Structural rearrangements in the membrane penetration protein of a non-enveloped virus. Nature. 2004;430(7003):1053–8. doi: 10.1038/nature02836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormitzer PR, Sun ZY, Wagner G, Harrison SC. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 2002;21(5):885–97. doi: 10.1093/emboj/21.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SJ, Cross TL, Greenberg HB. Comparison of the rotavirus nonstructural protein NSP1 (NS53) from different species by sequence analysis and northern blot hybridization. Virology. 1994;203(1):178–83. doi: 10.1006/viro.1994.1471. [DOI] [PubMed] [Google Scholar]
- Estes MK, Kapikian AZ. Fields Virology (5th Edition) Vol. 2. Lippincott Williams and Wilkens; Philadelphia: 2007. Rotaviruses; pp. 1917–1974. [Google Scholar]
- Gentsch JR, Das BK, Jiang B, Bhan MK, Glass RI. Similarity of the VP4 protein of human rotavirus strain 116E to that of the bovine B223 strain. Virology. 1993;194(1):424–30. doi: 10.1006/viro.1993.1280. [DOI] [PubMed] [Google Scholar]
- Glass RI, Bhan MK, Ray P, Bahl R, Parashar UD, Greenberg H, Rao CD, Bhandari N, Maldonado Y, Ward RL, Bernstein DI, Gentsch JR. Development of candidate rotavirus vaccines derived from neonatal strains in India. J Infect Dis. 2005;192 1:S30–5. doi: 10.1086/431498. [DOI] [PubMed] [Google Scholar]
- Haffejee IE. Neonatal rotavirus infections. Rev Infect Dis. 1991;13(5):957–62. doi: 10.1093/clinids/13.5.957. [DOI] [PubMed] [Google Scholar]
- Heaton PM, Ciarlet M. Vaccines: the pentavalent rotavirus vaccine: discovery to licensure and beyond. Clin Infect Dis. 2007;45(12):1618–24. doi: 10.1086/522997. [DOI] [PubMed] [Google Scholar]
- Heiman EM, McDonald SM, Barro M, Taraporewala ZF, Bar-Magen T, Patton JT. Group A human rotavirus genomics: evidence that gene constellations are influenced by viral protein interactions. J Virol. 2008;82(22):11106–116. doi: 10.1128/JVI.01402-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y, Jones RW, Ross J, Santos N, Kapikian AZ. Human rotavirus strains bearing VP4 gene P[6] allele recovered from asymptomatic or symptomatic infections share similar, if not identical, VP4 neutralization specificities. Virology. 2003;316(1):1–8. doi: 10.1016/s0042-6822(03)00543-9. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Saif LJ, Kang SY, Sereno MM, Chen WK, Kapikian AZ. Identification of group A rotavirus genes associated with virulence of a porcine rotavirus and host range restriction of a human rotavirus in the gnotobiotic piglet model. Virology. 1995;209(1):274–80. doi: 10.1006/viro.1995.1255. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Jones R, Ross J, Honma S, Santos N, Gentsch J, Kapikian AZ. Rotavirus serotype G9 strains belonging to VP7 gene phylogenetic sequence lineage 1 may be more suitable for serotype G9 vaccine candidates than those belonging to lineage 2 or 3. J Virol. 2004;78(14):7795–802. doi: 10.1128/JVI.78.14.7795-7802.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Nagesha HS, Dyall-Smith ML, Holmes IH. Comparative sequence analysis of VP7 genes from five Australian porcine rotaviruses. Arch Virol. 1989;109(3-4):173–83. doi: 10.1007/BF01311079. [DOI] [PubMed] [Google Scholar]
- Kirkwood CD, Coulson BS, Bishop RF. G3P2 rotaviruses causing diarrhoeal disease in neonates differ in VP4, VP7 and NSP4 sequence from G3P2 strains causing asymptomatic neonatal infection. Arch Virol. 1996;141(9):1661–76. doi: 10.1007/BF01718290. [DOI] [PubMed] [Google Scholar]
- Kirkwood CD, Palombo EA. Genetic characterization of the rotavirus nonstructural protein, NSP4. Virology. 1997;236(2):258–65. doi: 10.1006/viro.1997.8727. [DOI] [PubMed] [Google Scholar]
- Maes P, Matthijnssens J, Rahman M, Van Ranst M. RotaC: a web-based tool for the complete genome classification of group A rotaviruses. BMC Microbiol. 2009 Nov 23;9:238. doi: 10.1186/1471-2180-9-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi S, Cunliffe N, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor J, Gillard P, Chevart B, Han H, Neuzil K. Effect of human rotavirus vaccine on server diarrhea in african infants. N Eng J Med. 2010;362(4):289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- Martinez-Laso J, Roman A, Head J, Cervera I, Rodriguez M, Rodriguez-Avial I, Picazo J. Phylogeny of G9 rotavirus genotype: a possible explanation of its origin and evolution. J Clinical Virol. 2010;44(1):52–57. doi: 10.1016/j.jcv.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Mascarenhas JD, Linhares AC, Gabbay YB, Lima CS, Guerra Sde F, Soares LS, Oliveira DS, Lima JC, Macedo O, Leite JP. Molecular characterization of VP4 and NSP4 genes from rotavirus strains infecting neonates and young children in Belem, Brazil. Virus Res. 2007;126(1-2):149–58. doi: 10.1016/j.virusres.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, Heiman E, Arijs I, Delbeke T, McDonald SM, Palombo EA, Iturriza-Gomara M, Maes P, Patton JT, Rahman M, Van Ranst M. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J Virol. 2008a;82(7):3204–19. doi: 10.1128/JVI.02257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, Rahman M, Attoui H, Banyai K, Estes MK, Gentsch JR, Iturriza-Gomara M, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Patton JT, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Desselberger U, Van Ranst M. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch Virol. 2008b;153(8):1621–9. doi: 10.1007/s00705-008-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Bilcke J, Ciarlet M, Martella V, Banyai K, Rahman M, Zeller M, Beutels P, Van Damme P, Van Ranst M. Rotavirus disease and vaccination: impact on genotype diversity. Future Microbiol. 2009;4:1303–16. doi: 10.2217/fmb.09.96. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Joelsson D, Warakomski D, Zhou T, Mathis P, van Maanen M, Ranheim T, Ciarlet M. Molecular and biological characterization of the 5 human-bovine rotavirus (WC3)-based reassortant strains of the pentavalent rotavirus vaccine, RotaTeq®. Virology. 2010a doi: 10.1016/j.virol.2010.04.004. in press. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Rahman M, Ciarlet M, Zeller M, Heylen E, Nakagomi T, Uchida R, Hassan Z, Azim T, Nakagomi O, Van Ranst M. Reassortment of human rotavirus gene segments into G11 rotavirus strains. Emerg Infect Dis. 2010b;16(4):625–30. doi: 10.3201/eid1604.091591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Taraporewala ZF, Yang H, Rao S, Yuan L, Cao D, Hoshino Y, Mertens PP, Carner GR, McNeal M, Sestak K, Van Ranst M, Patton JT. Simian rotaviruses possess divergent gene constellations that originated from interspecies transmission and reassortment. J Virol. 2010c;84(4):2013–26. doi: 10.1128/JVI.02081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo EA, Bishop RF. Genetic analysis of NSP1 genes of human rotaviruses isolated from neonates with asymptomatic infection. J Gen Virol. 1994a;75(Pt 12):3635–9. doi: 10.1099/0022-1317-75-12-3635. [DOI] [PubMed] [Google Scholar]
- Palombo EA, Bishop RF. Sequences of VP6 genes of human rotavirus strain RV3 and its vaccine derivative. J Gen Virol. 1994b;75(Pt 9):2415–9. doi: 10.1099/0022-1317-75-9-2415. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12(2):304–6. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Schael I, Blanco M, Garcia D, White L, Alfonzo E, Crespo I, Cunto W, Pittman AL, Kapikian AZ, Flores J. Evaluation of the antigenicity and reactogenicity of varying formulations of the rhesus rotavirus-based quadrivalent and the M37 rotavirus vaccine candidates. J Med Virol. 1994;42(4):330–7. doi: 10.1002/jmv.1890420403. [DOI] [PubMed] [Google Scholar]
- Pesavento JB, Crawford SE, Estes MK, Prasad BV. Rotavirus proteins: structure and assembly. Curr Top Micro Immun. 2006;309:189–219. doi: 10.1007/3-540-30773-7_7. [DOI] [PubMed] [Google Scholar]
- Petterson EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera-A visualization system for exploratory research and analysis. J Comp Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Rao CD, Das M, Ilango P, Lalwani R, Rao BS, Gowda K. Comparative nucleotide and amino acid sequence analysis of the sequence-specific RNA-binding rotavirus nonstructural protein NSP3. Virology. 1995;207(1):327–33. doi: 10.1006/viro.1995.1087. [DOI] [PubMed] [Google Scholar]
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, Lopez P, Macias-Parra M, Ortega-Barria E, Richardson V, Rivera-Medina DM, Rivera L, Salinas B, Pavia-Ruz N, Salmeron J, Ruttimann R, Tinoco JC, Rubio P, Nunez E, Guerrero ML, Yarzabal JP, Damaso S, Tornieporth N, Saez-Llorens X, Vergara RF, Vesikari T, Bouckenooghe A, Clemens R, De Vos B, O'Ryan M. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- Santos N, Gouvea V, Timenetsky MC, Clark HF, Riepenhoff-Talty M, Garbarg-Chenon A. Comparative analysis of VP8* sequences from rotaviruses possessing M37-like VP4 recovered from children with and without diarrhoea. J Gen Virol. 1994;75(Pt 7):1775–80. doi: 10.1099/0022-1317-75-7-1775. [DOI] [PubMed] [Google Scholar]
- Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15(1):29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- Sukumaran M, Gowda K, Maiya PP, Srinivas TP, Kumar MS, Aijaz S, Reddy RR, Padilla L, Greenberg HB, Rao CD. Exclusive asymptomatic neonatal infections by human rotavirus strains having subgroup I specificity and “long” RNA electropherotype. Arch Virol. 1992;126(1-4):239–51. doi: 10.1007/BF01309698. [DOI] [PubMed] [Google Scholar]
- Tate J, Patel M, Steele AD, Gentsch J, Payne D, Cortese M, Nakagomi O, Cunliffe N, Jiang B, Neuzil K, deOliveira L, Glass R, Parashar U. Global impact of rotavirus vaccines. Expert Rev Vaccines. 2010;4:395–407. doi: 10.1586/erv.10.17. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CD, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Campens D, Karvonen A, Watt JP, O'Brien KL, DiNubile MJ, Clark HF, Boslego JW, Offit PA, Heaton PM. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Ruuska T, Koivu HP, Green KY, Flores J, Kapikian AZ. Evaluation of the M37 human rotavirus vaccine in 2- to 6-month-old infants. Pediatr Infect Dis J. 1991;10(12):912–7. doi: 10.1097/00006454-199112000-00006. [DOI] [PubMed] [Google Scholar]
- Ward RL. Possible mechanisms of protection elicited by candidate rotavirus vaccines as determined with the adult mouse model. Viral Immunol. 2003;16(1):17–24. doi: 10.1089/088282403763635410. [DOI] [PubMed] [Google Scholar]
- Wyatt RG, James HD, Jr, Pittman AL, Hoshino Y, Greenberg HB, Kalica AR, Flores J, Kapikian AZ. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983;18(2):310–7. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.