Abstract
This paper explores whether and how the behavioral impact of genotype disclosure can be disentangled from the impact of numerical risk estimates generated by genetic tests. Secondary data analyses are presented from a randomized controlled trial of 162 first-degree relatives of Alzheimer’s disease (AD) patients. Each participant received a lifetime risk estimate of AD. Control group estimates were based on age, gender, family history, and assumed ε4-negative apolipoprotein E (APOE) genotype; intervention group estimates were based upon the first three variables plus true APOE genotype, which was also disclosed. AD-specific self-reported behavior change (diet, exercise, and medication use) was assessed at 12 months. Behavior change was significantly more likely with increasing risk estimates, and also more likely, but not significantly so, in ε4-positive intervention group participants (53% changed behavior) than in control group participants (31%). Intervention group participants receiving ε4-negative genotype feedback (24% changed behavior) and control group participants had similar rates of behavior change and risk estimates, the latter allowing assessment of the independent effects of genotype disclosure. However, collinearity between risk estimates and ε4-positive genotypes, which engender high-risk estimates, prevented assessment of the independent effect of the disclosure of an ε4 genotype. Novel study designs are proposed to determine whether genotype disclosure has an impact upon behavior beyond that of numerical risk estimates.
Introduction
There are high expectations that using genotype to estimate the risk of common complex conditions will motivate health-related behavior change more strongly than other types of risk information (Collins et al., 2003; Gramling et al., 2003). Conversely, the detection of a genotype associated with a lowered risk of disease may lessen motivation to change behavior beyond the impact on motivation of the risk estimate associated with this genotype. Such expectations are consistent with the observations that the results of risk assessments that include genotype analyses are perceived as more accurate when a diagnosis is being confirmed (Marteau et al., 2004), and more reassuring when a risk conferring mutation is not found (La Russe et al., 2005). Such expectations are also consistent with theories of attitude change which predict that the greater the personal salience of information, the greater the impact (Chen and Chaiken, 1999).
In assessing risks of common complex conditions, a numerical risk estimate is often provided together with information about the presence or absence of a risk conferring mutation. So, for example, those undergoing a risk assessment for Crohn’s disease that includes a genetic test may be informed of their mutation status for CARD15 and of the likelihood that they will develop the disease (Lewis et al., 2007). Providing risk estimates of disease has a small, but significant, impact on risk perceptions (Slovic et al., 1980), which in turn have a small effect upon behavior to reduce the identified risk (Milne et al., 2000). It is therefore germane to consider the extent to which any motivating impact of genetic risk information is attributable to learning about the presence or absence of a risk conferring mutation or being given a numerical risk estimate of disease.
The few studies conducted in this area suggest that disclosure of genotypes indicating increased risk of disease are sometimes associated with an increased motivation to engage in behavior change (Lerman et al., 1997; Sanderson et al., 2008) but not always (McBride et al., 2002; Ito et al., 2006). Disclosure of the decreased risks associated with lower risk genotypes did not occur in two of the four trials designed to evaluate the motivational impact of DNA predictive testing for lung cancer in smokers (Lerman et al., 1997; McBride et al., 2002). There is, however, limited evidence to suggest that disclosure of such a genotype might reduce motivation beyond that following a lowered risk estimate (Marteau et al., 2005; Sanderson and Wardle, 2005). In each of these studies, in addition to genotype disclosure, participants were given a numerical risk estimate of the likelihood of developing lung cancer. The design of the studies was such that it was not possible to disentangle the effects of the numerical risk information from genotype disclosure. We report here secondary analysis from a randomized controlled trial in which we attempted to disentangle these effects, statistically.
The Risk Evaluation and Education for Alzheimer’s disease (REVEAL) study is a randomized controlled trial assessing the impact of genetic susceptibility testing and apolipoprotein E (APOE) disclosure on asymptomatic adult children of patients with Alzheimer’s disease (AD). Details of the study rationale, design, and main results have been published elsewhere (Green 2002; Cupples et al., 2004; Roberts et al., 2004; Marteau et al., 2005; Roberts et al., 2005). While there is no conclusive evidence that the risk of developing AD can be reduced by behavior change, diet, physical activity, and vitamin supplementation are under investigation for their potential to prevent or reduce risk of cognitive decline and dementias (Hendrie et al., 2006). One part of the trial assessed self-reported behavior change undertaken with the hope of reducing AD risk.
We have reported elsewhere the main analysis examining whether APOE disclosure motivates behavior change intended to reduce the risk of the disease (Chao et al., 2008). Adjusting for important baseline confounding variables (age, gender, years of education, and presence of a modifiable comorbidity), there was no effect on behavior change of the intervention group (all those whose genotype was disclosed whether positive or negative) compared with the control group (those whose genotype was not disclosed) (38% vs. 31%: adjusted odds ratio [OR] 1.45, 95% confidence interval [CI] [0.65, 3.21], p =0.36). Subgroup analyses showed that behavior change was more likely in those who were APOE ε4 positive than those who were APOE ε4 negative (53% vs. 24%: adjusted OR 2.9, 95% CI [1.19, 6.86], p =0.002). Behavior change was also more likely as lifetime risk estimates increased (per 1% increase in lifetime risk estimate: adjusted OR 1.05, 95% CI [1.01, 1.10], p =0.007). The aim of the secondary analyses reported in this paper was to explore whether and how the behavioral impact of DNA testing can be disentangled from the impact of the risk estimates generated by such tests.
We report here additional analyses of the REVEAL study data to test two hypotheses:
Hypothesis I
Given equivalent lifetime risk estimates, behavior change is more likely following the communication of DNA test results, indicating the presence of a genotype associated with increased risk than following risk communication when DNA testing is not performed.
Hypothesis II
Given equivalent lifetime risk estimates, behavior change is less likely following the communication of DNA test results, indicating the presence of a genotype associated with lowered risk than following risk communication when DNA testing is not performed.
Materials and Methods
Risk estimates
The methodology of the REVEAL clinical trial is described in detail elsewhere (Green, 2002; Roberts et al., 2004). We report here on details specifically relevant to this analysis. Prior to randomization, all participants attended an education session where they were informed about genetic susceptibility testing and told there was no proven preventive measure for AD. They were informed that while a number of interventions to prevent AD were under investigation, such as vitamin E, cholesterol lowering drugs, and mental stimulation, none was currently recommended. Eligible participants were randomized to either the intervention or the control groups in the ratio of 2:1 to achieve similarly sized groups for those testing positive and negative and for the control group (see Box 1 for REVEAL study design). Both groups received lifetime (up to age 85) risk estimates of AD based on gender, family history, and genotype, presented as a percentage (Cupples et al., 2004; Roberts et al., 2005). In addition, the intervention group had disclosed to them their APOE genotype. The intervention and control groups received their risk estimates during individual counseling sessions followed by a letter detailing the information presented.
Box 1. Summary of Design of the REVEAL Study.
| Group | Calculation of lifetime risk | Information made available to participants |
|
|---|---|---|---|
| Lifetime risk | APOE genotype | ||
| Intervention (n =103) | Based on gender, family history, and true APOE genotype (ε4 negative or ε4 positive) | Yes | Yes |
| Control (n =42) | Based on gender, family history, and assumed ε4-negative APOE genotype | Yes | No |
Intervention group
Lifetime risk assessments to the age of 85 were based upon age, gender, family history, and APOE genotype. Risk estimates were based on numerous sources, including a large-scale (n = ~13,000 families) study of the genetic epidemiology of AD; the generation of risk estimates used in the clinical trial are described in detail elsewhere (Cupples et al., 2004). In addition to being given their lifetime risk assessments, participants’ APOE genotype was disclosed.
Control group
Lifetime risk assessments to the age of 85 were based upon age, gender, family history, and an assumed ε4-negative genotype. As the most common genotype, APOE ε3/3, occurs in over 60% of the population (Farrer et al., 1997), the lifetime risk estimates given in the control group therefore resembled those given to participants who were tested and found to carry the APOE ε3/3 genotype.
Measures
The primary outcome in the analysis of behavioral change was a binary indicator of self-reported behavior change undertaken with the hope of preventing AD. Three questions were asked in which respondents were asked to indicate whether they had made changes in (a) their diet, (b) level of exercise, and (c) use of medication or vitamins, with the specific aim of preventing AD. Those reporting change at 12 months in one or more of diet, exercise level, and use of medication or vitamins were classified as having engaged in AD-specific behavior change. Those reporting no change in any of these were classified as not having engaged in AD-specific behavior change. Self-reported behavior change was relatively uncommon: 64% reported no change, 30% reported taking medication or vitamins with the aim of reducing their risks of AD, 13% reported changes to their diet, and 6% reported changes in exercise levels. As few participants reported two or more changes, responses were combined to produce a binary variable of behavior change (i.e., No or Yes).
Analysis
Multiple logistic regression was carried out to compare those receiving ε4-positive and ε4-negative test results with the control group, as appropriate, to test the two stated hypotheses. In an attempt to disentangle the effect upon behavior change of numerical lifetime risk estimate and APOE genotype, logistic regression models were fitted including either of these as independent variables. A combined model that included both APOE genotype and numerical lifetime risk estimates as independent variables was fitted subsequently to try to assess their independent effects, controlling for each other. All models were adjusted for age, gender, presence of a modifiable comorbidity, and number of years of education. Collinearity between genotype and risk estimate was assessed by Pearson’s correlation coefficient; principal components analysis of these two variables was used to attempt to address the effect of collinearity on the model.
All analyses were conducted using SPSS 12.0 for Windows (SPSS, Chicago, IL).
Results
One hundred and sixty-two participants were randomized. Seventeen participants were excluded due to drop out (n =14) or incomplete data at 1 year (n =3). The characteristics of the 145 participants included in the subsequent analyses are shown in Table 1.
Table 1.
Patient Characteristics
| All | Control | Intervention | ε4+ | ε4− | |
|---|---|---|---|---|---|
| Total number included in analysis | 145 | 42 | 103 | 49 | 54 |
| Age [years: mean (range)] | 53 (30–78) | 55 (37–78) | 52 (30–76) | 51 (34–72) | 53 (30–76) |
| Female gender | 107 (74%) | 34 (81%) | 73 (71%) | 40 (82%) | 33 (61%) |
| Years of education [mean (range)] | 17 (12–22) | 17 (12–21) | 17 (12–22) | 17 (12–21) | 17 (12–22) |
| Any modifiable comorbidity? % yes | 61 (42%) | 20 (48%) | 41 (40%) | 22 (45%) | 19 (35%) |
| Modeled % lifetime AD risk [mean (SD)] | 33 (13) | 27 (4) | 35 (14) | 48 (9) | 24 (5) |
| Behavior change at 12 months specific to AD prevention: % yes | 52 (36%) | 13 (31%) | 39 (38%) | 26 (53%) | 13 (24%) |
Seventeen patients excluded as follows: 15, no 1-year follow-up (8 control, 3 ε4+, 4 ε4−); 2, missing data (1 control, 1 ε4+).
Lifetime risk estimates in the different study groups are shown in Table 1 and Figure 1. Lifetime risks are similar in the ε4-negative and control groups, but higher for those who were tested and found to be ε4 positive.
FIG. 1.
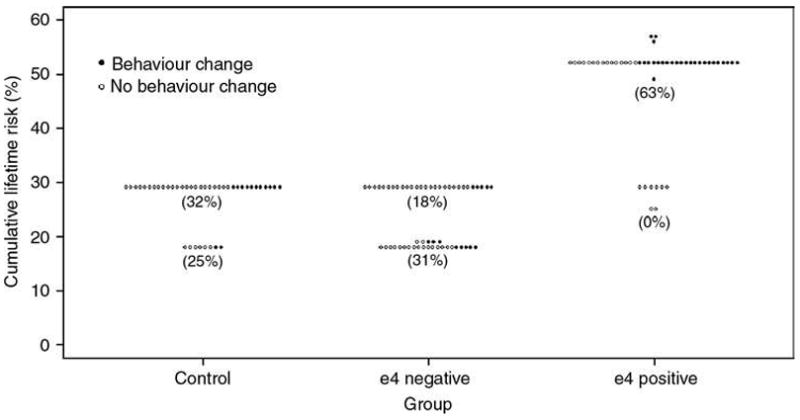
Cumulative lifetime risk estimates for Alzheimer disease by study group. Values in parentheses indicate percentages undergoing behavior change for given group/lifetime risk cross-classifications.
Hypothesis I
The rate of self-reported behavior change was higher, but not statistically significantly so, in the ε4 positive than in the control group (53% vs. 31%; adjusted OR =2.20, 95% CI [0.87, 5.56], p =0.10). Correspondingly, higher lifetime risk estimates were associated with significantly higher rates of self-reported behavior change (for an increase in lifetime risk estimate of 1%, adjusted OR =1.05, 95% CI [1.01, 1.09], p =0.02). There was high collinearity between group (ε4 positive or Control) and lifetime risk estimate (r =0.84, 95% CI [0.77, 0.89]), and very little overlap in the distributions of lifetime risk in these two groups (mean 48% in the ε4-positive group, 27% in control group, and 84% and 0% of participants in the respective groups having risk above 30%; Table 1 and Fig. 1). The OR for the former, adjusting for the latter, is required to test Hypothesis I. Because of the collinearity between the two, the least squares estimate of this OR was unstable, having opposite sign and considerably inflated standard error relative to that in the single-variable model; hence, it has not proved possible to test Hypothesis I. An attempt to re-fit the model by first conducting a principal components analysis on the two correlated variables, and then using the resulting orthogonal principal components as covariates, did not alter the result. If the collinearity were less marked, analysis of deviance could be used to compare models containing both variables with those containing only one.
Hypothesis II
Given that lifetime risk estimates were similar in the ε4-negative and control groups (Table 1), this Hypothesis could be tested by direct comparison between the proportions of participants reporting behavior change in these two groups. The rates of self-reported behavior change were similar in the ε4-negative and control groups (24% vs. 31%; adjusted OR =0.82, 95% CI [0.32, 2.11], p =0.68). We are unable to reject the null hypothesis: there is no evidence that behavior change is less likely following the communication of risk that incorporates analysis of DNA that is negative for a risk conferring mutation.
Discussion
Behavior change following risk assessment of AD was more likely the greater the numerical lifetime risk estimate and following disclosure of a genotype associated with increased risk of AD. We were not able to assess the effect of disclosing an ε4 genotype (relative to the control group) after controlling for numerical risk estimate. When numerical risk estimates were similar, as they were for those in the control group and for those in the intervention group informed they were ε4 negative, there was no difference in self-reported behavior change. This suggests that, contrary to predictions (Chen and Chaiken, 1999; Marteau and Senior, 2004; LaRusse et al., 2005), disclosure of genotype status associated with lower disease risk has no impact upon behavior beyond the impact of any associated numerical risk estimate.
The findings from these analyses illustrate a general problem in trying to isolate the motivational impact of genotype disclosure and indeed other biomarker risk information. The estimates derived from genotype disclosure differ from those derived from other sources both in provenance and the range of magnitudes of the risk estimated. So, for example, randomizing groups to undergo any additional biomarker test, in this case an analysis of APOE genotype, will result in a greater segregation of risk in those subjected to the additional biomarker, leading to the generation of both lower and higher risk magnitudes although overall the risk in the population tested remains the same. The interest, however, is in being able to disentangle the effects of type of test from numerical risk estimates to test the hypothesis that the salience of genotype has an impact on motivation beyond that produced by feedback of the risk estimates generated from genotype. Communicating the results of predictive genetic testing for common complex conditions is difficult, involving the communication of genotype and numerical risk estimates. If communicating genotype status has no motivating effect upon risk-reducing behavior beyond the motivating effect of disclosing the associated numerical risk estimate or if it has a demotivating effect (for example, if it instils a sense of fatalism), then it may be more effective and efficient to not disclose genotype but only the resultant numerical risk.
A further, more general problem with randomized trials designed to assess the behavioral impact of DNA predictive testing (McBride et al., 2002; Ito et al., 2006; Sanderson et al., 2008) is that the main comparison between the intervention and the control group is most often not informative. This is because the intervention group contains two subgroups—one of individuals receiving genotype-positive test results and one of individuals receiving genotype-negative test results. These different test results lead to higher and lower risk estimates that lead to higher and lower risk perceptions (Marteau et al., 2005). There is, therefore, an expectation that genotype-positive and genotype-negative test results will have opposite effects on behavior, and thus when the two subgroups are pooled to form a genotype feedback intervention group, there is unlikely to be any difference between this and a control group. The solution most often applied to this problem is to conduct subgroup analyses (McBride et al., 2002; Ito et al., 2006; Chao et al., 2008; Sanderson et al., 2008). However, given that subgroup allocation is not randomly determined, such comparisons are at risk of confounding (Pocock, 1983). They may also lack the statistical power to detect differences between subgroups.
The role of gender in explaining behavior change represents a further complexity in interpreting the results of the analyses presented in the current study. There is an association between gender and lifetime risk of AD (females tend to have higher risks than males) and, in this sample, between gender and genotype (a greater proportion of females were mutation positive). If behavior change is more likely in women undergoing AD risk assessment, then this may explain the higher rates of behavior change in those receiving higher lifetime risk estimates of AD and those who are mutation positive. While the effects of gender were controlled for in the analyses, the strengths of the gender variable’s associations with genotype and lifetime risk are such that we cannot be sure that some of the apparent effect of the latter pair of variables is not in part due to gender.
The solution to the problems of collinearity and subgroup analyses outlined above may be to consider alternative designs for studies of this type in preference to using increasingly complex methods of statistical analysis. We propose two possible designs. The first is to use explanatory as opposed to pragmatic trials (MacRae, 1989) in which the risk estimates given in the two trial arms are equivalent, but in only one arm is the provenance of the test revealed as emanating from genotypes. In the other arm the test could be described as an unspecified biomarker test or a test of protein. This would mean that both groups received comparable risk estimates allowing the variable of interest—namely, the provision of risks that stem from an analysis of genotype—to be assessed. While conceptually neat, this design raises questions concerning acceptability and feasibility. The acceptability would critically depend upon the views of clinical ethics committees reviewing such a study. The feasibility would be influenced by how plausible an unspecified bio-marker test would be for study participants. This would require piloting. In addition, further clinical studies assessing the behavioral impact of genotype feedback will provide stronger evidence if they include measures of actual behavior change.
The second type of design for addressing the problems of collinearity involves the use of analog studies, that is, those in which individuals are asked to respond as though they were in a particular situation. This allows variables of interest to be experimentally manipulated either prior to a clinical study or instead of one. While the internal validity of such studies is high, there is also an evidence that their external validity can be acceptable, provided the study mirrors closely the situation it is intended to mimic (Holt and Mazzuca 1992; Lanza et al., 1997) with greater validity likely with the use of video-based technologies (Lievens and Sackett, 2006). In the current context, an analog study might involve asking participants to imagine being given a lifetime risk for AD or indeed any other common complex disease. The risk estimate provided would then vary independently of the type of test and test result. So, for example, those given lifetime risk estimates of AD which were 35% would be randomly assigned to be told that this was based on their genotypes, or not. The outcome variables might include risk perceptions and intentions to engage in risk-reducing behaviors. Results from such studies provide an estimate of the extent to which cognitive and behavioral responses to risk information are predicted by risk estimations and type of test. The impact of describing test results as emanating from genotype-positive or genotype-negative test results could also be assessed by comparing the impact of presenting the risk estimate either with the genotype described as positive or negative, with the impact of leaving the genotype undisclosed. It should be noted, however, that even responses to hypothetical scenarios, however richly drawn, require validation in studies in which individuals respond to actual risk information, with behavior being measured objectively and not by self-report.
We are experimenting with both these designs in a series of studies investigating the motivational impact of DNA testing for common complex conditions (e.g., Wright et al., 2008).
If neither of these design options is possible, we would advocate opting for a design tailored to allow a test of the hypothesis in a subset of participants who have similar enough risk distributions in each study group to avoid collinearity. Pilot work could be used to assess the frequency distribution of risk in study groups and therefore the extent of collinearity, and through repeated simulation of the pilot data to identify a range of risk within which choices in study sample size and in optimal allocation ratio to trial arms provide reasonable power for an answer to the question in the restricted risk range. In the REVEAL study, the subset of ε4-positive participants with risk less than 30% was small (eight participants), the collinearity was very high, and the event rate was zero in this subset; this did not affect the ability to answer the primary trial questions, but did prohibit reliable estimation required to address one of the current hypotheses. Design options in future trials could be modified further to allow an assessment of both sets of hypotheses.
Concluding Comment
The collinearity between genotype and numerical lifetime risk estimates did not allow their independent effects to be assessed in those with a genotype associated with increased susceptibility. Novel study designs are needed to determine whether disclosure of genotype status has an impact upon behavior beyond that of the numerical risk estimates associated with them.
Acknowledgments
The GRaB (Genetic Risk and Behavior change) studies are funded by the Medical Research Council (G0500274). The REVEAL study was funded by National Institute of Health grants HG/AG02213 (The REVEAL study) and AG13846 (Boston University Alzheimer’s Disease Center).
References
- Chao S, Roberts JS, Marteau TM, et al. Health behaviour changes after genetic risk assessment for Alzheimer’s disease: The REVEAL Study. Alz Dis Assoc Dis. 2008;22:94–97. doi: 10.1097/WAD.0b013e31815a9dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Chaiken S. The heuristic-systematic model in its broader context. In: Chaiken S, Trope Y, editors. Dual Process Theories in Social Psychology. New York: Guilford Press; 1999. pp. 73–96. [Google Scholar]
- Collins FS, Green ED, Guttmacher AE, Guyer MS. A vision for the future of genomics research: a blueprint for the genomic future. Nature. 2003;422:835–847. doi: 10.1038/nature01626. [DOI] [PubMed] [Google Scholar]
- Cupples LA, Farrer LA, Sadovnick AD, et al. Estimating risk curves for first-degree relatives of patients with Alzheimer’s disease: The REVEAL study. Genet Med. 2004;6:192–196. doi: 10.1097/01.gim.0000132679.92238.58. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, et al. Effects of age, gender and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Gramling R, Nash J, Siren K, Culpepper L. Predictive genetics in primary care: expectations for the motivational impact of genetic testing affects the importance family physicians place on screening for familial cancer risk. Genet Med. 2003;5:172–175. doi: 10.1097/01.GIM.0000068986.03217.BB. [DOI] [PubMed] [Google Scholar]
- Green RC. Genetic testing for Alzheimer’s disease: has the moment arrived? Alzheimers Care Q. 2002;3:208–214. [Google Scholar]
- Hendrie HC, Albert MS, Butters MA, et al. The NIH Cognitive and Emotional Health Project. Report of the Critical Evaluation Study Committee. Alzheimers Dement. 2006;2:12–32. doi: 10.1016/j.jalz.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Holt WM, Mazzuca SA. A written case simulation of osteoarthritis as a predictor of prescribing behavior among family practitioners. Acad Med. 1992;67:414–415. doi: 10.1097/00001888-199206000-00020. [DOI] [PubMed] [Google Scholar]
- Ito H, Matsuo K, Wakai K, et al. An intervention study of smoking cessation with feedback on genetic cancer susceptibility in Japan. Prev Med. 2006;4:102–108. doi: 10.1016/j.ypmed.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Lanza ML, Carifio J, Pattison I, Hicks C. Validation of a vignette simulation of assault on nurses by patients. Image J Nurs Sch. 1997;29:151–154. doi: 10.1111/j.1547-5069.1997.tb01548.x. [DOI] [PubMed] [Google Scholar]
- LaRusse S, Roberts JS, Marteau TM, et al. Genetic susceptibility testing versus family history based risk assessment: impact on perceived risk of Alzheimer’s disease. Genet Med. 2005;7:48–53. doi: 10.1097/01.gim.0000151157.13716.6c. [DOI] [PubMed] [Google Scholar]
- Lerman C, Gold K, Audrain J, et al. Incorporating bio-markers of exposure and genetic susceptibility into smoking cessation treatment: effects on smoking-related cognitions, emotions, and behavior change. Health Psychol. 1997;16:87–99. doi: 10.1037//0278-6133.16.1.87. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Whitwell SCL, Forbes A, et al. Estimating risks of common complex diseases across genetic and environmental factors: the example of Crohn’s disease. J Med Genet. 2007;44:689–694. doi: 10.1136/jmg.2007.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens F, Sackett PR. Video-based versus written situational judgment tests: a comparison in terms of predictive validity. J Appl Psychol. 2006;91:1181–1188. doi: 10.1037/0021-9010.91.5.1181. [DOI] [PubMed] [Google Scholar]
- MacRae KD. Pragmatic versus explanatory trials. Int J Technol Assess Health Care. 1989;5:333–339. doi: 10.1017/s0266462300007406. [DOI] [PubMed] [Google Scholar]
- Marteau TM, Roberts S, LaRusse S, Green RC. Predictive genetic testing for Alzheimer’s disease: impact upon risk perception. Risk Anal. 2005;5:297–404. doi: 10.1111/j.1539-6924.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- Marteau TM, Senior V, Humphries S, et al. Psychological impact of genetic testing for familial hypercholesterolemia in a previously aware population: a randomized controlled trial. Am J Med Genet. 2004;128A:285–293. doi: 10.1002/ajmg.a.30102. [DOI] [PubMed] [Google Scholar]
- McBride DM, Bepler G, Lipkus IM, et al. Incorporating genetic susceptibility feedback into a smoking cessation program for African-American smokers with low income. Cancer Epidemiol Biomarkers Prev. 2002;11:521–528. [PubMed] [Google Scholar]
- Milne S, Sheeran P, Orbell S. Prediction and intervention in health-related behavior: a meta-analytic review of protection motivation theory. J Appl Soc Psychol. 2000;30:106–143. [Google Scholar]
- Pocock SJ. Clinical trials: A practical approach. John Wiley & Sons; New York: 1983. [Google Scholar]
- Roberts JS, Barber M, Brown TM, et al. Who seeks genetic susceptibility testing for Alzheimer’s disease? Findings from a multisite, randomized clinical trial. Genet Med. 2004;6:197–203. doi: 10.1097/01.gim.0000132688.55591.77. [DOI] [PubMed] [Google Scholar]
- Roberts JS, Cupples LA, Relkin NR, et al. Genetic risk assessment for adult children of people with Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2005;18:250–255. doi: 10.1177/0891988705281883. [DOI] [PubMed] [Google Scholar]
- Sanderson S, Humphries S, Hubbart C, et al. Psychological and behavioural impact of genetic testing smokers for lung cancer risk: a Phase II exploratory trial. J Health Psychol. 2008;13:481–494. doi: 10.1177/1359105308088519. [DOI] [PubMed] [Google Scholar]
- Sanderson SC, Wardle J. Will genetic testing for complex diseases increase motivation to quit smoking? Anticipated reactions in a survey of smokers. Health Educ Behav. 2005;32:640–653. doi: 10.1177/1090198105278756. [DOI] [PubMed] [Google Scholar]
- Slovic P, Fischhoff B, Lichenstein S. Facts and fears: understanding perceived risk. In: Schwing RC, Albers WA, editors. Societal Risk Assessment: How Safe is Safe Enough? New York: Plenum Press; 1980. pp. 181–216. [Google Scholar]
- Wright A, Takeichi C, Whitwell SCL, et al. The impact of genetic testing, for Crohn’s disease, risk magnitude and graphical format on motivation to stop smoking: An experimental analogue study. Clin Genet. 2008;73:306–314. doi: 10.1111/j.1399-0004.2008.00964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


